Ophthalmic pharmaceutical composition containing cyclosporine and preparation method and application thereof
The technology of a composition and cyclosporine is applied in the directions of drug combination, medical preparation of non-active ingredients, drug delivery, etc. Stable, good stability, reasonable prescription effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1: cyclosporine ophthalmic solution 1 and its preparation
[0081] 1) Prescription:
[0082]
[0083] 2) Preparation method:
[0084] (1) Mix medium-chain triglycerides, Tween-80 and propylene glycol, and dissolve them to obtain the first product;
[0085] (2) adding cyclosporine to the above-mentioned first product to dissolve it to obtain the second product;
[0086] (3) adding sodium hyaluronate and sorbitol to the water for injection to dissolve it to obtain the third product;
[0087] (4) Add the second product to the third product under stirring, and mix well;
[0088] (5) Replenish it with water for injection to the full amount, ready to use.
Embodiment 2
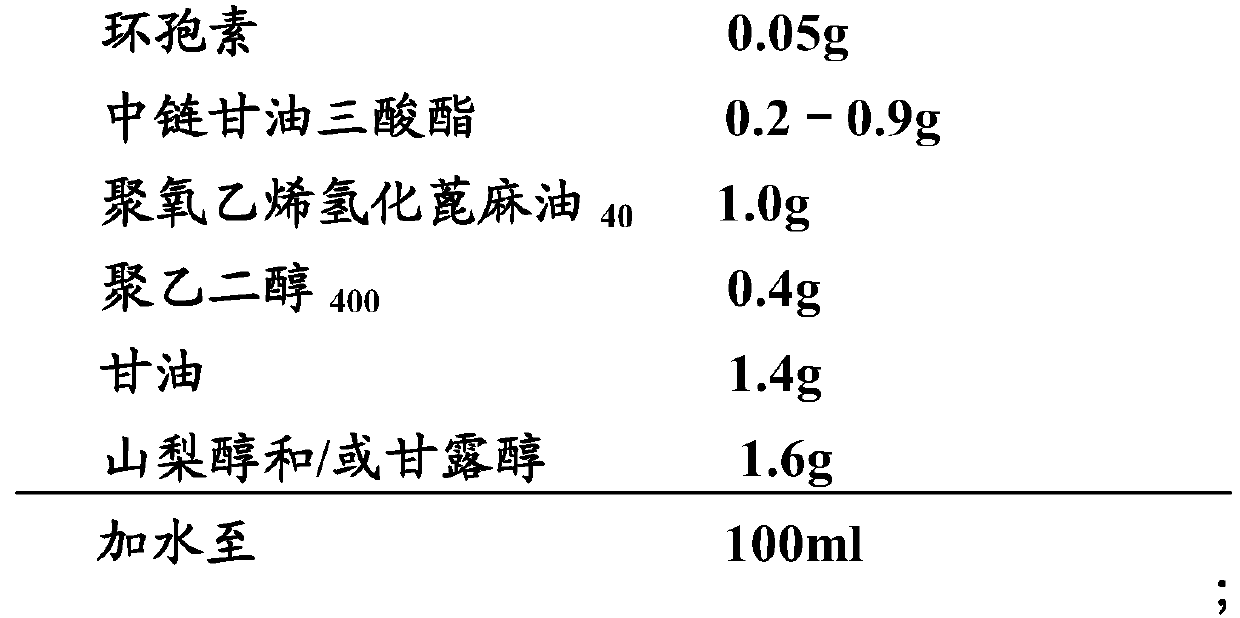
[0089] Embodiment 2: cyclosporine ophthalmic solution 2 and its preparation
[0090] 1) Prescription:
[0091]
[0092] 2) Preparation method:
[0093] (1) castor oil, polyoxyethylene hydrogenated castor oil 40 Mix with propylene glycol, make it dissolve, obtain the first product;
[0094] (2) adding cyclosporine to the above-mentioned first product to dissolve it to obtain the second product;
[0095] (3) adding glycerin and mannitol to the water for injection to dissolve it to obtain the third product;
[0096] (4) Add the second product to the third product under stirring, and mix well;
[0097] (5) Replenish it with water for injection to the full amount, ready to use.
Embodiment 3
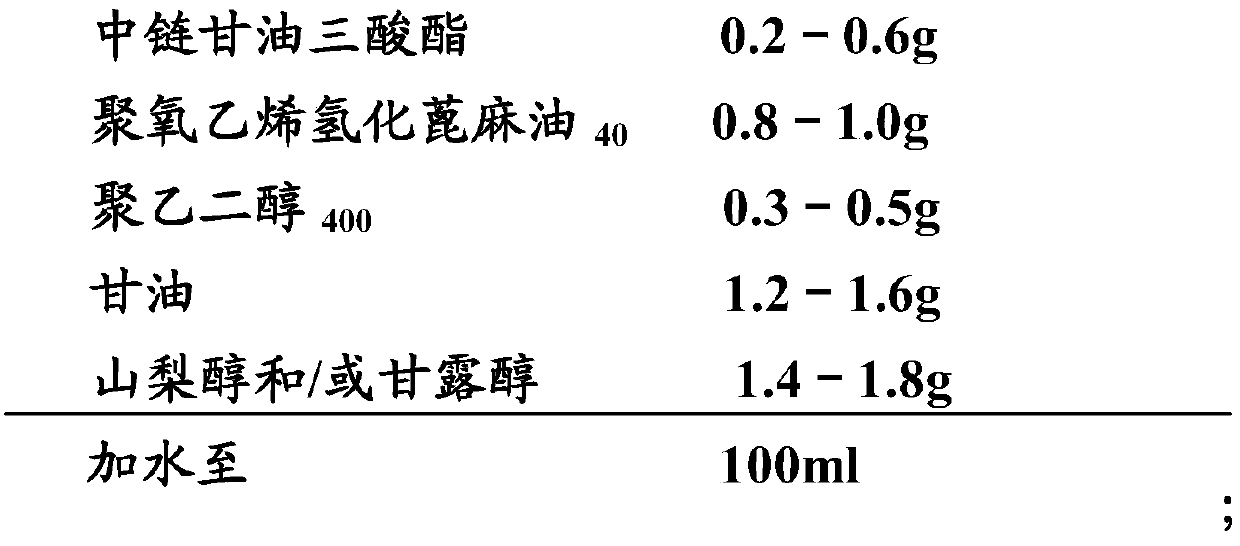
[0098] Embodiment 3: cyclosporine ophthalmic solution 3 and its preparation
[0099] 1) Prescription:
[0100]
[0101]
[0102] 2) Preparation method:
[0103] (1) Medium-chain triglycerides, polyoxyethylene hydrogenated castor oil 40 and polyethylene glycol 400 Mixing and dissolving to obtain the first product;
[0104] (2) adding cyclosporine to the above-mentioned first product to dissolve it to obtain the second product;
[0105] (3) Adding glycerin and sorbitol to the water for injection is dissolved to obtain the third product;
[0106] (4) Add the second product to the third product under stirring, and mix well;
[0107] (5) Replenish it with water for injection to the full amount, ready to use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com