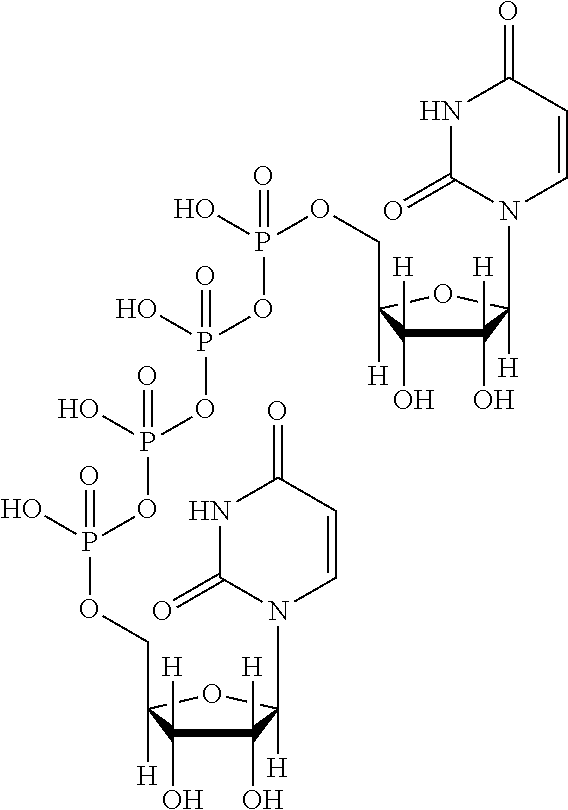
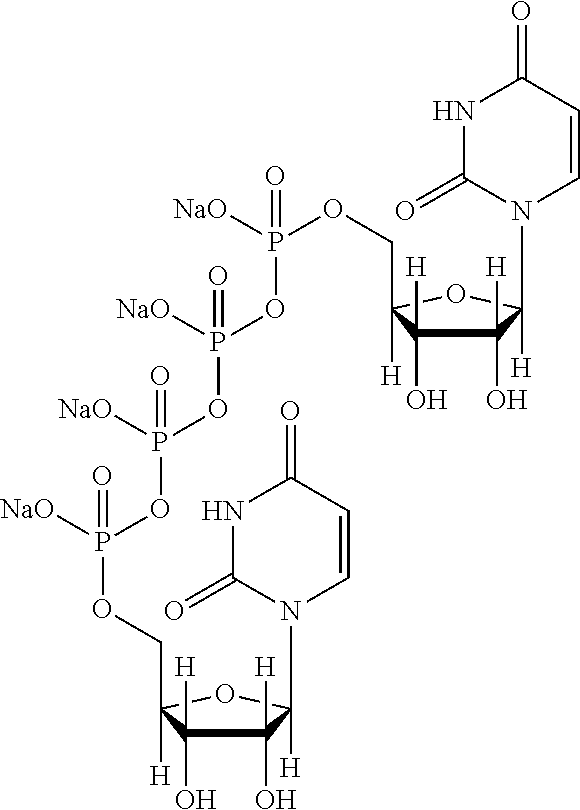
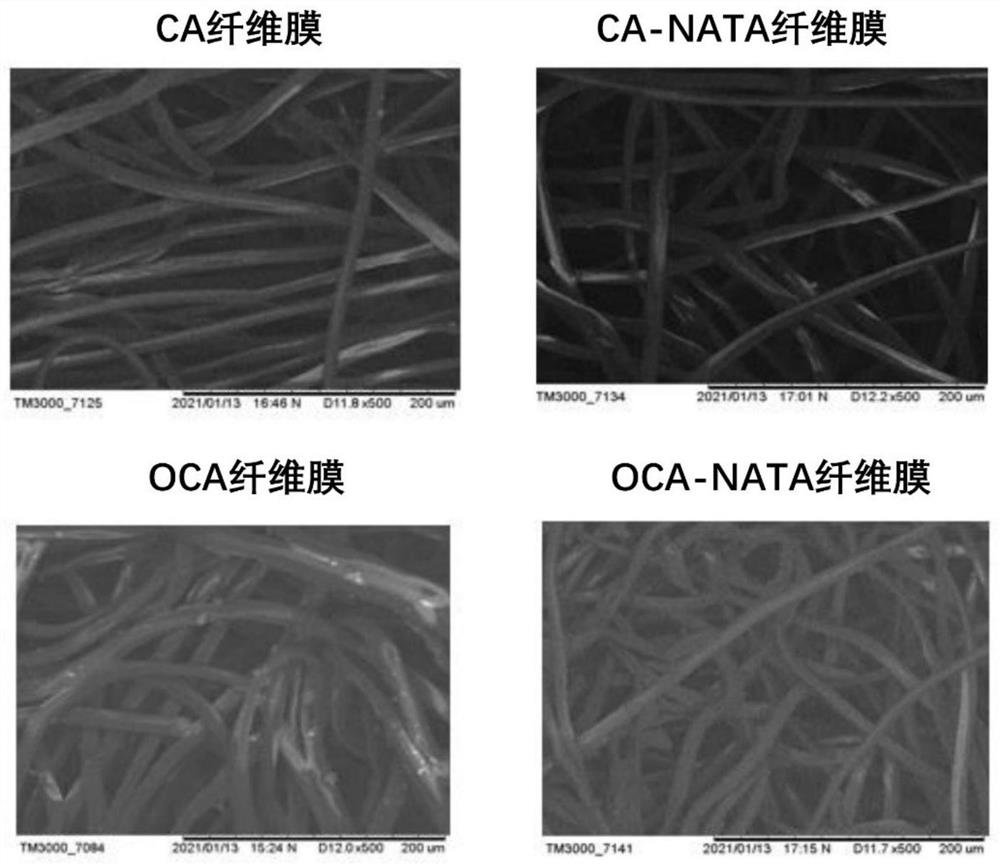
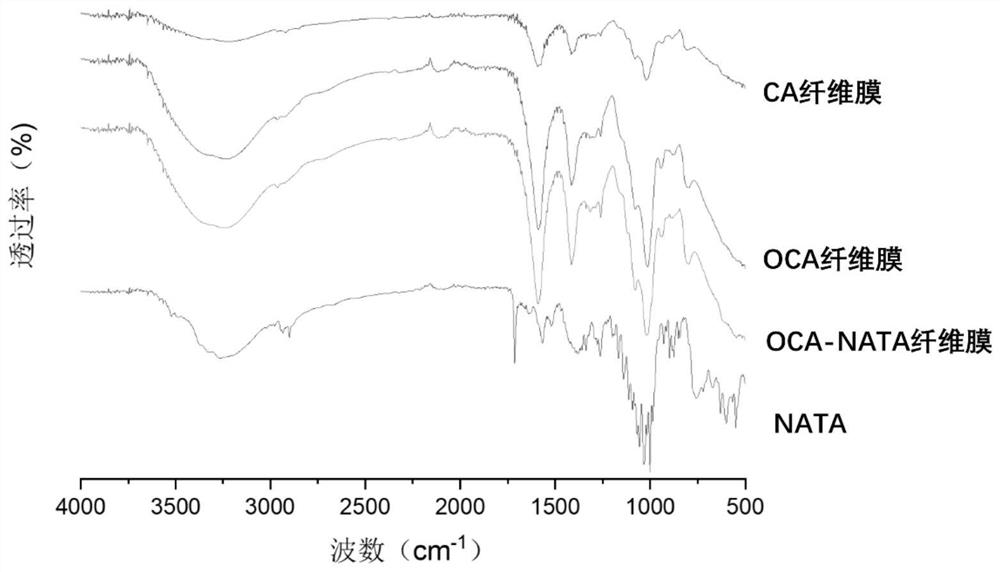
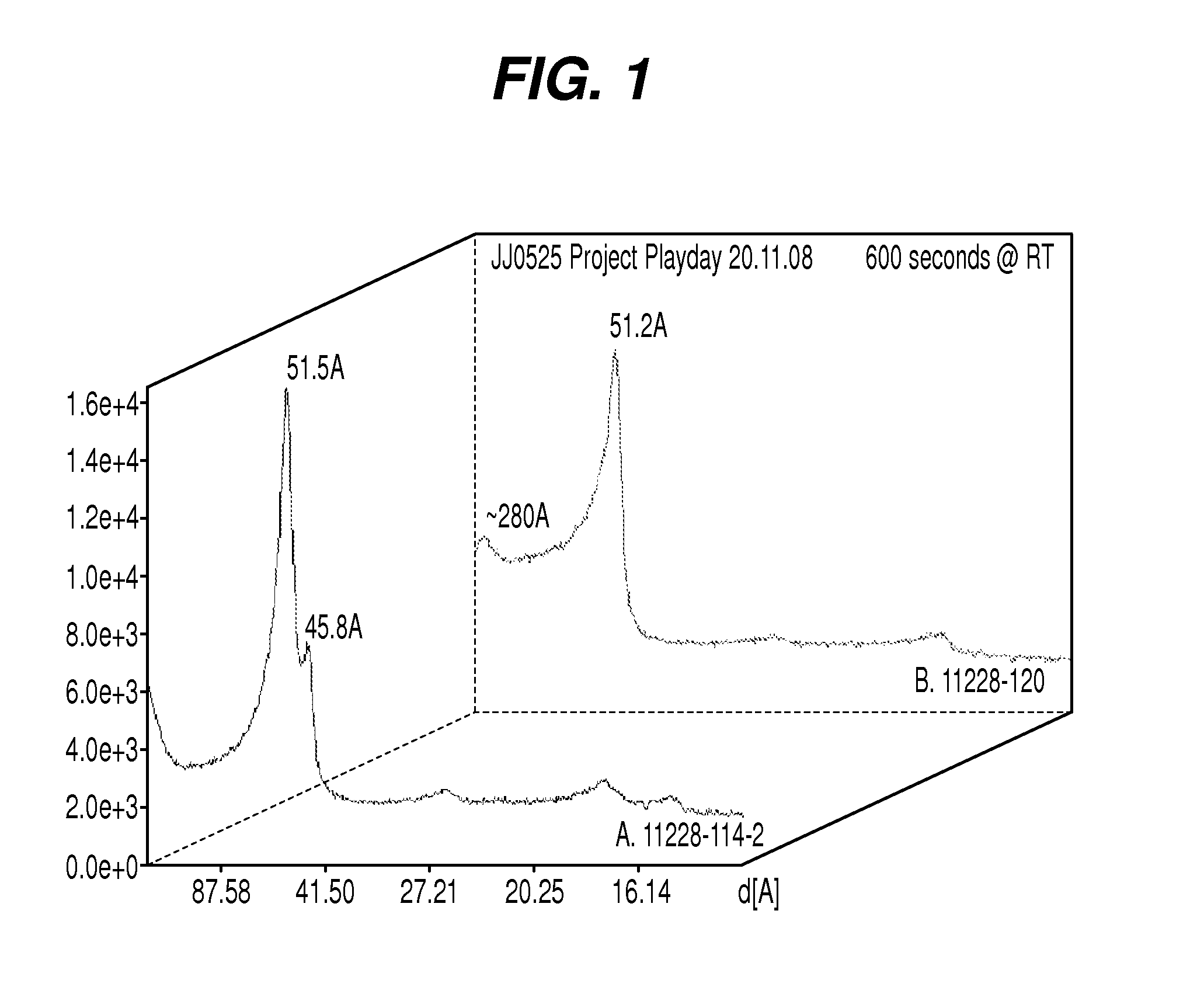
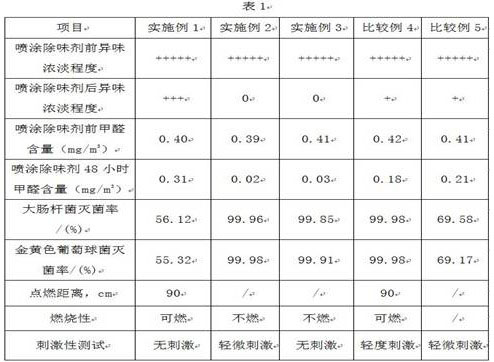
Patents
Literature
56 results about "Eye irritation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Many people complaining of eye irritation are diagnosed with seasonal eye allergies. Eye allergies sometimes cause significant discomfort, often interrupting daily activities with annoying symptoms. Eye allergies can feel miserable, as it affects vision and causes our eyes to itch uncontrollably.
Personal care sunscreen compositions having reduced eye irritation
The present invention is directed to a stable, minimal energy required self-assembling lamellar and spherulite composition comprising: mixture water, fatty alcohol, fatty acid, salt of fatty acid, polyglyceryl fatty acid ester and oils. The present invention relates to composition that can benefit eye mildness, even distribution of sunscreen physical filters on skin and enhanced stability.
Owner:HALIMI LAURENCE +1
Low-accumulation mild type shampoo composition
ActiveCN102824279AGood wash conditioning effectFine foamCosmetic preparationsHair cosmeticsScalpEye irritation
The invention provides a low-accumulation mild type shampoo composition which is formed by mixing water, a mild and safe surfactant, a low-accumulation conditioning agent and the like according to proper proportions. Compared with general shampoo, the shampoo composition has the advantages of favorable washing and conditioning effects on scalp and hairs, safe components, small stimulus to the skin and eyes and no accumulation after being repeatedly used for multiple times.
Owner:纳爱斯丽水日化有限公司
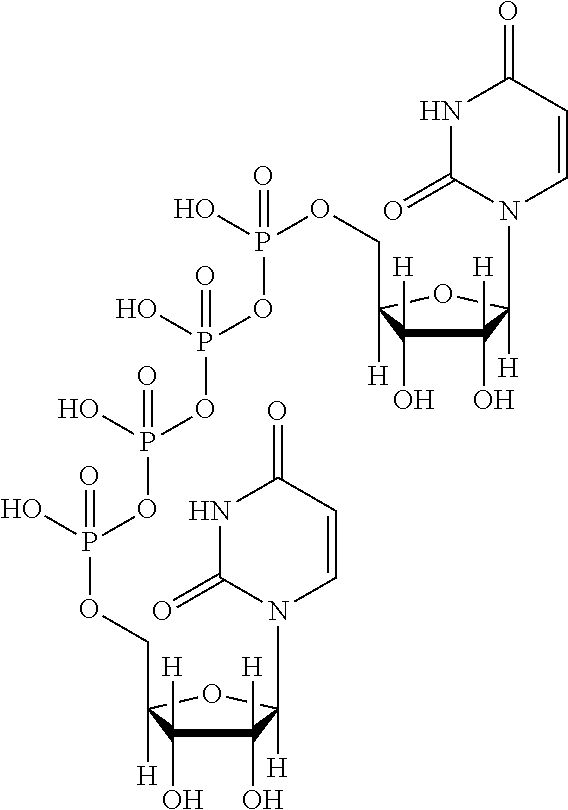
Ophthalmic solution comprising diquafosol
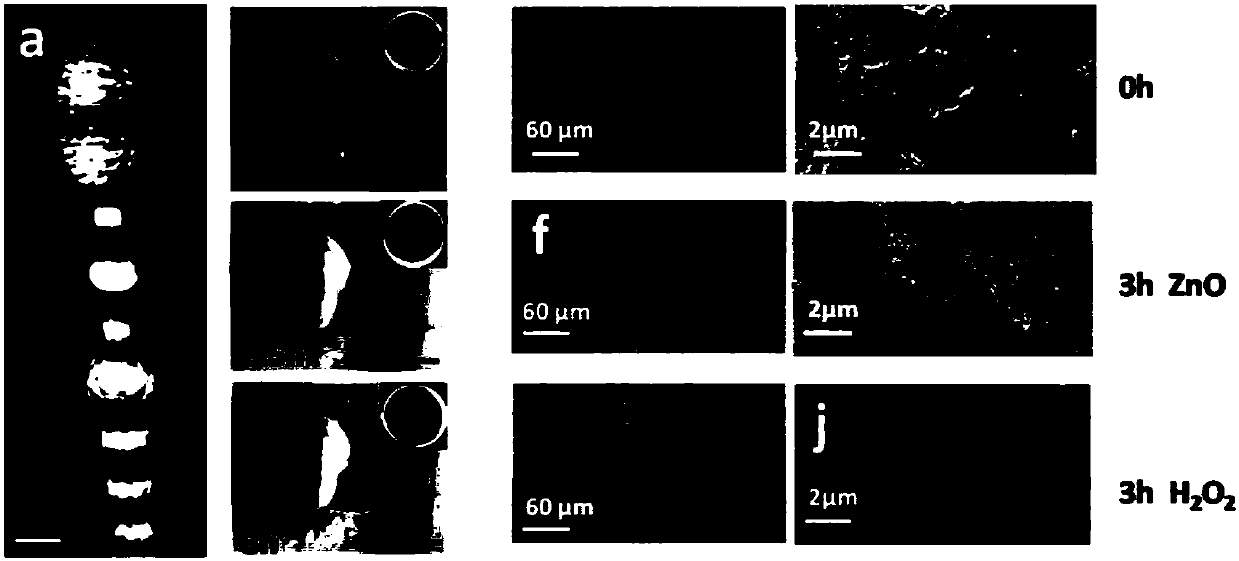
ActiveUS20150072951A1Reduce eye irritationImprove anti-corrosion performanceBiocideSenses disorderFiltrationEyes irritation
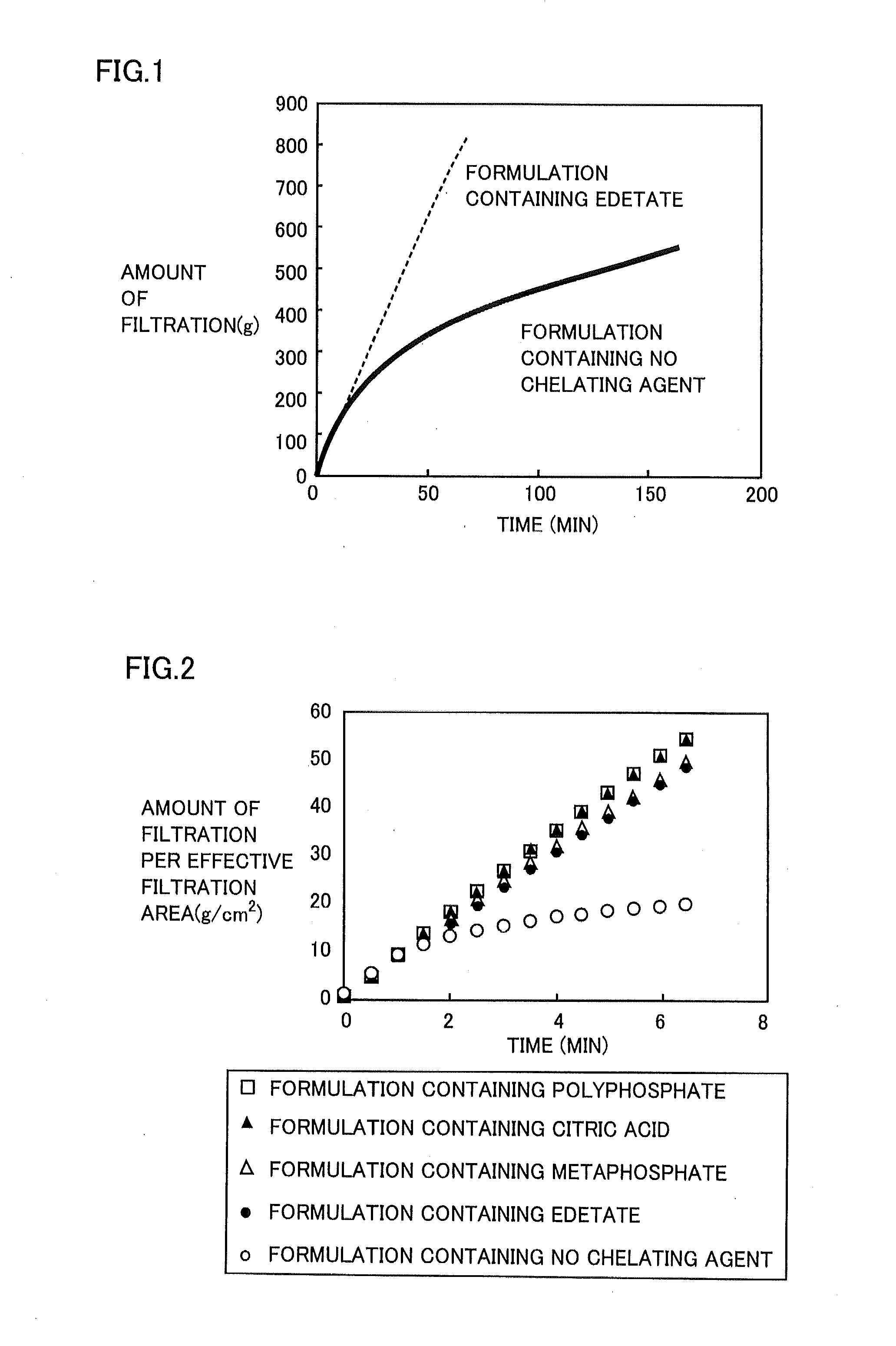
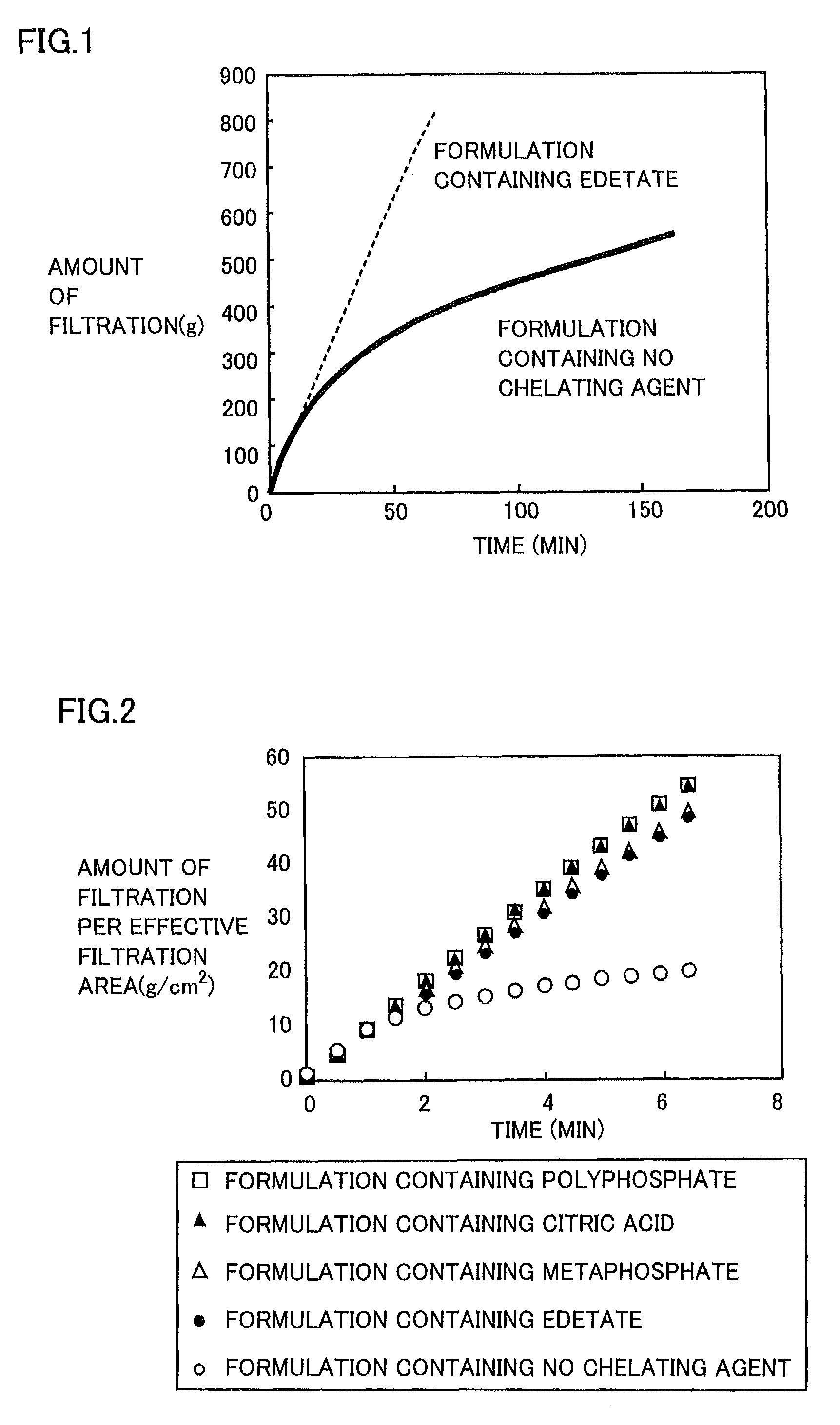
Regarding Diquafosol ophthalmic solution comprising a chelating agent at a concentration of 0.0001 to 1% (w / v), formation of insoluble precipitates found in Diquafosol ophthalmic solution during storage of the solution, as well as deterioration of the filtration performance in the course of production (course of filtration sterilization), have been inhibited. Further, in Diquafosol ophthalmic solution comprising a chelating agent, reduction of eye irritation and enhancement of the preservative effectiveness have been confirmed, in comparison to Diquafosol ophthalmic solution comprising no chelating agent. Accordingly, the present invention has been confirmed to provide physicochemical properties that are stable during the courses of production and distribution as well as the course of storage by a patient, and also reduce eye irritation and enhance preservative effectiveness.
Owner:SANTEN PHARMA CO LTD
Personal cleansing systems exhibiting low eye irritation potential
ActiveUS8470753B2Reduce eye irritationClear, viscous, effectiveCosmetic preparationsHair cosmeticsPersonal careCocamidopropyl hydroxysultaine
An ethylene oxide-free, dioxane-free, and formaldehyde-free personal care concentrate composition free of ethoxylated components which is non-irritating to eyes comprising water, sodium alkyl sulfate, propanediol, and a synthetic amphoteric surfactant selected from the group consisting of cocamidopropyl hydroxysultaine and cocamidopropyl betaine is disclosed. This composition is especially suitable for baby shampoos which are not irritating. In some embodiments the composition is free of formaldehyde.
Owner:SPECIALTY OPERATIONS FRANCE
Tear-free metabolism spray and preparation method thereof
InactiveCN105030552AAdjust water and oil balanceRelieve stressCosmetic preparationsToilet preparationsAllergySea salt
The invention relates to the field of cosmetics, and concretely relates to a nonirritant especially eye irritation-free metabolism moisture retention spray and a preparation method thereof. The spray is a compound moisture retention spray containing mineral matter components and other humectants, and the formula of the spray mainly comprises deep seawater, sea salt, beta-glucan and phenoxyethanol. The formula can be used for producing tear-free water locking and moisturizing spray. The spray has no obvious irritation to eyes, has no use precautions, can realize water supplementation and moistening of skins anytime and anywhere, can adjust water-oil balance of the skins, relieves the skin pressure, resists the allergy phenomenon and increases the natural protection function of the skins.
Owner:吴杰
Personal cleansing systems exhibiting low eye irritation potential
ActiveUS20120196783A1Easy to transportReduce eye irritationCosmetic preparationsHair cosmeticsPersonal careHydroxysultaine
An ethylene oxide-free, dioxane-free, and formaldehyde-free personal care concentrate composition free of ethoxylated components which is non-irritating to eyes comprising water, sodium alkyl sulfate, propanediol, and a synthetic amphoteric surfactant selected from the group consisting of cocamidopropyl hydroxysultaine and cocamidopropyl betaine is disclosed. This composition is especially suitable for baby shampoos which are not irritating. In some embodiments the composition is free of formaldehyde.
Owner:RHODIA OPERATIONS SAS
Method for evaluating eye irritation by using miniature pig erythrocytes
InactiveCN102359946ASmall batch-to-batch varianceClear genetic backgroundColor/spectral properties measurementsBiological testingFormularyOphthalmology
The present invention discloses an experimental method for evaluating eye irritation by using miniature pig erythrocytes. The method mainly comprises: (1) preparing a suspension of miniature pig erythrocytes; (2) carrying out a miniature pig erythrocyte hemolysis test; (3) carrying out a protein denaturation experiment; (4) predicting a model; (5) carrying out classification and judgment. According to the present invention, the source of the erythrocytes is reliable and safe; the tested material is easy to obtain, and has good uniformity; the test system is simple and cheap, and no special equipment is required; the detection method is rapid, sensitive and universal; the method of the present invention can be directly applicable for safety evaluations of chemicals, cosmetics, pesticides, eye medicines and other substances possibly contacting the eyes, wherein the chemicals comprise surfactants, disinfection products, detergent and the like, the cosmetics comprise shampoo, bath foam, eye cream and the like; the method further can be applicable for rapidly screening, classifying and identifying the eye irritations of the raw materials, the formulas or the products.
Owner:程树军 +1
Method for detecting irritation of cosmetic raw material and method for detecting anti-irritation of anti-irritation product
ActiveCN110018161AStimulating precisionStimulating objectiveMaterial analysis by optical meansMedicineEye irritation
The invention discloses a method for detecting anti-irritation of an anti-irritation product. The method for detecting the anti-irritation of the anti-irritation product comprises the following stepsof 1) obtaining an image of skin or a skin mimic obtained after patch treatment, wherein a patch is coated with a gel composition comprising the anti-irritation product and an irritant; and 2) measuring an erythema feature value of the skin or the skin mimic in the image. The invention also discloses a method for detecting the irritation of a cosmetic raw material, the gel composition and the patch. The detection method provided by the invention not only has good repeatability, but also can more accurately score the results of irritation antagonism; meanwhile, the subjective influence of people is avoided; and therefore, the irritation antagonism effect of the anti-irritation product is more accurately and objectively evaluated.
Owner:三立慧评(北京)检测技术有限公司
Use of plant extracts to prevent and/or reduce the signs of subjective discomfort and/or irritation in the topical application of cosmetic products
InactiveUS8221766B2Aesthetic appearance of skinImprove skin appearanceBiocideOrganic active ingredientsRhinacanthus nasutusEye irritation
The present invention describes compositions and methods for treating, preventing and improving the appearance of skin, particularly, treating, preventing, ameliorating, reducing and / or eliminating skin irritation, inflammation, and / or the signs of visible or subjective discomfort, wherein the compositions include natural plant constituents that inhibit at least one cytokine. The plant extracts are preferably derived from Populus nigra, Rhinacanthus nasutus, Sapindus rarak, and Thumbergia laurifolia, and any combinations thereof. The compositions are preferably applied to the skin, or are delivered by directed means, to a site in need thereof.
Owner:AVON PROD INC
Personal care composition having reduced eye irritation
The invention relates to a personal care composition having reduced eye irritation. The present invention is directed to a stable, minimal energy required self-assembling lamellar and spherulite composition comprising: mixture water, fatty alcohol, fatty acid, salt of fatty acid, polyglyceryl fatty acid ester and oils. The present invention relates to composition that can benefit eye mildness, even distribution of sunscreen physical filters on skin and enhanced stability.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Isolorydine clathrate and preparation method thereof
InactiveCN107007839AImprove solubilityAvoid irritating situationsOrganic active ingredientsSenses disorderSolubilityCyclodextrin derivative
The invention relates to an isolorydine clathrate and a preparation method thereof, particularly relates to an isolorydine cyclodextrin or cyclodextrin derivative clathrate, a preparation method thereof, a medicine composition comprising isolorydine cyclodextrin or cyclodextrin derivative clathrate and application to preparation of medicines thereof, and belongs to the field of medicine preparations. The isolorydine clathrate comprises isolorydine, cyclodextrin or cyclodextrin derivatives; the molecular molar ratio of isolorydine to cyclodextrin or cyclodextrin derivatives is (1 to 1)-(1 to 50). The isolorydine clathrate has the advantages that the solubility of isolorydine is improved, the clathrate has the characteristics of high water solubility and low eye irritation and the like, the problems in the prior art are solved, and the clathrate is suitably prepared into liquid preparations such as eye drops.
Owner:YUNNAN UNIV OF TRADITIONAL CHINESE MEDICINE
Camphol eye clearing pad and preparation method thereof
InactiveCN108743777AIngredient safetyReasonable formulaSenses disorderAerosol deliveryWrinkle skinAdditive ingredient
The invention provides a camphol eye clearing pad and a preparation method thereof. According to the eye clearing pad, kapur is utilized as a main ingredient and is matched with liquorice root extract, aloe extract, herba menthae extract, fructus forsythiae extract and bilberry fruit extract; high-purity kapur can quickly permeate and enter a deep layer of the eye skin to dredge eye meridians andcollaterals, skin channels can be quickly opened by the kapur, and other effective ingredients can enter eye base; medical effective ingredients are mutually matched to dredge eye meridians and collaterals to supplement moisture and nutrients which are needed by the eyes; thus, an eye metabolism speed is quickened, the problems of dry eyes and eye irritation are effectively relieved, and the problems of eye periphery cutis laxa, wrinkles around eyes, dark circles and the like are effectively prevented and improved. The preparation method comprises the steps: firstly, extracting the kapur and the bilberry fruit extract; preparing a medical layer; compounding the medical layer with a gel layer and a protection layer to obtain the camphol eye clearing pad. The eye pad prepared by the campholeye clearing pad preparation method disclosed by the invention has the advantages of comfortableness in use and good effect.
Owner:南京日光生物科技有限公司
Whitening toothpaste subjected to visible-light-stimulated oxidation reduction reaction and preparation method of whitening toothpaste
ActiveCN107582415AReduce stimulationPorousCosmetic preparationsToilet preparationsFreeze-dryingToothpaste
The invention provides whitening toothpaste subjected to a visible-light-stimulated oxidation reduction reaction and a preparation method of the whitening toothpaste. According to the technical scheme, zinc nitrate hexahydrate, ammonia water, and hexamethylene tetramine are taken as main raw materials and are subjected to a one-step reaction, the obtained product is reacted with sodium citrate andhydroxypropyl methyl cellulose, and the obtained product undergoes freeze drying and microwave processing to obtain the whitening toothpaste. The toothpaste contains porous zinc oxide, and can be fast subjected to an oxidation reduction reaction with color-changed teeth under yellow light stimulation. The whitening toothpaste has the following technical advantages: 1, the whitening toothpaste hasporosity and a good absorption effect; 2, the whitening toothpaste can respond to yellow light, is subjected to an oxidation reduction reaction without ultraviolet stimulation, and is safe during usefor the yellow light is mild and is less irritant to eyes; and 3, the process is simple and safe, no organic solvent is needed, the operation method is simple, and the equipment requirement is low, so that the preparation method is suitable for massive production and industrialization.
Owner:NANCHANG UNIV
Ophthalmic solution comprising diquafosol
ActiveUS9486529B2Improve anti-corrosion performancePerformance deteriorationOrganic active ingredientsSenses disorderFiltrationEye irritation
Regarding Diquafosol ophthalmic solution comprising a chelating agent at a concentration of 0.0001 to 1% (w / v), formation of insoluble precipitates found in Diquafosol ophthalmic solution during storage of the solution, as well as deterioration of the filtration performance in the course of production (course of filtration sterilization), have been inhibited. Further, in Diquafosol ophthalmic solution comprising a chelating agent, reduction of eye irritation and enhancement of the preservative effectiveness have been confirmed, in comparison to Diquafosol ophthalmic solution comprising no chelating agent. Accordingly, the present invention has been confirmed to provide physicochemical properties that are stable during the courses of production and distribution as well as the course of storage by a patient, and also reduce eye irritation and enhance preservative effectiveness.
Owner:SANTEN PHARMA CO LTD
Ophthalmic composition and preparation method and application thereof
ActiveCN110812323AImprove stabilityLess irritatingOrganic active ingredientsSenses disorderPreservative freeDrugs preparations
The invention belongs to the field of pharmaceutical preparations, and particularly relates to an ophthalmic composition. The composition comprises the following components in parts by weight: 0.5-5 parts of voriconazole and / or medicinal salt thereof, 8-35 parts of a solubilizer and 0.1-4 parts of polyvinyl alcohol. The invention also relates to a method for preparing the ophthalmic composition and application of the ophthalmic composition. The ophthalmic composition is high in stability and low in eye irritation, does not contain preservatives, and has a good prevention or treatment effect onfungal infectious diseases of eyes.
Owner:SHENYANG XINGQI PHARM CO LTD
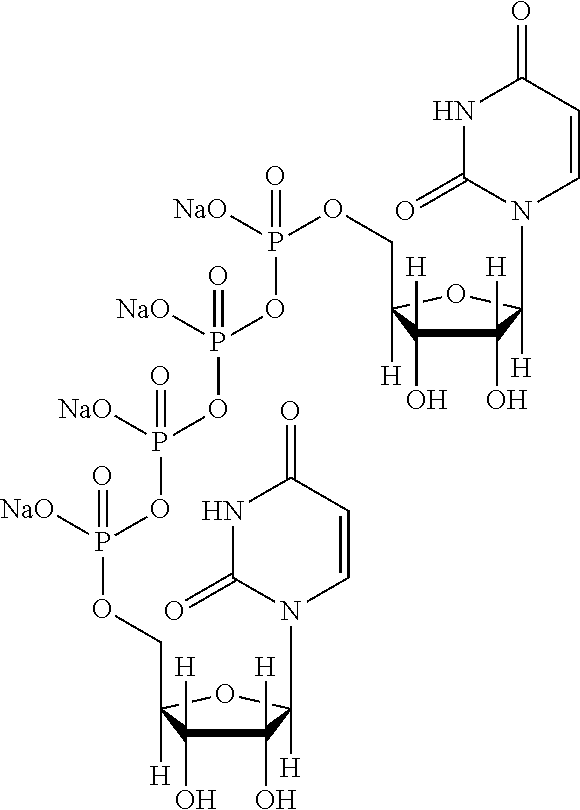
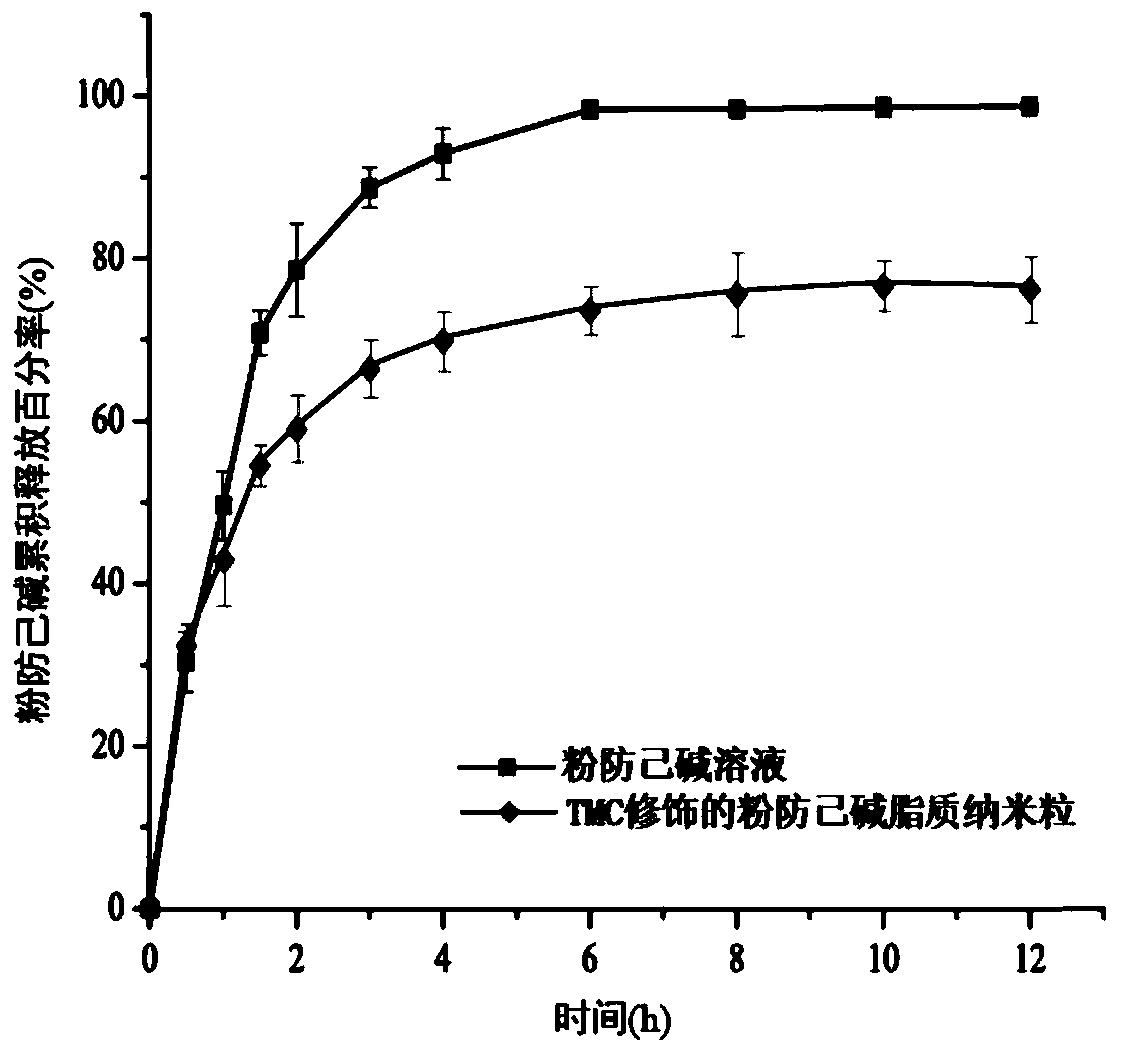
Tetrandrine lipid nanoparticle ophthalmic preparation and preparation method thereof
InactiveCN109985024AHuge membrane surface areaHigh drug loadingOrganic active ingredientsSenses disorderSolubilityHigh concentration
The invention discloses a tetrandrine lipid nanoparticle ophthalmic preparation and a preparation method thereof, wherein the preparation method comprises the following steps of: adding glycerol monooleate and tetrandrine into absolute ethyl alcohol, heating, carrying out ultrasonic dissolving and pressure-reducing rotary evaporation, and removing all the ethyl alcohol to obtain an oil phase; (2)placing a slow-release material, a penetration enhancer and a cationic material into ultrapure water, stirring, heating and dissolving to obtain a water phase; (3) adding the water phase into the oilphase, shearing, dispersing and cooling to the room temperature to obtain colostrum; and carrying out ultrasound to obtain the tetrandrine lipid nanoparticle ophthalmic preparation. The preparation has the advantages that the membrane superficial area is large, the drug loading capacity is big; the resistance time of drugs in eyes is prolonged, the bioavailability is improved, the eye irritation and the systemic toxicity caused by repeated administration and high concentration of the drugs are avoided. The cationic material can be degraded in vivo. The long-term stability of the preparation ishigh, the solubility is improved and the drug absorption is enhanced. The preparation has a microstructure similar to a biological membrane and is suitable as a carrier of a mucosal drug delivery system.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Traditional Chinese medicine eye drops for treating eyestrain and preparation method
InactiveCN104208437AGet rid of troublesSignificant effectSenses disorderPharmaceutical delivery mechanismModern medicineWater Chestnuts
The invention discloses traditional Chinese medicine eye drops for treating eyestrain and a preparation method and belongs to the field of traditional Chinese medicine. Active ingredients of the traditional Chinese medicine eye drops are composed of walnut twig, bodinier elsholtzia herb, betel nut, orchis, lotus leaf, sensitive joint vetch root, pepper, maidenhair, cassia seed, konjak, lygodium japonicum, bitter gourd, water-chestnut, gentian, litchi and chastetree fruit. The traditional Chinese medicine eye drops are proper in medicine compatibility, conform to the traditional Chinese medicine and pharmacy, and modern medicine and pharmacology theories, and play roles of removing stasis and relieving pain, reinforcing qi and nourishing blood, clearing heat and improving eyesight, and diminishing swelling and removing stasis; the traditional Chinese medicine eye drops have an obvious curative effect for eye drying, eye irritation, eye ache and swelling, blurred vision, vision diminution and the like due to eyestrain; the traditional Chinese medicine eye drops have short treatment course and quickly become effective to help an eyestrain patient out of trouble; the traditional Chinese medicine eye drops are safe and reliable, are convenient to use and do not have any toxic and side effects; people can work and live normally during the eye drops using period, and the total effective rate arrives at 97.1%.
Owner:李殿光
Cold compress eye patch for improving eye dryness, eye irritation and eye fatigue and preparation method thereof
InactiveCN109771528AImprove dry eyesImprove eyestrainSenses disorderHydroxy compound active ingredientsEye drynessBitter gourd
The invention discloses a cold compress eye patch for improving eye dryness, eye irritation and eye fatigue. The cold compress eye patch comprises active extract, menthol and hydrogel. The active extract is extract of notopterygium roots, Tripterygium wilfordii, houttuynia cordata, honeysuckles, roots of rehmannia, Radix Saposhnikoviae, bitter gourds, maguey, aloes, Coptis chinensis, sword beans,fennel, polygonum cuspidatum and eucalyptus leaves; the weight ratio of the active extract to the menthol to the hydrogel is 10:1-10:10-50. The invention further discloses a preparation method of thecold compress eye patch for improving eye dryness, eye irritation and eye fatigue. The cold compress eye patch for improving eye dryness, eye irritation and eye fatigue is used for solving the problems that existing medicine cures symptoms and does not cure diseases, is high in recurrence rate, and easily forms medicine dependence.
Owner:曲沃李时珍医药科技有限公司
Cation-modified pilocarpine hydrochloride flexible nano-liposome eye-drops preparation and preparation method thereof
ActiveCN107982219AHuge membrane surface areaImprove adhesionOrganic active ingredientsSenses disorderCholesterolBiocompatibility Testing
The invention discloses a cation-modified pilocarpine hydrochloride flexible nano-liposome eye-drops preparation and a preparation method thereof. The preparation method comprises the following steps:(1) adding lecithin and cholesterol into chloroform, and performing ultrasonic treatment to obtain suspension; and dissolving pilocarpine hydrochloride and a penetration enhancer in methanol to obtain a solution I; (2) uniformly mixing the suspension and the solution I, and removing an organic solvent to obtain a uniform lipid membrane; (3) dissolving a softener into purified water to obtain a solution II, and dissolving the lipid membrane into the solution II so as to obtain primary mixed emulsion; (4) performing ultrasonic treatment on the primary mixed emulsion, and adding into a warm bathso as to obtain a pilocarpine hydrochloride flexible nano-liposome preparation; and (5) dissolving a cationic material into the purified water to obtain a solution III, stirring the pilocarpine hydrochloride flexible nano-liposome preparation, dropping the solution III, and stirring, thereby obtaining the eye-drops preparation in the invention. The preparation disclosed by the invention has hugemembrane surface area and high adhesion, and the drug penetration is increased. The eye-drops preparation has excellent biocompatibility and low eye irritation.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Infant hair shampoo
InactiveCN106821835AProtect the skinImprove the symptoms of dryness and itchingCosmetic preparationsHair cosmeticsSodium lactateTectorial membrane
The invention relates to the field of personal care articles, in particular to infant hair shampoo which consists of the following substances in parts by weight: 15-25 parts of olive oil, 15-20 parts of a fructus kochiae extract, 10-15 parts of a loquat leaf extract, 15-25 parts of a barley extract, 10-15 parts of a barley extract liquid, 5-10 parts of protein hydrolysate, 8-12 parts of sodium lactate, 10-15 parts of a matricaria chamomilla extract, 5-8 parts of citric acid, 0.5-1 part of flavoring rose essence, 5-8 parts of pure sorbide, 8-12 parts of natural glycine betaine and 5-12 parts of plant essential oil. The infant hair shampoo provided by the invention is made from purely natural plant extracts, is free of chemical substance, does no harm to infants, is gentle and tearless in formula, is free of infant eye irritation, is faintly acid as the pH value is 6.5, can form a protection membrane to protect head skin of the infants, and is capable of remarkably reducing skin eczema, improving symptoms of dryness and itching of hair of the infants, providing hair nutrition, balancing grease and deeply cleaning scalps.
Owner:青岛宏致复合织造有限公司
Method for evaluating eye irritation by means of mouse kidney cell system
ActiveCN106834410AStable subcultureDifficult to differentiateDiagnosticsMicrobiological testing/measurementMouse KidneyFluorescein
The invention discloses a method for evaluating eye irritation by means of a mouse kidney cell system, and the method is applicable to evaluation of cosmetic raw material irritation, in particular to evaluation of eye irritation of surfactants. According to the method, when irritation of a test material to cells exists, the cells contract, fully-fused cells are damaged, and when fluorescein acts on damaged cell membranes, the damage degree of the cell membranes is indirectly reflected according to the amount of leaked fluorescein, so that irritation of the test material is represented. The test material used by the method is stable in population, reliable in source and high in fusing speed, a cell growth culturing membrane is easy to form, and a guarantee is provided for stability of a test; and when the mouse kidney cell system is used for evaluation of irritation damage of the surfactants, a result is in good correlation with a result of a rabbit eye animal test.
Owner:SOUTH CHINA INST OF COLLABORATIVE INNOVATION
Silicone prepolymer solutions
In one aspect, the invention relates to silicone prepolymer compositions comprising a silicone prepolymer and a solvent. A soluble silicone prepolymer can be provided having increased average silicon content, thereby attaining a desired oxygen permeability. A solvent can be provided with a desired balance between hydrophilicity and hydrophobicity by selection and modification of the solvent molecular structure, resulting in molded polymer films and articles that exhibit minimal or nonexistent eye irritation and exhibit highly transparent products. This abstract is intended as a scanning tool for purposes of searching in the particular art and is not intended to be limiting of the present invention.
Owner:JOHNSON & JOHNSON VISION CARE INC +1
Natamycin-loaded alginic acid gel medicine membrane and a preparation method thereof
ActiveCN112891326AReduce eye irritationLess discomfortOrganic active ingredientsSenses disorderEye irritationDrug release
The invention relates to the technical field of medical materials, in particular to a natamycin-loaded alginic acid gel medicine membrane and a preparation method thereof. The natamycin-loaded alginic acid gel medicine membrane comprises the following components and contents: every 1mL of the medicine membrane contains 0.01-0.03 g of sodium alginate, 0.0025-0.0075 g of polyoxyethylene and 0.005-0.015 g of natamycin. Alginic acid gel is used as a carrier, natamycin is loaded, and an ethanol solution containing calcium ions is used for crosslinking, so that the spatial structure of the material is more cohesive, the medicine-loaded gel is more stable after membrane formation, the size of pores in a medicine membrane is reduced, membrane release is delayed, and the action time of natamycin is prolonged; alginic acid is good in histocompatibility, and eye irritation and discomfort of natamycin are reduced; the medicine membrane can be better attached to the cornea ulcer surface, and has long medicine action time, good effects, no irritation and discomfort and high acceptability of patients; and the medicine membrane is a novel ophthalmic medicine membrane which is simple in preparation method, low in cost and wide in market prospects.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
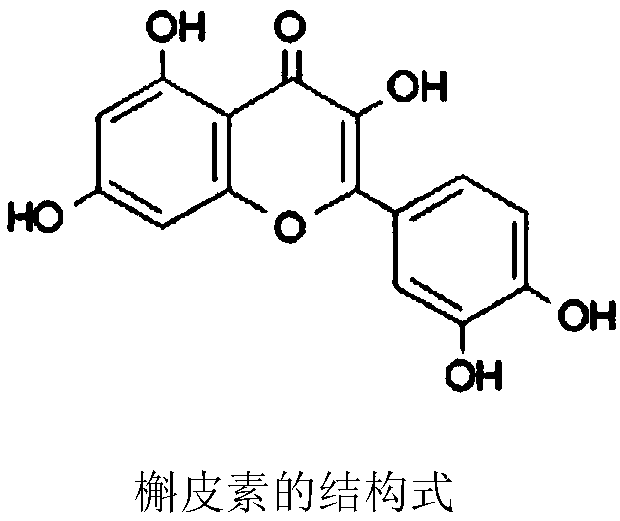
Quercetin eye drops and preparation method thereof
ActiveCN108078919AImprove corneal permeabilityAdhesive to the corneaOrganic active ingredientsSenses disorderBiocompatibility TestingEye irritation
The invention belongs to the technical field of the medicine, relates to quercetin eye drops and a preparation method thereof, and specifically relates to quercetin hydroxypropyl-beta-cyclodextrin inclusion compound eye drops. The quercetin eye drops is prepared from the following components in weight volume percent: 0.1%-0.8% of quercetin, 0.025%-0.3% of penetration enhancer, 7%-30% of hydroxypropyl-beta-cyclodextrin, 0.005%-0.02% of bacteriostatic agent, 0.6%-1.5% of isoosmotic adjusting agent, 0.001%-0.05% of pH regulator, and the balance of water for injection. The penetration enhancer isone of the thiolated chitosan, chitosan or cell-permeable peptide (R8). The penetration enhancer is combined with the inclusion compound of the hydroxypropyl-beta-cyclodextrin of the quercetin to prepare the eye drops with good effect of preventing and treating cataract; the eye drops has the advantages of being good in cornea permeability, high in bioavailability, good in biocompatibility, and small in eye irritation, and has extensive development prospect.
Owner:SHENYANG PHARMA UNIVERSITY
Natamycin-grafted oxidized alginic acid fiber membrane and preparation method thereof
ActiveCN113069556AReduce eye irritationLess discomfortOrganic active ingredientsSenses disorderFiberEye irritation
The invention relates to the technical field of medical materials, and relates to a natamycin-grafted oxidized alginic acid fiber membrane and a preparation method thereof. The preparation method comprises specific preparation process as follows: preparing oxidized alginic acid fiber: reacting sodium periodate with alginic acid fiber to obtain the oxidized alginic acid fiber; putting the oxidized alginic acid fiber into a glycerol aqueous solution to be soaked, conducting rinsing and drying, and putting the oxidized alginic acid fiber into a dry and sterile centrifugal tube to be stored; and soaking the oxidized alginic acid fiber with a natamycin aqueous solution, oscillating and incubating the oxidized alginic acid fiber at 50 DEG C in a dark place for 36 hours, then washing the oxidized alginic acid fiber twice with an ethanol aqueous solution and deionized water, and drying to obtain the oxidized alginic acid fiber membrane. The surface of the oxidized alginic acid fiber membrane is grafted with natamycin, so that the eye irritation and discomfort caused by the natamycin can be reduced, the half-life period of the medicine is prolonged, the utilization rate of the medicine is increased, and the treatment effect of the fungal keratitis is improved; and the preparation process is simple, the cost is low, and the product is easy to preserve and has wide market prospects.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Personal care compositions having reduced eye irritation
The present invention is directed to a stable, minimal energy required self-assembling lamellar and spherulitic composition comprising: mixture water, fatty alcohol, fatty acid, salt of fatty acid, polyglyceryl fatty acid ester and oils. The present invention relates to composition that can benefit eye mildness, even distribution of sunscreen physical filters on skin and enhanced stability.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Treatment method for improving swallowing function by using sensory irritation of sacculus pharyngeal cavity
The invention discloses a treatment method for improving a swallowing function by using the sensory irritation of the sacculus pharyngeal cavity, and relates to the technical field of medical treatment. The method comprises seven steps, adopts the theory of a Rood technology, is a new technology derived from catheter balloon dilatation, and performs cool and hot temperature stimulation and pressure stimulation on the throat wall by using sacculi; swallowing start in a swallowing period can be effectively promoted by combining the conventional treatment for promoting swallowing start and a newtechnology, thereby reducing leakage or missing suction which is caused by start delay of a patient; various sensory irritation methods such as temperature stimulation and pressure stimulation can promote the repair of a sensory feedback path, continuously correct the sensory irritation, strengthen the reorganization of the brain function of the patient, reflexively cause the motor response, and be conductive to restoring the feeling and movement function.
Owner:福建中医药大学附属人民医院(福建省人民医院)
Aerosol type air deodorant and preparation method thereof
InactiveCN111821838AEfficient captureSimple processGas treatmentDispersed particle separationBiotechnologySkin Injury
The invention provides an aerosol type air deodorant and a preparation method thereof. The deodorant is prepared from the following components in parts by weight: 0.1 to 5.0 parts of a wetting agent,0.01 to 1 part of a long-acting bactericide, 1.0 to 5.0 parts of a deodorant, 0.01 to 1.0 part of a preservative, 0.05 to 3.0 parts of a corrosion inhibitor, 60.0 to 70.0 parts of deionized water and30.0 to 50.0 parts of a propellant. The air deodorant has the following beneficial effects that 1, the aerosol type air deodorant can effectively capture and adsorb odor molecules and neutralize and decompose the odor molecules into odorless and harmless substances; 2, the aerosol type air deodorant is simple in process and convenient to use, the product is safe and non-toxic, and after the aerosol type air deodorant is used, spots, skin injury, eye irritation and the like are avoided; and 3, the aerosol type air deodorant can quickly remove indoor air peculiar smell, such as smoke smell, musty smell, kitchen peculiar smell, toilet peculiar smell and formaldehyde peculiar smell, and eliminate bacteria and the like. The invention also discloses a preparation method of the aerosol type air deodorant.
Owner:广东阜和实业有限公司
Cattera modified coat hydrochloric acid -based nano -lipid -oriented eye preparation and preparation method
ActiveCN107982219BHuge membrane surface areaImprove adhesionOrganic active ingredientsSenses disorderOphthalmologyPILOCARPINE HCL
The invention discloses a cation-modified pilocarpine hydrochloride flexible nano-liposome eye-drops preparation and a preparation method thereof. The preparation method comprises the following steps:(1) adding lecithin and cholesterol into chloroform, and performing ultrasonic treatment to obtain suspension; and dissolving pilocarpine hydrochloride and a penetration enhancer in methanol to obtain a solution I; (2) uniformly mixing the suspension and the solution I, and removing an organic solvent to obtain a uniform lipid membrane; (3) dissolving a softener into purified water to obtain a solution II, and dissolving the lipid membrane into the solution II so as to obtain primary mixed emulsion; (4) performing ultrasonic treatment on the primary mixed emulsion, and adding into a warm bathso as to obtain a pilocarpine hydrochloride flexible nano-liposome preparation; and (5) dissolving a cationic material into the purified water to obtain a solution III, stirring the pilocarpine hydrochloride flexible nano-liposome preparation, dropping the solution III, and stirring, thereby obtaining the eye-drops preparation in the invention. The preparation disclosed by the invention has hugemembrane surface area and high adhesion, and the drug penetration is increased. The eye-drops preparation has excellent biocompatibility and low eye irritation.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
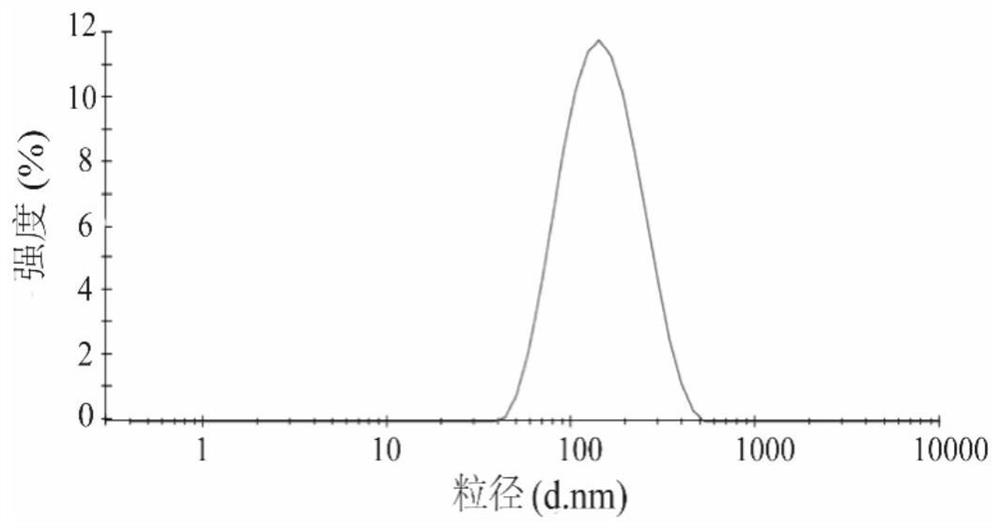
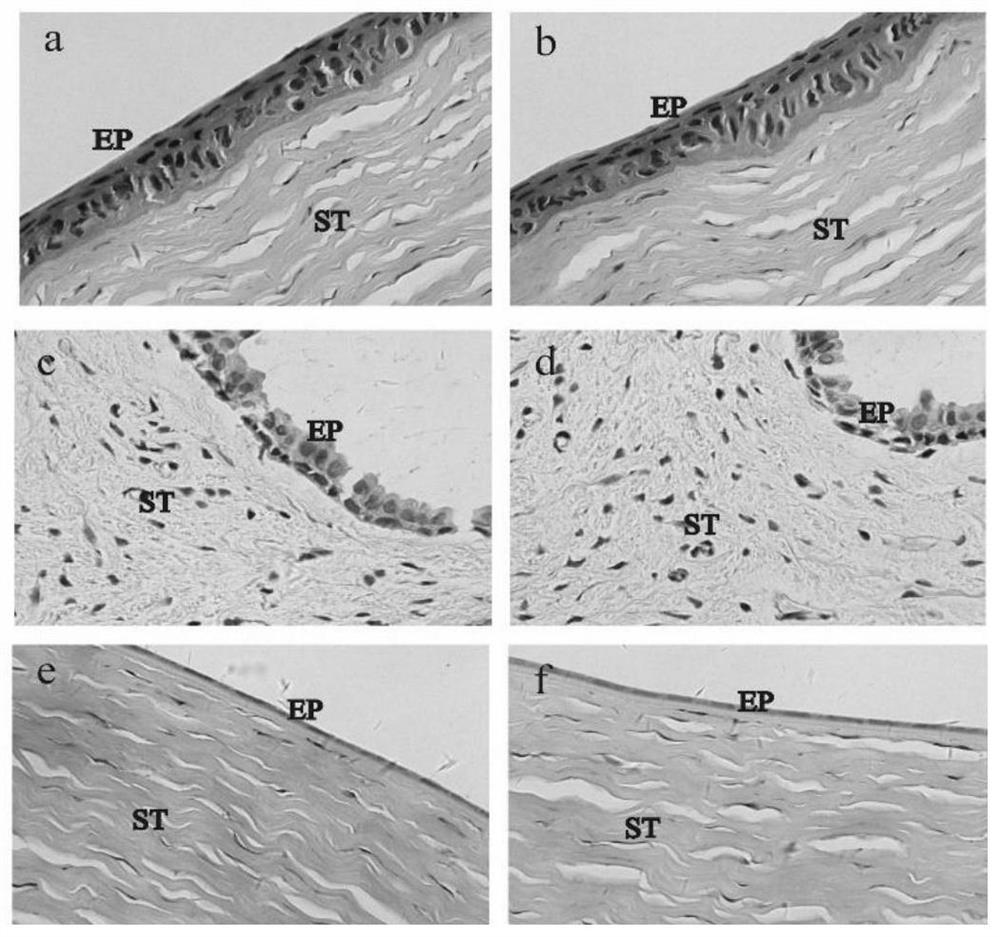
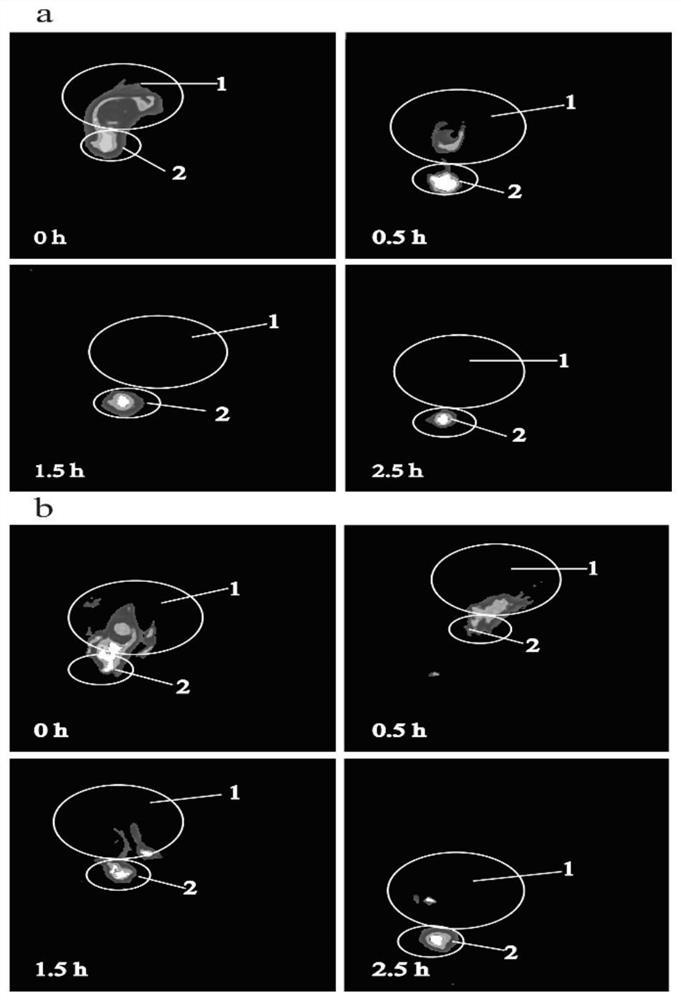
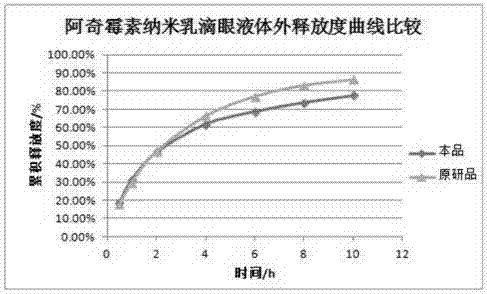
Preparation method for azithromycin micro-emulsion eye drops
InactiveCN107412246AAchieve slow releaseImprove bioavailabilityAntibacterial agentsOrganic active ingredientsOrganic acidAzithromycin
The invention discloses a preparation method for azithromycin micro-emulsion eye drops. The preparation method comprises the following steps: under the condition of 40-50 DEG C of the temperature, slowly dropwise adding an oil phase using midchain oil as a matrix into a water phase in which azithromycin, an organic acid and buffer salt thereof are dissolved, adding an emulgator, and uniformly stirring to obtain the azithromycin micro-emulsion eye drops. The preparation method is capable of, under the heating condition, using the midchain oil as the oil phase matrix which is dropwise added into water solution in which the azithromycin and the organic acid and the buffer salt thereof are dissolved, embedding the acidized azithromycin dissolved in the water, and remarkably improving the bioavailability of the eye drops. In addition, O / W microemulsion is formed by a self-emulsifying method under the situation without adding an organic solvent, the permeability of cell membranes is greatly increased, the storage stability of the eye drops is improved, the slow release of the azithromycin in the eyes is realized, the untoward effect of the larger eye irritation by drugs is relieved, the eye drops has the good biocompatibility, the drug loss is reduced, the vision is not affected, the administration frequency is reduced, and the patient adaptability is improved.
Owner:沈小玲
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com