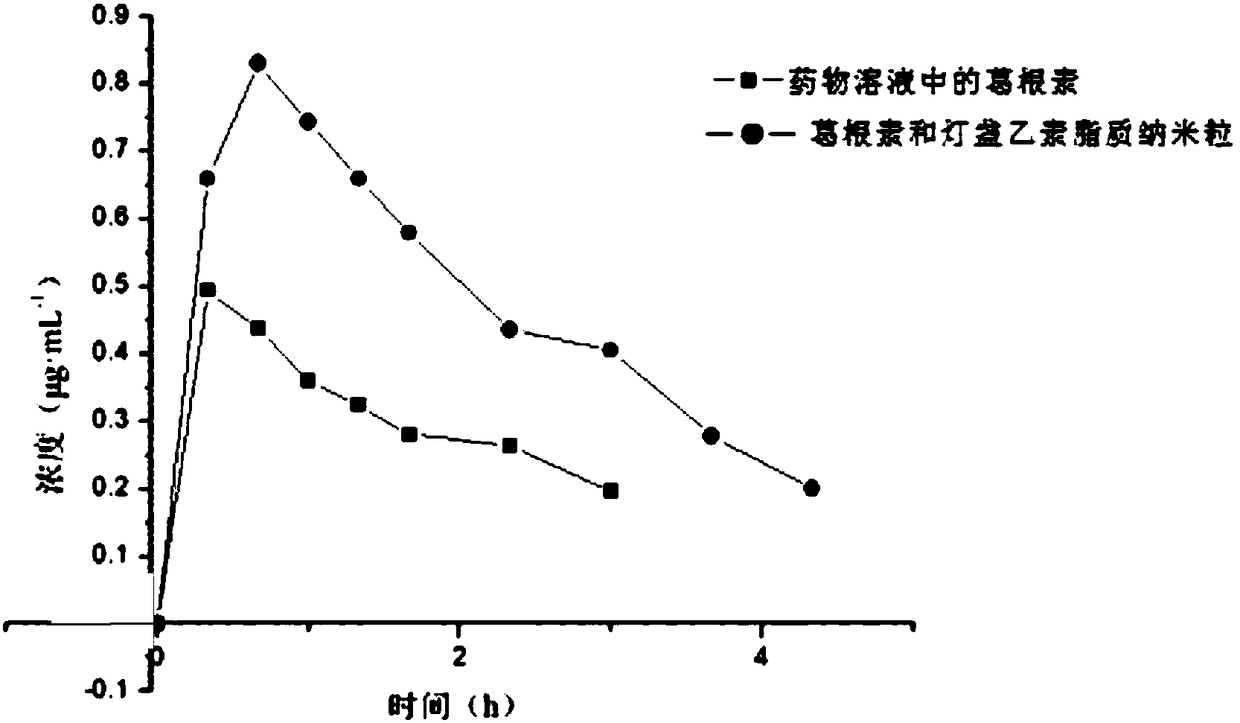
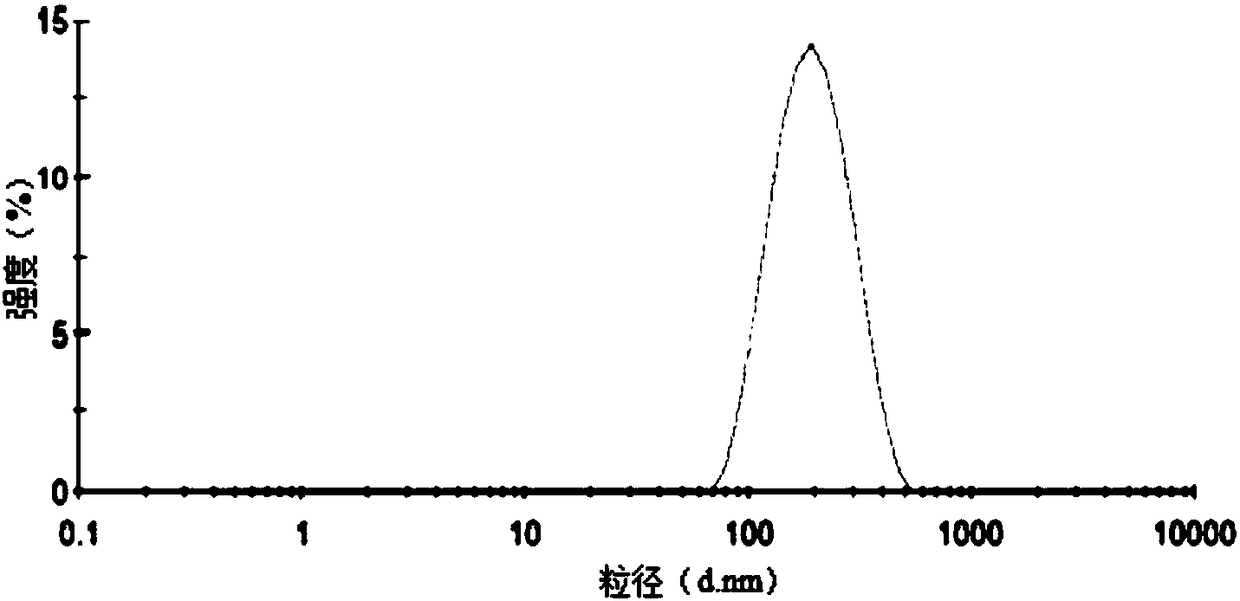
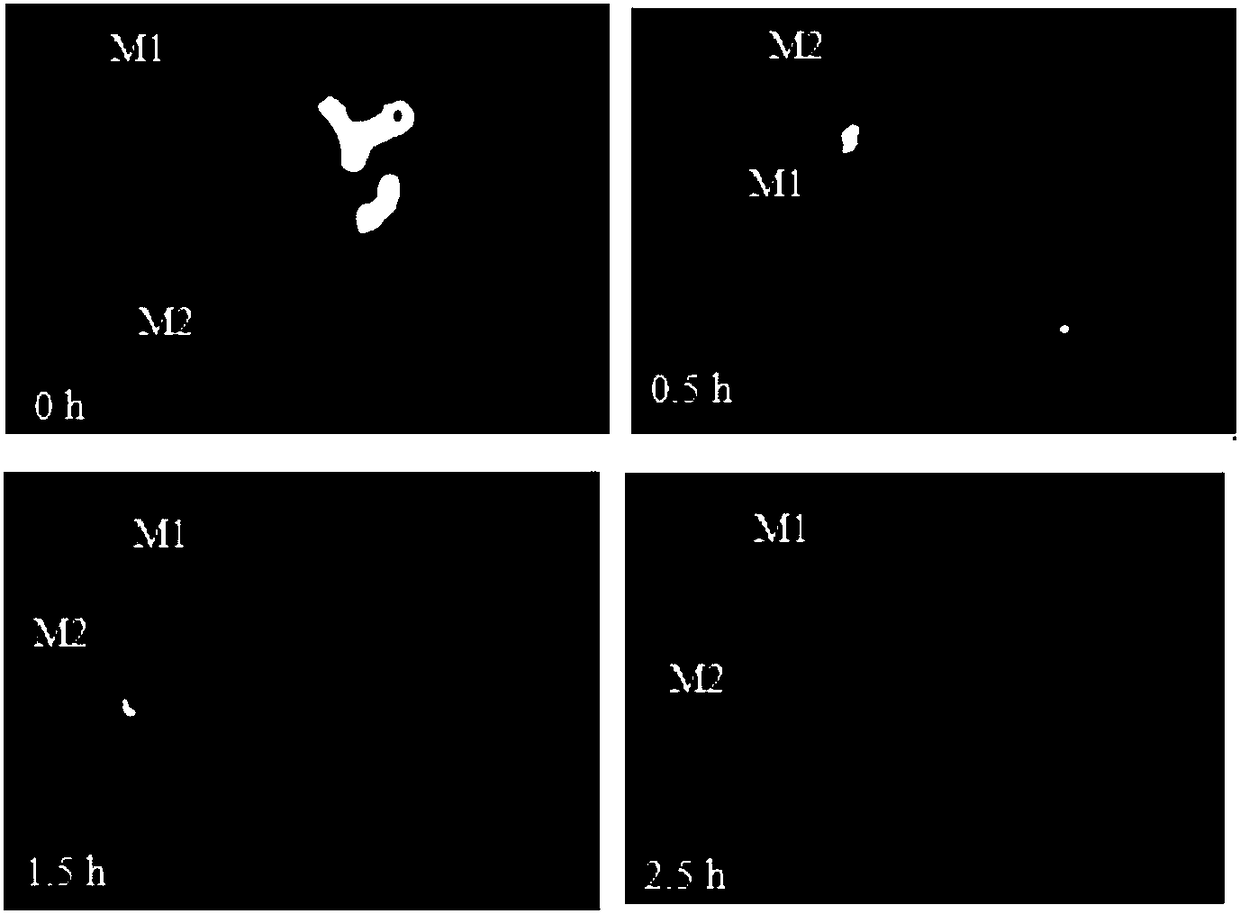
Puerarin and scutellarin lipid nanoparticle ophthalmic preparation and preparation method thereof
A technology of lipid nanoparticles and scutellarin, applied in the field of medicine, can solve problems such as difficulty in achieving long-term effects, large drug dose loss, and poor visual acuity, and achieve improved compliance, high long-term stability, and drug-loading capacity strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation method of puerarin and scutellarin lipid nanoparticle ophthalmic preparation comprises the following steps:
[0038] (1) Take 32.0 mg of glycerol monooleate, 1 mg of scutellarin, add 1 mL of absolute ethanol as a solvent, heat to 60°C, and dissolve under stirring to obtain a uniform first oil phase; distilled water is the first water phase;
[0039] (2) Take another 1mg of lecithin and 1mg of cholesterol, add 1mL of methanol-ethanol mixture with a volume ratio of 1:1 for ultrasonic dissolution, and add 1mL of Tween 80 to obtain a uniform second oil phase; then add 1mg of puerarin, 4.44mg Pluronic F127, 0.5mg Gelucire 44 / 14 and 20mg octadecyl carboxymethyl chitosan quaternary ammonium salt were added to 2mL phosphate buffer with pH 6.8 and stirred until completely dissolved to obtain the second aqueous phase;
[0040] (3) Add the first oil phase to the first water phase, stir at 400 rpm for 2 hours to obtain the first primary emulsion; then add the second...
Embodiment 2
[0045] The preparation method of puerarin and scutellarin lipid nanoparticle ophthalmic preparation comprises the following steps:
[0046] (1) Take 15mg glycerol monolinoleate, 15mg diglycerol oleate, 1mg scutellarin, add 0.67mL solvent absolute ethanol, heat to 50°C, and dissolve under stirring to obtain a uniform first oil phase; distilled water is first aqueous phase;
[0047] (2) Take another 1mg of lecithin and 1mg of cholesterol, add 1mL of methanol-ethanol mixture with a volume ratio of 1:1 for ultrasonic dissolution, and add 1mL of glycerin to obtain a uniform second oil phase; then add 1mg of puerarin, 4.44mg of Pulang Nick F127, 0.3mg Labrasol and 20mg hydroxypropyl chitosan were added to 2mL pH 7.0 phosphate buffer and stirred until completely dissolved to obtain the second aqueous phase;
[0048] (3) Add the first oil phase to the first water phase, stir at 200 rpm for 1 hour to obtain the first primary emulsion; then add the second oil phase to the second water ...
Embodiment 3
[0052] The preparation method of puerarin and scutellarin lipid nanoparticle ophthalmic preparation comprises the following steps:
[0053](1) Take 10mg of phosphatidylethanolamine, 20mg of dilinoleoylphosphatidylethanolamine, 12mg of 1-palmitoyl-2-oleoylphosphatidylcholine, 1mg of scutellarin, add 1.66mL of solvent absolute ethanol, heat to 60°C, Dissolve under stirring to obtain a uniform first oil phase; distilled water is the first water phase;
[0054] (2) Take another 1mg of lecithin and 1mg of cholesterol, add 1mL of methanol-ethanol mixture with a volume ratio of 1:1 for ultrasonic dissolution, and add 1mL of polyethylene glycol 400 to obtain a uniform second oil phase; then add 1mg of puerarin, 12mg of Pluronic F127, 0.2mg of Solutol HS 15 and 20mg of carboxymethyl chitosan were added to the phosphate buffer with a pH of 6.0 and stirred until completely dissolved to obtain the second aqueous phase;
[0055] (3) Add the first oil phase to the first water phase, stir f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com