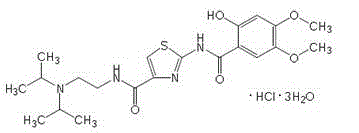
Acotiamide compound
A technology of acotiamide hydrochloride and water compound, which is applied in the application field of acotiamide hydrate and its preparation, and medicine, and can solve the problems of uncharacterized crystal structure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add 60 grams of acotiamide hydrochloride and 300 milliliters of water to a 500-ml reaction flask equipped with stirring, a thermometer and a condenser, add 0.5 grams of dimethylformamide (DMF) to the above aqueous solution, and stir for 30 minutes. Filter and cool the filtrate to 13°C for later use.
[0053] Cool 1150ml of methyl ethyl ketone-ethanol=5:5 mixture to 13°C, add the above standby solution while stirring, keep it warm for 18 hours, crystals precipitate out, filter, and dry to obtain 54.3 grams of white crystals. Measured 3 times by Karl Fischer method, get the average value, contain 8.93% (weight percent) moisture. Purity 99.9% (HPLC normalization method), optical purity 99.96% ee (chiral HPLC).
[0054] Elemental Analysis Results:
[0055] Measured value (calculated value), C: 47.15(47.10), H: 6.94(6.92), N: 10.45(10.47),
[0056] S: 5.95 (5.98), Cl: 6.60 (6.64)
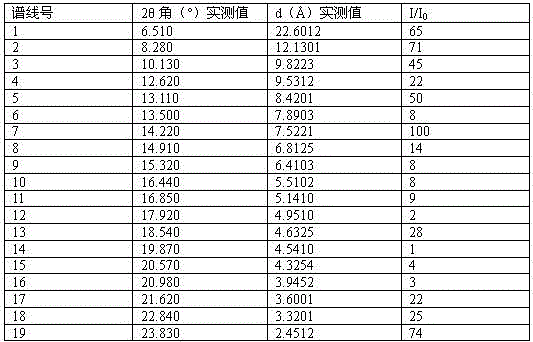
[0057] X-ray powder diffraction characteristic absorption peaks (2θ) and D values are as...
Embodiment 2
[0062] Tablets containing acotiamide hydrochloride trihydrate
[0063] Prescription: 20 grams of acotiamide hydrochloride trihydrate, 100 grams of lactose, 36 grams of corn starch, 30 grams of crystalline cellulose, 10 grams of carboxypropyl cellulose, and 4 grams of magnesium stearate.
[0064] The ingredients described above were uniformly mixed, and the mixture was formed into 100 mg / tablet tablets with a single punch tablet machine through a hole of 9.1 mm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com