Valsartan medicine composition and preparation method thereof
A composition and technology of valsartan, applied in the field of valsartan pharmaceutical composition and its preparation, can solve the problems of incomplete drug release and decreased release rate during storage, and achieve high release rate in vitro and good quality stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The preparation of embodiment 1 valsartan capsule
[0057] A preparation of drug-containing granules
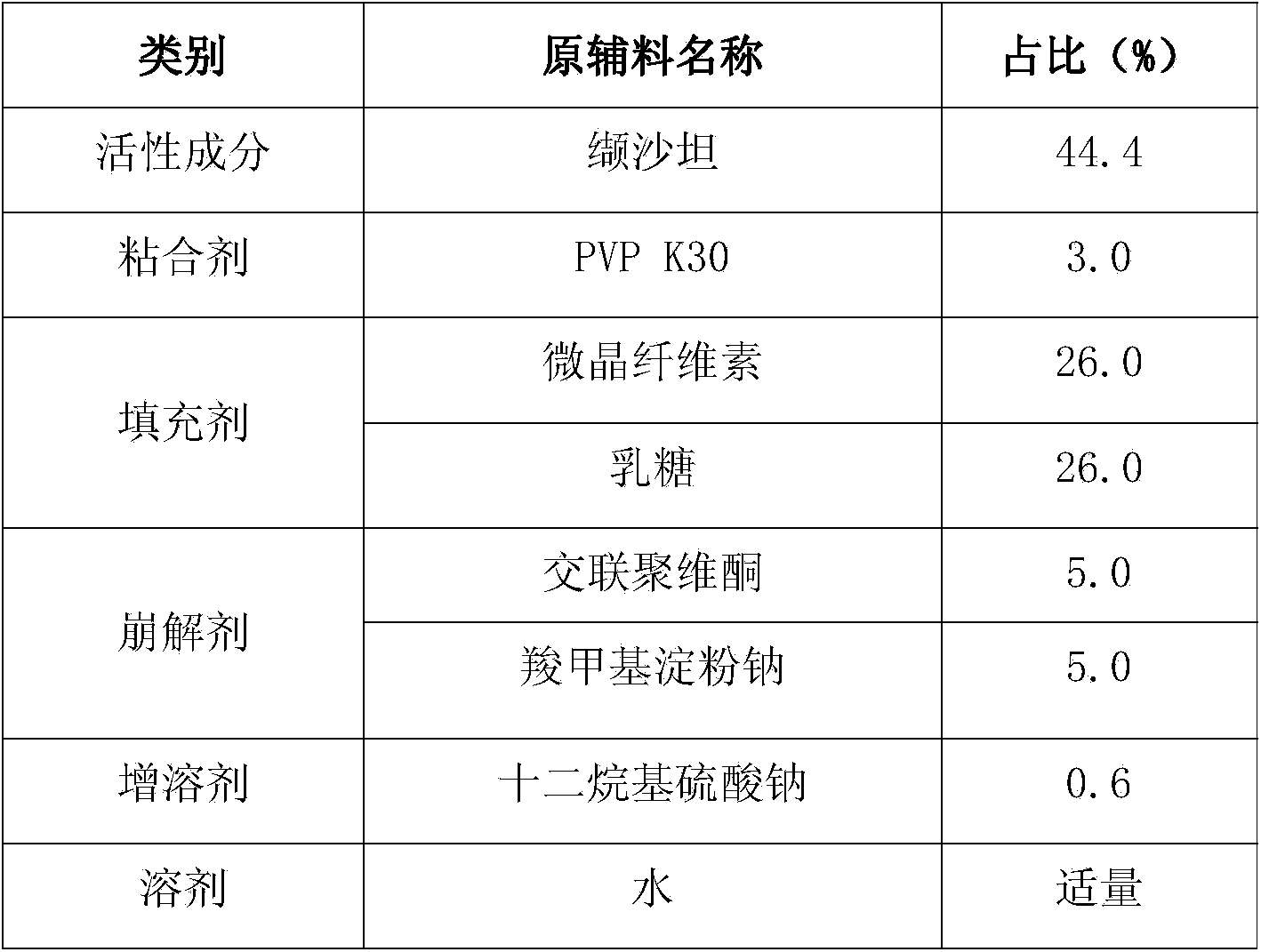
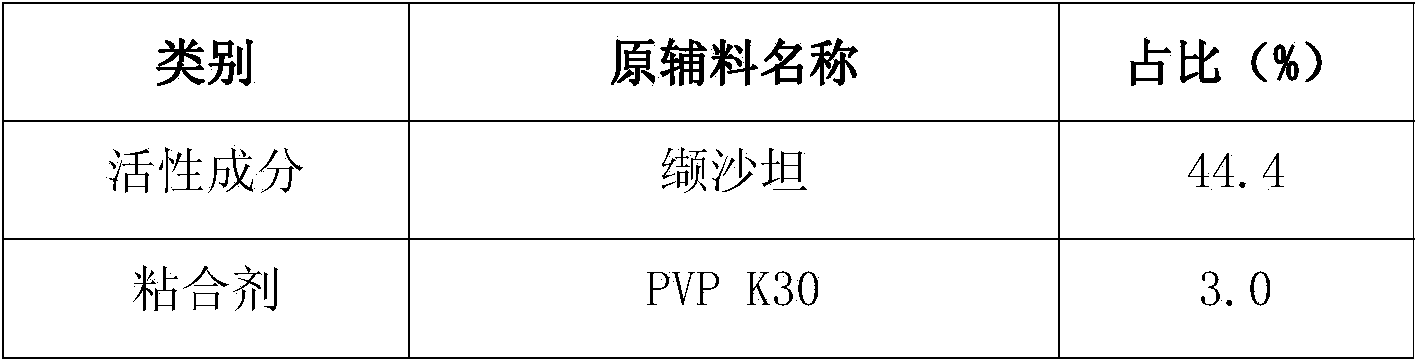
[0058] Add 54g of PVP K30 into 2000g of purified water, stir until clarified, add sodium lauryl sulfate while stirring, stir until dissolved and set aside; weigh the raw and auxiliary materials according to the prescription in Table 4, pass through an 80-mesh sieve, and mix the processed raw and auxiliary materials Put it into a wet granulator (G6 experimental multifunctional wet mixing granulator, Shenzhen Xinyite Technology), dry mix for 5-10 minutes, slowly add the prepared binder solution, and stir at a speed of 3-5r / min , Cutting speed 3-5r / min, wet mixing 3-6min, discharging, put in a swing granulator (YK160A swing granulator, Jiangsu Changzhou Shuangdong Pharmaceutical Machinery Factory) 18 mesh granules. Put the wet granules in a drying oven at 55°C (JC101 electric blast drying oven, Shanghai Chengshun Instrument Co., Ltd.), and dry for 5-7 hours until the moi...
Embodiment 2
[0067] The preparation of embodiment 2 valsartan tablets
[0068] A preparation of drug-containing granules
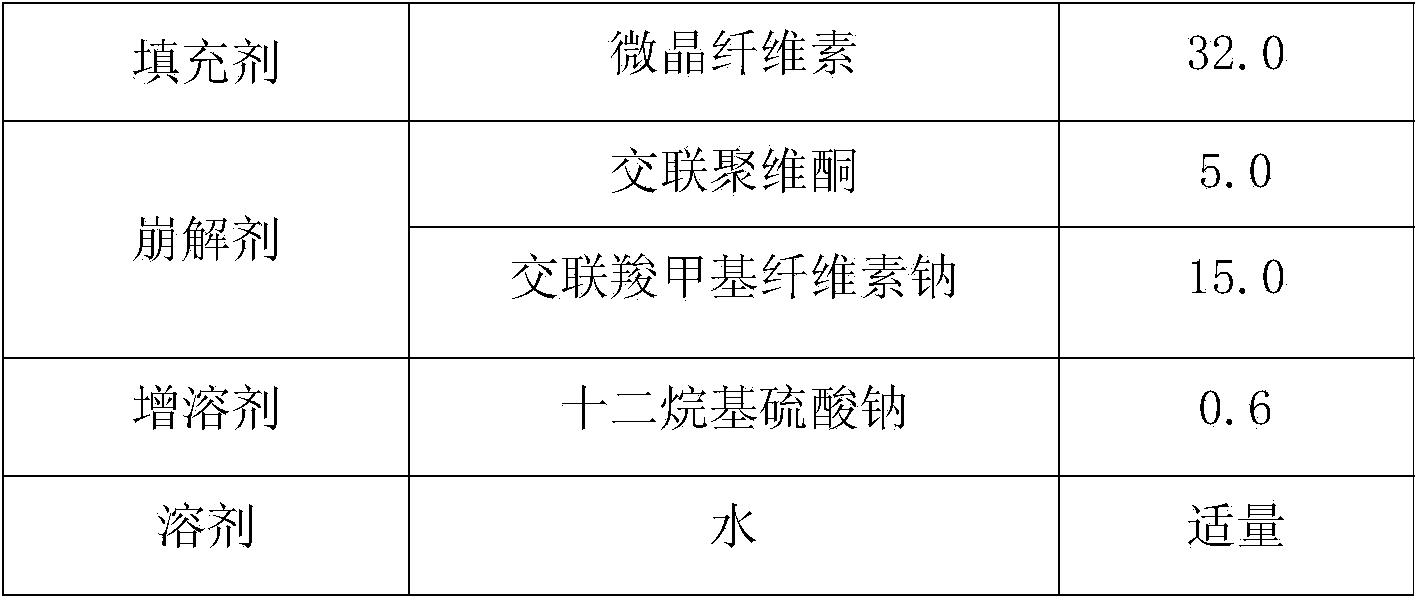
[0069] Add 54.0g of PVP K30 into 2000g of purified water, stir until clarified, add sodium lauryl sulfate while stirring, stir until dissolved and set aside; weigh the raw and auxiliary materials according to the prescription in Table 6, pass through an 80-mesh sieve, and process the raw Put the auxiliary materials into the wet granulator (G6 experimental multifunctional wet mixing granulator, Shenzhen Xinyite Technology), dry mix for 5-10 minutes, slowly add the prepared binder solution, and stir at a speed of 3-5r / min, cutting speed 3-5r / min, wet mixing for 3-6min, discharge, put in a swing granulator (YK160A swing granulator, Jiangsu Changzhou Shuangdong Pharmaceutical Machinery Factory) to make 20 mesh granules. Put the wet granules in a drying oven at 55°C (JC101 electric blast drying oven, Shanghai Chengshun Instrument Co., Ltd.), and dry for 5-7 hours until th...
Embodiment 3
[0082] The preparation of embodiment 3 valsartan tablets
[0083] A preparation of drug-containing granules
[0084] Take 54.0g of PVP K30 and add it into 2000g of purified water, stir until clarified, add sodium lauryl sulfate while stirring, stir until dissolved and set aside; weigh the raw and auxiliary materials according to the prescription in Table 9, pass through an 80-mesh sieve, and process the raw Put the auxiliary materials into the wet granulator (G6 experimental multifunctional wet mixing granulator, Shenzhen Xinyite Technology), dry mix for 5-10 minutes, slowly add the prepared binder solution, and stir at a speed of 3-5r / min, cutting speed 3-5r / min, wet mixing for 3-6min, discharge, put in a swing granulator (YK160A swing granulator, Jiangsu Changzhou Shuangdong Pharmaceutical Machinery Factory) to make 20 mesh granules. Put the wet granules in a drying oven at 55°C (JC101 electric blast drying oven, Shanghai Chengshun Instrument Co., Ltd.), and dry for 5-7 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com