Patents
Literature
161results about How to "Avoid sudden release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Photosensitive PRP (platelet-rich plasma) gel and preparation method and application thereof
ActiveCN106822183ATightly boundSeamless integrationSurgical adhesivesAerosol deliveryChemical reactionBlood plasma
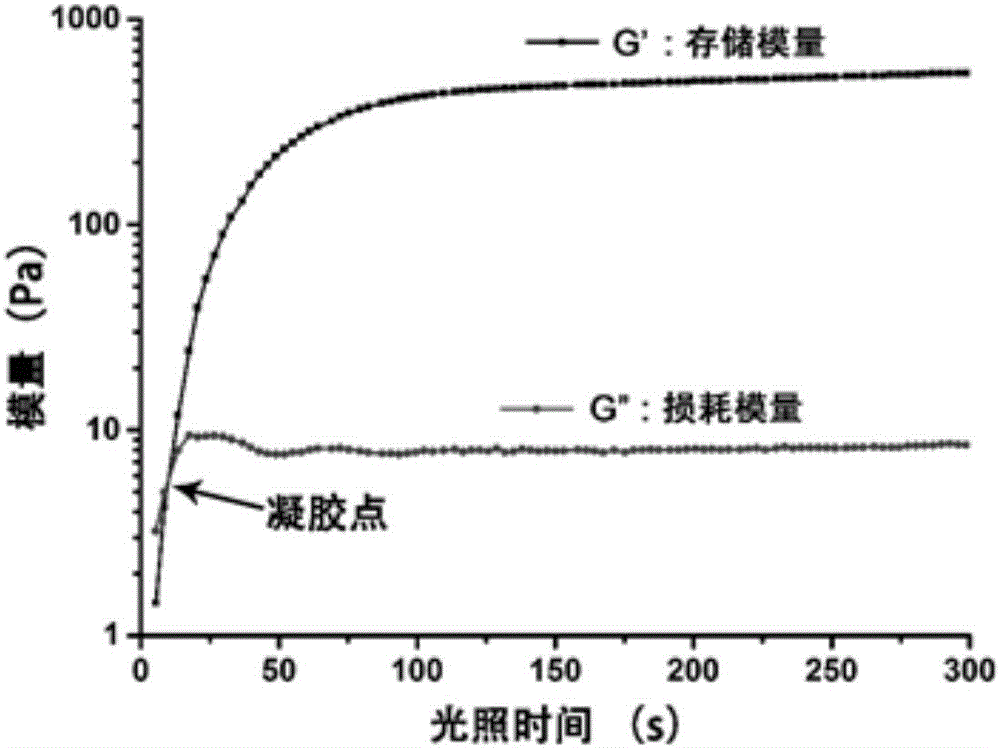
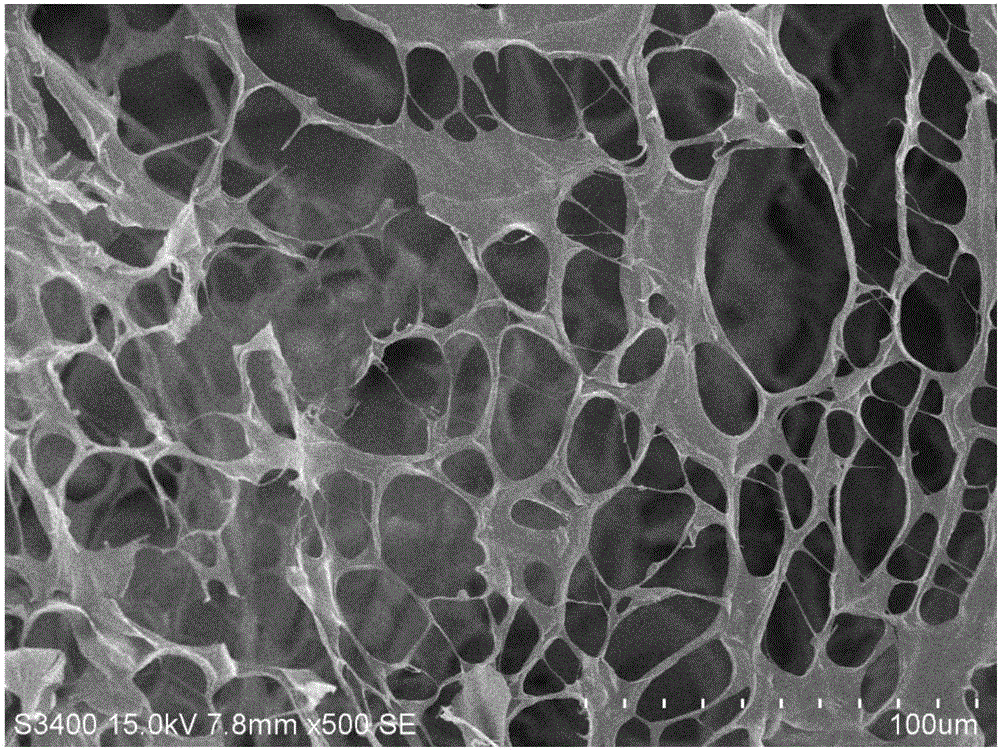
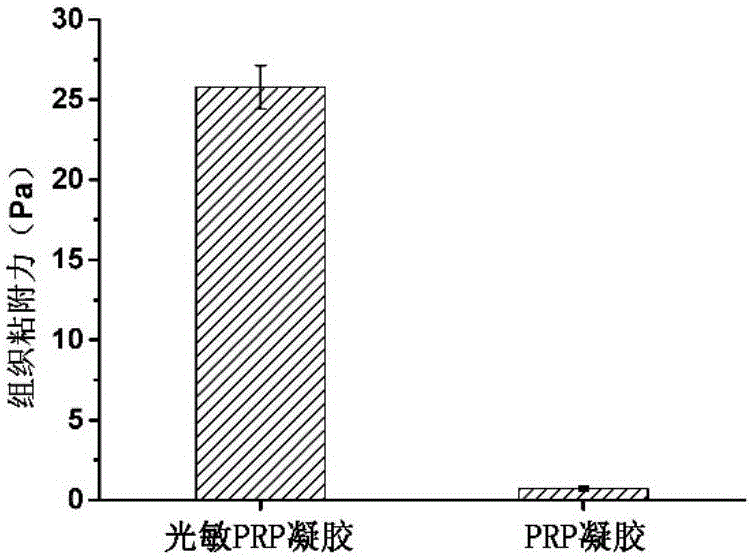
The invention relates to a photosensitive PRP (platelet-rich plasma) gel and a preparation method and application thereof. The preparation method comprises the following steps of mixing a biocompatible medium solution of a macromolecule modified by an o-nitrobenzyl photo response group and an extracted PRP according to a certain ratio, so as to form a gel precursor solution; then, radiating the gel precursor solution by light, and enabling the o-nitrobenzyl group in the macromolecule modified by the o-nitrobenzyl photo response group to generate photochemical reaction under the excitation action of a light source, so as to produce an aldehyde functional group; generating coupling reaction with amino distributed at the surface of the protein in the PRP to form an imine bond, so as to realize the preparation of the photosensitive PRP gel. Compared with the prior art, the photosensitive PRP gel prepared by the method has the advantages that the internal cell factors can be slowly released, and the tight and seamless bonding between the PRP gel and wounds is realized.
Owner:ZHONGSHAN GUANGHE MEDICAL TECH CO LTD
Cisplatin complex and preparation method thereof
ActiveCN102863627AGood biocompatibilityGood solubilityHeavy metal active ingredientsPharmaceutical non-active ingredientsSide chainSolubility
The invention provides a cisplatin complex which is formed by complexing cisplatin with polymer with a structure as a formula (I), and further provides a preparation method of the cisplatin complex. The cisplatin and the polymer with the structure as the formula (I) are in complex reaction in aqueous media to generate the cisplatin complex. The cisplatin complex has fine biocompatibility and is degradable, and polyethylene glycol is grafted on a side chain of the polymer, so that the prepared cisplatin complex has fine dissolvability. When the cisplatin complex is dissolved in the aqueous media, the cisplatin is protected by a hydrophilic polyethylene glycol chain segment and a hydrophobic amino acid chain segment, and accordingly sudden release of cisplatin caused by influences of a blood circulation system after intravenous injection can be effectively avoided, and stability of the cisplatin complex is improved. In addition, carboxyl contained in the cisplatin complex has pH sensitivity and deprotonation tendency in a low-pH environment, medicine release is facilitated, and medicine curative effect can be improved.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
A covering assembly for a seat and seat adapted for protecting a user
ActiveCN102822015APrevent exitAvoid sharpVehicle seatsPedestrian/occupant safety arrangementBraced frameEngineering
A covering assembly for a seat, including a cover adapted to cover a support frame of the seat and an inflatable member adapted to assume a resting deflated condition and an active inflated condition is described. The inflatable member is located on a side of the cover intended to be faced towards the support frame. The cover is adapted to contain the inflatable member both in the deflated condition and in the inflated condition.
Owner:DAINESE
Core-shell type pre-charged chemotherapy medicine embolization microsphere and preparation method thereof
InactiveCN108721684ATargetedWith controlled releaseSurgical adhesivesTissue regenerationEmulsionArterial Embolization
The invention provides a core-shell type pre-charged chemotherapy medicine embolization microsphere and a preparation method thereof. The method comprises the following steps of using water / oil / water(W / O / W) complex emulsion type or oil / water / oil (O / W / O) complex emulsion type emulsified micro liquid drips as templates; coating tiny liquid drips containing medicine into outer layer liquid drips; high molecular polymers in the inner and outer layer liquid drips are converted through measures such as physical measures and chemical measures to form an inner layer core and an outer layer shell. Theformed core-shell structure medicine microsphere is formed by polymers of two-layer structures; the inner core contains medicine; the outer shell is favorable for maintaining the activity of the inner core medicine; the stable medicine release is realized; the sudden release is prevented; the safety is enhanced. The medicine carried microsphere prepared by the method has the advantages that the particle diameter range is 10 to 1000 micrometers; the sphere degree is good; the particle diameter is uniform; (the dimension deviation is 5 percent); the medicine encapsulation rate is high; the structure / carried medicine is controllable; meanwhile, the preparation method of the pre-charged chemotherapy medicine embolization microsphere can provide a novel idea for the clinic application study ofthe transcatheter arterial embolization.
Owner:ENERGY RES INST OF SHANDONG ACAD OF SCI
Cholesterol modified amphiphilic pH response pennicuius copolymer as well as preparation and micelle of copolymer
ActiveCN104231155AQuick releaseControlled releaseOrganic active ingredientsPharmaceutical non-active ingredients(Hydroxyethyl)methacrylateCholesterol
The invention belongs to the technical field of preparation of biomedical high molecular polymer materials and discloses a cholesterol modified amphiphilic pH response pennicuius copolymer and a preparation method and a micelle system prepared based thereof. The copolymer has a structure shown in the formula I in the specification, wherein x is 10-36, y is 15-40 and z is 8-30. The copolymer is obtained by virtue of irregular copolymerization of a hydrophilic block hydroxyethyl methylacrylate, hydrophobic cholesterol and pH response block methacrylic acid N, N-diethyl aminoethyl combined with hydrophilic poly(ethylene glycol) methyl ether methacrylate. The copolymer is dissolved in a solvent to obtain a nanoscale micelle system, wherein the inner layer is a cholesterol modified hydrophobic chain segment, the middle layer is a pH response chain segment and the shell is a hydrophilic chain segment, so that the function of high entrapment performance, stable existence of a neutral condition and quick release in a weak acidic condition is achieved. By adjusting the proportions of the blocks in the polymer, the release rates of medicines can be regulated to satisfy the release requirements on different drugs.
Owner:SOUTH CHINA UNIV OF TECH
Ticagrelor solid dispersion and preparation method thereof
ActiveCN104434805ASolve insolubleEvenly dispersedOrganic active ingredientsPowder deliveryPolyvinylpyrrolidoneChemistry
A ticagrelor solid dispersion and preparation method thereof; the solid dispersion is prepared via dispersing the ticagrelor into a carrier material, the carrier material comprising one or more of polyvinylpyrrolidone, copovidone and crospovidone.
Owner:SUNCADIA PHARM CO LTD +1
Water-retention and slow-release fertilizer based on coal gangue micron-grade hollow sphere and preparation method of fertilizer
InactiveCN103570463APromote sustainable developmentLarge drug loading capacity in the cavityFertilizer mixturesOrganic fertilizerMaterials science
The invention discloses water-retention and slow-release fertilizer based on a coal gangue micron-grade hollow sphere and a preparation method of the fertilizer. The novel water-retention and slow-release fertilizer based on the coal gangue micron-grade hollow sphere consists of a coal gangue hollow sphere shell layer and a fertilizer core layer, wherein the shell layer is a coal gangue micron-grade hollow sphere or a coal gangue micron-grade hollow sphere coated with an organic coating material on the surface, and the core layer consists of a fertilizer material or a composite material simultaneously containing organic fertilizer and a water-retention material. The material of the core layer is dissolved in water, and is loaded in a hollow cavity of the coal gangue hollow sphere through vacuum, ultrasonic or pressurized impregnation; and the organic coating material covers the coal gangue hollow sphere to seal a shell porous structure, so as to enhance a slow-release effect. The water-retention and slow-release fertilizer disclosed by the invention obviously enhances slow-release performance of the fertilizer, improves utilization rate of the fertilizer and makes full use of solid waste coal gangue, and has relatively strong water-absorption and water-retention properties; and the fertilizer is low in cost, simple in process and environment-friendly and has wide application prospect.
Owner:TSINGHUA UNIV
Growth factor porous micro-sphere compound system coated by injectable hydrogel
InactiveCN105288594AHigh drug loadingImprove stabilityNervous disorderPeptide/protein ingredientsCompound systemHyaluronic acid
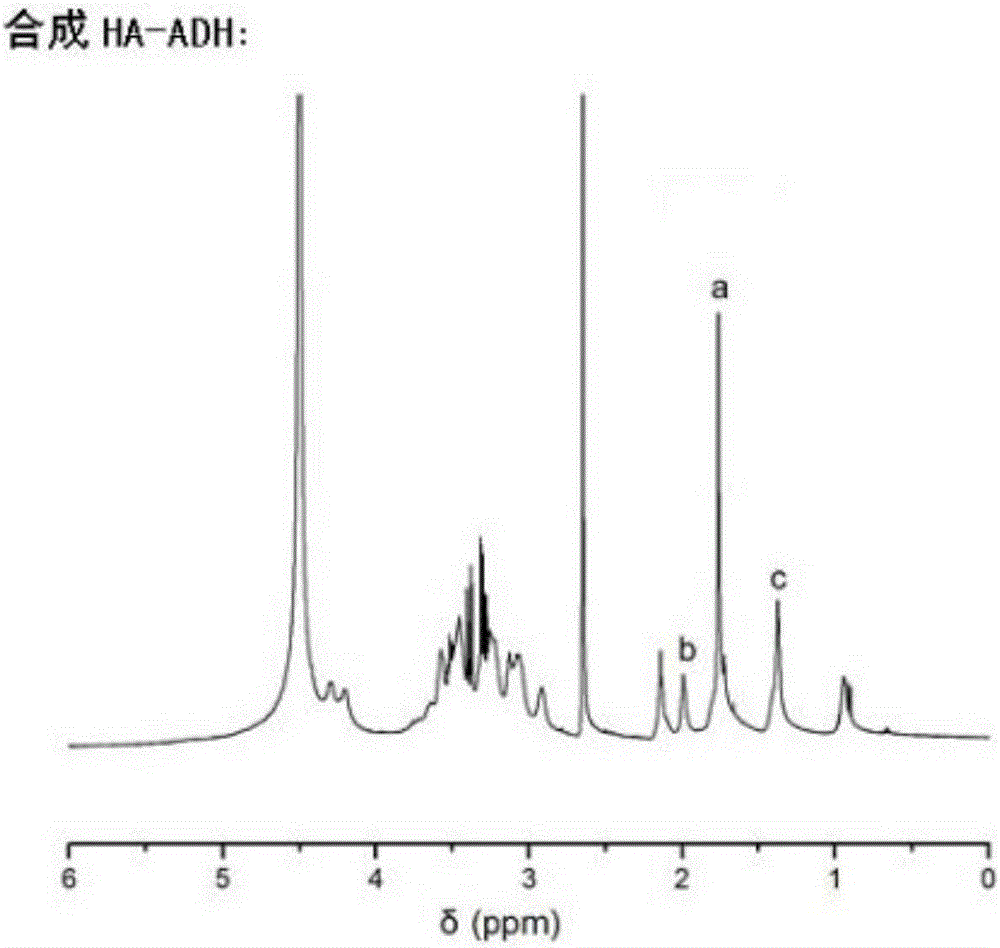
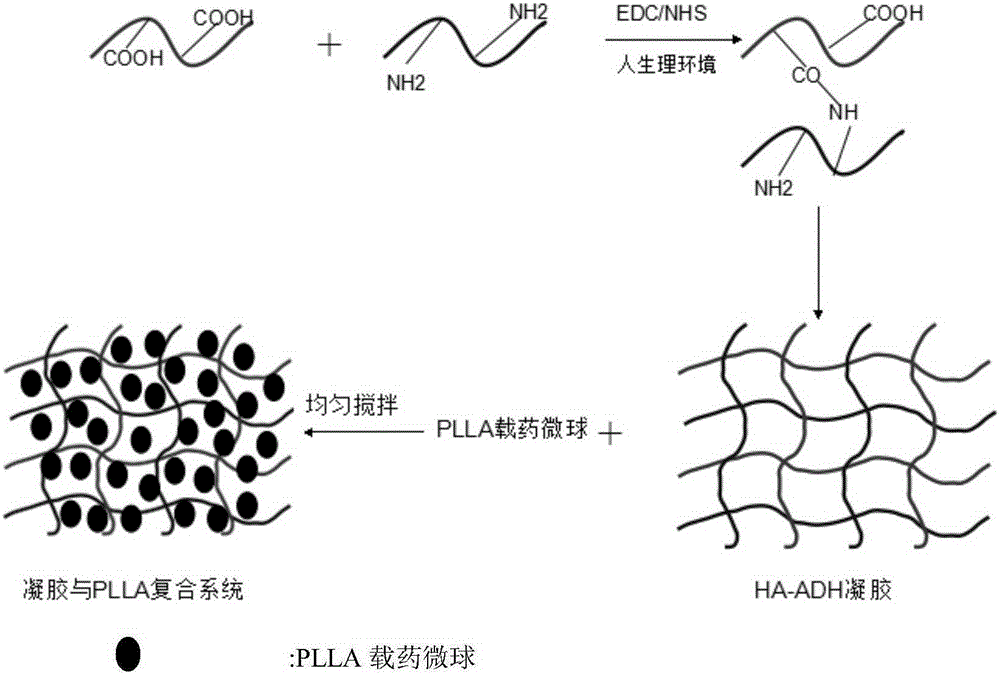
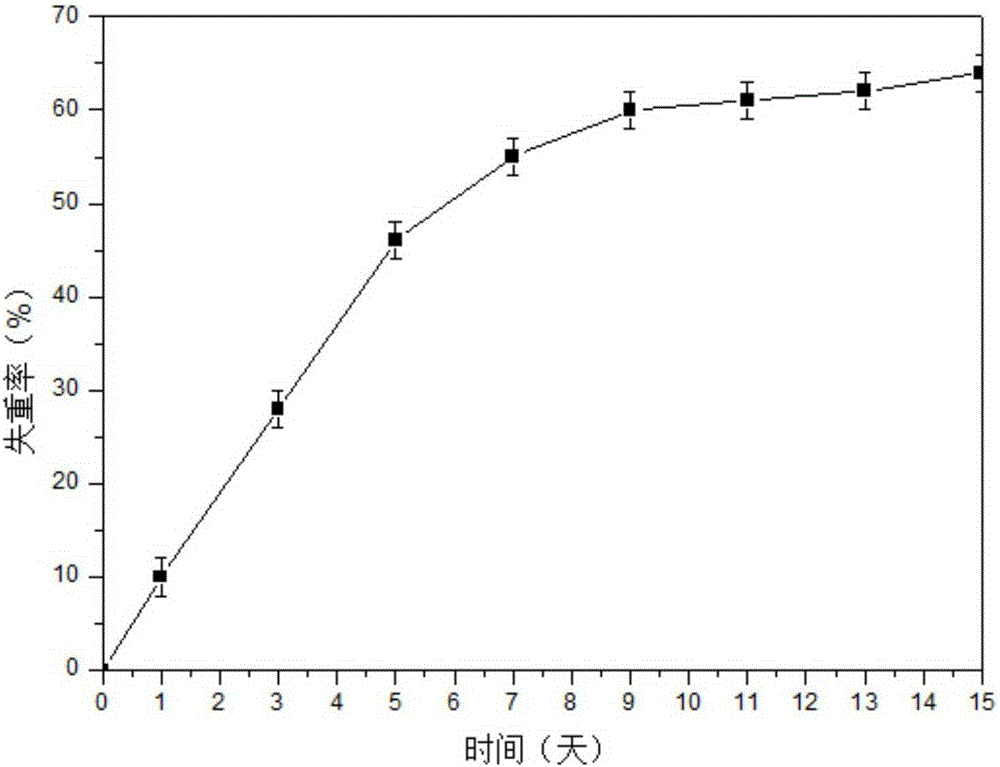
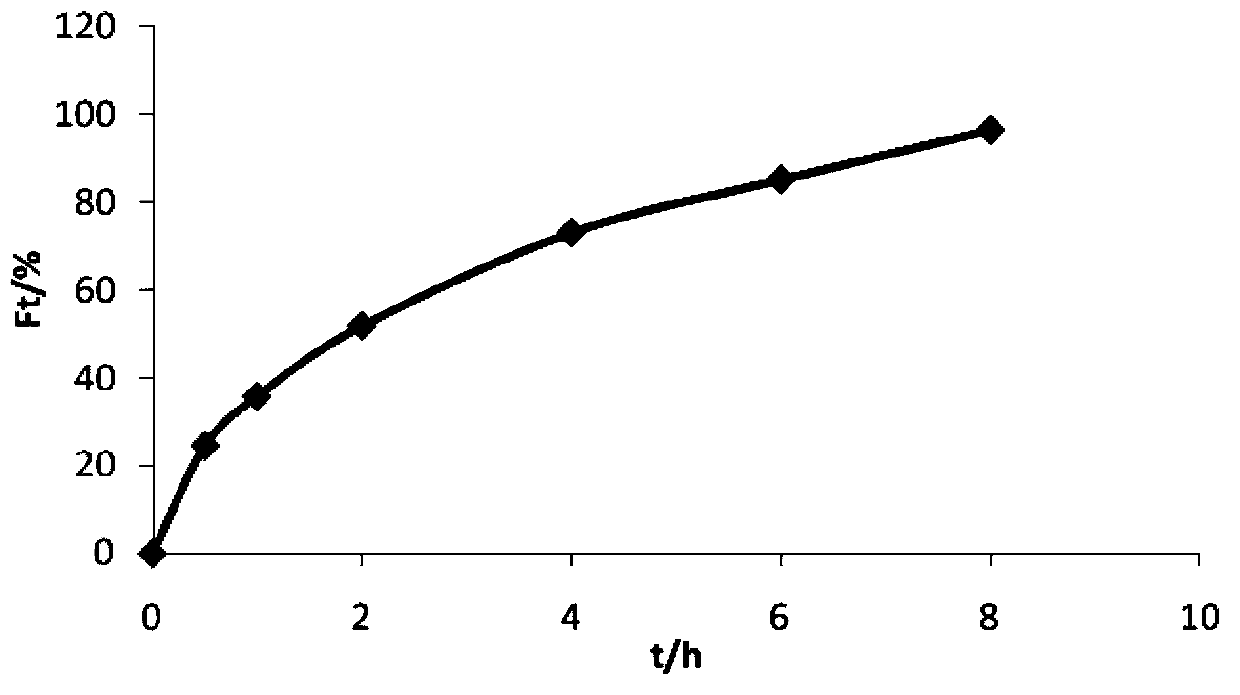
The invention relates to the preparation of a growth factor porous micro-sphere compound system coated by an injectable hydrogel. The preparation is characterized by comprising a first component, namely HA-ADH sol obtained by cross-linking of hyaluronic acid (HA) and adipic dihydrazide (ADH), and polylactic acid (PLLA) porous micro-spheres which are carried with growth factors and loaded in the first component. The compound system is capable of degrading and slowly releasing the growth factors under physiological conditions, so that the slow releasing of the growth factors in a body is realized, and the problems that the growth factors are released too fast and an action period is too short are solved, thereby reaching the effect of neural restoration.
Owner:WUHAN UNIV OF TECH
Trimetazidine hydrochloride sustained release tablet and preparation method thereof
InactiveCN103385861AQuality improvementSmooth releaseOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletAdhesive
The invention discloses a trimetazidine hydrochloride sustained release tablet prepared from the following ingredients in percentage by weight: 10-25% of trimetazidine hydrochloride, 20-60% of hydrophilic gel skeleton material, 1-20% of a hydrophobic blocker, 15-55% of filler, 1-10% of a lubricant, 0 or 1-10% of a coating material and a proper dosage of adhesive. A wet granulation method is adopted in the preparation process. After the trimetazidine hydrochloride sustained release tablet disclosed by the invention touches water, the hydrophilic gel skeleton material can expand rapidly to form a gel layer with a plurality of pores, a drug can be slowly released through the pores, and the hydrophobic blocker can partially block the pores to avoid the burst release of the drug. Proper mass and proper proportion of hydrophilic gel skeleton material and hydrophobic blocker are added to realize slow and uniform release of the drug under the cooperation of the two ingredients and well avoid the burst release phenomenon so as to beneficially ensure the safety and effectiveness of drug therapy.
Owner:SHANDONG UNIV
Injectable PLGA porous composite microsphere preparation embedded with BMP-2 containing particles and preparation method and application thereof
The invention belongs to the technical field of medicine, and provides an injectable PLGA porous composite microsphere preparation embedded with BMP-2 containing particles. A drug-carrying particle is formed by wrapping BMP-2 with hydrophilic materials such as chitosan, collagen or albumin; a microsphere with a 3D porous structure is constructed by the drug-carrying particle and a porous microsphere material PLGA; the drug-carrying particle is embedded inside the porous microsphere. The invention also provides a preparation method of the porous composite microsphere preparation, and an application in preparation of a repair material for defects of bone or cartilage tissue. The porous composite microsphere preparation of the invention can not only realize slow release of drugs at an administration site, but also facilitate proliferation and differentiation of porous supports of seed cells.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Doxorubicin hydrochloride loaded temperature-sensitive self-healing hydrogel and preparation method thereof
InactiveCN105520906AReduce releaseAvoid sudden releaseOrganic active ingredientsAerosol deliverySelf-healingSide effect
The invention relates to a doxorubicin hydrochloride loaded temperature-sensitive self-healing hydrogel and a preparation method thereof. The doxorubicin hydrochloride loaded temperature-sensitive self-healing hydrogel comprises chitosan, sodium beta-glycerophosphate, and polyethylene glycol with two ends respectively carrying a benzaldehyde group. The preparation method comprises the following steps: forming an imide bond with a self-healing performance through using a reaction of an amino group in the structure of chitosan and the benzaldehyde groups at two ends of the polyethylene glycol, combining the imide bond with chitosan / sodium beta-glycerophosphate temperature-sensitive gel, and loading doxorubicin hydrochloride into the obtained material through physical clathration to prepare the novel temperature-sensitive self-healing hydrogel administration system. The administration system is a liquid at room temperature, and rapidly gelates at a human body temperature after being injected to a tumor position in order to form a medicine reservoir ; and compared with traditional in situ gels, the temperature-sensitive self-healing hydrogel has the advantages of strong mechanical strength, realization of rapid self restoration under the action of outside force or tissue damages to avoid burst release of a medicine, further delay of release of the medicine and prolongation of the detention time of the medicine in the tumor position, medicine effect enhancement and reduction of toxic and side effects.
Owner:CHINA PHARM UNIV
Manufacturing method for flexible display screen and preparing device for flexible display screen
InactiveCN106373917AAvoid sudden releaseAvoid curlSolid-state devicesSemiconductor/solid-state device manufacturingNon destructiveEngineering
The invention, which is suitable for the technical field of the flexible display screen, discloses a manufacturing method for a flexible display screen and a preparing device for a flexible display screen. The manufacturing method comprises: preparing a carrier substrate and a stripping device; setting a stripping separation layer on the carrier substrate; arranging a flexible display screen layer on the stripping separation layer; arranging a support membrane adhering to the flexible display screen layer on the surface of the stripping device; and driving the stripping device, enabling the support membrane to adhering to the flexible display screen layer, and stripping the flexible display screen layer from the stripping separation layer. In addition, the preparing device consists of a stripping device and a flexible display screen layer forming component for forming a flexible display screen layer on a stripping separation layer; and a support membrane that adheres to the flexible display screen layer to strip the flexible display screen layer from the stripping separation layer is arranged on the surface of the stripping device. According to the invention, non-destructive stripping is realized; the product yield is high; the performances are excellent; and the cost is low.
Owner:TCL CORPORATION
Compound of co-carried cis-platinum and adriamycin, micelle and preparation method of micelle
InactiveCN103055324AGood stabilityAvoid quick releaseHeavy metal active ingredientsOrganic active ingredientsCis-platinumDoxorubicin
The invention provides a compound of co-carried cis-platinum and adriamycin, micelle and a preparation method of the micelle. The compound is compounded by cis-platinum, adriamycin and a segmented copolymer, wherein the cis-platinum and the segmented copolymer are compounded through coordination; the adriamycin and the segmented copolymer are compounded through electrostatic interaction; and the segmented copolymer has the structure of formula (I) or formula (II). The compound micelle of the co-carried cis-platinum and adriamycin comprises the compound of the co-carried cis-platinum and adriamycin and an aquosity medium. The preparation of the compound micelle comprises the step of reacting the adriamycin, the cis-platinum and the segmented copolymer having the structure of formula (I) or formula (II) in the aquosity medium so as to obtain the micelle of the co-carried cis-platinum and the adriamycin. The compound micelle can stably carry the cis-platinum and the adriamycin under physiological conditions, and the release of the compound micelle has pH value sensitivity.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Preparation method of intelligent controlled release essence capsule
InactiveCN104307446ASmart Control ReleaseAvoid sudden releaseEssential-oils/perfumesMicroballoon preparationTemperature responseOil phase
The invention provides a preparation method of an intelligent controlled release essence capsule with an ambient temperature response function. The preparation method comprises the following steps: (1) fully stirring and dissolving (methyl) alkyl acrylate monomers with different alkyl chain lengths, an oil-soluble initiator and essence to prepare a homogeneous oil phase; (2) adding the prepared organic oil into an aqueous solution containing an emulsifier for full stirring and shearing emulsification to obtain emulsified drops with the particle size of 10nm-5mm; (3) heating emulsion to generate polymerization reaction by the (methyl) alkyl acrylate monomers to form essence-wrapped capsule wall, and performing filtering and washing to obtain the essence capsule with a nuclear shell structure. The synthesis technology is simple, and the energy consumption is low; the requirement on equipment is low, and reaction conditions are easy to control; the preparation method has a large-scale production prospect. The capsule capable of intelligently adjusting the release speed has a great application prospect in the field of cosmetics and the field of intelligent heat storage and temperature adjustment textiles.
Owner:TIANJIN POLYTECHNIC UNIV
Microsphere preparation encapsulating hydrophilic medicine and preparation method thereof
ActiveCN102266294BAvoid sudden releaseImprove hydrophilicityPharmaceutical non-active ingredientsGranular deliveryParaffin waxMicrosphere
The invention discloses a microsphere preparation encapsulating a hydrophilic medicine, and the microsphere preparation comprises the following components in percentage by weight: a hydrophilic medicine 0.1-40%, methoxy poly(ethylene glycol)-poly(lactic acid) block copolymers 0.1-99%, and poly(lactic acid) or poly(lactic acid-glycollic acid) copolymers 0.1-99%. The microsphere preparation has round and intact surface, uniform and controllable particle diameter, high encapsulation rate, and controllable drug release behaviors. The invention also discloses a preparation method of the microsphere preparation encapsulating the hydrophilic medicine, and the preparation method has simple process and good controllability and comprises the following steps: adding a water solution of the hydrophilic medicine into an organic solution of the methoxy poly(ethylene glycol)-poly(lactic acid) block copolymers, and vortexing to form water-in-oil type primary emulsion; adding the primary emulsion intoliquid paraffin containing emulsifier I, and vortexing to form water-in-oil type multiple emulsion; and adding the multiple emulsion into liquid paraffin containing emulsifier II, continuing stirringfor 4-24 h, centrifuging, collecting pellets, washing, and drying to obtain the microsphere preparation encapsulating the hydrophilic medicine.
Owner:ZHEJIANG UNIV
Adriamycin nano-particles and preparation method thereof
ActiveCN102552934AAvoid sudden releaseInhibition releaseOrganic active ingredientsPharmaceutical non-active ingredientsPolyethylene glycolL glutamate
The invention provides adriamycin nano-particles. The adriamycin nano-particles are prepared by compounding adriamycin and a block copolymer with a structure shown as a formula (I) or a formula (II) through electrostatic interaction. A preparation method for the adriamycin nano-particles comprises the following step of: electrostatically compounding the adriamycin and the block copolymer with thestructure shown as the formula (I) or the formula (II) in an aqueous medium to obtain the adriamycin nano-particles. In the aqueous medium, a poly(gamma-propargyl-L-glutamate-g-mercaptosuccinic acid)segment and a polyethylene glycol segment which are contained in the block copolymer wrap the adriamycin into cores of the nano-particles, so the adriamycin composite particles are high in stability.The nano-particles are expected to be gathered at tumor sites in blood circulation through an 'enhanced permeability and retention effect' so as to improve the targeting effect of the adriamycin on the tumor sites. Meanwhile, the electrostatic interaction between a carboxyl group of the block copolymer and an amino group of the adriamycin is easy to eliminate under the condition of low intracellular pH values, so that the intracellular release can be accelerated, and the medicinal effect can be improved.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
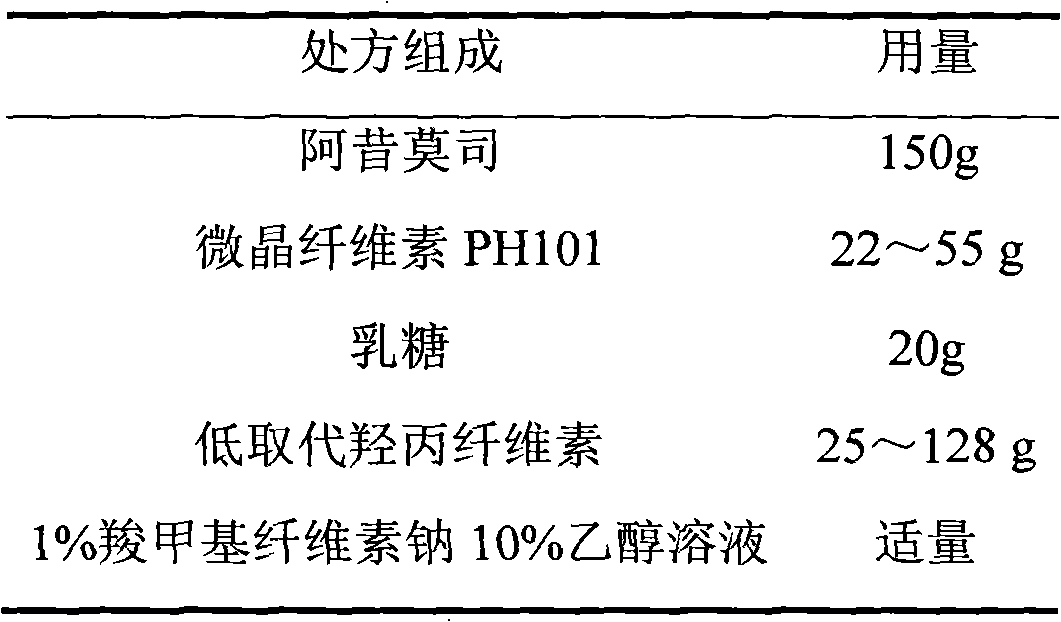
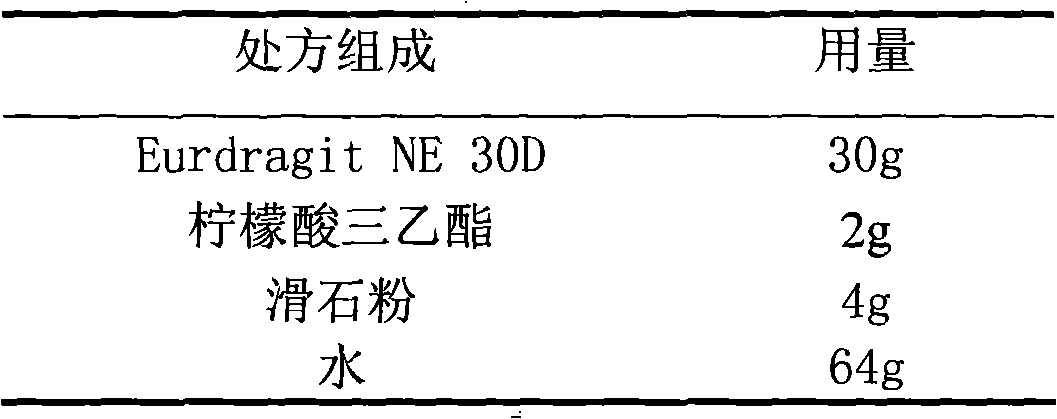
Acipimox film-controlled slow-release pellet capsule
ActiveCN103211785AAccelerated agingReduce permeabilityOrganic active ingredientsMetabolism disorderMedicineLactose
The invention relates to an acipimox film-controlled slow-release pellet capsule. A slow-release film of the acipimox film-controlled slow-release pellet utilizes Eurdragit NE 30D as a film-formation material. A pellet core of the acipimox film-controlled slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains pharmaceutically acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises the Eurdragit NE 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit NE 30D to triethyl citrate to talcum powder is 30: 2: 4 and coating weight gain is in a range of 20 to 39%. The acipimox film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the acipimox film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the acipimox film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept prior to expiration date.
Owner:北京天衡药物研究院有限公司
Preparation method of high-strength self-healing hydrogel
InactiveCN107057246AGood biocompatibilityFast self-healingPharmaceutical delivery mechanismProsthesisChemistryPolyacrylamide
The invention discloses a preparation method of high-strength self-healing hydrogel and belongs to the technical field of preparation of medical materials. The preparation method comprises the following steps: dissolving polyvinyl alcohol, adding an acrylamide solution, by taking N,N'-methylene bisacrylamide as a crosslinking agent and nano titanium dioxide as a free radical initiator, firstly polymerizing acrylamide under ultraviolet light condition to form polyacrylamide network gel, then carrying out freeze-thraw cycle to form polyvinyl alcohol network gel, then carrying out oxidation crosslinking on the surface of a prefabricated hydrogel network by utilizing self-polymerization effect of dopamine, so that a polydopamine composite layer strongly adhered on the surface of a gel network is formed, and self-made nano hydroxy calcium phosphate is adsorbed, and the aim of further strengthening the hydrogel network is achieved. Meanwhile, the formed polydopamine surface layer and the adsorbed nano hydroxy calcium phosphate have good biocompatibility, and the problem that the traditional self-healing hydrogel is poor in mechanical strength and biocompatibility is effectively solved, so that the high-strength self-healing hydrogel has better application prospect.
Owner:CHANGZHOU YAHUAN ENVIRONMENTAL PROTECTION TECH
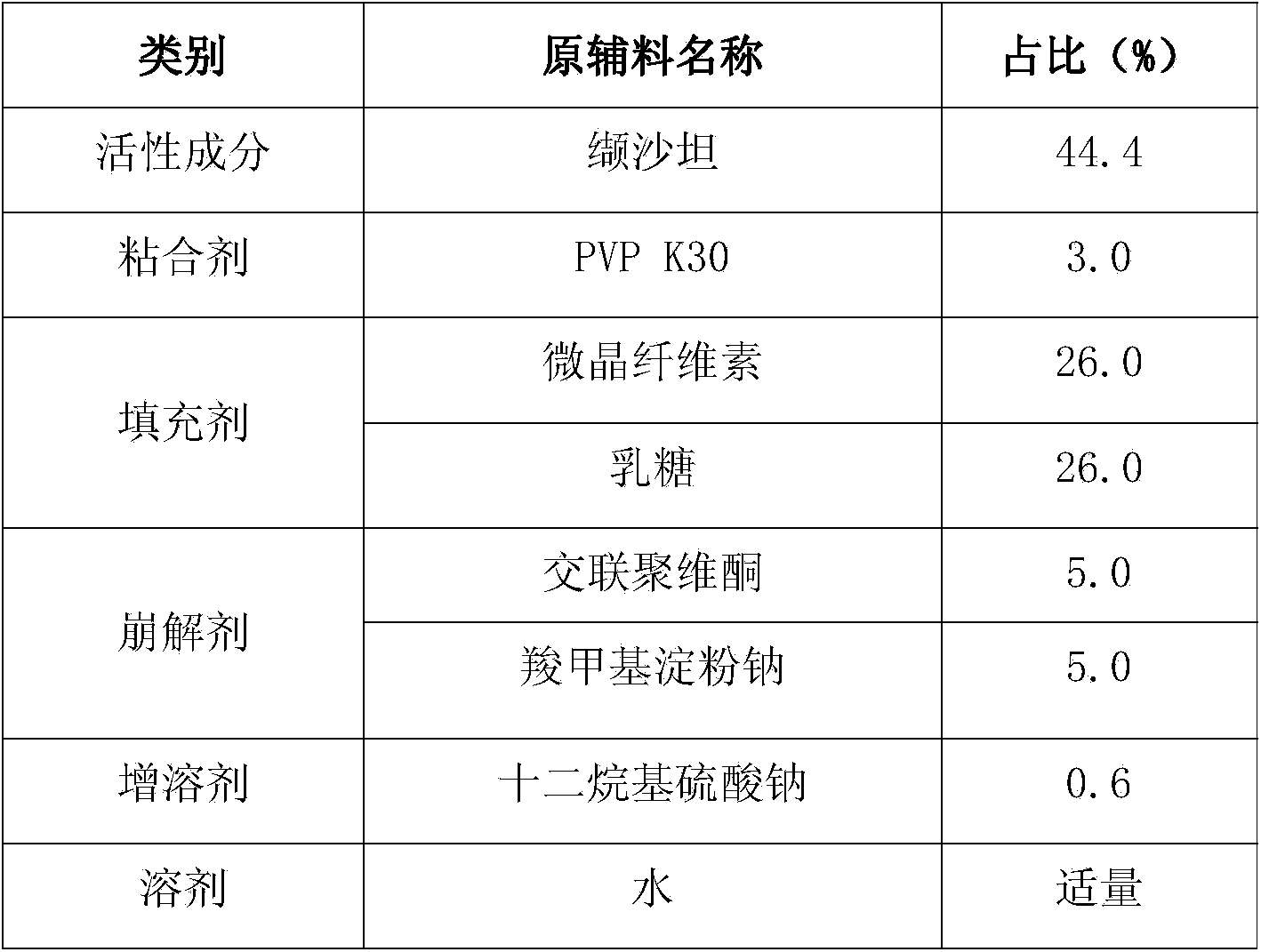
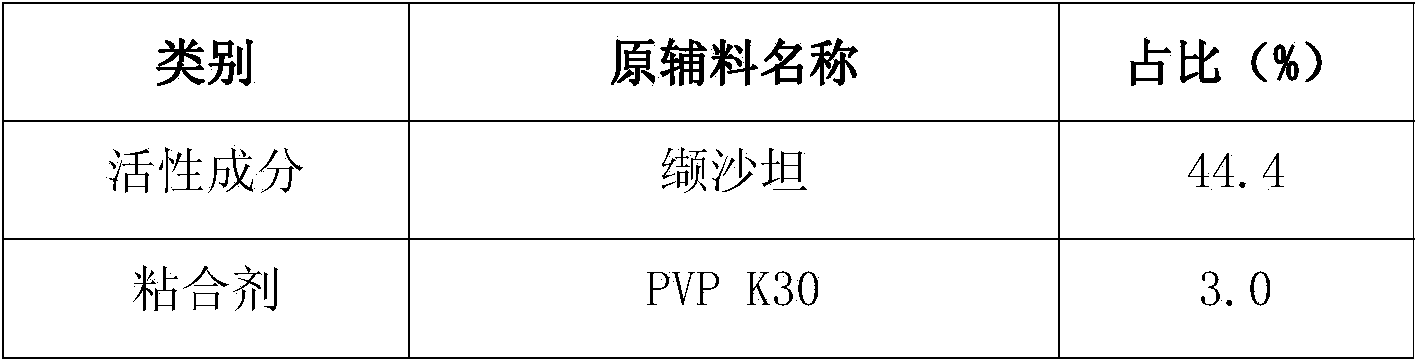
Valsartan medicine composition and preparation method thereof
ActiveCN103816134AEnhanced in vitro releaseImprove stabilityPharmaceutical non-active ingredientsCapsule deliveryValsartanAdhesive
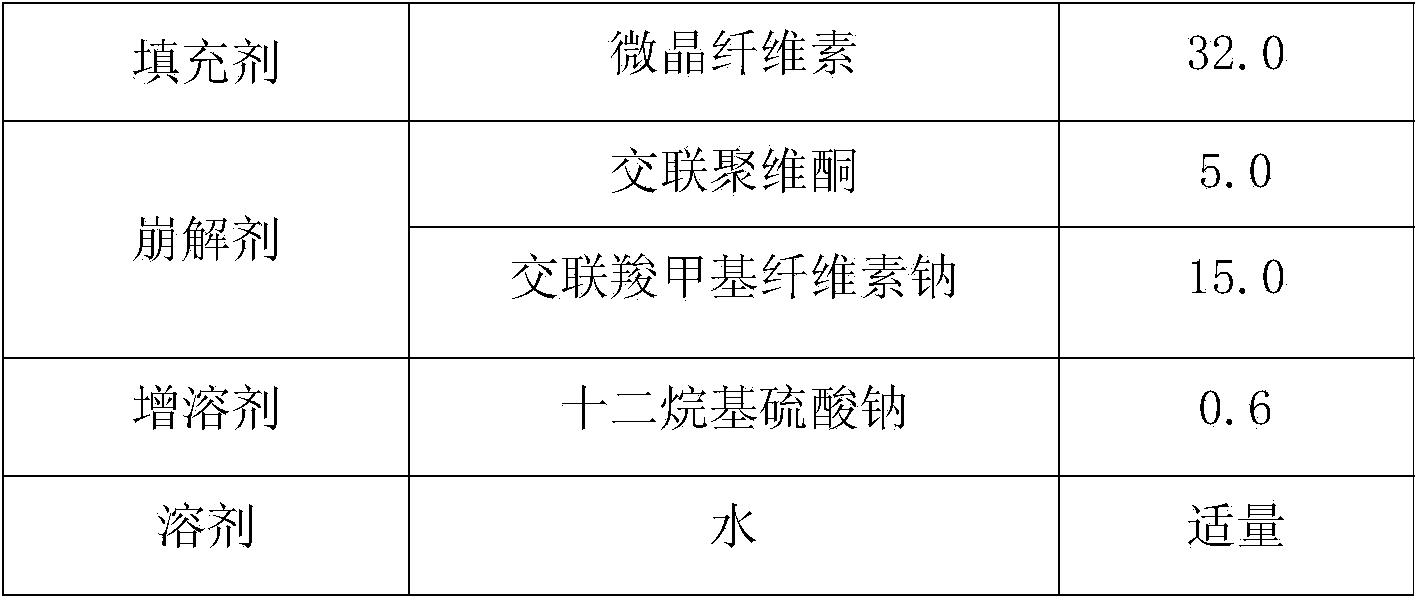
The invention relates to a medicine composition, and particularly relates to a valsartan medicine composition and a preparation method thereof. The medicine composition disclosed by the invention comprises the following components: valsartan, an adhesive, filler, a disintegrating agent, a solubilizer and a solvent.
Owner:珠海润都制药股份有限公司
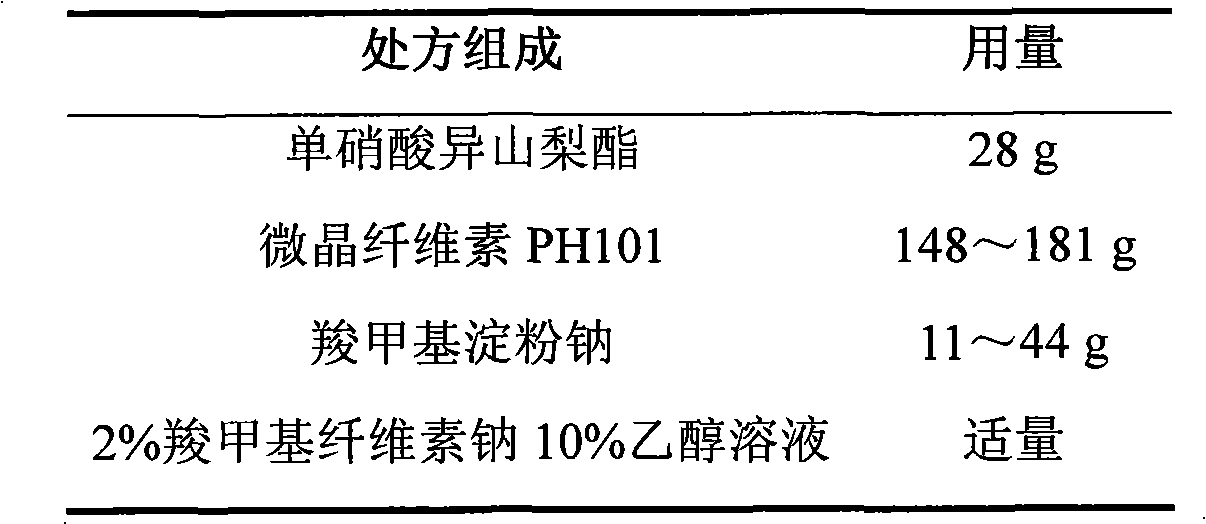
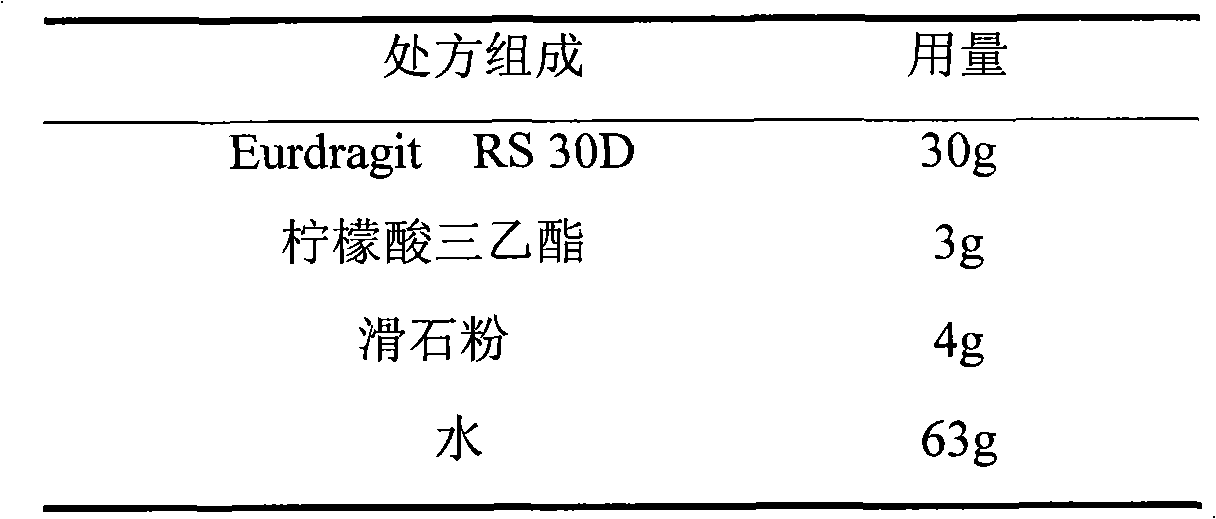
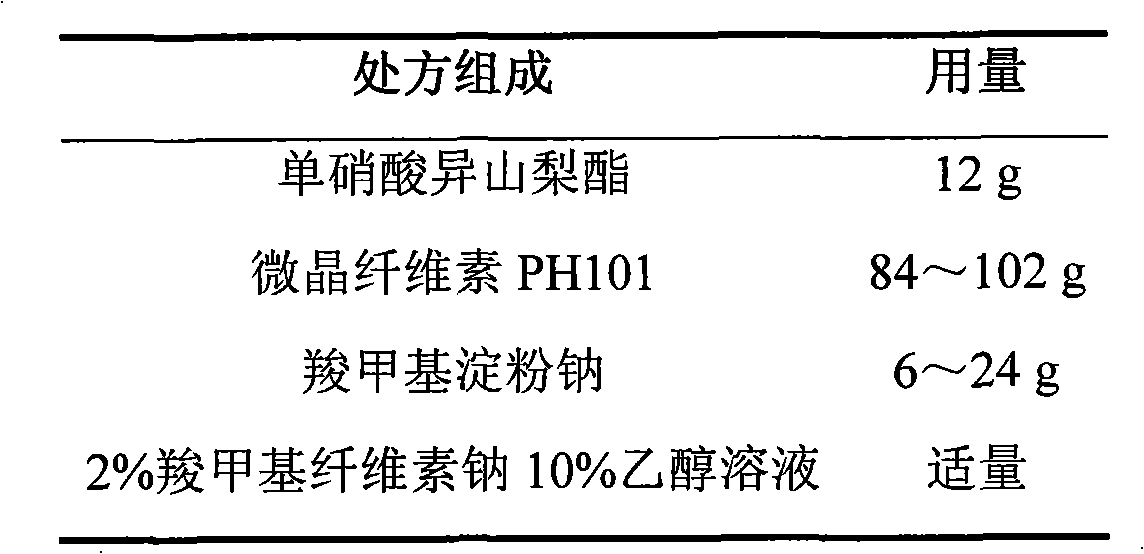
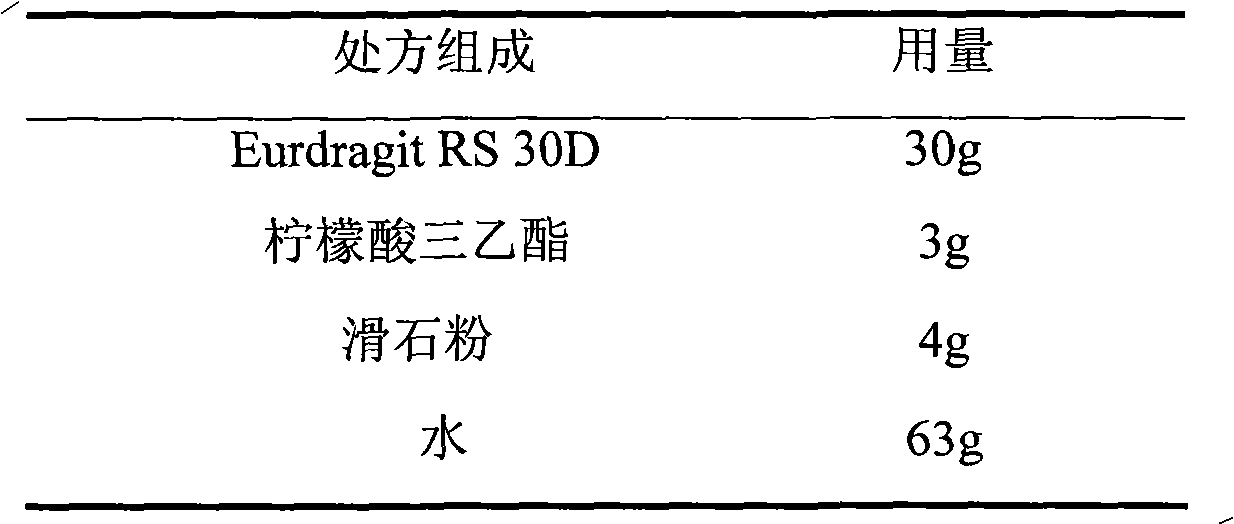
Isosorbide mononitrate sustained-release pellet, and isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it
ActiveCN103211768AAccelerated agingReduce permeabilityPharmaceutical non-active ingredientsGranular deliverySustained release pelletsMedicine
The invention relates to an isosorbide mononitrate sustained-release pellet, and an isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it. The sustained-release film of the sustained-release pellet adopts Eurdragit RS 30D as a film forming material, the core of the sustained-release pellet contains high-expansibility sodium carboxymethyl starch and a pharmaceutically-acceptable excipient commonly used for sustained-release pellets, and optimally, the excipient is microcrystalline cellulose, wherein the weight percentage of sodium carboxymethyl starch in the core of the sustained-release pellet is 5-20%. The sustained-release film of the sustained-release pellet includes the Eurdragit RS 30D, a plasticizer triethyl citrate and an anti-adherent talcum powder, the optimal ratio of the Eurdragit RS 30D to triethyl citrate to the talcum powder is 30:3:4, and the optimal coating weight gain is 19-38%. The core will obviously expand after absorbing water because of the containment of sodium carboxymethyl starch highly expanding after contacting with water, so the sustained-release film is stretched, the thickness of the film is thinned, the apertures of water-pervious micro-pores are increased, the permeability is good, and the permeability decrease caused by film ageing is compensated, thereby the middle and later stage release speed is basically constant, the last stage residue is small, and a stable release performance is always maintained prior to the expiration date.
Owner:北京天衡药物研究院有限公司
Injectable PLGA (poly(lactic-co-glycolic acid)) porous composite microsphere preparation embedded with BMP-2 and TGF-beta1 containing microspheres as well as preparation method and application of composite microsphere preparation
The invention belongs to the technical field of medicine, and provides a PLGA (poly(lactic-co-glycolic acid)) porous composite microsphere preparation embedded with BMP-2 and TGF-beta1 containing microspheres therein, which not only can bring about benefits for the adhesion and the growth of seed cells by virtue of a 3D porous structure but also can undergo controlled- and sustained-release and can be applied to injection. The invention also provides a preparation method of the porous composite microsphere preparation as well as application of the porous composite microsphere preparation in preparing bone or cartilage tissue defect repairing materials. The porous composite microsphere disclosed by the invention, which can serve as a good carrier for cell adhesion and proliferation and can be directly injected to a target part, can offer a possibility of non-surgical repair to bone and cartilage tissue injuries; and the porous composite microsphere preparation is extensive in clinical application and market prospect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
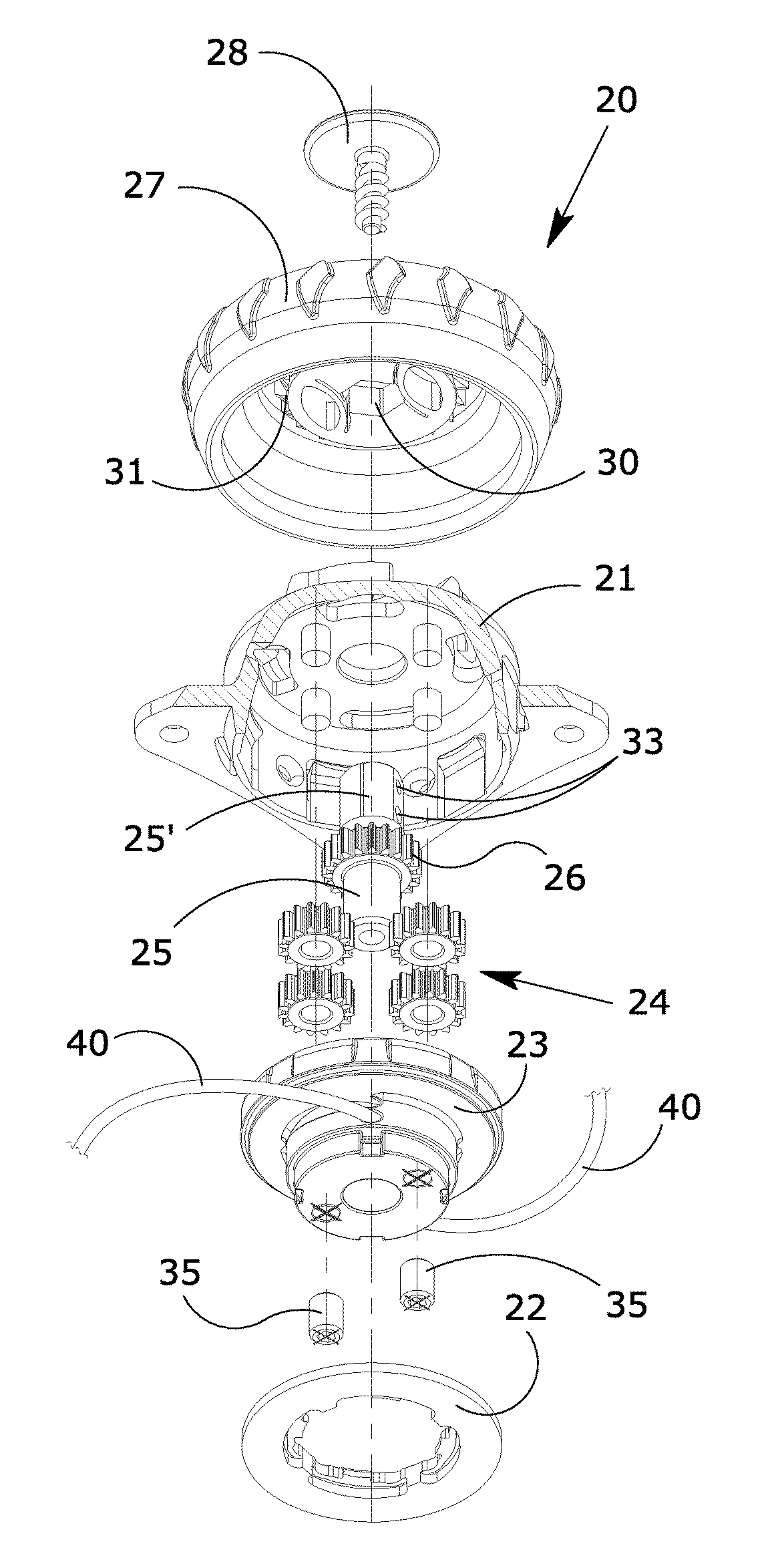
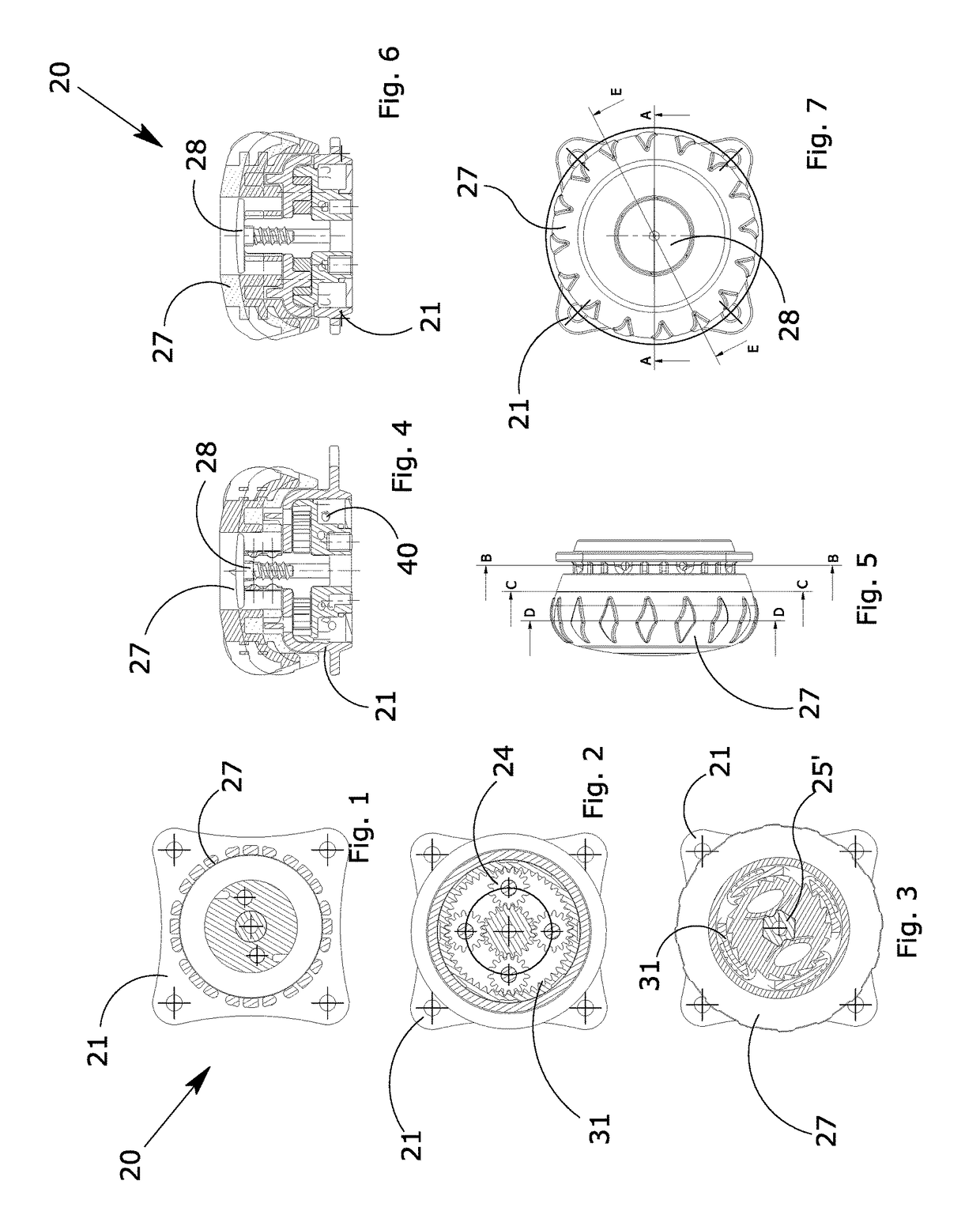
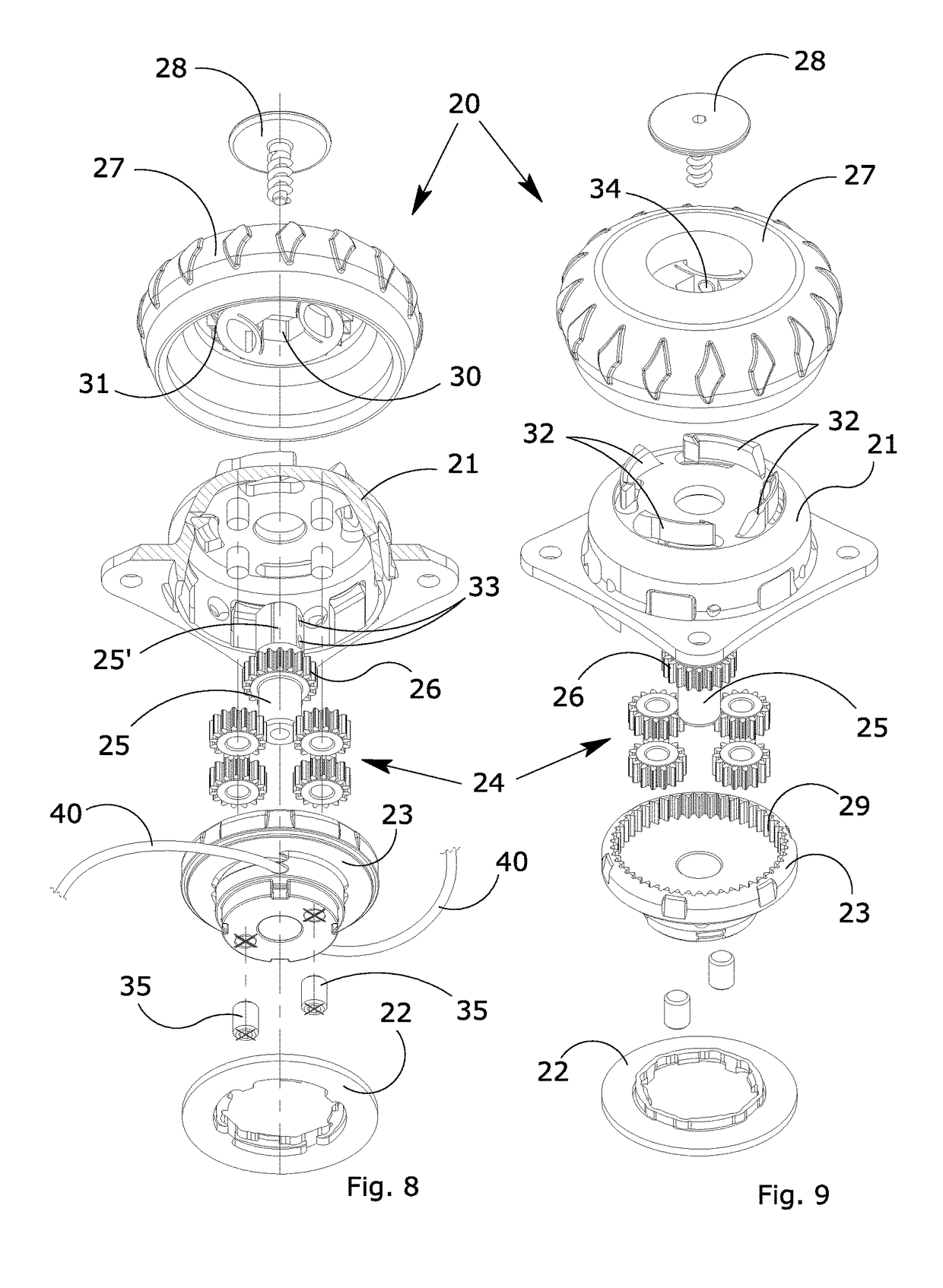
Manually operated rotative locking device for orthopedic orthoses or braces
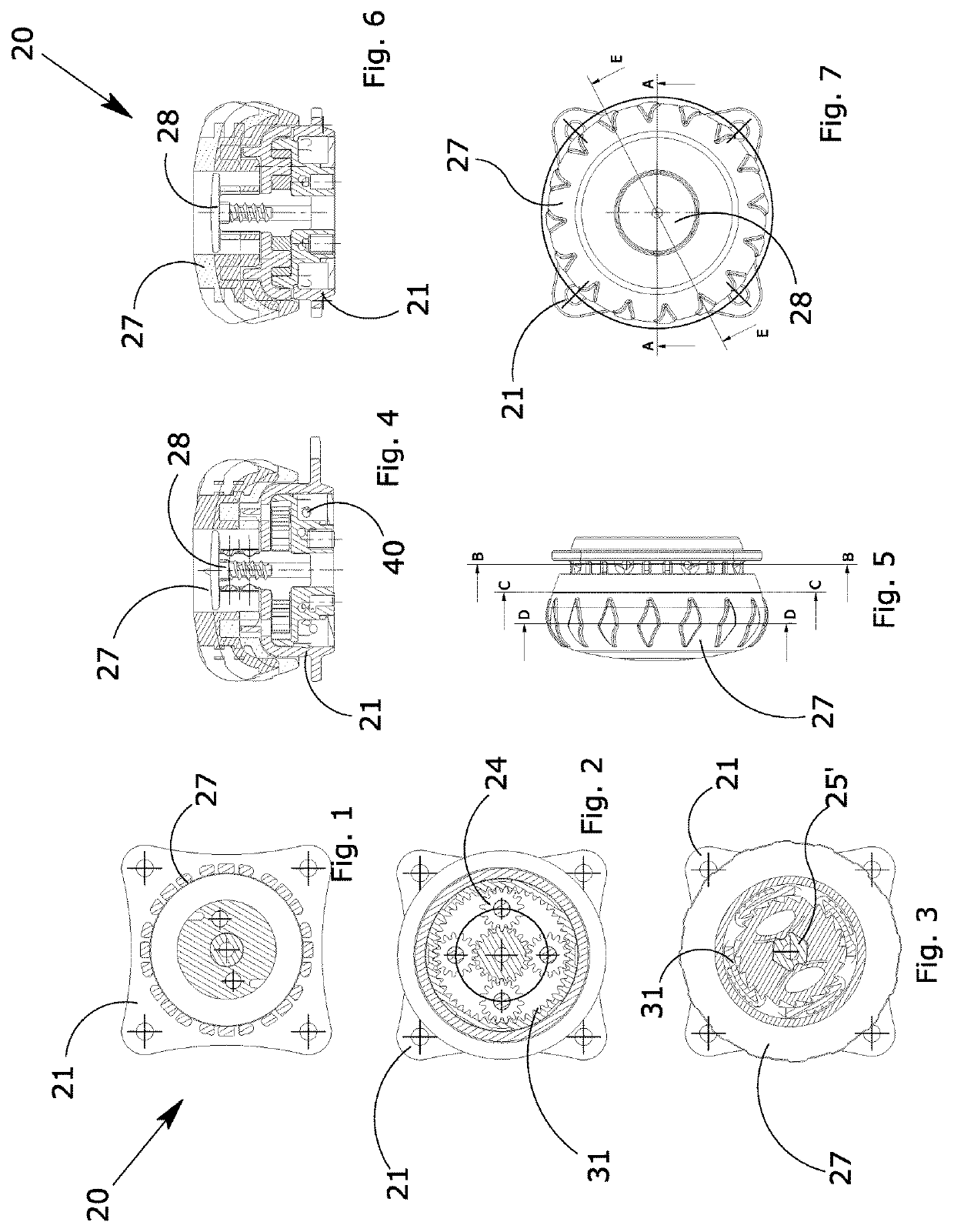
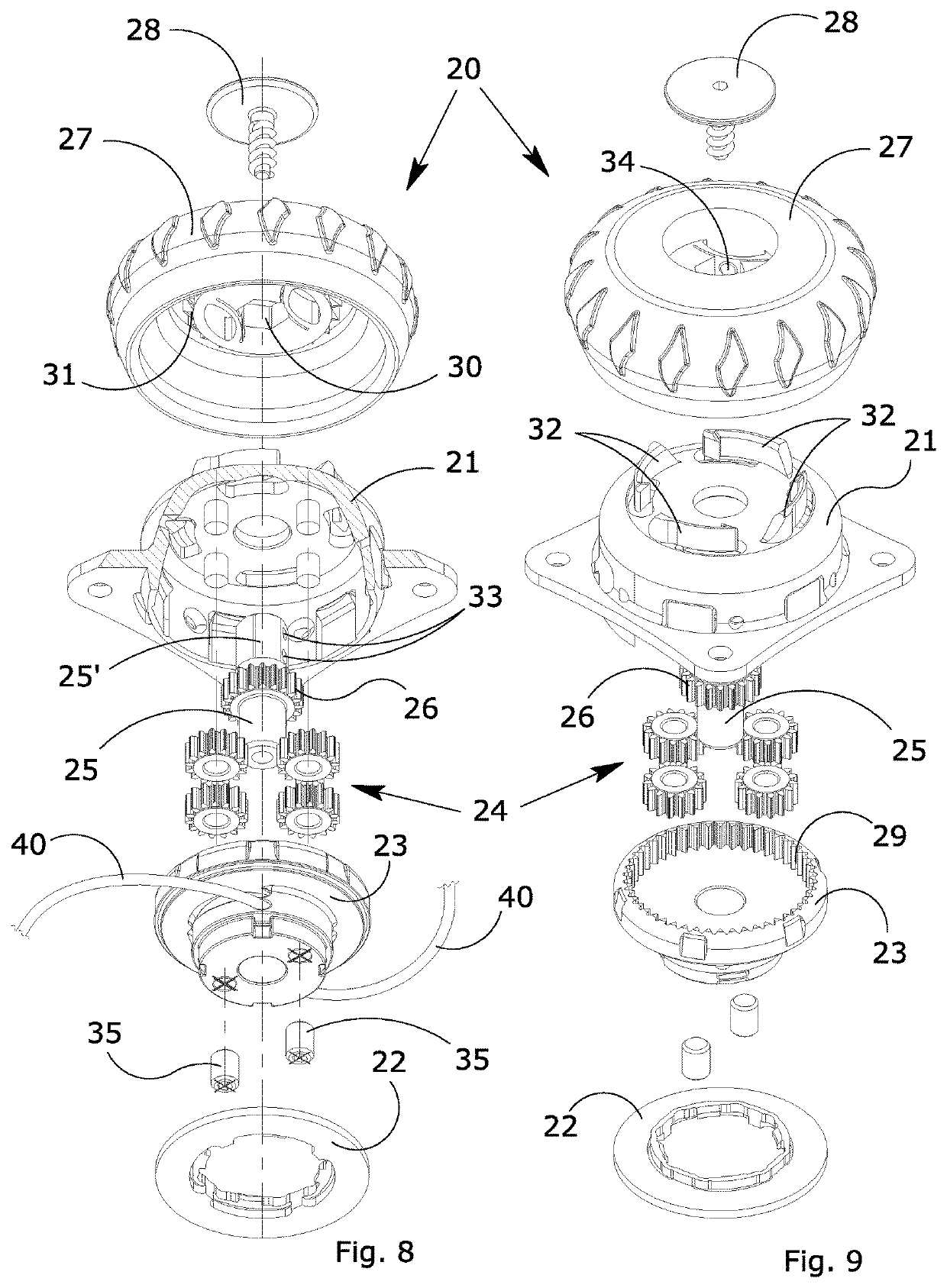
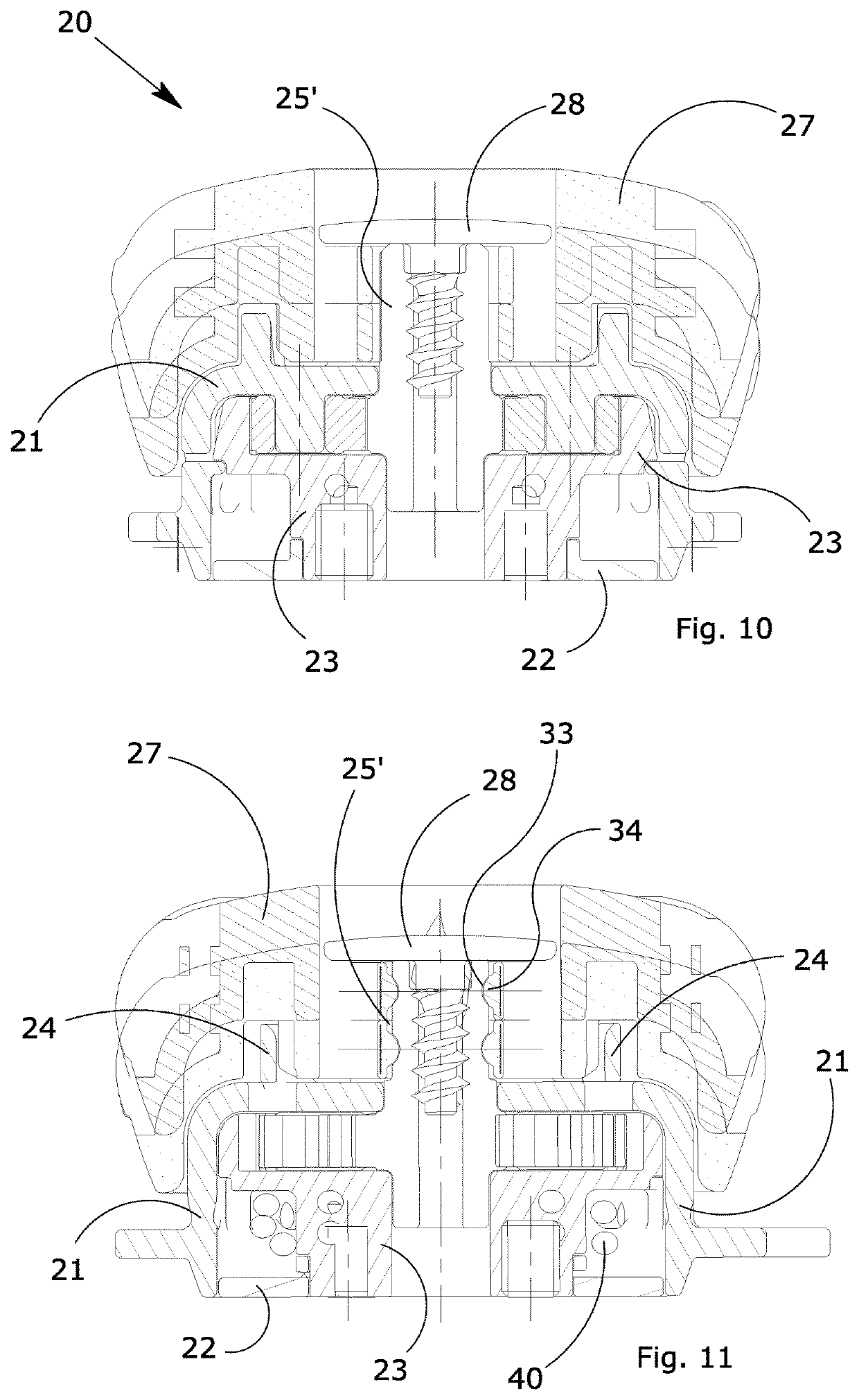
ActiveUS20170304102A1Eliminate orMitigate such drawbackNon-surgical orthopedic devicesFasteningsEngineeringOrthotic device
A blocking device (20) for fastening orthoses used in the orthopedic-rehabilitation sector to parts of the body of a person comprises a casing (21) housing a cable (40) wound on a reel (23) by the rotation of a knob (27) restrained to the casing (21) during the winding stage and partially releasable from the casing by pushing and / or pulling the knob, in order to operate with respect to a ratchet-type non-return system, wherein the ratchet-type non-return system consists of elastic tabs (32) which are part of the casing (21), and an inner toothed edge (31) of the knob (27). The knob (27) is restrained in a rotating direction and unrestrained in an axial direction with respect to the reel (23) by a pin (25) with a shaped head (25′), whereby the restraint between the knob (27) and the reel is achieved by a shaped axial opening (30) in the knob, which reflects the shape of the head (25′) of the pin (25), wherein maintaining the knob (27) in the gripping / release positions is ensured by the concave profiles (33) present on the shaped head (25′) of the pin (25) and convex surfaces (34) present in the opening (30) of the knob (27), thereby allowing the knob to be moved from a gripping condition with the ratchet-type non-return system in the tightening and blocking stage to a release condition in the opening stage while maintaining control of the cable (40) wound on the reel.
Owner:F G P
Preparation method of anti-adhesion medical antibacterial gauze for treating burns
ActiveCN107375997AAvoid stickingImprove antibacterial abilityAntibacterial agentsAbsorbent padsGlycerolFreeze dry
The invention provides a preparation method of an anti-adhesion medical antibacterial gauze for treating burns, and belongs to the field of medical dressing processing. The preparation method comprises the following steps of antibacterial extracting solution preparation, gauze activation and modification treatment, mixed soak solution preparation and gel layer infusion. According to the preparation method, natural antibacterial matters in the traditional Chinese medicines are extracted through a boiling mode and are then filtered and concentrated to obtain an antibacterial medical solution, then the antibacterial medical solution, citric acid, glycerol and a sodium alginate solution are mixed, then an ordinary medical gauze with polyacrylic acid surface modification is soaked in mixed liquid, and the gauze soaked with the mixed liquid is taken out after being fully soaked and is treated by freeze drying to obtain a novel anti-adhesion high moisture-absorbent medical antibacterial gauze for treating the burns. The preparation method is simple and is liable in industrialization.
Owner:ZHENDE MEDICAL CO LTD
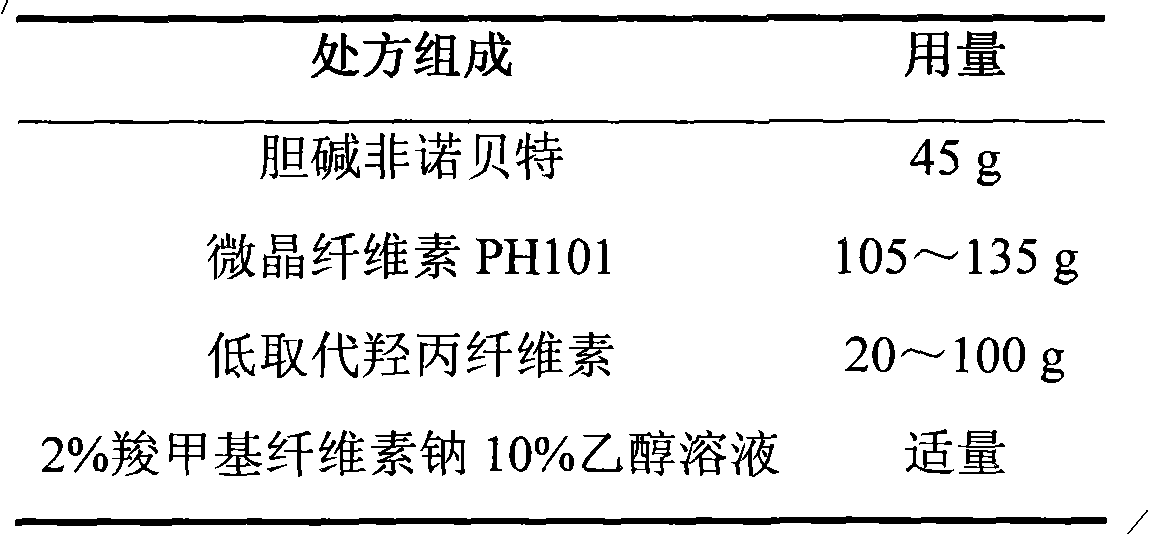
Choline fenofibrate film-controlled enteric slow-release pellet capsule
ActiveCN103211786AAccelerated agingReduce permeabilityOrganic active ingredientsMetabolism disorderSustained release pelletsMedicine
The invention relates to a choline fenofibrate film-controlled enteric slow-release pellet capsule. A slow-release film of the choline fenofibrate film-controlled enteric slow-release pellet utilizes Eurdragit RS 30D as a film-formation material. A pellet core of the choline fenofibrate film-controlled enteric slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains a pharmaceutically acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises Eurdragit RS 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of urdragit RS 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 20 to 38%. The choline fenofibrate film-controlled enteric slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the choline fenofibrate film-controlled enteric slow-release pellet expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the choline fenofibrate film-controlled enteric slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
Preparation method of medical antibacterial bandage
InactiveCN108159476AAchieving sustained releaseImprove antibacterial propertiesAbsorbent padsBandagesFreeze-dryingAqueous solution
The invention discloses a preparation method of a medical antibacterial bandage. The preparation method comprises spinning, weaving of a blank towel, preparation of an antibacterial liquid, preparation of an antibacterial soaking liquid, modification treatment, softening and drying. The modification treatment process comprises dipping a blank towel obtained by the step b into a washing machine, carrying out cleaning through water at 95-100 DEG C according to a bath ratio of 1: 20-27 for 25-36min, cooling the blank towel to 5-10 DEG C, washing the blank towel through water for 4-8min, adding anantibacterial soaking solution into the water, adjusting the temperature of the water solution to 65-75 DEG C, and carrying out soaking through the washing machine for 40-50min. The drying process comprises taking out a bandage and carrying out freeze drying to obtain gel on the surface of the bandage. The invention provides the preparation method of the medical antibacterial bandage. The medicalantibacterial bandage has the traditional sterilization and antibacterial functions and a cleaning function, has effects of improving blood circulation, promoting metabolism, eliminating fatigue andrefreshing brain, feels smooth, supple and comfortable, absorbs moisture and is breathable and comfortable.
Owner:JIANGSU GOLDEN AUTUMN ELASTIC FABRICS
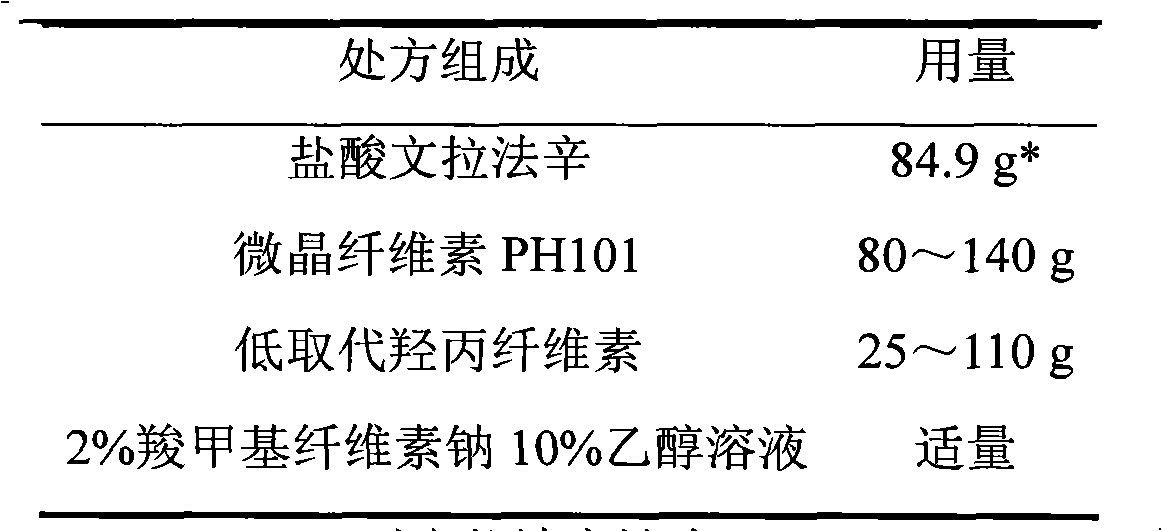
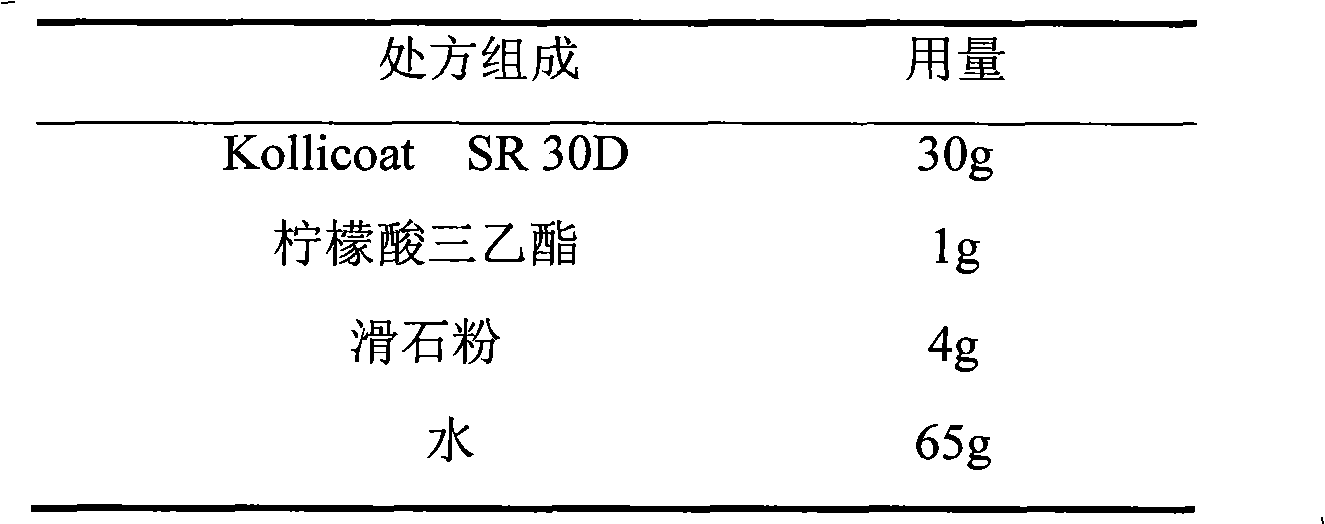
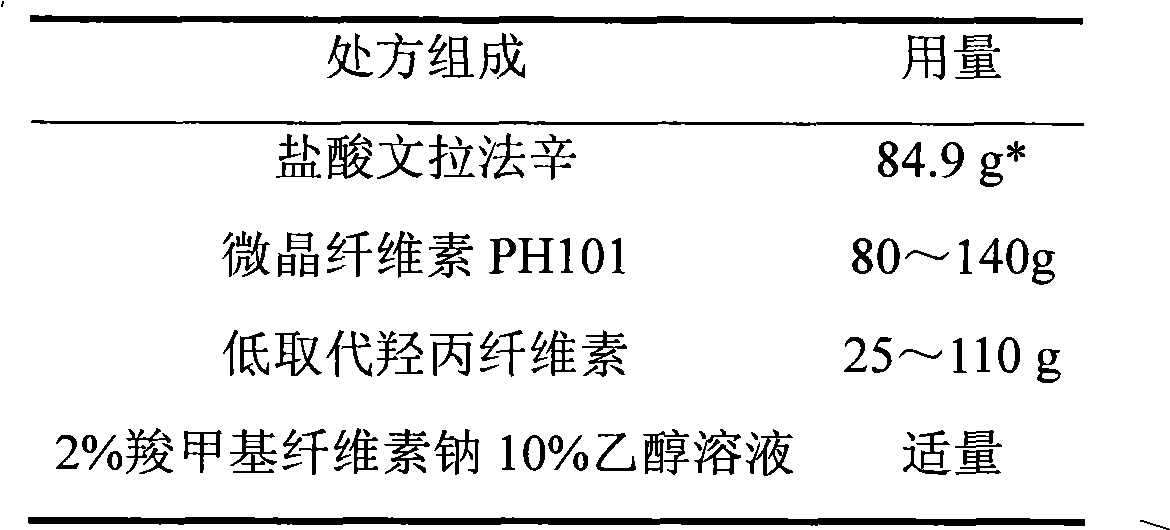
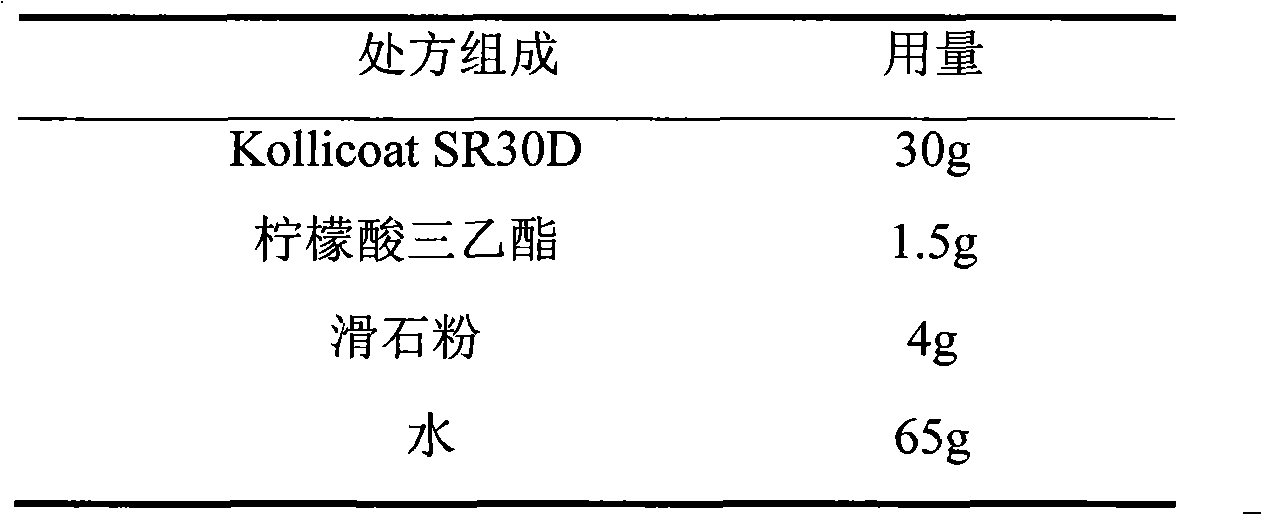
Venlafaxine hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211791AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderSustained release pelletsLow-substituted hydroxypropylcellulose
The invention relates to a venlafaxine hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the venlafaxine hydrochloride film-controlled slow-release pellet utilizes Kollicoat SR30D as a film-formation material. A pellet core of the venlafaxine hydrochloride film-controlled slow-release pellet contains low-substituted hydroxyprepyl cellulose having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of low-substituted hydroxyprepyl cellulose. The slow-release film comprises Kollicoat SR30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Kollicoat SR30D to triethyl citrate to talcum powder is 30: 1: 4 and a film weight increasing ratio is in a range of 21 to 39%. The venlafaxine hydrochloride film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxyprepyl cellulose having high water expansibility and thus after absorbing water, the venlafaxine hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the venlafaxine hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
Biodegradable drug-loaded nanofiber medical bandage for burn department and preparation method thereof
ActiveCN111298184AGood biocompatibilityPromote degradationPharmaceutical delivery mechanismAbsorbent padsBULK ACTIVE INGREDIENTBiocompatibility
The invention provides a biodegradable drug-loaded nanofiber medical bandage for burn department and a preparation method thereof. The medical bandage is of a double-layer structure composed of a surface supporting layer and an inner hydrophobic layer. A common gauze bandage subjected to carboxymethylation modification is used as a main body structure to serve as the surface supporting layer; a coaxial electrostatic spinning method is adopted, the surface supporting layer serves as a receiving layer of coaxial electrostatic spinning, and the inner hydrophobic layer with shell / core structure coaxial nanofibers is constructed on the fiber surface of the common gauze bandage subjected to carboxymethylation modification; in the coaxial nanofiber with the shell / core structure, a shell layer material is a mixture of gelatin and zein, and a core layer material is a mixture of chitosan and active ingredients. The medical bandage has excellent biocompatibility, degradability, hemostatic and antibacterial properties, anti-adhesion performance and drug sustained release performance. The preparation method is simple and controllable, and has huge application value.
Owner:安徽迈德普斯医疗科技有限公司
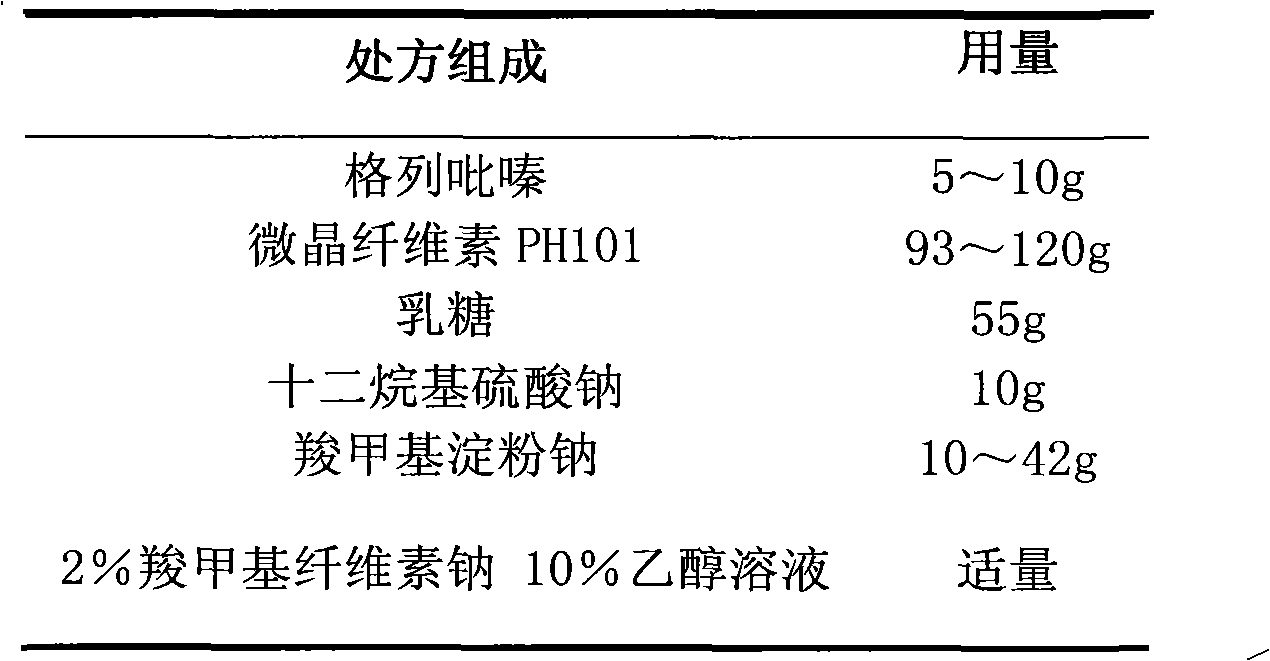
Glipizide film-controlled slow-release pellet capsule
ActiveCN103211787AAccelerated agingReduce permeabilityMetabolism disorderSulfonylurea active ingredientsSustained release pelletsMedicine
The invention relates to a glipizide film-controlled slow-release pellet capsule. A slow-release film of the glipizide film-controlled slow-release pellet utilizes Eurdragit RL 30D as a film-formation material. A pellet core of the glipizide film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose, lactose and sodium dodecyl sulfate, and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit RL 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 17 to 36%. The glipizide film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the glipizide film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the glipizide film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:内蒙古天衡医院管理有限公司
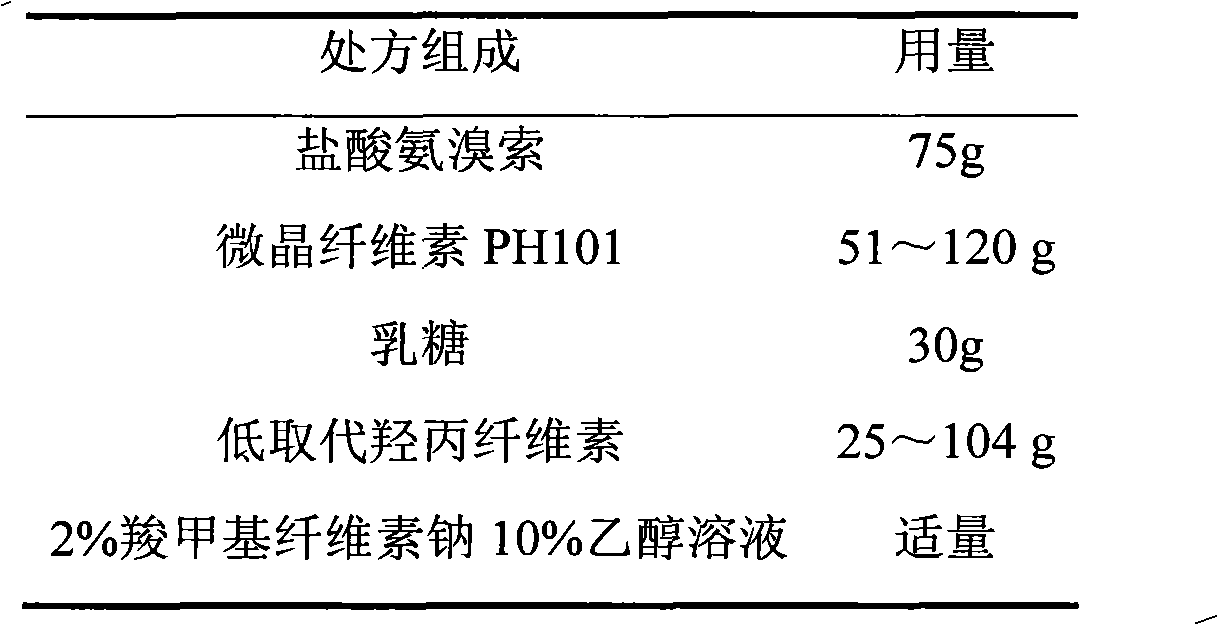
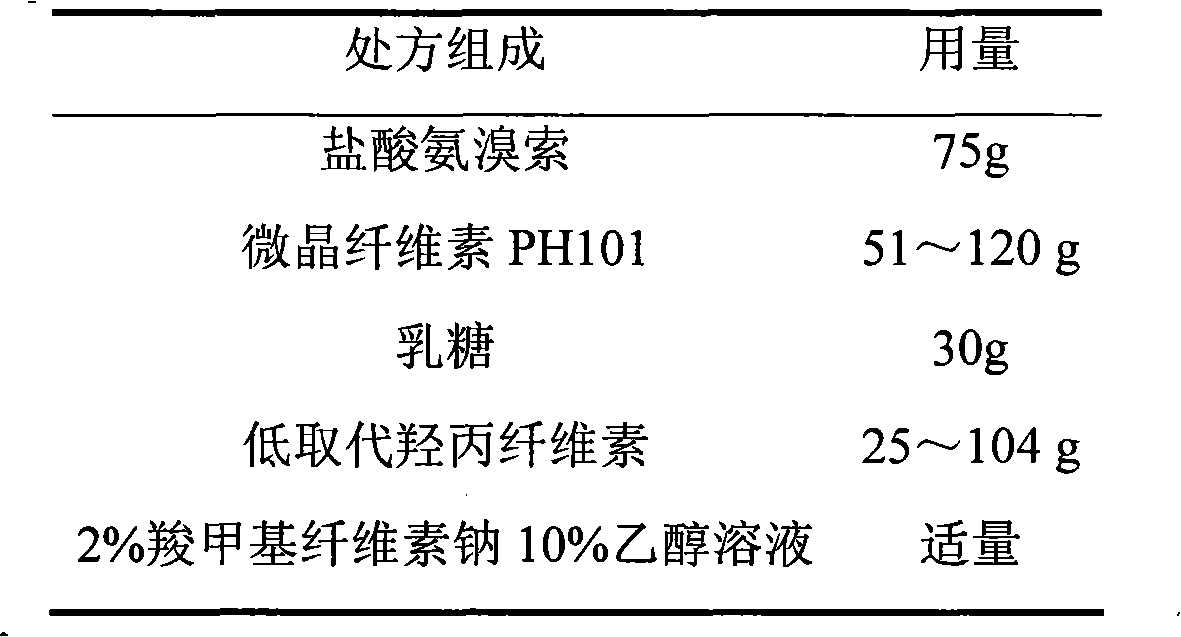
Ambroxol hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211789AAccelerated agingReduce permeabilityOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsLactose
The invention relates to an ambroxol hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the ambroxol hydrochloride film-controlled slow-release pellet utilizes Kollicoat SR 30D as a film-formation material. A pellet core of the ambroxol hydrochloride film-controlled slow-release pellet contains low-substituted hydroxyprepyl cellulose having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 10 to 40wt% of low-substituted hydroxyprepyl cellulose. The slow-release film comprises Kollicoat SR 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Kollicoat SR 30D to triethyl citrate to talcum powder is 30: 1.5: 4 and a film weight increasing ratio is in a range of 20 to 36%. The ambroxol hydrochloride film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxyprepyl cellulose having high water expansibility and thus after absorbing water, the ambroxol hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the ambroxol hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
Manually operated rotative locking device for orthopedic orthoses or braces
ActiveUS10617546B2Eliminate orMitigate such drawbackNon-surgical orthopedic devicesFasteningsRatchetEngineering
A blocking device for fastening orthoses used in the orthopedic-rehabilitation sector to parts of the body of a person comprises a casing housing a cable wound on a reel by the rotation of a knob restrained to the casing during the winding stage and partially releasable from the casing by pushing and / or pulling the knob, in order to operate with respect to a ratchet-type non-return system, wherein the ratchet-type non-return system consists of elastic tabs which are part of the casing, and an inner toothed edge of the knob. The knob is restrained in a rotating direction and unrestrained in an axial direction with respect to the reel by a pin with a shaped head, whereby the restraint between the knob and the reel is achieved by a shaped axial opening in the knob, which reflects the shape of the head of the pin, wherein maintaining the knob in the gripping / release positions is ensured by the concave profiles present on the shaped head of the pin and convex surfaces present in the opening of the knob, thereby allowing the knob to be moved from a gripping condition with the ratchet-type non-return system in the tightening and blocking stage to a release condition in the opening stage while maintaining control of the cable wound on the reel.
Owner:F G P
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com