Slow-releasing micro-pills of sophocarpidine and its preparing method
A technology of slow-release pellets and matrine, which is applied in the direction of pill delivery, bulk delivery, digestive system, etc., can solve the problems of difficult to control drug release rate, unsteady drug effect, short maintenance time, etc. To achieve the effect of easy quality control, wide range of drug-containing percentage, and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 Preparation of pill core containing drug
[0042] A. Prescription:
[0043] Matrine 500g
[0044] Microcrystalline Cellulose (MCC) 500g
[0045] Total 1000g
[0046] B. Preparation process:
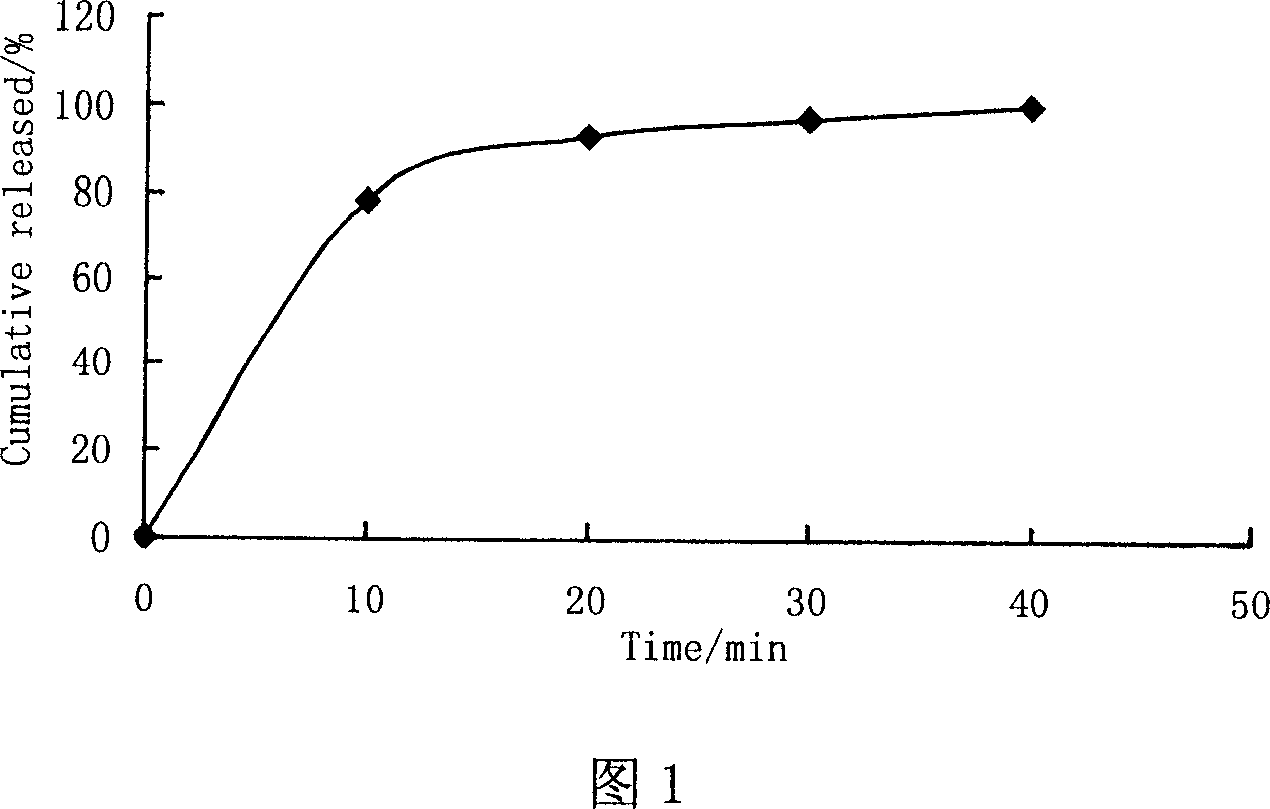
[0047] Take matrine raw material, pulverize and pass through a 120-mesh sieve, add microcrystalline cellulose, pass through an 80-mesh sieve and mix thoroughly; add a certain amount of wetting agent (such as 50% ethanol) to the mixed material to make a soft material , passed through a 16-mesh sieve to obtain wet granules; put the wet granules into the extruder and extrude them into strip-shaped extrudates; quickly add the extrudates to the spheronizer for spheronization, and take them out after a certain period of time to obtain pellets ;Dry the prepared pellets at 45°C for 8 hours to obtain dry pellets; as a result, the appearance of the pellets is smooth and spherical, and the dissolution test results are shown in Figure 1. It can be seen that t...
Embodiment 2
[0049] The core of the pills containing the medicines prepared in Example 1 is coated with ethyl cellulose slow-release film coating, and the matrine sustained-release pellets are obtained.
[0050] A. Prescription of coating solution:
[0051] Ethyl cellulose 60g
[0052] PEG1500 6g
[0053] Diethyl phthalate 5g
[0055] 95% ethanol to 1000mL
[0056] B. Preparation process:
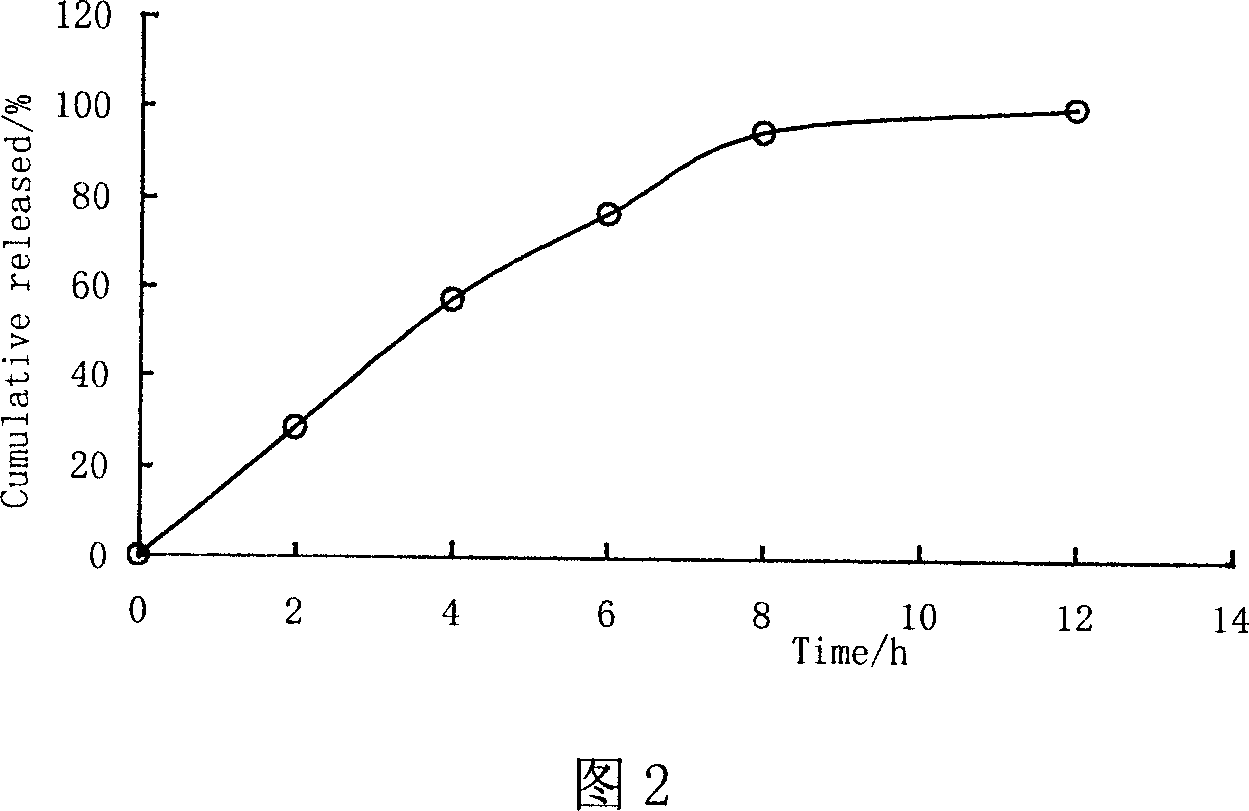
[0057] Mix ethyl cellulose and porogen PEG1500 at a ratio of 10:1, add ethanol and stir until clear, then add appropriate amount of plasticizer diethyl phthalate and talcum powder, stir at a high speed and homogenize, and then coat and polymerize The weight gain is between 7% and 8%. The release rate of the drug in water was determined by spectrophotometry, and the results are shown in Figure 2.
Embodiment 3
[0059] The drug-containing pellet core prepared in Example 1 was coated according to the following coating liquid prescription to obtain matrine sustained-release pellets.
[0060] A. Prescription of coating solution:
[0061] Surelease 600g
[0062] 5% PEG6000 solution 75g
[0063] Water up to 1000g
[0064] B. Preparation process:
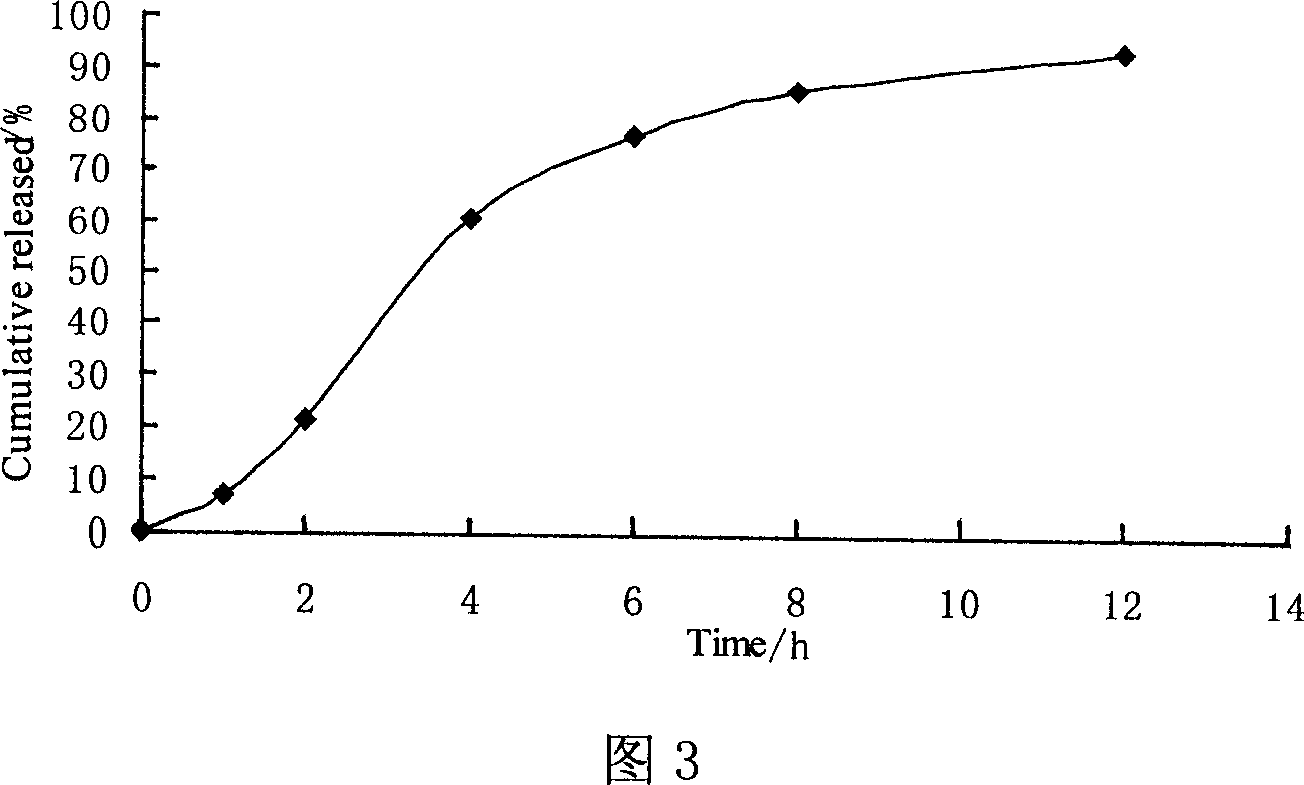
[0065] According to the above prescription, take Surelease and porogen 5% PEG6000 solution, mix it at a ratio of 8:1, dilute it with water to a solid content of about 15%, stir at a high speed to coat evenly, and the weight gain is between 10% and 12%. . The release rate of the drug in water was determined by spectrophotometry, and the results are shown in Figure 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com