Quatemary ammonium chalcone derivatives resistant to drug-resistance bacteria activities, preparation method and application of quatemary ammonium chalcone derivatives
A technology of chalcone derivatives and drug-resistant bacteria, which is applied in the preparation of organic compounds, antibacterial drugs, and carboxylic acid amides, and can solve problems such as weak selectivity, difficult mass production, and high toxicity of antibacterial peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
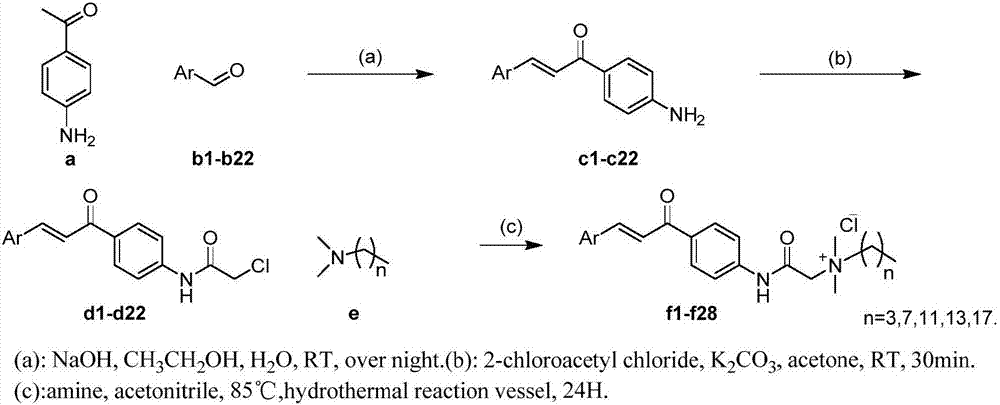
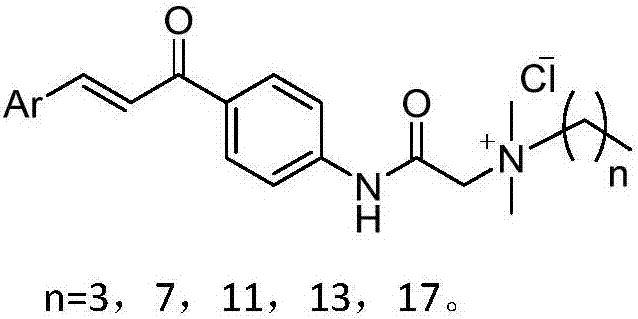
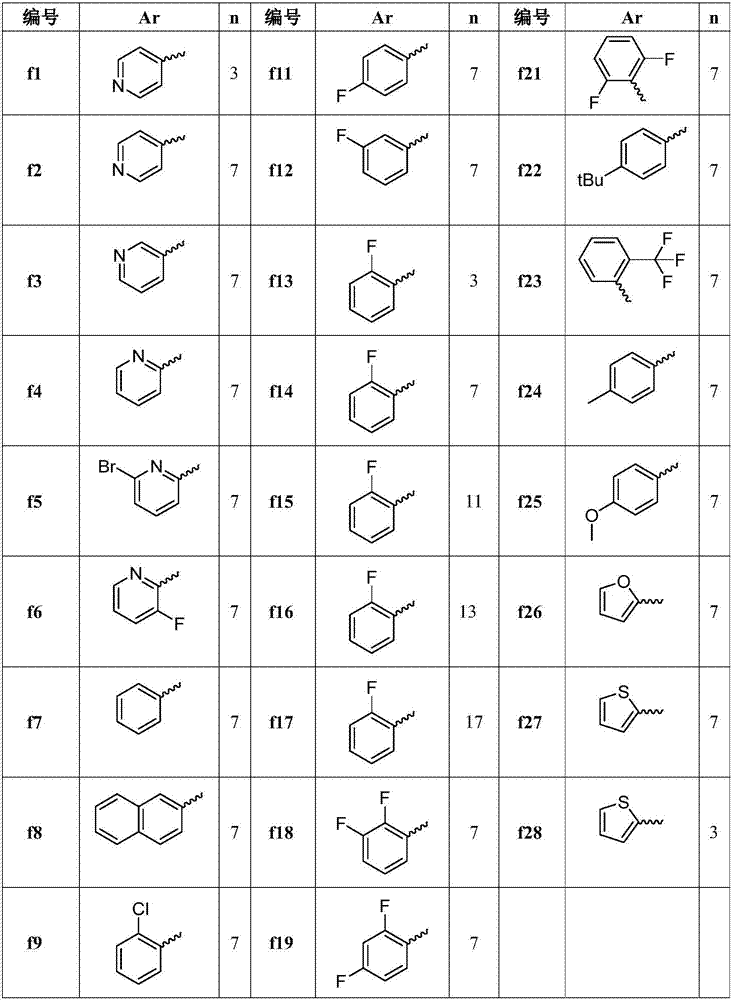
[0024] The preparation of embodiment 1 compound f1-f28
[0025] (1) Preparation of compound c1-c22
[0026] Take sodium hydroxide (110.97mg, 2.77mmol) in a single-necked round bottom flask (200mL), add water (25mL) and dissolve under magnetic stirring at room temperature; then compound a p-aminoacetophenone (300.00mg, 2.22mmol) and compound Add b1-b22 (2.26mmol) into the Erlenmeyer flask (100mL), add absolute ethanol (25mL) and sonicate until the system is clear, then add the clear solution dropwise (1 drop / second) to the above-mentioned stirred hydrogen In the single-necked bottle of sodium oxide solution, after the dropwise addition, the reaction was continued at room temperature, and the system was brownish yellow and clear. After about 6 hours, TLC (PE:EA=1:1) detection showed that the reaction was complete. The reaction was stopped, and the reaction system was poured into ice water (50 mL), and a large amount of yellow solid was precipitated immediately, filtered with s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com