Sustained-release capsules capable of clearing heat and resolving lumps and preparation method thereof
A slow-release capsule, heat-clearing and stagnation-clearing technology, which is applied in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of complex components of traditional Chinese medicine extracts and difficulties in the preparation of sustained-release preparations, and improve the dissolution rate , Reduce the dosage and frequency of medication, and reduce the effect of taking the frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
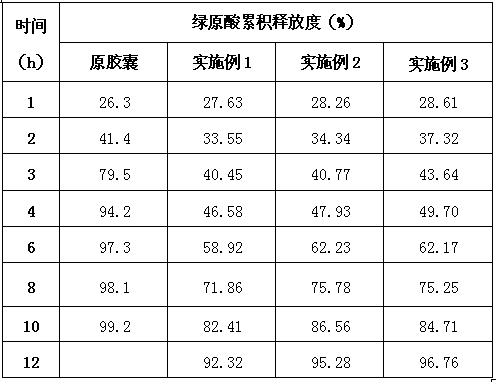
[0020] 1) Preparation of Senecio extract: The preparation method disclosed in the patent application document CN101249126A is used to prepare the Senecio extract. The specific method is as follows: Take 3000g of Senecio, add water to decoct twice, add 10 times the amount of water for the first time, add 8 times the amount of water for the second time, decoct for 2 hours each time, filter, combine the filtrates, and concentrate to 25°C When measuring the thick paste with a relative density of 1.18-1.19, add ethanol equivalent to 1 times the amount of thick paste, let it stand for 12 hours, filter, recover ethanol from the filtrate, and concentrate it to 80 ° C to measure the relative density of 1.30 Thick paste, dried at 70-80°C, ground into fine powder, set aside.
[0021] 2) Formula: Senecio extract 230.8g;
[0022] Hydroxypropylmethylcellulose K4M 75.7g;
[0023] Resistant starch 37.9g;
[0024] PVP-K30 18.9g;
[0025] PEG 6000 15.2g.
[0026] 3) Preparation process of ...
Embodiment 2
[0028] 1) Preparation of Senecio extract: Same as Example 1.
[0029] 2) Formula: Senecio extract 1154.0 g;
[0030] Hydroxypropylmethylcellulose K4M 291.0g;
[0031] Sodium carboxymethylcellulose 87.5g;
[0032] Resistant starch 189.5g;
[0033] PVP-K30 57.0g;
[0034] PEG 6000 113.5 g.
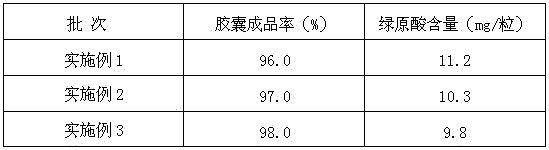
[0035] 3) Preparation process of sustained-release capsules: Take Senecio extract, hydroxypropylmethylcellulose K4M, carboxymethylcellulose, and resistant starch in the formula amount and mix evenly. Dissolve PVP-K30 and PEG 6000 with an appropriate amount of 80% ethanol, spray into the ethanol solution to make soft materials by wet method, granulate with 40 mesh; degas and dry under reduced pressure at 65℃~70℃, moisture <2%, granulate with 30 mesh, and fill , 0.5g / capsule, should get 3768 sustained-release capsules, actually get 3655 capsules.
Embodiment 3
[0037] 1) Preparation of Senecio extract: Same as Example 1.
[0038] 2) Formula: Senecio extract 2308.0g;
[0039] Hydroxypropylmethylcellulose K4M 632.0g;
[0040] Sodium Carboxymethyl Cellulose 315.0g
[0041] Resistant starch 189.0g;
[0042] PVP-K30 265.0g;
[0043] PEG 6000 76.0 g.
[0044] 3) Preparation process of sustained-release capsules: Take Senecio extract, hydroxypropylmethylcellulose K4M, carboxymethylcellulose, and resistant starch in the formula amount and mix evenly. Dissolve PVP-K30 and PEG6000 with an appropriate amount of 80% ethanol, spray into the ethanol solution to make soft materials by wet method, granulate with 40 mesh; degas and dry under reduced pressure at 65℃~70℃ until the water content is less than 2%, granulate with 30 mesh, fill, 0.5g / capsule, should get 7570 sustained-release capsules, actually get 7419 capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com