Tetrandrine liquid crystal nanoparticle ophthalmic preparation and preparation method thereof
A technology for tetrandrine and ophthalmic preparations, applied in the field of medicine, can solve problems such as difficulty in achieving long-term effect, large loss of drug dose, systemic toxicity, etc., and achieve high compliance, high long-term stability and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
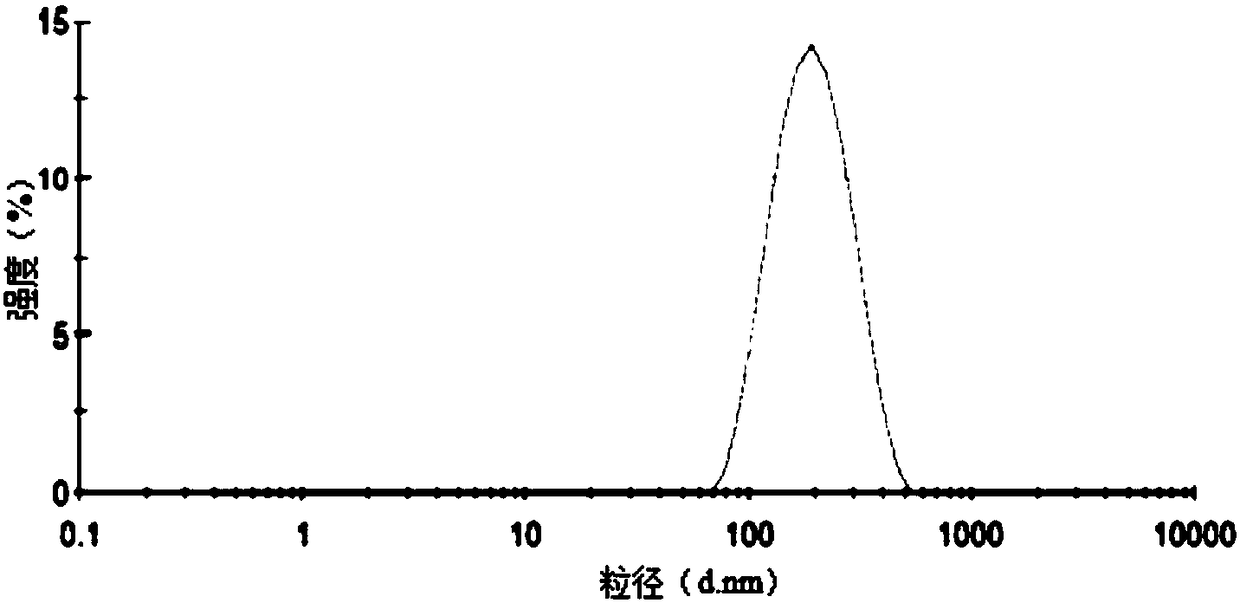
Image
Examples
Embodiment 1
[0032] A preparation method for tetrandrine liquid crystal nanoparticle ophthalmic preparation, comprising the steps of:
[0033] (1) Take 30.8mg glycerol monooleate, 3.4mg Pluronic F127, 1mg Tetrandrine, add 1mL absolute ethanol, heat to 75°C, dissolve under stirring, rotary steam under reduced pressure, remove all ethanol, and obtain a uniform oil phase;
[0034] (2) Add Gelucire 44 / 14 and 3 mg of octadecyl carboxymethyl chitosan quaternary ammonium salt to 3 mL (3 g) of distilled water and stir until completely dissolved to obtain an aqueous phase; the amount of Gelucire 44 / 14 is monooleic acid Glycerides, Pluronic F127, tetrandrine, octadecyl carboxymethyl chitosan quaternary ammonium salt and 0.2% of the total weight of distilled water;
[0035] (3) Add the water phase to the oil phase, at 10000r·min -1 Shear and disperse for 3 minutes, cool to room temperature to obtain colostrum;
[0036] (4) Ultrasound the colostrum, the ultrasonic power is 200w, the ultrasonic wave...
Embodiment 2
[0039] A preparation method for tetrandrine liquid crystal nanoparticle ophthalmic preparation, comprising the steps of:
[0040] (1) Take 15mg glycerol monolinoleate, 15mg diglycerol oleate, 3.3mg pluronic F127, 1mg tetrandrine, add 0.67mL absolute ethanol, heat to 75°C, dissolve under stirring, spin under reduced pressure Steam to remove ethanol to obtain a homogeneous oil phase;
[0041] (2) Add Labrasol and 3mg carboxymethyl chitosan into 3mL (3g) distilled water and stir until completely dissolved to obtain the water phase; the amount of Labrasol added is glycerol monolinoleate, diglyceride oleate, pluronic F127, 0.1% of the total weight of tetrandrine, carboxymethyl chitosan and distilled water;
[0042] (3) Add the water phase to the oil phase, at 5000r·min -1 Shear and disperse for 3 minutes, cool to room temperature to obtain colostrum;
[0043] (4) Ultrasound the colostrum, the ultrasonic power is 200w, the ultrasonic wave is 5s, the interval is 5s, and the ultras...
Embodiment 3
[0045] A preparation method for tetrandrine liquid crystal nanoparticle ophthalmic preparation, comprising the steps of:
[0046] (1) Take 10mg of phosphatidylethanolamine, 20mg of dilinoleoylphosphatidylethanolamine, 12mg of 1-palmitoyl-2-oleoylphosphatidylcholine, 5mg of pluronic F127, 1mg of tetrandrine and add 1.66mL of absolute ethanol, Heat to 60°C, dissolve under stirring, rotary steam under reduced pressure, remove all ethanol, and obtain a homogeneous oil phase;
[0047] (2) Add Solutol HS 15 and 5 mg carboxymethyl chitosan to 5 mL (5 g) of distilled water and stir until completely dissolved to obtain an aqueous phase; the amount of Solutol HS 15 added is phosphatidylethanolamine, dilinoleoylphosphatidylethanolamine, 0.05% by total weight of 1-palmitoyl-2-oleoylphosphatidylcholine, Pluronic F127, tetrandrine, carboxymethyl chitosan and distilled water;
[0048] (3) Add the water phase to the oil phase, at 8000r·min -1 Shear and disperse for 3 minutes, cool to room t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com