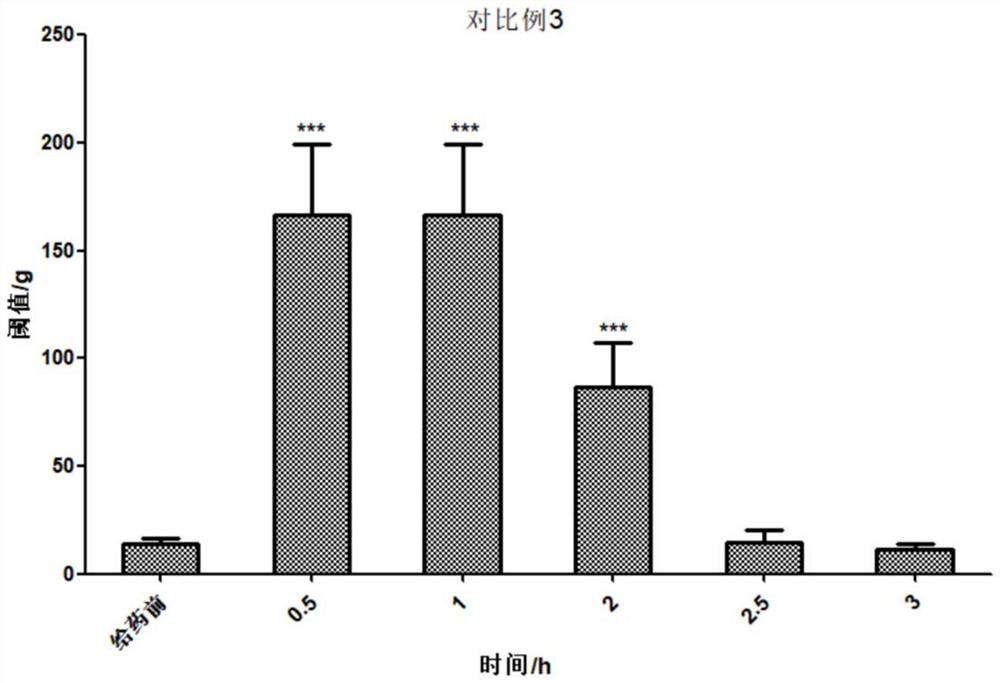
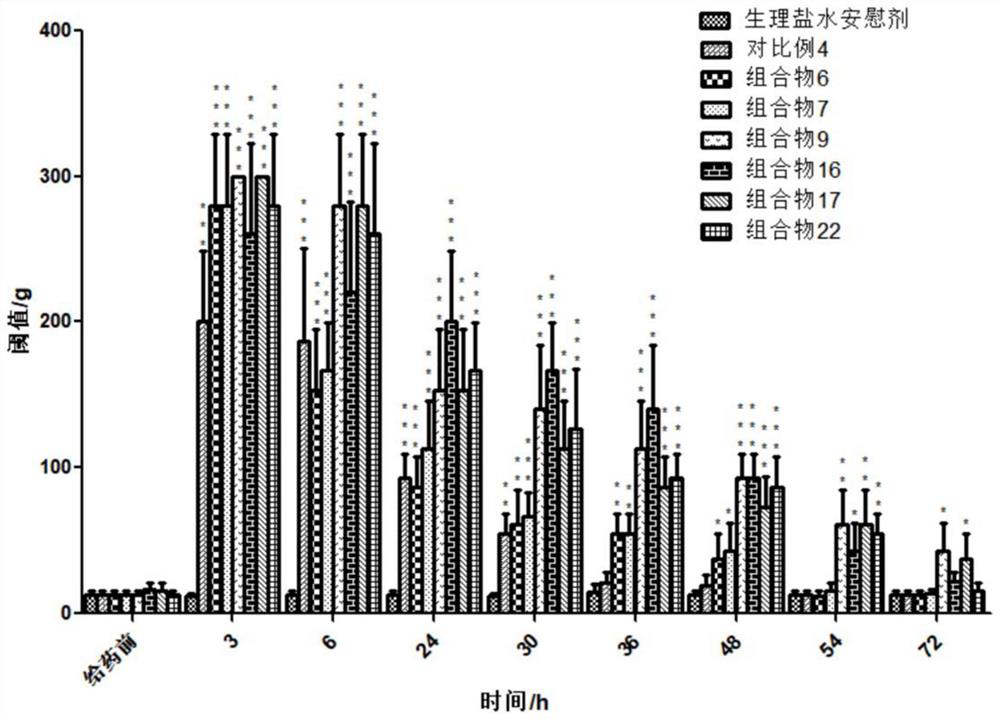
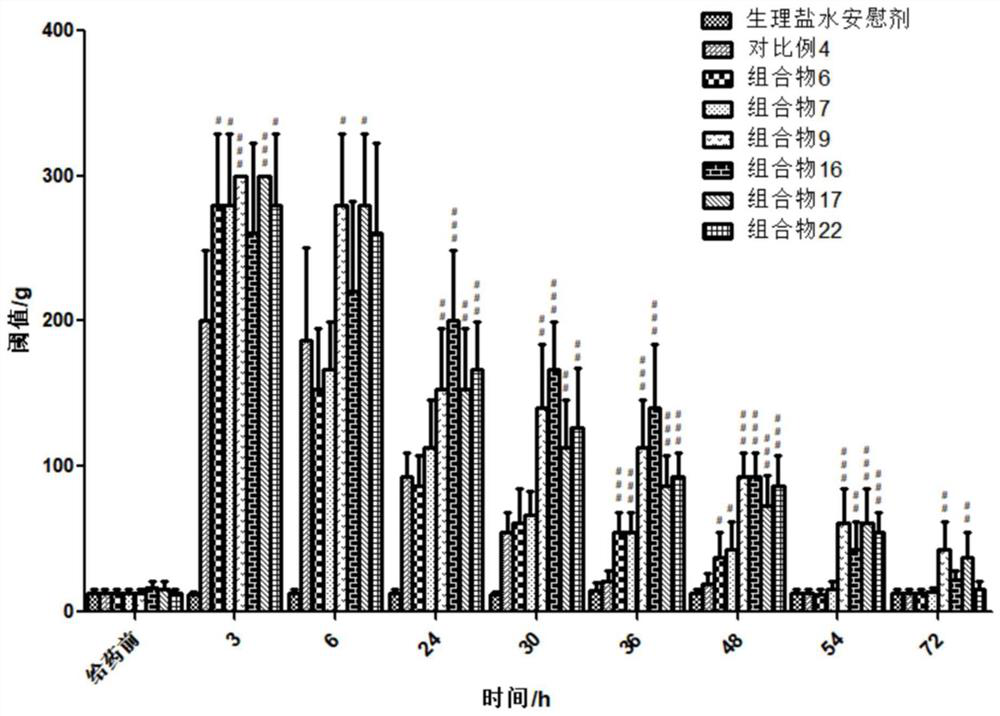
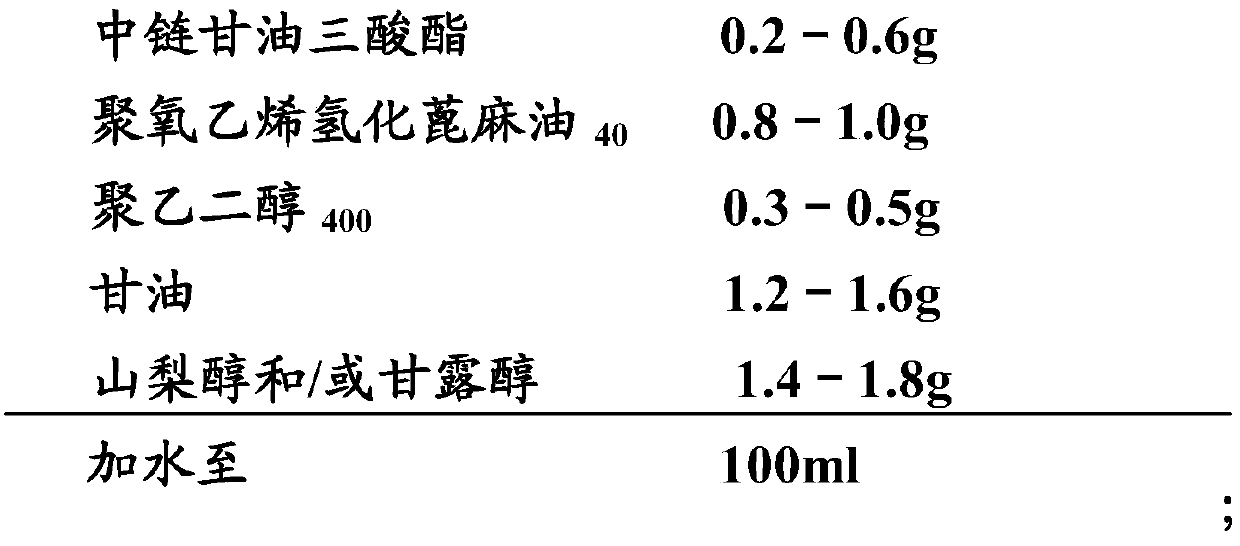
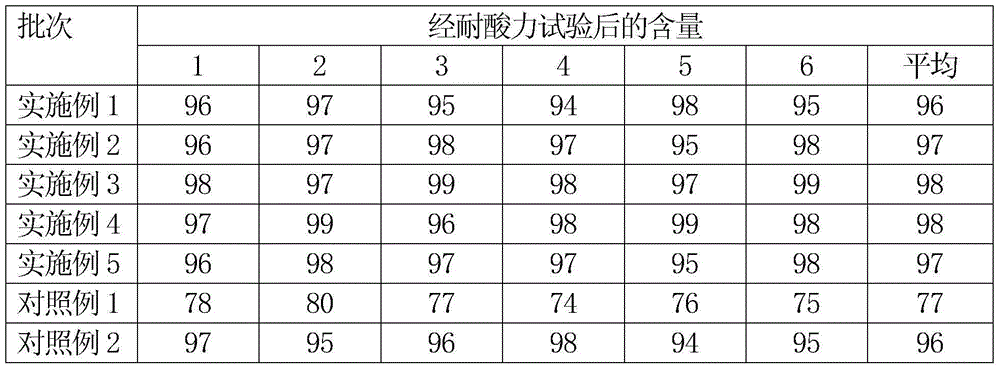
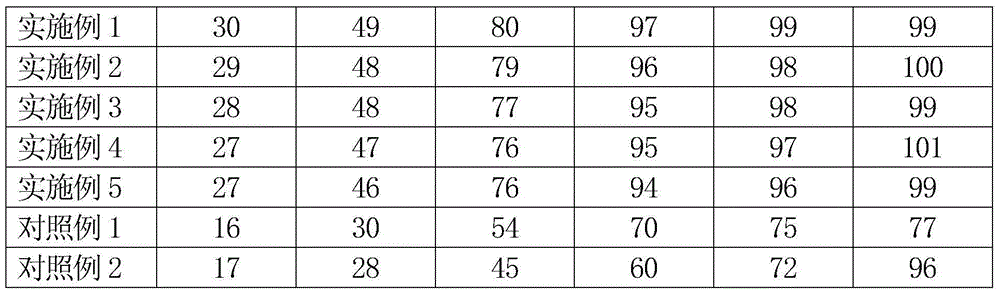
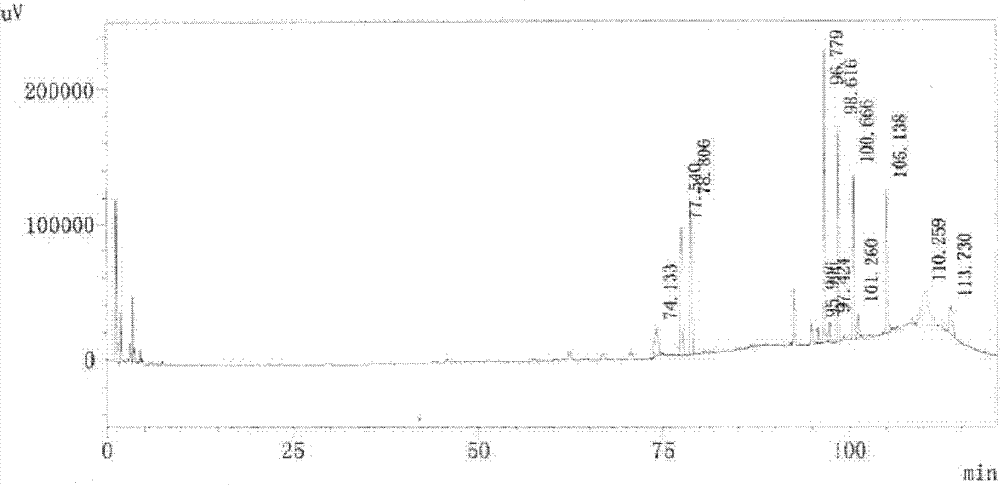
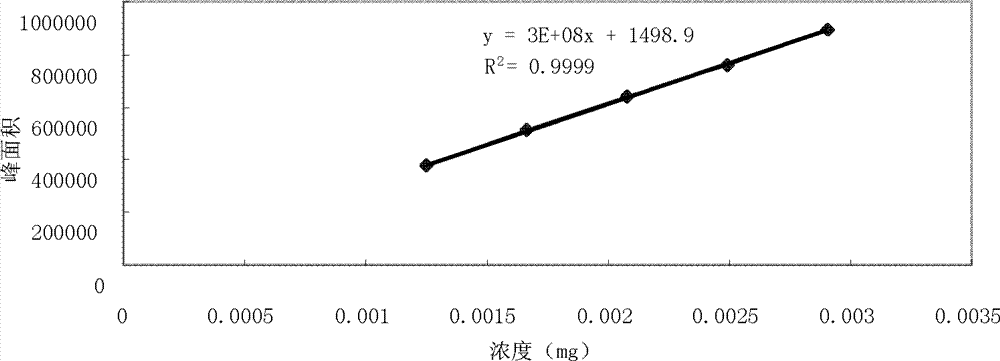
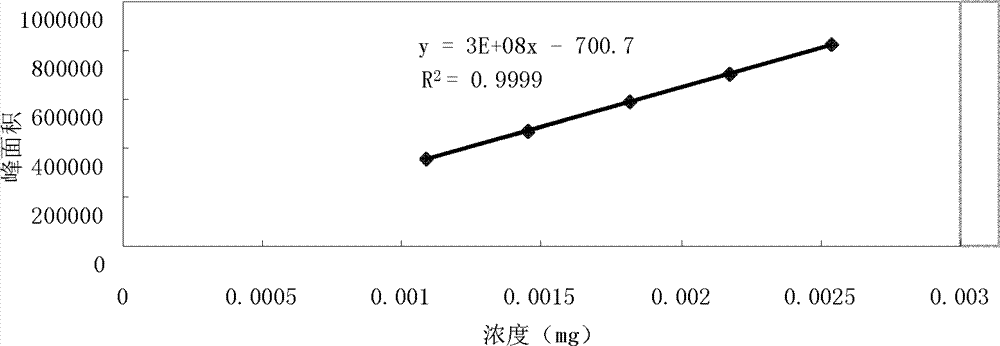
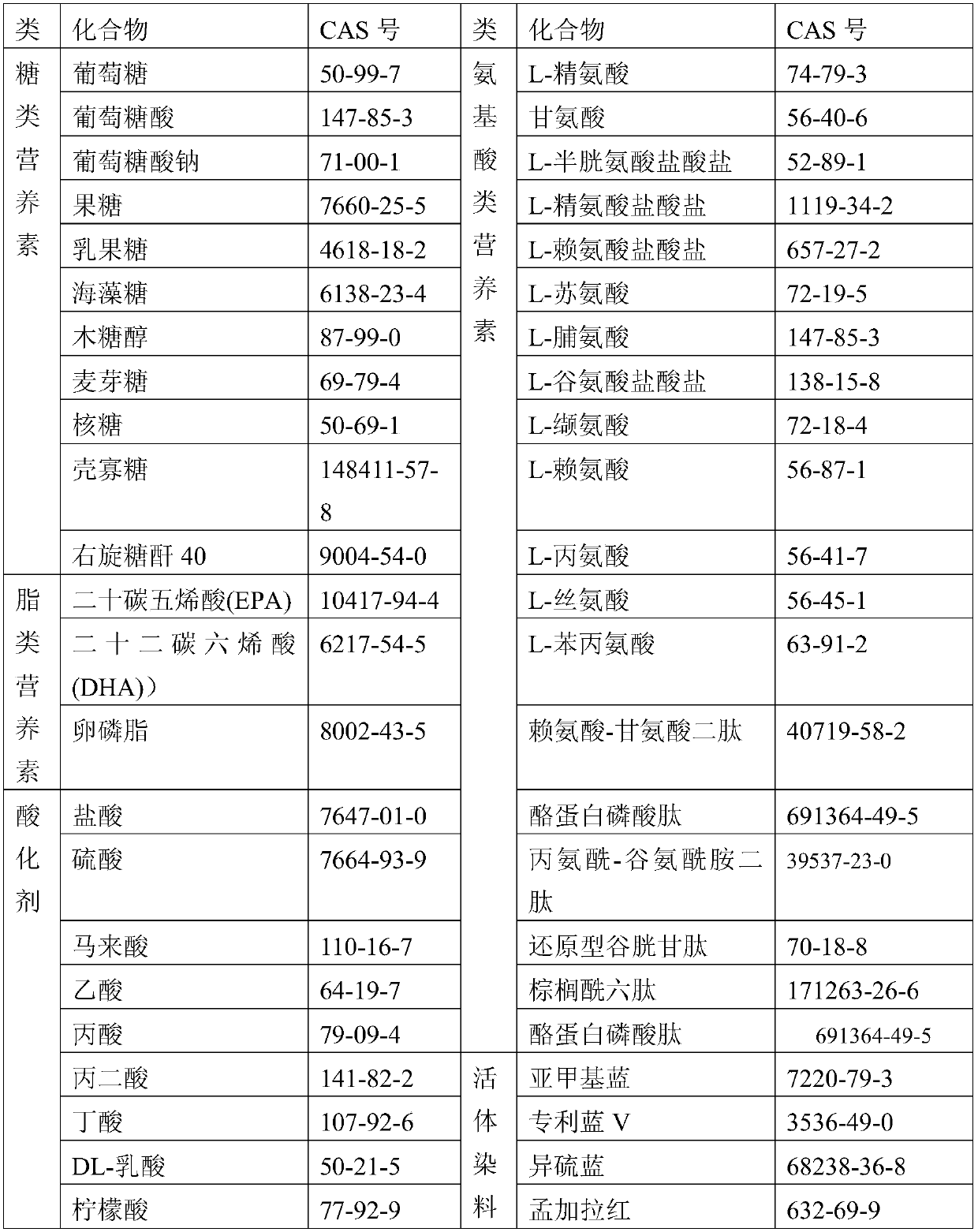
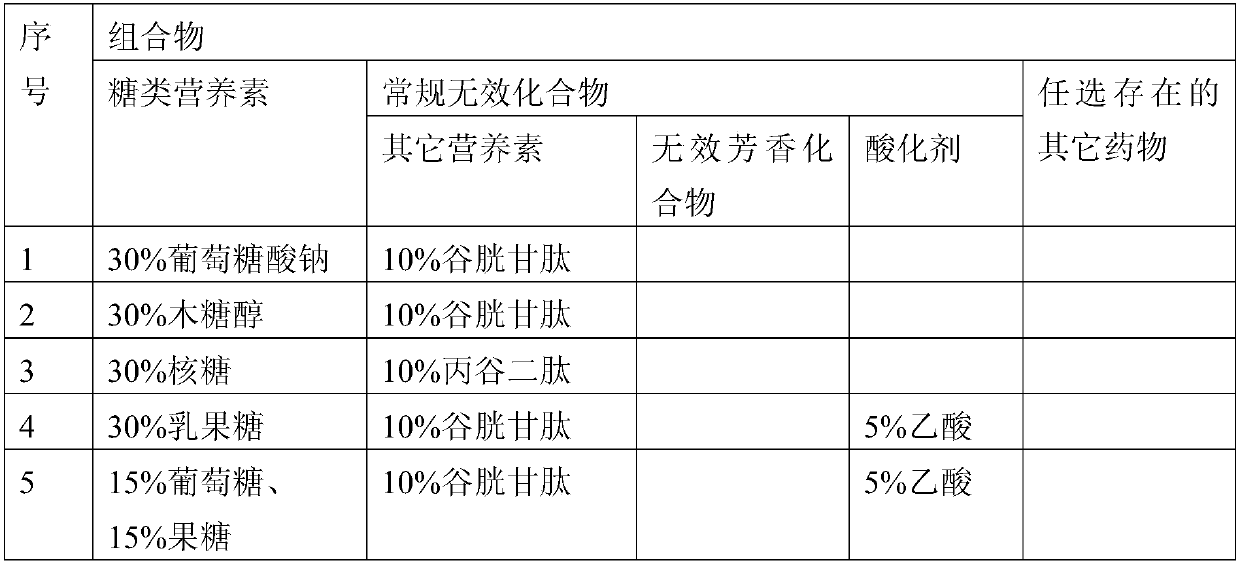
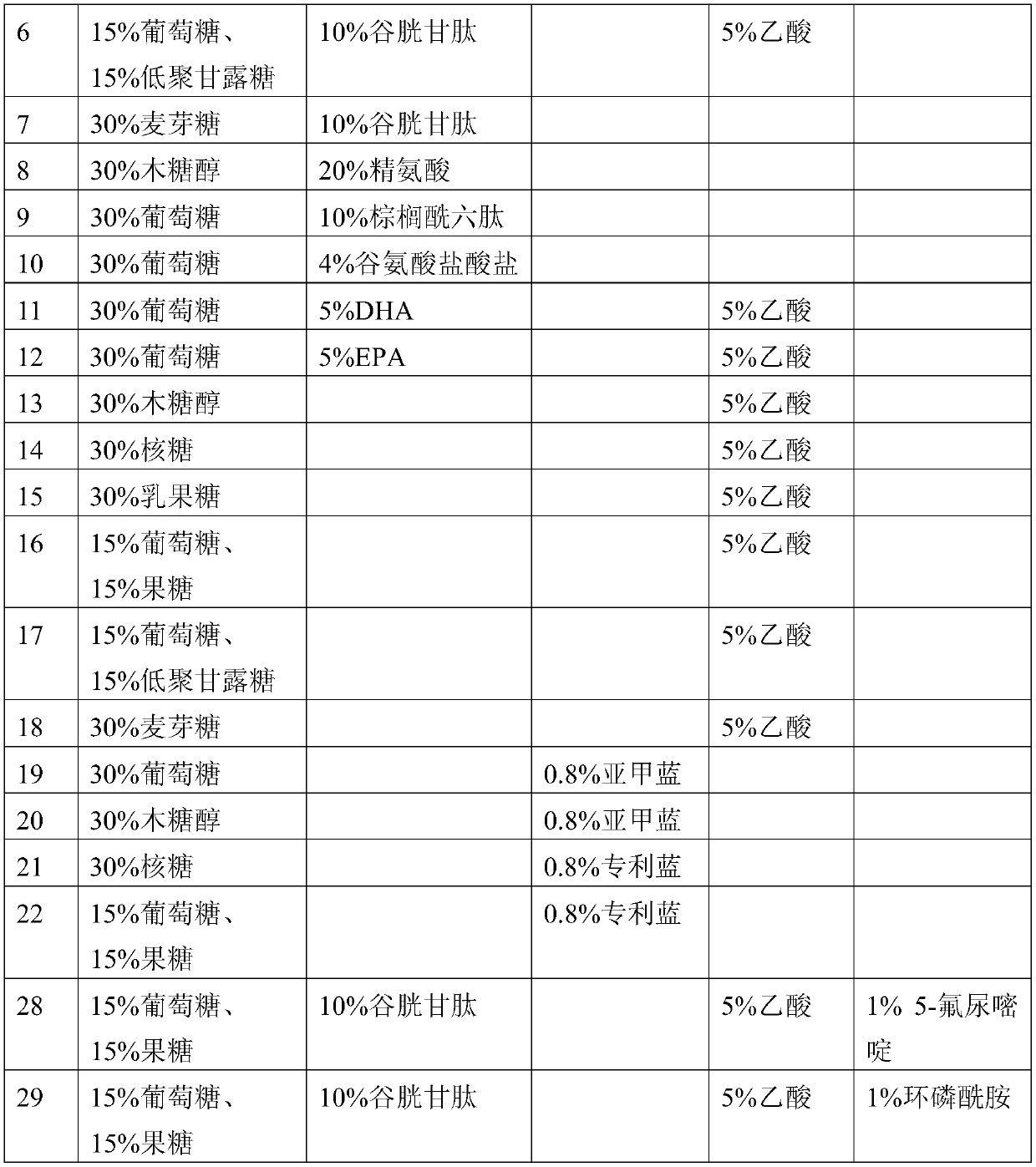
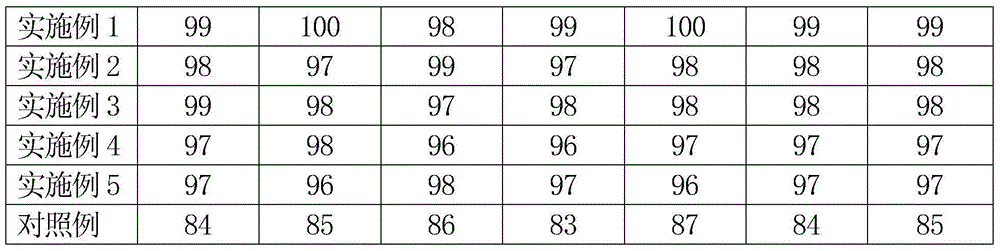
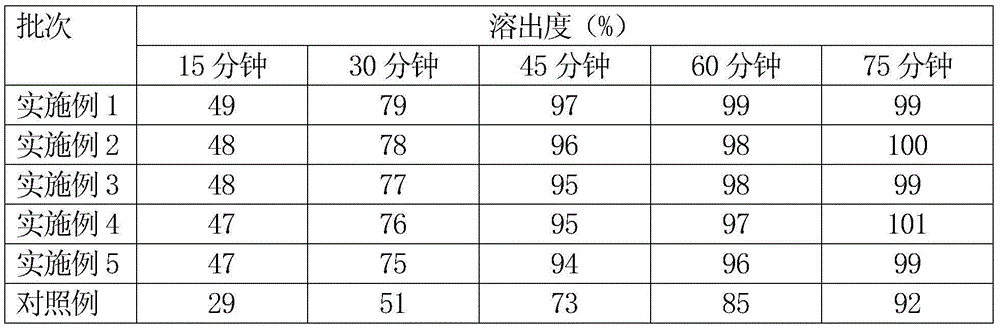
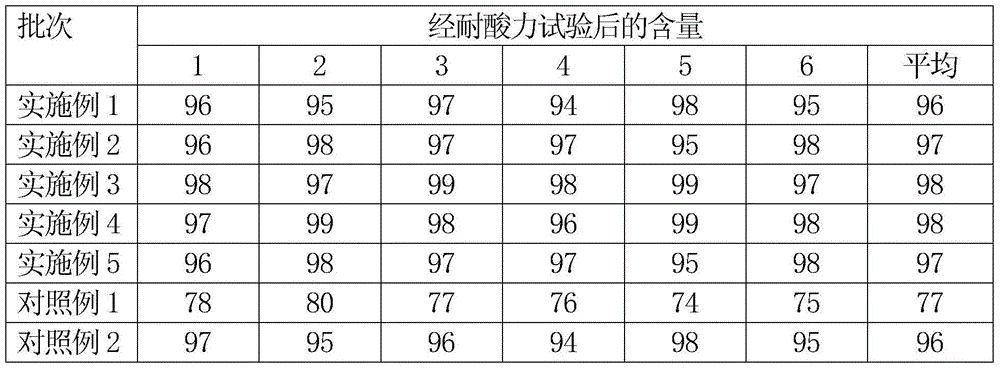
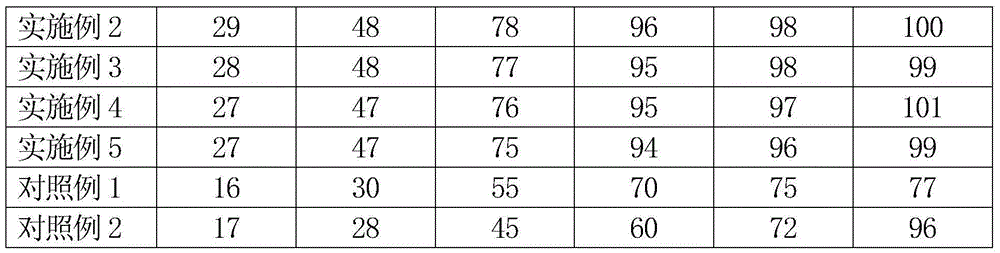
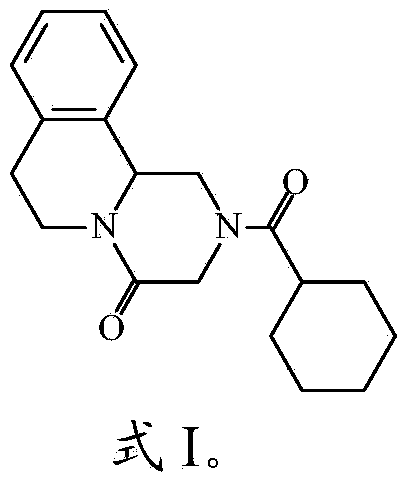
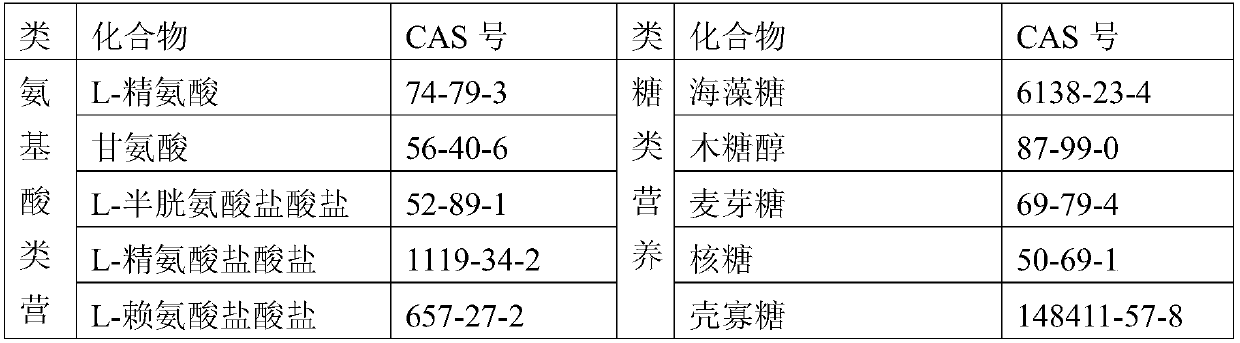
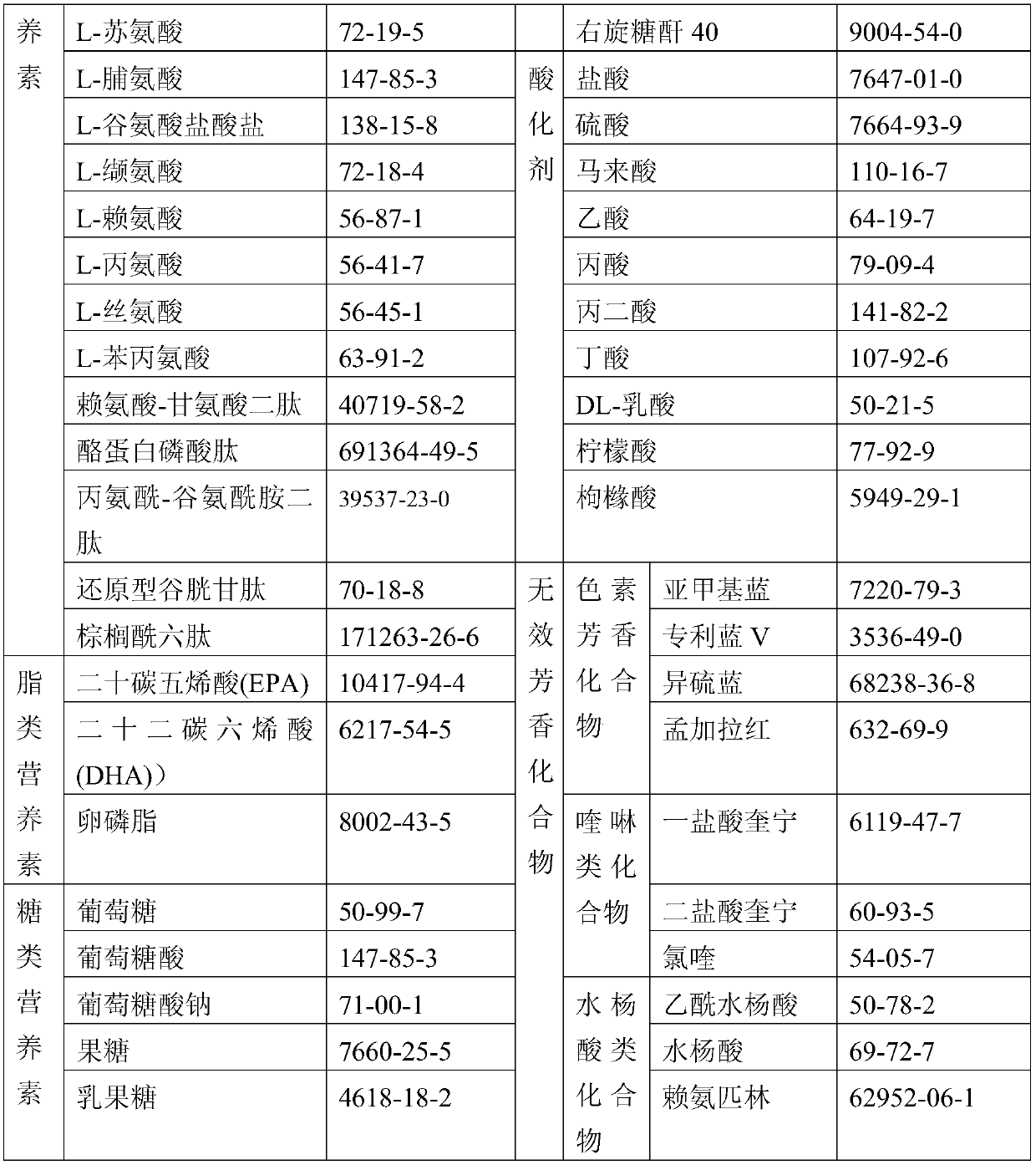
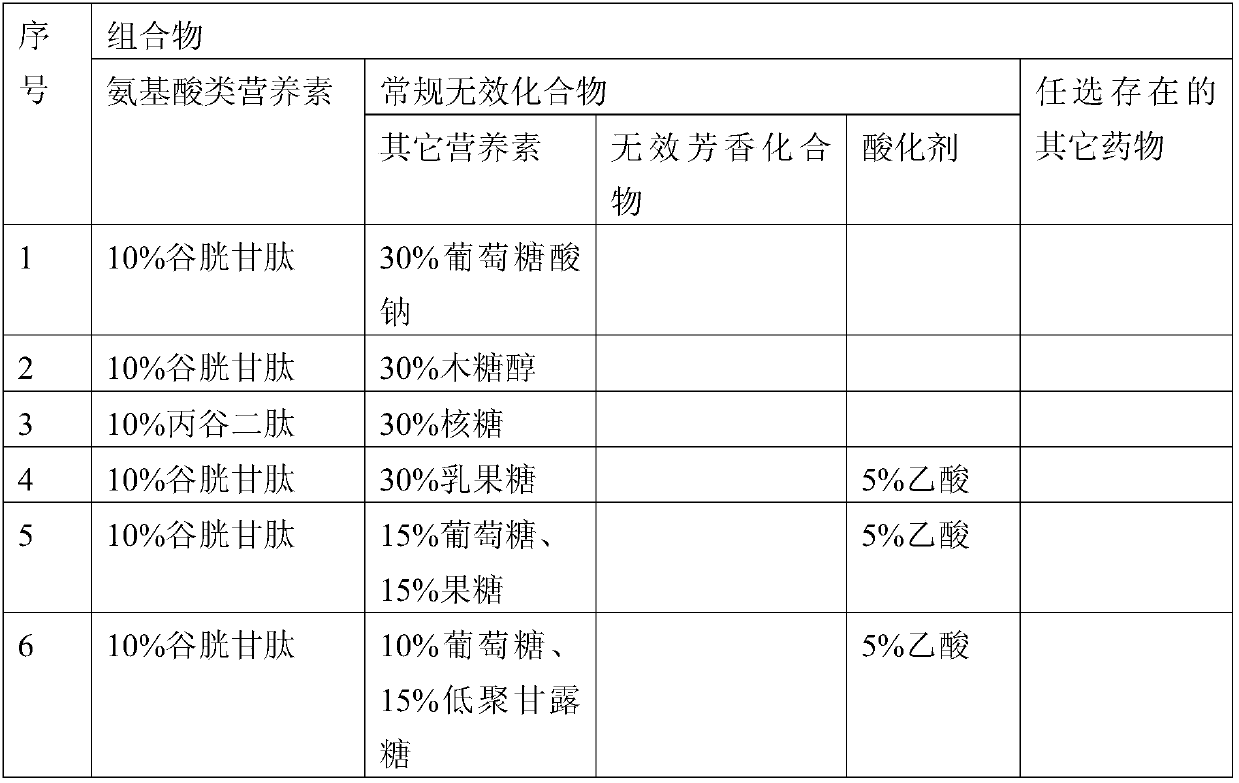
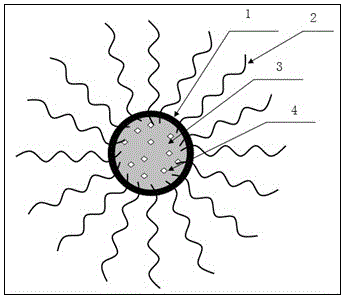
Patents
Literature
72results about How to "Small local irritation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Long acting drug delivery system for treating breast cancer and preparation method and applications thereof
InactiveCN108159055AEnsure safetyIncrease concentrationOrganic active ingredientsPharmaceutical delivery mechanismProcainePhospholipid
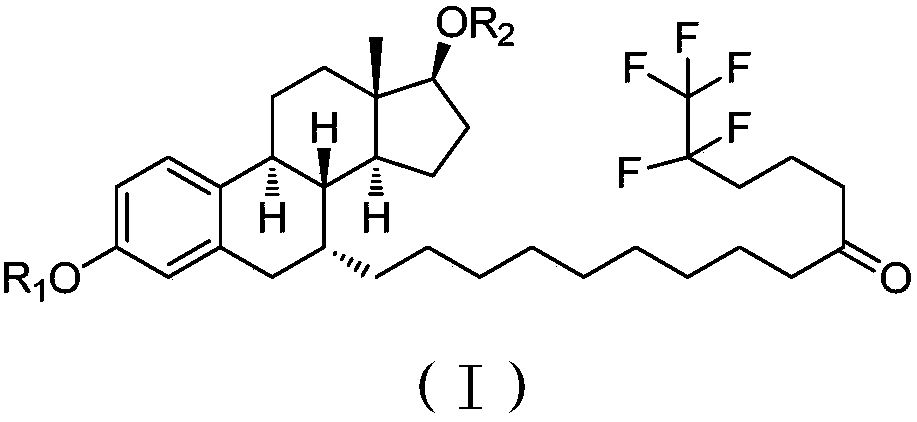
The invention provides a long acting drug delivery system, which comprises fulvestrant or derivatives thereof and takes phospholipid as a sustained release material. The invention mainly discloses a high concentration formula of fulvestrant or derivatives thereof. The main component is fulvestrant or derivatives thereof. The sustained release material is different phospholipids or a mixture of phospholipids and different kinds of plant oil. The solvent is at least one of ethanol, ethyl lactate, 1,2-propylene glycol, and ethyl acetate. The analgesic is benzyl alcohol, lidocaine, procaine, or ropivacaine. The viscosity of the formula is 20-45 mPa.s, and the concentration of the formula is 60-300 mg / mL. The antioxidant of the formula is Ve, lipoic acid, and the like. The phospholipids can increase mutual solubility and has a sustained release function. The preparation is prepared in an aseptic filtration mode.
Owner:XIAN LIBANG PHARMA TECH
Crushing stirring machine for preparing megestrol acetate suspension
InactiveCN108479492AEvenly dispersedSmall local irritationMixing methodsRotary stirring mixersMegestrol acetateEngineering
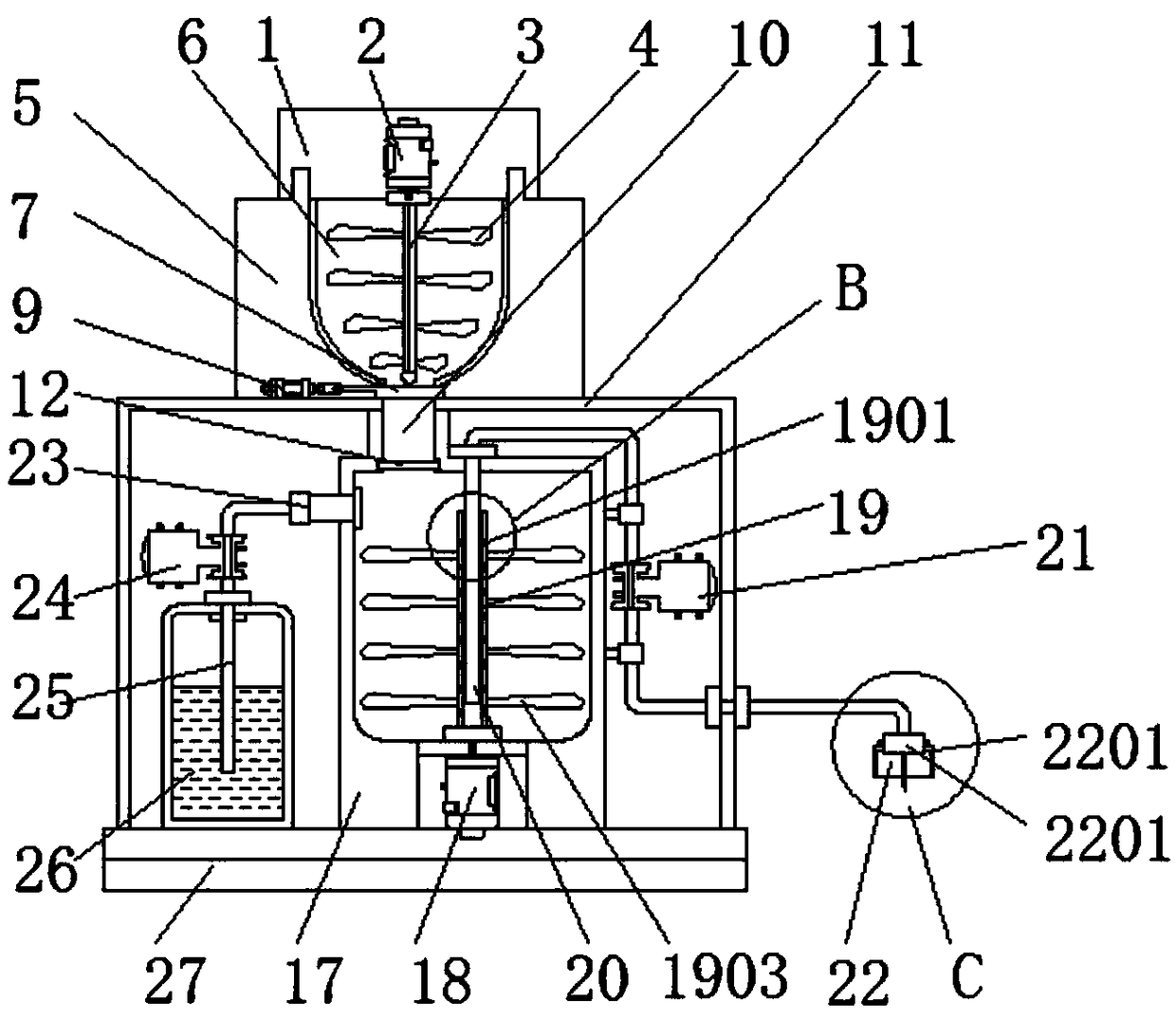
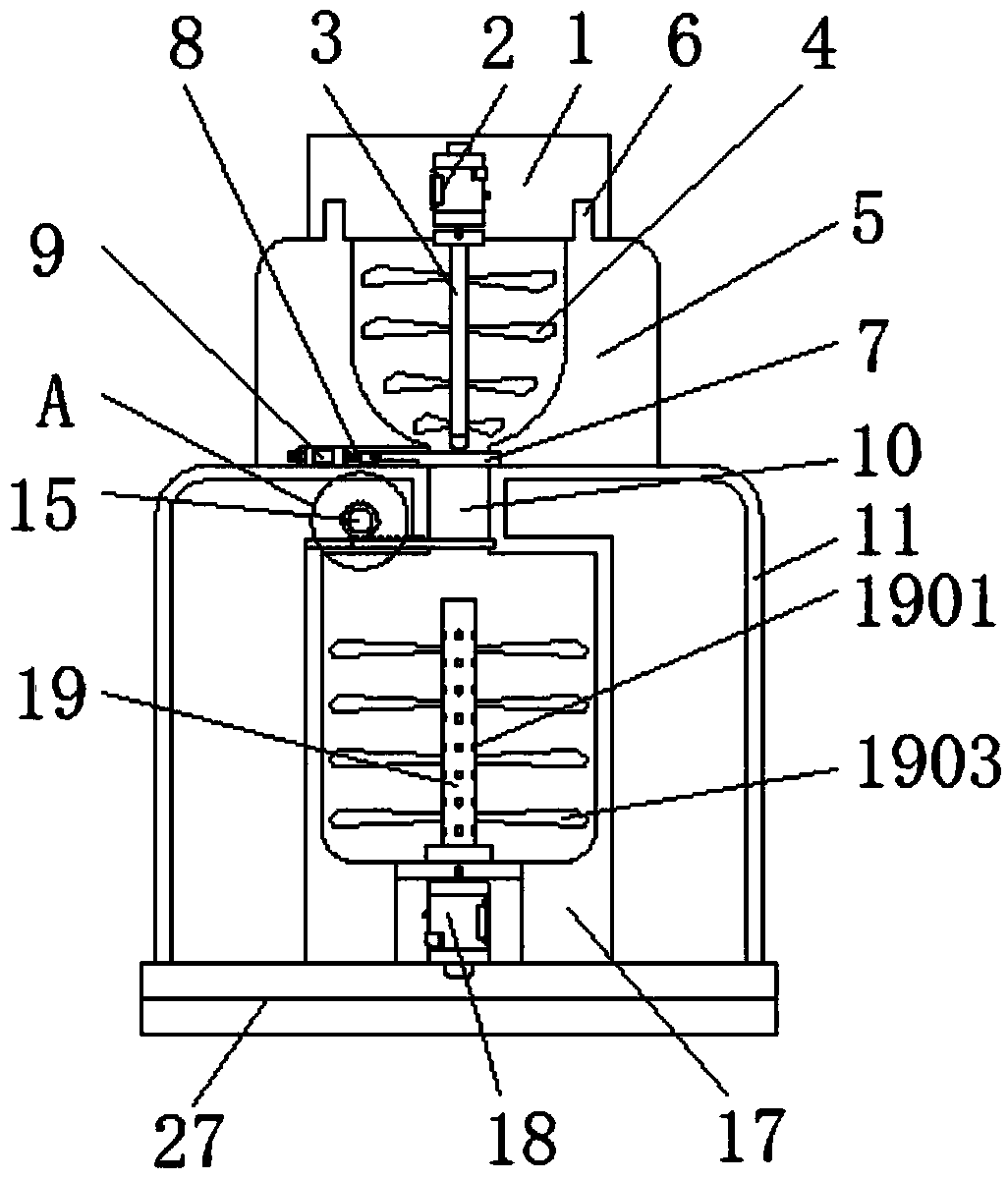
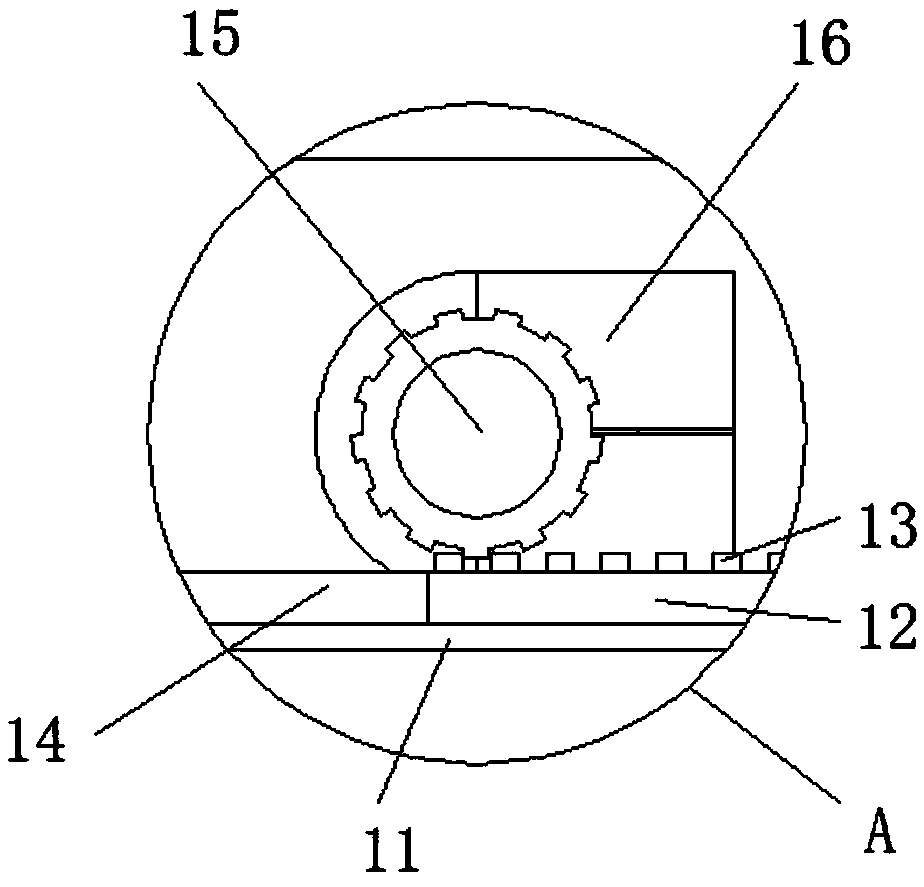
The invention discloses a crushing stirring machine for preparing a megestrol acetate suspension. The crushing stirring machine comprises a first machine body, a second machine body, a crushing cavity, a first valve, a stirring machine body, a water tube, a discharge mechanism, a second feeding hole and a base, wherein a first motor is arranged inside the first machine body; a first rotating shaftis arranged below the first motor; crushing blades are fixed on the outer side of the first rotating shaft; the second machine body is arranged below the first machine body; the crushing cavity is formed inside the second machine body; crushing blades are arranged inside the crushing cavity; the first valve is mounted below the crushing cavity. By adopting the crushing stirring machine for preparing the megestrol acetate suspension, megestrol acetate tablets can be uniformly crushed, and after being mixed with water and taken by a patient, the medicine of uniform granules is large in distribution area in a gastrointestinal tract, rapid to absorb, high in bioavailability, and in addition small in partial irritation to the gastrointestinal tract since suspension granules are uniformly dispersed.
Owner:青岛国海生物制药有限公司
Niclosamide ethanolamine salt-hydroxypropyl-beta-cyclodextrin inclusion compound and preparation thereof
PendingCN110833623AGood water solubilityStrong solubilizing abilityOrganic active ingredientsPharmaceutical non-active ingredientsEthanolaminesCombinatorial chemistry
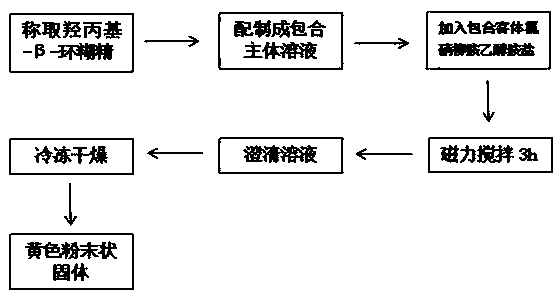
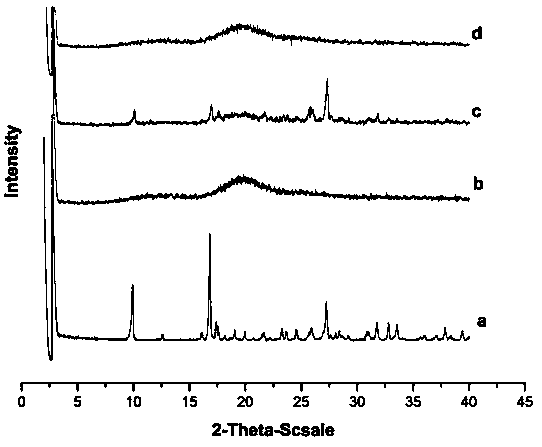
The invention provides niclosamide ethanolamine salt-hydroxypropyl-beta-cyclodextrin inclusion and a preparation method thereof; the inclusion is prepared from niclosamide ethanolamine salt and hydroxypropyl-beta-cyclodextrin as a raw material and belongs to the field of pharmacy. In the invention, a molar ratio of hydroxypropyl-beta-cyclodextrin to niclosamide ethanolamine salt is 7:(1-16):1, theinclusion is prepared by: adding niclosamide ethanolamine salt to a hydroxypropyl-beta-cyclodextrin solution prepared, stirring in the dark to obtain a clear solution, and lyophilizing to obtain theniclosamide ethanolamine salt-hydroxypropyl-beta-cyclodextrin inclusion; after the inclusion is formed, the water solubility of the niclosamide ethanolamine salt is obviously improved. The inclusion has high inclusion rate, the preparation method is simple, the efficiency is high, the application range is wide, and the inclusion is suitable for large-scale production.
Owner:SHENYANG PHARMA UNIVERSITY
Application of amino acid nutrient, and pharmaceutical composition comprising amino acid nutrient
PendingCN110870914AImprove effectivenessImprove securityPowder deliveryPeptide/protein ingredientsDiseasePharmaceutical Substances
The present application discloses an application of an amino acid nutrient in preparing a local pharmaceutical composition for treating local lesion diseases, the local pharmaceutical composition comprising the amino acid nutrient and a synergist of the amino acid nutrient for treating local lesion diseases, and a device comprising the composition.
Owner:CHENGDU KUACHANGAOPU MEDICAL TECH CO LTD
Reservoir type ropivacaine pharmaceutical composition as well as preparation method and application thereof
ActiveCN113116813AGood injectabilityControllable release rateAntipyreticAnalgesicsAntioxidantDrug efficiency
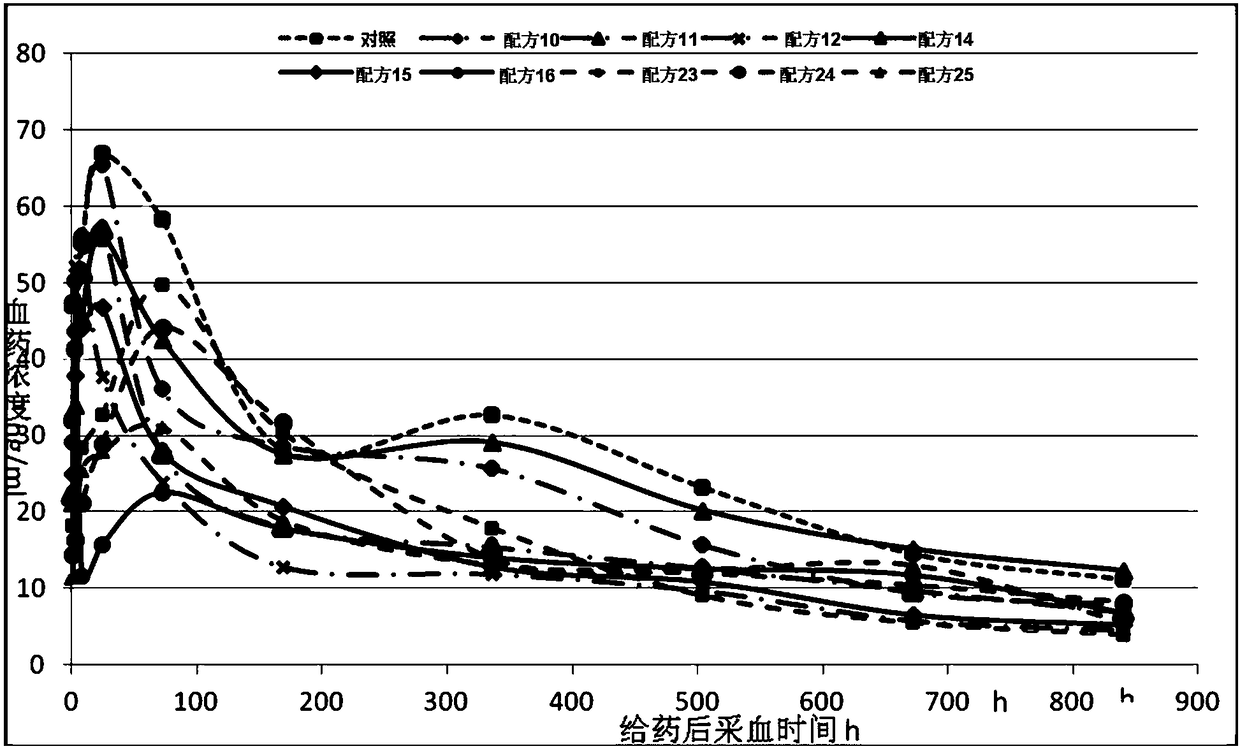
The invention provides a reservoir type ropivacaine pharmaceutical composition as well as a preparation method and application thereof. The pharmaceutical composition comprises: ropivacaine, a pharmaceutical solvent, a medicinal phospholipid, medicinal oil, and pharmaco enhancers, a non-essential antioxidant and a non-essential acid-base regulator. The pharmaceutical composition has a controllable release behavior and a sustained release effect, can significantly reduce the peak serum concentration of the drug, maintain a stable plasma concentration in the body, prolong the effective treatment time, reduce the effective therapeutic dose, improve the utilization of the drug, and reduce the risk of neurotoxicity. The pharmaceutical composition has a long-lasting analgesic effect and can be used for pain treatment.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Blood-nourishing and cephalocathartic micropills and its preparation technology
InactiveCN101053607AExcellent disintegration and solubilityThe curative effect is fastNervous disorderGranular deliveryBiotechnologyCassia
The invention relates to a micro-pill preparation and its producing craft, especially a micro-pill for tonifying blood and clearing liver heat and its producing craft. The material drug is produced according to following weight shares: angelica 250-420; ligusticus wallichii franchet 280-40; Paeonia lactiflora pallas 200-380; ripe radix rehmanniae 200-400; gambir plant 580-750; suberect spatholobus stem 550-780; brunella vulgaris linne 600-720; cassia seed 500-750; nacre 560-750; corydalis tuber 200-420; manchurian wildginger 30-100. The invention has a good resolvability, a smart curative effect, a stable medicine releasing, a high bio-availability, a small local thrill, a beautiful pill appearance, a small moisture absorption, and a good feeling for patients, further it is easy to transport, store and carry.
Owner:马鸿森
Medical product containing solution-type triamcinolone acetonide acetate
ActiveCN104971039ASimple production processSmall local irritationOrganic active ingredientsAerosol deliveryIrritationMedicine
The invention provides a medical product containing solution-type triamcinolone acetonide acetate. The medical product contains triamcinolone acetonide acetate, sulfobutyl ether-beta-cyclodextrin and sodium hyaluronate. The medical product containing solution-type triamcinolone acetonide acetate is small in local irritation in clinic use, especially when the medical product is applied to intra-articular injection, the product is easy to absorb, the particle deposition on the surface of the periosteum existing in the suspension type triamcinolone acetonide acetate medical product is avoided, and the periosteum injury caused by particle deposition is reduced.
Owner:上海正大通用药业股份有限公司
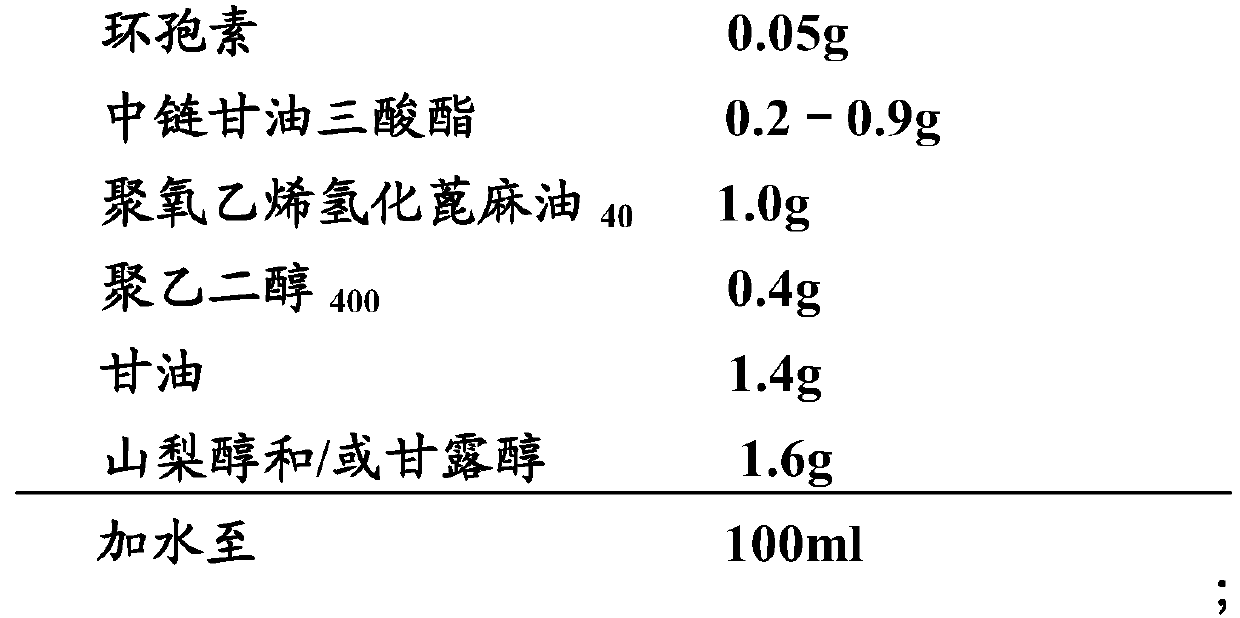
Ophthalmic pharmaceutical composition containing cyclosporine and preparation method and application thereof
ActiveCN110237233ASolve solubilitySmall local irritationSenses disorderAntimycoticsOil phaseSURFACTANT BLEND
The invention belongs to the field of medicines and preparations and relates to an ophthalmic pharmaceutical composition containing cyclosporine, a preparation method and application thereof. Specifically, the ophthalmic pharmaceutical composition containing the cyclosporine is prepared from, by weight, 0.01%-0.5% of cyclosporine, 0.2%-0.9% of an oil phase and 0.1%-5.0% of a surfactant. The ophthalmic solution can be stably stored for 24 months, is good in stability and low in impurity content.
Owner:SHENYANG XINGQI PHARM CO LTD
'Gushukang' micropills and its preparation technology
InactiveCN101053608AExcellent disintegration and solubilityThe curative effect is fastSkeletal disorderGranular deliverySalvia miltiorrhizaLongspur
The invention relates to a micro-pill preparation and its producing craft, especially the Gushukang micro-pill and its producing craft. The Gushukang micro-pill is produced according to following weight shares: longspur epimedium 100-1000; ripe radix rehmanniae 200-400; drynaria 60-660; astragalus root 130-1400; radix salviae miltiorrhizae 60-660; dried edible fungus 50-530; cucumber-seed 50-530. The invention has a good resolvability, a smart curative effect, a stable medicine releasing, a high bio-availability, a small local thrill, a beautiful pill appearance, a small moisture absorption, and a good feeling for patients, further it is easy to transport, store and carry.
Owner:杨义澄
Heart-benefiting Yixinshu micropill and its prepn process
InactiveCN101088546AExcellent disintegration and solubilityThe curative effect is fastPill deliveryCardiovascular disorderMANNITOL/SORBITOLMedicine
The present invention is heart-benefiting Yixinshu micropill and its preparation process. The heart-benefiting Yixinshu micropill is prepared with ginseng 100-500 weight portions, ophiopogon root 100-500weight portions, schisandra 100-300weight portions, astragalus root 150-450weight portions, red sage 200-600weight portions, Chuanxiong rhizome 100-300weight portions, haw 200-400weight portions, avicel 300-500weight portions, and mannitol 50-200weight portions. It has diameter smaller than 0.05mm, excellent disintegrating and dissolving performance, fast acting, high bioavailability, and other advantages, and possesses functions of promoting blood circulation to disperse blood clots, benefiting qi, nourishing yin, etc.
Owner:葛浩斌
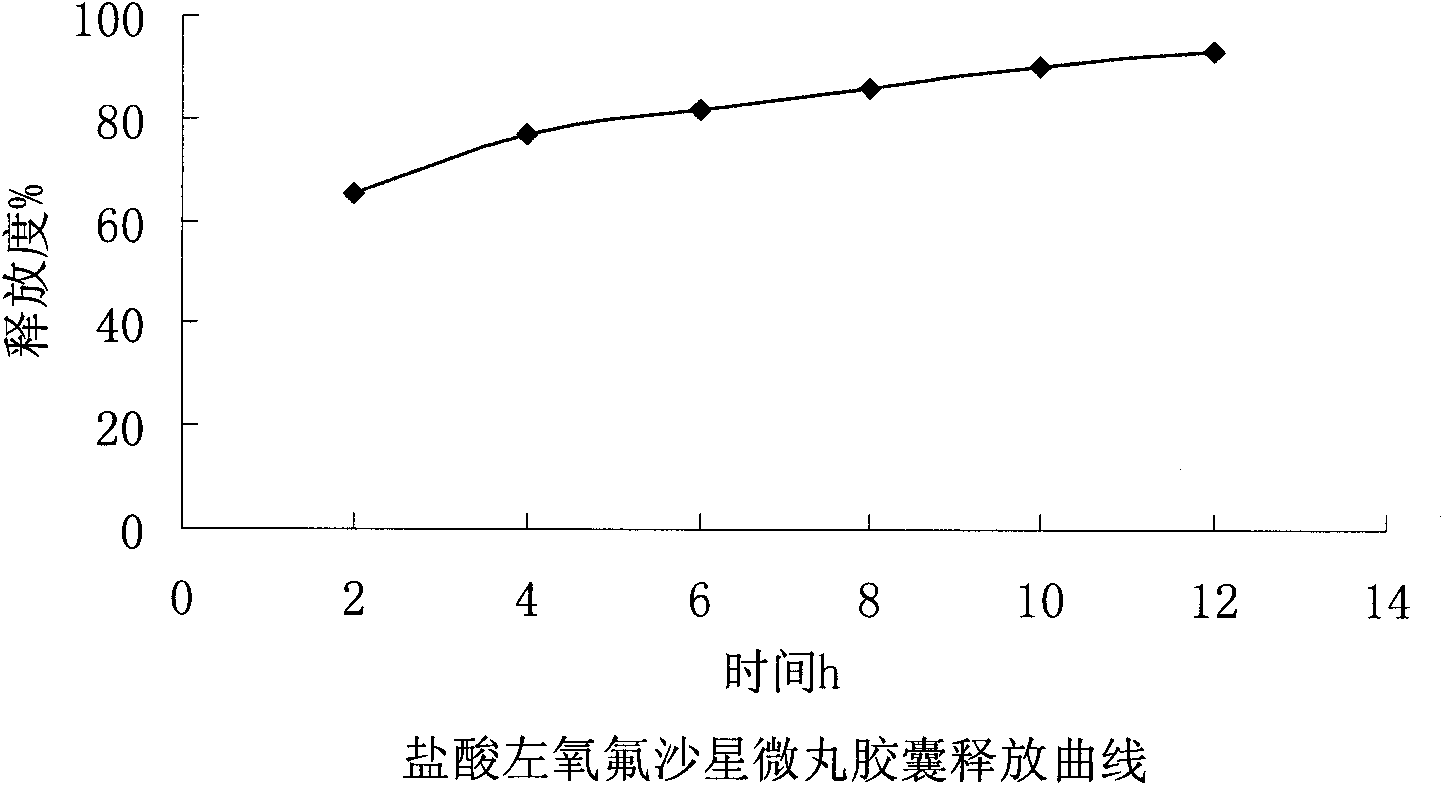
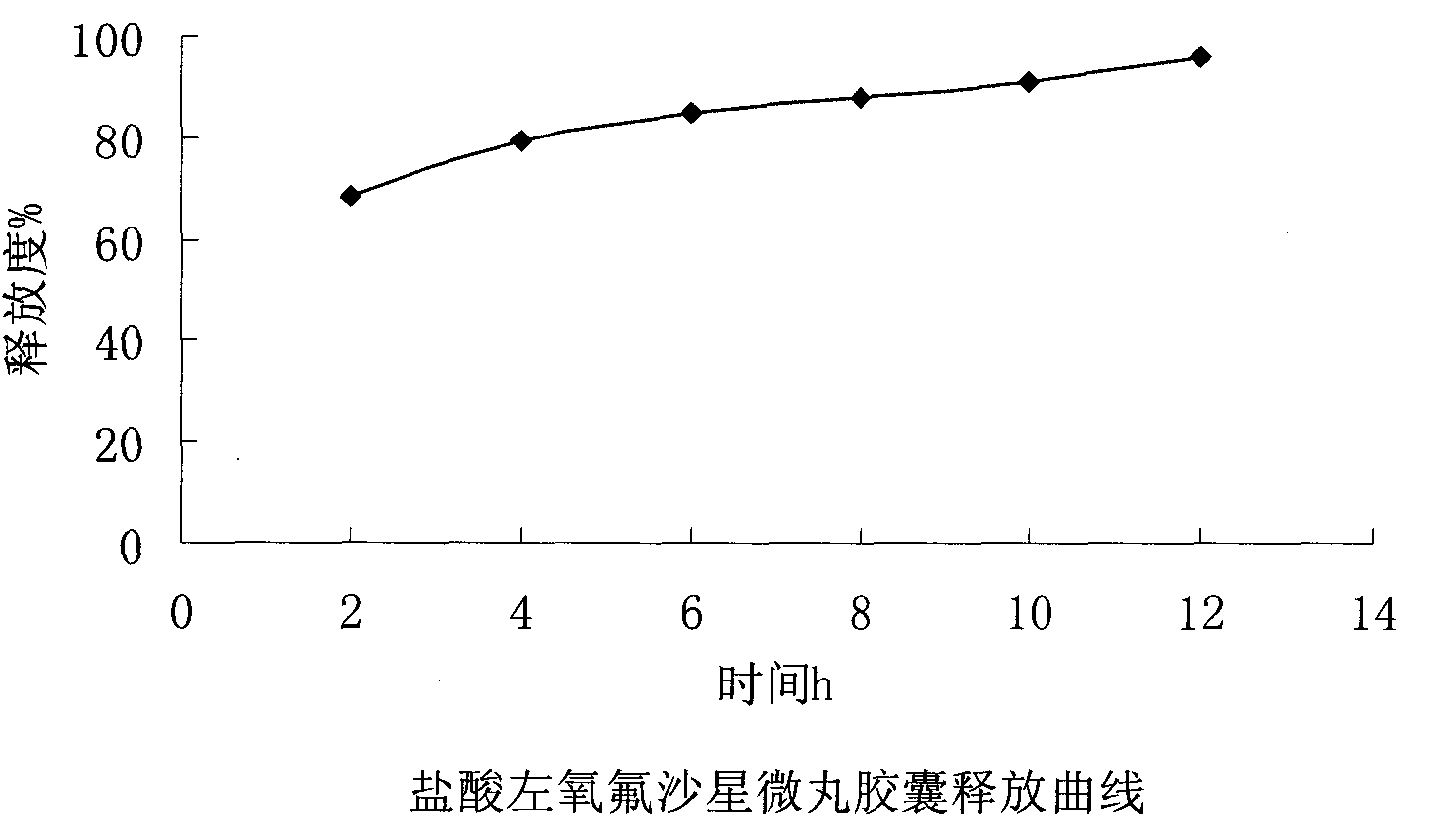
Levofloxacin hydrochloride micropill capsule and preparation method thereof
ActiveCN102106842AUniform sizeImprove liquidityAntibacterial agentsOrganic active ingredientsPlasticizerFluidized bed
The invention relates to a levofloxacin hydrochloride micropill capsule and a preparation method thereof. The levofloxacin hydrochloride micropill capsule comprises a pill core, a medicament-containing layer, a sustained-release coating layer and a quick-release layer, wherein levofloxacin hydrochloride is contained in the medicament-containing layer and the quick-release layer; and the sustained-release coating layer comprises the following materials in percentage by weight: 20 to 60 percent of levofloxacin hydrochloride, 30 to 55 percent of pill core, 10 to 25 percent of binding agent, 3 to 5 percent of sustained-release coating material, 0.3 to 3 percent of pore-forming agent and 0.1 to 1 percent of plasticizer. The levofloxacin hydrochloride micropill capsule has the advantages of high stability, small local stimulation of medicaments, high bioavailability and the like. Due to the adoption of a fluidized bed, the problems of large dust and low yield in a method of powder agglomerating in the background technology are solved.
Owner:HAINAN PULIN PHARMA +1
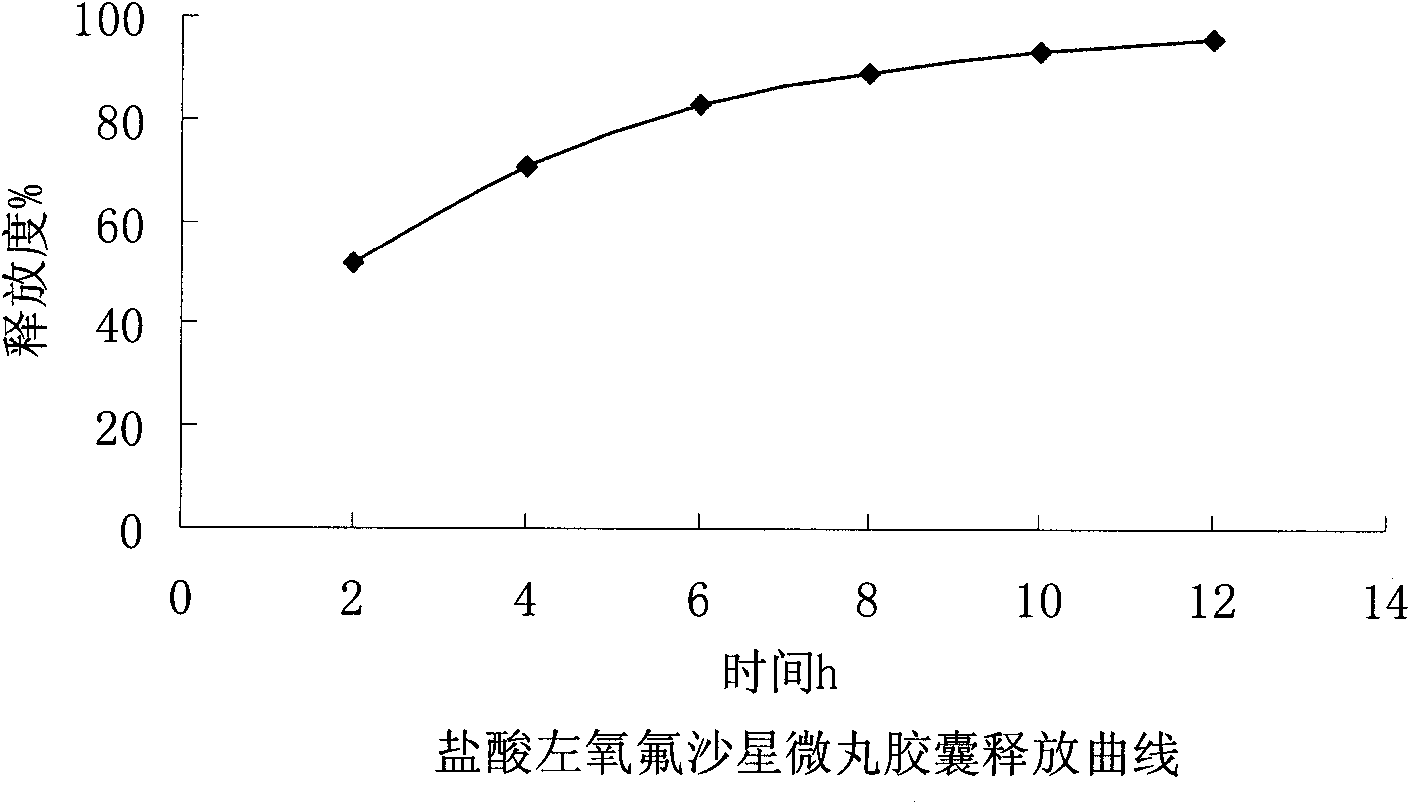
Levofloxacin hydrochloride micro-pill capsule and its preparing method
InactiveCN1813758AUniform sizeImprove liquidityAntibacterial agentsOrganic active ingredientsLevofloxacinGastrointestinal irritation
The present invention relates to a levofloxacin hydrochloride micropill capsule and its preparation method. It is composed of capsule external shell and micropill, the micropill is formed from levofloxacin hydrochloride, blank pill core and adhesive, every pill contains (by wt%) 10%-80% of levofloxacin hydrochloride, 15%-60% of medicine micropill pill core and 5%-30% of adhesive. Besides, said invention also provides the concrete steps of its preparation method.
Owner:范敏华
Pellet type pantoprazole sodium enteric capsule and preparation method thereof
InactiveCN104644616AImprove stabilityAvoid photolysisOrganic active ingredientsDigestive systemGranularityIsolation layer
The invention discloses a pellet type pantoprazole sodium enteric capsule and a preparation method thereof. The pellet type pantoprazole sodium enteric capsule is a sustained-release preparation composed of a capsule shell and uniformly mixed pantoprazole sodium pellets held in the capsule shell; the pantoprazole sodium pellet from inside to outside in turn comprises a blank pellet core, a main drug layer, an isolation layer and an enteric layer, wherein the weight of the main drug layer is 14-55% of that of the blank pellet core, the weight of the isolation layer is 9-10% of that of the total weight of the blank pellet core and the main drug layer, the weight of the enteric layer is 28-32% of that of the total weight of the blank pellet core, the main drug layer and the isolation layer; 40-60% of that of the pantoprazole sodium pellet is coated with a pigmented layer on the outside of the enteric layer; the weight of the pigmented layer is 0.5-1% of that of the total weight of the blank pellet core, the main drug layer, the isolation layer and the enteric layer; the diameter of the blank pellet core is 0.8-1.0 mm; the granularity of the pantoprazole sodium pellet is 14-20 mesh; each capsule contains 20-40 mg of the pantoprazole sodium. The pellet type pantoprazole sodium enteric capsule has the advantages of acidproof, rapid release and stable.
Owner:ZHEJIANG CHANGDIAN PHARMA +1
Detection method of medicament for treating angina pectoris
InactiveCN102768247AExcellent disintegration and solubilityThe curative effect is fastComponent separationCardiovascular disorderAnginaCoronary heart disease
The invention provides a detection method of a medicament for treating angina pectoris. According to the detection method, ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1 in the medicament are detected by high performance liquid chromatography. Conditions of the high performance liquid chromatography comprise: octadecylsilane bonded silica gel is used as a chromatographic column for a filler; and an acetonitrile solution is used as a mobile phase A and water is used as a mobile phase B for gradient elution. The gradient elution process is as follows: during the first 25 minutes, the mobile phase A acetonitrile solution is 15% by volume and the mobile phase B water is 85% by volume; from the 25th minute to the 70th minute, the mobile phase A acetonitrile solution is 15-22% by volume and the mobile phase B water is 85-78% by volume; from the 70th minute to the 80th minute, the mobile phase A acetonitrile solution is 22-30% by volume and the mobile phase B water is 78-70% by volume; from the 80th minute to 105th minute, the mobile phase A acetonitrile solution is 30-40% by volume and the mobile phase B water is 70-60% by volume; and from the 105th minute to 120th minute, the mobile phase A acetonitrile solution is 40-15% by volume and the mobile phase B water is 60-85% by volume.
Owner:梁丽
Preparation method of lung-soothing concentration pill
InactiveCN103417593AWith heat-clearing and expectorantHigh content of active ingredientsRespiratory disorderDrageesIrritationObstructive chronic bronchitis
The invention discloses a preparation method of a lung-soothing concentration pill. The preparation method comprises the following steps: taking hempleaf groundsel herb as raw materials, adding water to soak the hempleaf groundsel herb, performing extraction through an ultrasonic extraction tank, and merging the extracts for decoction, filtering and concentration to obtain an extractum; drying and crushing extractum to sieve by a 100-mesh sieve, so as to obtain hempleaf groundsel herb extractum powder; mixing with starch, microcrystalline cellulose and sodium carboxymethyl starch to form mixture powder; preparing the concentration pill by a centrifugal coating palletizing machine. The concentration pill has the benefits of reducing fever, eliminating phlegm, relieving cough and relieving asthma, can be used for lungs infection, chronic bronchitis, asthmatoid bronchitis, acute respiratory tract infection and the like. According to the invention, as ultrasonic extraction is adopted, the dissolving-out amount of effective component of materials to be extracted can be improved in unit time, and the prepared concentration pill is high in effective component content, small in taking dose and small in local irritation.
Owner:LIAONING PENGJIAN PHARMA CO LTD
Pharmaceutical composition containing carbohydrate nutrient and conventional ineffective compound and application of pharmaceutical composition
PendingCN110870869AImprove effectivenessImprove securityHydroxy compound active ingredientsAntipyreticDiseasePharmaceutical drug
The present application discloses an application of a carbohydrate nutrient as a local active ingredient in preparing a local pharmaceutical composition for treating local lesion diseases, the local pharmaceutical composition containing the carbohydrate nutrient as a local active ingredient and a conventional ineffective compound, and a device comprising the composition.
Owner:CHENGDU KUACHANGAOPU MEDICAL TECH CO LTD
Mini-pill type nicergoline capsule and preparation method thereof
InactiveCN104622850AImprove solubilityWidely distributedSenses disorderNervous disorderSide effectBiomedical engineering
The invention discloses a mini-pill type nicergoline capsule and a preparation method thereof. The mini-pill type nicergoline capsule is a sustained-release preparation and is prepared from a capsule shell and a nicergoline mini-pill accommodated in the capsule shell, wherein the nicergoline mini-pill sequentially comprises an empty pill core, a main drug layer and a pigmented layer from inside to outside; the main drug layer accounts for 15-60 percent of the empty pill core, and the pigmented layer accounts for 0.5-1 percent of the total weight of the empty pill core and the main drug layer. The mini-pill type nicergoline capsule has the advantages of small stimulation to intestines and stomach, high utilization rate, high stability, less toxic or side effects and capability of fully releasing effective components in time.
Owner:ZHEJIANG CHANGDIAN PHARMA +1
Pellet type omeprazole enteric capsule and preparation method thereof
InactiveCN104546737AWidely distributedIncrease local concentrationOrganic active ingredientsDigestive systemGranularityIsolation layer
The invention discloses a pellet type omeprazole enteric capsule and a preparation method thereof. The pellet type omeprazole enteric capsule is a sustained-release preparation, and is composed of a capsule shell and evenly mixed omeprazole pellets received in the capsule shell; each omeprazole pellet is composed of a blank pill core, a main drug layer, an isolation layer and an enteric layer in order from inside to outside, wherein the weight of the main drug layer accounts for 15-60% of that of the blank pill core, the weight of the isolation layer accounts for 10% of the total weight of the blank pill core and the main drug layer, the weight of the enteric layer accounts for 28-32% of the total weight of the blank pill core, the main drug layer and the isolation layer; besides, the enteric layers of 40-60% of omeprazole pellets are further coated with pigment layers; the weight of the pigment layer accounts for 0.5-1% of the total weight of the blank pill core, the main drug layer, the isolation layer and the enteric layer; the diameters of the blank pill cores are 0.8-1.0mm; the granularity of the omeprazole pellets is 14-20 meshes; each capsule contains 15-30mg of omeprazole. The pellet type omeprazole enteric capsule has the advantages of good acid resistance, sufficient active ingredient release without delay, good stability and the like.
Owner:ZHEJIANG CHANGDIAN PHARMA +1
Spraying agent for treating asthma and preparation method thereof
InactiveCN102302528AVolatileSmall local irritationAerosol deliveryRespiratory disorderIrritationXanthine
The invention discloses a spraying agent for treating asthma and a preparation method thereof. The spraying agent contains angelica volatile oil serving as an effective part for relieving asthma, and pharmaceutically allowable auxiliaries, wherein a spraying agent emulsion is prepared through weighing, mixing and setting volume, emulsifying and the like; and 0.2 to 15ml of the angelica volatile oil is contained in each 100ml of the spraying agent emulsion. The angelica volatile oil is easily volatized, has small local irritation, and is more suitable for administration in a local spraying inhalation, so that the medicament can directly reach a pathological position and has quick acting and good curative effect; and less drug is absorbed and enters blood circulation, and the adverse reaction of the whole body is light, so that the spraying agent is more suitable for long-term preventive administration in intermission phase of asthma. The volatile oil has the treatment effect on pulmonary hypertension, pulmonary heart disease, arrhythmia and other complications caused by chronic asthma while overcoming adverse reactions of beta2 receptor agonists and xanthine medicaments.
Owner:GANSU UNIV OF CHINESE MEDICINE
Double hydrochloride vertebral gel spray for purifying nose and its use
InactiveCN101073552AEasy to useSmall local irritationAerosol deliveryAntiparasitic agentsNasal cavityVein
The invention is concerned with the Vertebral double net hydrochloride clean gelatin nebula pressurized spray for nose, which consists of Vertebral double net hydrochloride and gel substrate. It is uses for anti-trypanosomiasis. It is: stays in liquid form, in order to control to spray into nasal cavity easily and separates on the surface of Nasal evenly and forms gelatin by interdiffusing with nose grume. With its long lasting inside the nasal cavity, it can direct input the medicine to the brain that is 2-5 times over injection through vein.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Intragastric floating sustained release tablet of praziquantel composition and preparation method thereof
ActiveCN103800297AImprove bioavailabilityGood water solubilityOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletBlood concentration
The invention belongs to the technical field of a medicine preparation, and discloses an intragastric floating sustained release tablet of a praziquantel composition and a preparation method thereof. The intragastric floating sustained release tablet of the praziquantel composition comprises the following raw materials: 65-85 parts of praziquantel, 50-60 parts of hydroxypropyl-beta-cyclodextrin, 40-60 parts of hydroxypropyl methyl cellulose, and 30-50 parts of xanthan gum. The intragastric floating sustained release tablet of the praziquantel composition provided by the invention is good in sustained release effect, and the blood concentration curve of praziquantel after medicine administration is smoother, the bioavailability of the praziquantel is significantly improved, and popularization and application of the praziquantel are facilitated.
Owner:SICHUAN AGRI UNIV
Slow releace tablets of clarithromycin, and its preparing method
ActiveCN101002790AGuaranteed releaseStable blood concentrationAntibacterial agentsOrganic active ingredientsAdhesiveClarithromycin
A slowly-released tablet of clarithromycin contains proportionally clarithromycin, high-molecular adhesive, flow-assistant lubricant, pH regulator and sweetening agent. Its preparing process is also disclosed.
Owner:HAINAN PULIN PHARMA +1
Cis-platinum lung cancer-resistant active targeting stealth liposomes and preparation method thereof
ActiveCN102008438AAvoid phagocytosisNo toxicityHeavy metal active ingredientsMacromolecular non-active ingredientsIrritationCholesterol
The invention relates to a cis-platinum active lung targeting stealth liposome drug delivery system. Injection or a lyophilized product prepared from cis-platinum, a self-prepared polymer-monoclonal antibody F(ab') 2 fragment modifier, a self-prepared cholesterol-polymer conjugate, cholesterol and the like in a self-assembly mode is applied to treating solid tumors of human lung squamous carcinoma. The drug delivery system has the encapsulation rate of 71.25+ / -1.14 percent, and the loading rate of 8.81+ / -0.23 percent; and results of cell experiments and animal experiments show that the drug delivery system has obvious medical sustained-release effect, can avoid removal of a reticuloendothelial system (RES) in blood, has the inhibition rate for growth of carcinoma blocks of a corresponding lung cancer-loaded nude mouse improved by 80.34 percent compared with that of cis-platinum solution, has obvious effect of treating corresponding lung cancer in a targeting way, does not have obvious toxin and also can reduce the local irritation of a technical material.
Owner:SUZHOU UNIV
Externally applied traditional Chinese medicine composition for treating gynecological diseases, transdermal medicine delivery patch and application
PendingCN112315945AGreat psychologyHuge physical burdenPharmaceutical non-active ingredientsSexual disorderGYNECOLOGIC DISORDERSHomalomena
The invention discloses an externally applied traditional Chinese medicine composition for treating gynecological diseases. The composition comprises the following traditional Chinese medicine components: cortex acanthopanacis, obscured homalomena rhizome, garden balsam stem, notopterygium root, radix angelicae pubescentis, daemonorops draco, frankincense, myrrh, safflower, folium artemisiae argyi, parasitic loranthus, teasel root, rhizoma sparganii, curcuma zedoary, radix paeoniae rubra, pericarpium zanthoxyli, dandelions, radix angelicae dahuricae and radix saposhnikoviae. A preparation method of a transdermal medicine delivery patch prepared from the traditional Chinese medicine components comprises the steps of preparing superfine medicine powder and preparing the patch. Besides, the invention further discloses application steps of the transdermal medicine delivery patch to treatment of gynecological diseases. The traditional Chinese medicine composition provided by the invention has a good curative effect and few side effects; According to the transdermal medicine delivery patch prepared from the traditional Chinese medicine composition, by means of an ultrasonic introductiontechnology, effective components in medicines permeate into deep tissues through local skin, so that relatively high medicine concentration is rapidly achieved, and the purpose of treatment is furtherachieved. A transdermal medicine delivery technology is good in directionality, accurate and controllable in action range, high in treatment efficiency, small in local irritation and good in clinicalcompliance.
Owner:FIRST PEOPLES HOSPITAL OF YUNNAN PROVINCE +1
Pharmaceutical composition containing amino acid nutrient and conventional ineffective compound and application of pharmaceutical composition
InactiveCN110870860AImprove effectivenessImprove securityAntibacterial agentsAntimycoticsBULK ACTIVE INGREDIENTPharmaceutical Substances
The invention discloses an application of an amino acid nutrient as a topical active ingredient in preparation of a topical pharmaceutical composition for treating topical lesion diseases, a topical pharmaceutical composition, which contains the amino acid nutrient topical active ingredient and conventional ineffective compound, for treating topical lesion diseases, and a device comprising the composition.
Owner:CHENGDU KUACHANGAOPU MEDICAL TECH CO LTD
Paclitaxel lipid nanoparticle injection liquid with anti-tumor activity
InactiveCN102871963AImprove solubilityReduce eliminationOrganic active ingredientsPowder deliveryPaclitaxel InjectionActive agent
The invention provides paclitaxel lipid nanoparticle injection liquid, which comprises paclitaxel medicine, lipid materials, water for injection, surfactant, stabilizers and metal ion chelating agents. Most medicine is wrapped inside lipid, the direct contact between the medicine and blood or other liquid is avoided, and the local irritation of the medicine on the injection position is reduced, and the toxicity is reduced. No any organic solvents are contained, no solvents with toxicity and irritation on human bodies are contained, and no irritation and no sensitization are caused on the human bodies. The adverse reaction of the existing preparation caused by the recipe problem can be eliminated, and the goals of safety and efficiency are reached. Meanwhile, the paclitaxel lipid nanoparticle injection liquid has the excellent characteristic that the tumor medicine resistance is overcome, and the cure efficiency of the paclitaxel on the existing various tumors is greatly improved.
Owner:ZHEJIANG UNIV
Wuji gastric floating sustained-release pellet and preparation method thereof
InactiveCN105434619ATo achieve a sustained release effectLarge distribution areaDigestive systemMicrocapsulesSustained release pelletsLiver stomach
The invention relates to a pharmaceutical composition for treating liver-stomach disharmony, bitter taste, epigastric upset, vomiting, acid regurgitation, stomachache and dysentery, especially to an oral gastric floating pellet preparation prepared mainly on the basis of a set traditional Chinese medicine prescription. The pellet preparation is mainly composed of a lightweight drug-loaded pellet core and a retardant layer coating, wherein the lightweight drug-loaded pellet core comprises 10 to 15% of extract of a Wuji prescription, 0 to 20% of a filler, 60 to 65% of an adhesive, a skeletal material and a floating aid, 5 to 10% of a hydrophilic gel material and 5 to 10% of a floating sustaining agent, and the retardant layer coating comprises 90 to 95% of a coating material and 5 to 10% of an anti-adherent. According to the invention, a high-speed fusion and stirring method is employed to prepare a pellet; the prepared pellet floats upon entering water and continuously floats for 6 h, so drugs in the pellet are slowly released; the prepared pellet has the characteristics of a great distribution area, small local irritation, etc., and due to the floating characteristic of the pellet, retention time of the pellet in the stomach is substantially prolonged, which enables bioavailability of the drugs to be greatly improved; and since the high-speed fusion and stirring method is utilized to prepare the pellet, the advantages of easiness, economic performance and no risks of fire outbreak and explosion are obtained compared with a conventional wet-rolling preparation method.
Owner:QINGDAO UNIV OF SCI & TECH
Pharmaceutical composition containing chemical ablating agent and biologically-active glycosides, and application thereof
PendingCN108685926ASmall local irritationGood curative effectHeavy metal active ingredientsSalicyclic acid active ingredientsGlycosideDrug biological activity
Owner:CHENGDU KUACHANG SCI & TECH CO LTD
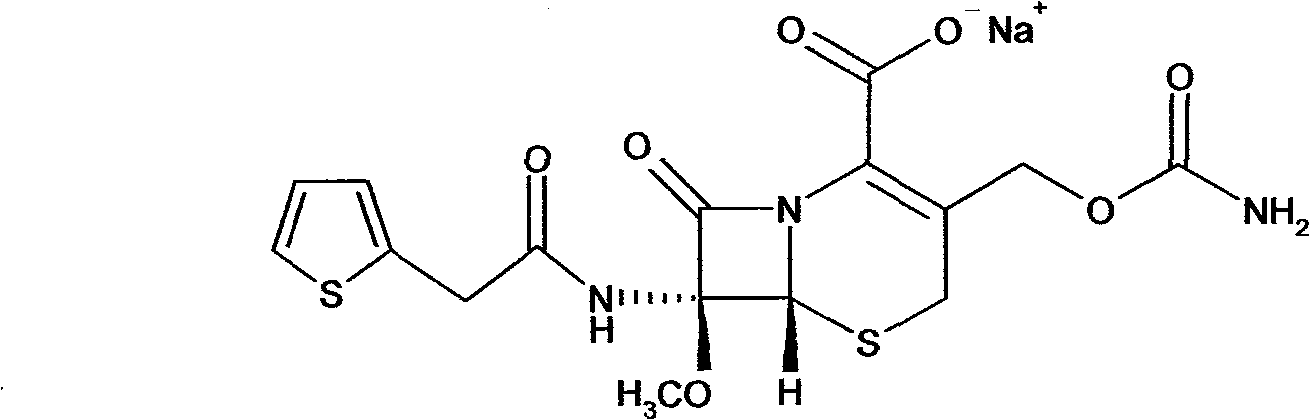
Composition of cefoxitin acid
InactiveCN101669956ASolve the problem of insoluble in waterSolve instabilityAntibacterial agentsOrganic active ingredientsGeneration rateArginine
The invention relates to a cefoxitin acid composition which is characterized by comprising the combination of cefoxitin acid and arginine. The preparation method of the composition mainly comprises the following steps: weighing the aseptic raw material medicines of the cefoxitin acid and the arginine according to the proportion of a recipe in an aseptic environment, mixing evenly and then subpackaging. The composition solves the problem that the active component of the cefoxitin acid is not dissolved in water, also solves the problem that the original medicine of cefoxitin sodium is unstable,can effectively lower the impurity content and obviously enhance the stability of a product, thereby reducing the generation rate of side reactions, such as anaphylactic reactions, effectively; a safety testing result shows that compared with the original pharmaceutical preparation, the preparation has smaller local irritability and very important clinical application value.
Owner:CHANGSHA KINGDAY BIO PHARMA TECH
Drug for post-perineotomy local anesthesia and analgesia
InactiveCN102908527BThe composition is uniquePlay an analgesic roleAnaestheticsPlant ingredientsMedicinal herbsCurative effect
Owner:陈为宝
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com