Acotiamide hydrochloride sustained-release pellet and preparation method thereof
A technology of acotiamide hydrochloride and sustained-release pellets, which is applied in the field of medicine, can solve the problems of high incidence of adverse reactions, large fluctuations in blood drug concentration, and many times of taking drugs, and achieve small differences in individual bioavailability and reduce Dosing frequency and effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] formula:
[0065] Ball core: acotiamide hydrochloride 2g, microcrystalline cellulose 80g, lactose 18g, hypromellose 3g.
[0066] Coating ingredients: EudragitNE30D5g, PEG60001g, triethyl citrate 0.2g, talc powder 0.5g, titanium dioxide 0.1g.
[0067] Preparation method: take acotiamide hydrochloride, microcrystalline cellulose, and lactose, pass through a 100-mesh sieve, mix evenly, add hypromellose to make a soft material, and then move the soft material into an extrusion machine to extrude into a high-quality Density strips, and finally in the centrifugal spheroidization machine, the strips are broken into granules and rounded to make pellets, which are coated with coating ingredients and finally packed into bags. Get the prepared acotiamide hydrochloride sustained-release assay pellets with a diameter of ≤2.5mm.
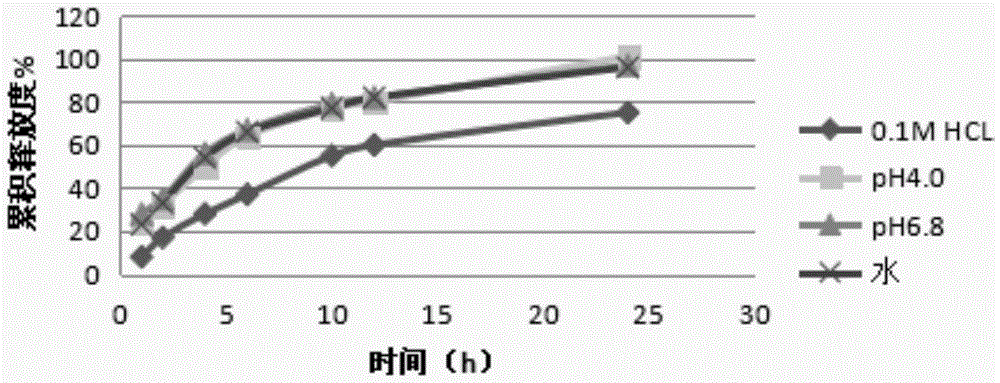
[0068] Get the prepared acotiamide hydrochloride sustained-release pellets, measure the release curve in water, 0.1M hydrochloric acid solution, pH4.0 ca...
Embodiment 2
[0071] formula:
[0072] Ball core: acotiamide hydrochloride 10g, microcrystalline cellulose 70g, lactose 18g, hypromellose 4g.
[0073] Coating ingredients: Eudragit NE30D 5g, PEG60001g, triethyl citrate 0.2g, talc powder 1g, titanium dioxide 0.1g.
[0074] Preparation method: Take acotiamide hydrochloride, microcrystalline cellulose, and lactose and pass through a 100-mesh sieve, mix evenly, and use hypromellose solution as a binder in a centrifugal coating granulator to make pellets , coat the pellets with coating ingredients, and finally pack into capsules. Get the prepared acotiamide hydrochloride sustained-release assay pellets with a diameter of ≤2.5mm.
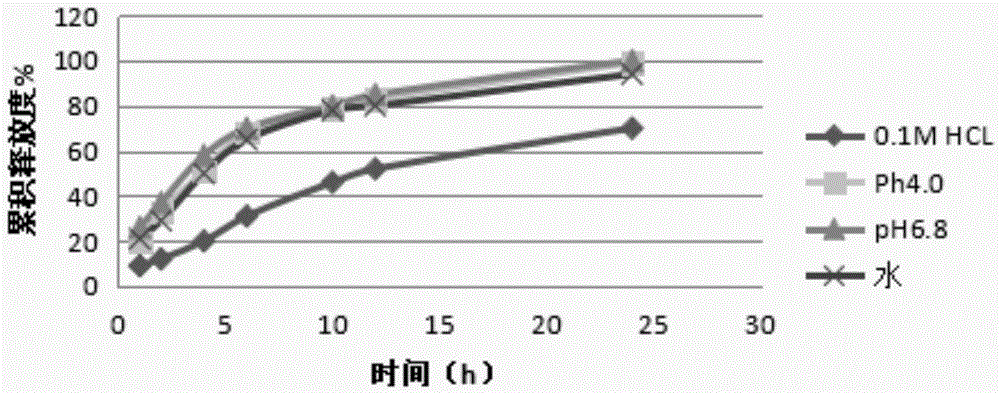
[0075] Get the prepared acotiamide hydrochloride sustained-release pellets, measure the release curve in water, 0.1M hydrochloric acid solution, pH4.0 carbonate buffer, pH6.8 phosphate buffer respectively, the results are shown in figure 2 .
[0076] The results showed that the prepared acotiamide hydrochloride su...
Embodiment 3
[0078] formula:
[0079] Ball core: 20g of acotiamide hydrochloride, 70g of microcrystalline cellulose, 10g of lactose, 4g of hypromellose. Coating ingredients: 7 g of ethyl cellulose, 300.5 g of povidone K, 0.3 g of triethyl citrate, 0.8 g of talc, and 0.2 g of titanium dioxide.
[0080] Preparation method: take acotiamide hydrochloride, microcrystalline cellulose, and lactose, pass through a 100-mesh sieve, mix evenly, add hypromellose to make a soft material, and then move the soft material into an extrusion machine to extrude into a high-quality Density strips, and finally in the centrifugal spheroidization machine, the strips are broken into granules and rounded to make pellets, which are coated with coating ingredients, and finally loaded into capsules. Get the prepared acotiamide hydrochloride sustained-release assay pellets with a diameter of ≤2.5mm.
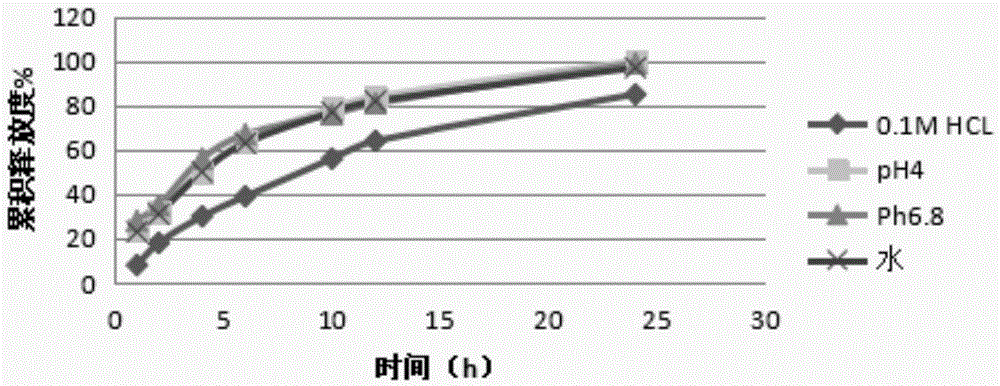
[0081] Get the prepared acotiamide hydrochloride sustained-release pellets, measure the release curve in water, 0.1M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com