Construction of long-circulating liposome of fraxetin and its research on anti-enteritis effect
A technology of long-circulating liposomes and qupretin is applied in the directions of liposome delivery, medical preparations of non-active ingredients, and active ingredients of heterocyclic compounds, which can solve the problems of low in vitro release and bioavailability, and achieve Low cost, simple preparation method, and the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The construction of a long-circulating liposome of afracetin and its anti-intestinal inflammatory effect The mass ratio of phospholipid to cholesterol in the prescription (A), the ratio of drug to phospholipid (B) and the dosage of DSPE-PEG 2000 (C) and other influencing factors OK for:
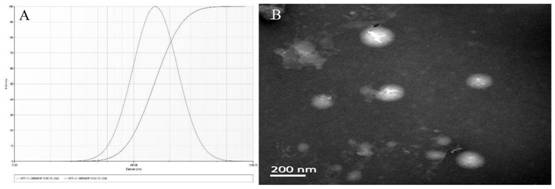
[0032] Taking the mass ratio of phospholipid to cholesterol (A), the ratio of drug to phospholipid (B) and the amount of DSPE-PEG 2000 (C) as the influencing factors, the construction of a long-circulating liposome of afracetin was optimized by orthogonal experiments and its anti-enteritis effect research prescription. Secondly, the quality of the construction of a frucetin long-circulating liposome and its anti-enteritis effect was investigated with the particle size and encapsulation efficiency as indicators. The factor level table is shown in Table 1, and the experimental results are shown in Table 2.
[0033] Table 1 Influencing factors and levels of orthogonal test
[0034] ...
Embodiment 2
[0039] Construction of a long-circulating liposome of fencetin and its preparation for anti-enteritis research:
[0040] The formulation factors affecting EE% and particle size were determined by orthogonal experimental design. In the experimental design, K1, K2 and K3 were used to represent the sum of each level (Table 2). The order of the influence of various factors on the EE% and particle size of afracetin long-circulating liposomes is B>A>C>D, and the best prescription is A 2 B 1 C 2 . The results showed that the frucetin long-circulating liposomes prepared at this time had the smallest particle size and the highest EE%.
[0041] Long-circulating liposomes were successfully prepared by thin-film dispersion method. Lecithin, cholesterol, DSPE-PEG and afracetin were co-dissolved in 20mL absolute ethanol and put into a round bottom flask. The ratio of phospholipids to cholesterol is 10:1, the ratio of fencetin to phospholipids is 1:8, and the dosage of DSPE-PEG2000 is 8%...
Embodiment 3
[0045] Study on Pharmacokinetics of Long-Circulating Liposomes of Frucetin
[0046] (1) In vivo studies
[0047] Sprague-Dawley (SD) rats (200±20g, male) were provided by the Animal Experiment Center of Zhangjiagang Hospital of Traditional Chinese Medicine Affiliated to Nanjing University of Traditional Chinese Medicine. Before the experiment, all mice were fasted for 12 h, but allowed to drink water freely. The 12 rats were randomly divided into two groups, ie, the free frucetin group and the frucetin long-circulating liposome group, with 6 rats in each group. The two groups of rats were given the same dose (200 mg / kg) of free afrescetin (suspended in 0.5% sodium carboxymethylcellulose solution) and afrescetin long-circulating liposomes. Whole blood samples (0.5 mL each) were collected at different time points (0.08, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24 h) after administration. Plasma was collected after whole blood was centrifuged (3700rpm, 10min), and store...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com