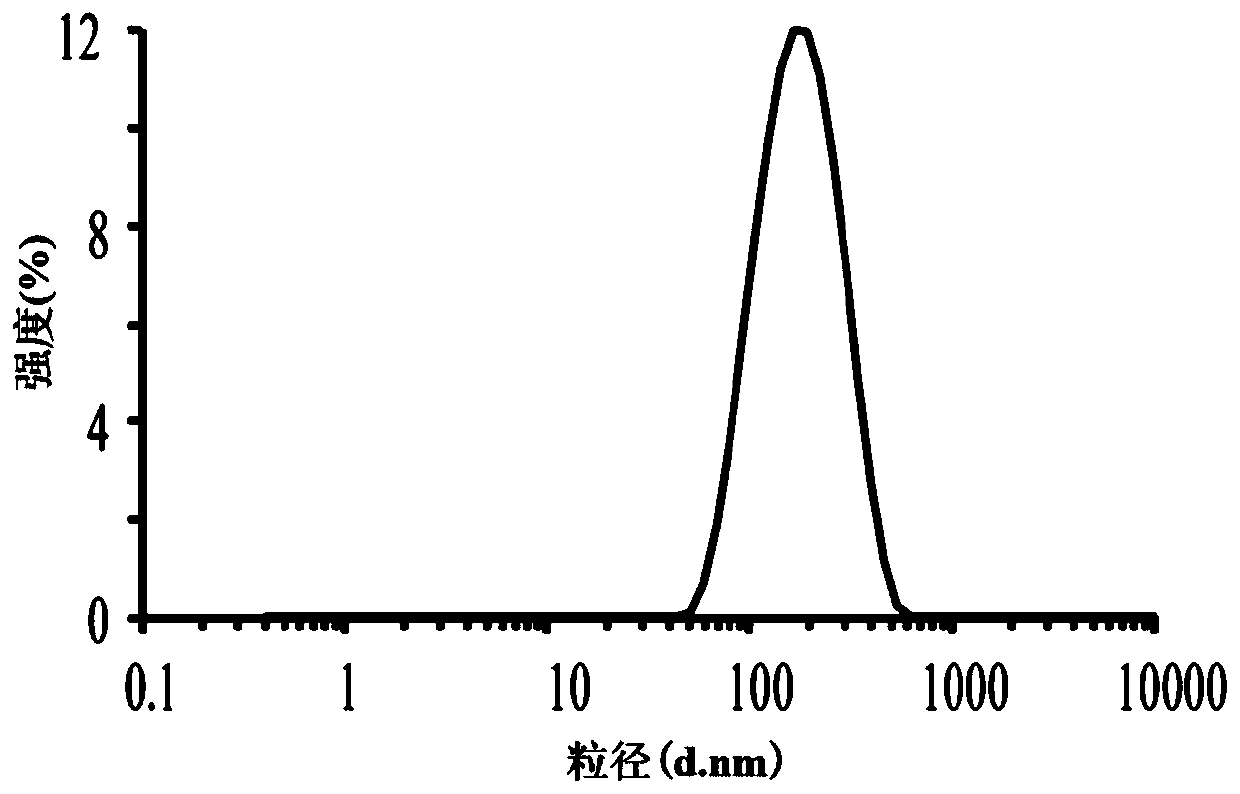
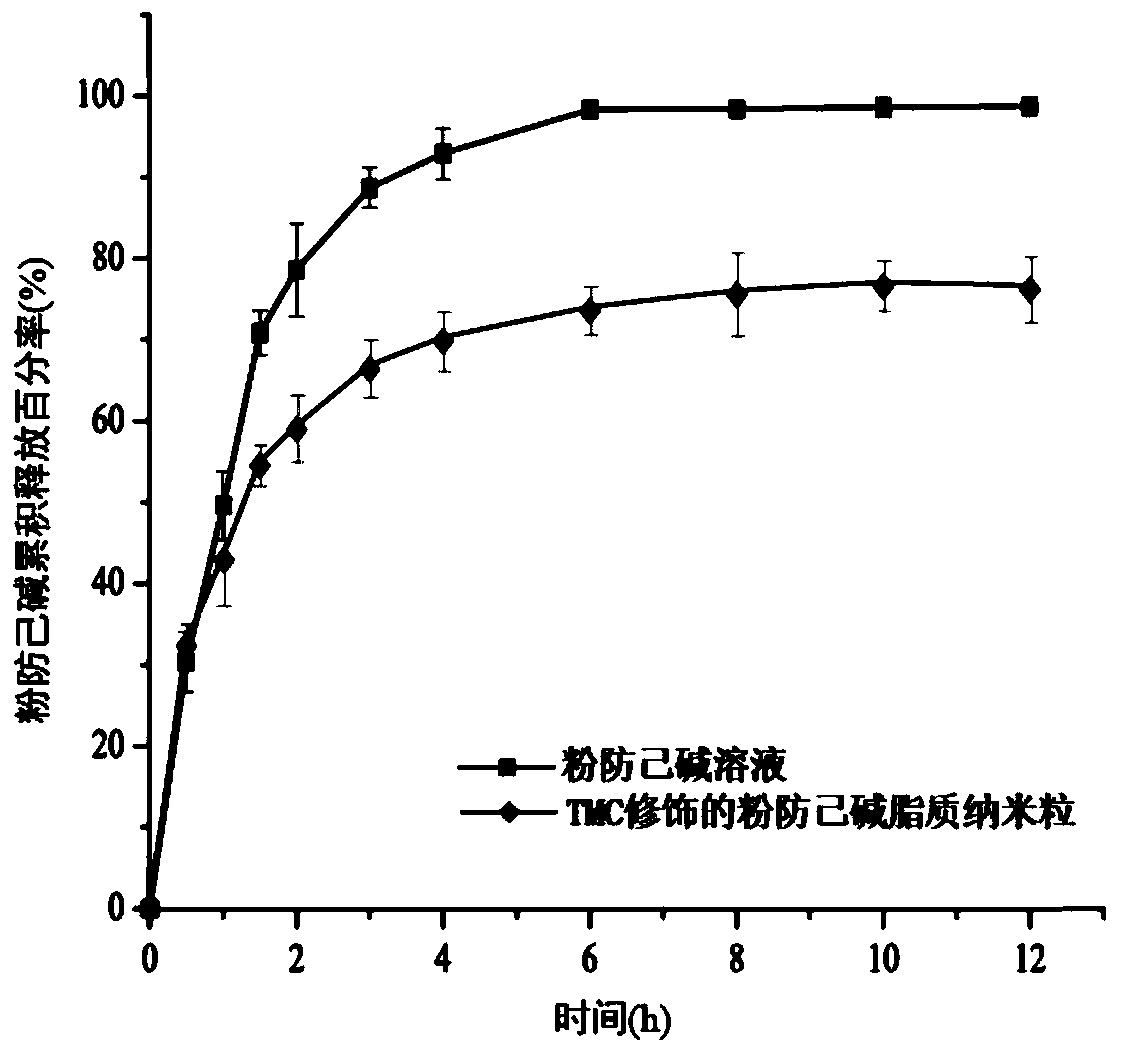
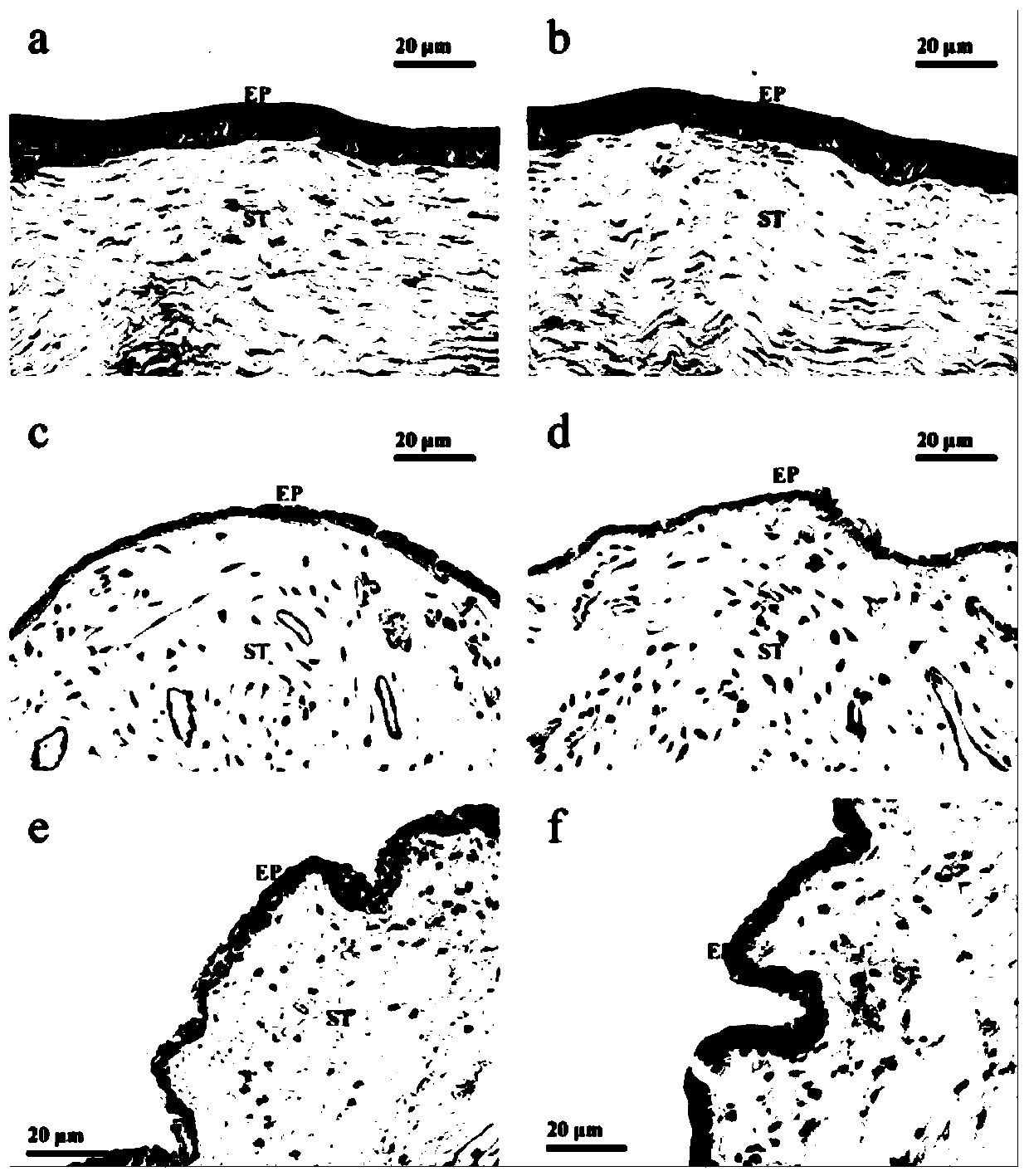
Tetrandrine lipid nanoparticle ophthalmic preparation and preparation method thereof
A technology of lipid nanoparticles and tetrandrine, which is applied in the field of medicine, can solve problems such as difficulty in exerting biological activity of drugs, limited distribution of intraocular tissues, and short residence time in the cornea, so as to improve bioavailability, promote drug absorption, prolong The effect of residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation method of tetrandrine lipid nanoparticle ophthalmic preparation includes the following steps:
[0041] (1) Take 350mg monooleic acid glyceride, 10mg tetrandrine, add an appropriate amount of absolute ethanol, heat to 75°C, dissolve it ultrasonically at a power of 190W, rotate under reduced pressure at 45°C, and remove the ethanol to obtain the oil phase ;
[0042] (2) Take another 42 mg poloxamer 407, 0.46 mg Gelucire 44 / 14 and 60 mg N-trimethyl chitosan with a viscosity average molecular weight of 400,000 and a quaternization degree ≥ 60%, and place them in ultrapure water and stir. , Raise the temperature to 65℃ to completely dissolve, and then get the water phase;
[0043] (3) Add the water phase to the oil phase, shear and disperse at 10000 rpm for 2 minutes, and cool to room temperature to obtain colostrum; ultrasonic wave for 5 seconds at a power of 150 W and ultrasonic wave for 5 minutes at an interval of 5 seconds to obtain tetrandrine lipid nano Opht...
Embodiment 2
[0046] A preparation method of tetrandrine lipid nanoparticle ophthalmic preparation includes the following steps:
[0047] (1) Take 350 mg of glyceryl monooleate and 5 mg of tetrandrine, add an appropriate amount of absolute ethanol, heat to 65°C, dissolve it ultrasonically at a power of 150W, rotate under reduced pressure at 45°C, and remove all ethanol to obtain an oil phase;
[0048] (2) Take another 20mg poloxamer 407, 0.04mg Labrasol and 40mg carboxymethyl chitosan with a viscosity average molecular weight of 350,000 and a degree of substitution ≥90%, place them in ultrapure water, stir, and heat to 75℃ to complete Dissolve, get the water phase;
[0049] (3) Add the water phase to the oil phase, shear and disperse at 10000rpm for 2.5min, cool to room temperature to obtain colostrum; under the condition of 190W, sonicate for 5s with 5s interval for 10min to obtain tetrandrine Lipid nanoparticle ophthalmic preparation.
Embodiment 3
[0051] A preparation method of tetrandrine lipid nanoparticle ophthalmic preparation includes the following steps:
[0052] (1) Take 350mg of glyceryl monooleate and 20mg of tetrandrine, add an appropriate amount of absolute ethanol, heat to 85°C, dissolve it ultrasonically at a power of 250W, rotate under reduced pressure at 45°C, and remove the ethanol to obtain the oil phase;
[0053] (2) Take another 50 mg poloxamer 407, 1 mg Plurol Oleique CC497 and 80 mg hydroxypropyl chitosan with a viscosity average molecular weight of 370,000 and a degree of substitution ≥80%, put them in ultrapure water, stir, and heat to 85°C To completely dissolve, get the water phase;
[0054] (3) Add the water phase to the oil phase, shear and disperse at 10000rpm for 3min, and cool to room temperature to obtain colostrum; under the condition of 250W, ultrasonic wave for 5s, 5s interval, 15min for tetrandrine Nanoparticle ophthalmic preparations.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com