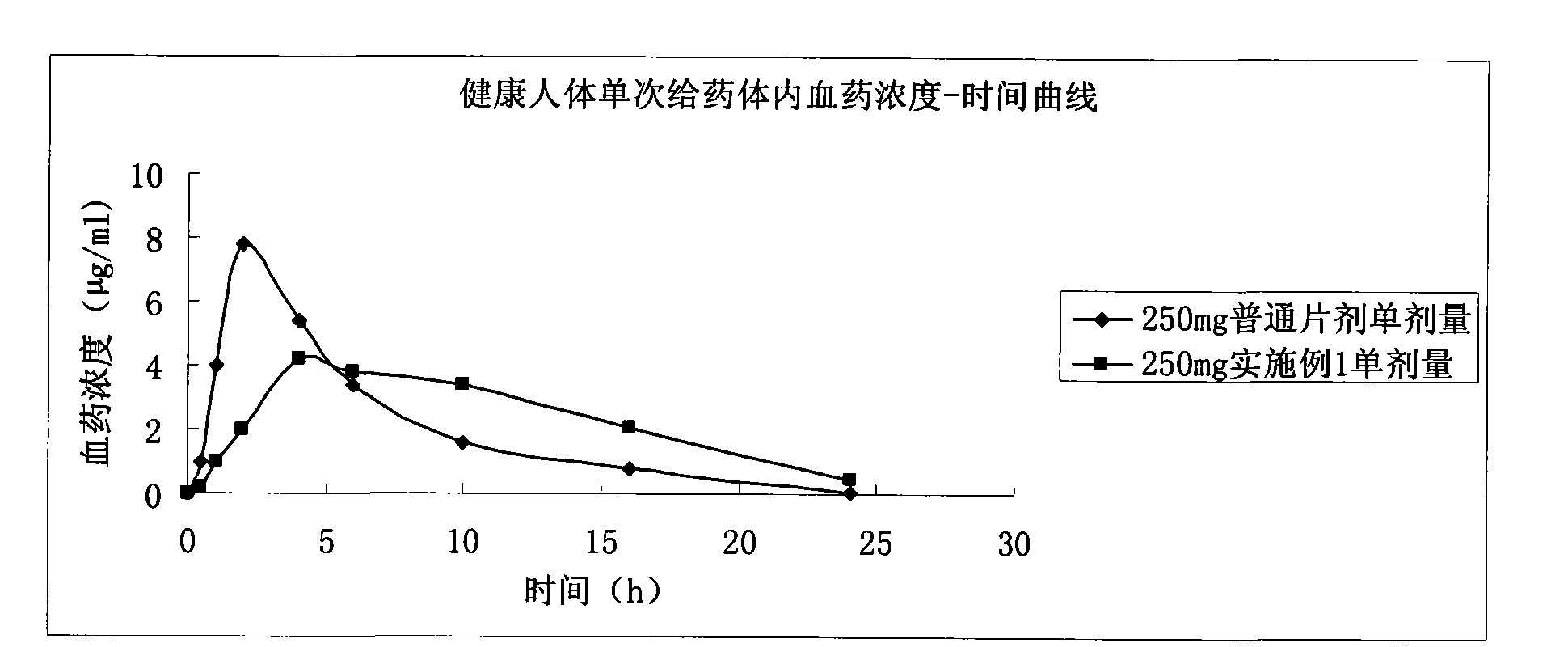
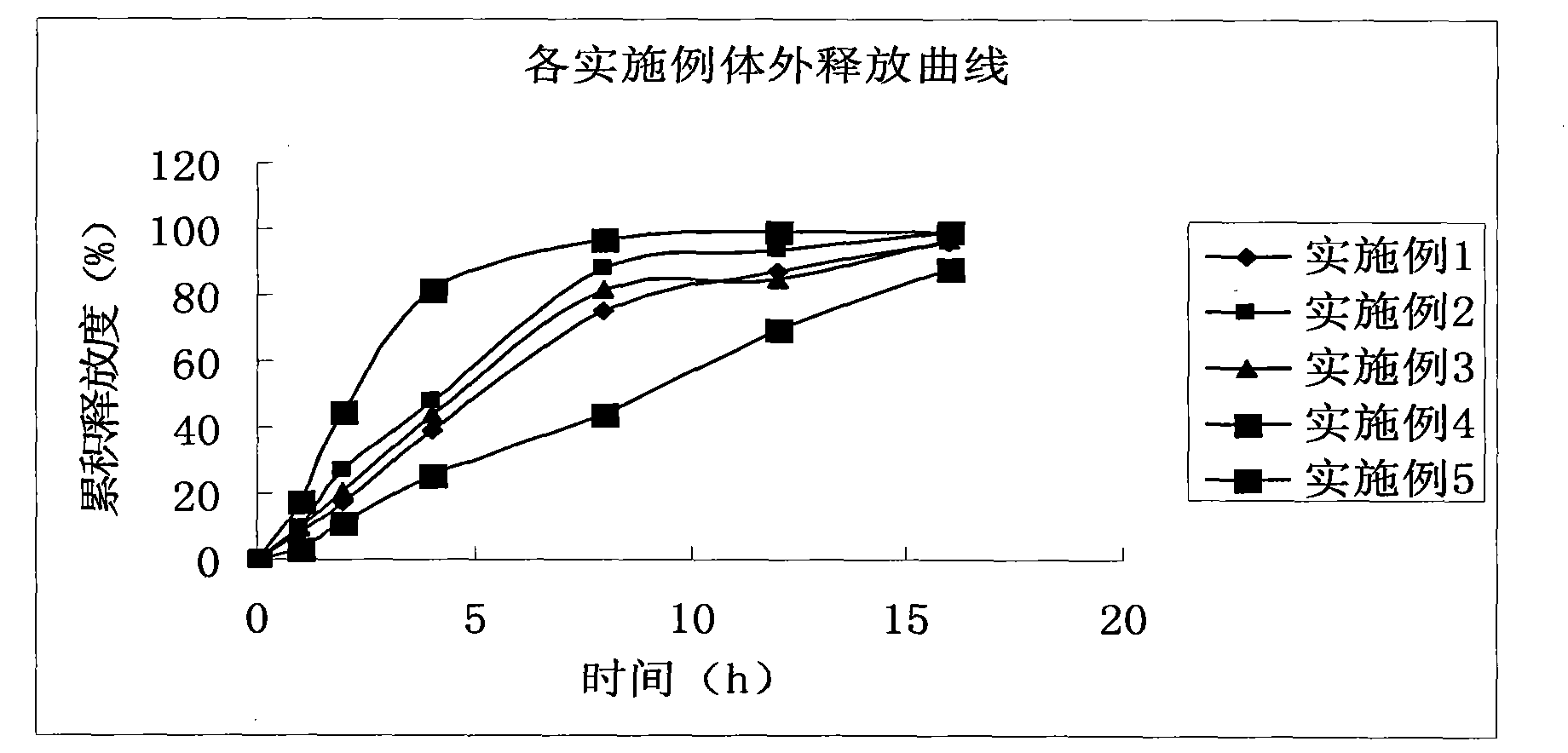
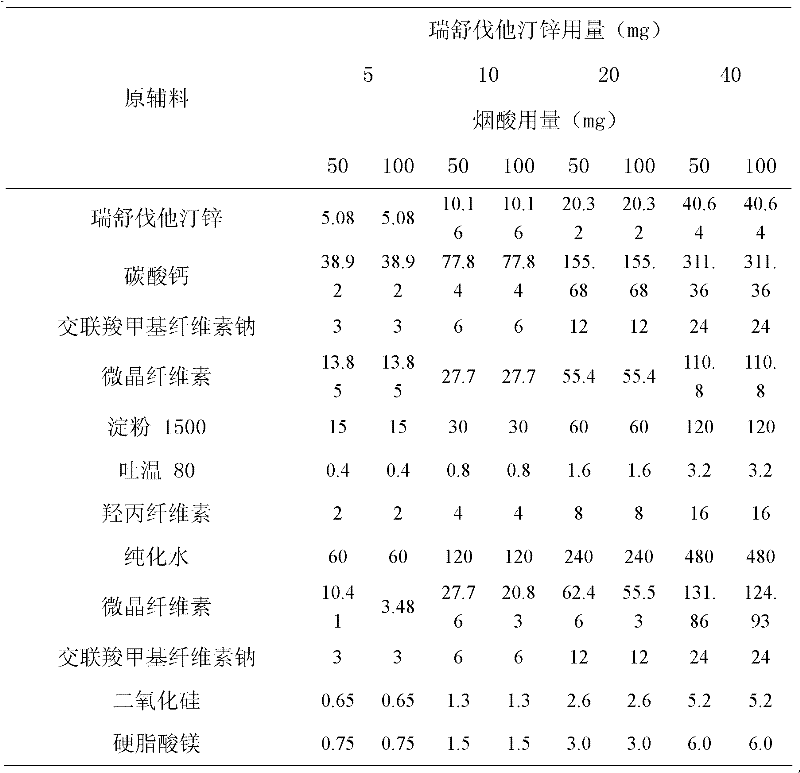
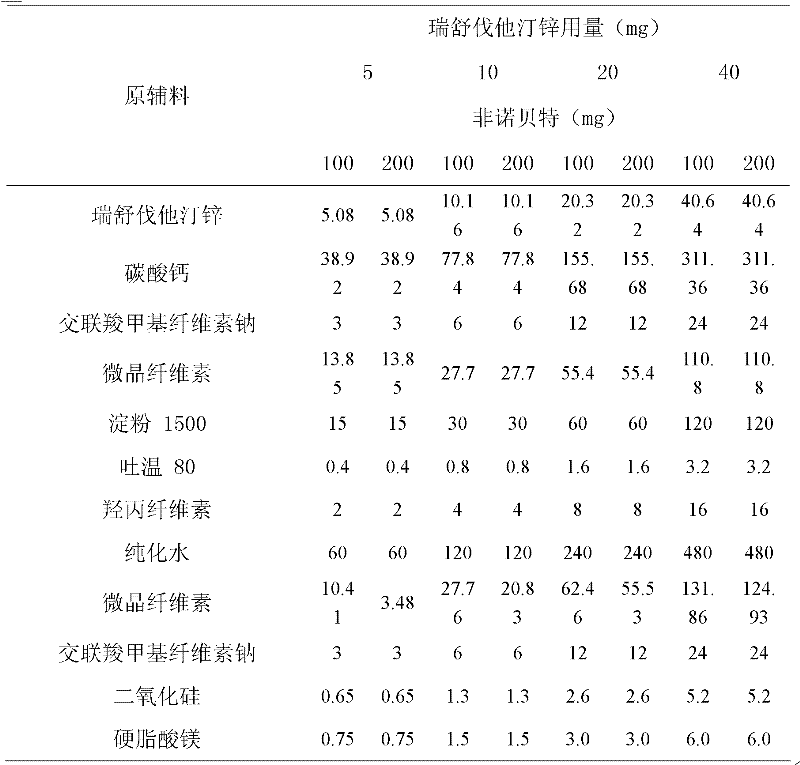
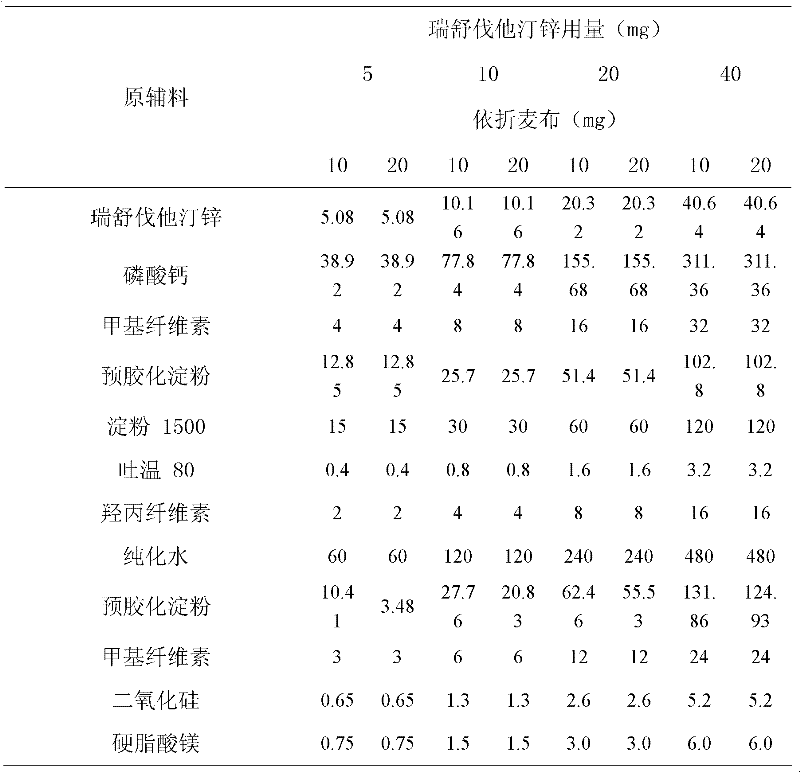
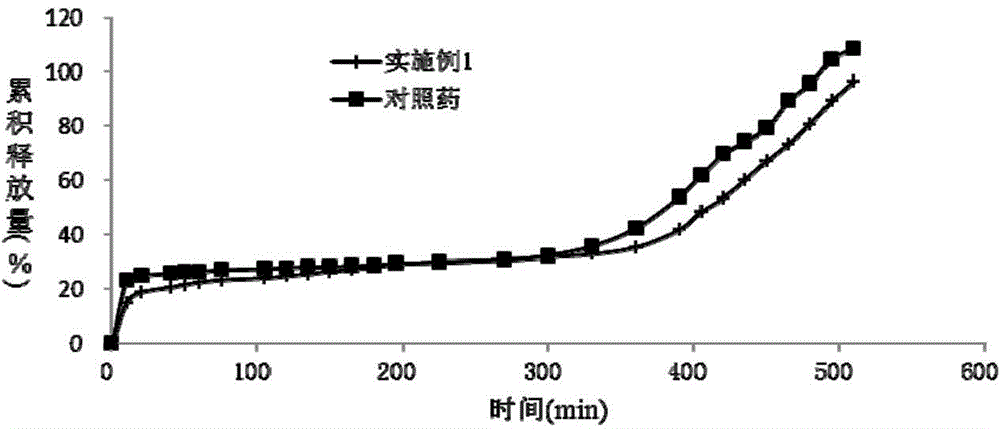
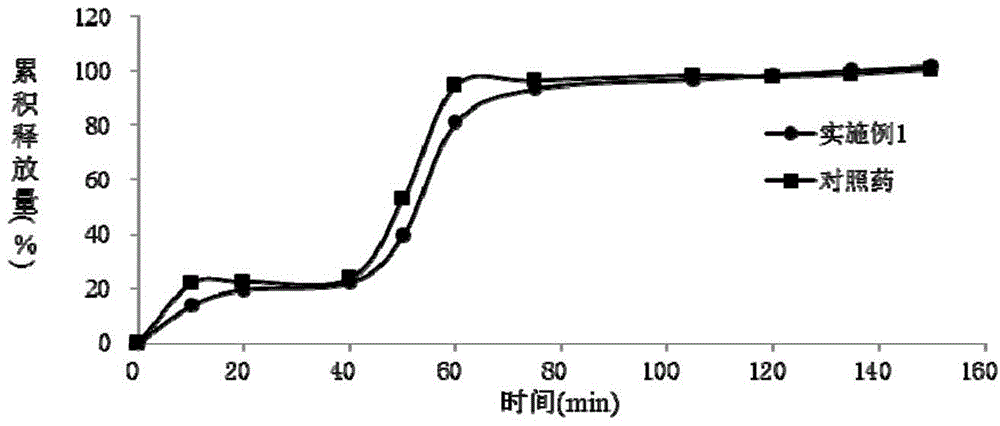
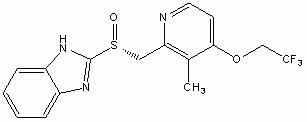
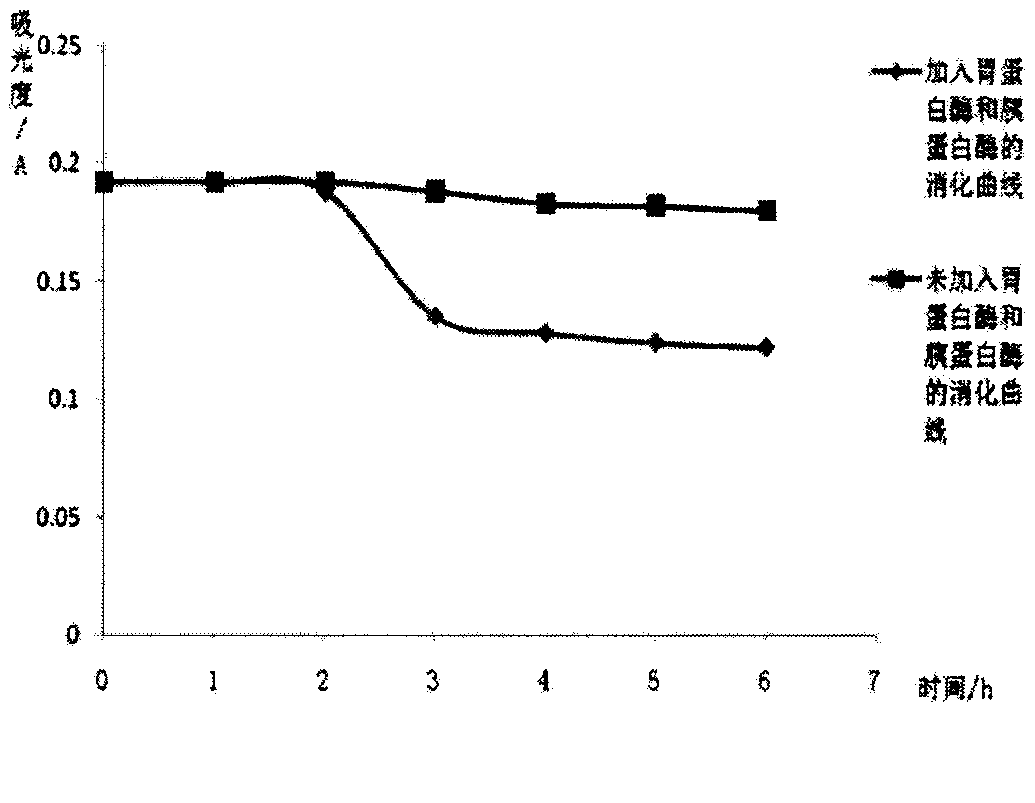
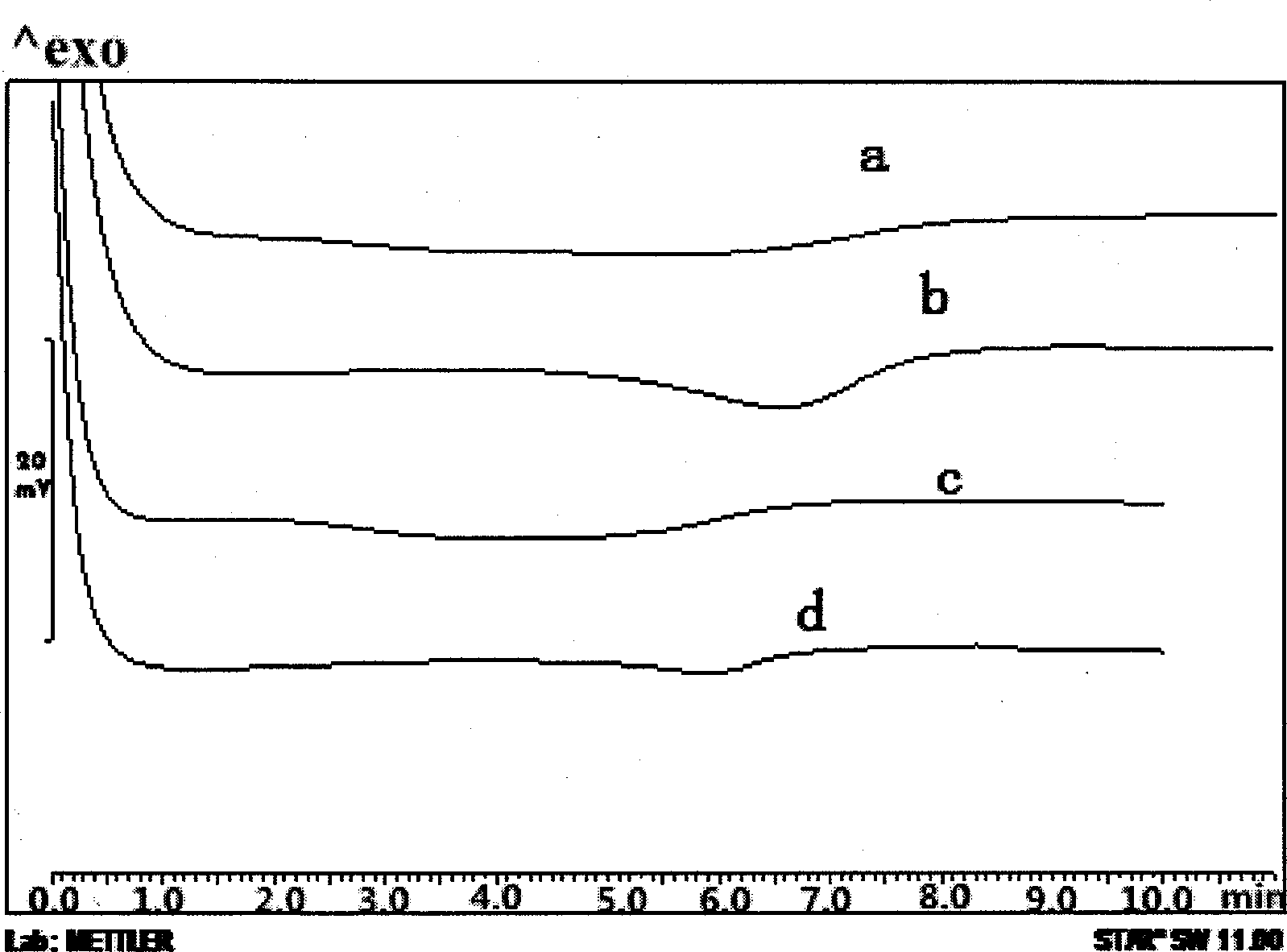
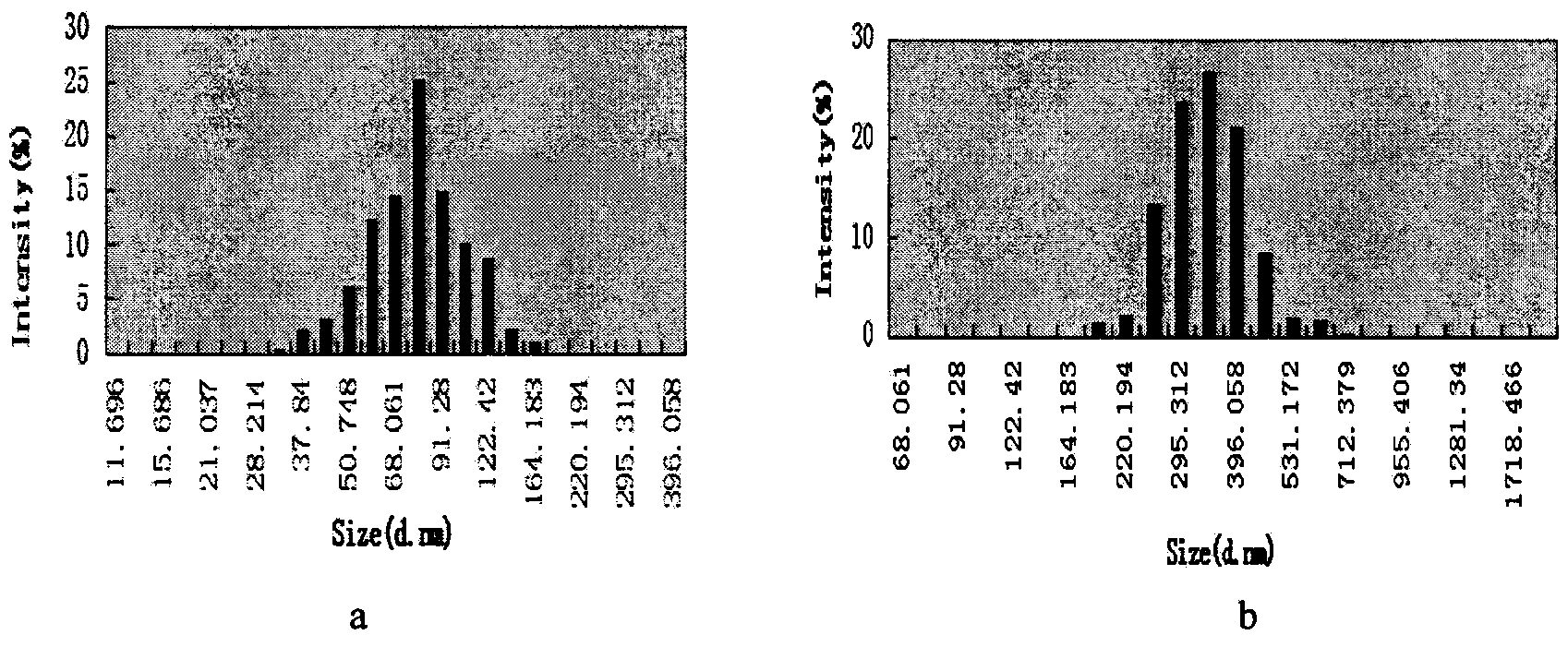
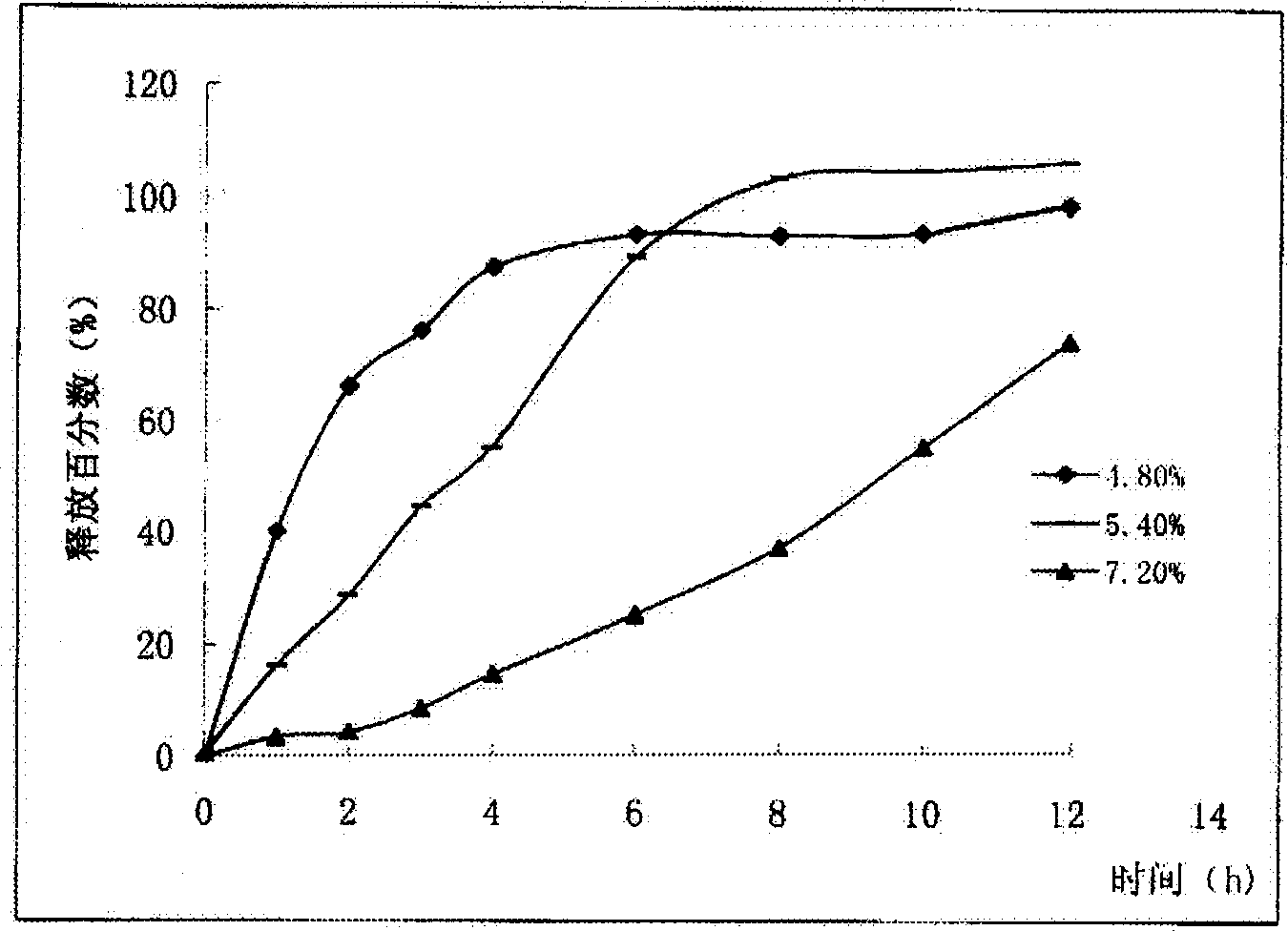
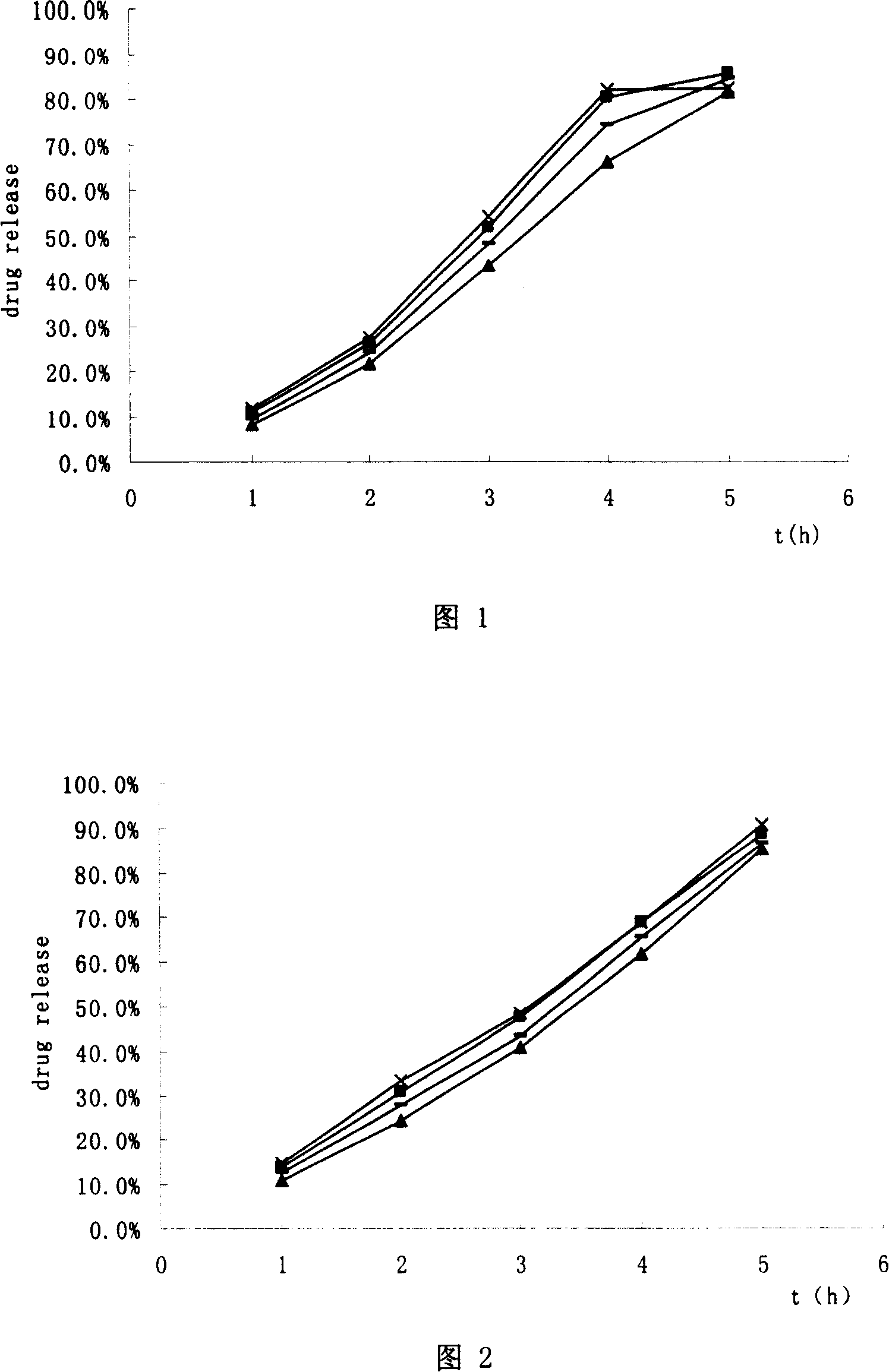
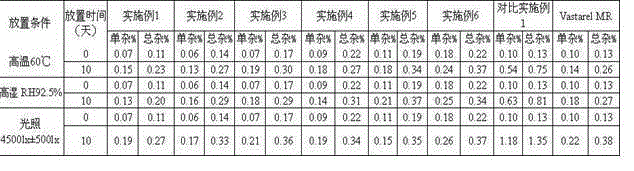
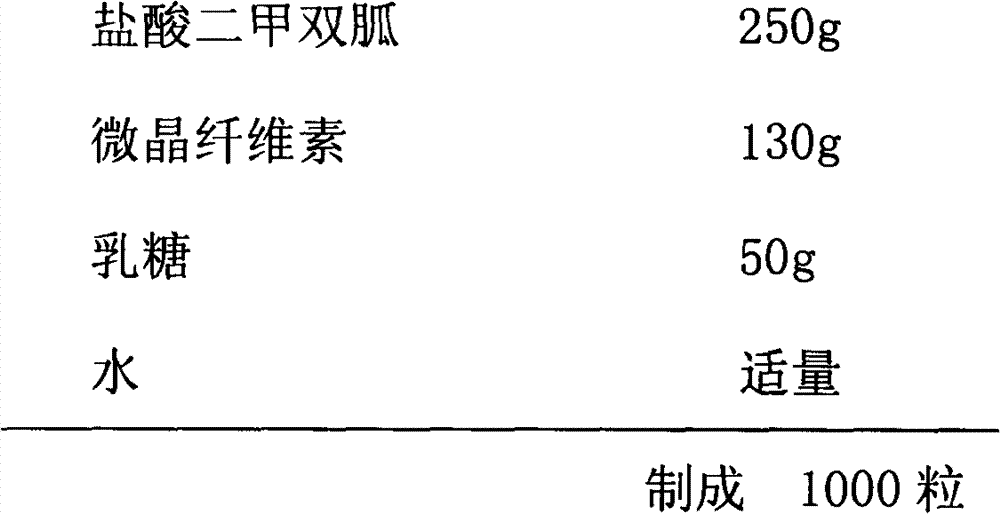
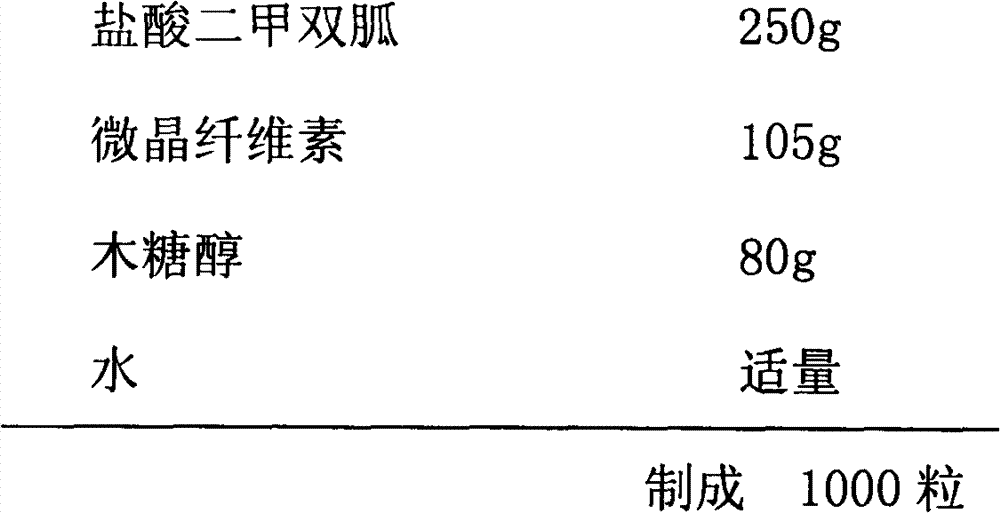
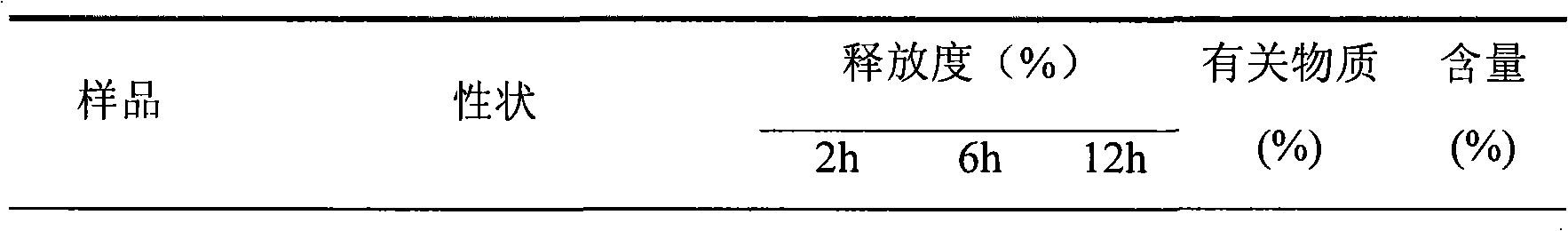
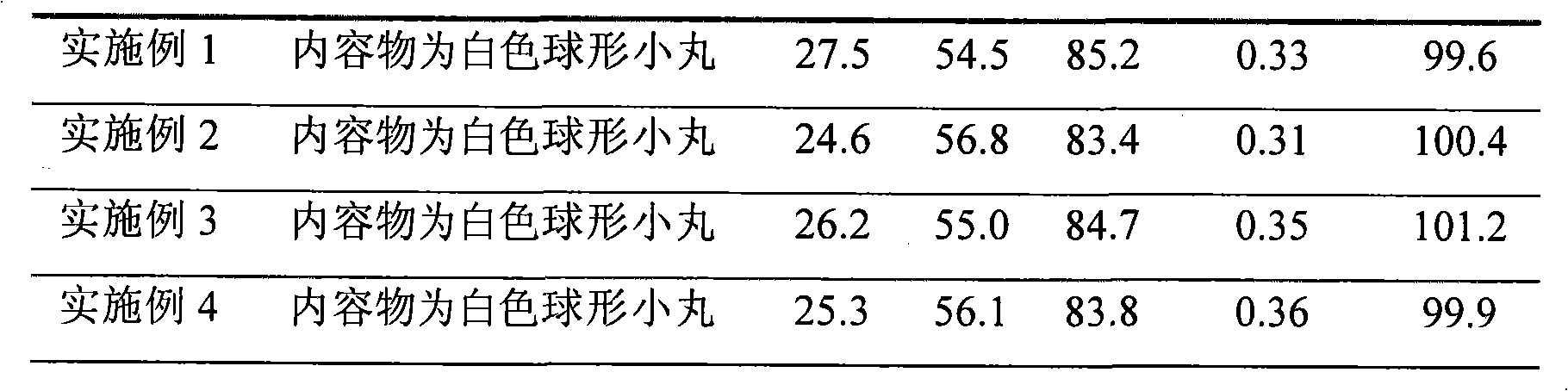
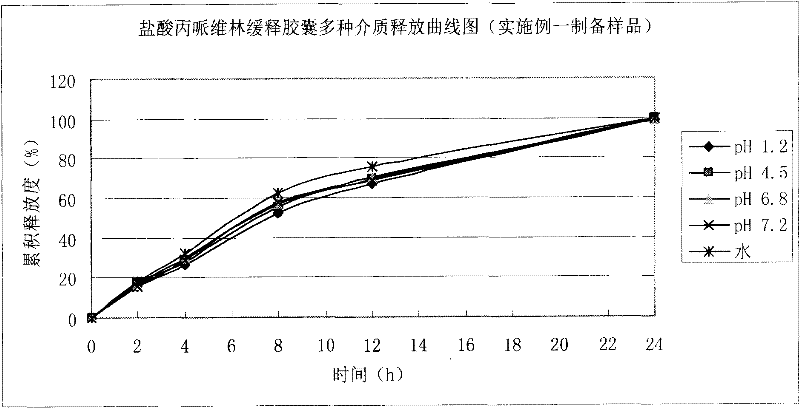
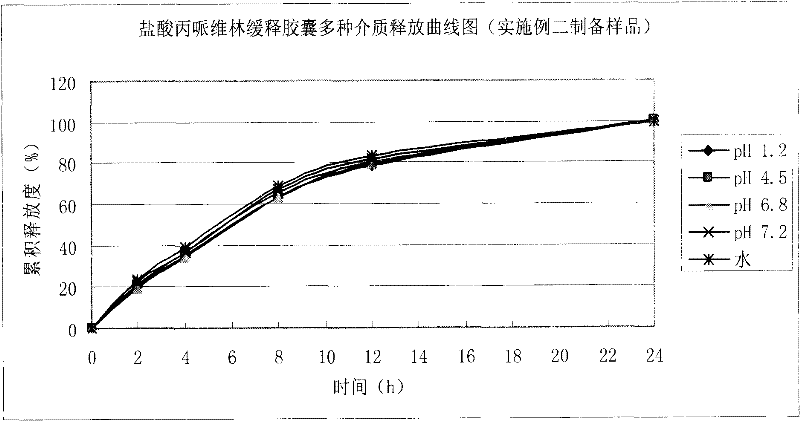
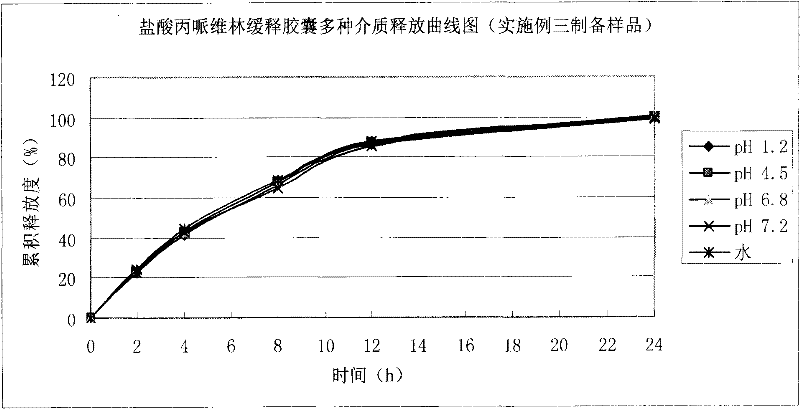
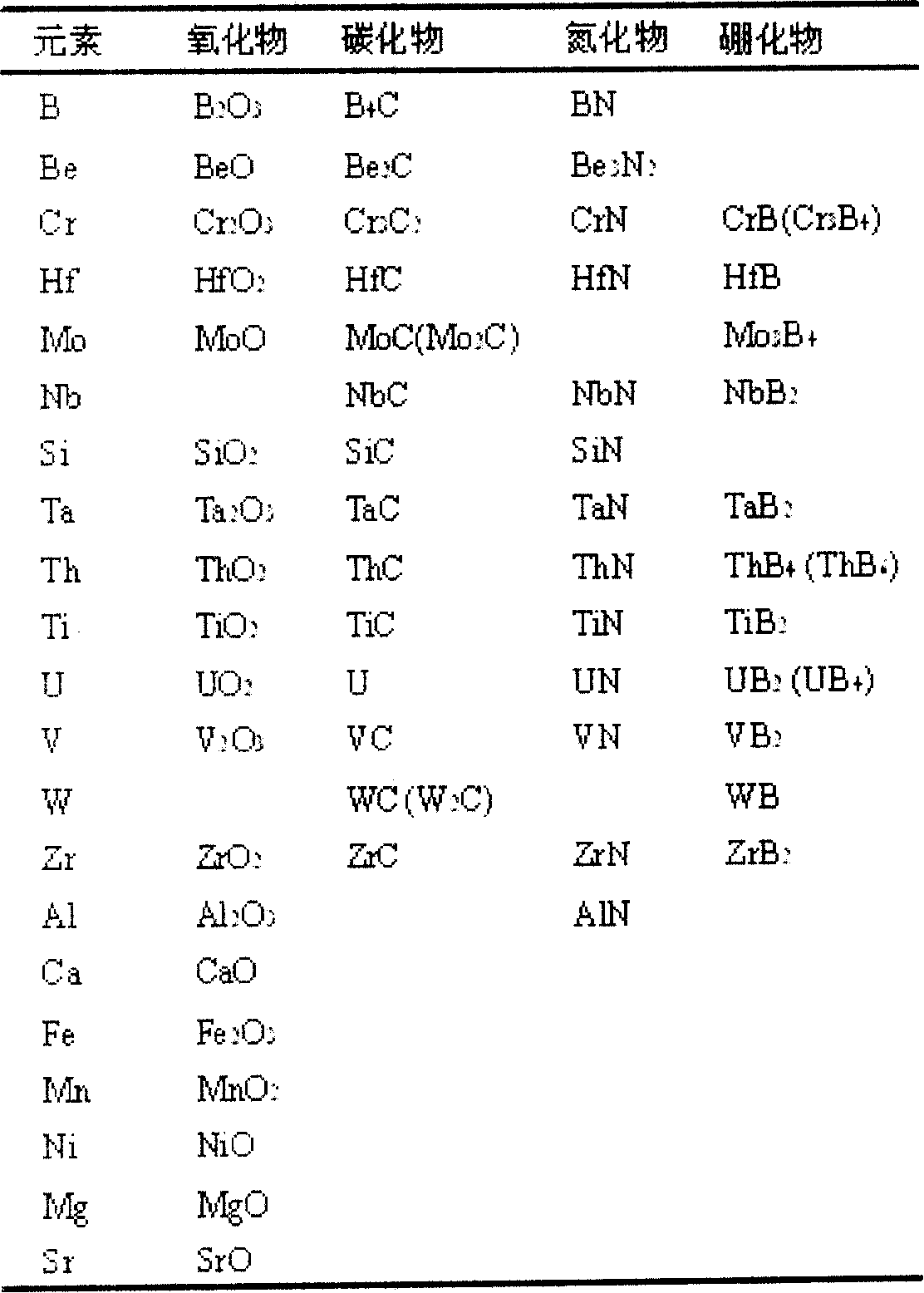
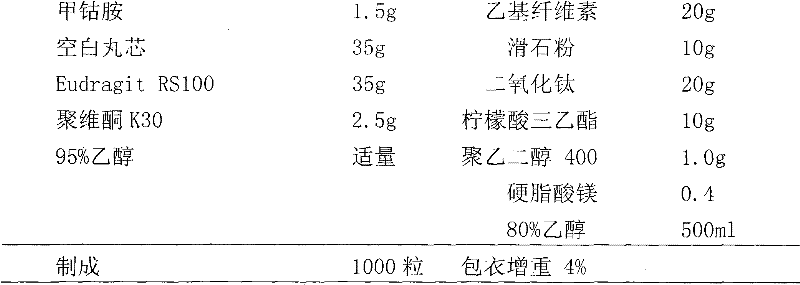
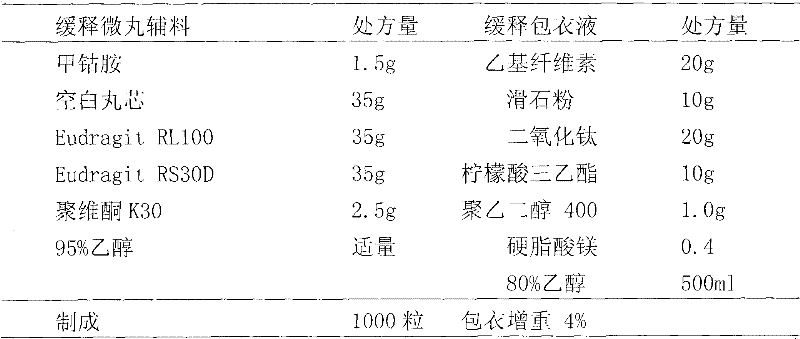
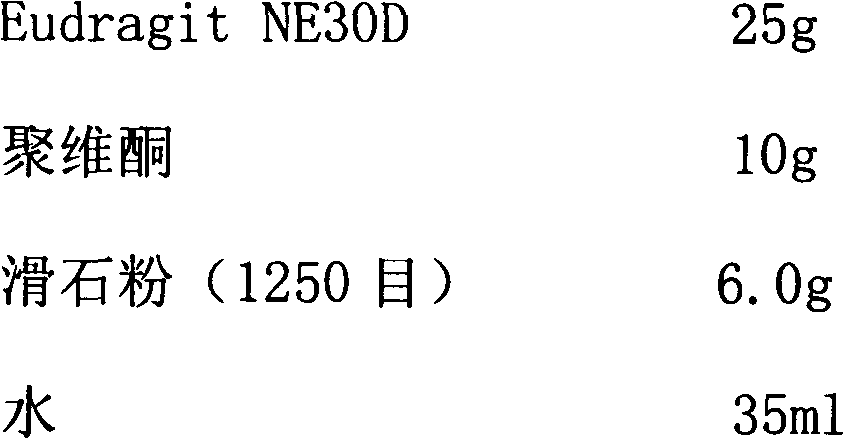Patents
Literature
423 results about "Sustained Release Capsule" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The risk of these health problems can happen as soon as the first weeks of using this medicine (indomethacin sustained-release capsules) and may be greater with higher doses or with long-term use. Do not use this medicine (indomethacin sustained-release capsules) right before or after bypass heart surgery.
Sustained release preparation of licardipine hydrochloride and its preparing process
InactiveCN101011395AGood slow releaseQuick effectOrganic active ingredientsPharmaceutical product form changeSide effectSustained Release Capsule
The invention relates to a method for preparing Licardipine Hydrochloride slow-release agent, which can be used to treat hypertension, coronary disease or the like. The inventive slow-release agent is formed by quick-release stomach-soluble micro drop and slow-release enteric-soluble micro drop at 1:0.5-5 ratios in the hollow capsule. The inventive capsule has slow-release effect in 12 hours. The slow-release enteric-soluble micro drop comprises Licardipine Hydrochloride, medical macromolecule materials, drug release adjusting agent and some medical finding. The micro drops are prepared by extruding-rolling technique. The invention can quickly approach the blood drug density to treatment object and hold the density stably, with low side effect.
Owner:SOUTHEAST UNIV
Method for treating irritable bowel syndrome and other functional gastrointestinal disorders
The invention relates to methods and compositions for the treatment of irritable bowel syndrome (IBS) and other functional gastrointestinal disorders. The methods of the invention involve the administration, in a delayed-release capsule or tablet containing a mixture of peppermint oil and chlorophyll. Other ingredients that may also be effective treatments may be included. This method may be useful as a new and safer treatment for IBS and other functional gastrointestinal disorders. The action of the peppermint oil is effective as a modulator of gastrointestinal motility and sensation. Chlorophyll may improve bowel activity by stimulation of secretion and motility. This is the first description of this unique mixture of these natural products for the treatment of gastrointestinal conditions.
Owner:EHRENPREIS BEN Z +1
Medicinal composition containing ibuprofen sodium salt
InactiveCN102389423AOrganic active ingredientsNervous disorderSustained Release TabletBULK ACTIVE INGREDIENT
The invention relates to a medicinal composition containing ibuprofen sodium salt, which is prepared by taking ibuprofen sodium salt and other one or a plurality of medicinal components as active ingredients, and combing the active ingredients with pharmaceutically appropriate auxiliary materials. The medicinal composition can be made into oral preparations, including conventional tablets, dispersible tablets, sustained release tablets, capsules, sustained release capsules, soft capsules, particles, dry suspension, suspension and the like, and used for symptomatic treatment of being antipyretic, antiphlogistic, analgesic and anti-allergic, and improving sleeping.
Owner:FUKANGREN BIO PHARMA
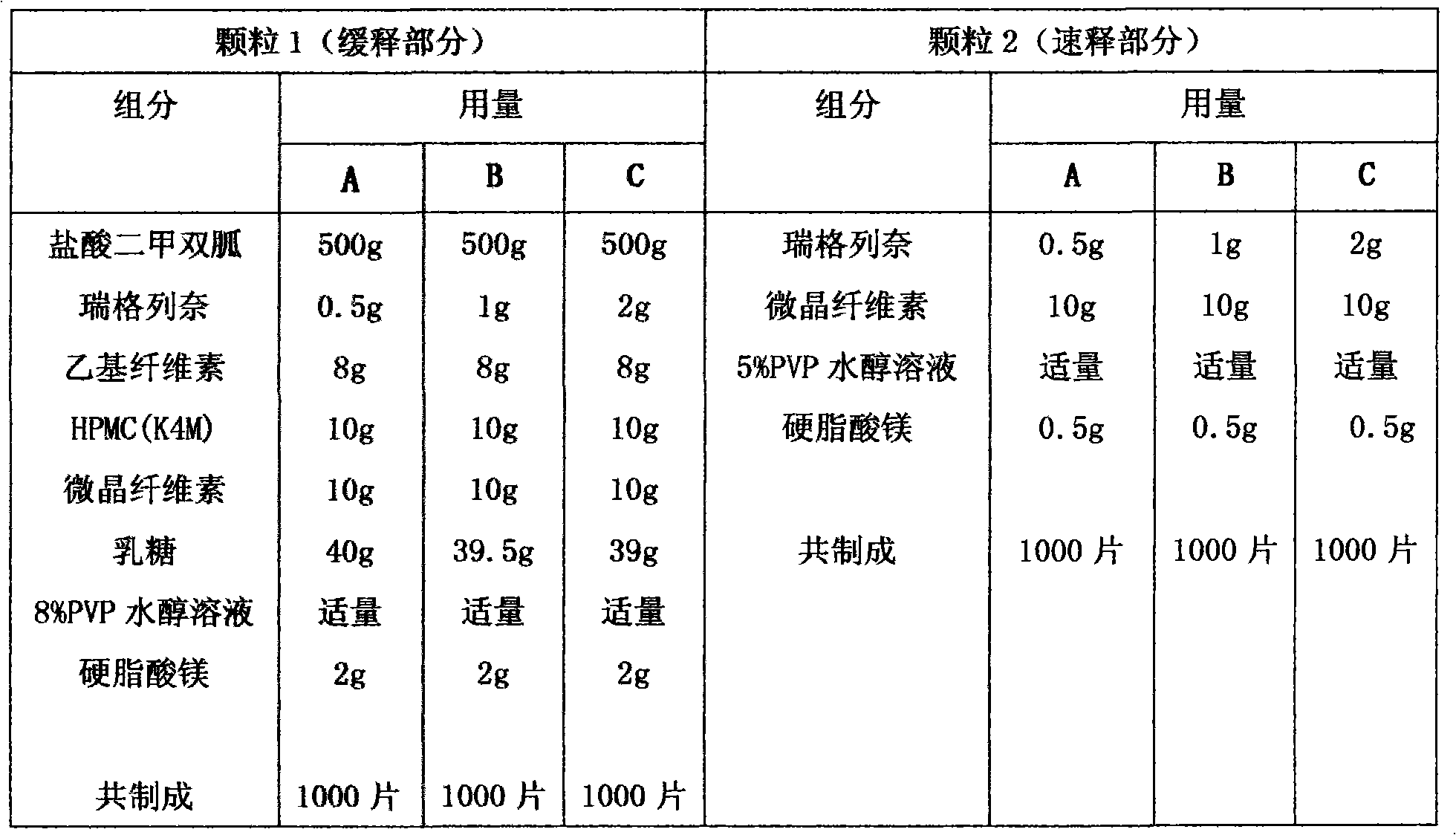
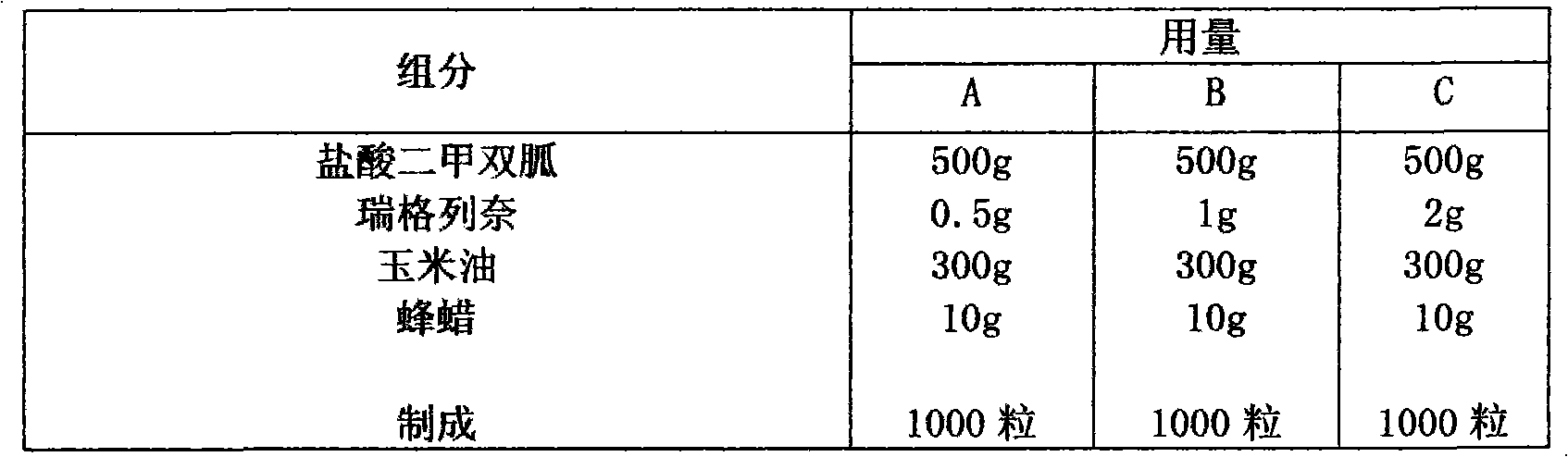
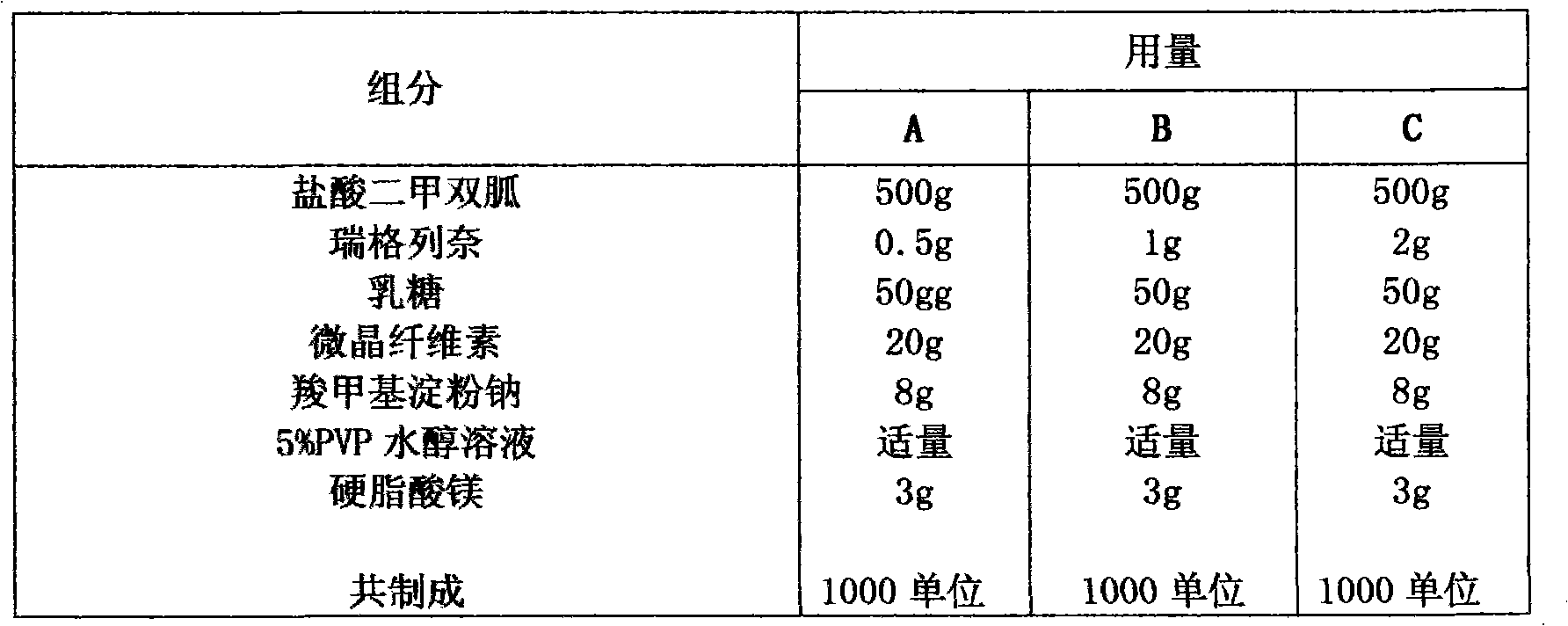
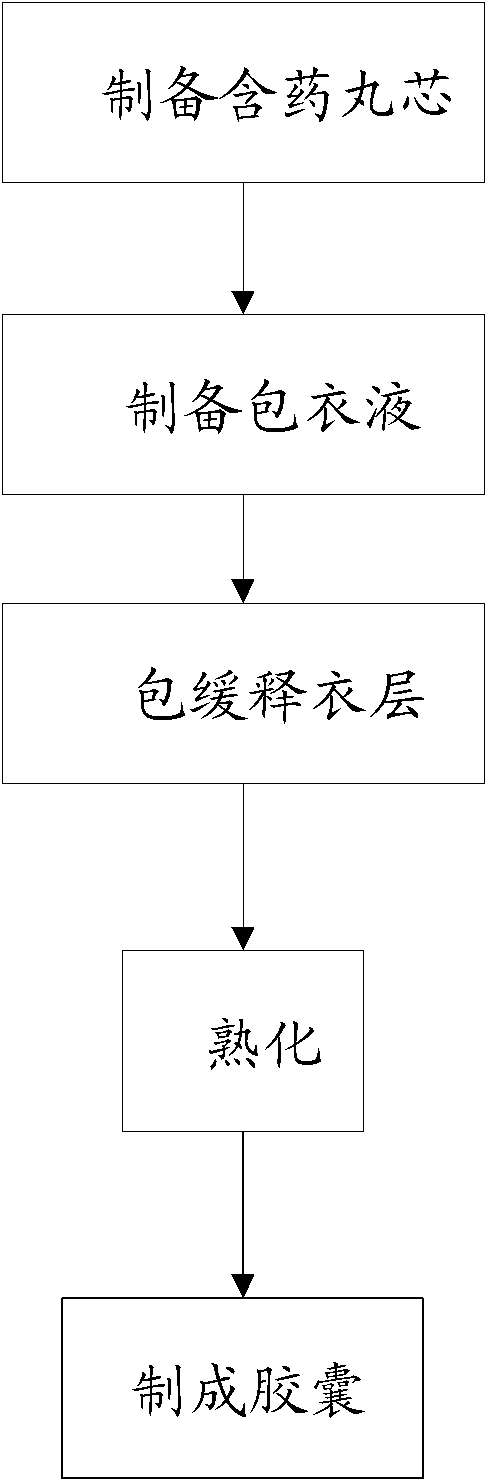
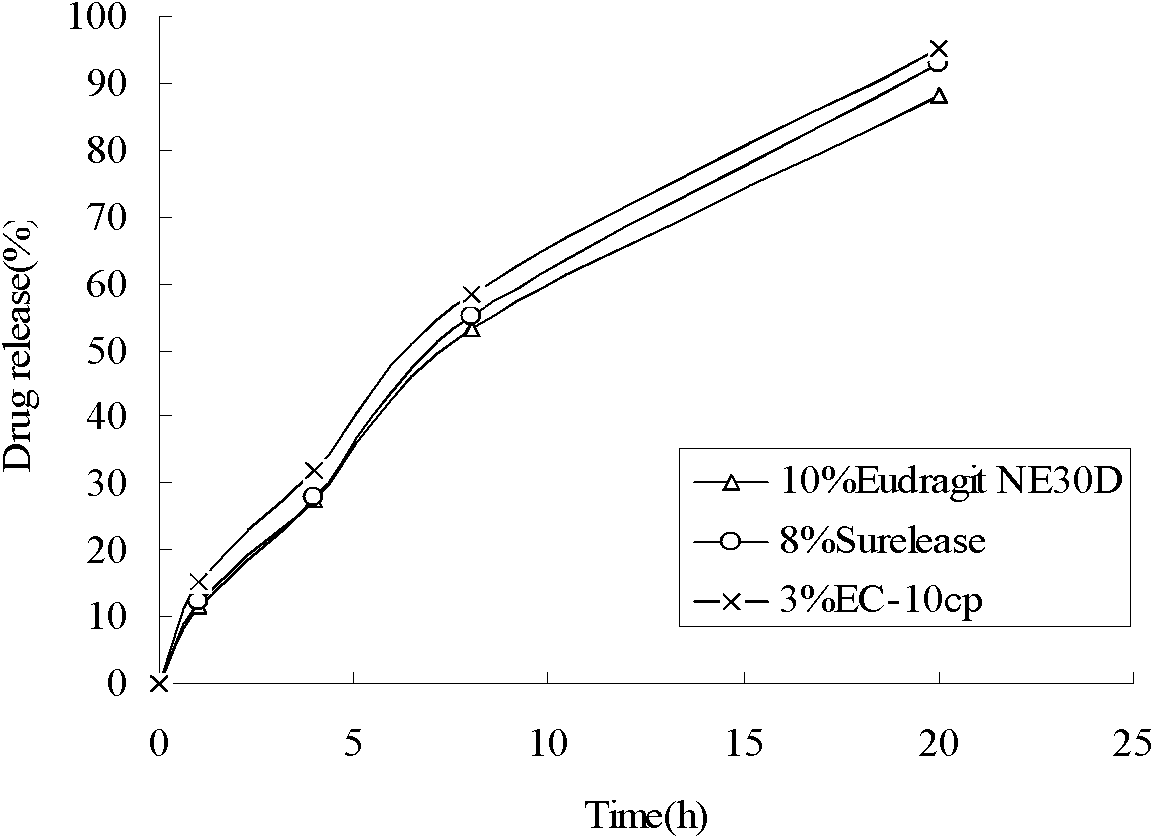
Compound with metformin and repaglinide, preparation method thereof and application thereof
InactiveCN101822672ALower glucose toleranceLower natural responsesOrganic active ingredientsMetabolism disorderInsulin dependent diabetesOrally disintegrating tablet
The invention relates to a composite composition with metformin hydrochloride and repaglinide as active ingredients, a preparation method thereof and application thereof and belongs to the technical field of medicaments. The composite composition is a medicinal composition which is mixed by using the metformin hydrochloride and repaglinide as the active ingredients and by using a carrier and can be prepared into sustained-release tablets, sustained-release granules, sustained-release capsules, common troches and capsules, granules, dispersible tablets, chewable tablets, orally disintegrating tablets, buccal tablets, liquid capsules, soft capsules, drop pills and other oral preparations. The composite composition is used for treating patients with I-type diabetes or II-type diabetes (non-insulin-dependent diabetes) and has synergistic effect on controlling blood sugar.
Owner:深圳南方盈信制药有限公司 +1
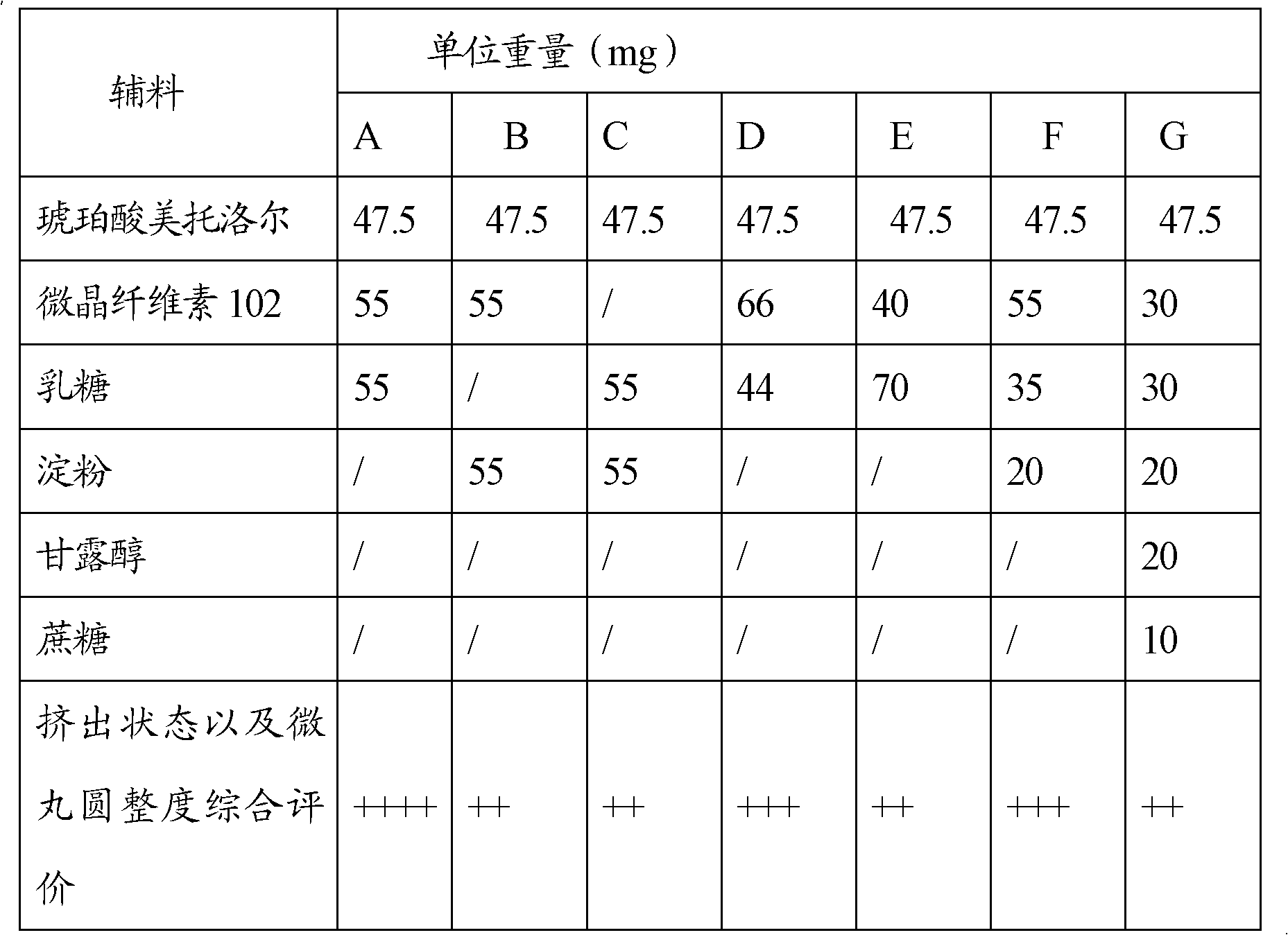
Sustained-release capsules of metoprolol succinate and preparation method thereof
ActiveCN102274205AStable drug releaseSimple processOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsMedicine
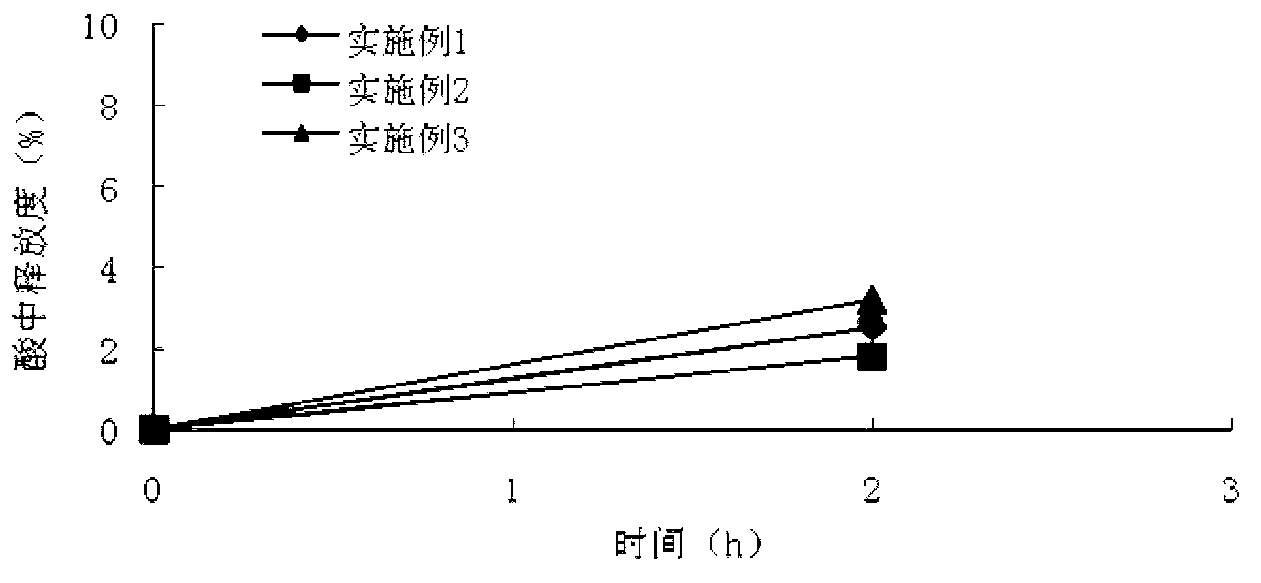
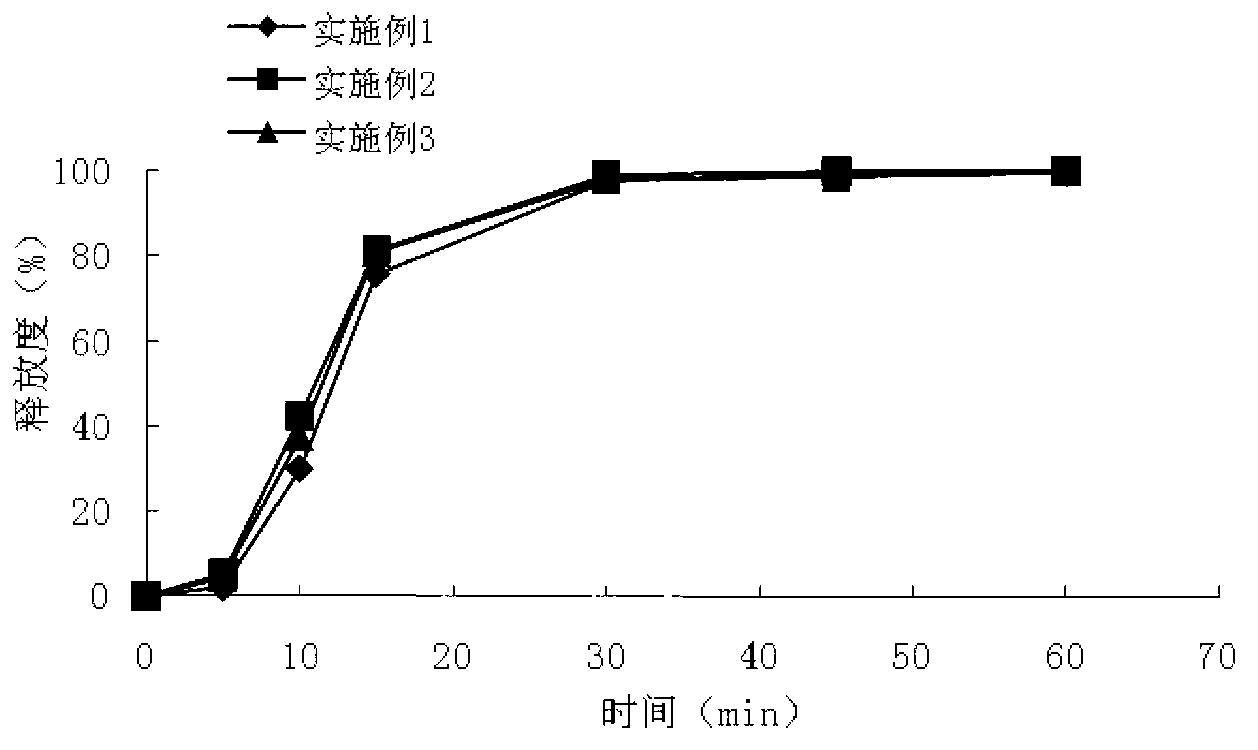
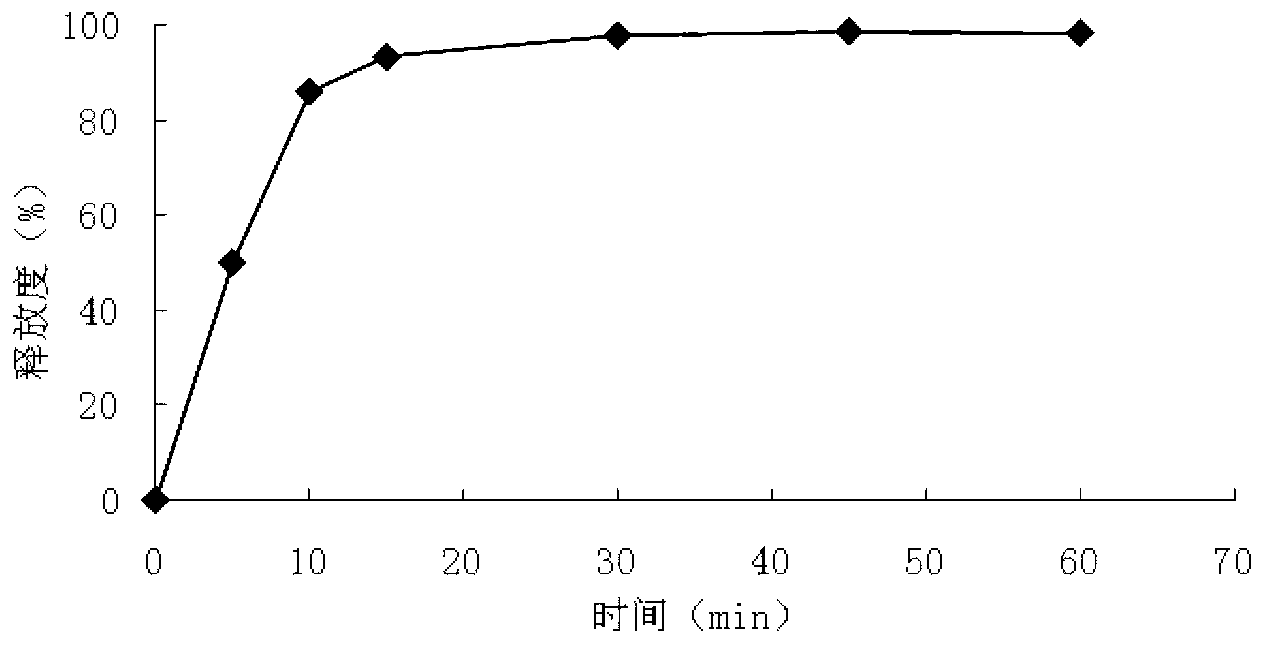
The invention discloses a metoprolol succinate sustained-release capsule, consisting of a capsule shell and contents; the contents comprise a metoprolol succinate sustained-release pellet; the metoprolol succinate sustained-release pellet comprises a metoprolol succinate contained pill core and a sustained-release coating layer coated outside the metoprolol succinate contained pill core; the metoprolol succinate contained pill core is prepared by using metoprolol succinate as the active ingredient and mixing with an auxiliary material, wherein the weight ratio of the metoprolol succinate to the auxiliary material is 1:1.5 to 1:3. The metoprolol succinate sustained-release medicine disclosed by the invention is stable and the expected effects of all release behaviors in acid, alkaline and water can be achieved. The invention further discloses a method for preparing the metoprolol succinate sustained-release capsule; compared with the prior art, the process is simpler and has good operability and reproducibility.
Owner:佛山市隆信医药科技有限公司
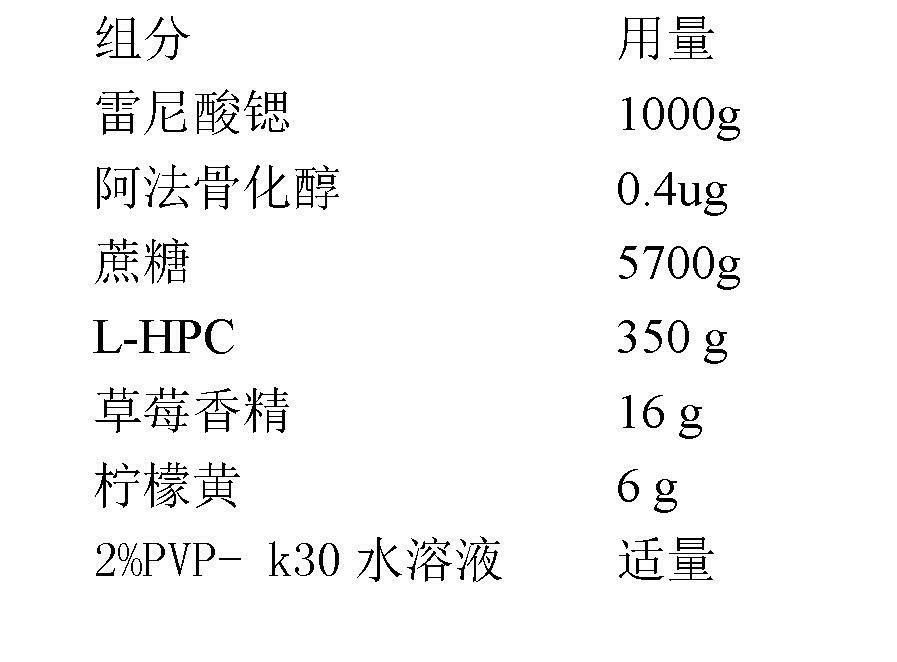
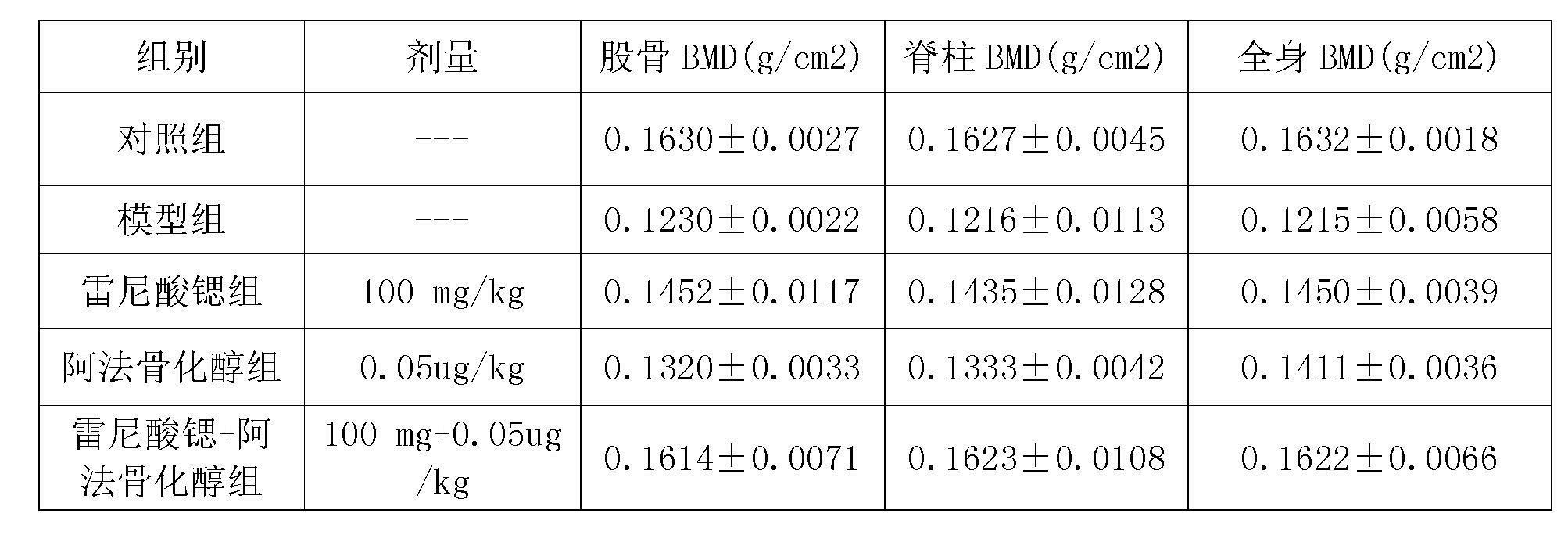
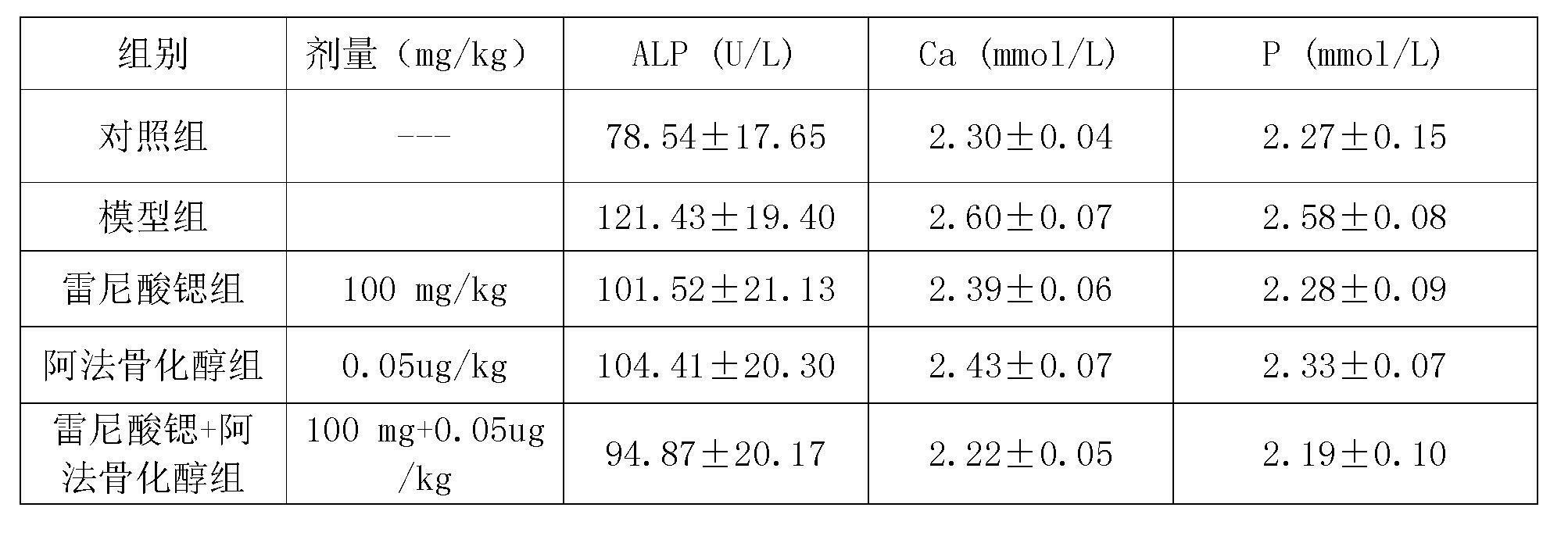
Medicinal composition containing strontium salt
The invention relates to a medicinal composition containing strontium salts and vitamin D derivatives. Mixed with auxiliary materials acceptable on pharmacy, the medicinal composition of the invention can be prepared into oral formulations such as particulate granules, common tablets, chewable tablets, dispersible tablets, orally disintegrating tablets, effervescent tablets, buccal tablets, capsules, softgels, sustained release tablets, sustained release capsules, oral solutions, syrups, etc., and can be used to prevent and treat various primary or secondary osteoporosis.
Owner:FUKANGREN BIO PHARMA
Memantine hydrochloride sustained-release capsule and preparation method thereof
ActiveCN103181914ASustained releaseSmooth releaseNervous disorderPharmaceutical non-active ingredientsSustained release pelletsMemantine Hydrochloride
The invention provides a preparation method of a memantine hydrochloride sustained-release preparation. According to the present invention, sustained-release pellets are obtained by sustained-release coating of drug-containing pellets containing memantine hydrochloride. The drug-containing pellets are obtained by extrusion spheronization or solution medicine-feeding or suspension medicine-feeding, and are nearly circular in shape. The shape and granularity-controllable drug-containing pellets are subjected to sustained-release coating and the thickness of the film formed by coating can also be controlled. The invention realizes the controllability of the coating film and the spherical pellets, and reproducibility of stability of releasing the memantine hydrochloride is controlled under the circumstance of guaranteeing nonoccurrence of crystal form of memantine hydrochloride. The preparation of the invention can provide sustained release in the form of a single dose within 24 hours. The drug penetrates and diffuses to the release medium through the film pores. Because the size is small, the medicine taking is less susceptible to foods and the efficacy is improved. The production method of the present invention is simple, is suitable for industrial production, and has a great application value.
Owner:SHANGHAI FOSUN PHARMA DEV CO LTD
Load-controllable hydrophobic pesticide sustained-release microcapsule and preparation method thereof
ActiveCN102939964AIncrease coverageDrug loading can be controlledBiocideAnimal repellantsSustained Release CapsuleDivalent metal ions
The invention discloses a load-controllable hydrophobic pesticide sustained-release microcapsule and a preparation method and applications thereof. The microcapsule comprises a core material and a wall material, wherein the wall material is prepared from hydroxyl acrylate polymers with carboxyl groups under the crosslinking of divalent metal ions, and the hydrophobic pesticide core material is fed into the microcapsule. The preparation method comprises the following steps of: firstly, dissolving a hydrophobic pesticide and hydroxyl acrylates so as to form a polymer solution; then, adding water into the polymer solution, and carrying out high-speed stirring on the obtained mixture so as to form an emulsion; and finally, adding a divalent metal ion solution into the emulsion so as to obtain a granular condensation product, and filtering and drying the obtained product. The size of the microcapsule can be flexibly controlled at 0.5-50 microns, and the mass of the core material accounts for 5-70% of the total weight of a sustained-release capsule. According to the sustained-release capsule disclosed by the invention, through adjusting parameters such as the compositions of the wall material, the solvent types, the ratio of the core material to the wall material, and the like, the effective loading of one or more hydrophobic pesticides can be realized, and the obtained drug-carrying microcapsule has an advantage that the drug-carrying capacity and the sustained-release performance are controllable, therefore, the preparation method is applicable to the preparation of multiple hydrophobic pesticide sustained-release microcapsules.
Owner:ZHONGKAI UNIV OF AGRI & ENG
Auxiliary composition and application thereof for low-temperature one-bath scouring and bleaching process of cotton textiles
ActiveCN102268814AAvoid affecting the effect of bleachingBleach smoothBleaching apparatusTextile printerSustained Release Capsule
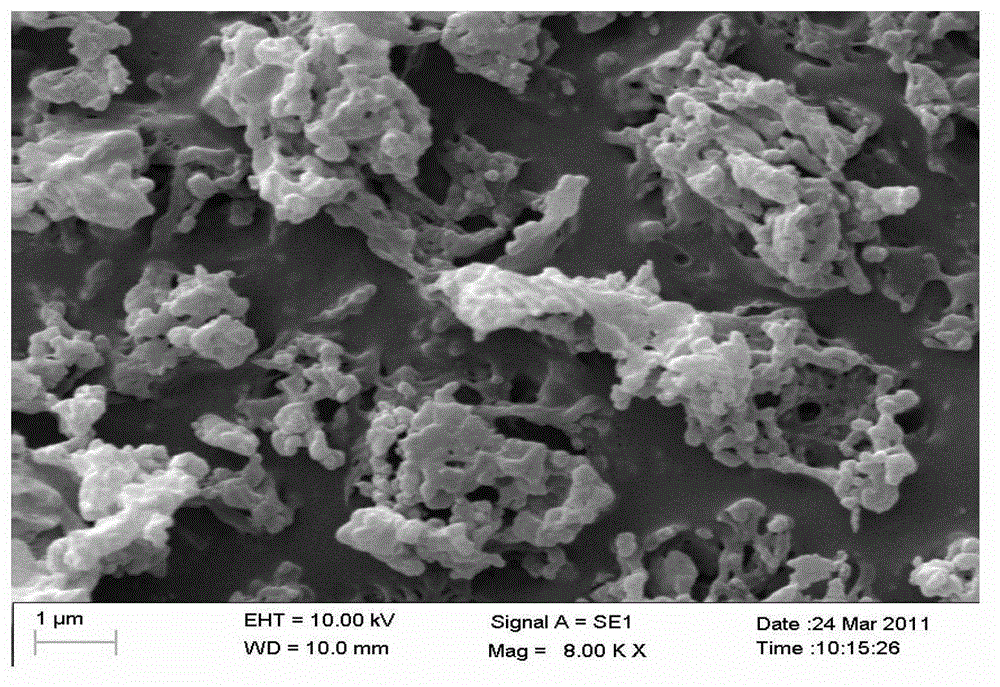
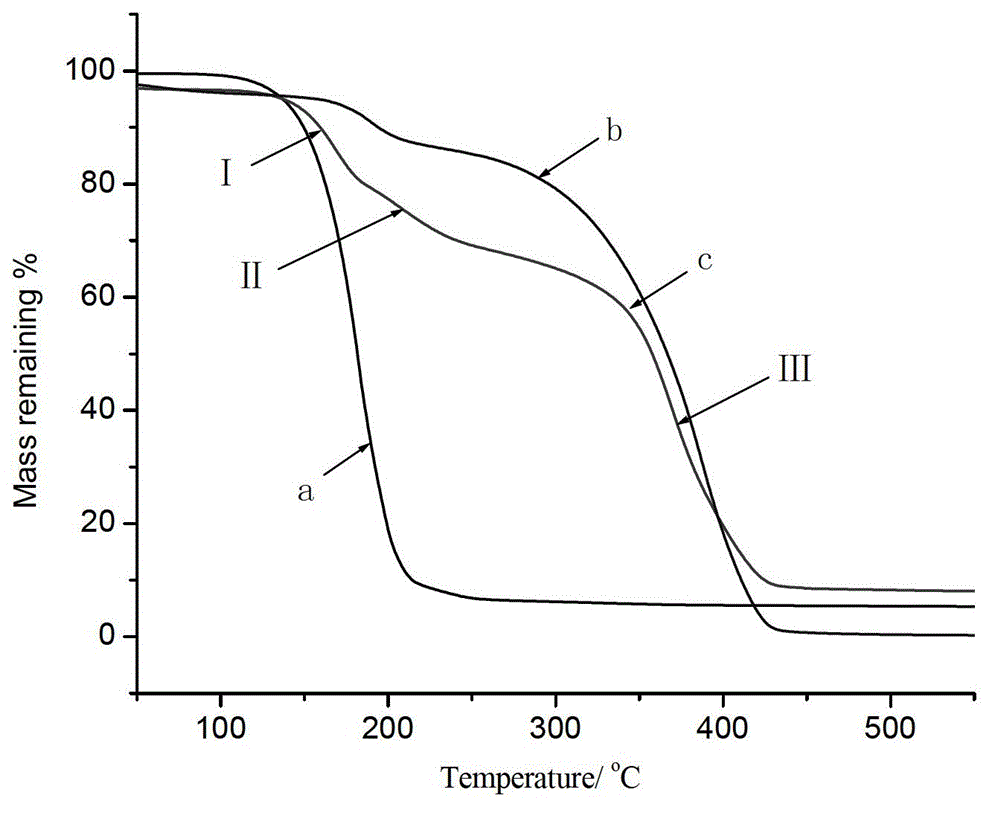
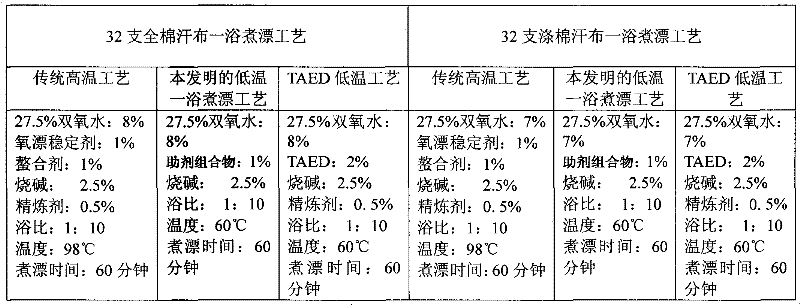
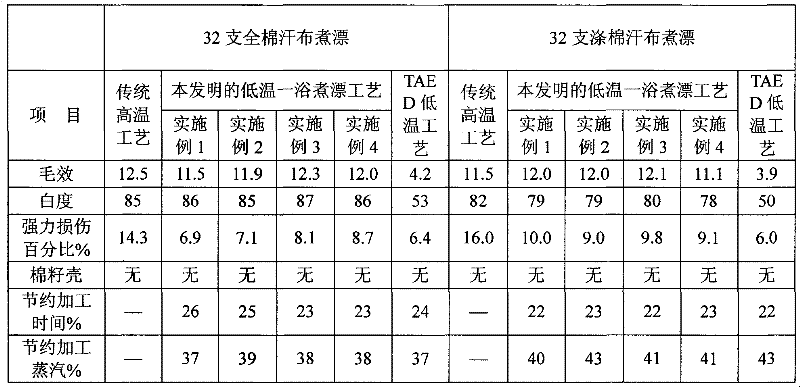
The invention relates to an auxiliary agent composition used in a low temperature one-bath scouring and bleaching process for cotton textile and application thereof, belonging to the technical field of textile printing and dyeing. The auxiliary agent composition comprises, by weight, 10 to 15% of a hydrogen peroxide activator, 2 to 5% of low temperature activating catalyst sustained release capsule, 10 to 15% of a chelating agent, 30 to 40% of diatomite, 10 to 15% of a surfactant and 19 to 31% of a bulking agent. The invention also provides a low temperature one-bath scouring and bleaching process for cotton textile. The process comprises the steps of adding an effective amount of the auxiliary agent composition, hydrogen peroxide and caustic soda into cotton textile and carrying out scouring and bleaching at a temperature of 55 to 60 DEG C for 45 to 60 minutes. According to the invention, cooperative utilization of the auxiliary agent composition, hydrogen peroxide H2O2 and caustic soda enables the low temperature one-bath scouring and bleaching process in the invention to be realized and scouring and bleaching one-bath treatment at or below a temperature 60 DEG C to be carried out, and the effects of scouring and bleaching by using the process provided in the invention are identical with the whiteness and capillary effects of conventional high temperature alkali hydrogen peroxide one-bath process carried out at a temperature of 98 to 100 DEG C, or even at 130 DEG C.
Owner:义乌市中力工贸有限公司 +1
Prepn of medicine for treating hepatosis
InactiveCN1403134AImprove bioavailabilityProlong the action timeDigestive systemAntiviralsOrganic solventMedicine
The present invention relates to the preparation of medicine for treating hepatosis. Coarse Ganhuangcao powder is extracted with water or organic solvent to obtain extractive and the extractive is mixed with supplementary material to prepare the medicine in different forms, including tablet, capsule, dropping pill, injection, delayed releasing capsule, etc. The medicine is easy to take, high in biological utilization and long in the time for the active component of Ganhuangcao to act in the body.
Owner:江云 +1
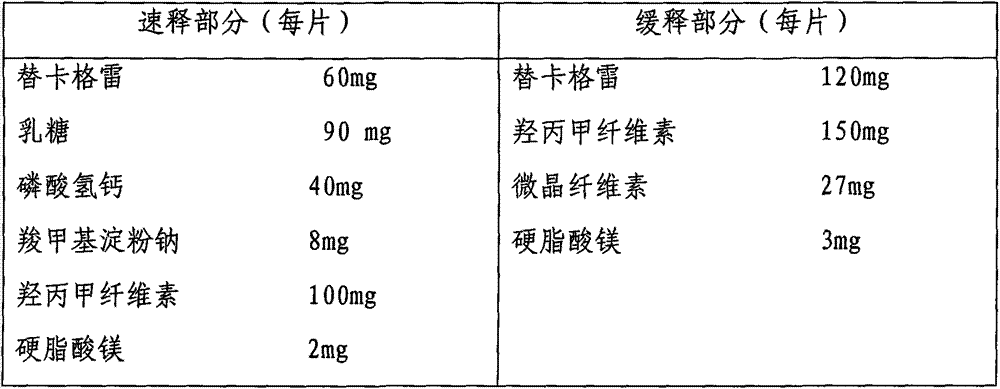
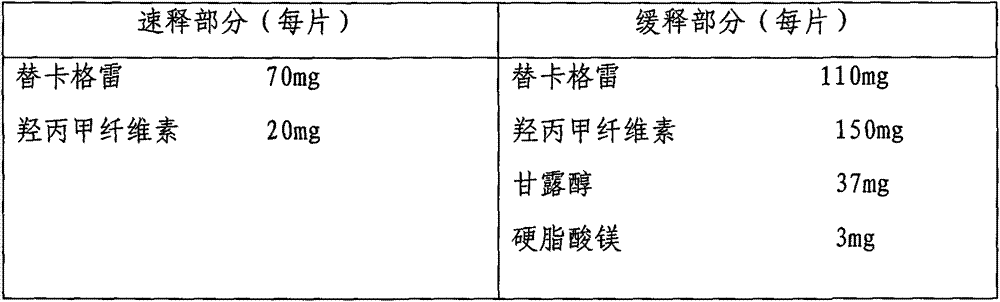
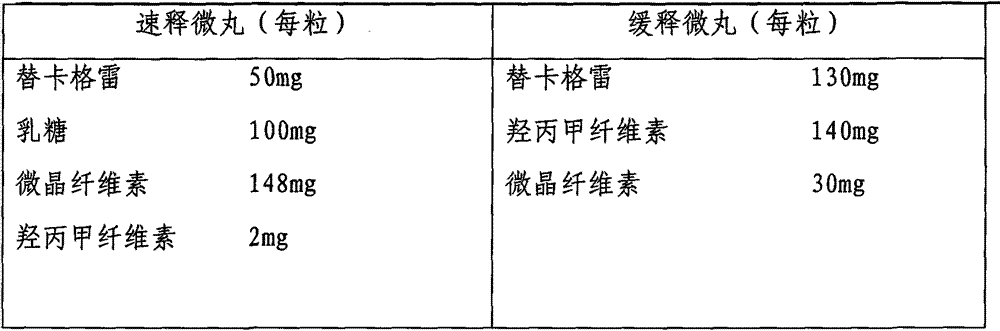
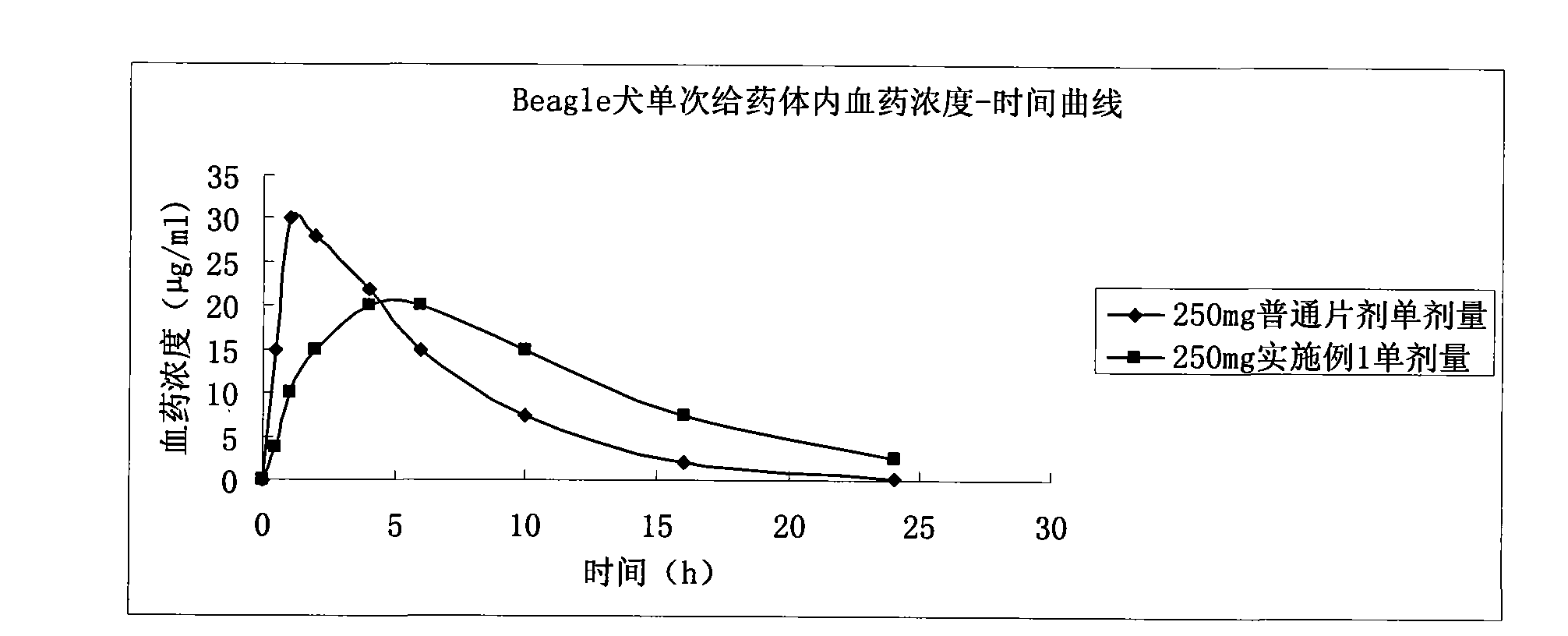
Ticagrelor sustained-release preparation
ActiveCN103520164AOrganic active ingredientsGranular deliveryImmediate releaseSustained Release Capsule
The invention relates to a sustained-release preparation composed of ticagrelor, a pharmaceutically acceptable sustained-release material, and other pharmaceutically acceptable auxiliary materials. The sustained-release preparation has an immediate-release part and a sustained-release part. The preparation can be double-part tablets obtained by compression by using a double-part tabletting machine, or tablets with the sustained-release medicine as a tablet core and the immediate-release medicine as outer coating, or sustained-release capsules composed of the immediate-release part and the sustained-release part. With the sustained-release preparation provided by the invention, medicine effect is fast, and medicine effective concentration can be maintained for a long time. Therefore, an ideal treatment effect can be provided.
Owner:BEIJING KANG LISHENG PHARMA TECH DEV
Levetiracetam slow release pellet capsule preparation and preparation method thereof
InactiveCN101647789AImprove liquidityReduce or eliminate irritationNervous disorderPharmaceutical delivery mechanismMass compositionSide effect
The invention relates to a levetiracetam slow release pellet capsule preparation and a preparation method thereof. The levetiracetam slow release pellet capsule preparation comprises the following components in percentage by mass: 50-70 percent of levetiracetam, 15-30 percent of blank pellet, 5-10 percent of hydroxypropylmethyl cellulose or polyvidone, 7-15 percent of ethylcellulose or acrylic resin, 1-8 percent of talc powder and 0.5-2 percent of cataloid. The preparation method comprises the following steps: coating levetiracetam fine powder on the blank pellet by a binding agent to be madeinto a medicine-contained pellet; coating an isolating layer on the medicine-contained pellet; coating a slow release coating film on the medicine-contained pellet coated with the isolating layer to prepare a slow release pellet; and mixing the slow release pellet, the talc powder and the cataloid and filling the mixture into a capsule. The levetiracetam is made into the slow release capsule preparation by a pellet and slow release technology, and the slow release preparation can stabilize blood medicine concentration, reduce the generating frequency and degree of the side effect and fundamentally solve the problems of great influence on a tablet by gastric pyloric sphincter and big differences of gastric emptying individuals.
Owner:天津药物研究院药业有限责任公司
Statins zinc salt-containing blood fat-reducing composite
InactiveCN102357096AReduce cholesterolReduce contentOrganic active ingredientsMetabolism disorderSustained Release CapsuleOrally disintegrating tablet
The invention relates to an oral drug composite for reducing the blood fat, in particular to a drug composite which takes any 1-2 of statins zinc salt and nicotinic acidniacin blood fat reducing medicine or fibrates blood fat reducing medicine or cholesterol restraining and absorbing medicine as active ingredients, the preparation of the drug composite, and the use of the drug composite. The drug composite takes the statins zinc salt and the nicotinic acidniacin blood fat reducing medicine or the fibrates blood fat reducing medicine or the cholesterol restraining and absorbing medicine as the active ingredients and takes the corresponding ingredients as the drug auxiliary materials. The oral preparation which is prepared according to the technology and the method of the patent description comprises granular formulation, conventional tablet, chewable tablet, dispersible tablet, orally disintegrating tablet, buccal tablet, capsule, soft capsule, pill, sustained release tablet, sustained release capsule and the like. The oral drug composite is quick and durable in effect, and is convenient to take, thereby being used for treating various hyperlipemias.
Owner:FUKANGREN BIO PHARMA
Sutained release formulation for venlafaxine hydrochloride
InactiveUS20060182797A1Quick releaseOrganic active ingredientsNervous disorderSustained Release CapsuleMini tablets
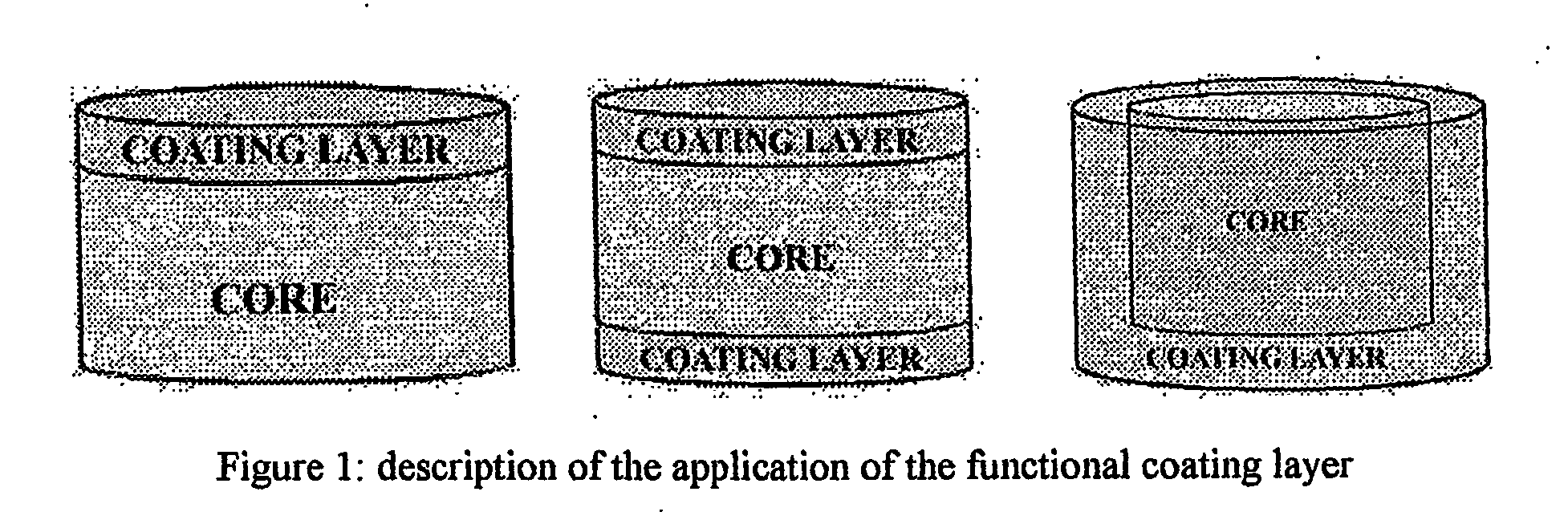
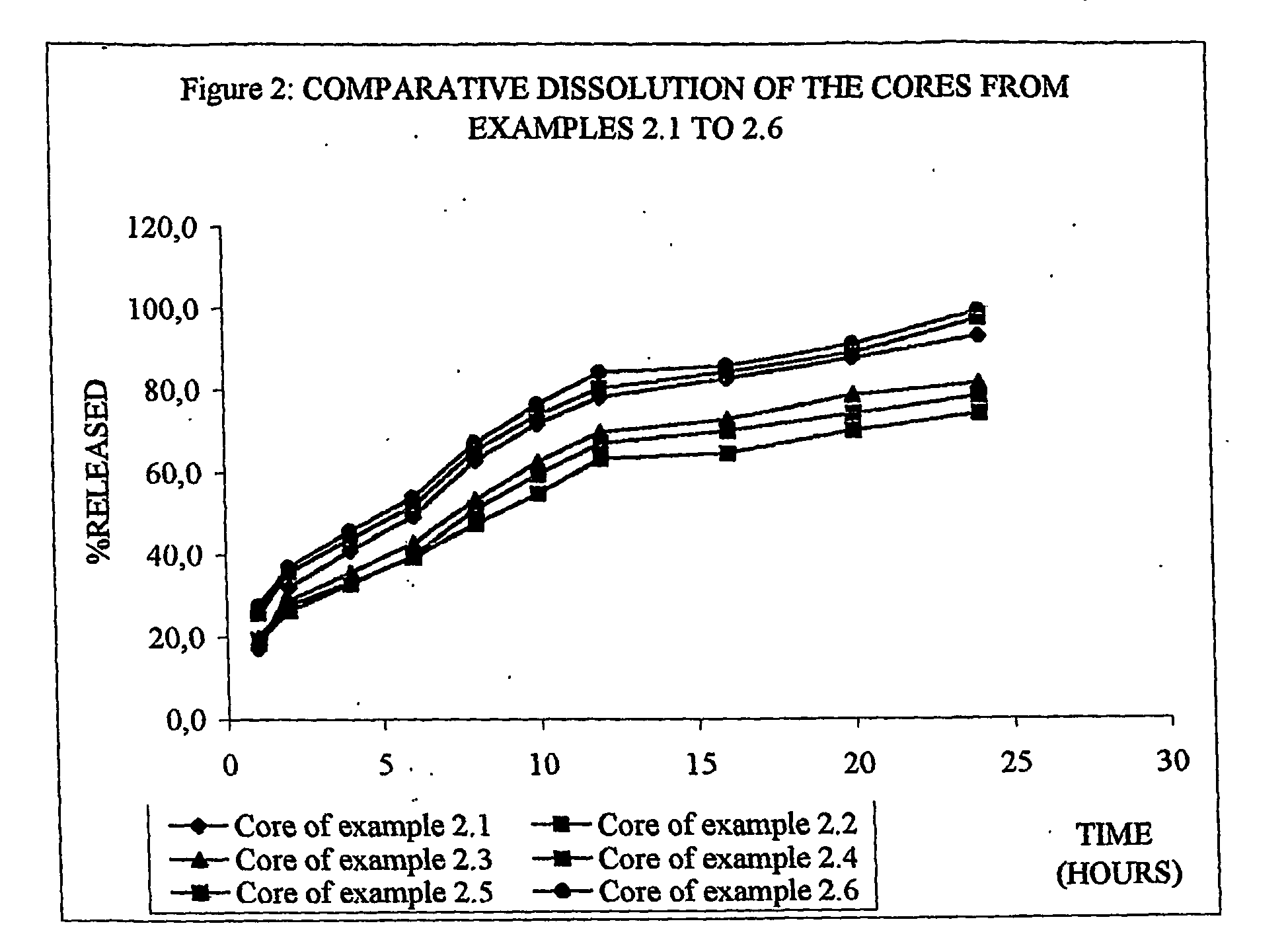
The invention provides a sustained release composition that; 1. Is free of initially increased drug delivery that occurs (in sustained release systems containing the water soluble drug venlafaxine HCl, known as burst phenomenon, by using a functional core partially or totally coated by a functional coating layer or film. 2. Delivers the drug substance within 24 hours and is therefore suitable for once daily administration of the said drug substance. 3. Exhibits linearity between the strength dosage form and the (total mass of the dosage form, by proportional increase of the amounts of the drug substance and the excipients in the formulation. 4. Is possible to be divided in smaller doses, without affecting the release of the drug substance. The invention provides a sustained release capsule formulation containing an appropriate number of functional complex mini tablets comprising of: I. A functional core comprising the active ingredient, especially the water-soluble drug Venlafaxine HCl and appropriate excipients. 2. A functional coating layer or film that reduces the initial surface of the core that is available for the release of the water-soluble drug Venlafaxine HClt phenomenon.
Owner:PHARMATHEN
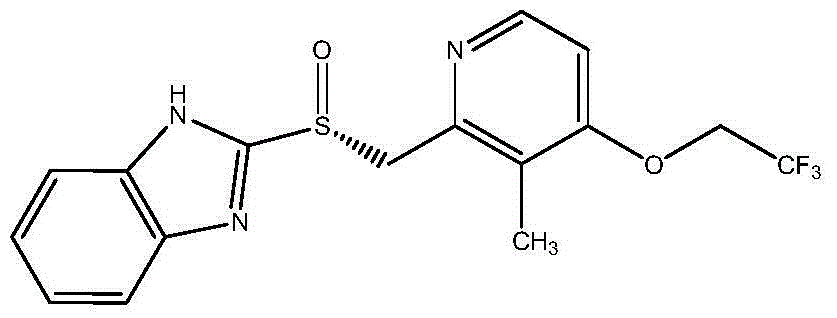
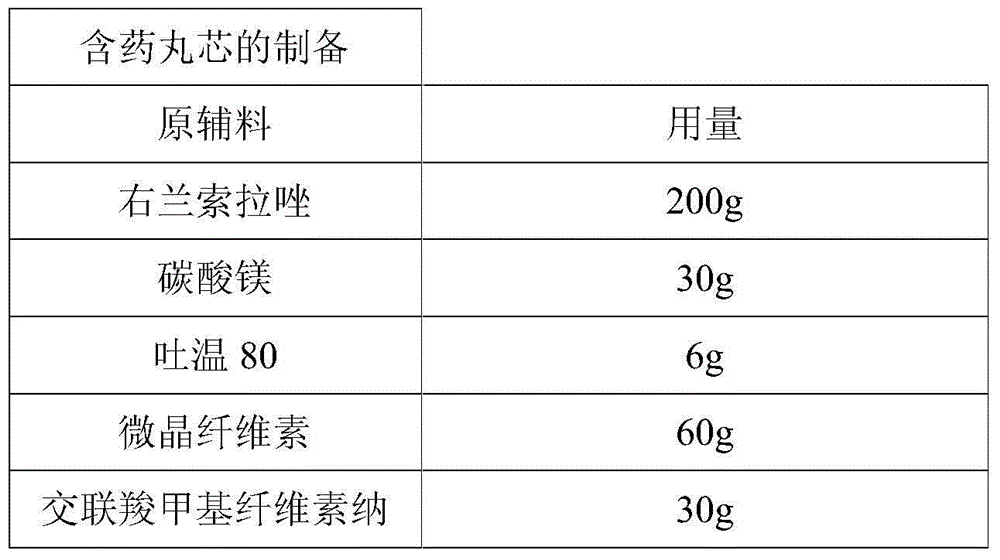
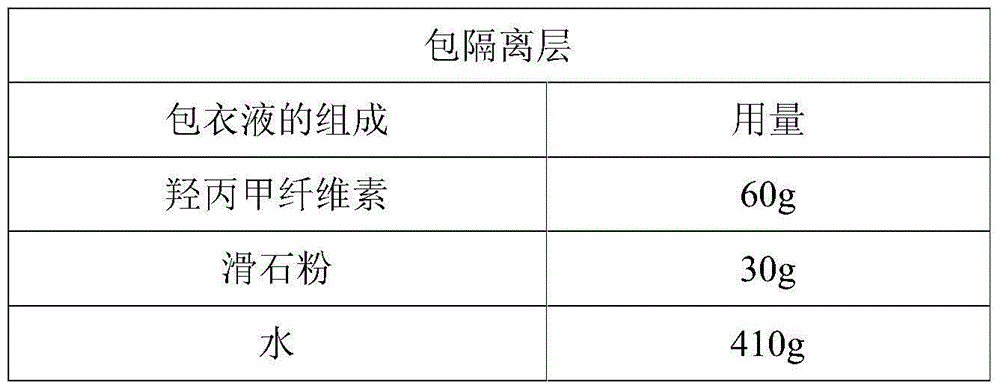
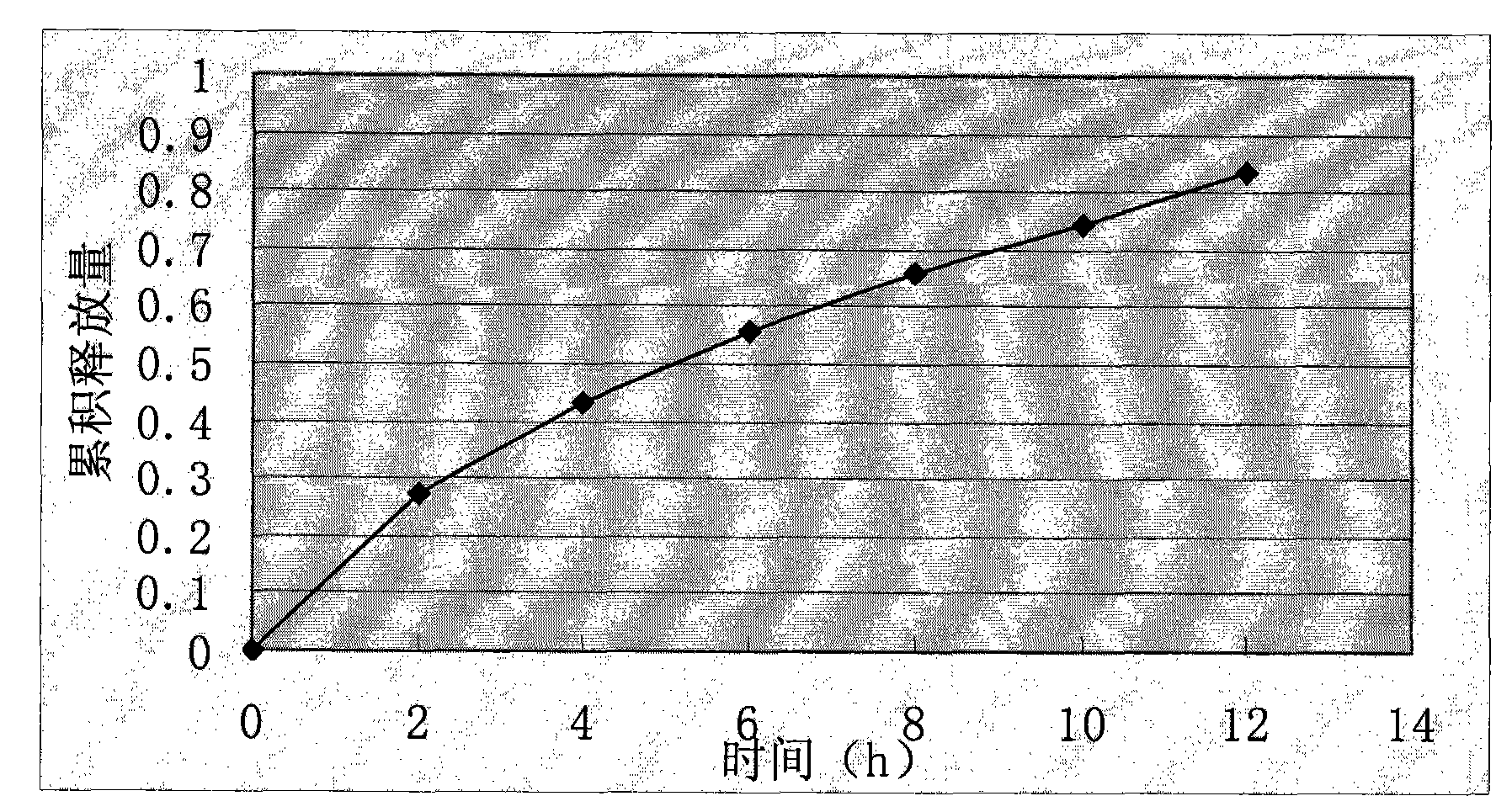
Dexlansoprazole sustained-release capsule and preparation method thereof
The invention relates to a dexlansoprazole sustained-release capsule and a preparation method thereof. The capsule comprises two dexlansoprazole micropills, wherein the two micropills consist of hollow pill cores, active medicine layers, isolating coating layers and sustained-release coating layers. The dexlansoprazole sustained-release capsule provided by the invention can be released in the intestinal tract in position so as to persistently inhibit acid release.
Owner:BEIJING RED SUN PHARMA
Dexlansoprazole sustained release capsule and preparation method thereof
ActiveCN102600109ASolve the speed problemSolve the problem of bioavailabilityOrganic active ingredientsDigestive systemSustained Release CapsuleDexlansoprazole
The invention belongs to the technical field of pharmaceutical preparation, and particularly relates to a dexlansoprazole sustained release capsule and a preparation method thereof. Two kinds of pellets are arranged in the capsule, and the weight ratio of dexlansoprazole contained in the first kind of pellets and the second kind of pellets is 1:3-4. The dexlansoprazole sustained release capsule disclosed by the invention can avoid the destruction of the dexlansoprazole in gastric acid, and is released in the intestinal tract in a fixed position so as to achieve the purposes of taking effect rapidly and improving bioavailability.
Owner:乐普药业科技有限公司
Preparation method of waxy corn starch nano particle-insulin sustained-release capsules
InactiveCN104337795AHigh yieldLow costPeptide/protein ingredientsMetabolism disorderSustained Release CapsuleCrystallinity
The invention discloses a preparation method of waxy corn starch nano particle-insulin sustained-release capsules. Debranched starch nano particles are prepared by adopting a biological enzyme method; an insulin solution is embedded into an amylase double helix cavity, and thus the waxy corn starch nano particle-insulin sustained-release capsules are prepared. The nano starch prepared by the method is high in yield, low in cost, and relatively safe and environmentally-friendly in method, and is used as an embedded material of insulin. Through determination of the characteristics in the sustained-release capsules, such as particle size, endothermic character, crystallinity and digestibility, the digestibility of the waxy corn starch nano particle-insulin sustained-release capsules in a stomach is low, and the waxy corn starch nano particle-insulin sustained-release capsules are mainly used for releasing drugs in intestines.
Owner:QINGDAO AGRI UNIV
Hydrochloric tamsulosin sustained-release capsule and its preparation method
ActiveCN101125134APrecise Controlled ReleaseControl releaseOrganic active ingredientsPharmaceutical delivery mechanismSide effectOral medication
The present invention provides a tamsulosin hydrochloride sustained-release capsule. The tamsulosin hydrochloride sustained-release capsule of the present invention can avoid the sudden release of the drug tablets and the performance differences generated from the gastric emptying differences, display minor food effect or do not display food effect, and obtain the stable curve of the plasma drug concentration and longer action time simultaneously, so as to reduce the occurrence of cardiovascular side effects, greatly improve the safety, effectiveness and compliance of the medication for the patients. The tamsulosin hydrochloride sustained-release capsule of the present invention can ensure the sustained and regular release of the main ingredient tamsulosin hydrochloride after the oral administration, and the present invention is characterized by convenient administration, durable function, stable efficacy, fewer side effects and so on.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Trimetazidine hydrochloride sustained-release capsule and preparation method thereof
InactiveCN104473905AOrganic active ingredientsSenses disorderControlled releaseSustained release pellets
The invention discloses a trimetazidine hydrochloride sustained-release capsule and a preparation method thereof, belonging to the technical field of medicines. The sustained-release capsule is prepared by filling sustained-release pellets containing trimetazidine in capsules, wherein the sustained-release pellets are a framework control preparation. The trimetazidine hydrochloride sustained-release capsule prepared by the preparation method is simple in process and low in cost. As the capsule is in a multiunit control release system of drug, the user does not need to worry about abrupt release of drug. Compared with preparations such as sustained release tablets, the trimetazidine hydrochloride sustained-release capsule is safe and reliable in medication and high in medication compliance.
Owner:万全万特制药(厦门)有限公司
Tegasevod maleate oral preparation and its preparation process-for curing intestinal irritability syndrome
InactiveCN1443535AQuality improvementQuality is easy to controlOrganic active ingredientsDigestive systemEffervescent tabletOral medicine
The present invention relates to an oral medicine preparation for curing intestinal irritability syndrome using constipatino as main sympton-tijaseluo maleate. It is made up by using tijaseluo maleate as active component and adding proper auxiliary material through a certain preparation process, and can be made into various oral dosage forms of tablet, oral disintegrant table, effervescent tablet, capsule, suspension gel and powder preparation, etc.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Metformin hydrochloride sustained-release capsule and its preparation method
InactiveCN103239424AEasy to swallowAvoid disintegrationOrganic active ingredientsMetabolism disorderSide effectPatient compliance
The invention relates to a metformin hydrochloride sustained-release capsule and its preparation method. The metformin hydrochloride sustained-release capsule is prepared by steps of coating a metformin hydrochloride granule sustained-release material and putting into an enteric capsule. In comparison with the prior art, sustained-release granules and enteric capsule filling technology are combined together to prepare the new dosage form of metformin hydrochloride sustained-release (enteric-coated) capsule. As the sustained-release granule coating and enteric capsule filing technology is adopted, metformin hydrochloride will not be disintegrated and will not stimulates gastric mucosa, and adverse reactions such as nausea, stomachache, diarrhoea and the like caused by medication can be avoided. Meanwhile, metformin hydrochloride will not be damaged by gastric juice, and bioavailability of metformin hydrochloride is raised. In addition, the product provided by the invention is also a sustained-release enteric-coated preparation. The medicine can be stably released in a body, effective plasma concentration is maintained for a long time, and toxic and side effect which might be caused by higher plasma concentration within a short time are avoided. The frequency for taking the medicine is reduced, and patient compliance is also raised.
Owner:BOSEN BIO PHARMA SHANXI PROVINCE
Dexlansoprazole sustained release capsule and preparation method thereof
ActiveCN104940169AAvoid destructionAvoid residueOrganic active ingredientsDigestive systemSustained Release CapsuleDexlansoprazole
The invention belongs to the technical field of pharmaceutical preparation and aims at improving bioavailability of dexlansoprazole in vivo. The dexlansoprazole sustained release capsule provided by the invention is hardly released in gastric acid, and can be disintegrated in intestines, and active ingredients are dissolved out, so that destruction of dexlansoprazole in the gastric acid is avoided; the dexlansoprazole sustained release capsule provided by the invention contains two different types of enteric micropelets, so that two-time dual drug release (DDR) is realized; in a process of preparing eudragit S100 aqueous dispersion, different amounts of alkaline substances are added, different mol numbers of carboxyls in polymers are neutralized, and an enteric-coating material is controlled to be dissolved at different pH values, so that two-time release is realized; besides, an aqueous dispersion coating is adopted, so that ethanol residue is effectively avoided.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
New type medicina preparation with one chinese medicinal herb extract ant its preparation method
InactiveCN1559550ASuit one's needsGood curative effectAntipyreticDigestive systemMedicinal herbsSoftgel
Owner:SHENYANG PHARMA UNIVERSITY
Piclofenac potassium sustained release capsule and preparing technique thereof
InactiveCN101322695ASmall toxicityGood formulation stabilityOrganic active ingredientsAntipyreticAnalgesics drugsSustained release pellets
The invention provides a sustained-release capsule of diclofenac potassium, which is an anti-inflammation and analgesic drug, and a production method thereof. The diclofenac potassium sustained-release capsule is produced by preparing diclofenac potassium into sustained-release pellets and then filling the pellets into the capsule; the diclofenac potassium sustained-release pellet consists of a blank pellet core, a main drug layer coated outside of the pellet core and containing diclofenac potassium and a sustained-release coating layer covered on the main drug layer. The diclofenac potassium sustained-release capsule of the invention has fine preparation stability and prominent release effect.
Owner:海南华旗药业销售有限公司
Slow released diacetyl rheinic acid prepn and its prepn process
InactiveCN101019847ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsAntipyreticSustained Release CapsulePolyethylene glycol
The present invention is slow released diacetyl rheinic acid preparation and its preparation process. The slow released diacetyl rheinic acid preparation, which may be granule, tablet or capsule, is prepared with diacetyl rheinic acid as main material and other supplementary material. The slow released diacetyl rheinic acid preparation of the present invention has excellent slow releasing effect and simple preparation process.
Owner:上海慈瑞医药科技股份有限公司
Sustained-release capsule containing propiverine hydrochloride and preparation method of sustained-release capsule
InactiveCN102579404ALasting effectReduce the number of dosesPharmaceutical delivery mechanismPharmaceutical non-active ingredientsSustained Release TabletSustained Release Capsule
The invention discloses a sustained-release capsule containing propiverine hydrochloride and a preparation method of the sustained-release capsule. The sustained-release capsule comprises sustained-release micropills and an empty capsule, wherein, the sustained-release micropills comprise pill cores containing drugs accounting for 75 to 97 percent and sustained-release coating layers accounting for 3 to 25 percent by weight percentage. The sustained-release capsule containing propiverine hydrochloride comprises hundreds of the sustained-release micropills with uniform particle sizes, and preparation errors or preparation defects of individual micropills cannot influence the drug release behavior of the whole preparation seriously, so that the sustained-release capsule is safer than a sustained-release tablet, the irritant activity to gastrointestinal tracts is smaller, plasma concentration is smoother, the bioavailability is higher, and the sustained-release capsule can continuously release the drugs for 24 hours if being taken for one time per day so as to treat overactive bladder. The preparation method of the sustained-release capsule prepares the pill cores containing the drugs in an extrusion and spheronization method or a drug added manner, adopts a fluidized bed to coat the sustained-release coating layers, achieves simple technology, and is easy to achieve industrialized mass production.
Owner:广州科的信医药技术有限公司
A novel enteron pharmaceutical preparation and its preparing method
InactiveCN101161250AImprove qualityRich choiceDigestive systemInorganic non-active ingredientsDigestive canalOral medication
The present invention relates to a novel digestive canal pharmaceutical preparation and its preparation method, the first contribution of the present invention is to give more choices to people in general oral administration preparations, wherein, oral administration preparations such as honey pill, micropill, concentration pill, water pill, gel preparation, cream, tablet, chewing tablet, powder agent, granule, capsule, slow release capsule etc. is convenient, capable of suitable for different consumption level people; the second contribution of the present invention is to upgrade the general oral administration preparations into stomach retention floating oral administration preparation by inducting corresponding supplementary, greatly prolonging medicine retain time in stomach, greatly increasing the local focal medicine effective concentrate in stomach, in order to short period of treatment, promote clinic effective rate and cure rate to provide necessary condition; the third contribution of the present invention is to upgrade the oral administration preparations and the stomach retention floating oral administration preparation into loading far infrared auxiliary therapeutic function oral administration preparation by inducting corresponding supplementary nanocrystallization, leading product curative effect to promote further.
Owner:丛繁滋
Novel mecobalamin sustained-release capsule and preparation method thereof
InactiveCN102232939ALow costEasy to prepareOrganic active ingredientsNervous disorderSustained release pelletsBlood concentration
The invention relates to a novel mecobalamin sustained-release capsule and a preparation method thereof. The mecobalamin sustained-release capsule comprises mecobalamin, povidone, eudragit and other commonly used auxiliary materials. The preparation method comprises the following steps of: preparing a medicated enveloping solution, coating medicated sustained-release membranes, preparing a sustained-release enveloping solution outside the medicated layer, coating the sustained-release membranes outside the medicated layer, preparing sustained-release pellets and capsules and filling. The novel mecobalamin sustained-release capsule can maintain a long-lasting and stable blood concentration after oral administration.
Owner:广州艾格生物科技有限公司
Compound isosorbide mononitrate aspirin sustained-release capsule preparation and preparation method
ActiveCN103285017AReduce adverse reactionsAvoid adverse reactionsPharmaceutical delivery mechanismHeterocyclic compound active ingredientsSustained release pelletsSustained Release Capsule
The invention discloses a compound isosorbide mononitrate aspirin sustained-release capsule preparation. The compound isosorbide mononitrate aspirin sustained-release capsule preparation is characterized by comprising an isosorbide mononitrate sustained-release capsule preparation and an aspirin enteric-coated preparation, wherein the isosorbide mononitrate sustained-release capsule preparation contains 40-80 parts by weight of isosorbide mononitrate and comprises an immediate-release pellet with 30 percent of isosorbide mononitrate and a sustained-release pellet with 70 percent of isosorbide mononitrate, and the aspirin enteric-coated preparation contains 50-90 parts by weight of aspirin. The invention also provides a preparation method of the compound capsule preparation. With the adoption of the compound isosorbide mononitrate aspirin sustained-release capsule preparation, the curative effects are better improved, the adverse reaction due to stimulation from the aspirin to a gastric mucosa is better reduced, and meanwhile, the isosorbide mononitrate can also satisfy the requirement of stable release in a stomach.
Owner:吉林天衡药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com