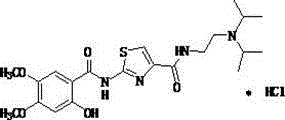
Preparation method of acotiamide hydrochloride
A technology of acotiamide hydrochloride and alkyl formate, which is applied in the field of preparing acotiamide hydrochloride, and can solve the problems of non-utilization of industrialized production, danger, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0021] Synthesis of 2-hydroxy-4,5-dimethoxybenzoyl chloride (compound of formula I)
[0022] Take 19.8g (0.1mol) of 2-hydroxy-4,5-dimethoxybenzoic acid in a three-necked flask (with thermometer, drying tube and tail gas absorption device), then add 50ml of thionyl chloride to it, and heat to reflux After 3 hours, TLC tracking showed that the reaction was complete, and the excess thionyl chloride was distilled off. After post-treatment, 18.8 g of the product 2-hydroxy-4,5-dimethoxybenzoyl chloride was obtained, and the yield was about 86.8%.
Embodiment 1b
[0024] Synthesis of 2-hydroxy-4,5-dimethoxybenzoyl chloride (compound of formula I)
[0025] Take 19.8g (about 0.1mol) of 2-hydroxy-4,5-dimethoxybenzoic acid in a three-necked flask (with thermometer, drying tube and tail gas absorption device), and then add 50ml of thionyl chloride and tetrahydrofuran to it (THF) 1ml, DMF0.05ml was added dropwise and heated to reflux for 2.5h, followed by TLC, the reaction was complete, the excess thionyl chloride was distilled off, and the product 2-hydroxy-4,5-dimethoxybenzoyl chloride was obtained after post-treatment. 20.1 g, about 92.8% yield.
Embodiment 2a
[0027] Synthesis of ethyl 2-[(2-hydroxy-4,5-dimethoxybenzoyl)amino]-1,3-thiazole-4-carboxylate
[0028] Take 21.7g (about 0.1mol) of 2-hydroxy-4,5-dimethoxybenzoyl chloride prepared according to the method of Example 1a in a three-necked flask, add 17.2g (about 0.1mol) of 2-aminothiazole-4 -Ethyl formate and 100ml of dichloromethane, heated to reflux, reacted for 4h. After the reaction, the solvent was evaporated to dryness, and 70ml of methanol was added to reflux and suction filtered to obtain about 32.6g of solid, with a yield of 92.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com