Sustained-release capsule containing propiverine hydrochloride and preparation method of sustained-release capsule
A technology of propiverine hydrochloride and sustained-release capsules, which can be used in pharmaceutical formulations, medical preparations with inactive ingredients, urinary system diseases, etc. Small, high bioavailability, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
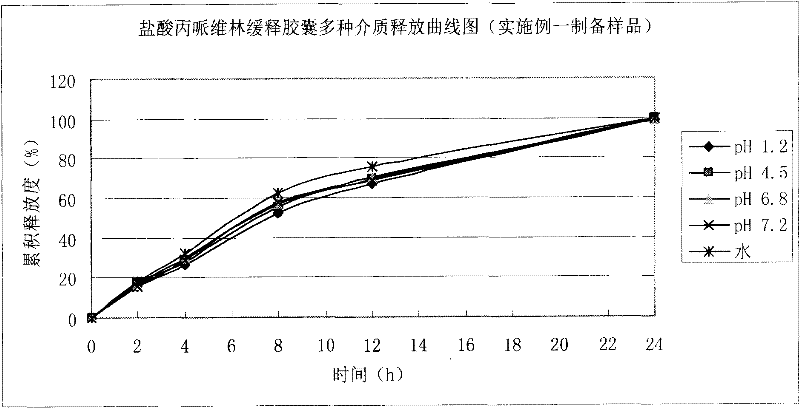
Embodiment 1
[0033] 1. Preparation of pill-containing core:
[0034]
[0035] Preparation process: Propiverine hydrochloride, microcrystalline cellulose, and lactose are mixed uniformly in equal increments, purified water is used to make soft material, and pellets are extruded and spheronized. The sieve particle size is 0.8mm, 60 Dry in an oven at ℃, sieving, and select 20-40 mesh-containing pellet cores for coating.
[0036] 2. Package slow release layer:
[0037]
[0038] The prescription amount of Surisi was added to purified water to fully disperse it, stirred for 45 minutes, put the pill-containing core in a fluidized bed, adjusted various process parameters to ensure the fluidized state, and the coating weight increased to 11%.
[0039] The process parameters of the fluidized bed: inlet air volume: 20-30HZ, inlet air temperature: 45-55°C, material temperature: 40-45°C, atomization pressure: 1.0bar, spraying speed: 2~4g / min.
[0040] 3. Curing: Curing in an oven at 50°C for 12 hours.
[0041] 4...
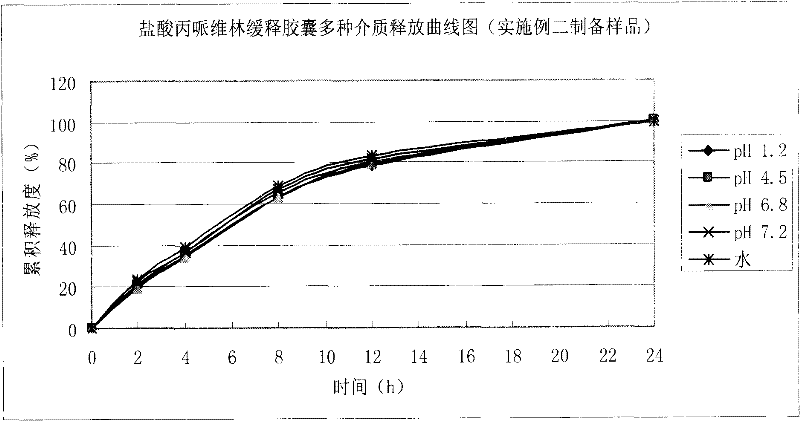
Embodiment 2
[0045] 1. Preparation of pill-containing core:
[0046]
[0047] Preparation process: Propiverine hydrochloride and lactose, microcrystalline cellulose, and anhydrous citric acid are mixed uniformly in equal increments, purified water is used to make soft material, and pellets are extruded and spheronized, and the particle size of the sieve plate It is 0.8mm, dried in an oven at 60°C, sieved, and selected 20-40 mesh-containing pellet cores for coating.
[0048] 2. Package slow release layer:
[0049]
[0050] Add the prescribed amount of talcum powder to purified water and stir for 45 minutes, add Eudragit NE30D water dispersion to make it fully dispersed, continue to stir for 45 minutes, and set aside. The pill core containing medicine is placed in a fluidized bed, and various process parameters are adjusted to ensure a fluidized state, and the coating weight increases to 3.5%.
[0051] The process parameters of the fluidized bed: inlet air volume: 20-30HZ, inlet air temperature: 35...
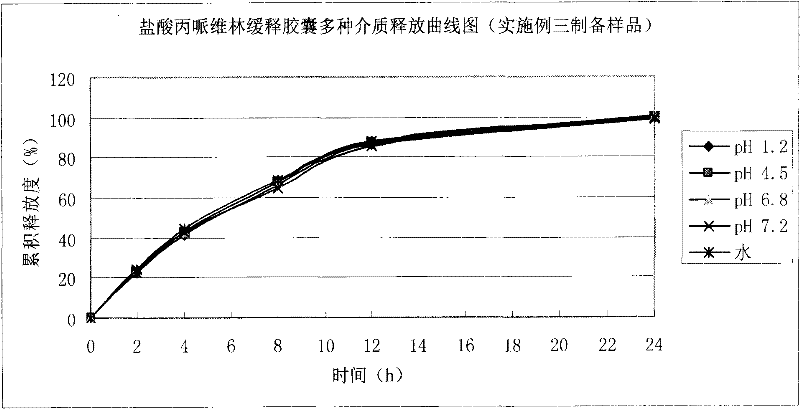
Embodiment 3
[0057] 1. Preparation of pill-containing core:
[0058]
[0059] Preparation process: Dissolve propiverine hydrochloride, PVP-K30 and citric acid in purified water, stir and use to dissolve completely to prepare a drug-containing solution. The microcrystalline cellulose blank pill core is placed in a fluidized bed, the above-mentioned drug-containing solution is sprayed on the surface of the blank pill core, and the pill-containing core is prepared by the drug application method, dried, and screened to take 20-30 mesh pill-containing cores, and then coated use.
[0060] 2. Package slow release layer:
[0061]
[0062] Add the prescription amount of talcum powder to purified water and stir for 45 minutes, add triethyl citrate, Eudragit RS30D and RL30D water dispersion to make it fully dispersed, continue to stir for 45 minutes, and set aside. Put the drug-containing pellet core in a fluidized bed, adjust various process parameters to ensure the fluidized state, and the coating weigh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com