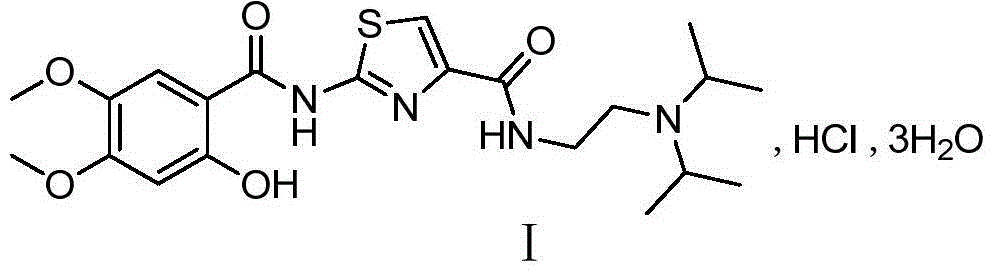
Preparation method of acotiamide hydrochloride intermediate
A technology for acotiamide hydrochloride and intermediates, which is applied in the field of preparation of acotiamide hydrochloride intermediates, can solve the problems of low overall yield, cumbersome post-processing, difficult to scale up production and the like, and achieves good yield and high purity , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
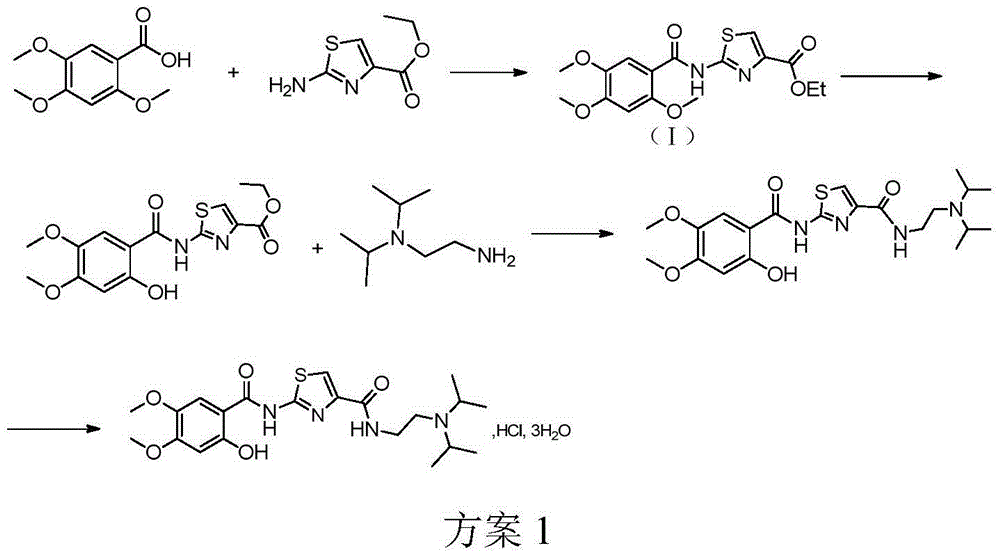
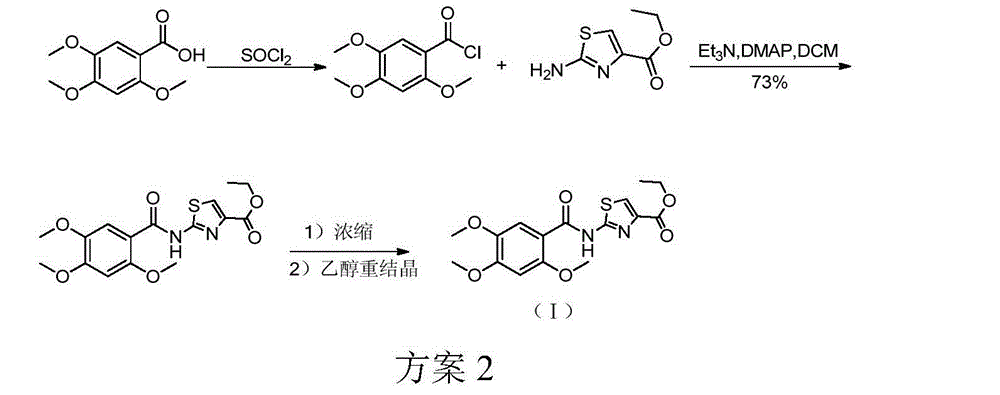
[0023] Example 1: Preparation of 2-[N-(2,4,5-trimethoxybenzoyl)amino]-1,3-thiazole-4-carboxylic acid ethyl ester
[0024] Put 2,4,5-trimethoxybenzoic acid 400g (1.887mol), TBTU664g (2.069mol), DIEA685ml, toluene 4L, 2-aminothiazole-4-ethyl carboxylate 340g (1.977mol) into a 10L reaction flask in sequence , heating and stirring the reaction solution to reflux state. TLC detected that the reaction of the raw materials was basically complete (about 8h), the heating was stopped, the reaction solution was stirred and cooled to room temperature (25°C) naturally. 4L of ethanol was added to the reaction solution, slurried for 2 hours, filtered, and the filter cake was washed with ethanol and dried at 45°C overnight (about 12 hours) to obtain 530 g of an off-white solid with a molar yield of 76%.
[0025] 1 H NMR (400MHz, CDCl 3 ):δ=1.29-1.32(t,J=7.2Hz,3H),3.77(s,3H),3.91(s,3H),4.01(s,3H),4.29(q,J=7.6Hz,2H) ,6.85(s,1H),7.40(s,1H),8.09(s,1H),11.66(s,1H);
[0026] 13 C NMR (100MHz)...
Embodiment 2
[0029] Example 2: Preparation of 2-[N-(2,4,5-trimethoxybenzoyl)amino]-1,3-thiazole-4-carboxylic acid ethyl ester
[0030] 5.3 g (0.025 mol) of 2,4,5-trimethoxybenzoic acid, 5.0 g (0.03 mol) of CDI, 1.9 g of DBU, 53 mL of toluene, and 4.3 g of ethyl 2-aminothiazole-4-carboxylate were put into 250 mL of The reaction flask was stirred at 90°C. TLC detected that the reaction of the raw materials was basically complete (about 8h), the heating was stopped, the reaction solution was stirred and cooled to room temperature (25°C) naturally. 53 mL of ethanol was added to the reaction solution, slurried for 2 hours, filtered, and the filter cake was washed with ethanol, and dried at 45°C overnight (about 12 hours) to obtain an off-white solid with a molar yield of 62%.
[0031] The resulting product was determined to be the same as Example 1 through the analysis of the structure of the spectrum.
Embodiment 3
[0032] Example 3: Preparation of 2-[N-(2,4,5-trimethoxybenzoyl)amino]-1,3-thiazole-4-carboxylic acid ethyl ester
[0033] 5.3 g (0.025 mol) of 2,4,5-trimethoxybenzoic acid, 5.0 g (0.03 mol) of CDI, 1.9 g of DBU, 53 mL of toluene, and 4.3 g of ethyl 2-aminothiazole-4-carboxylate were put into 250 mL of The reaction flask was heated and stirred to reflux. TLC detected that the reaction of the raw materials was basically complete (about 8h), the heating was stopped, the reaction solution was stirred and cooled to room temperature (25°C) naturally. 53 mL of ethanol was added to the reaction solution, slurried for 2 hours, filtered, the filter cake was washed with ethanol, and dried at 45°C overnight (about 12 hours) to obtain an off-white solid with a molar yield of 67%.
[0034] The resulting product was determined to be the same as Example 1 through the analysis of the structure of the spectrum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com