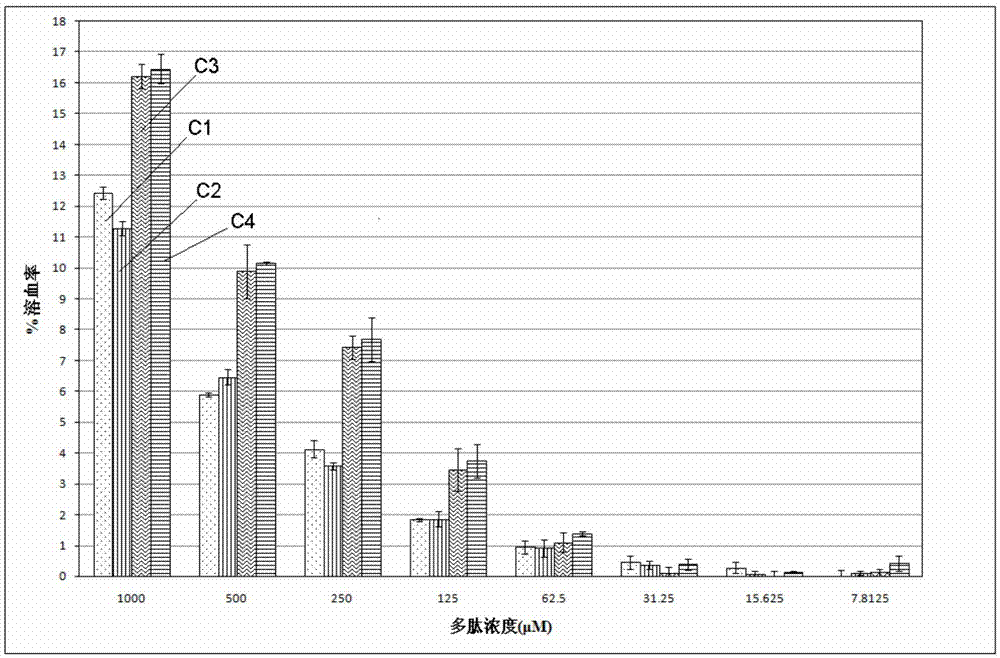
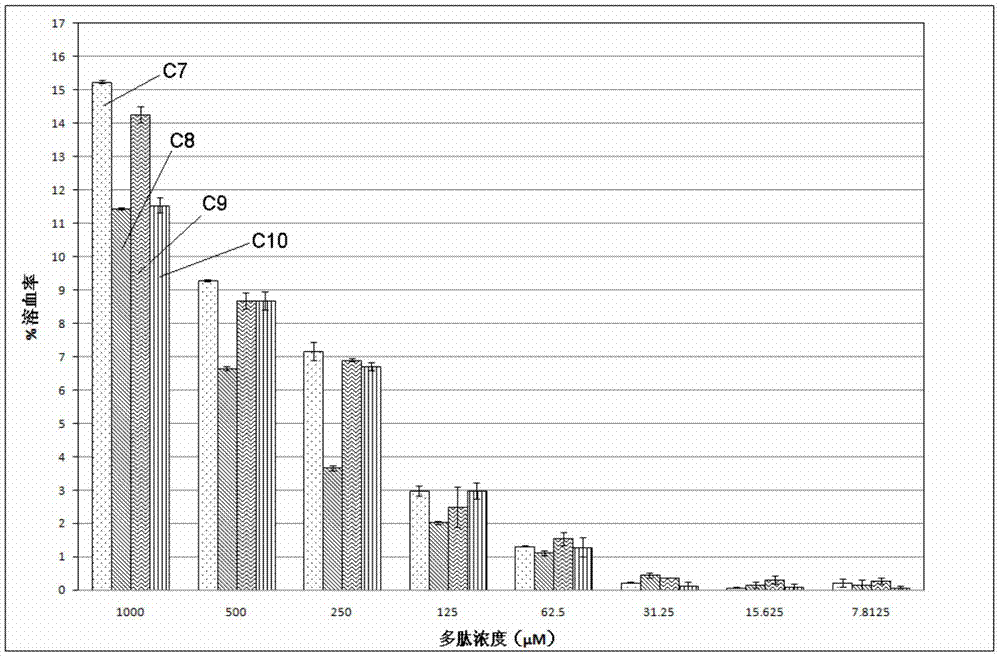
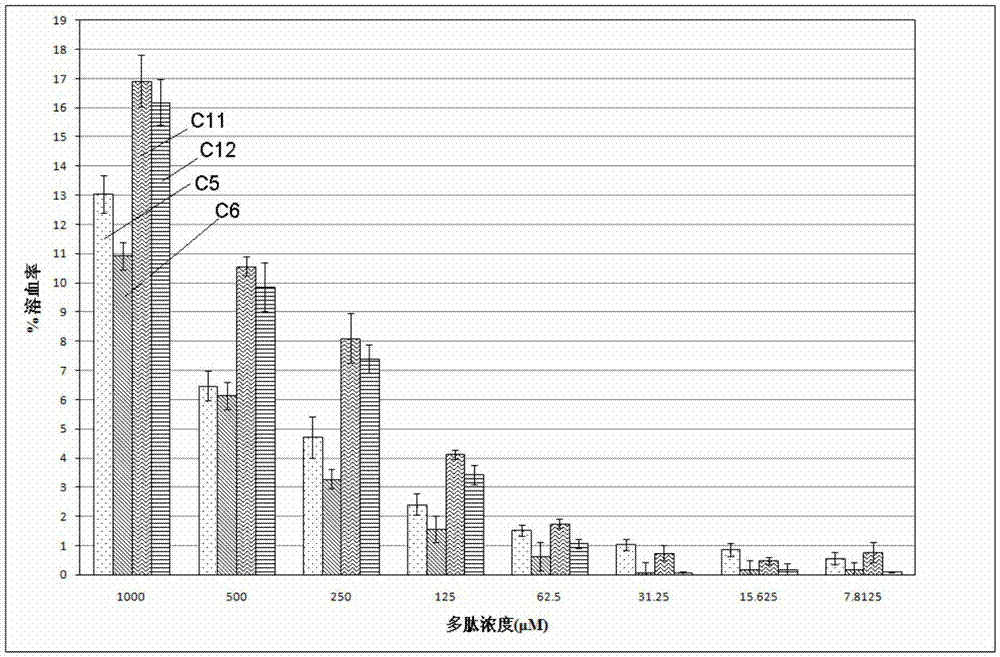
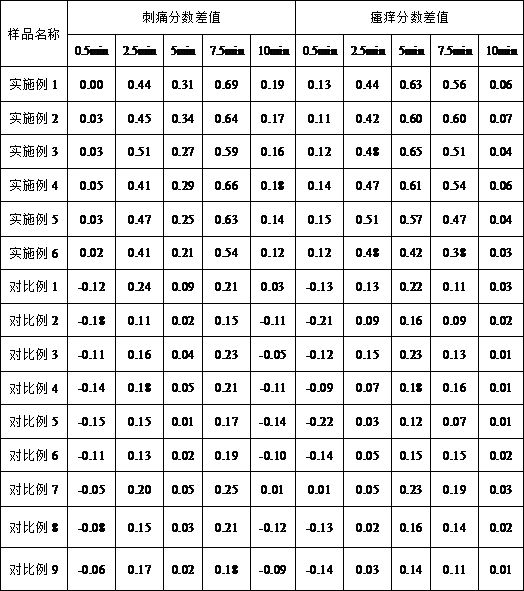
Patents
Literature
87 results about "Erythrocyte hemolysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Erythrocyte fragility refers to the propensity of erythrocytes (red blood cells, RBC) to hemolyse (rupture) under stress. It can be thought of as the degree or proportion of hemolysis that occurs when a sample of red blood cells are subjected to stress (typically physical stress, and most commonly osmotic and/or...
Cryopreservation of human red blood cells
InactiveUS20060127375A1Lower Level RequirementsAvoid hemolysisBiocideDead animal preservationFreeze thawingPhosphorylation
A red blood cell storage composition includes a composition of red blood cells and biochemistry altering reagents, the biochemistry altering reagents being present at a concentration so as to reduce the percent hemolysis of the red blood cells during the freeze-thaw cycle below that of the percent hemolysis of the red blood cells in the absence the biochemistry altering reagents. The red blood cell storage composition preferably includes reagents selected from: modifiers of glycolytic / metabolic components, modifiers of antioxidant potential, effectors of intracellular ionic distribution, modifiers of membrane fluidity, modifiers of cytoskeletal structure, effectors of the cyclooxygenase second messenger pathway, effectors of the lipoxygenase second messenger pathway, effectors of the hexose monophosphate second messenger pathway, effectors of the phosphorylation second messenger pathway, modifiers of specific messenger molecules, and combinations thereof.
Owner:LIFECELL
Soft PVC plastic in use for apparatus transfusions
A soft polyvinyl chloride plastics for blood transfusion device, such as blood bag, is proportionally prepared from PVC resin, phthalate, trimellitate, citrate, epoxy soybean oil, composite Ca-P stabilizer and silicon oil. Its advantages are no erythrocatalysis, high stability of trimellitate, and low toxin.
Owner:威海威高创新有限公司
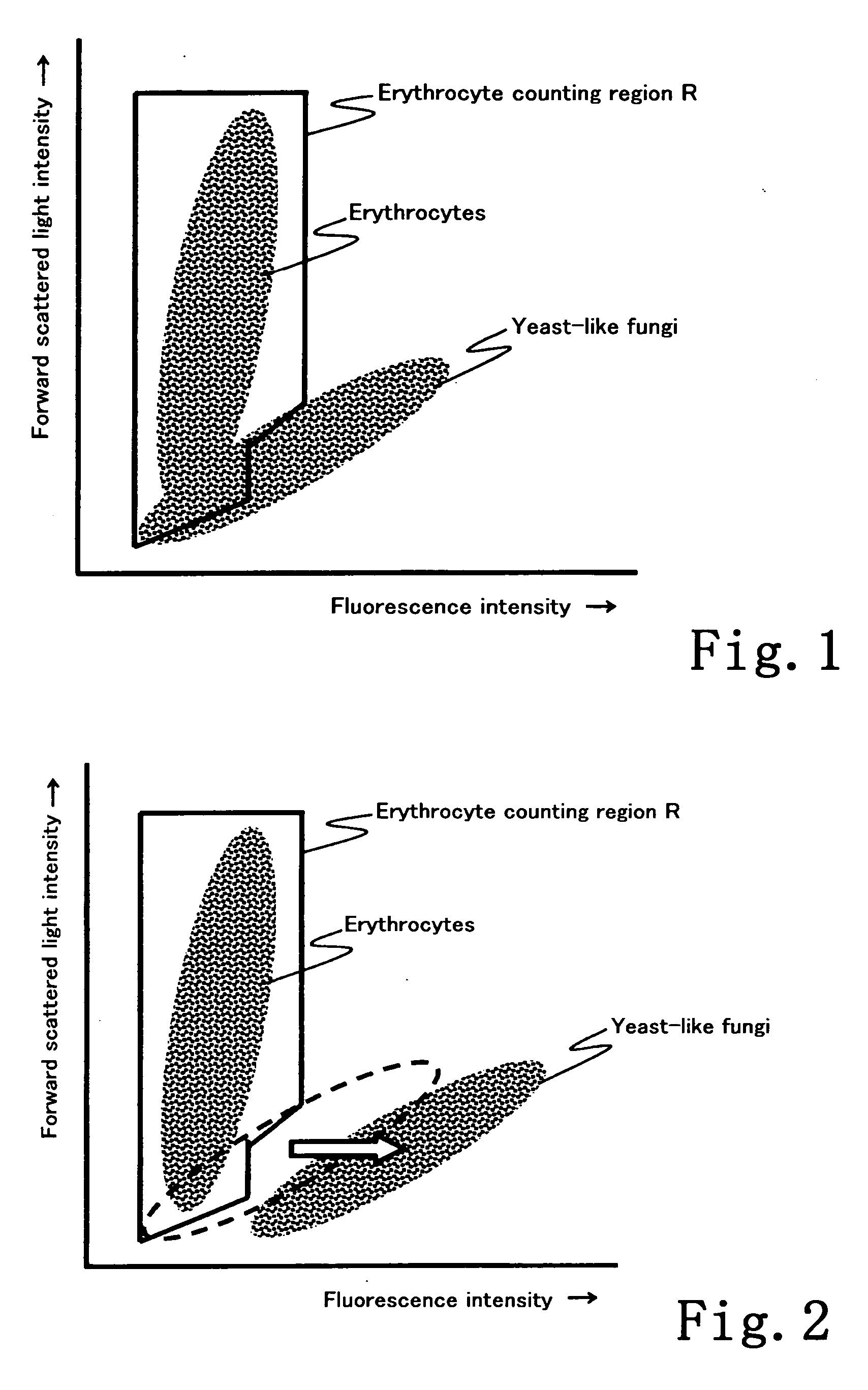
Method, apparatus, reagent kit and reagent for distinguishing erythrocytes in a biological speciment
ActiveUS20060073601A1Good precisionEffective distinctionMicrobiological testing/measurementChemiluminescene/bioluminescenceFungal microorganismsFluorescence
A method for distinguishing erythrocytes in a biological specimen, comprising the steps of preparing a sample liquid by performing to give a damage to a cell membrane of yeast-like fungi without hemolyzing erythrocytes in a biological specimen and to stain the yeast-like fungi with a fluorescent dye; detecting a first information and a second information from a particle in the sample liquid, wherein the first information reflects a size of the particle and the second information reflects a degree of fluorescent staining of the particle; and distinguishing the erythrocytes from the yeast-like fungi based on the first information and second information detected, is disclosed. An apparatus, a reagent kit and a reagent for carrying out the method are also disclosed.
Owner:SYSMEX CORP
Pretreating liquid and freeze protecting liquid for cryopreservation of erythrocyte and their application
InactiveCN1857312AAchieve deep cryopreservationAchieve protectionMammal material medical ingredientsPharmaceutical non-active ingredientsErythrocyte membraneRed blood cell
The present invention discloses cryopreservation method of erythrocyte and its erythrocyte pretreating liquid and erythrocyte freeze protecting liquid. The method includes first hatching erythrocyte in erythrocyte pretreating liquid, then protecting erythrocyte in erythrocyte freeze protecting liquid and finally freezing erythrocyte. The erythrocyte pretreating liquid is basic buffering liquid with small molecular sugar component added, and the erythrocyte freeze protecting liquid is macro molecular protecting liquid with small molecular sugar and albumin component added. The present invention protects the inner erythrocyte membrane and outer erythrocyte membrane to raise erythrocyte recovering rate and lower the hemolysis rate of erythrocyte after thawing.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Cichorium endivia L. extract and application thereof
ActiveCN101869299AImprove immunityEasy extractionCosmetic preparationsToilet preparationsDPPHFood additive
The invention relates to extraction of a natural plant, in particular to a cichorium endivia L. extract. The cichorium endivia L. is formed by ethanol extraction, extraction and purification of petroleum ether and ethyl acetate, concentration and drying, and a substance with strong anti-oxidation activity is finally obtained. The invention belongs to the field of natural product extraction. Tests of ORAC oxidation resistance, DPPH free radical removing capability and folin-phenol reduction capability show that the anti-oxidation activity of the extract is stronger than that of vitamin C, and the extract can effectively inhibit red cell hemolysis at the same time. A method for preparing a strong anti-oxidation active substance by adopting the cichorium endivia L. as a raw material has simple process and equipment and low investment, is suitable for large-scale production, and is applicable in the industries of medicaments, food additives, health-care products, cosmetics and the like.
Owner:BEIJING GUIQIANJIN MEDICAL TECH
Antioxidation active researching method for positioning chitoglycansulfate
InactiveCN1740789AWith anticoagulationAntithromboticColor/spectral properties measurementsTesting medicinal preparationsSuperoxide radicalStudy methods
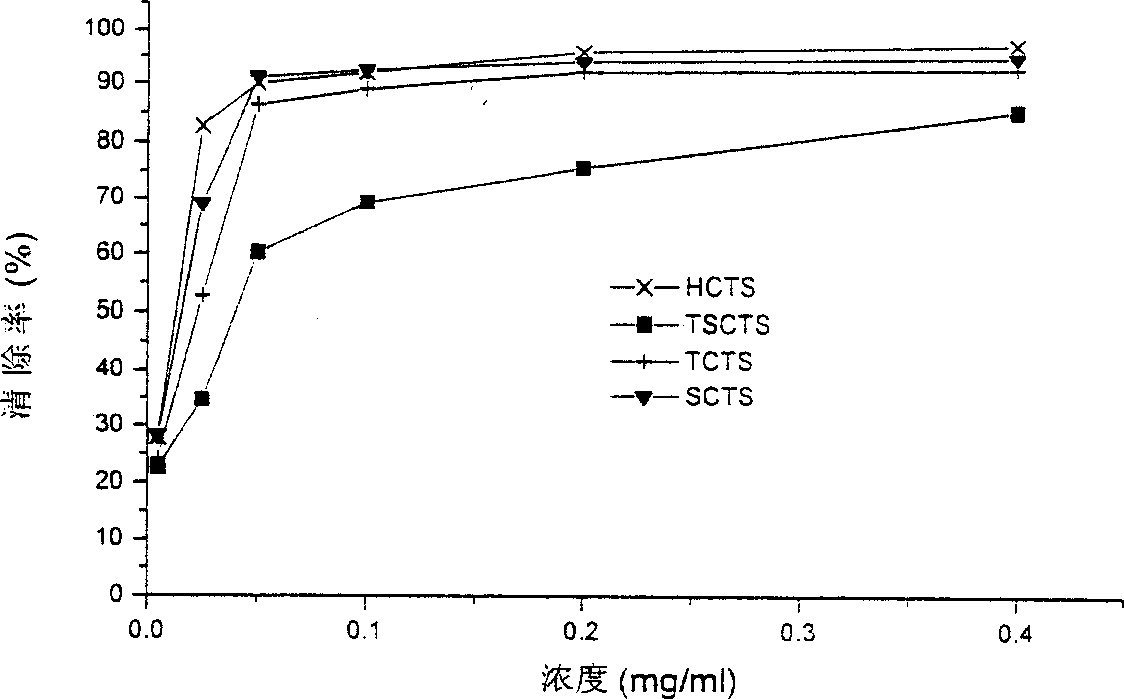
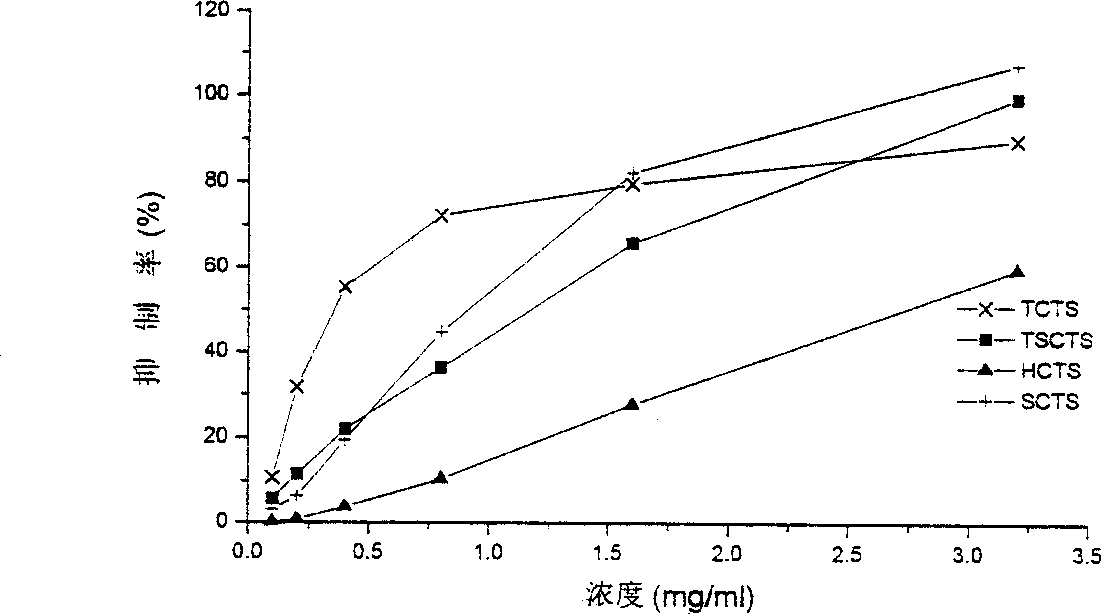
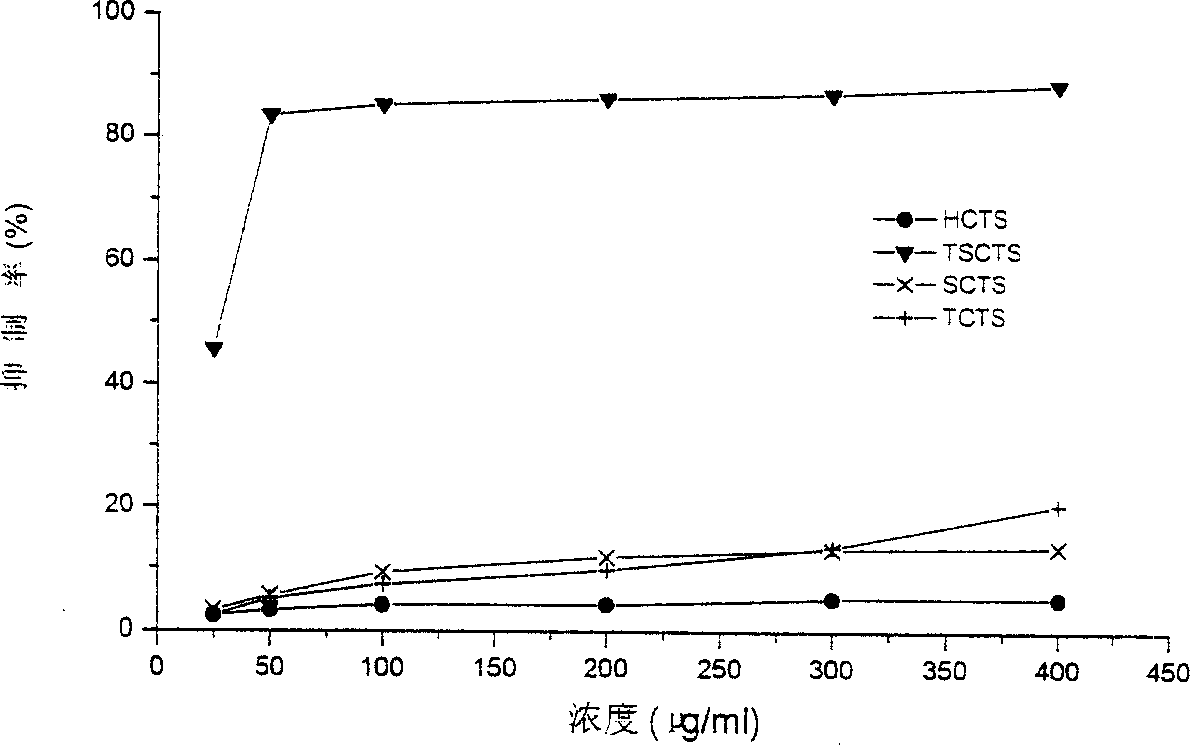
The present invention discloses a method for researching oxidation-resisting activity of localized chitosan sulfate. Said invention researches the oxidation-resisting activity of chitosan sulfates (HCTS,TSCTS,SCTS and TCTS) substituted in different position points, and finds that said four kinds of sulfate derivatives have the obvious action for inhibiting superoxide radical and hydroxyl radical, and have strong reducing power. Said four kinds of derivatives also have the strong action for inhibiting hemolysis induced by H2O2. Said invention can be used as basis for developing medicine for resisting senility.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Method for classifying/counting leukocytes, reagent kit for classifying leukocytes, and reagent for classifying leukocytes
ActiveCN103460041AMicrobiological testing/measurementScattering properties measurementsOrganic acidFluorescence
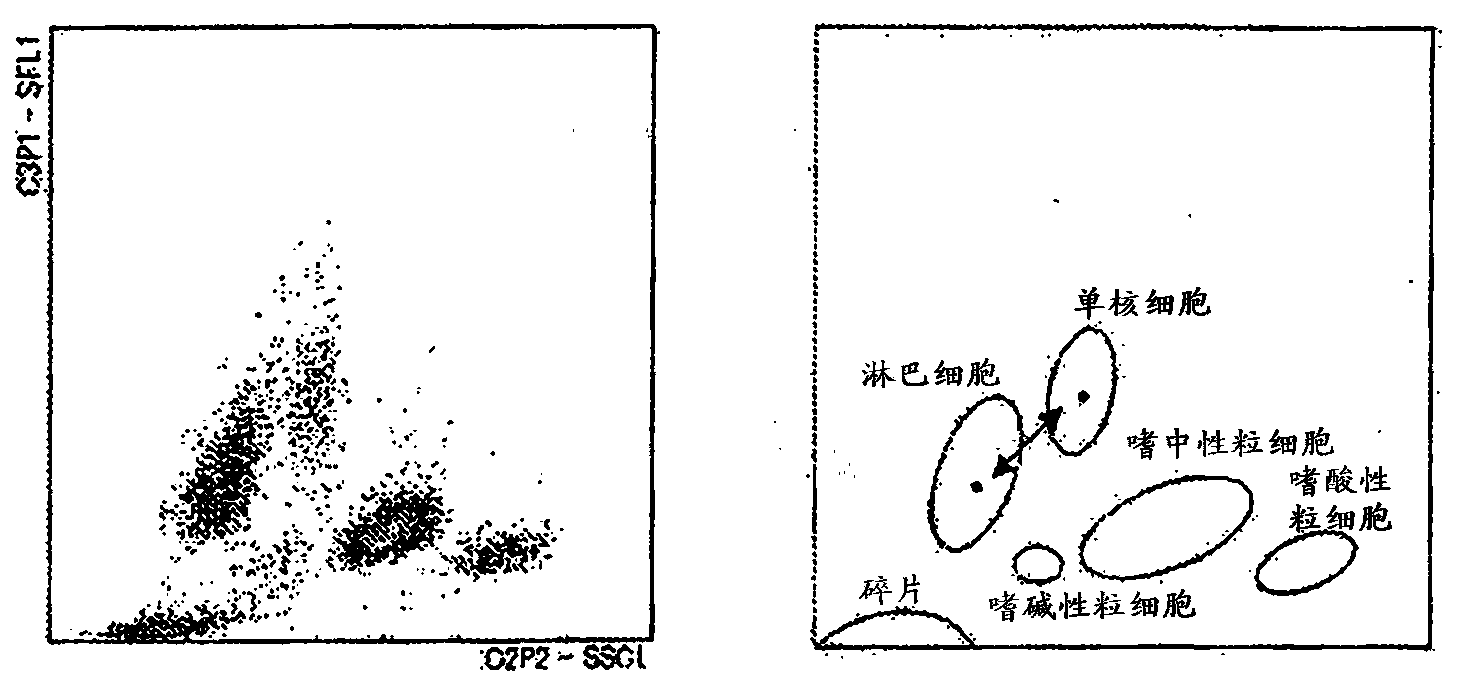
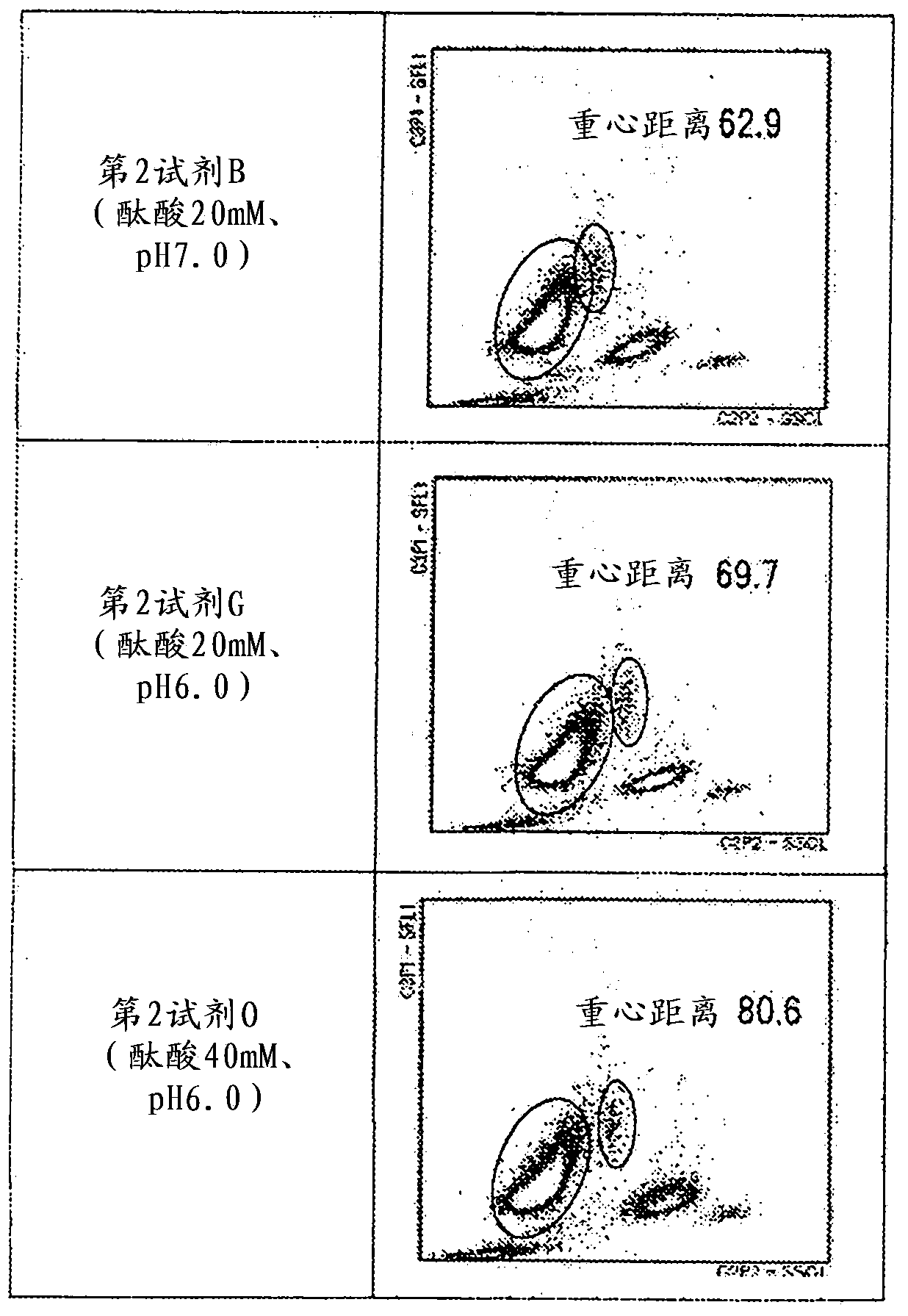
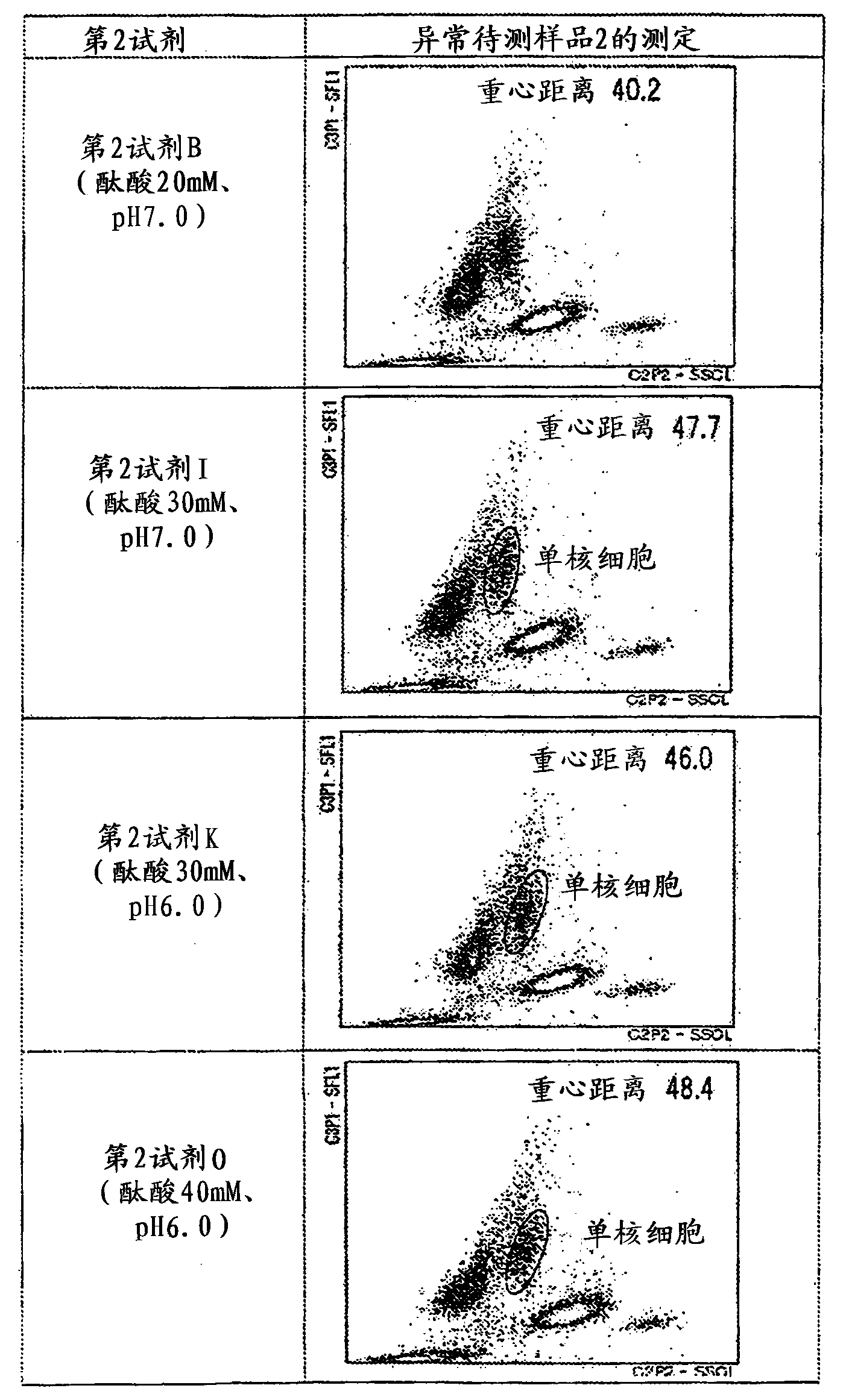
Provided is a method for classifying / counting leukocytes, which can classify / count normal leukocytes and enables the distinction between blasts and atypical lymphocytes. The method for classifying / counting leukocytes comprises: a step of mixing a biological sample, a first reagent and a second reagent together to prepare a measurement sample, wherein the first reagent can stain a nucleic acid and the second reagent can hemolyze erythrocytes and can damage cell membranes of leukocytes to the extent that a fluorescent dye can go through the cell membranes and comprises a cationic surfactant, a nonionic surfactant and an aromatic organic acid at a concentration of 20 to 50 mM inclusive; a step of irradiating the measurement sample with light to obtain information on scattered light and information on fluorescence; and a step of classifying the leukocytes in the biological sample on the basis of the information on scattered light and the information on fluorescence obtained, and detecting blasts and atypical lymphocytes separately. In the method, the pH value of the second reagent is 5.5 to 6.4 inclusive when the concentration of the aromatic organic acid in the second reagent is 20 mM or more and less than 30 mM, and the pH value is 5.5 to 7.0 inclusive when the concentration is 30 to 50 mM inclusive.
Owner:SYSMEX CORP
Anti-irritation composition and application thereof
ActiveCN113018224AAntagonistic stimulationLess irritatingCosmetic preparationsHair cosmeticsIrritationActive agent
The invention relates to an anti-irritation composition and an application thereof. The anti-irritation composition comprises a peach gum extract and a cactus extract. The anti-irritation composition disclosed by the invention has a remarkable effect of antagonizing irritation of a surfactant. The efficacy evaluation experiments prove that the anti-irritation composition has an obvious inhibiting effect on erythrocyte hemolysis caused by the surfactant, the irritation to chick chorioallantoic membrane caused by the surfactant is obviously reduced, skin texture damage caused by the surfactant can be effectively improved, the skin lipid arrangement disorder caused by the surfactant is obviously improved, and the adverse reaction of different surfactants on the human skin is obviously reduced.
Owner:BEIJING ACAD OF TCM BEAUTY SUPPLEMENTS
Preparation method of red blood cell membrane-coated gelatin-loaded berberine hydrochloride gold nanoparticles and application thereof
ActiveCN108113977AHigh encapsulation efficiencyHigh drug loadingAntibacterial agentsOrganic active ingredientsErythrocyte membraneHemangiectasis
The invention belongs to the technical field of a pharmaceutical, and particularly relates to an red blood cell membrane-coated gelatin-loaded berberine hydrochloride gold nanoparticles, as well as apreparation method and application thereof. The RBCM-BH-GNPs prepared through the method is uniform in particle size distribution and favorable in stability, and shows a remarkable shell-core structure; a single-layer erythrocyte membrane coats spherical BH-GNPs. The RBCM-BH-GNPs cannot cause erythrocytes hemolysis or agglutination in vitro, and can be used for intravenous injection. In addition,the RBCM-BH-GNPs prepared through the invention has a remarkable in vitro sustained release effect on BH in vitro and the sustained release effect is superior to the BH-GNPs, the RBCM-BH-GNPs solves the problems of hemangiectasis, fall of blood pressure, heart inhibition and side reaction caused by excessive plasma drug peak concentration since BH common injection is short of slow release, and provides an idea for expanding clinical application of the BH and developing new traditional Chinese medicine preparations of the BH.
Owner:SHANGHAI JIAO TONG UNIV
Erythrocyte preservation fluid and preservation apparatus
InactiveCN109566602AReduce agglutinationLong storage timeDead animal preservationRed blood cellD-Glucose
The invention relates to an erythrocyte preservation fluid. The erythrocyte preservation fluid comprises the following components: 0.2-0.6mg / ml adenine, 20-50mg / ml glucose, 5-15mg / ml sodium citrate, 0.02-0.06mg / ml sodium dihydrogen phosphate, 1.0-3.0mg / ml anticoagulation agents, and 5-20mg / ml film protective agents, wherein the anticoagulation agents are selected from one or more of EDTA-3K and EDDHA salts. By the aid of the erythrocyte preservation fluid, the coagulability of erythrocytes in the preservation process can be reduced, erythrocyte preservation time can be prolonged, and erythrocyte hemolysis can be reduced.
Owner:NINGBO AJCORE BIOSCIENCES INC
Peripheral blood lymphocyte micronucleus detection kit and detection method thereof
InactiveCN106525699AEnsure objectivityImprove detection efficiencyPreparing sample for investigationIndividual particle analysisFluorescenceBiology
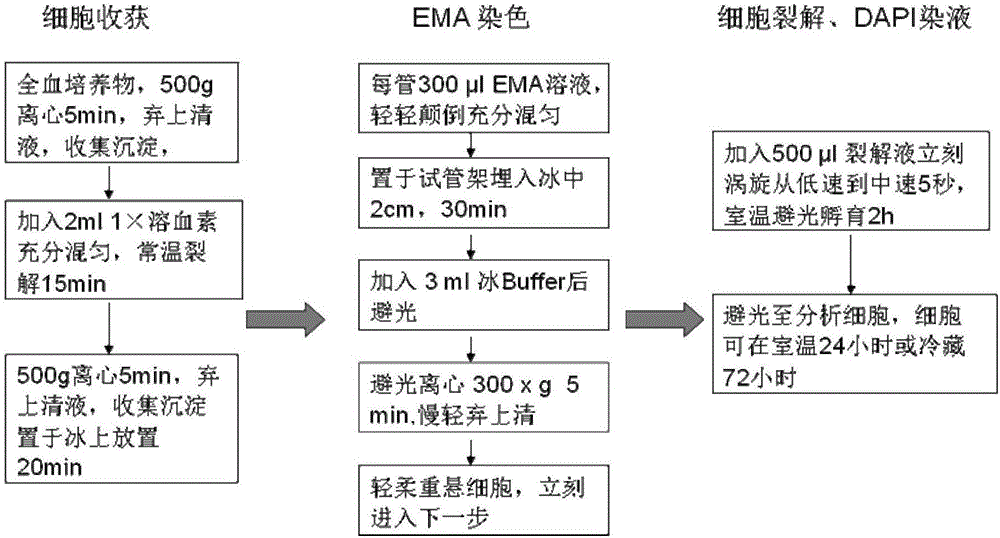
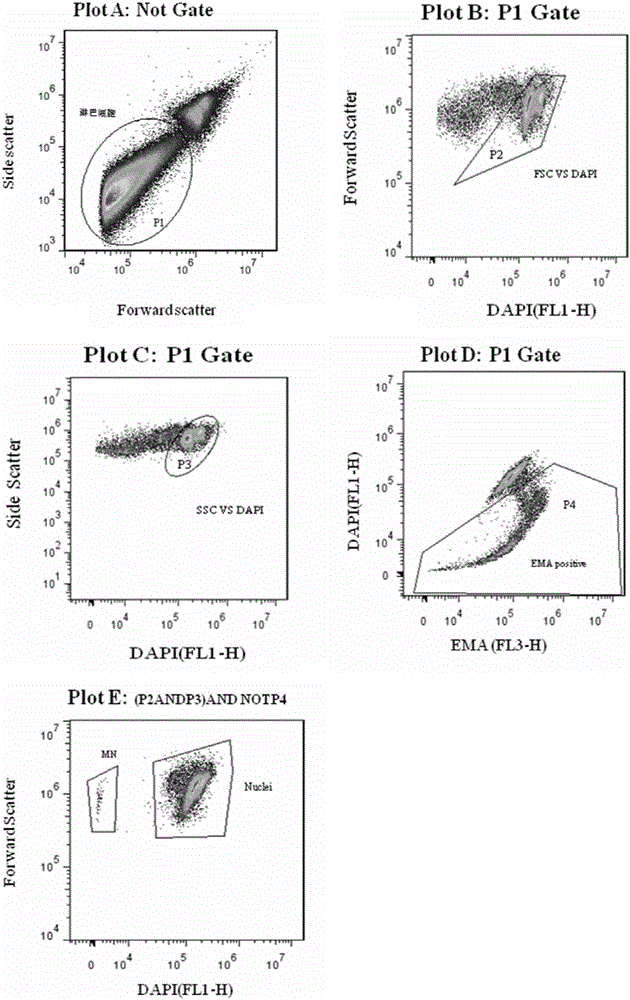
The invention discloses a peripheral blood lymphocyte micronucleus detection kit and a detection method thereof. The detection kit provided by the invention comprises erythrocyte hemolysin, a red fluorescent dye solution, a lysis solution and a positive standard product, wherein the lysis solution comprises sodium chloride, sodium citrate, Triton X-114, green fluorescent dye and RNase; and a peripheral blood lymphocyte micronucleus detection method based on the kit and a flow cytometry detection method is provided. An experiment proves that the peripheral blood lymphocyte micronucleus detection kit and the peripheral blood lymphocyte micronucleus detection method are quick and accurate; on the premise of guaranteeing objective and true experimental results, the detection cost is reduced, and the experiment has repeatability and can be widely applied to clinical diagnosis and monitoring of occupational exposure genetic damage of healthy people, rehabilitation detection of genetic damage of cancer patient and cellular genetic science research.
Owner:SHENZHEN PREVENTION & TREATMENT CENT FOR OCCUPATIONAL DISEASES
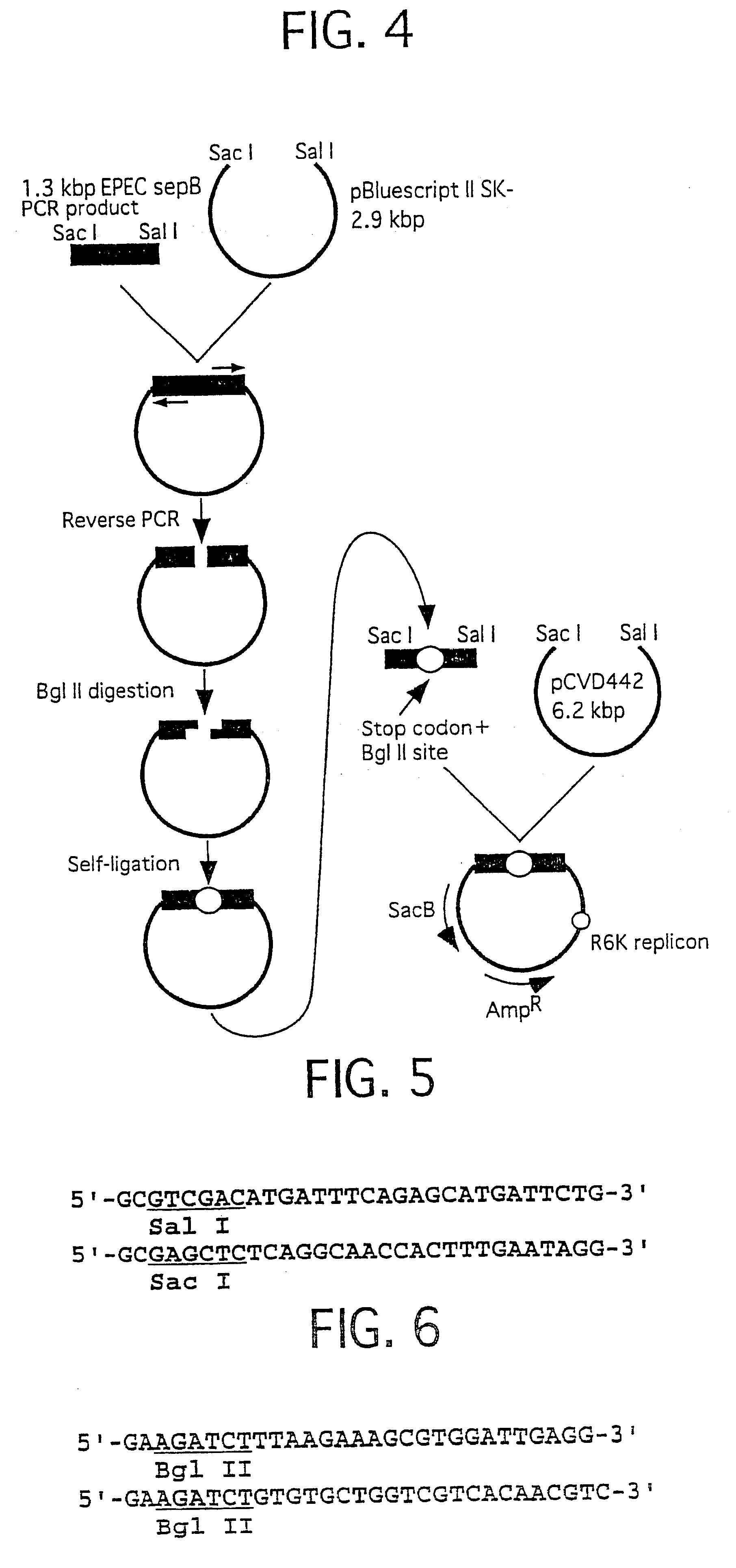
Method for detecting substances inhibiting the bacterial type III secretion mechanism and function of secretory proteins thereof
InactiveUS6586200B2Microbiological testing/measurementBiological material analysisRed blood cellSecreted substance
The present invention relates to a method for detecting substances specifically inhibiting a type III secretion mechanism and functions of the type III secretory proteins, within short time and large amounts thereof, without depending upon animal infectious experiments. Namely it relates to the method for detection of a type III secretory mechanism inhibitor comprising mixing a bacterium having the type III secretory mechanism and an erythrocyte suspension, adding the type III secretory mechanism inhibitor thereto, and detecting changes in the thus formed hemolytic activity. The method for detecting substances can be treated large amount of samples within short time by exhibiting the substances inhibiting the type III secretion mechanism or the functions of the type III secretory proteins as numerical index of the hemolytic activity of erythrocytes. Consequently, the present invention is useful for development of drugs.
Owner:THE KITASATO INST
Method for evaluating eye irritation by using miniature pig erythrocytes
InactiveCN102359946ASmall batch-to-batch varianceClear genetic backgroundColor/spectral properties measurementsBiological testingFormularyOphthalmology
The present invention discloses an experimental method for evaluating eye irritation by using miniature pig erythrocytes. The method mainly comprises: (1) preparing a suspension of miniature pig erythrocytes; (2) carrying out a miniature pig erythrocyte hemolysis test; (3) carrying out a protein denaturation experiment; (4) predicting a model; (5) carrying out classification and judgment. According to the present invention, the source of the erythrocytes is reliable and safe; the tested material is easy to obtain, and has good uniformity; the test system is simple and cheap, and no special equipment is required; the detection method is rapid, sensitive and universal; the method of the present invention can be directly applicable for safety evaluations of chemicals, cosmetics, pesticides, eye medicines and other substances possibly contacting the eyes, wherein the chemicals comprise surfactants, disinfection products, detergent and the like, the cosmetics comprise shampoo, bath foam, eye cream and the like; the method further can be applicable for rapidly screening, classifying and identifying the eye irritations of the raw materials, the formulas or the products.
Owner:程树军 +1
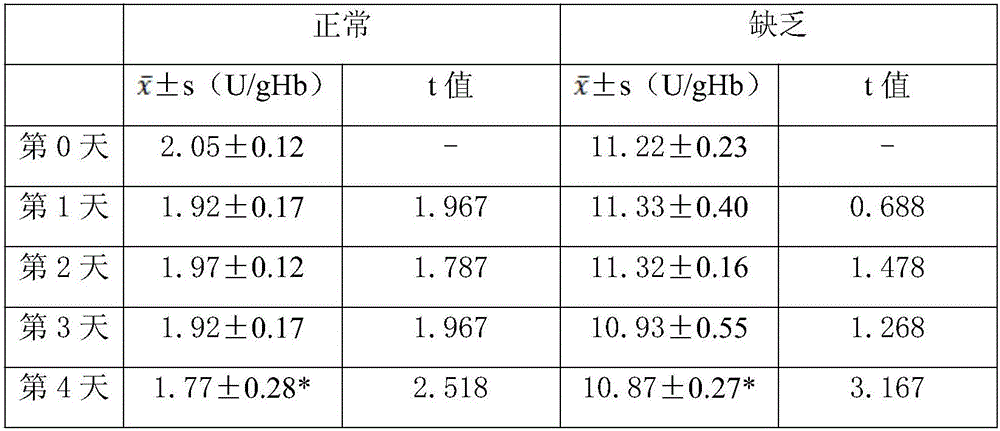
Lyophilized whole blood controls for G6PD (glucose-6-phosphate dehydrogenase) and preparation method of lyophilized whole blood controls
ActiveCN105758700AImprove uniformityImprove stabilityPreparing sample for investigationMedicineNormal level
The invention discloses lyophilized whole blood controls for G6PD (glucose-6-phosphate dehydrogenase) and a preparation method of the lyophilized whole blood controls. The controls are lyophilized products prepared from CPD whole blood matrixes and G6PD. The preparation method of the lyophilized whole blood controls comprises steps as follows: red blood cells and plasma of the CPD whole blood matrixes are separated centrifugally; red blood cell hemolysates are prepared with a physical method and are mixed with hemolysates in different ratios, and the CPD whole blood matrixes are obtained; different quantities of G6PD are added to the whole blood matrixes, the control containing G6PD in a normal level and the control containing G6PD in a deficient level are prepared, the two controls are subpackaged and subjected to lyophilization and vacuum capping, and the lyophilized whole blood controls for G6PD are obtained. The controls have good product homogeneity, good long-term sample storage stability and good stability after redissolving, are not influenced by factors such as transportation, temperature and the like, are wide in applicable range, can be suitable for different brands of quantitative detection kits, can meet the clinical quality control requirement for G6PD detection, and can improve the accuracy of a clinical sample detection result.
Owner:THE PEOPLES HOSPITAL OF GUANGXI ZHUANG AUTONOMOUS REGION
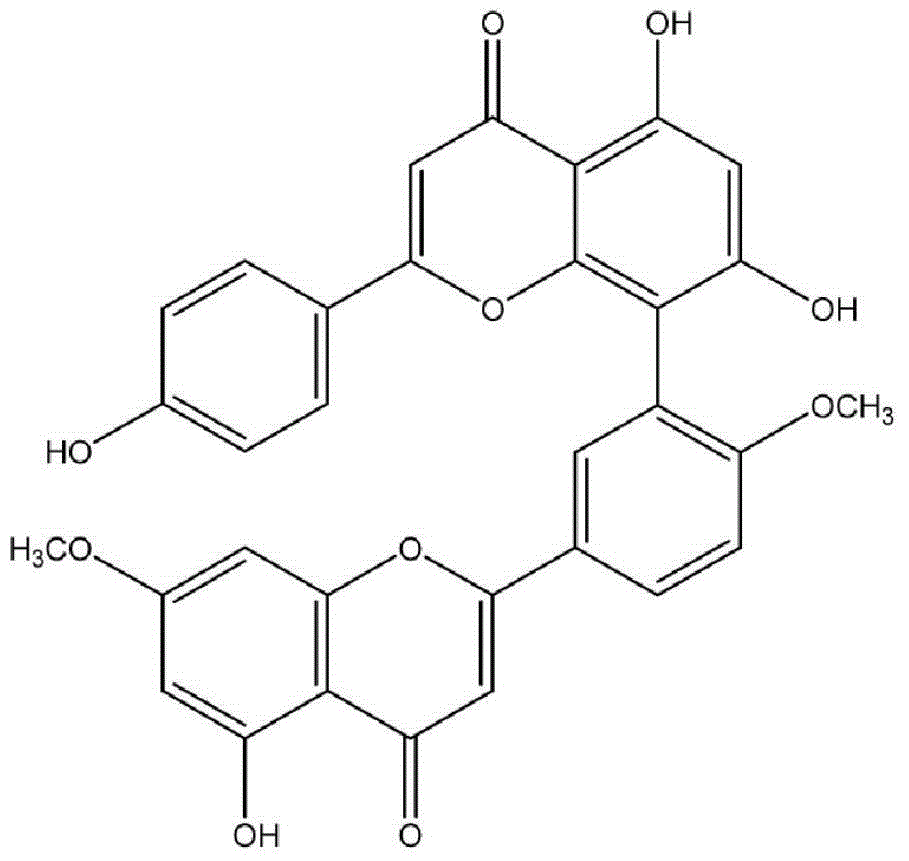
Application of ginkgetin in preparation of drugs for treating streptococcus suis infection
ActiveCN105193784ATherapeutic effect hasInhibition of pore-forming activityAntibacterial agentsOrganic active ingredientsTreatment effectRed blood cell
The invention discloses application of ginkgetin in preparation of drugs for treating streptococcus suis infection. A rabbit red blood cell hemolytic test, a macrophage (J774) damage protection test and a therapeutical effect test on mouse streptococcus suis infection prove that ginkgetin has a better curative effect on streptococcus suis infection. According to the application of ginkgetin in preparation of drugs for treating streptococcus suis infection, provided by the invention, compared with treatment with antibiotics, treating streptococcus suis infection with ginkgetin has the advantages of no drug resistance and high cure rate, therefore, ginkgetin can be used for development of new drugs for resisting streptococcus suis infection, and is of great significance in determination of drug target.
Owner:JILIN UNIV FIRST HOSPITAL
Method for extracting antioxidation active substance from Cichorium endivia L.
ActiveCN101869348AGood inhibitory effectEnhanced inhibitory effectCosmetic preparationsToilet preparationsFood additiveCichorium
The invention relates to a method for extracting an antioxidation active substance from Cichorium endivia L., which adopts ethanol extraction, petroleum ether and ethyl acetate extraction for purification, macroporous absorption resin elution, concentration and drying to finally obtain the substance with high antioxidation activity and belongs to the field of natural product extraction. The Cichorium endivia L. extract has high radical-removing, antioxidation and reducing capabilities, the activity of the extract is higher than the activity of water-soluble vitamine E, and an erythrocyte hemolysis experiment indicates that the Cichorium endivia L. extract also has the antioxidation capability at the cell level. The method, which adopts Cichorium endivia L. as raw material to prepare the high-antioxidation active substance, has the advantages of simple technique and equipment and less investment, and is suitable for mass production and applicable to industries such as medicine and food additives, health-care products and cosmetics.
Owner:BEIJING UNION GENIUS PHARMA TECH
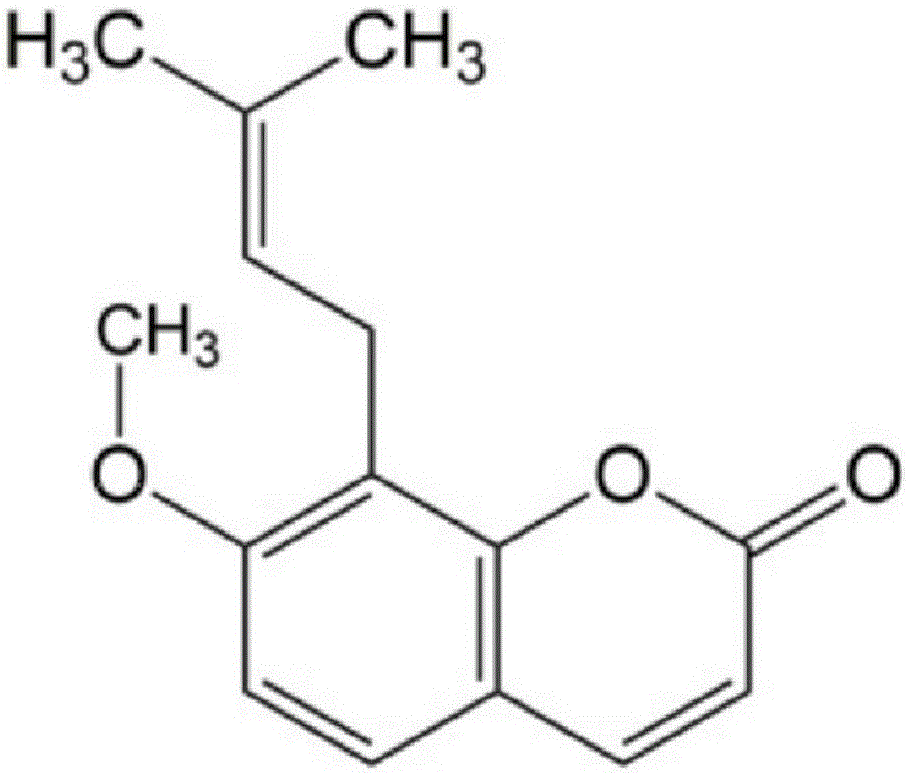
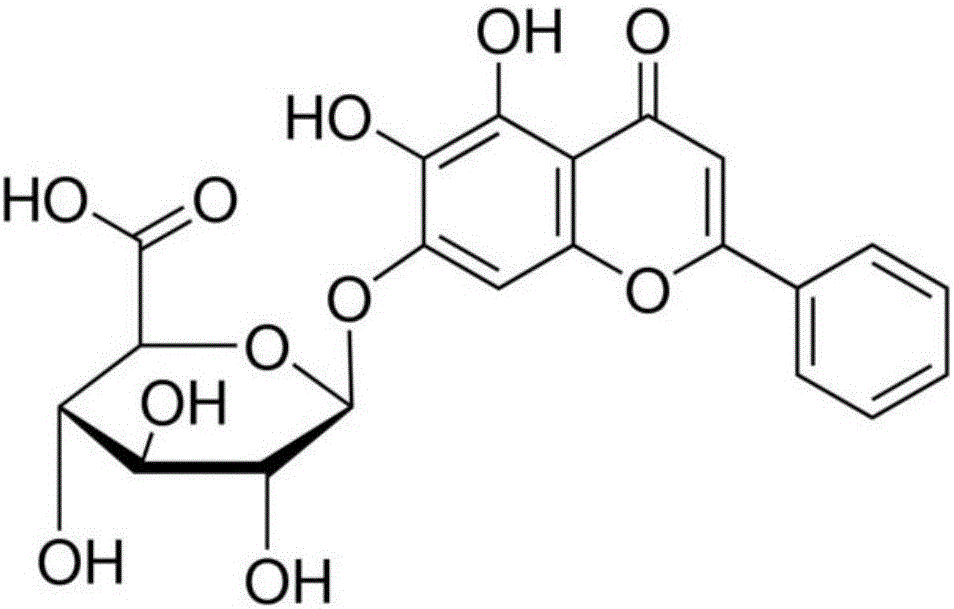
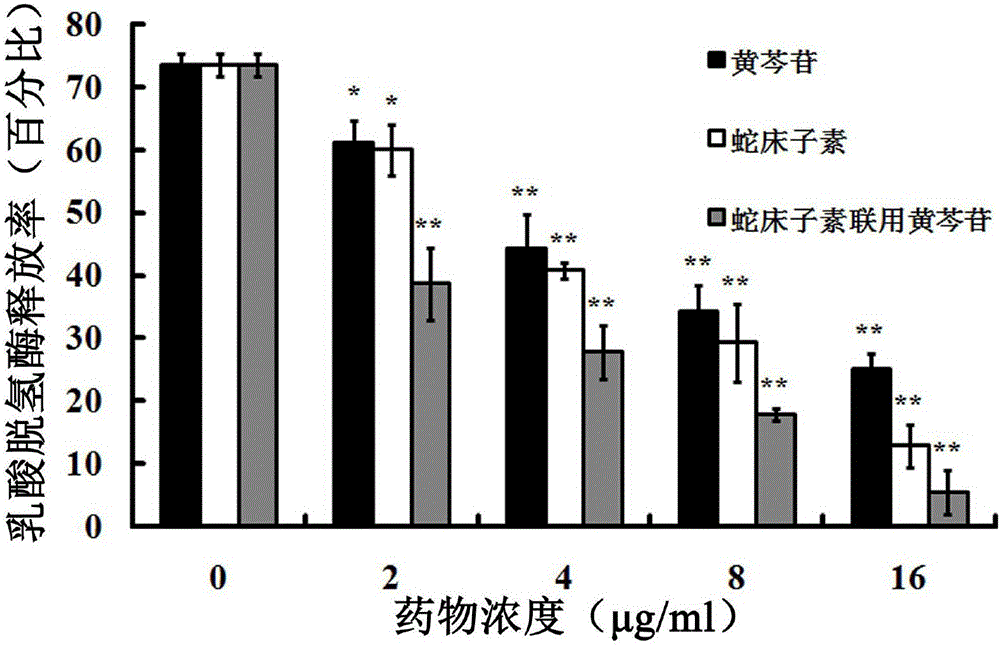
Application of combination of cnidium lactone and baicalin to preparation of drugs for treating pneumonia
The invention relates to application of combination of cnidium lactone and baicalin to preparation of drugs for treating pneumonia. Rabbit erythrocyte haemolysis test, human pulmonary epithelial cell (A549) injury protection test and a mouse staphylococcus aureus pneumonia model prove that the treatment effect of the combination of cnidium lactone and baicalin is obviously better than the treatment effect of singly using each of the cnidium lactone and baicalin. Conventional antibiotics are abused and the bacterial drug resistance is increasingly enhanced; the combination of cnidium lactone and baicalin has the characteristics of high curative rate and no drug resistance, so that the combination of cnidium lactone and baicalin is capable of enhancing selectivity of application of drugs and has great significance on development of new drugs.
Owner:JILIN UNIV
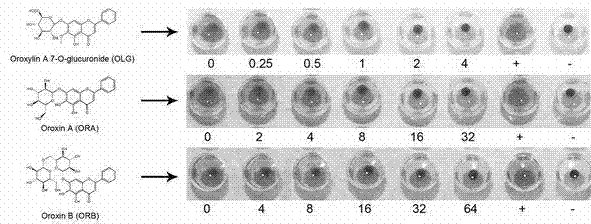
Application of three kinds of oroxin in preparing drug for resisting staphylococcus aureus infection
The invention relates to application of three kinds of oroxin in preparing a drug for resisting staphylococcus aureus infection. The staphylococcus aureus infection resistance of three kinds of oroxin can be proved by virtue of a sheep red blood cell haemolysis test, molecular dynamics simulation and a human pulmonary epithelial cell (A549) injury protective test. Compared with treatment by antibiotics, treatment by using three kinds of oroxin has the characteristics of no drug resistance and high cure rate, so that the three kinds of oroxin can be used in development of new drugs, and has an important meaning to drug target confirmation.
Owner:JILIN UNIV
Antimicrobial peptide derived from scorpion venom as well as preparation method and application of antimicrobial peptide
ActiveCN111423501AHigh selectivityLow hemolytic activityAntibacterial agentsPeptide/protein ingredientsEucaryotic cellArginine
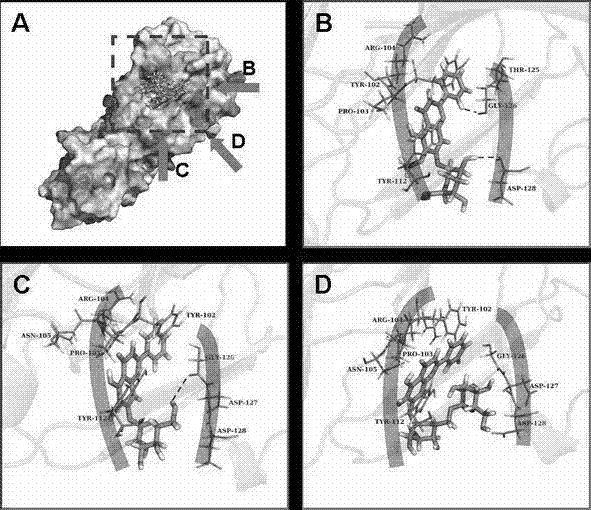
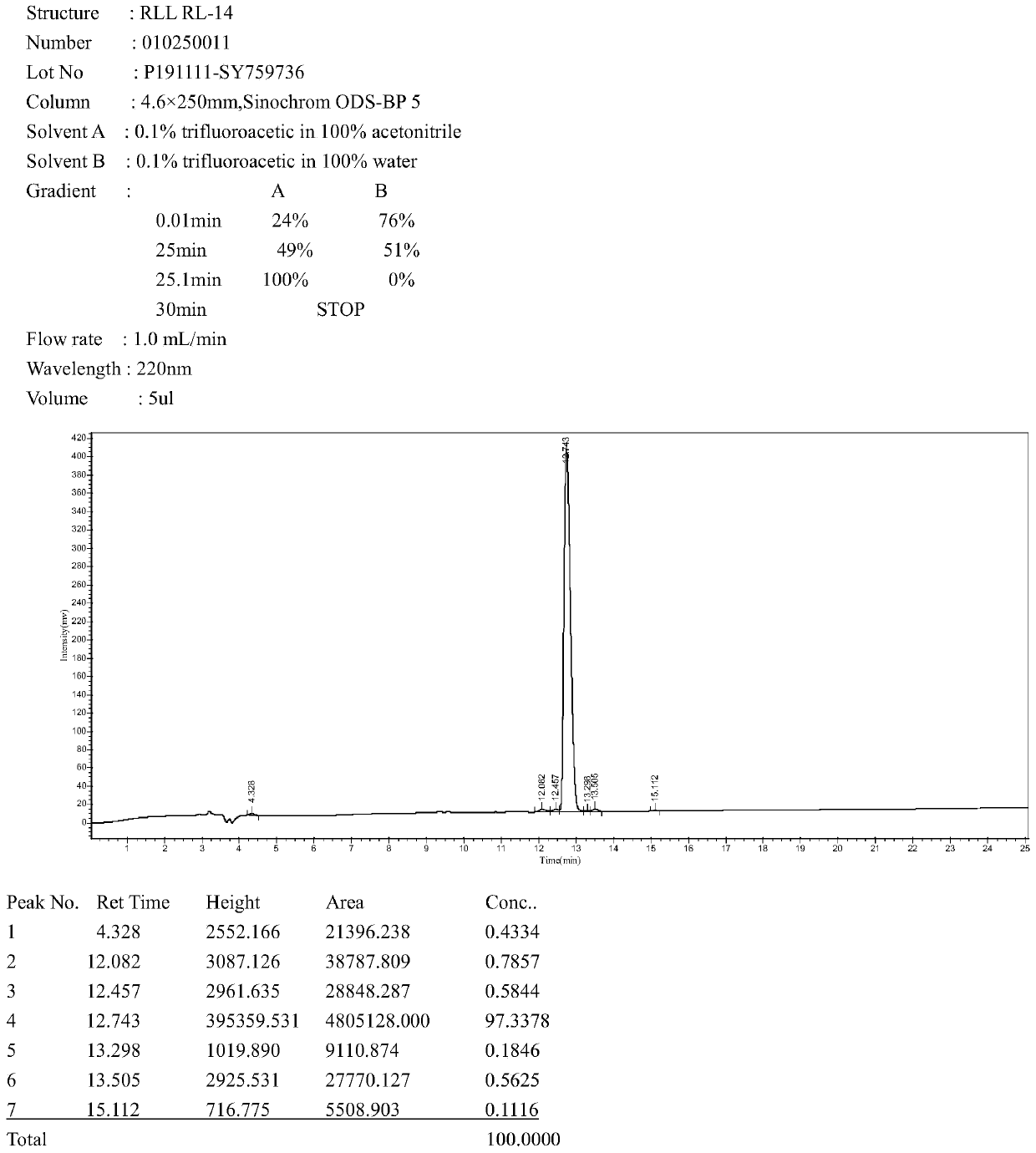
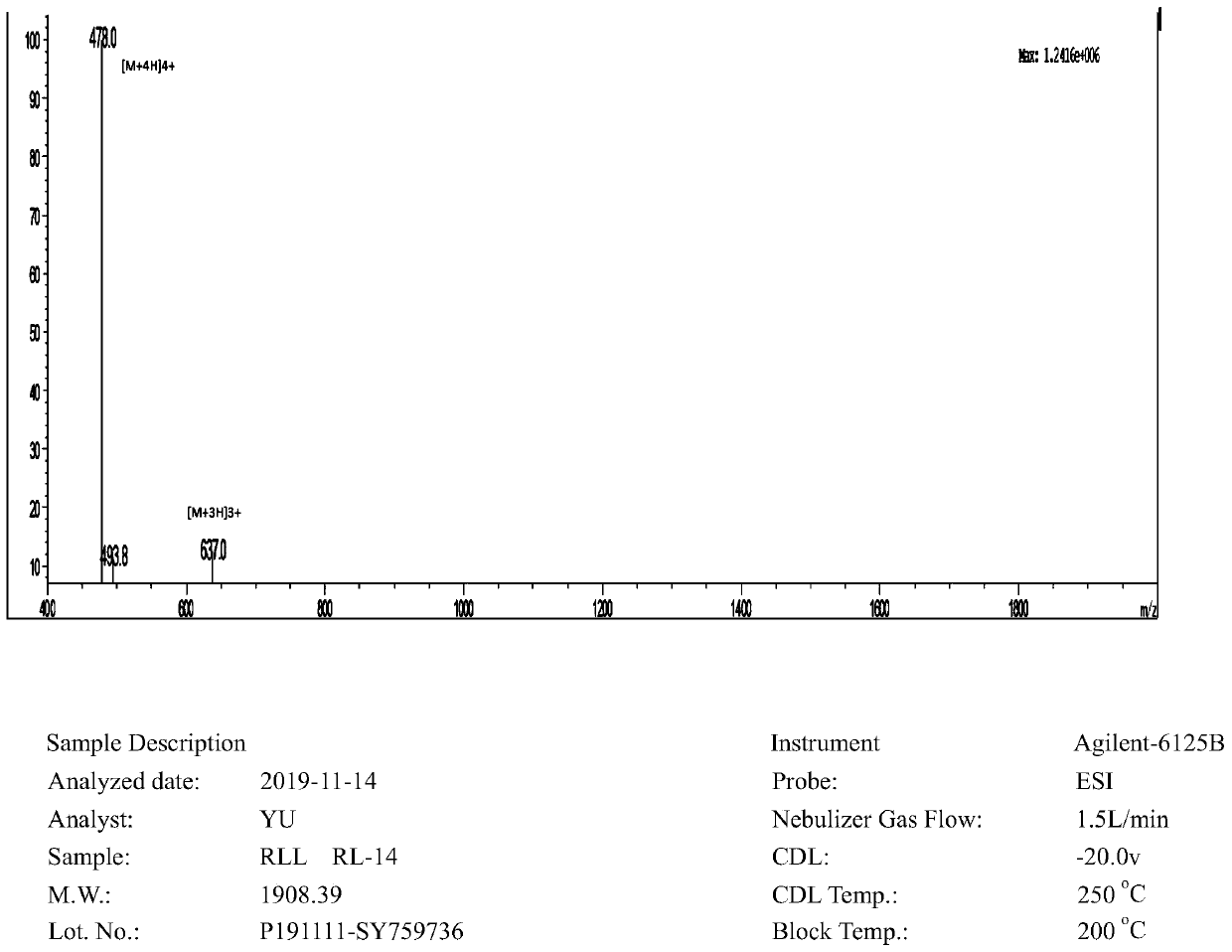
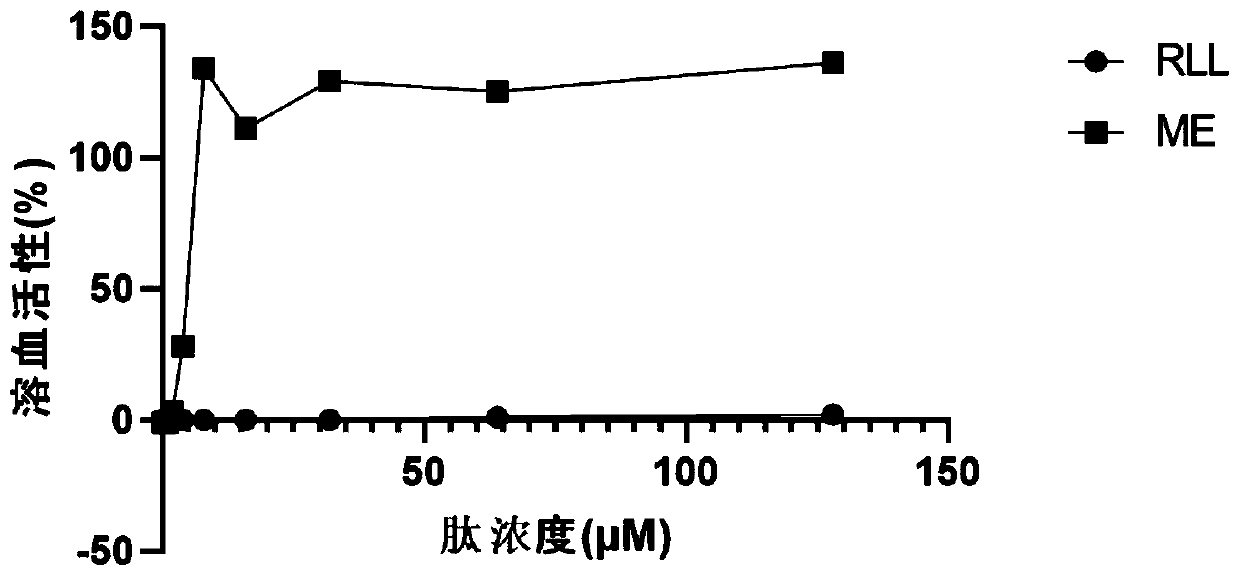
The invention provides an antimicrobial peptide derived from scorpion venom as well as a preparation method and application of the antimicrobial peptide. The antimicrobial peptide RLL has a sequence shown as SEQ ID No.1. A peptide RSN is obtained by intercepting 14 amino acids in the middle of the scorpion toxin, negatively charged aspartic acid in the peptide RSN is replaced with positively charged arginine, and serine, glycine and asparagine in a peptide chain are replaced with leucine to obtain the antimicrobial peptide RLL; and the antimicrobial peptide RLL has very low hemolytic activityand eukaryotic cytotoxicity, the antimicrobial peptide cannot cause 10% erythrocyte hemolysis at a concentration of 128 [mu]mol / L, the survival rate of mouse macrophages RAW 264.7 reaches 80% or more,and the antimicrobial peptide has the development potential of becoming an antibiotic substitute.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Cryopreservation of human red blood cells
InactiveUS20120141974A1Lower Level RequirementsAvoid hemolysisDead animal preservationMammal material medical ingredientsHexosephosphatesPhosphorylation
A red blood cell storage composition includes a composition of red blood cells and biochemistry altering reagents, the biochemistry altering reagents being present at a concentration so as to reduce the percent hemolysis of the red blood cells during the freeze-thaw cycle below that of the percent hemolysis of the red blood cells in the absence the biochemistry altering reagents. The red blood cell storage composition preferably includes reagents selected from: modifiers of glycolytic / metabolic components, modifiers of antioxidant potential, effectors of intracellular ionic distribution, modifiers of membrane fluidity, modifiers of cytoskeletal structure, effectors of the cyclooxygenase second messenger pathway, effectors of the lipoxygenase second messenger pathway, effectors of the hexose monophosphate second messenger pathway, effectors of the phosphorylation second messenger pathway, modifiers of specific messenger molecules, and combinations thereof.
Owner:LIVESEY STEPHEN A +3
Human red blood cell source depressor factor and use thereof
InactiveCN1450085AAvoid interferenceHigh purityMammal material medical ingredientsAnimals/human peptidesDiseaseWater baths
The present invention relates to an erythrocyte duoparental depressor factor (EDDF), its preparation method and application. Its preparation method includes the following steps: using human blood andadopting centrifugal separation process to obtain erythrocyte, using deionized water to hemolyze erythrocyte, after the hemolysate is stood still at 4 deg.C, heating the hemolysate at water bath with100 deg.C, then cooling in ice bath, centrifugalizing obtained product more than 12000g at 4 deg.C to take supernatant, ultrafiltering supernatant to collect the ultrafiltrate whose molecular weight is less than 500D, freeze-drying said ultrafiltrate, making the freeze-dried sample undergo the process of column chromatograph for sorting and separation so as to obtain the invented product which can be used for preparing medicine for curing hypertension disease.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
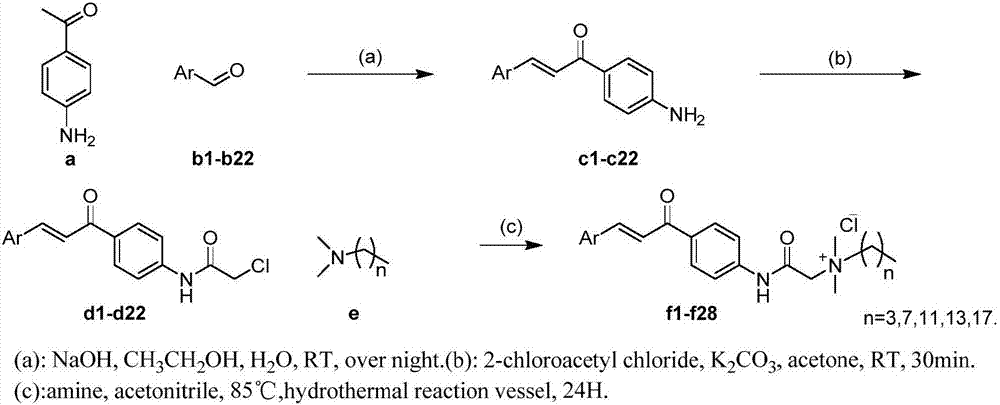
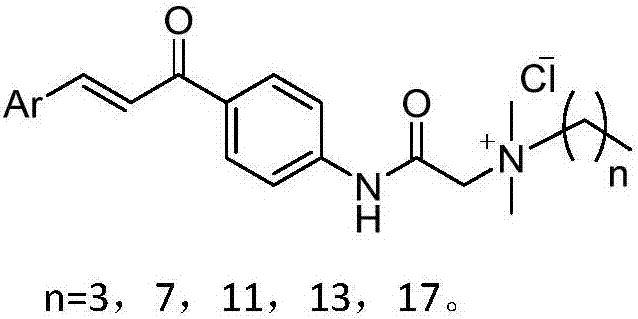
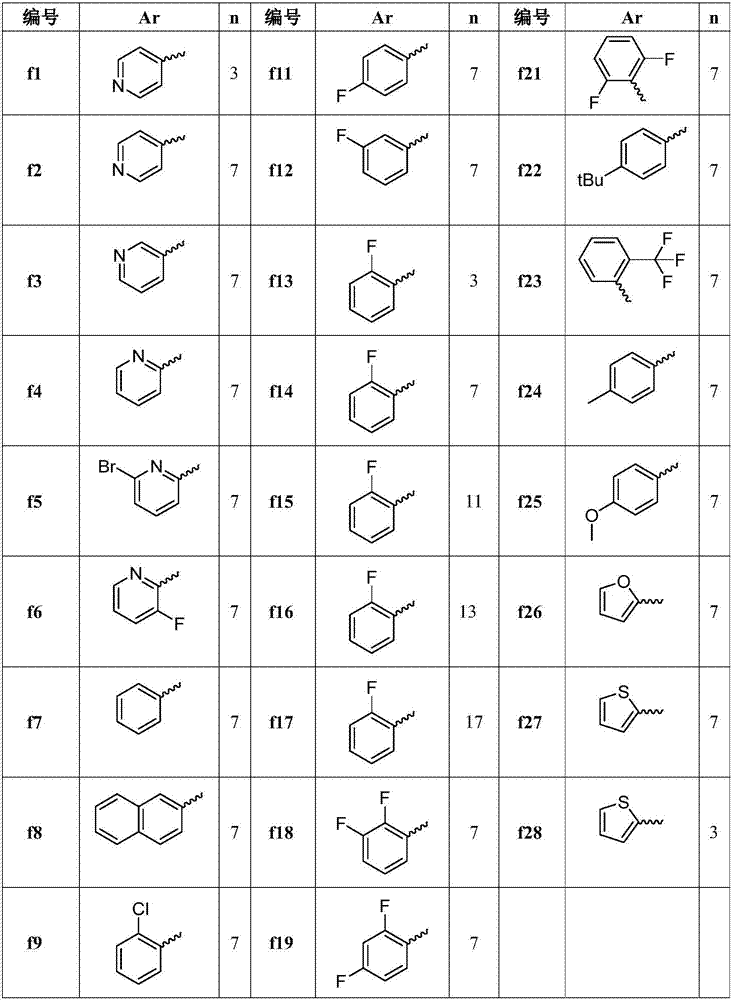
Quatemary ammonium chalcone derivatives resistant to drug-resistance bacteria activities, preparation method and application of quatemary ammonium chalcone derivatives
ActiveCN107235894AHigh activityGood antibacterial effectAntibacterial agentsOrganic compound preparationBacteroidesEscherichia coli
The invention belongs to the technical field of medicinal chemistry, and discloses quatemary ammonium chalcone derivatives resistant to drug-resistance bacteria activities, a preparation method and application of the quatemary ammonium chalcone derivatives. A structural formula is as shown in the description. Proved by invitro antibacterial activity experiments and erythrocyte hemolytic experiments, a large part of compounds in the series of the derivatives have great antibacterial effects and selections on staphylococcus aureus and colonic enterococcus faecalis of gram-positive bacteria, escherichia coli and pseudomonas aeruginosa of gram-negative bacteria. The selected part of compounds show great antimicrobial activities on a plurality of 'superbacteria' including methicillin-resistant staphylococcus aureus (MRSA), vancomycin resistant enterococcus (VRE), carbapenem-resistant enterobacteriaceae (CRE) and new delhi metallo (NDM) carrying NDM genes, and especially show excellent activities on the MRSA. Proved by invitro erythrocyte toxicity experiments, the series of compounds are low in erythrocyte toxicity and can be adopted as new anti-microbial drug candidates.
Owner:ZHENGZHOU UNIV
Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same
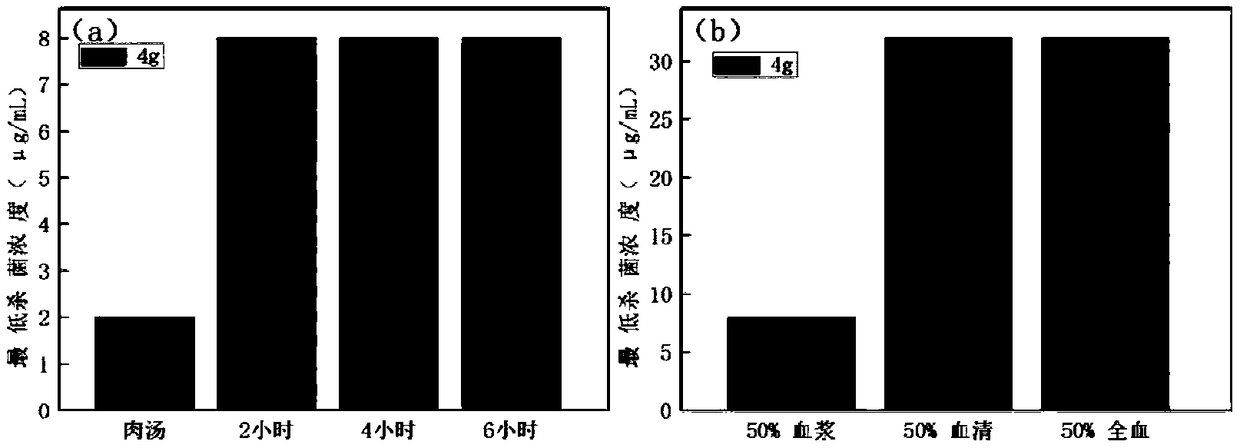
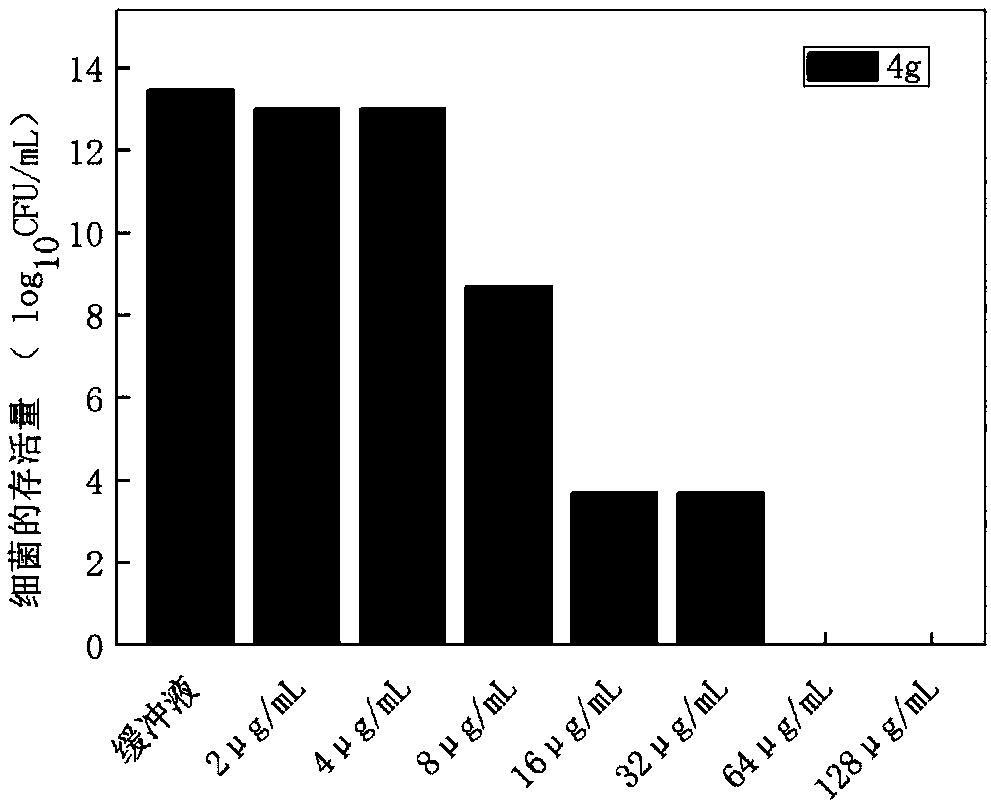
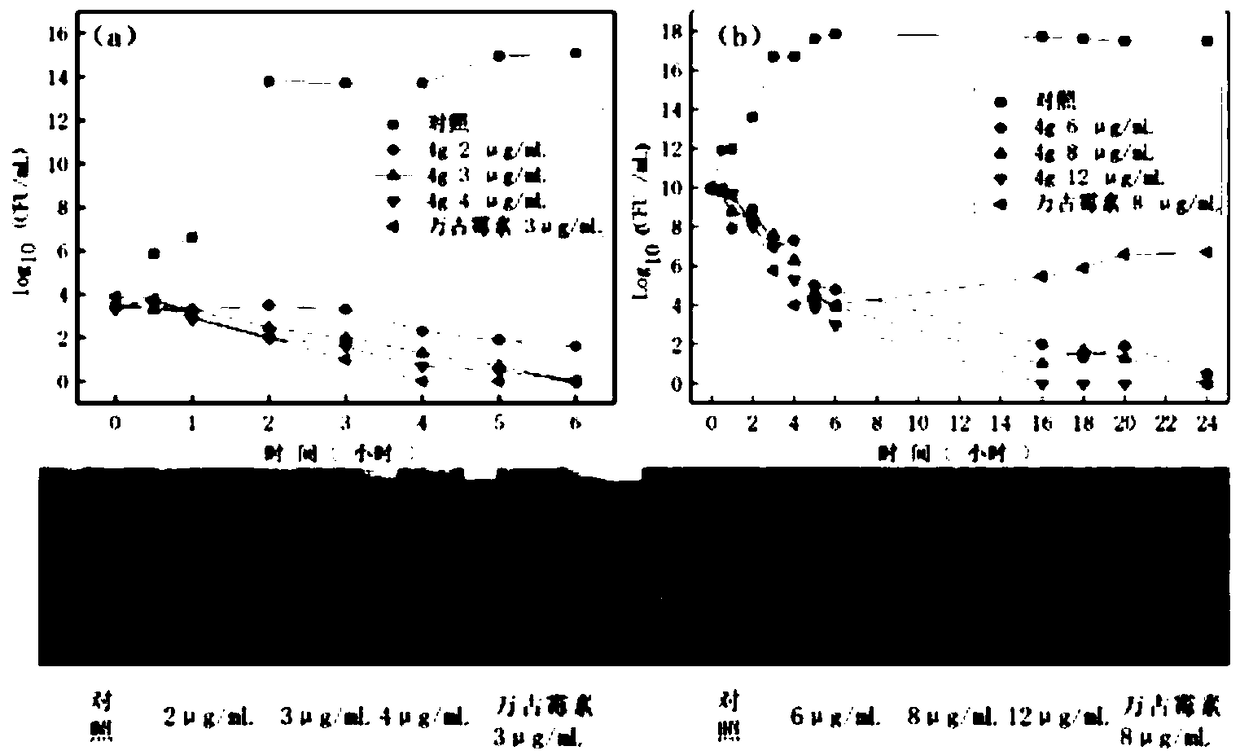
ActiveCN106220588ALow toxicityHigh antibacterial activityAntibacterial agentsOrganic chemistryRed blood cellMeropenem
The invention belongs to the field of medical chemistry and discloses a metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and a preparation method of the same. The compound is represented as the structural formulas 1, 2 or 3 as follows, wherein R is sodium, a methyl group, an n-hexyl group, an n-decyl group or an n-dodecyl group. The inhibitor is combined with a carbapenem antibiotic (e.g. meropenem) to achieve the inhibition activity of metal [beta]-lactamase, so that the sensitivity to the carbapenem antibiotic of a bacterial strain having drug resistance against the carbapenem is recovered. The inhibitor has significant effects on a bacterial strain producing NDM-1 enzyme and having drug resistance against the carbapenem. A red blood cell haemolytic test and a cytotoxicity test prove that the compound is low in toxicity. The series of the compounds is hopeful to serve as a potential candidate medicine of the metal [beta]-lactamase inhibitor.
Owner:ZHENGZHOU UNIV
Anti-candida polypeptide and use thereof and anti-candida medicine
ActiveCN107021999ASimple structureHuman red blood cell hemolysis rate is lowAntimycoticsPeptide/protein ingredientsRed blood cellCandida famata
The present invention provides an anti-candida polypeptide and use thereof and an anti-candida medicine, and the amino acid sequence of the polypeptide comprises any amino acid sequence as shown in SEQIDNO:1 to SEQIDNO:12. Experiment shows that the polypeptide designed by the invention is simple in structure and has the function of inhibiting Candida, and the polypeptide has the advantages of low hemolysis rate of human red blood cells.
Owner:SHENZHEN RES INST THE CHINESE UNIV OF HONG KONG
Anti-irritation composition, and preparation method and application thereof
ActiveCN113041193ASimple compositionStimulates effective resistanceCosmetic preparationsToilet preparationsActive agentAcid substances
The invention discloses an anti-irritation composition, and a preparation method and application thereof. The anti-irritation composition comprises peach gum, cactus, centella asiatica and semen plantaginis; the material composition is simple; the preparation process is simple; all the components interact; and skin irritation caused by cosmetic raw materials can be effectively resisted. Effect evaluation experiments prove that the anti-irritation composition in the invention can antagonize lactic acid stimulation, improve the inhibition rate of red blood cell hemolysis caused by sodium dodecyl sulfate (SDS), reduce chick chorioallantoic membrane irritation caused by SDS and antagonize various irritation sources in cosmetics. When the composition is applied to cosmetics, the composition is expected to have an anti-irritation effect and can antagonize skin irritation caused by various irritation sources such as acidic substances, surfactants, flavours and fragrances, preservatives and the like in cosmetic raw materials.
Owner:BEIJING DONGFANG MIAOSEN BIOTECH CO LTD
Camellia flower essential oil skin care lotion and preparation technology thereof
InactiveCN105726420AImprove permeabilityMoisturizingCosmetic preparationsAntipyreticBiotechnologyCamellia cuspidata
The invention belongs to the technical field of skin care products, and particularly relates to a camellia flower essential oil skin care lotion and a preparation technology thereof. The camellia flower essential oil skin care lotion is prepared from a camellia flower extracting solution, tea oil, prinsepia utilis royle oil and camellia flower essential oil. The skin care lotion is natural in fragrance and rich in natural nutritional ingredients such as unsaturated fatty acid, camellia dulcin, protein, barringtogenol, vitamin E and mineral substances including potassium, phosphorus, magnesium and calcium, can speed up repairing of a skin barrier function and can be applied to daily accidental skin damage to control inflammations and reduce secondary skin damage; in addition, the structure of the contained fatty acid is very close to that of the human body lipid, the very significant protection and anti-radiation effects on erythrocyte hemolysis caused by ultraviolet rays are achieved, and the significant effect on skin care is achieved.
Owner:GUANGDONG FANLONG AGRI TECH DEV CO LTD
Screening method for erythrocyte osmotic fragility and application of the same
InactiveCN107290294AGood repeatabilityThe detection method is simpleColor/spectral properties measurementsRed blood cellScreening method
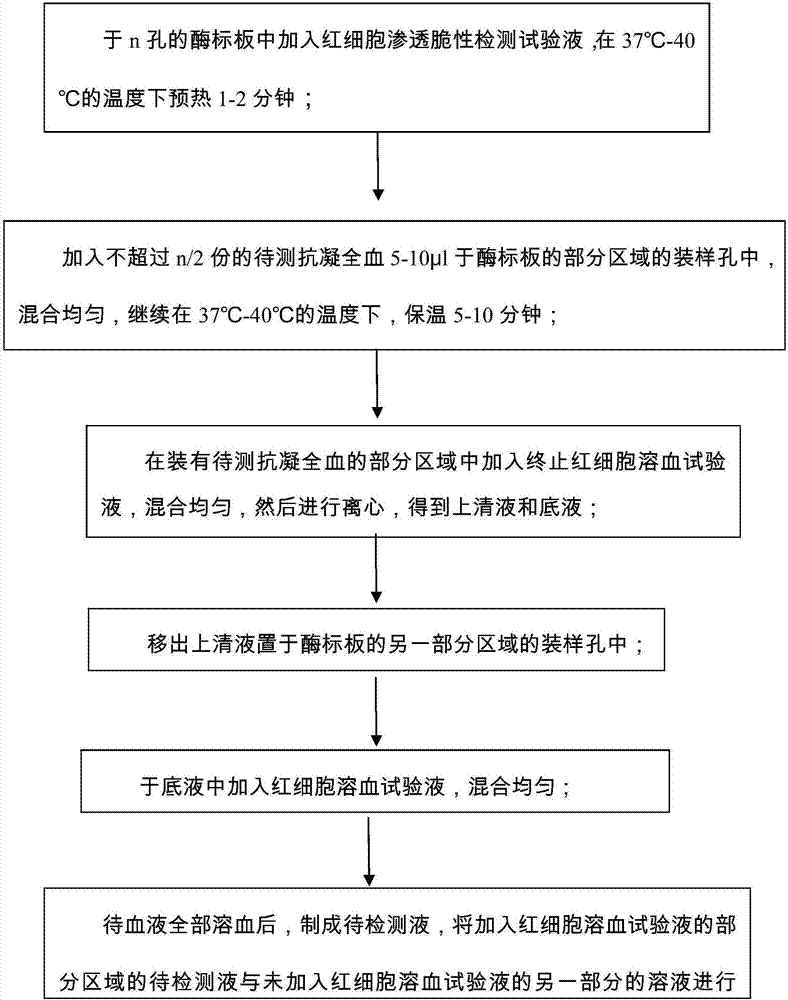
The invention provides a screening method for erythrocyte osmotic fragility, which comprises the following steps: S1, test solutions of the erythrocyte osmotic fragility are added into an enzyme plate and preheated at a temperature of 37 to 40 DEG C for 1-2 minutes; S2, 5-10 Mu l of anticoagulant whole blood to be measured is added into holding sample holes of partial areas of the enzyme plate, and then the test solution of the erythrocyte osmotic fragility and the anticoagulant whole blood are mixed evenly; S3, test solutions for stopping erythrocyte hemolysis are added into the partial areas filled with the anticoagulant whole blood to be measured, all solutions are mixed evenly and centrifugation is carried out; S4, the supernatant is removed and placed in the holding sample holes of the other partial areas of the enzyme plate; S5, erythrocyte hemolysis solutions are added into the base solution, and all solutions are mixed evenly; S6, after the hemolysis process is finished, colorimetry is carried out between the solution to be measured of the partial areas filled with the erythrocyte hemolysis test solution and the solution of the other partial areas not filled with the erythrocyte hemolysis test solution. With the advantages of simplicity, trace amounts, fast speed and large samples, the screening method for the erythrocyte osmotic fragility further has the advantages that the screening workload can be reduced, the screening can be completed fast and accurately, the reproducible results can be obtained and the experimental results can be automatically calculated.
Owner:杭州金域医学检验所有限公司
Extractive of Abalone mushroom, extraction method and application thereof
InactiveCN1911253AImprove disease resistanceEnhanced inhibitory effectPowder deliveryAntinoxious agentsDiseaseHuman health
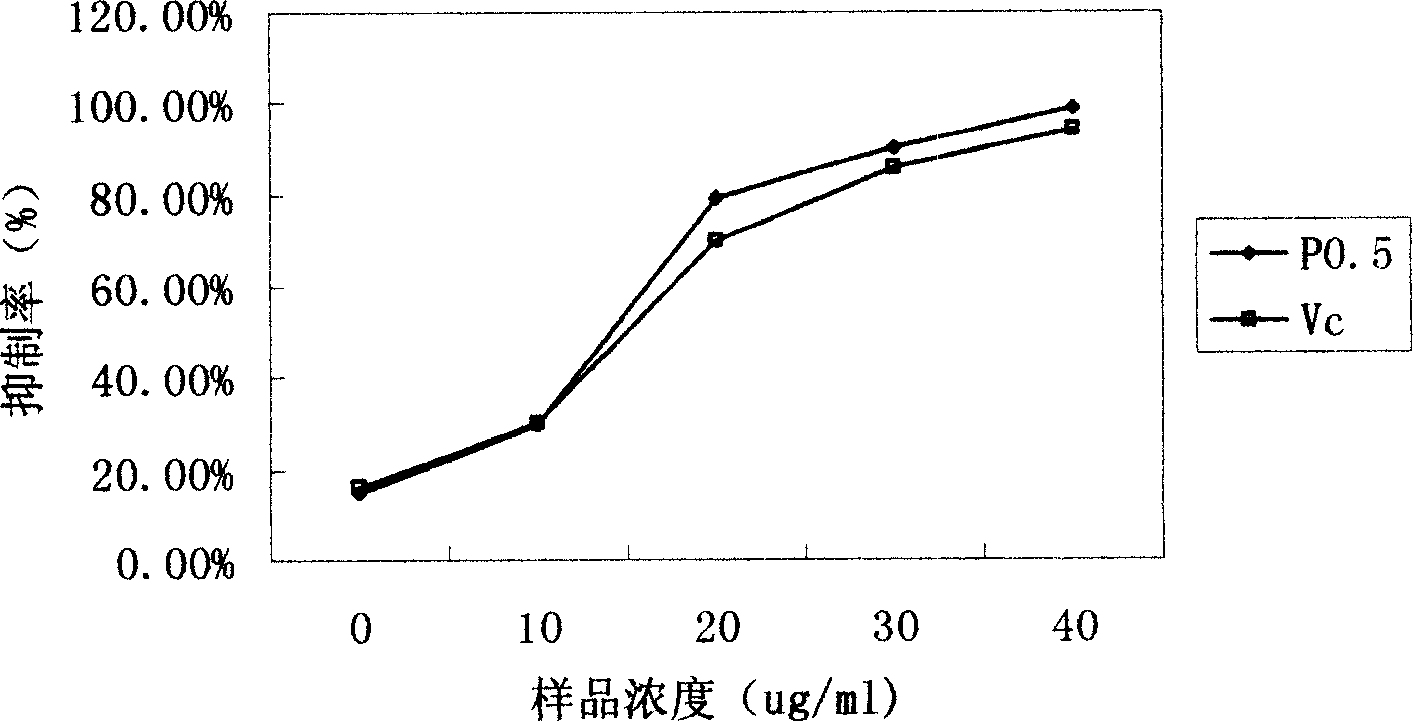
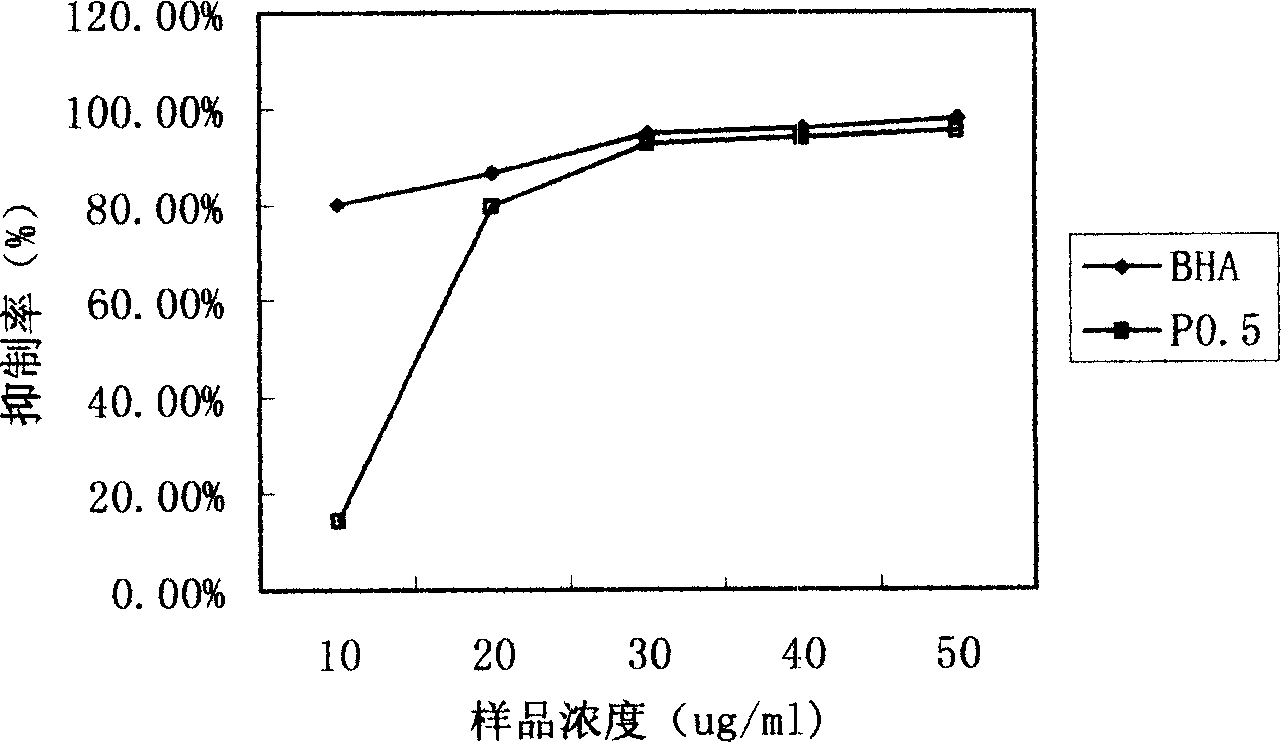
An extract of the abalone mushroom for improving immunity, preventing diseases and taking care of human health is composed of the extract P0.5a containing polyose (82.64%) and protein (0.63%) and the extract P0.5b-1 containing polyose (45.48%) and protein (12.63%). Its extracting method is also disclosed.
Owner:NANKAI UNIV
Amide aromatic phenol antibacterial peptide analogue with antibacterial activity and preparation method thereof
ActiveCN108794343AHigh activityHigh antibacterial activityAntibacterial agentsOrganic compound preparationEscherichia coliStaphylococcus aureus
The invention belongs to the technical field of pharmaceutical chemistry, and discloses an amide aromatic phenol antibacterial peptide analogue with drug-resistance bacteria resistant activity and without obvious toxicity and a preparation method thereof. The target product is obtained by 3-4 reaction steps, and the main structure of the product is shown as follows. In-vitro antibacterial activityexperiments prove that most of the series of compounds have excellent activity on Gram-positive staphylococcus aureus and enterococcus faecalis, Gram-negative Escherichia coli and stenotrophomonas maltophilia, and the compounds have excellent broad spectrum antibacterial activity; moreover, in-vitro red cell hemolytic data is low in toxicity and has excellent selectivity. One part of the compounds also have excellent antibacterial activity on 'superbacteria' comprising drug-resistant methicillin staphylococcus aureus (MRSA), clinical strains producing enzymes NDM-1 and KPC-2 and the like. Therefore, the series of compounds are expected to serve as novel antibacterial candidate drugs.
Owner:ZHENGZHOU UNIV
Method of producing electrically conductive polymers and removing protein-bound substances
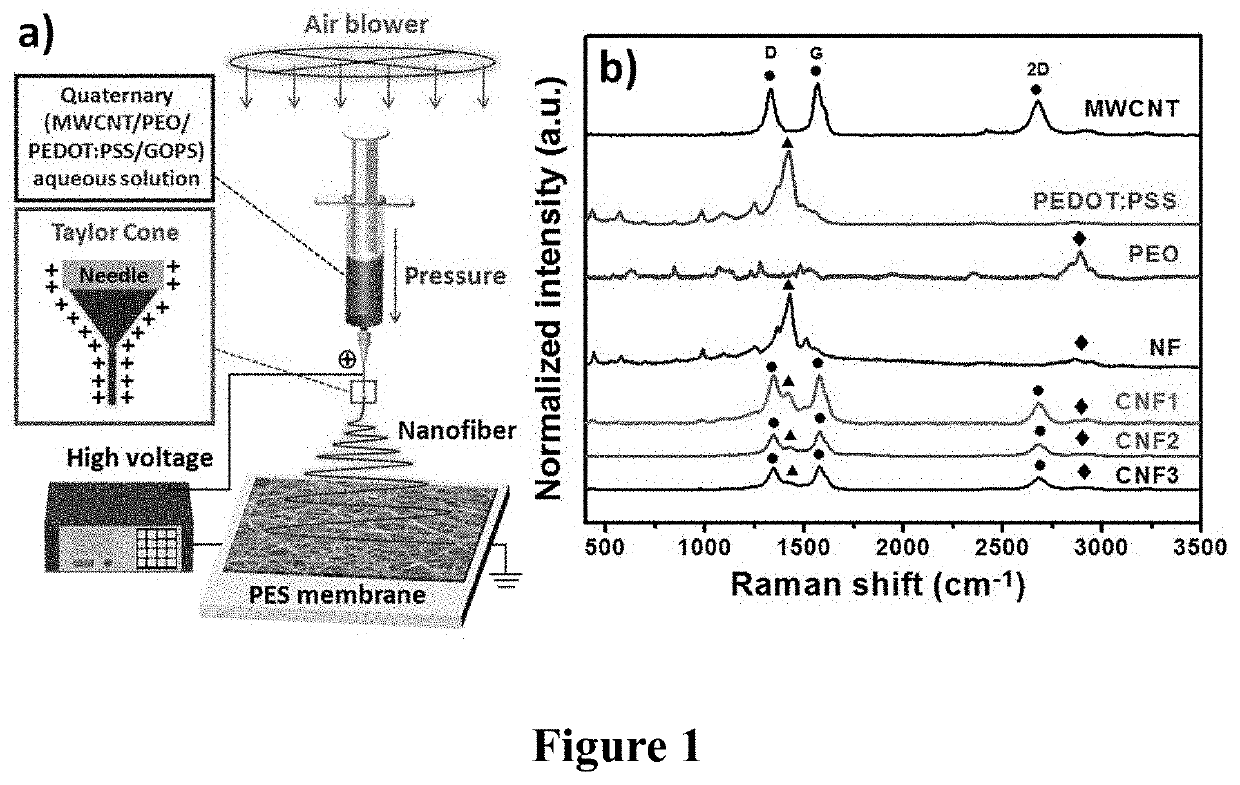
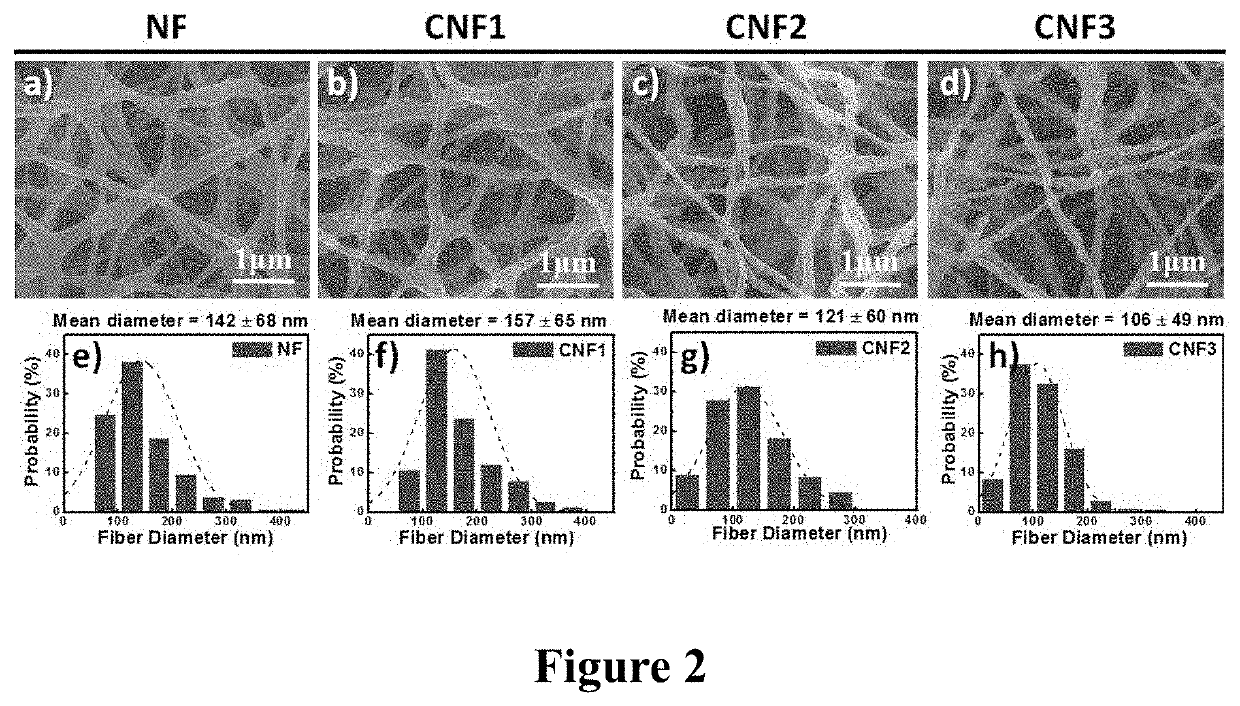
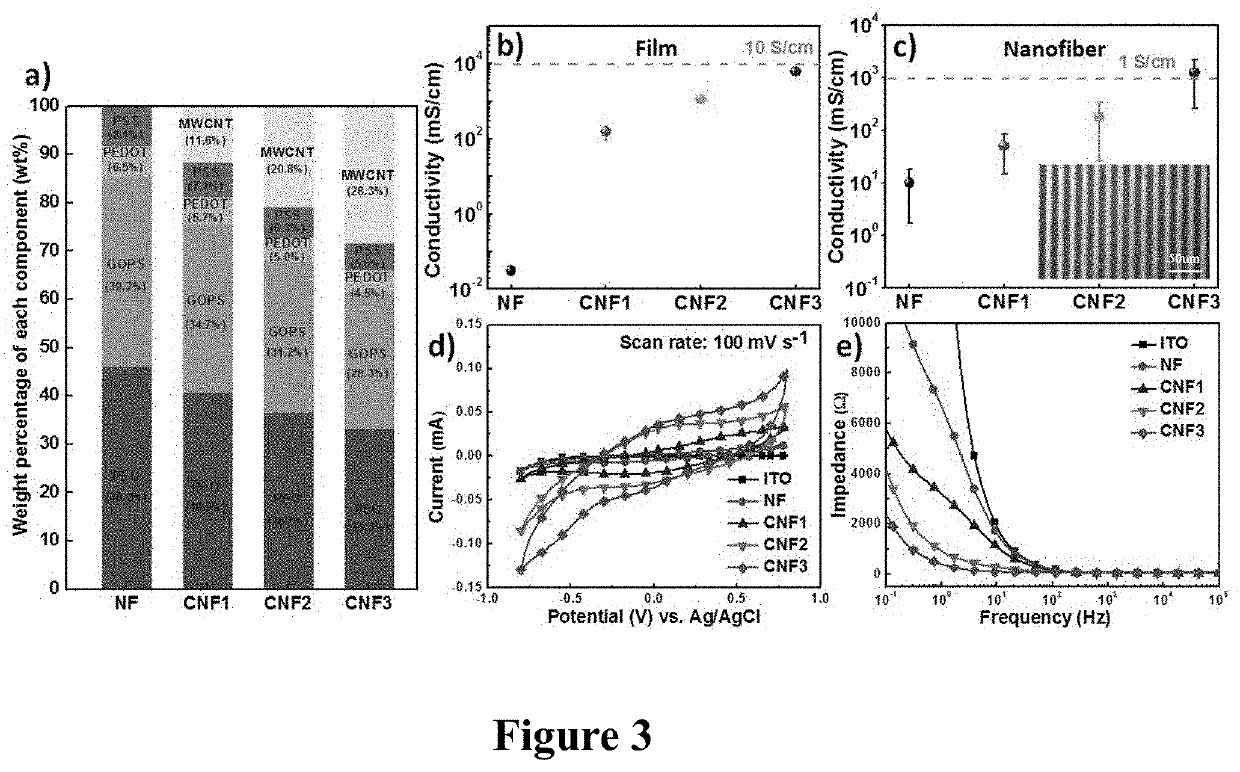
PendingUS20210086144A1Reduce percentageImprove dialysis effectElectroconductive/antistatic filament manufactureMembranesPolyethylene oxideCarbon nanotube
The present invention provides an organic bioelectronic HD device system for the effective removal of protein-bound substances, comprising PEDOT:PSS, a multiwall carbon nanotube, polyethylene oxide (PEO), and (3-glycidyloxypropyl)trimethoxysilane (GOPS). The composite nanofiber platform exhibited (i) long-term water-resistance; (ii) high adhesion strength on the PES membrane; (iii) enhanced electrical properties; and (iv) good anticoagulant ability and negligible hemolysis of red blood cells, suggesting great suitability for use in developing next-generation bioelectronic medicines for HD.
Owner:MING CHI UNIVERSITY OF TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same](https://images-eureka.patsnap.com/patent_img/8e3380f8-46f7-4f90-9f36-80b500392661/160722101833.PNG)
![Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same](https://images-eureka.patsnap.com/patent_img/8e3380f8-46f7-4f90-9f36-80b500392661/160722101844.PNG)
![Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same](https://images-eureka.patsnap.com/patent_img/8e3380f8-46f7-4f90-9f36-80b500392661/160722101849.PNG)