Compound of co-carried cis-platinum and adriamycin, micelle and preparation method of micelle
A doxorubicin and compound technology, applied in the field of polymer drugs, can solve the problems of large differences in hydrophilicity, hydrophobicity, charge properties and molecular weight, and cannot meet the needs of the market, avoiding rapid release, improving curative effect, and good stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
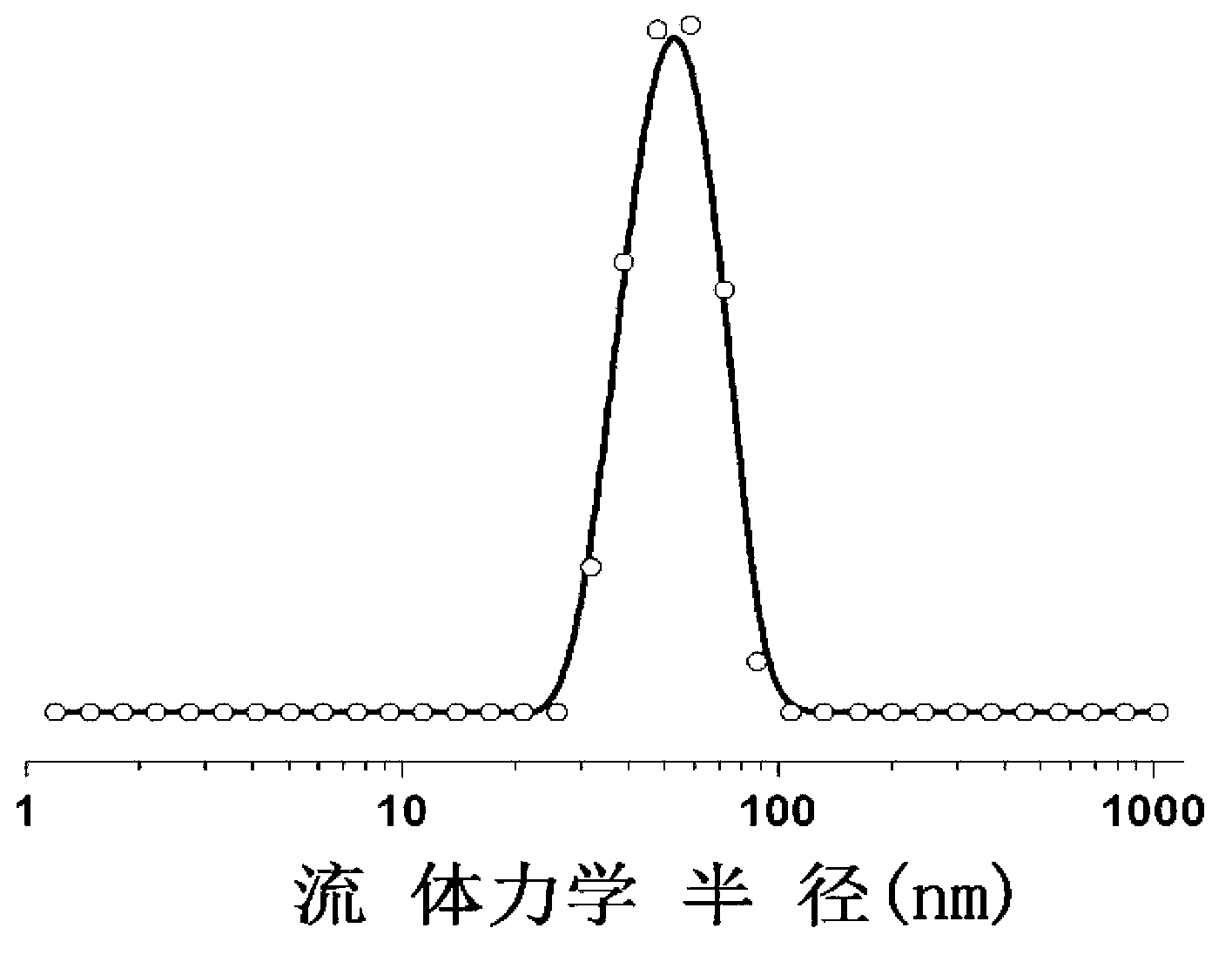
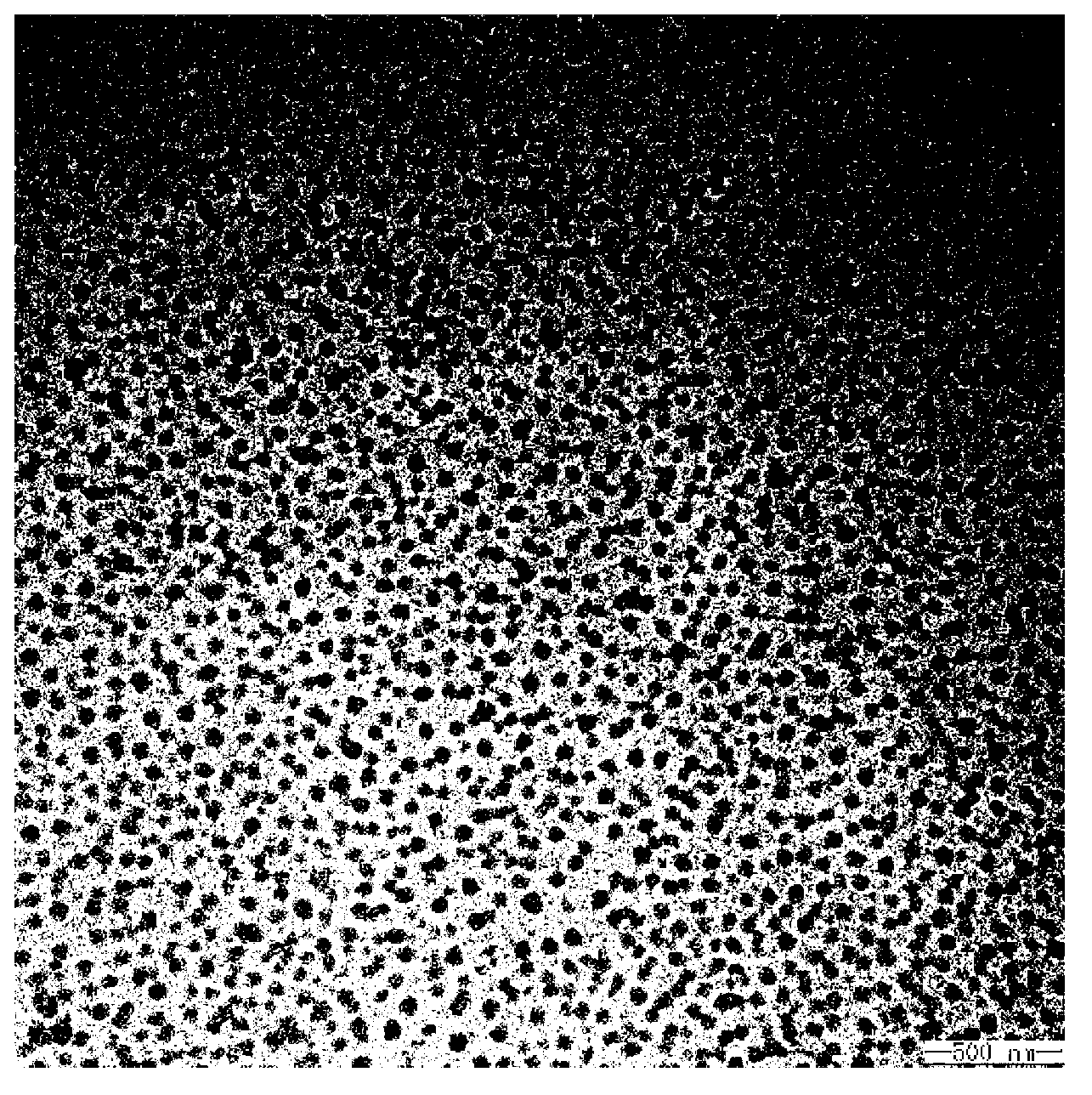
[0062] The present invention also provides a method for preparing micelles co-loading cisplatin and doxorubicin, comprising the following steps:
[0063] reacting doxorubicin, cisplatin and a block copolymer with a structure of formula (I) or formula (II) in an aqueous medium to obtain micelles co-loading cisplatin and doxorubicin;
[0064] In the present invention, doxorubicin, cisplatin and block copolymers having the structure of formula (I) or formula (II) are used as raw materials, and the order of addition of doxorubicin, cisplatin and block copolymers in the present invention has no With special restrictions, doxorubicin can be reacted with block copolymer first, and then cisplatin can be added for reaction; cisplatin can also be reacted with block copolymer first, and then doxorubicin can be added for reaction; Platinum is added to the reaction with the block copolymer. The reaction process is preferably carried out under dark conditions. The electrostatic complex ti...
Embodiment 1
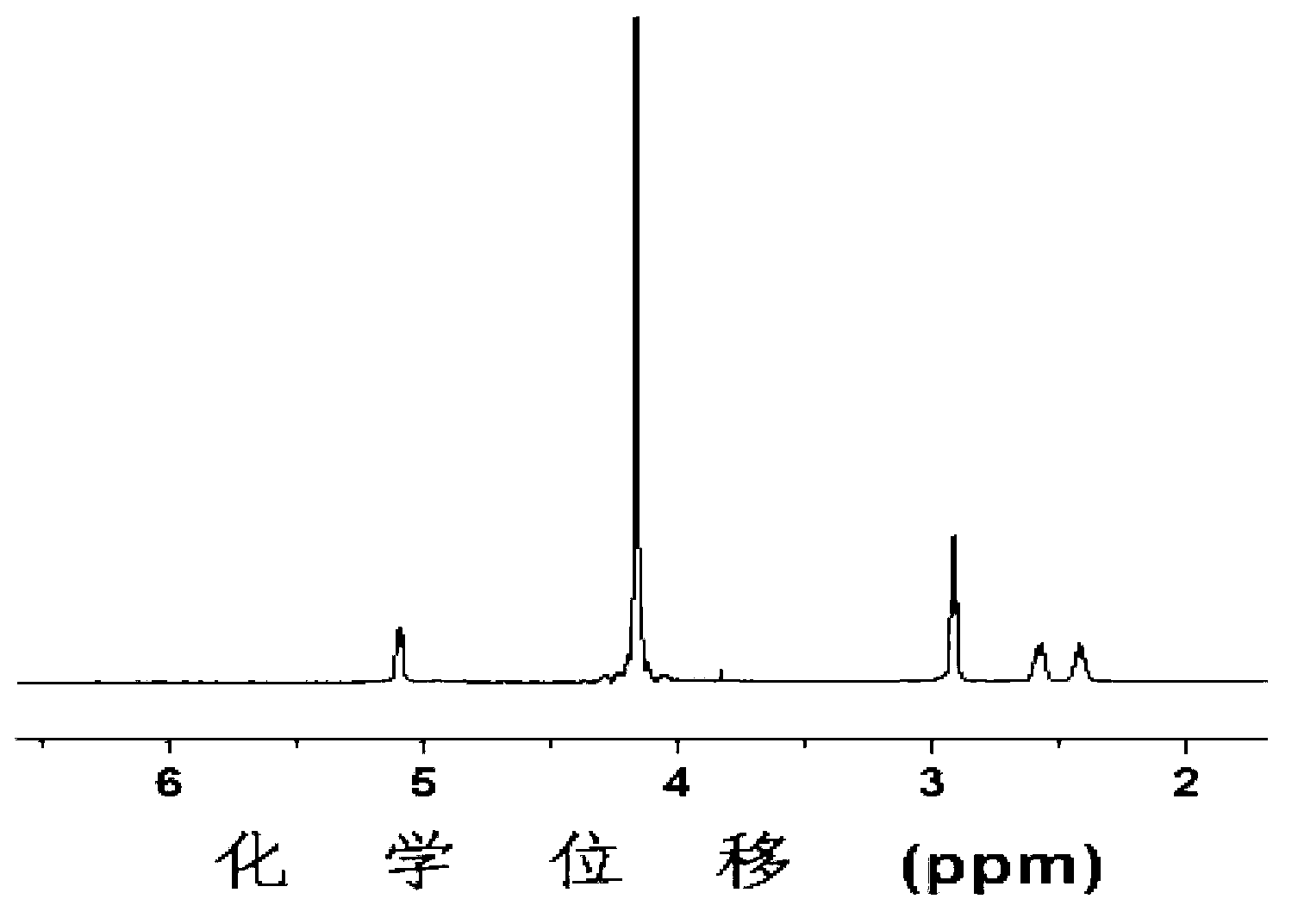
[0083] Add 2.4454g of polyethylene glycol monomethyl ether having a structure of formula (III) with a number average molecular weight of 5000 to the dry reaction bottle, and remove water by azeotroping with 60mL of anhydrous toluene at 130°C for 3 hours, then pump it to dryness under reduced pressure The remaining toluene; the obtained solid was dissolved in 25 mL of dry N,N-dimethylformamide to obtain the first solution; 6.4460 g of γ-benzyl-L-glutamate-N-carboxylic acid anhydride was dissolved in In 40mL of dry N,N-dimethylformamide, the second solution was obtained; in a nitrogen atmosphere, the first solution was mixed with the second solution, and stirred and reacted for 72h at room temperature under nitrogen protection conditions; The N,N-dimethylformamide was dried under pressure, and then the obtained solid was dissolved in dichloromethane, then settled with diethyl ether, filtered with suction, and dried to obtain a compound with a protective group.
[0084] Take 6.63...
Embodiment 2
[0087] Add 1.6251g of polyethylene glycol with a number average molecular weight of 4000 having the structure of formula (V) into the dry reaction bottle, and remove water by azeotroping with 50mL of anhydrous toluene at 130°C for 3 hours, then vacuum the remaining toluene to dryness ; The obtained solid was dissolved in 15 mL of dry N,N-dimethylformamide to obtain a first solution; 4.2790 g of γ-benzyl-L-glutamate-N-carboxylic acid anhydride was dissolved in 30 mL of dry In N,N-dimethylformamide, the second solution was obtained; in a nitrogen atmosphere, the first solution was mixed with the second solution, and stirred and reacted for 72 hours at room temperature under nitrogen protection conditions; after the reaction, vacuum pumping to dryness N,N-dimethylformamide, and then the obtained solid was dissolved in dichloromethane, then settled with diethyl ether, filtered with suction, and dried to obtain an intermediate product.
[0088] Take 4.4241 g of the prepared interme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com