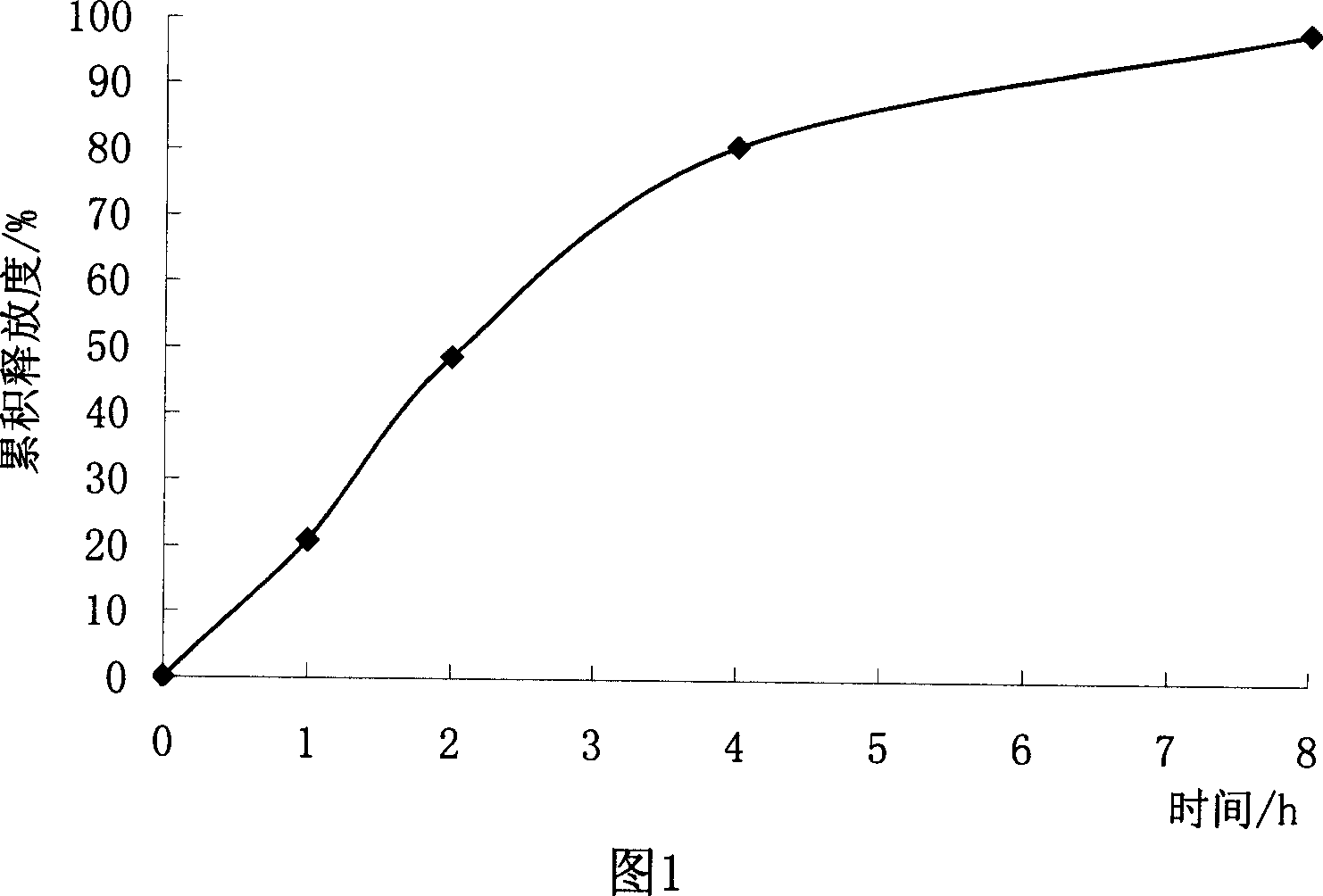
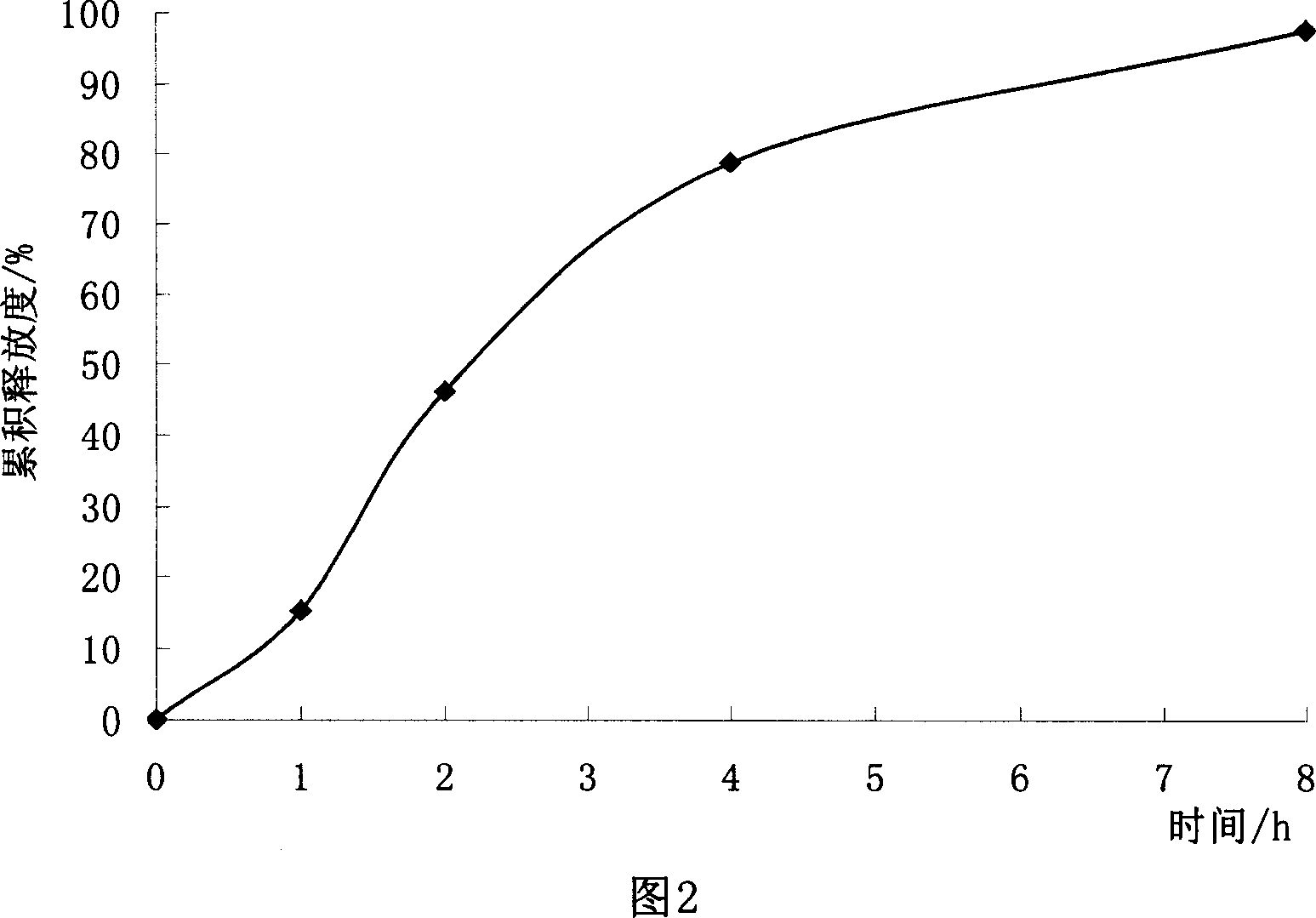
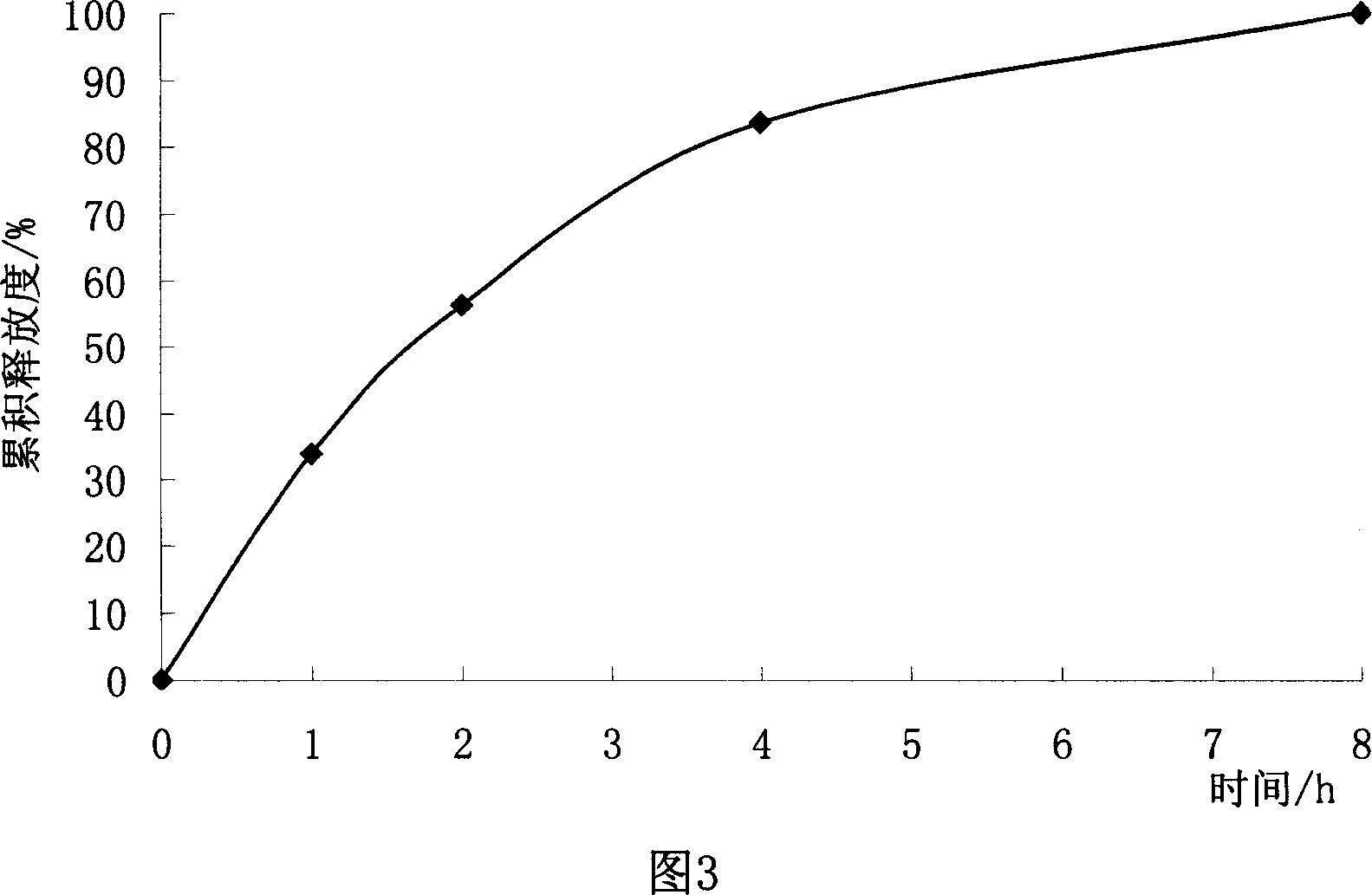
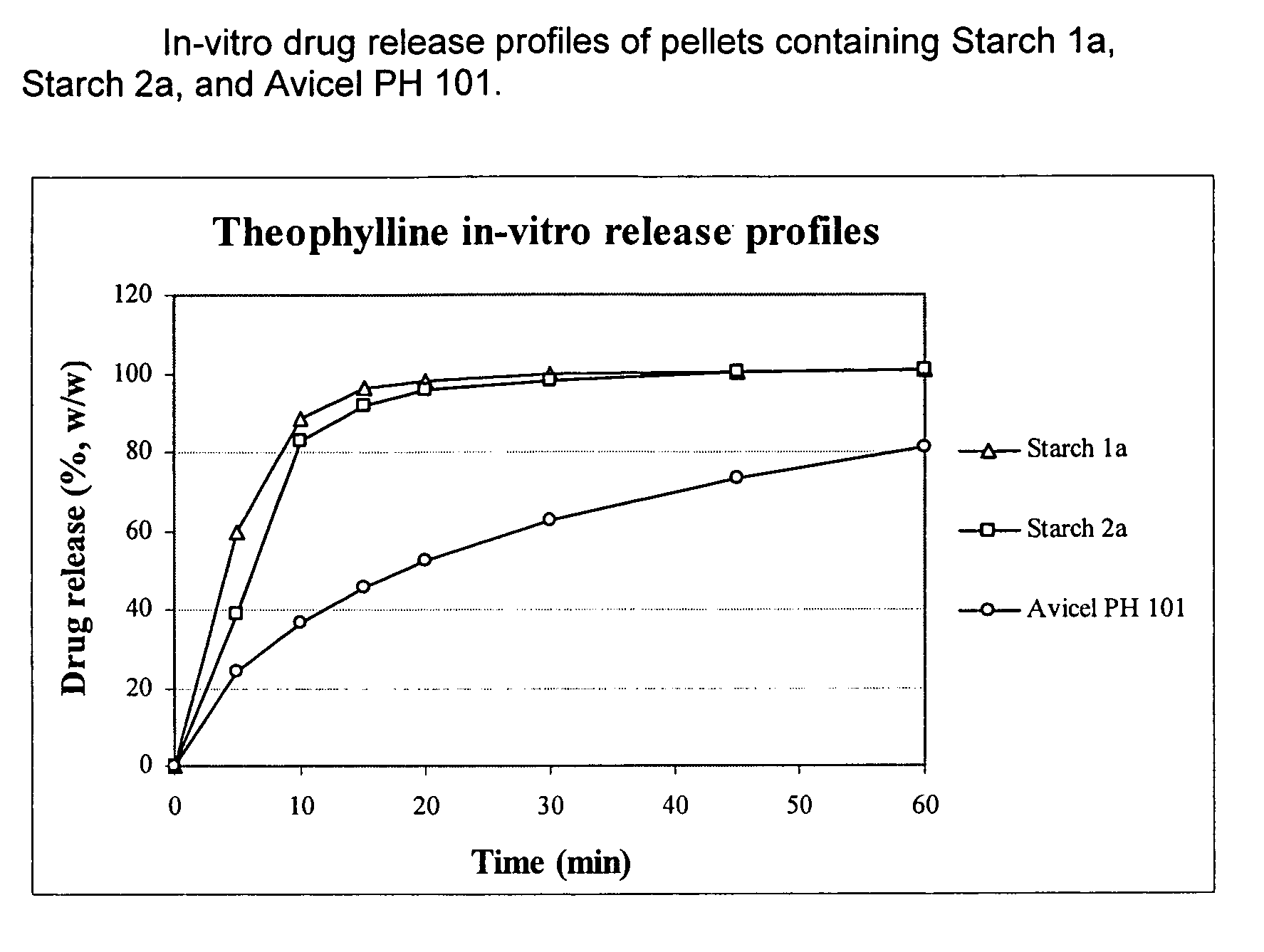
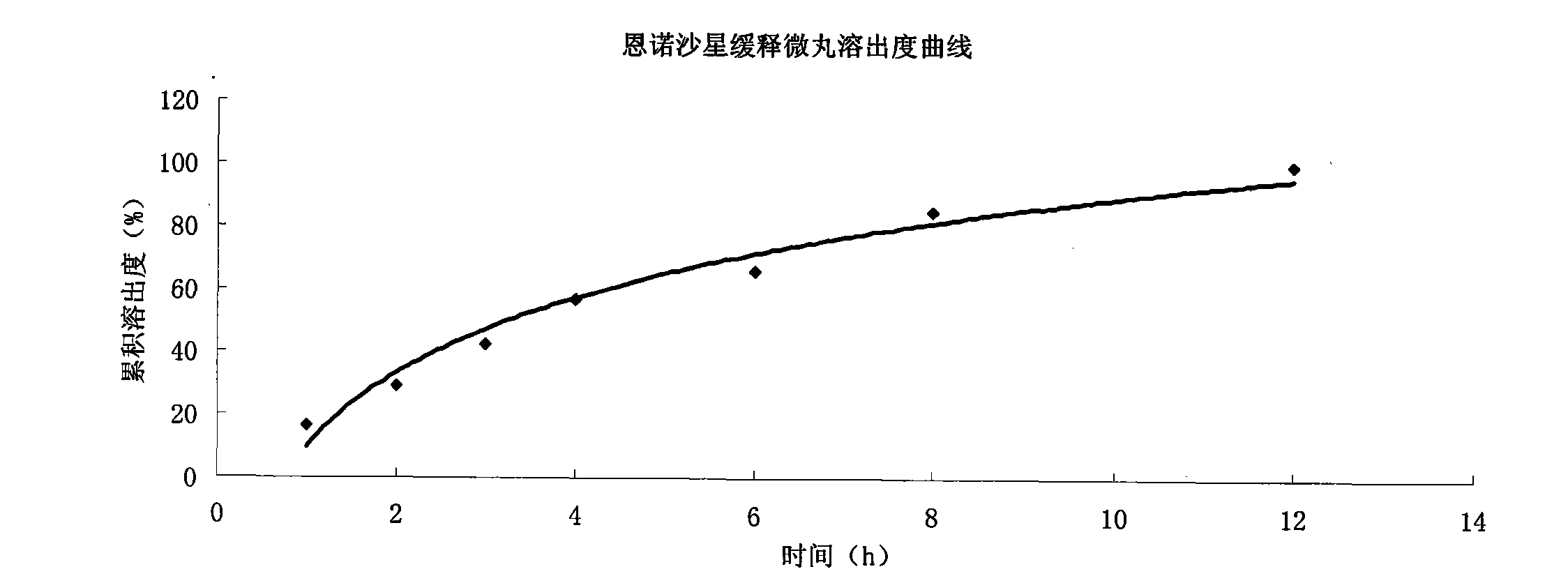
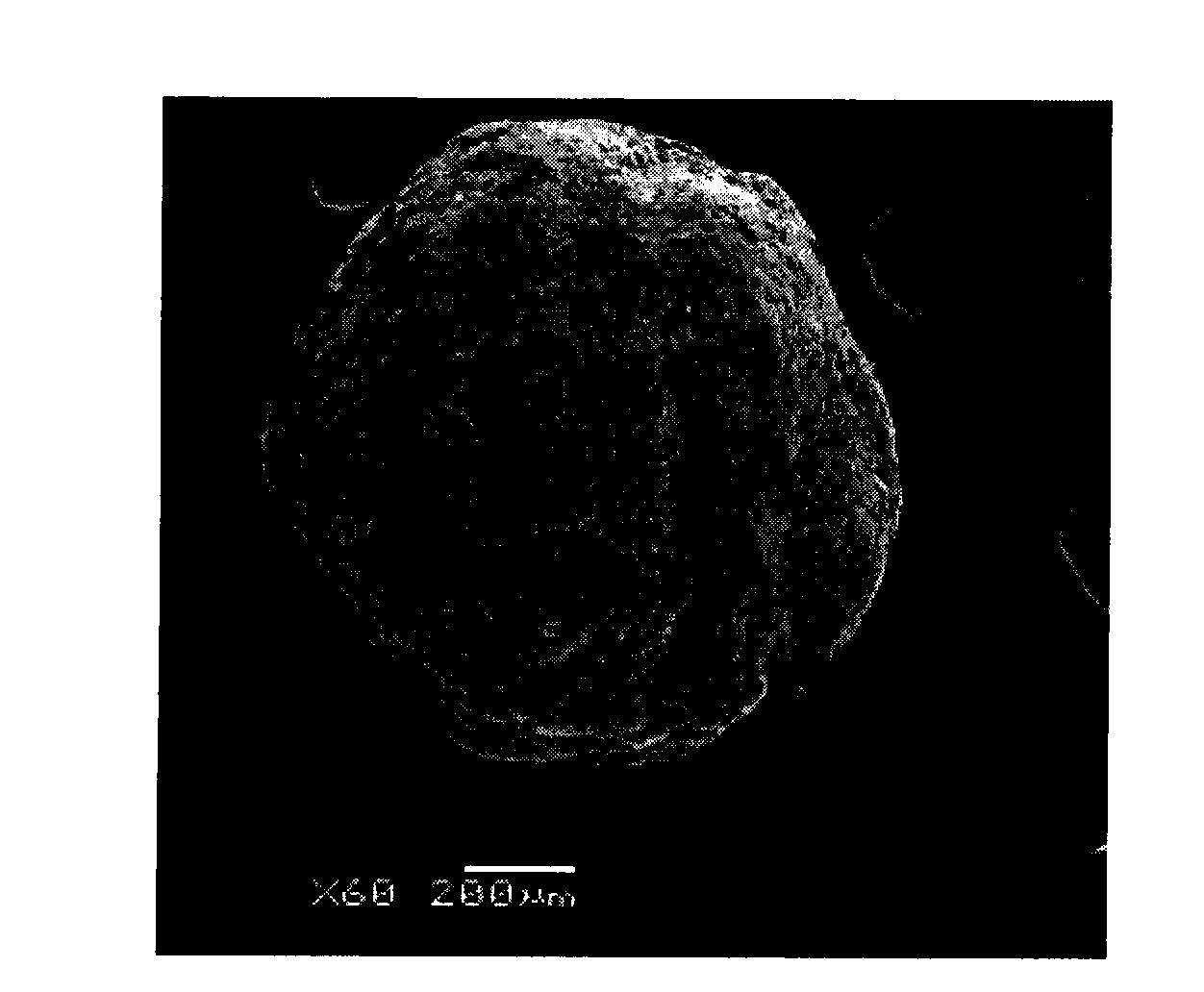
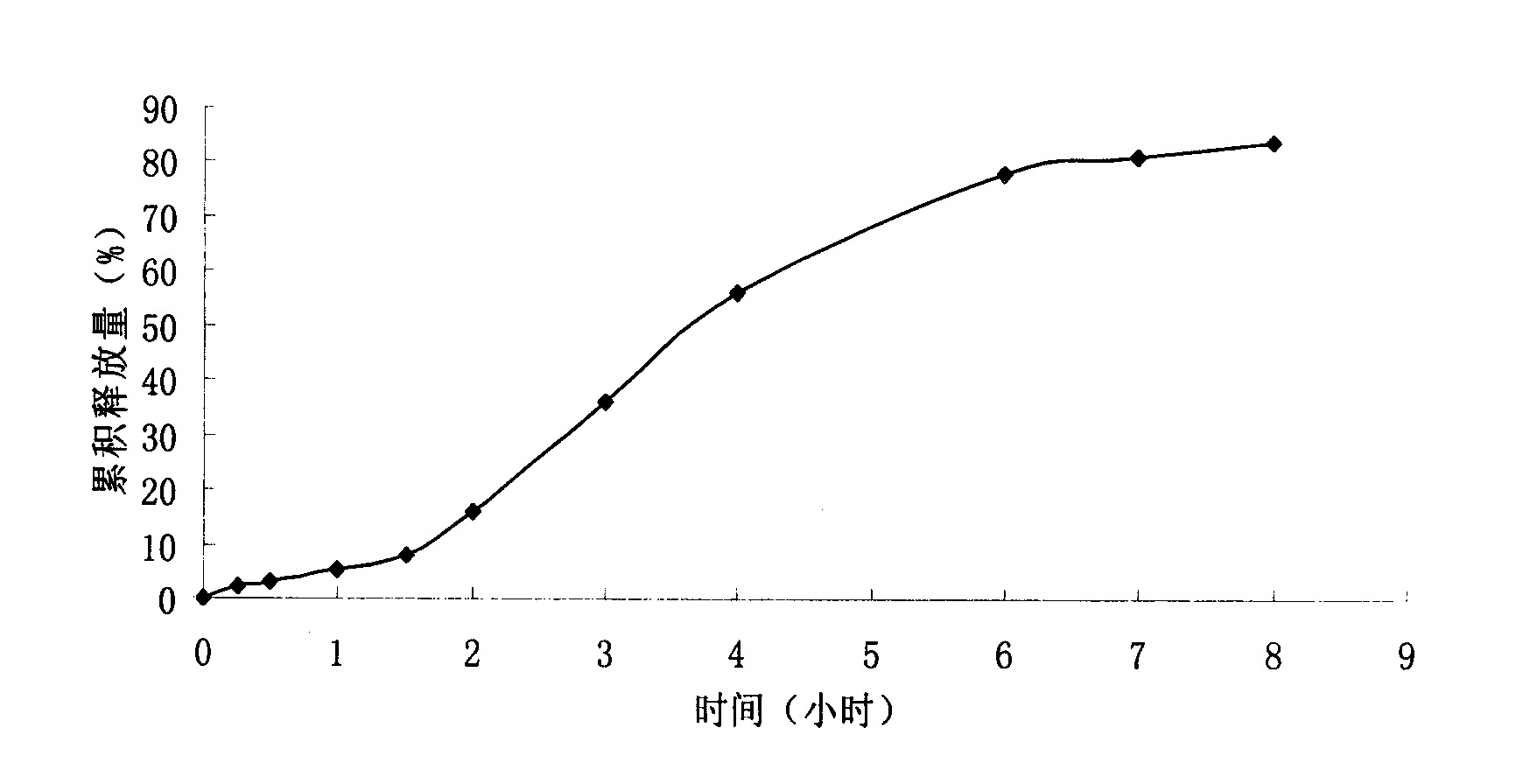
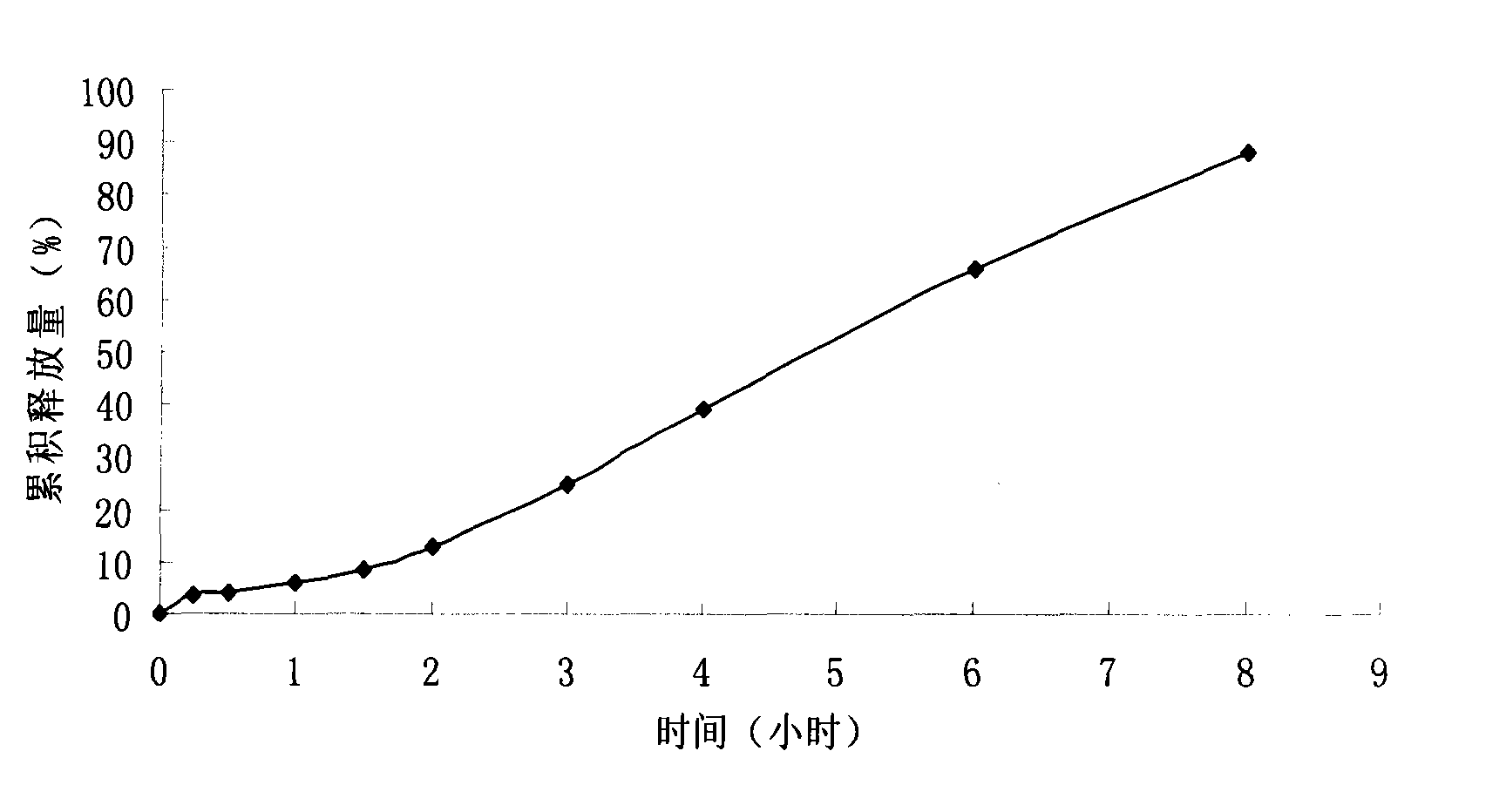
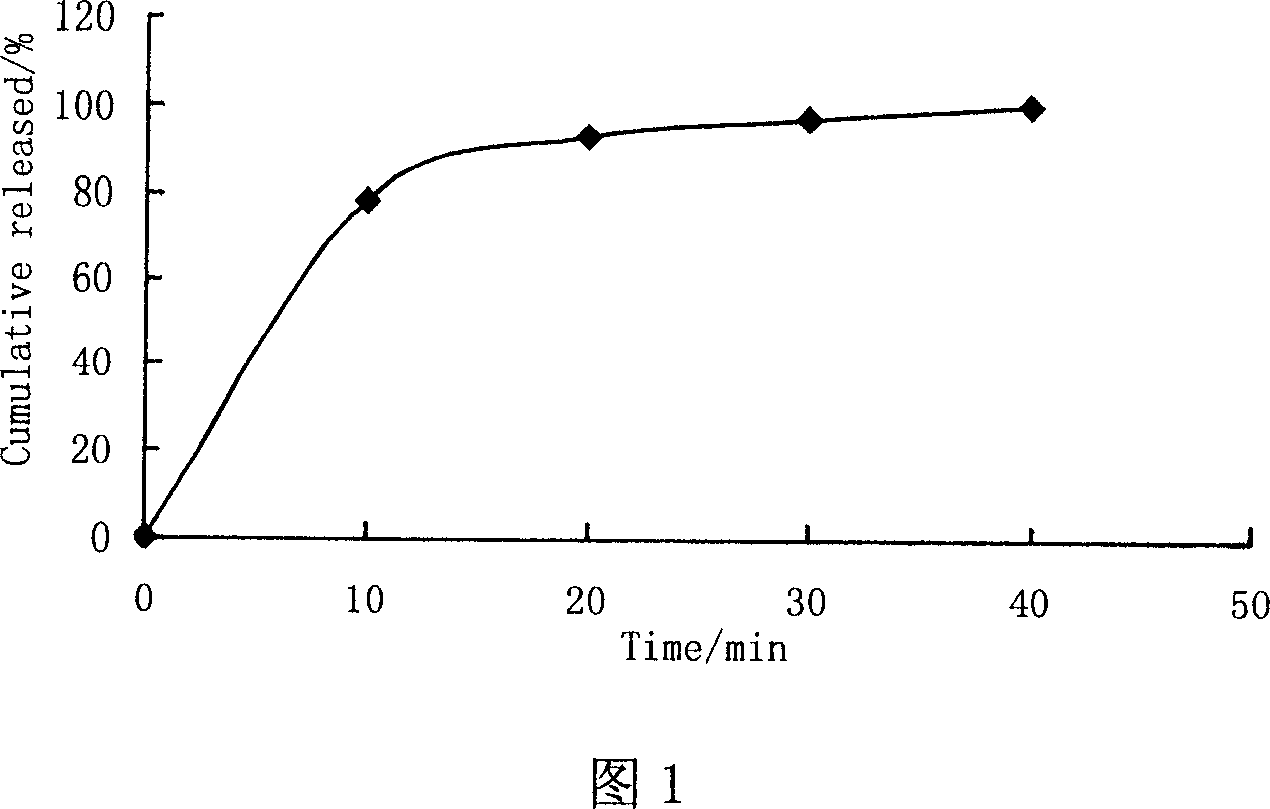
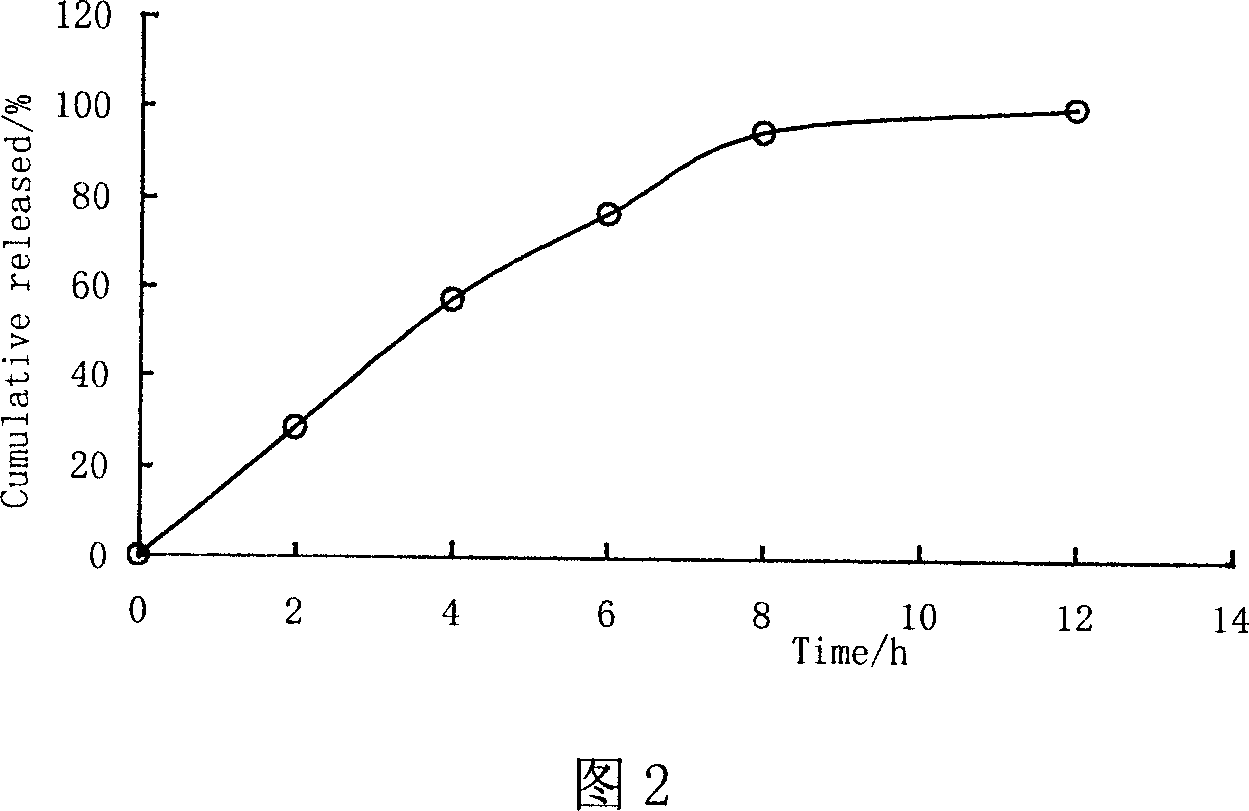
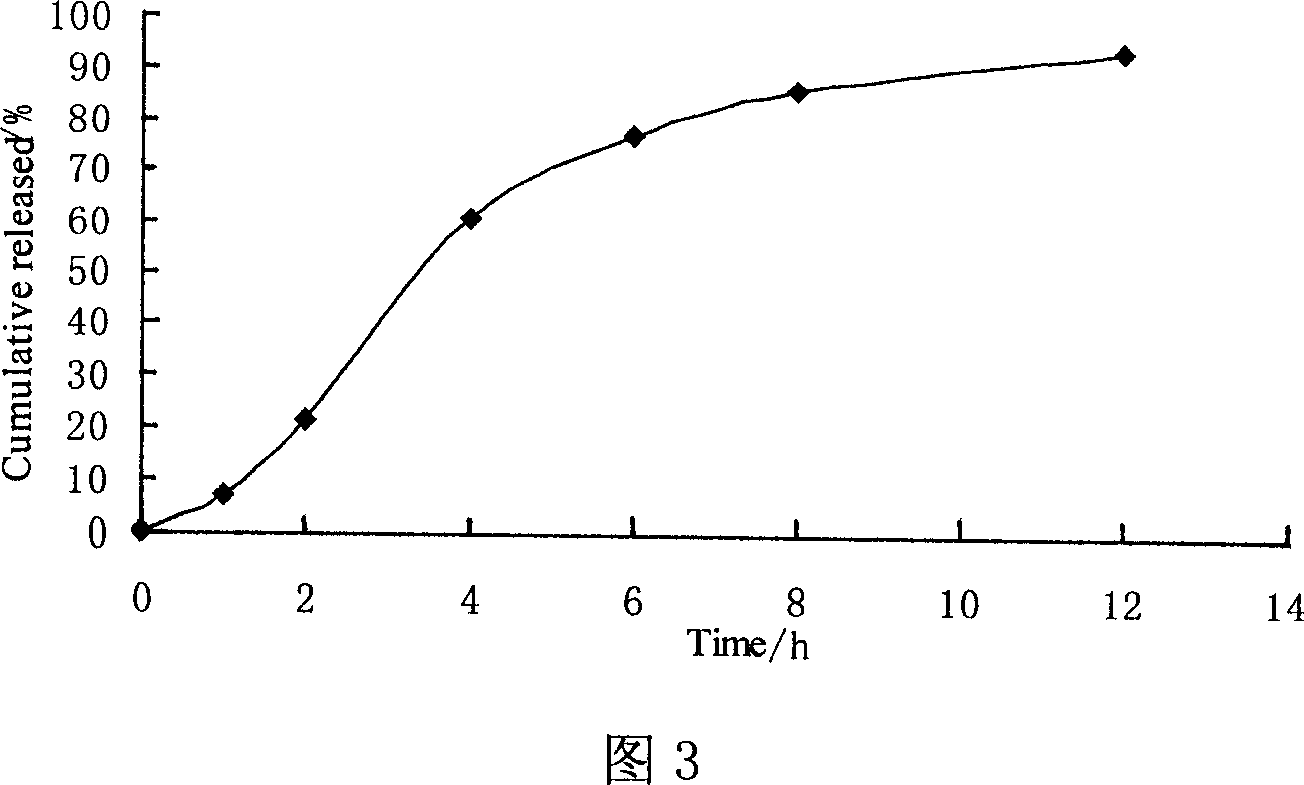
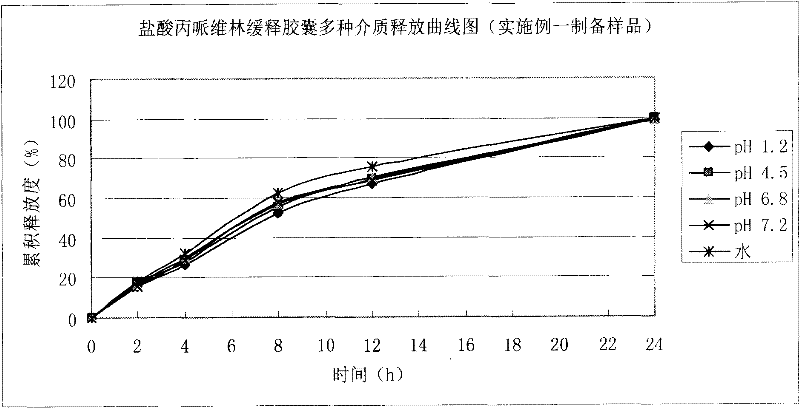
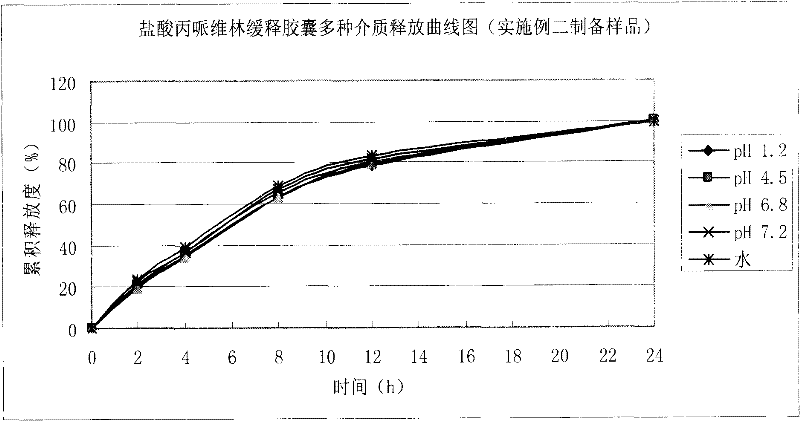
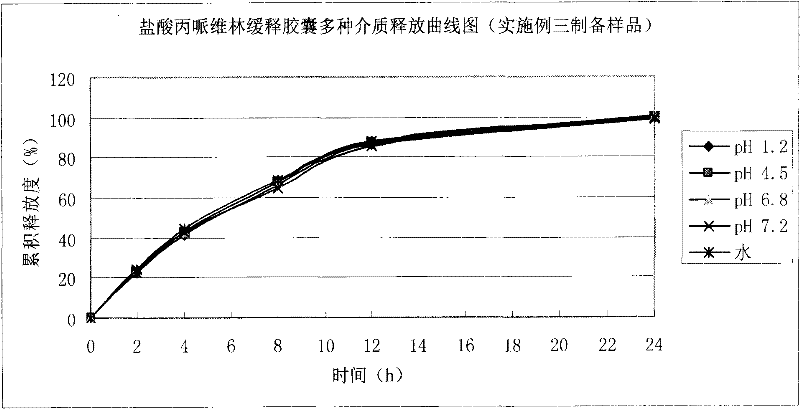
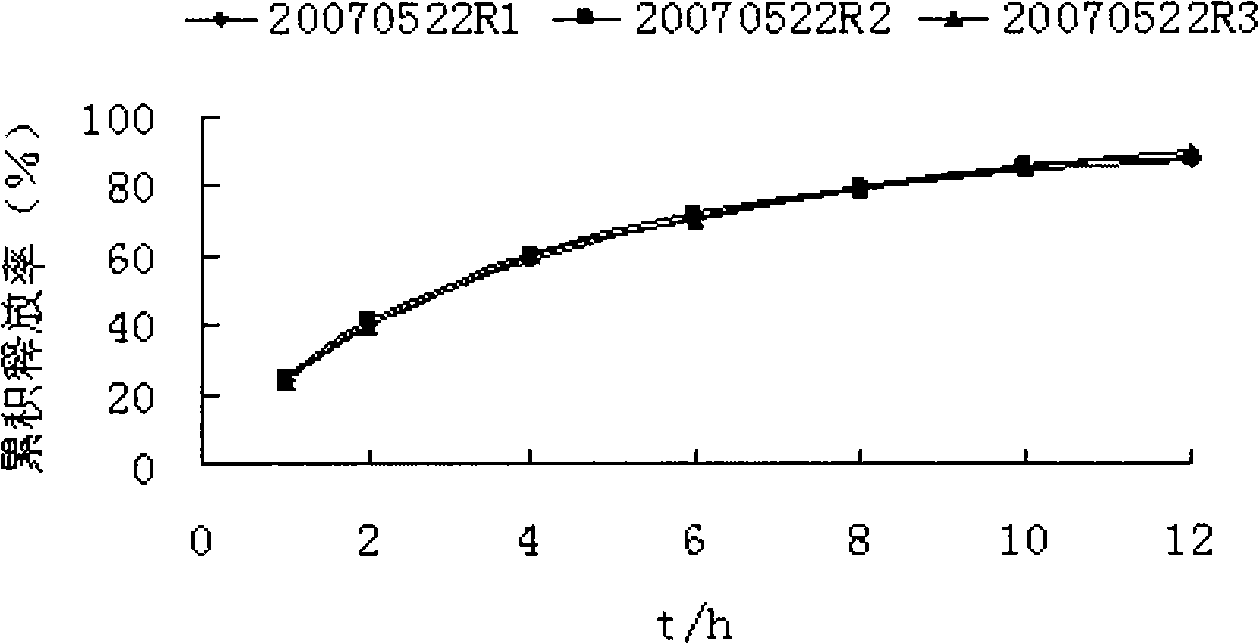
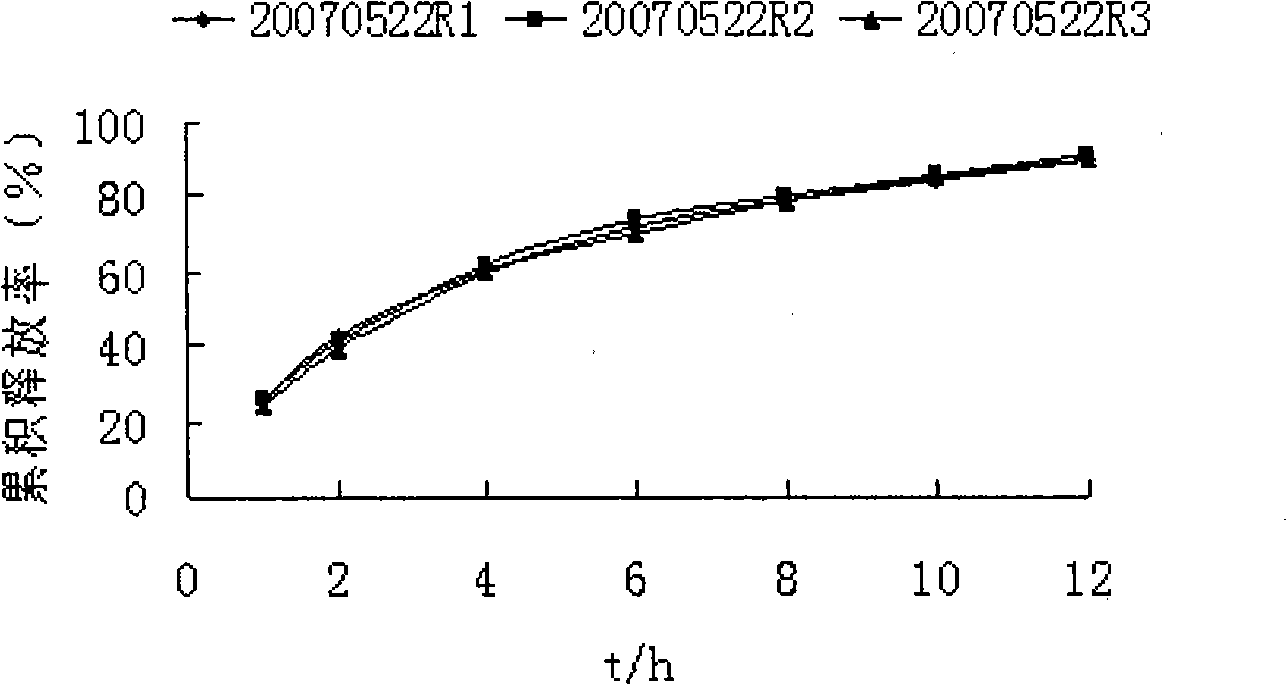
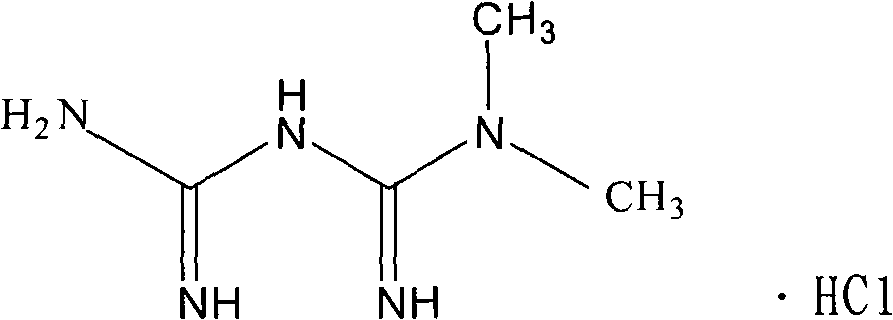
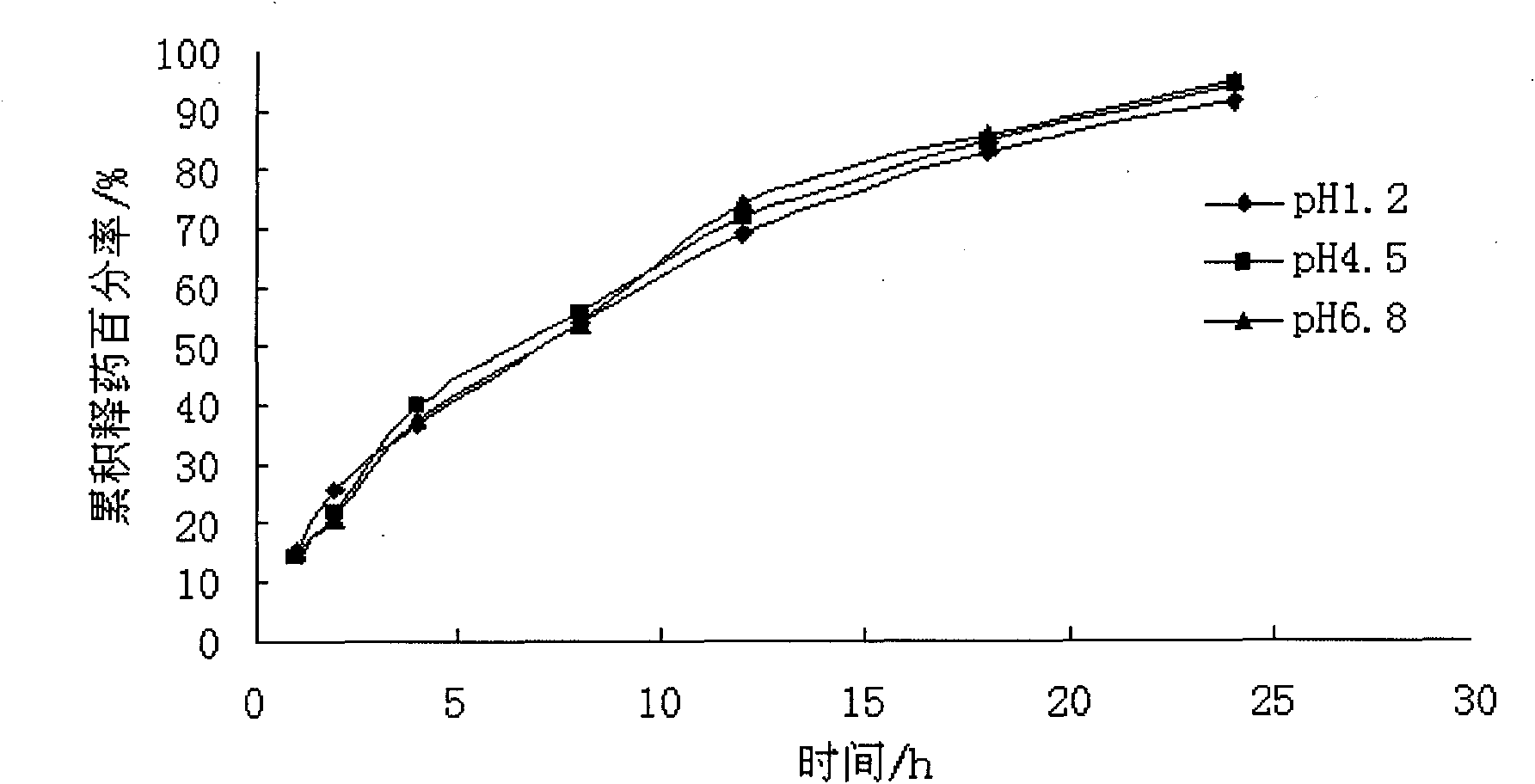
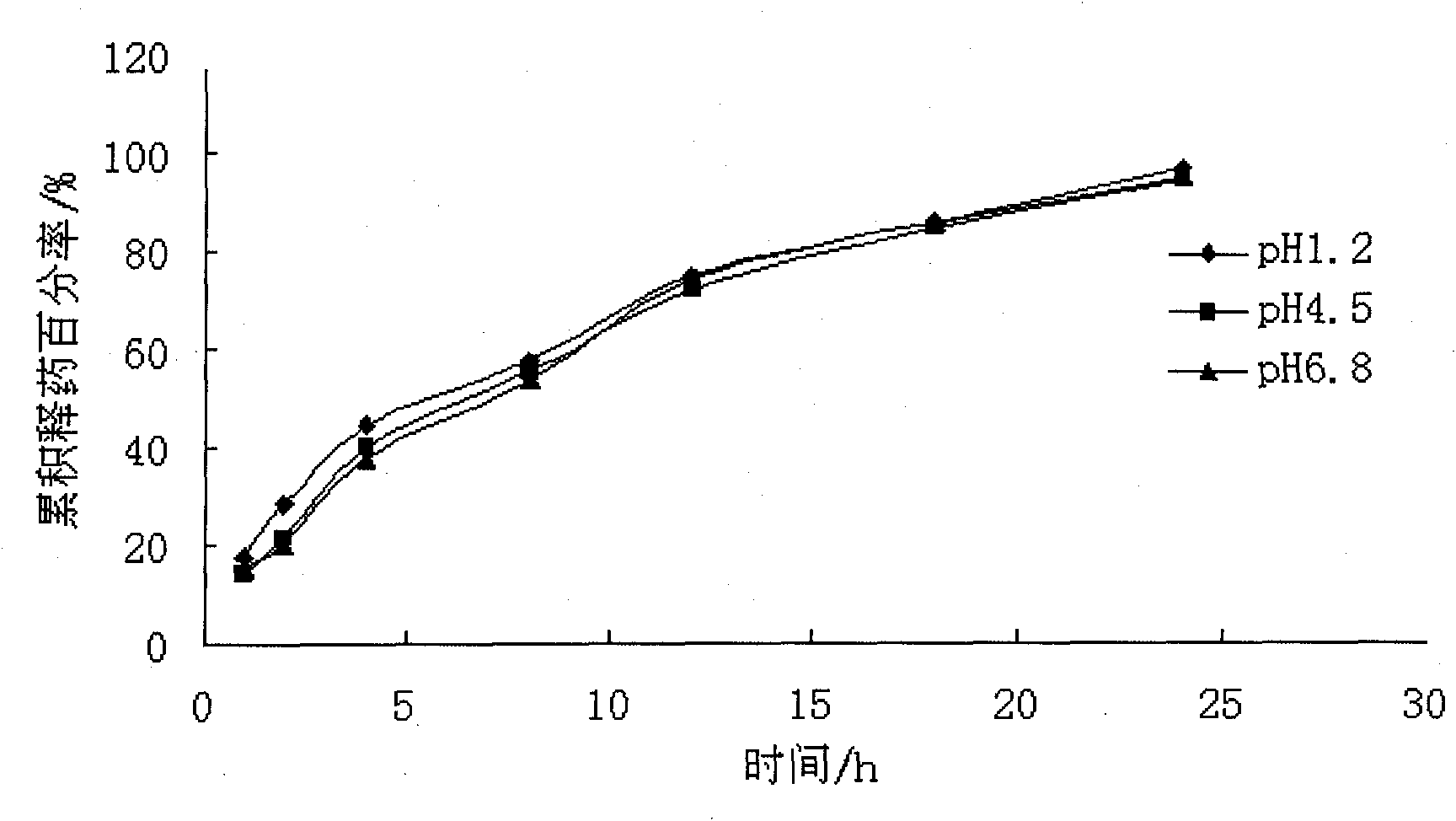
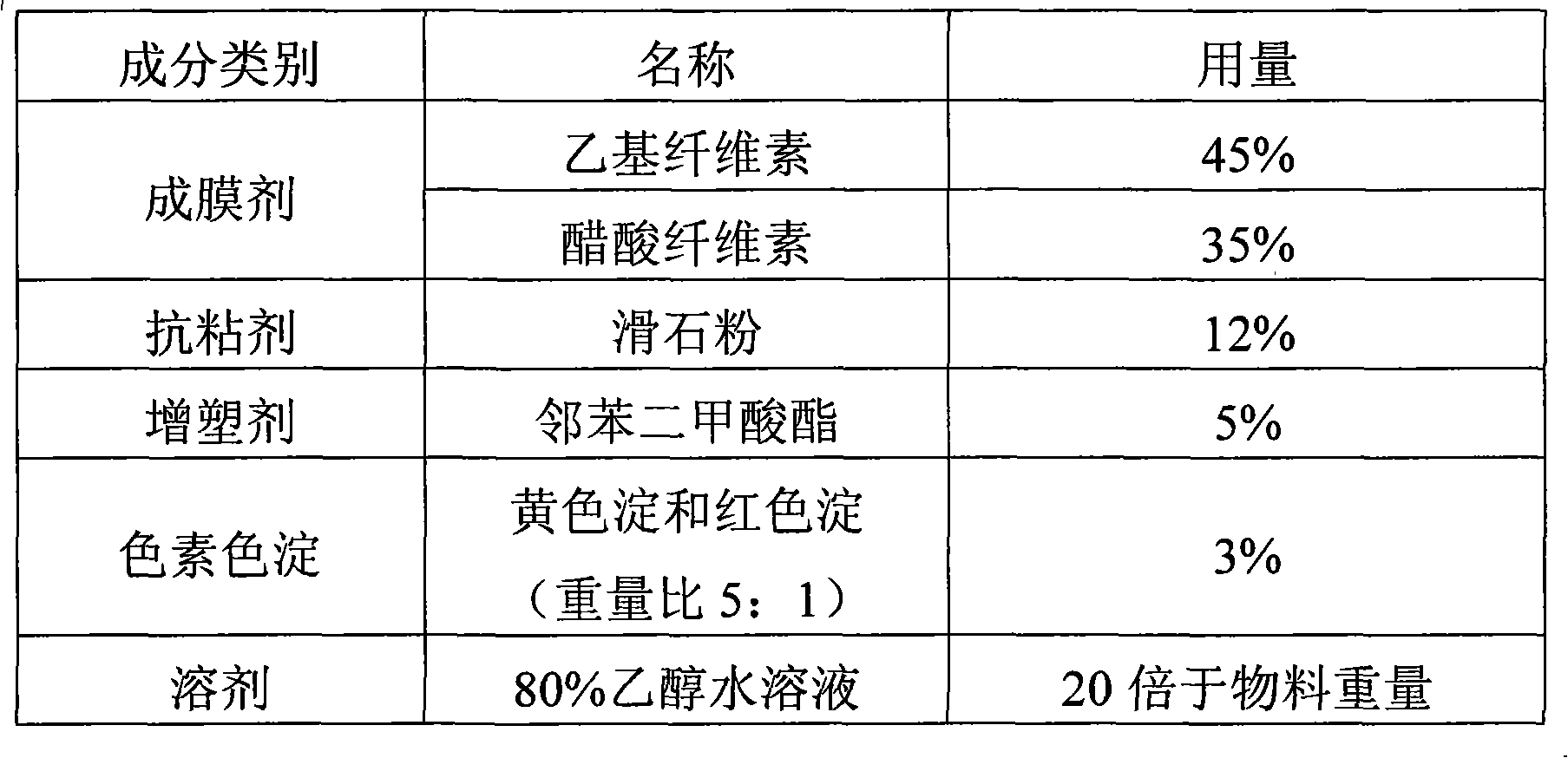
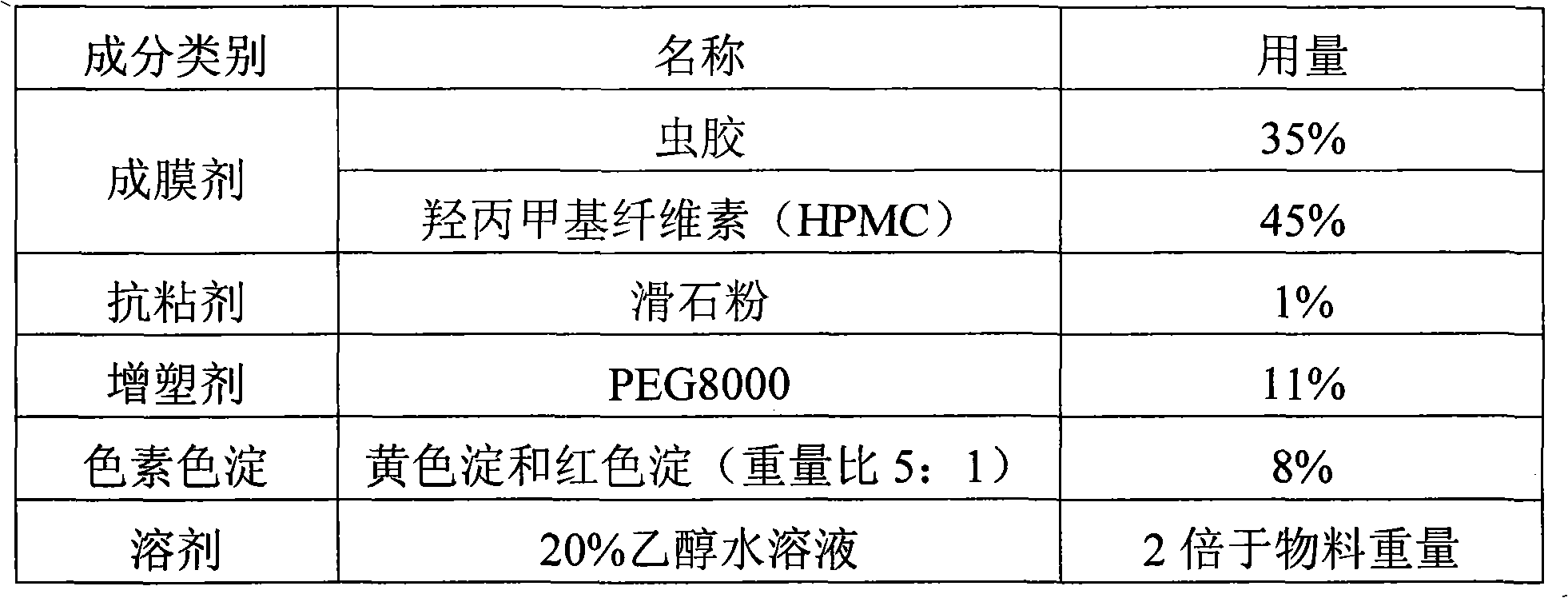
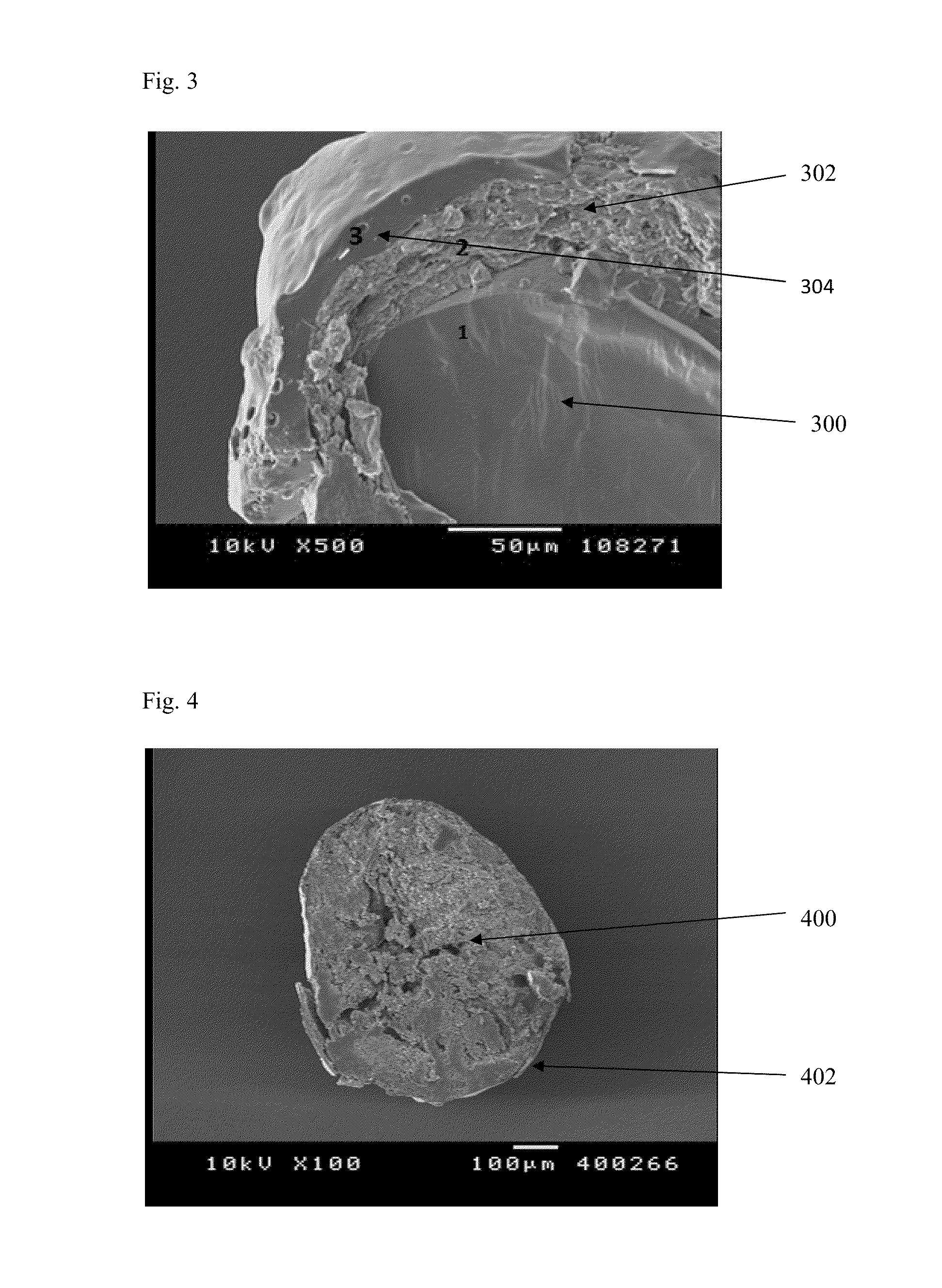
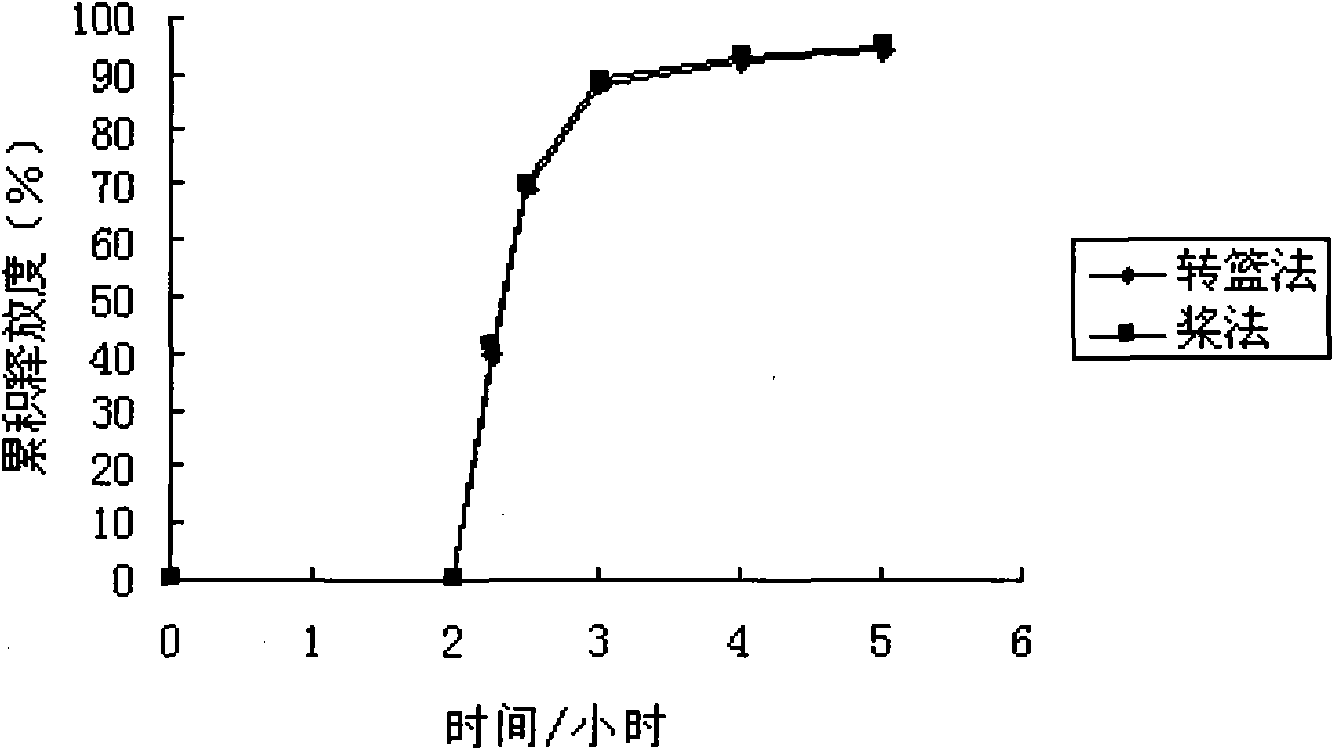
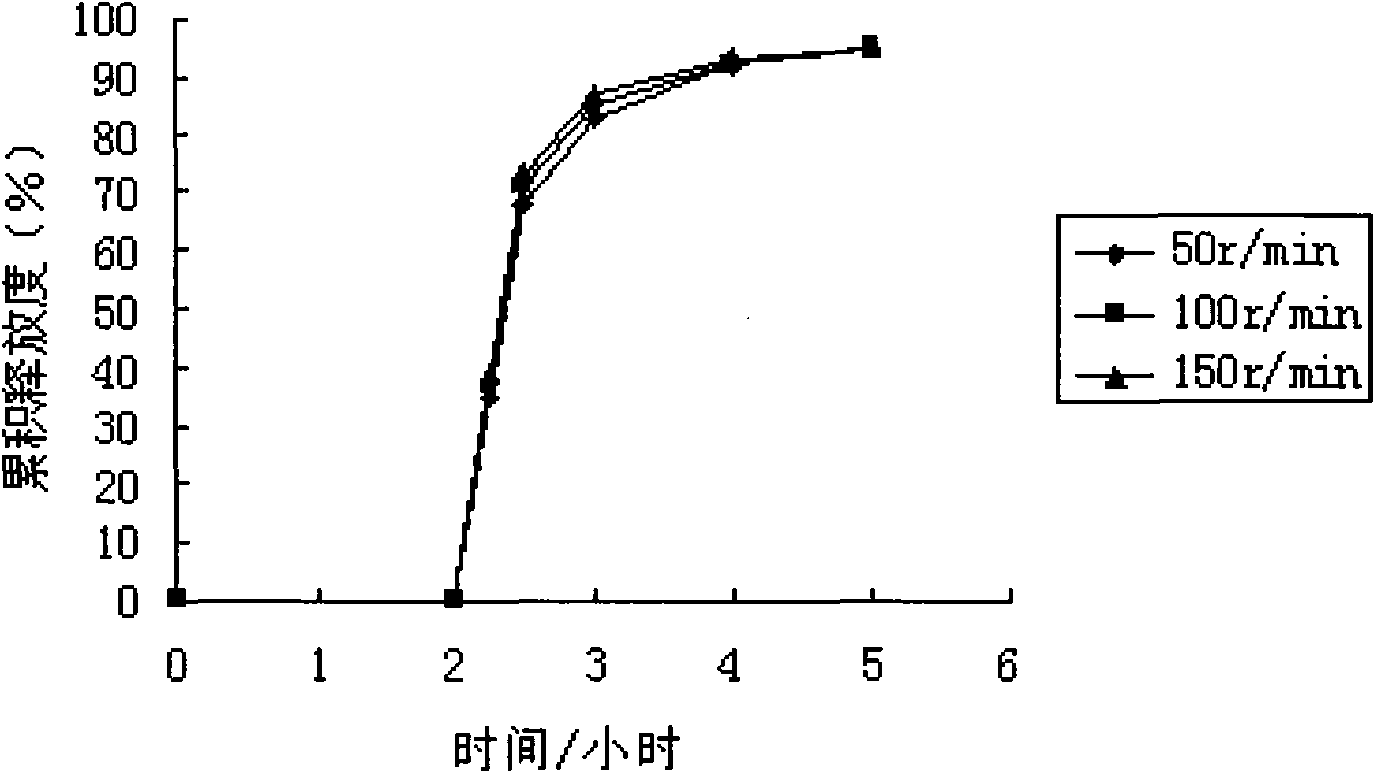
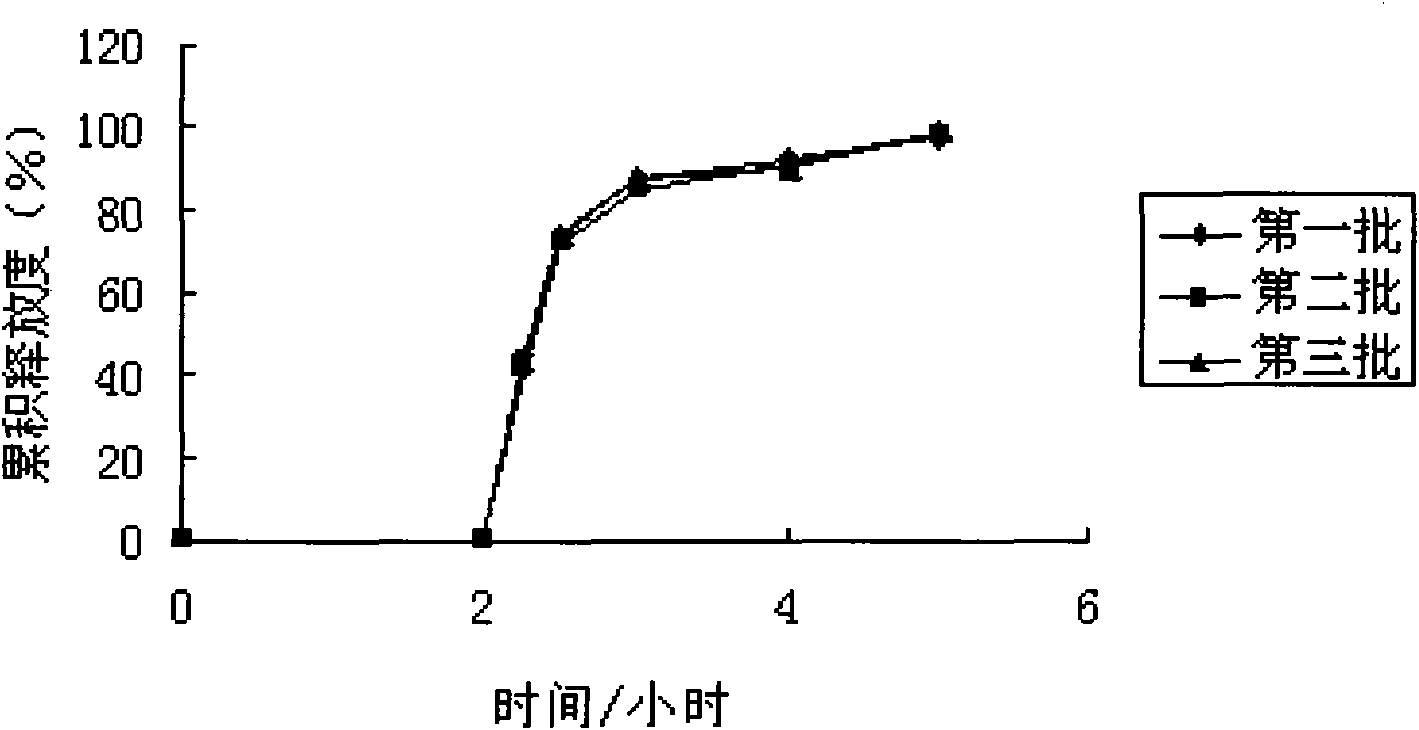
Patents
Literature
136 results about "Extrusion spheronization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
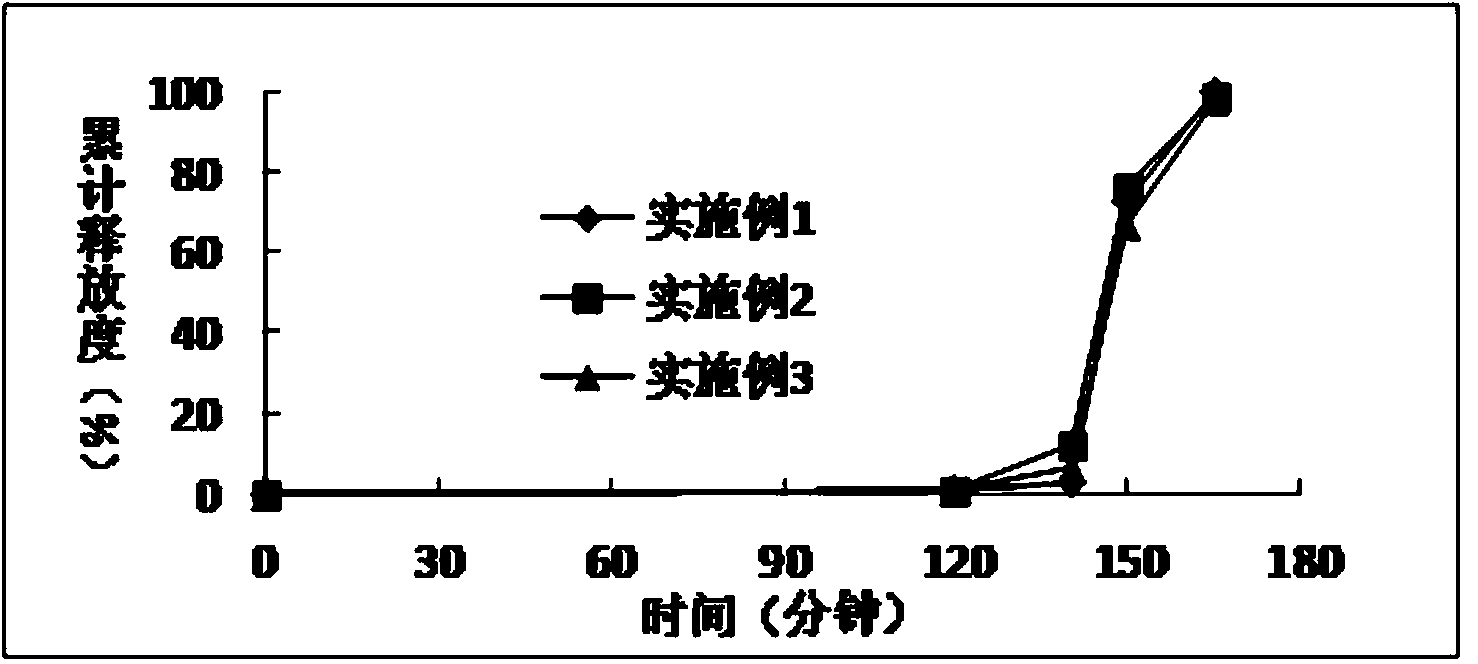
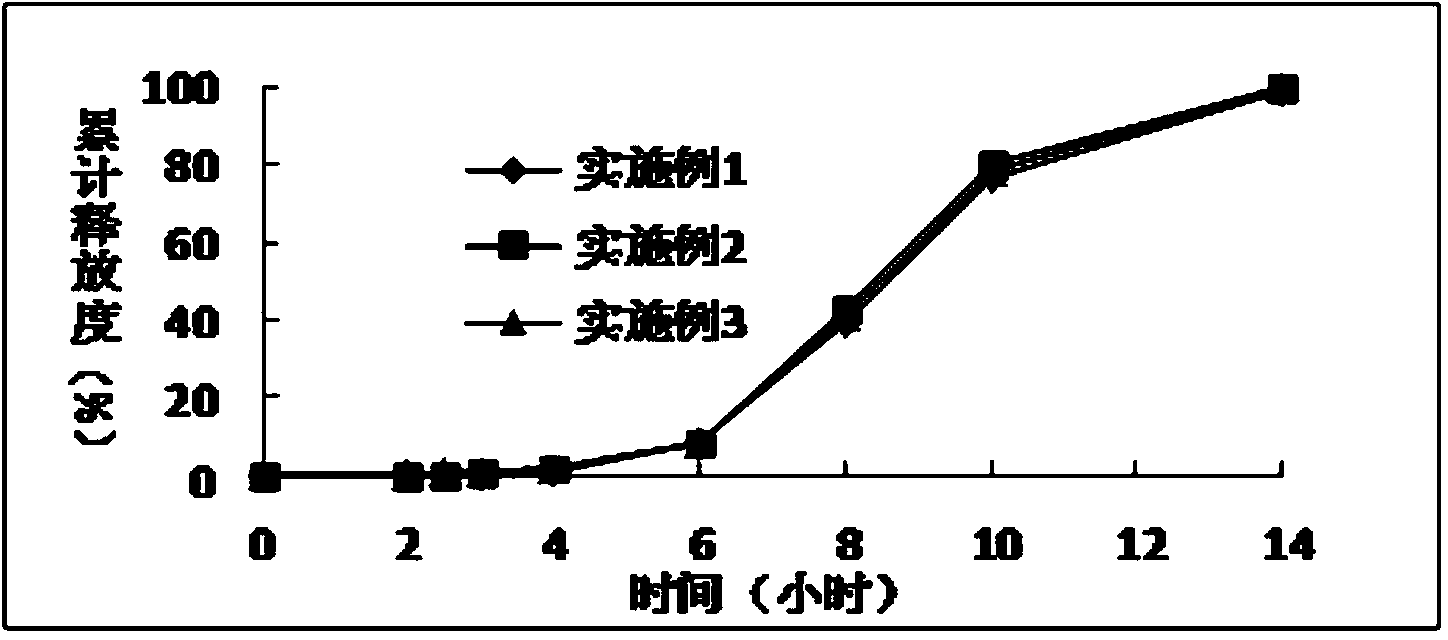
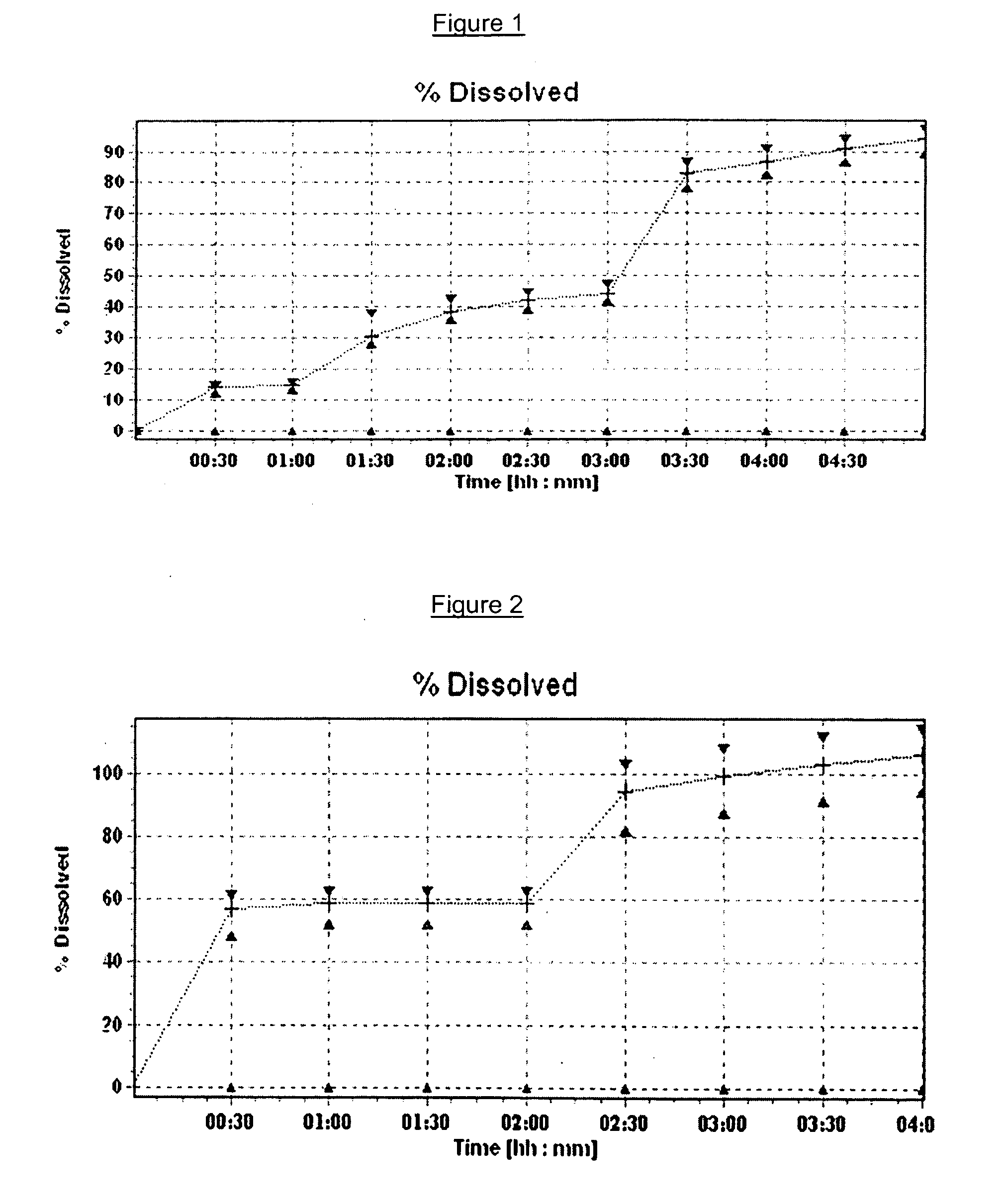
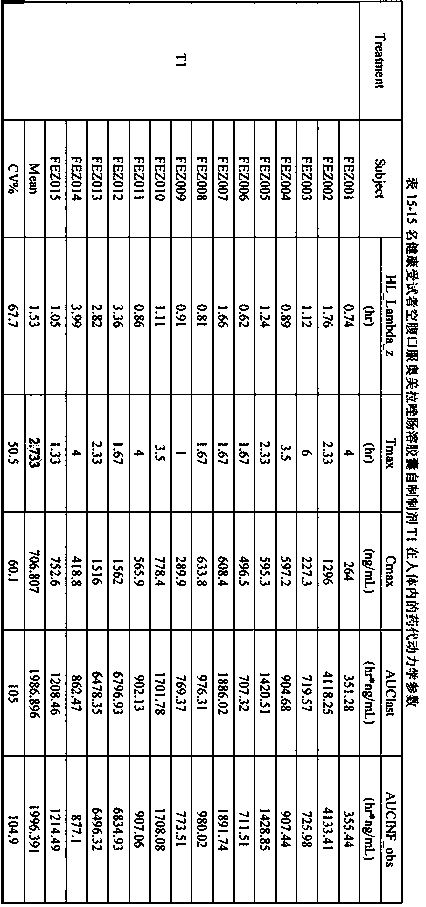
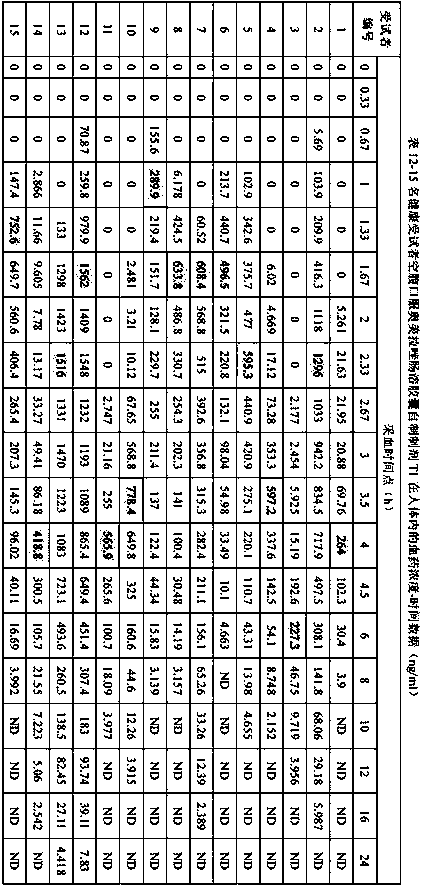
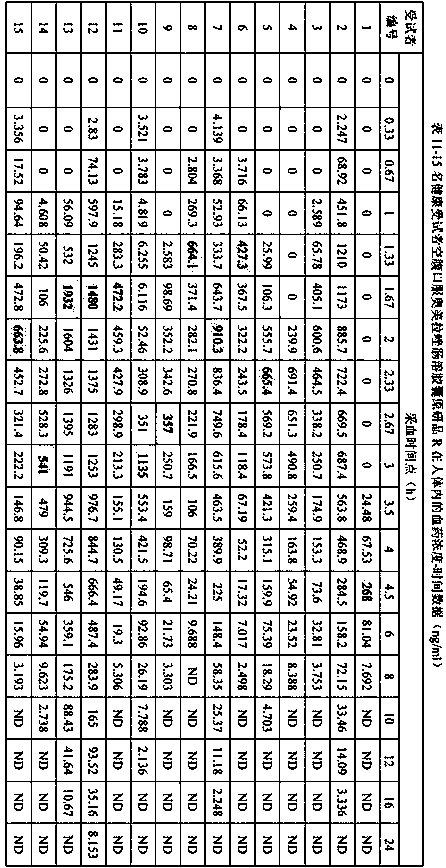
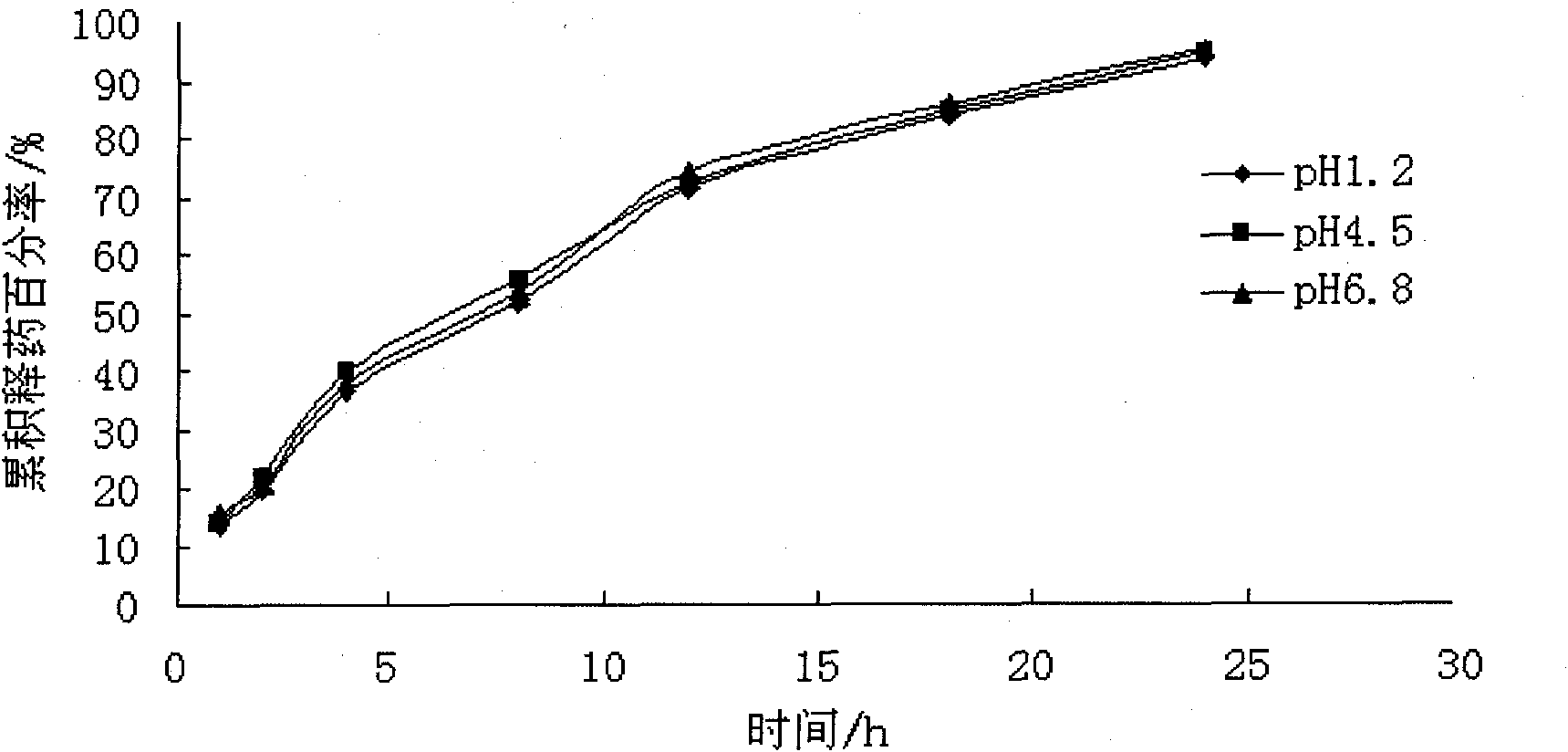
Extrusion Spheronization has been used in agrochemicals, detergent additives, sweeteners, food and now it is used in pharmaceuticals. Extrusion spheronization is primarily used for the production of multiparticulates for oral controlled drug delivery system.
Slow-releasing preparation containing metformin hydrochloride and glipizide and its preparation method
InactiveCN101057849AEvenly distributedReduce local irritationOrganic active ingredientsMetabolism disorderMedicineMetformin Hydrochloride
The invention discloses a diabecron and glipizide -containing slow-release agent and the method for preparing the same. The glipizide micro-pill takes blank micro-pill as carrier, and combines glipizide and other medical findings with it. The diabecron-containing slow-release micro-pill comprises diabetosan pill, slow-release coating membrane material or other medical findings. The method for preparing diabecron-containing slow-release micro-pill takes extrusion rolling method or blank micro-pill loading method. The product is characterized by safety, high efficient, low toxicity and convenient usage. It can be used to treat non-insulin-dependent diabetes mellitus.
Owner:QIQIHAR MEDICAL UNIVERSITY
Sustained-release micro-pellet of trimetazidine and preparation process thereof
InactiveCN1994280AImprove liquidityUniform absorption rate in the bodyOrganic active ingredientsPharmaceutical non-active ingredientsTrimetazidine DihydrochlorideSustained release pellets
The invention relates to a slow-release micro drop whose active component is Humeitashen or other salt, wherein it is formed by element and film layer that controlling the drug release, whose weight ratio is 20:1-5:1; the Humeitashen content of element is 10-60%. The invention mainly uses protrusion method to prepare drop, and uses fluidize bed to pack.
Owner:SHANDONG INST OF PHARMA IND
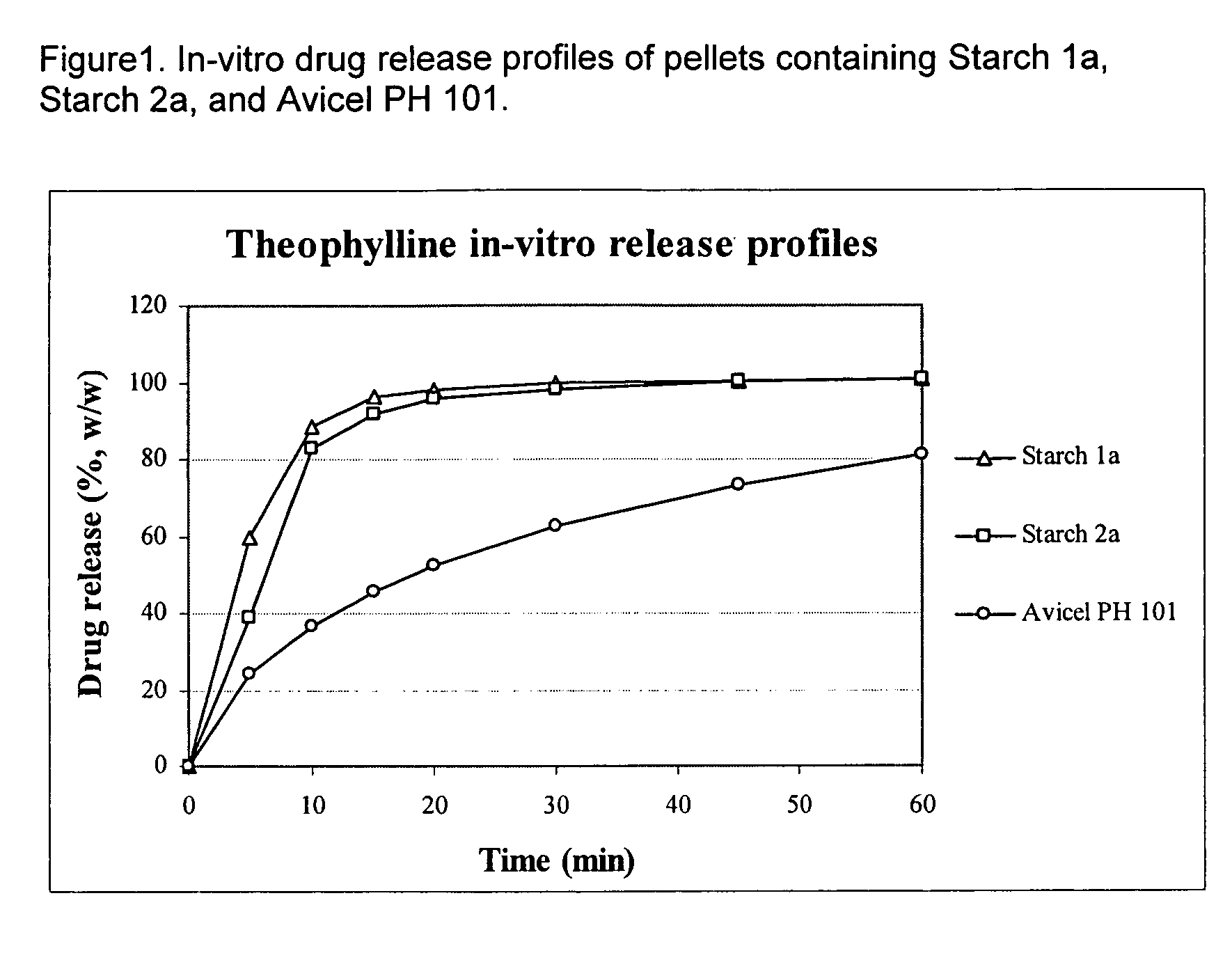
Use of debranched starch in extrusion-spheronization pharmaceutical pellets
InactiveUS20060246192A1Milk preparationPharmaceutical non-active ingredientsExcipientPharmaceutical preservatives
This patent pertains to the use of debranched starch in the preparation of pharmaceutical pellets by extrusion spheronization. Such excipients are useful in any dry dosage form, including tablets and capsules, for either immediate or sustained release.
Owner:HENKEL KGAA +1
Enveloping process for micro-capsule animal medicament
ActiveCN102319181AMeet the needs of productionReduce energy consumptionOrganic active ingredientsMicrocapsulesBiotechnologyFluidized bed drying
The invention relates to the field of processing of animal medicaments, in particular to an enveloping process for a micro-capsule animal medicament. The enveloping process for the micro-capsule animal medicament comprises the following steps of: weighing materials; preparing soft materials; granulating by extruding and rolling; drying with a fluidized bed; coating; polishing; drying with the fluidized bed; sieving; detecting a product and the like to prepare the micro-capsule animal medicament. Compared with a fluidized bed liquid atomizing enveloping process, the enveloping process related to be invention has the most outstanding advantages of high production efficiency, simple product enveloping process, difficulty in causing the phenomenon of sticking, reduction in the energy consumption of a unit product by over 60 percent, capability of meeting the practical requirements of the animal medicament on large production amount and low production cost and contribution to wide popularization and application of the micro-capsule animal medicament.
Owner:GUANGDONG WENS DAHUANONG BIOTECH +1
DMF (dimethyl fumarate) enteric-coated micropellet and preparation method thereof
ActiveCN104352441AReduce gastrointestinal side effectsAvoid sublimationOrganic active ingredientsPharmaceutical non-active ingredientsMedicineExtrusion spheronization
The invention relates to a DMF (dimethyl fumarate) enteric-coated micropellet and a preparation method thereof. The DMF enteric-coated micropellet comprises a pellet core and a double-layer enteric coating, wherein the pellet core is coated with the double-layer enteric coating. The enteric-coated micropellet is prepared by the following steps: the pellet core is prepared with the extrusion-spheronization method; and the pill-containing pellet core is coated with the double-layer enteric coating to form the enteric-coated micropellet. The obtained DMF enteric-coated micropellet is simple in preparation technology, complete in drug release and good in reproducibility, and external dose dumping risk is reduced.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Drug delivery composition
A drug delivery composition that comprises extruded spheroids. The spheroids comprise at least one active pharmaceutical ingredient; at least one extrusion-spheronization aid; at least one superdisintegrant; and at least one glidant, at least one lubricant, and / or at least one oil. The spheroids may also be coated. In a further aspect, a drug delivery composition that comprises coated spheroids that have inert spheroids and at least one coating for the spheroids. The coating comprises at least one active pharmaceutical ingredient and at least one superdisintegrant.
Owner:INTELLIPHARMACEUTICS
Memantine hydrochloride sustained-release capsule and preparation method thereof
ActiveCN103181914ASustained releaseSmooth releaseNervous disorderPharmaceutical non-active ingredientsSustained release pelletsMemantine Hydrochloride
The invention provides a preparation method of a memantine hydrochloride sustained-release preparation. According to the present invention, sustained-release pellets are obtained by sustained-release coating of drug-containing pellets containing memantine hydrochloride. The drug-containing pellets are obtained by extrusion spheronization or solution medicine-feeding or suspension medicine-feeding, and are nearly circular in shape. The shape and granularity-controllable drug-containing pellets are subjected to sustained-release coating and the thickness of the film formed by coating can also be controlled. The invention realizes the controllability of the coating film and the spherical pellets, and reproducibility of stability of releasing the memantine hydrochloride is controlled under the circumstance of guaranteeing nonoccurrence of crystal form of memantine hydrochloride. The preparation of the invention can provide sustained release in the form of a single dose within 24 hours. The drug penetrates and diffuses to the release medium through the film pores. Because the size is small, the medicine taking is less susceptible to foods and the efficacy is improved. The production method of the present invention is simple, is suitable for industrial production, and has a great application value.
Owner:SHANGHAI FOSUN PHARMA DEV CO LTD
Enrofloxacin slow-release micropill for livestocks, and preparation method of same
InactiveCN102648896AExpand the scope of clinical applicationImprove the characteristics of easy color change when exposed to lightAntibacterial agentsOrganic active ingredientsPharmaceutical formulationSodium carboxymethyl starch
The invention relates to the field of pharmaceutical preparations and particularly relates to a slow-release micropill preparation containing enrofloxacin and a preparation method of the preparation. The slow-release micropill provided by the invention is formed by coating an enrofloxacin micropill; the enrofloxacin micropill comprises enrofloxacin and auxiliary material and is formed through extruding and rounding; the auxiliary material is any one or more of microcrystalline cellulose, starch, cane sugar, artificial gum, lactose and sodium carboxymethyl starch; according to weight percent, the auxiliary material in the micropill accounts for 70% to 95%; and coating is made of high-molecular enteric material, film forming material, opaquer and the like. The slow-release micropill preparation has the characteristics of slow release and high bioavailability, can be used for treating bacteria and mycoplasma infection of livestocks, and has better curative effect on chronic respiratory diseases, colibacillosis and salmonellosis, and the frequency of medicine taking can be reduced; and in addition, the slow-release micropill provided by the invention has the advantages that the stability of medicine is improved, and peculiar bitter of enrofloxacin can be covered completely, so that feeding intake of the animals is not influenced, and the recovery rate is improved.
Owner:ZHENGZHOU FUYUAN ANIMAL PHARMA
Intragastric floating slowly releasing micropill and preparation method thereof
InactiveCN101579317ASmall local irritationTo achieve a sustained release effectInorganic non-active ingredientsGranular deliveryFluidized Bed Coating MethodIrritation
The invention discloses an intragastric floating slowly releasing micropill preparation and a preparation method thereof. The micropill preparation mainly comprises three parts, namely a light medicament-carrying pill core, a gas-producing layer coating and a blocking layer coating, wherein the light pill core comprises 1 to 40 percent of medicament, 40 to 70 percent of wax material, and 20 to 30 percent of filling agent; the gas-producing layer coating comprises 3 to 7 percent of coating material, 5 to 20 percent of plasticizer, 2 to 8 percent of gas-producing component, and 5 to 20 percent of antiplastering aid; and the blocking layer coating comprises 20 to 50 percent of coating material, 0 to 30 percent of plasticizer, and 10 to 30 percent of antiplastering aid. A spheronization methodand a fluidized bed coating method are adopted to prepare the micropills, the prepared micropills float after entering water and persistently float for 24 hours, and medicaments of the micropills rea lize the slowly releasing effect. The prepared micropills have the characteristics of large distribution area, small local irritation and the like; and the micropill has floating characteristics, greatly prolongs the retention time in stomachs, and ensures that the bioavailability of the medicaments which have best absorption in the stomachs and at special positions of upper parts of small intestines is greatly improved. The micropill is prepared by adopting the prior method, and is easy to realize industrialized production.
Owner:SHENYANG PHARMA UNIVERSITY
Sustained release beadlets containing stavudine
InactiveUS7135465B2Sufficient amountPowder deliveryCosmetic preparationsBlood levelRetroviral infection
Extended dosage forms of stavudine are provided comprising beadlets formed by extrusion-spheronization and coated with a seal coating. The beadlets are also coated with a modified release coating such that a hard gelatin capsule containing such beadlets will provide blood levels of stavudine over approximately 24 hours. The beadlets are prepared from a dry blend of stavudine, a spheronizing agent, a suitable diluent and a stabilizing amount of magnesium stearate. The magnesium stearate, in contrast to other similar pharmaceutical adjuncts, has been found to stabilize stavudine against degradation due to hydrolysis in the presence of the limited amount of water necessary for the extrusion-spheronization process. Also included in the scope of the invention are hard gelatin capsules containing, in addition to the stavudine beadlets, similar beadlets containing other therapeutic agents utilized to treat retroviral infections.
Owner:BRISTOL MYERS SQUIBB CO
Slow-releasing micro-pills of sophocarpidine and its preparing method
InactiveCN101045053AImprove liquidityImprove stabilityOrganic active ingredientsDigestive systemSophocarpidineFluidized Bed Coating Method
Owner:SHANDONG INST OF PHARMA IND
Sustained-release capsule containing propiverine hydrochloride and preparation method of sustained-release capsule
InactiveCN102579404ALasting effectReduce the number of dosesPharmaceutical delivery mechanismPharmaceutical non-active ingredientsSustained Release TabletSustained Release Capsule
The invention discloses a sustained-release capsule containing propiverine hydrochloride and a preparation method of the sustained-release capsule. The sustained-release capsule comprises sustained-release micropills and an empty capsule, wherein, the sustained-release micropills comprise pill cores containing drugs accounting for 75 to 97 percent and sustained-release coating layers accounting for 3 to 25 percent by weight percentage. The sustained-release capsule containing propiverine hydrochloride comprises hundreds of the sustained-release micropills with uniform particle sizes, and preparation errors or preparation defects of individual micropills cannot influence the drug release behavior of the whole preparation seriously, so that the sustained-release capsule is safer than a sustained-release tablet, the irritant activity to gastrointestinal tracts is smaller, plasma concentration is smoother, the bioavailability is higher, and the sustained-release capsule can continuously release the drugs for 24 hours if being taken for one time per day so as to treat overactive bladder. The preparation method of the sustained-release capsule prepares the pill cores containing the drugs in an extrusion and spheronization method or a drug added manner, adopts a fluidized bed to coat the sustained-release coating layers, achieves simple technology, and is easy to achieve industrialized mass production.
Owner:广州科的信医药技术有限公司
Metformin hydrochloride and Glipizide sustained-release pellet and method of preparing the same
ActiveCN101278919AAvoid Difficulty ScreeningAvoid screening timeOrganic active ingredientsMetabolism disorderSustained release pelletsBlood concentration
The invention discloses a metformin hydrochloride and glipizide sustained-release pellet and a preparation method thereof. In the preparation method, a sustained release coated pellet of the metformin hydrochloride and a sustained release pellet of the glipizide are prepared respectively; and the two pellets are filled in capsules in a proportion of 250g-500g of the metformin hydrochloride and 2.5g-10g of the glipizide in every 1000 capsules; wherein, the coated pellet of the metformin hydrochloride is prepared by pill pericardium sustained release coating membrane; the sustained release pellet of the glipizide is prepared directly by extrusion-spheronization method. In the invention, the two pellets are filled into one capsule, thereby being convenient for quality control; octodecyl alcohol is taken as sustained release material for the sustained-release pellet of the glipizide, which is convenient for the forming of the pellet so as to reduce bursting release effectively. The metformin hydrochloride and the glipizide slowly release a drug within 12 hours, which reduces the frequency of taking medicine, stabilizes blood concentration better and reduces untoward effect, thereby having good marketing prospect.
Owner:国药控股星鲨制药(厦门)有限公司
Salvia miltiorrhiza effective-component synchronous site-specific drug delivery mini-pill and preparation method thereof
InactiveCN101773546APromote absorptionGood dispersionPill deliveryPharmaceutical non-active ingredientsDiseaseFluidized Bed Coating Method
The invention discloses a salvia miltiorrhiza effective-component synchronous site-specific drug delivery mini-pill and a preparation method thereof, belonging to the technical field of drugs. The effective salvia miltiorrhiza component synchronous site-specific drug delivery mini-pill is a pill-shaped preparation obtained by the following steps of: respectively adding a right amount of excipients to salvianolic acid and tanshinone to prepare a mini-pill with the diameter being smaller than 2.5 mm; then respectively coating stomachic coating and enteric coating on the mini-pill with the diameter being smaller than 2.5 mm; and mixing according to the ratio of 1:1. The preparation method can adopt an extrusion-spheronization method and a fluidized bed coating method. Compared with the prior art, the invention solves the problem of effective-component synchronous site-specific delivery, can enhance the bioavailability and is beneficial to stabilizing the effective components; the salvia miltiorrhiza effective-component synchronous site-specific drug delivery mini-pill has outstanding curative effect on cardiovascular and cerebrovascular diseases, such as hypertension, cerebral thrombosis, coronary heart disease, and the like; in addition, the preparation method can adopt conventional process equipment and has the advantages of high production efficiency, stable product quality, validity period more than two years and easy industrialized production.
Owner:SHENYANG PHARMA UNIVERSITY
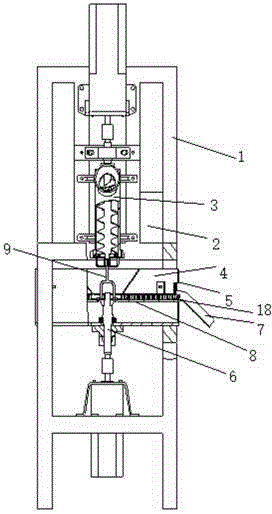
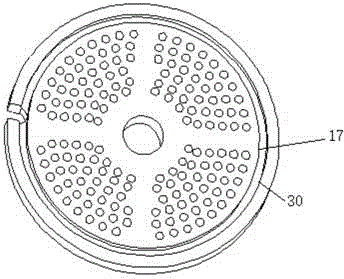
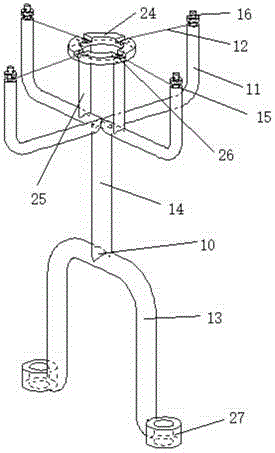
Extrusion-spheronization integrated granulating machine
InactiveCN104549045AReduce areaSmall footprintGranulation in rotating dishes/pansGranulation by material expressionEngineeringSmall footprint
The invention discloses an extrusion-spheronization integrated granulating machine. A support cutter mechanism is arranged between a screw extruder and a spheronizator of the granulating machine; the support cutter mechanism at least comprises a filament propping rod, a filament and a base support; filament winding slots are formed at two ends of each filament propping rod, the filament is fixed on the filament propping rod by the filament winding slot, two ends of each filament propping rod are in clearance fitting with grooves of a pore plate by pins, a spheronization disc cover is arranged above a spheronization disc and is in clearance fitting with the spheronization disc; a spheronization feeding hole, a lifting lug and a single-screw guide groove are respectively formed in and arranged on the spheronization disc cover; the spheronization disc cover is fixed on a spheronization barrel by the lifting lug; the spheronization feeding hole and the single-screw guide groove are arranged on the outer surface and the inner surface of the spheronization disc cover respectively, the spheronization feeding hole is communicated with a through hole, the inlet and the outlet of the single-screw guide groove are communicated with the through hole and a discharge hole respectively, friction lines are formed on the upper end surface of the spheronization disc, and the base support is arranged on the spheronization disc. The extrusion-spheronization integrated granulating machine is small in occupied area, high in granulating efficiency, short in time and good in granulating effect.
Owner:HUAZHONG AGRI UNIV
Omeprazole enteric capsule and preparation method thereof
ActiveCN109125282AImprove in vitro dissolutionTime to peak blood concentrationAntibacterial agentsOrganic active ingredientsFluidized Bed Coating MethodOmeprazole
The invention discloses an omeprazole enteric capsule and a preparation method thereof. The omeprazole enteric capsule comprises omeprazole enteric pellets filled into a gelatin capsule shell. Each omeprazole enteric pellet sequentially comprises an omeprazole carrying pellet core, an isolation layer and an enteric layer from inside to outside, wherein the omeprazole carrying pellet core is prepared through an extrusion spheronization method, and the isolation layer and the enteric layer are prepared through a fluidized bed coating method. The omeprazole enteric capsule is different from original products in a way that Tween 80 and low-substituted hydroxypropyl cellulose are added to the omeprazole carrying pellet cores on the basis of omeprazole, lactose anhydrous, microcrystalline cellulose, mannitol, disodium hydrogen phosphate, hydroxypropyl cellulose and lauryl sodium sulfate. The prepared omeprazole enteric capsule does not change the stability and reproducibility of the capsule,increases the in-vitro dissolution rate of the capsule, especially increases capsule homogeneity to allow the blood drug concentration peak reaching time of different health volunteers to be consistent and has a good application value in clinical application.
Owner:珠海润都制药股份有限公司
Method for preparing high-porosity ozone oxidation nbsCOD catalyst from fenton iron sludge
InactiveCN108855084AEfficient removalHigh compressive strengthCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsPorosityThermal insulation
The invention relates to a method for preparing a high-porosity ozone oxidation nbsCOD catalyst from fenton iron sludge. According to the method, treated fenton iron sludge and aluminum oxide are utilized as main raw materials, the defined amount of pore forming agent is added to be evenly stirred and mixed with water, an extruding and rounding method is utilized, the mixed materials are put intoan extruding rod machine, and cylindrical particles with a length to diameter ratio as 0.85 to 1.00 can be obtained by extruding and cutting into particles; the cylindrical particles are put into a balling disk of a centrifuging rounding machine to be formed into balls under the rotation speed of 40 to 50r / min, the balls are dried and put into a chamber type electric resistance furnace to be roasted under 450 to 550 DEG C, thermal insulation is performed for 2 to 4 h, and the high-porosity ozone oxidation nbsCOD catalyst is achieved. The density of the high-porosity ozone oxidation nbsCOD catalyst prepared by the method disclosed by the invention is 0.70 to 1.00 g / cm<3>, the porosity is 65 to 82%, the water absorption is 55 to 75%, and the compressive strength is higher than 80N. The catalyst prepared by the method disclosed by the invention effectively improves capacity of ozone to degrade nbsCOD.
Owner:SHANDONG UNIV OF TECH
Atenolol non-pH-dependent sustained release pellets and preparation method thereof
InactiveCN101804033AStable release rateOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsOrganic acid
The invention relates to the field of pharmaceutical preparations, in particular to a non-pH-dependent sustained release pellet preparation containing atenolol and a preparation method thereof. The atenolol non-pH-dependent sustained release pellet preparation mainly consists of atenolol-containing pellet cores and coating materials, and is characterized in that the atenolol-containing pellet cores contain a certain proportion of solid organic acid, wherein the solid organic acid is one or more selected from fumaric acid, sorbic acid and adipic acid. The atenolol-containing pellet cores are prepared by the extrusion-spheronization method. The results show that the prepared pellets have good roundness, and the sustained release preparation can be smoothly released in different pH release media.
Owner:CHINA PHARM UNIV
Para-aminosalicylate colon positioning drug delivery system
ActiveCN101590020ASmall toxicityIncrease concentrationOrganic active ingredientsDigestive systemSide effectAdhesive
The invention relates to a para-aminosalicylate colon positioning drug delivery system which is a pellet or a particle. The inner part of the system is a kernel which contains the following components according to the mass percent: 50-98 percent of para-aminosalicylate, 1-30 percent of adhesive and 1-20 percent of skeleton material; and the surface of the kernel is coated with a layer of coatings which is 1 percent-40 percent of the mass of the whole pellet or the particle and contains film forming agent, sticking resistant agent and plasticizer. The invention also discloses a method for preparing the formulation, which comprises a sugar coating pot method, an extrusion-rolling method and a fluidized bed preparation method. Compared with the prior common tablet and a colon positioning tablet, the formulation has the better colon positioning effect, has the characteristics of increased specific surface area and good dissolving effect, is beneficial to absorbing and can reduce the poison and side effect caused by fast drug release by an individual difference of the formulation with a large dose, such as tablets, and the like.
Owner:上海新菲尔生物制药工程技术有限公司
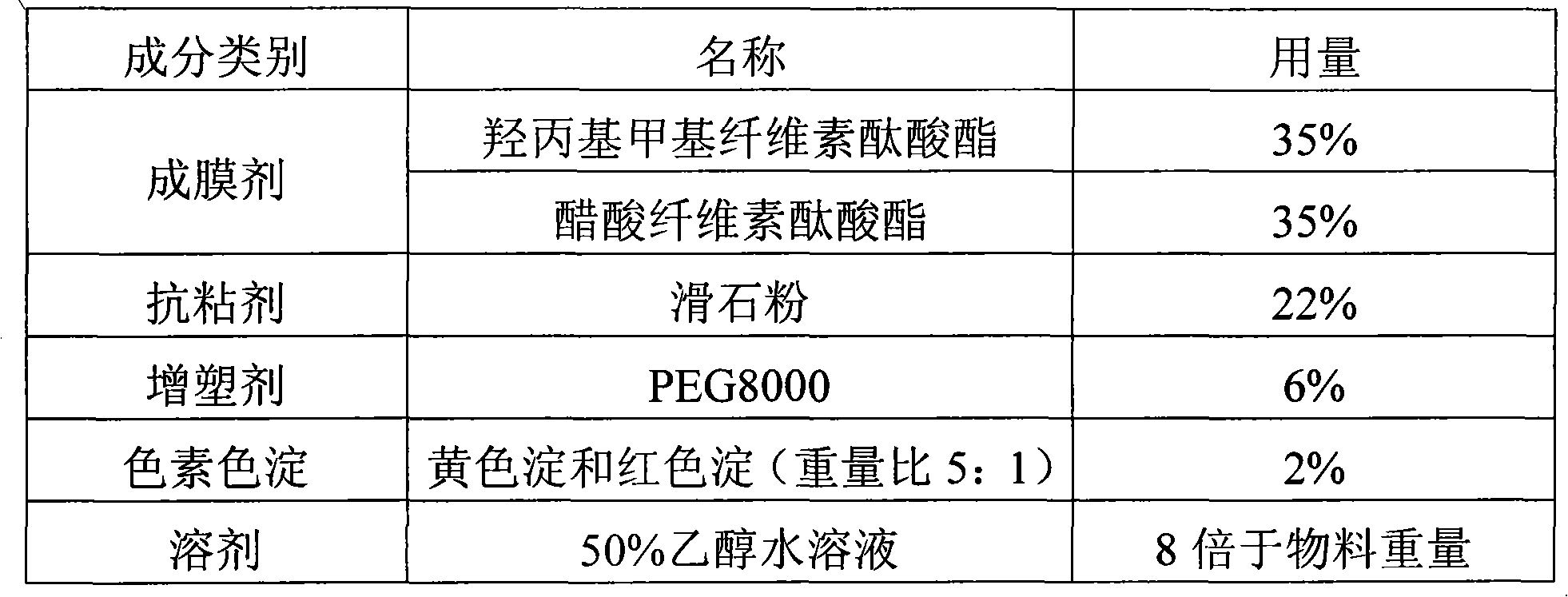
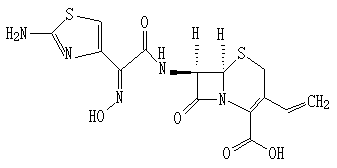
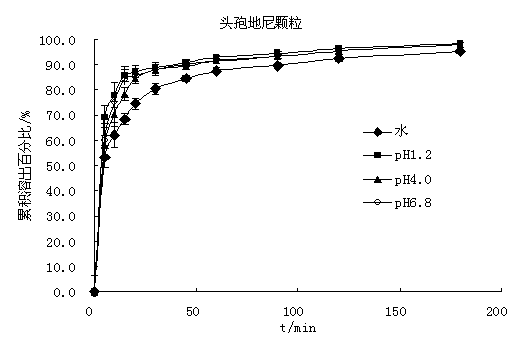
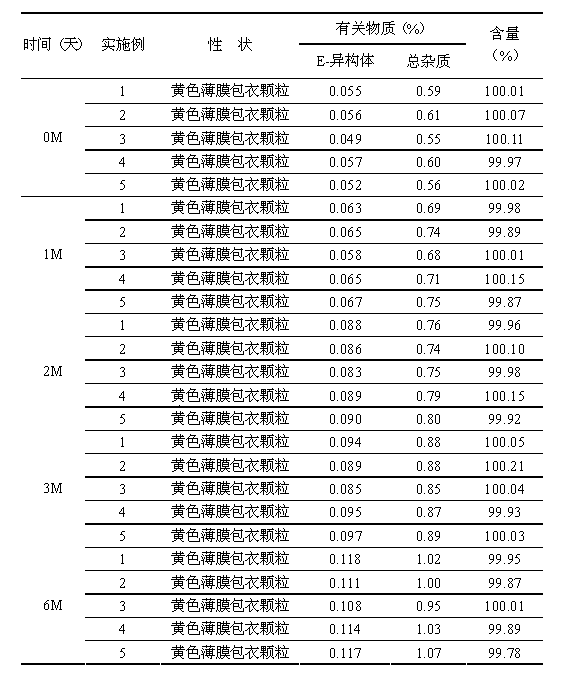
Cefdinir granule and preparation method thereof
InactiveCN104116713AGreat tasteRapid oral absorptionAntibacterial agentsOrganic active ingredientsActive componentCentrifugation
The invention relates to the technical field of medicinal preparations, and discloses a cefdinir granule and a preparation method thereof. The cefdinir granule comprises an active component cefdinir, a filler, a disintegrating agent, a flavoring, a perfume and a flow aid. The cefdinir granule is characterized in that small balls with the size of about 40 meshes are prepared by adopting an extrusion-spheronization technology or a centrifugation granulation technology, the small balls are coated by a colored film, and packaging is carried out by adopting a line-shaped band. The cefdinir granule prepared in the invention has the advantages of good mouthfeel, fast oral absorption, high bioavailability, and improvement of the medication compliance of patients especially children patients by the mixed use of the flavoring, the perfume and the colored film coating.
Owner:南京亿华药业有限公司
Sirolimus preparation and preparation process thereof
ActiveCN101632662AGood dissolution effectLow dissolutionOrganic active ingredientsAntimycoticsOral medicationBioavailability
The invention provides a Sirolimus preparation and a preparation process thereof. The process comprises the following steps: preparing an intermedium by a solid dispersion technology; preparing pellets by an extrusion spheronization process; and finally filling capsules. The preparation and the process of the preparation ensure the high leaching ability of Sirolimus, achieve the aim of slow release, have smaller leaching difference among the capsules and better solve the application limit caused by low bioavailability and narrow therapeutic window of the oral administration of the Sirolimus.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Aroma-enhancing and cooling particles for cigarettes as well as preparation method and application thereof
PendingCN112273717AReduce the temperatureLess irritatingTobacco smoke filtersCellulosePolymer science
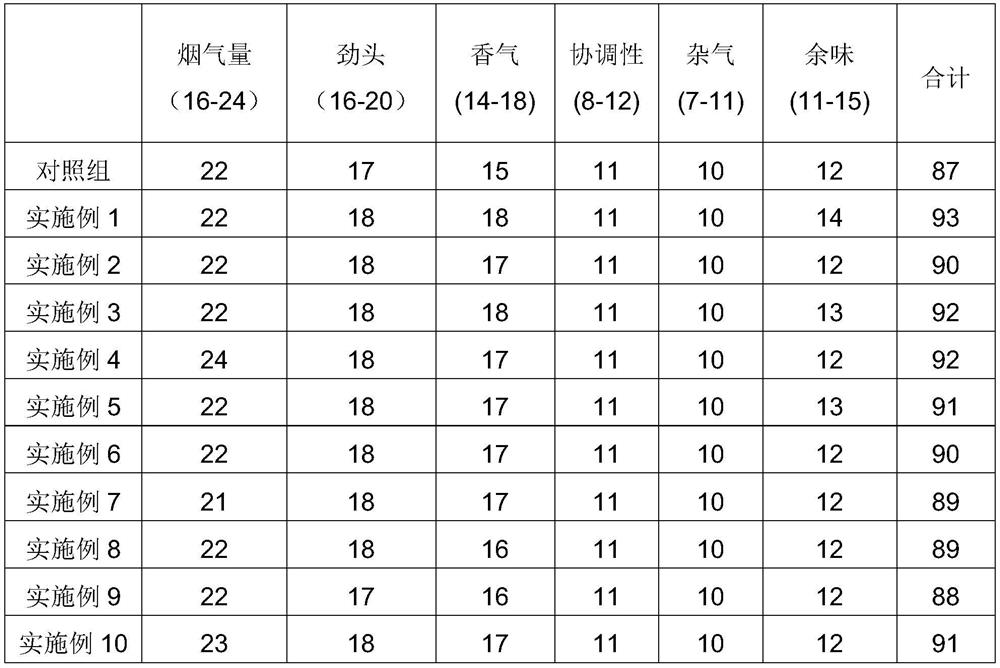
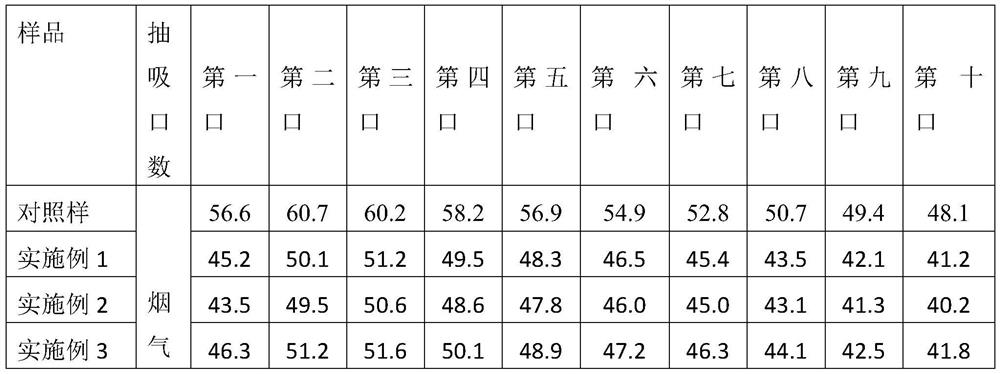
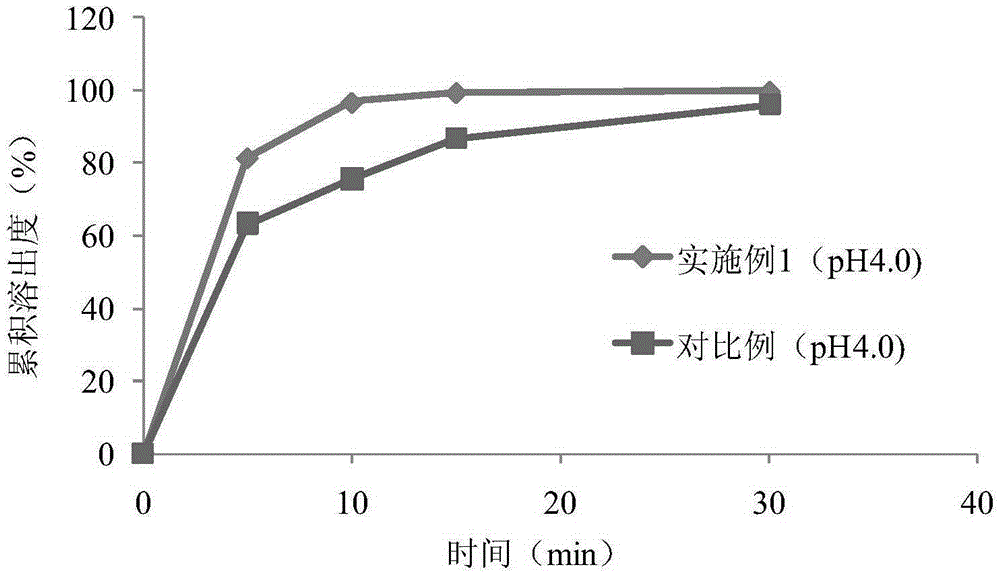
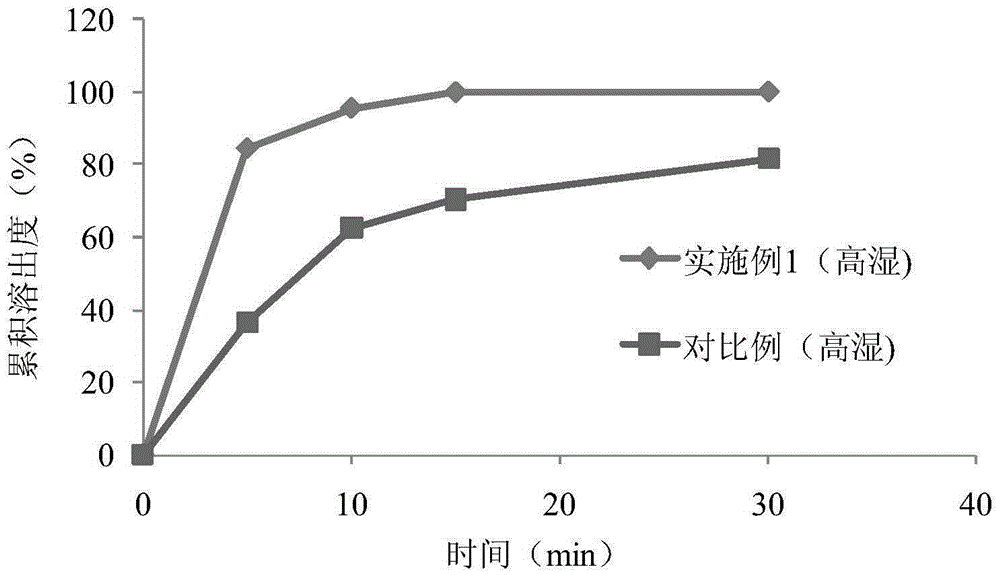
The invention discloses aroma-enhancing and cooling particles for cigarettes, which comprise cellulose, a derivative and an aroma substance, wherein the particles are formed by mixing aroma substancepowder and cellulose and derivative powder, and the mass content of the natural aroma substance powder is 20%-100%. According to the preparation method of the aroma-enhancing and cooling particles forcigarettes, the aroma substance powder is mixed with the cellulose and derivative powder, and the aroma-enhancing and cooling particles are prepared through a wet extrusion spheronization process. According to the preparation method of the aroma-enhancing and cooling particles with the coating for the cigarettes, the cellulose and derivative powder is used as a material, and cellulose and derivative particles are prepared through a wet extrusion spheronization process; and the surfaces of the cellulose and derivative particles are coated with a coating containing the aroma substance in a fluidized film coating manner. When the aroma-enhancing and cooling particles are applied to a cigarette filter stick, the temperature of smoke can be reduced, the irritation of the smoke is reduced at the same time, no offensive odor is brought, the smoke aroma quantity, prolong the aroma lasting time are improved, and the cigarette smoking quality is improved.
Owner:NANTONG CELLULOSE FIBERS CO LTD +2
Medicine composition with norfloxacin and method for preparing medicine composition
ActiveCN105343028AEvenly dispersedUniform contentAntibacterial agentsOrganic active ingredientsNorfloxacinTraditional medicine
The invention relates to a medicine composition with norfloxacin and a method for preparing the medicine composition. The medicine composition comprises an effective quantity of the norfloxacin and pharmaceutical excipients. Proportions of main medicines are 50-80%; the ball forming excipients are selectively one type of microcrystalline cellulose, powdered cellulose, low-substituted hydroxypropyl cellulose and corn starch or combinations of the microcrystalline cellulose, the powdered cellulose, the low-substituted hydroxypropyl cellulose and the corn starch. The medicine composition with the norfloxacin and the method have the advantages that on the one hand, the dissolving-out speeds of oral preparations can be controlled, and on the other hand, the stability of the preparations can be improved.
Owner:ZHEJIANG WANBANG PHARMA
Micro-pills of Niuhuang Shangqing Wan Contg. calculus Bovis, and its prepn. method
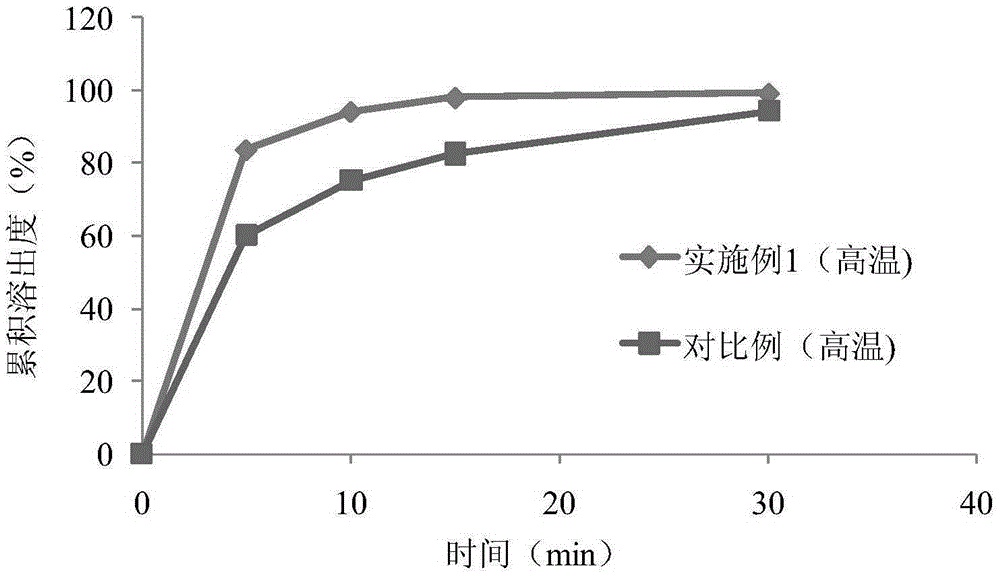
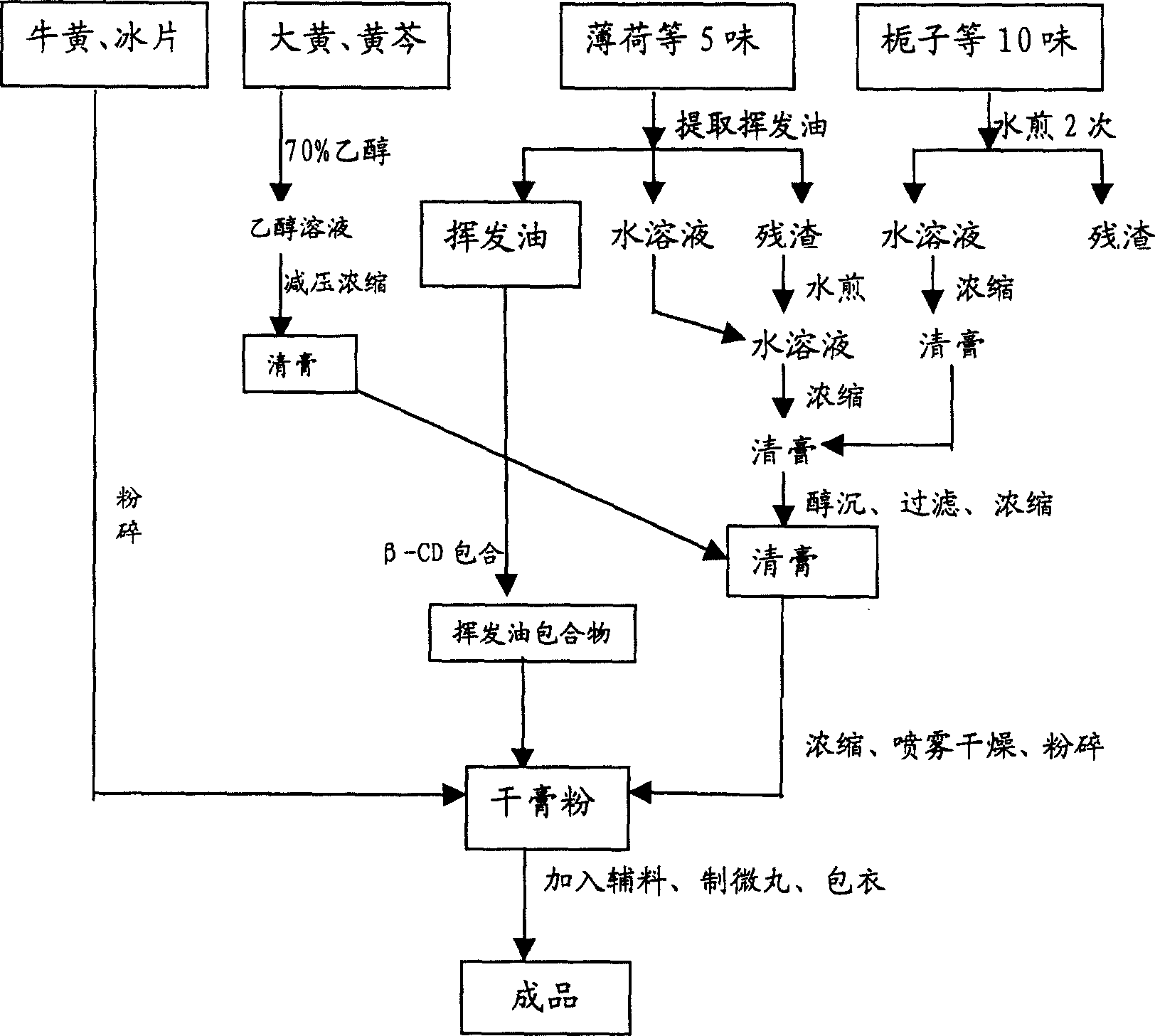
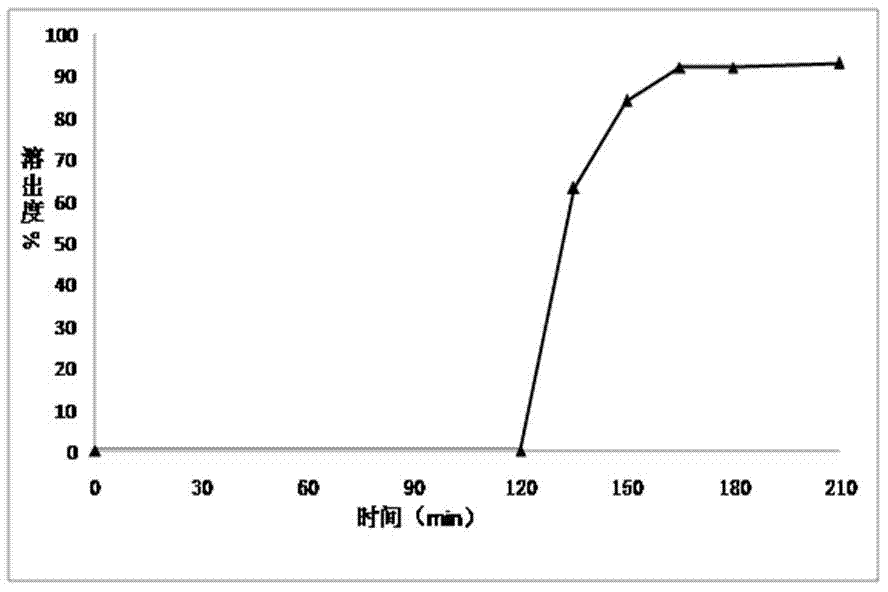
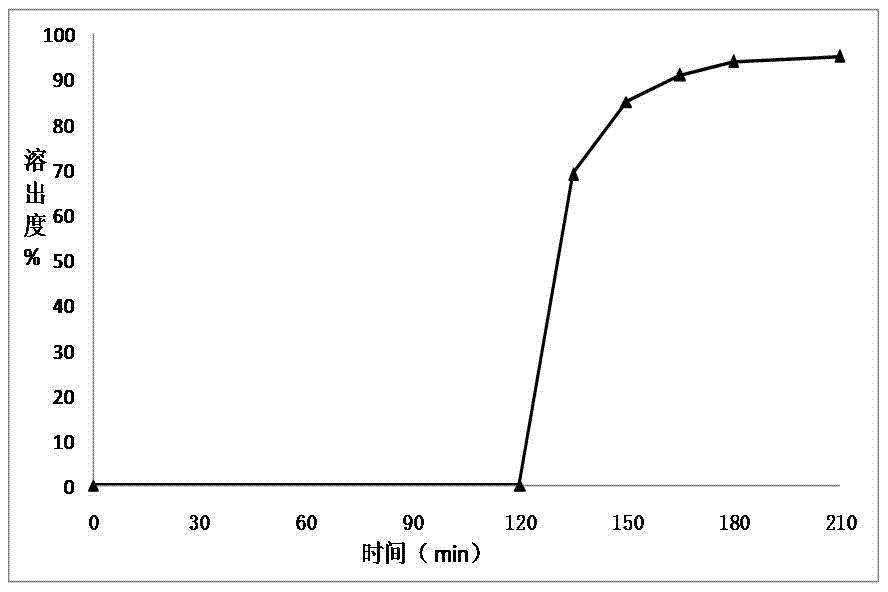
ActiveCN1903284AInhibit pathological changesSmall diameterHydroxy compound active ingredientsAntipyreticAdditive ingredientCurative effect
A cow-bezoar microbolus for clearing away heat of the upper part of the body, an improvement on cow-bezoar bolus for higher curative effect, features that is contains the promoter chosen from microcrystalline cellulose, superfine silica gel powder, polyvidone, cross-linking polyvidone and compressible starch beside the components said cow-bezoar bolus has. Its preparing process is also disclosed.
Owner:BEIJING TONGRENTANG CO LTD +1
Delayed release preparation containing safe and reliable plasticizer and preparation method thereof
ActiveCN102755302AImprove securityIncrease dosageOrganic active ingredientsNervous disorderPlastic materialsPharmaceutical Aids
The invention relates to the technical field of medicines and discloses a delayed release preparation containing a safe and reliable plasticizer and a preparation method thereof. The preparation method comprises the following steps of: firstly preparing a medicine-loading pill core, and then coating an isolating layer and an enteric layer containing the specific plasticizer, wherein the medicine-loading pill core can be prepared by preparing active medicine ingredients and auxiliary materials into soft materials and then adopting an extrusion rounding method and also can be prepared by dissolving the active medicine ingredients and the auxiliary materials and directly coating on a hollow pill core by a fluidized bed. The delayed release preparation selects the specific plastic material, and does not contain phthalic acid ester and citrate plastic materials, so that the safety is better.
Owner:华益泰康药业股份有限公司
Clindamycin palmitate hydrochloride particle and preparation method thereof
The invention provides a clindamycin palmitate hydrochloride particle and a preparation method thereof. The clindamycin palmitate hydrochloride particle provided by the invention consists of a drug pill core and a coating layer covering the outside of the drug pill core, wherein the drug pill core comprises clindamycin palmitate hydrochloride, a carrier and a water-soluble pore-forming agent; andthe coating layer comprises a pH-dependent coating material. According to the clindamycin palmitate hydrochloride particle provided by the invention, a soft material is prepared by virtue of a wet process / the drug pill core is prepared by virtue of an extrusion-spheronisation process firstly, and then the soft material or the drug pill core is coated by coating liquid by virtue of a spray coatingprocess. The clindamycin palmitate hydrochloride particle provided by the invention is applicable to children; the integrity of the particle can be kept before the particle is taken, and the particle,after entering human bodies, can be slowly released, so that a stable and lasting blood concentration can be provided, and clinical demands of reducing the times of administration, reducing dose-related toxicity and improving patient compliance can be satisfied; and meanwhile, the bad taste of the medicine (the particle) can be masked without adding a great amount of flavoring agents.
Owner:GUANGZHOU DAGUANG PHARMA
Skeleton Diclofenac Potassium Sustained-release Pellet Capsules and Production Process
ActiveCN102266292ALittle side effectsReduce the number of dosesOrganic active ingredientsAntipyreticSustained release pelletsDiclofenac Acid
The invention relates to matrix diclofenac potassium sustained-release pellet capsules and a production process thereof. The matrix diclofenac potassium sustained-release pellet capsules are prepared by filling matrix diclofenac potassium sustained-release pellets into gastric-soluble capsule shells. The matrix diclofenac potassium sustained-release pellets are prepared in one step by extrusion and rolling. The formula of the matrix diclofenac potassium sustained-release pellets comprises basic remedy diclofenac potassium 10-50%, matrix agent 1-20%, diluent 20-80%, antioxidant 0.1-5%, antisticking agent 0.1-5%, absorption promoting agent 1-10%, and any available wetting agent as balance, wherein the matrix agent is hydrophilic gel matrix agent, or hydrophilic gel matrix agent and erodiblematrix agent, or hydrophilic gel matrix agent and insoluble matrix agent. The matrix diclofenac potassium sustained-release pellet capsules provided by the invention have the advantages of high bioavailability, long in vivo holdup time, regular drug release, good in vivo (Beagle dogs) absorption and reproducibility, good sustained-release effect, simple production process, short production period, and no flying of dust during production, and are suitable for industrial production.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD
Hydrophilic matrix beadlet compositions with enhanced bioavailability
ActiveUS9399020B2Improved stable beadlet formulationsImprove bioavailabilityPowder deliveryHydroxy compound active ingredientsZeaxanthinDissolution
The instant invention provides hydrophilic matrix beadlet compositions that include at least one fat soluble nutrient and an effective amount of cellulose polymer with low viscosity. The invention also provides a process for the preparation of the hydrophilic matrix composition by employing a fluid bed system or extrusion spheronization technique. This hydrophilic matrix beadlet composition is comprised of at least 5% to about 25% carotenoid wherein free lutein is present in combination with zeaxanthin. The free flowing nature of the composition allows it to be compressed into tablets or to be filled into two piece capsules or blended as a dry premix for beverage applications. These hydrophilic matrix compositions exhibit desired dissolution characteristics and at least 1.6 times more bioavailability as compared with marketed reference formulations containing modified starch, thus making it advantageous for nutraceutical applications.
Owner:OMNIACTIVE HEALTH TECH
Recipe of valnemulin hydrochloride enteric-coated pellet and preparation method thereof
InactiveCN101874785AMask bitter off-flavorsImprove stabilityAntibacterial agentsPharmaceutical non-active ingredientsHigh concentrationPrill
The invention provides a recipe of a valnemulin hydrochloride enteric-coated pellet and a preparation method thereof, wherein, acrylic resin water dispersion is taken as coating liquid, and water is taken as a solvent, thus changing the method that the existing resin coating liquid selects high concentration alcohol as the solvent, lowering cost and overcoming the defect of environmental influence due to evaporation of a large amount of alcohol during the coating process. The preparation method of the valnemulin hydrochloride enteric-coated pellet comprises the steps of preparation of the valnemulin hydrochloride medicine carrying pellet, preparation of the coating liquid and preparation of valnemulin hydrochloride coating. By adopting an extrusion-spheronization process procedure, the preparation method solves the defects of a one-step fluidized bed granulating method such as coarse and loose particles, large particle size range, a large amount of dust; and the pellet prepared by the method has even particle size and narrower distribution range, thus being beneficial to uniform saturation of the coating liquid on the particles, and improving the taste masking effect.
Owner:广东大华农动物食品保健品股份有限公司 +1
Gastrodia elata film coated micro-pill preparation and preparation method thereof
InactiveCN103933372AImprove bioavailabilityLess irritatingNervous disorderGranular deliveryGastrodiaAdhesive
The invention discloses a gastrodia elata film coated micro-pill preparation and a preparation method thereof. The gastrodia elata film coated micro-pill preparation is characterized by comprising a pill core and a coating layer, wherein the pill core comprises the following raw materials in parts by weight: 40-60 parts of gastrodia elata, 30-70 parts of filler and proper adhesive; the ratio of the weight of the film coating to the total weight of gastrodia elata powder and filler is (0.15-0.25):1. The gastrodia elata micro-pill is prepared by adopting an extrusion rolling method, and then related film coating materials are screened. Compared with the commercially available ordinary gastrodia capsule, the finally obtained gastrodia elata film coated micro-pill preparation has excellent humidity resistance and good stability.
Owner:GUIZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com