Ticagrelor sustained-release preparation
A technology of ticagrelor and preparation, applied in the field of medicine, can solve problems such as poor compliance, myocardial infarction or stroke, and increase the risk of acute thrombosis in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
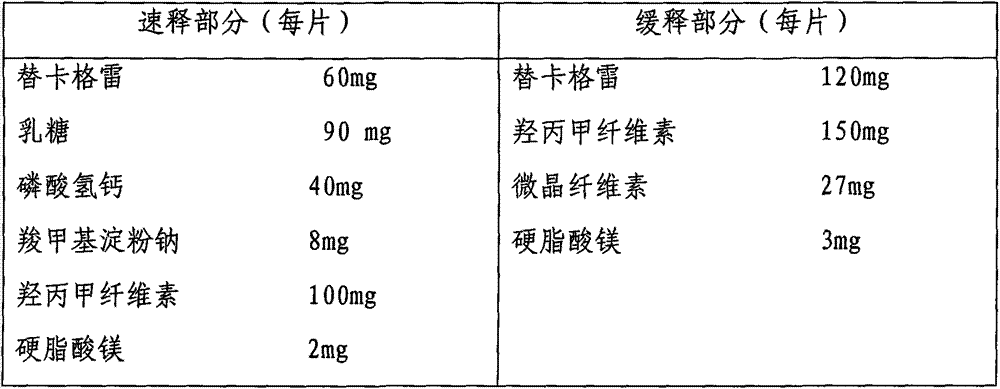
[0021] Example 1: Preparation of Ticagrelor Sustained Release Tablets
[0022]
[0023] Preparation process: pass the main drug and auxiliary materials through a 100-mesh sieve. Mix the main drug in the immediate-release part and the auxiliary materials except magnesium stearate evenly, make soft material with 70% ethanol, granulate with 16 mesh, dry at 60°C, granulate with 16 mesh, and add magnesium stearate as the instant release part. Release part of the granules; mix the main drug in the slow-release part and the excipients except magnesium stearate evenly, make soft material with 70% ethanol, granulate with 16 mesh, dry at 50°C, granulate with 16 mesh, add stearin Magnesium sulfate was used as the sustained-release part granules; this bilayer sustained-release tablet of ticagrelor was prepared on a bilayer tablet press.
Embodiment 2
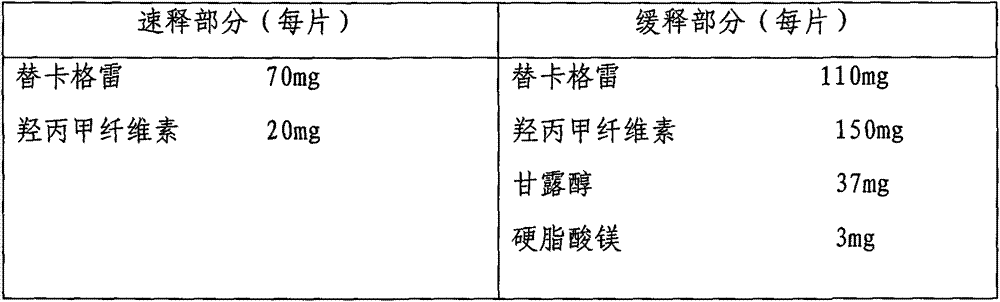
[0024] Example 2: Preparation of Ticagrelor Sustained Release Tablets
[0025]
[0026] Preparation process: pass the main drug and auxiliary materials through a 100-mesh sieve. Mix the main drug in the slow-release part and the excipients except magnesium stearate evenly, make soft material with 70% ethanol, granulate with 16 mesh, dry at 50°C, granulate with 16 mesh, add magnesium stearate and press into tablets As the sustained-release part; the hypromellose in the immediate-release part is made into a 5% aqueous solution, the main drug is added and suspended evenly, and the immediate-release part is wrapped on the sustained-release part by coating equipment.
Embodiment 3
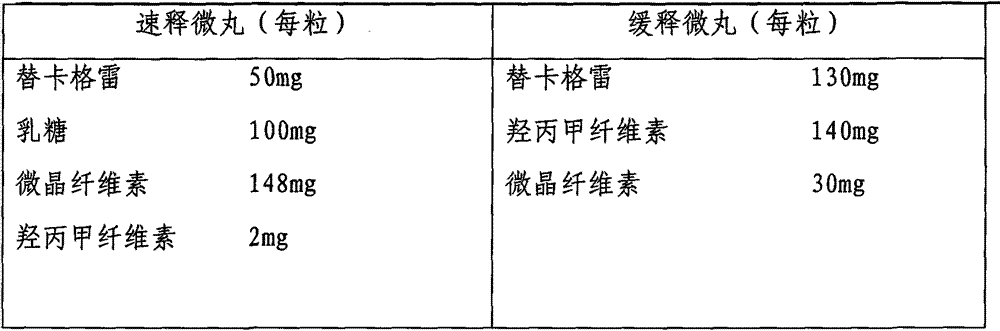
[0027] Embodiment 3: Preparation of ticagrelor sustained-release capsules
[0028]
[0029] Preparation process: pass the main drug and auxiliary materials through a 100-mesh sieve. Mix the main drug in the quick-release part with lactose and microcrystalline cellulose evenly, make hypromellose into a 3% aqueous solution as a binder, and use extrusion spheronization equipment to prepare quick-release pellets; The main drug in the mixture is mixed evenly with hypromellose and microcrystalline cellulose, and 70% ethanol is used as a binder to prepare slow-release pellets with extrusion and spheronization equipment; the two kinds of pellets are respectively coated with film, and mixed Then pack into capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com