Antimicrobial peptide derived from scorpion venom as well as preparation method and application of antimicrobial peptide
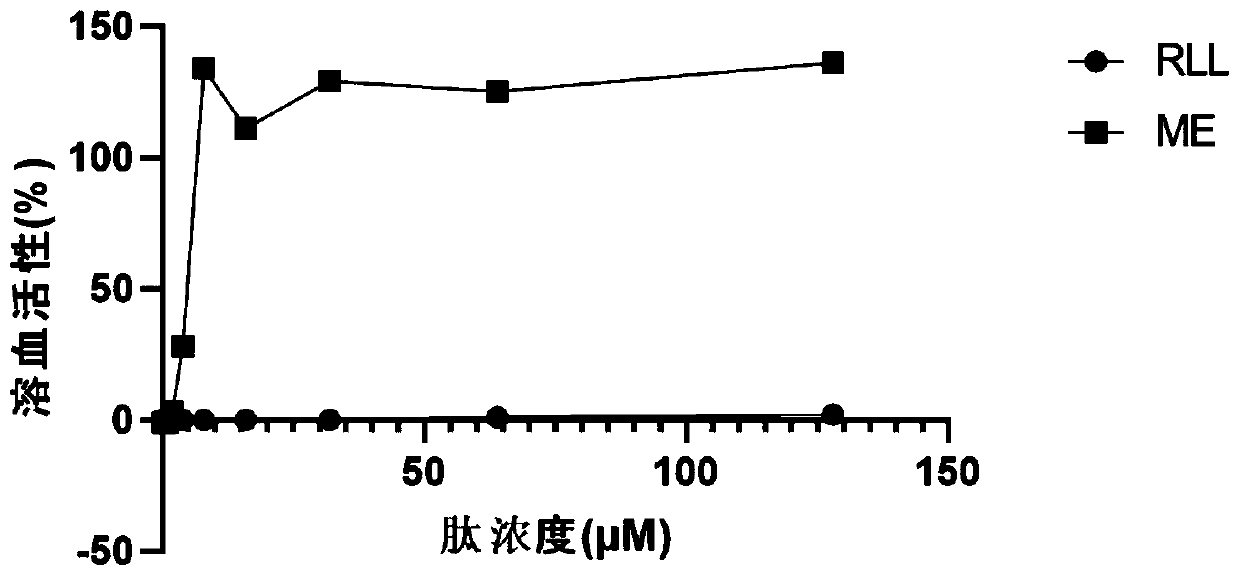
A technology of antimicrobial peptides and self-scorpion toxins, which is applied in the direction of antibacterial drugs, medical preparations containing active ingredients, peptides, etc., can solve the problems of unfavorable use of antibacterial drugs, high cytotoxicity, and high hemolytic value, and achieve low hemolytic activity and Effects of eukaryotic cytotoxicity, reduced hemolytic activity, and high inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Design of Antimicrobial Peptides
[0016] The amino acid sequence of scorpion venom Buthinin is:
[0017] SIVPIRCRSNRDCRRFCGFRGGRCTYARQCLCGY;
[0018] By intercepting the 14 amino acids in the middle of the scorpion venom Buthinin, a polypeptide containing 5 positive charges and a hydrophobic value of -4.22 was obtained. The amino acid sequence of a peptide RSN obtained is: RSNRDCRRFCGFRG;
[0019] Replace negatively charged aspartic acid in RSN with positively charged arginine, and then replace serine, glycine and asparagine in the peptide chain with leucine to obtain RLL, whose amino acid sequence is: RLLRRCRRFCLFRL;
[0020] Its positive charge content is 6, and its hydrophobic value is increased to 0.448.
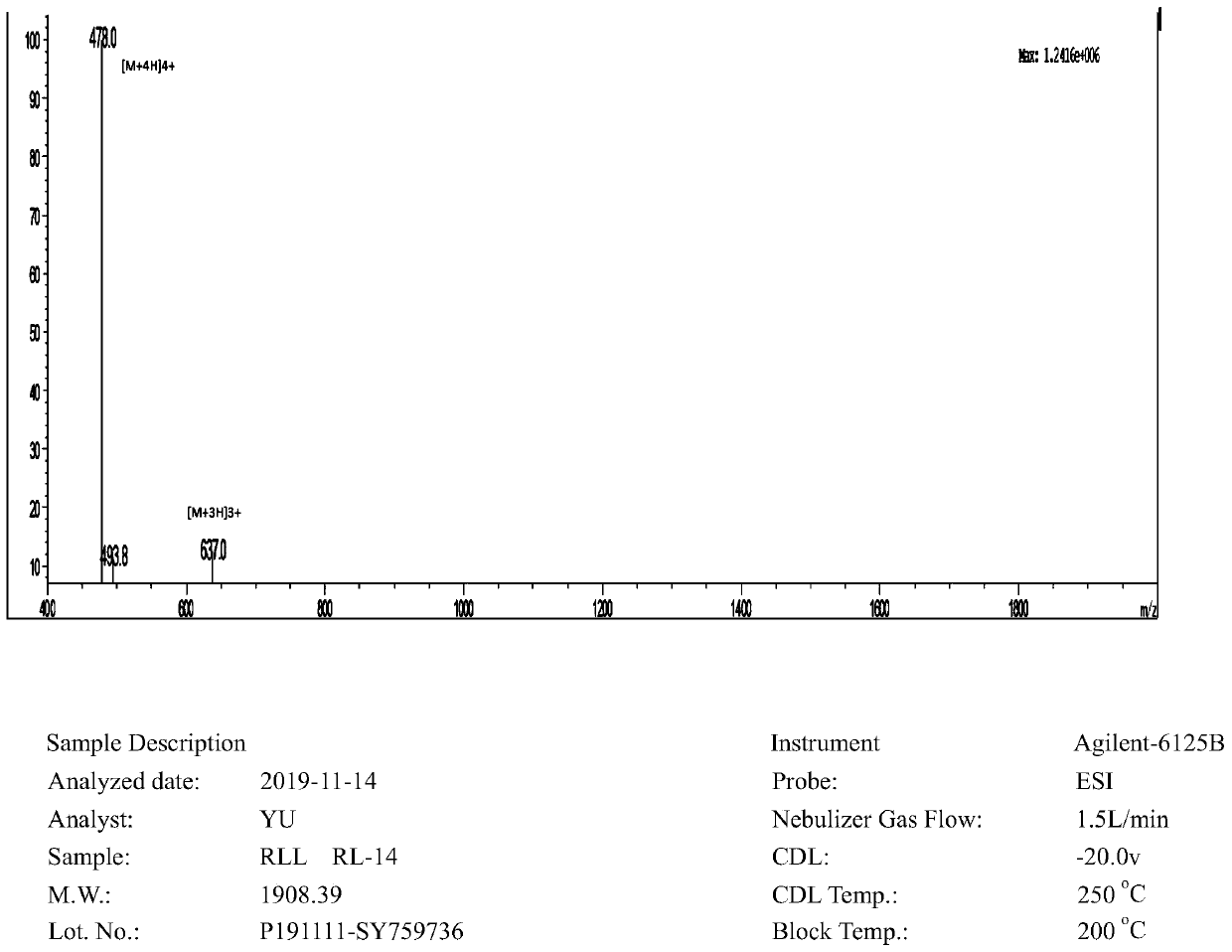
[0021] The RLLs obtained through transformation all have high cell selectivity. The N-terminus of RLL is acetylated to raise a positive charge and increase the stability of the peptide. The sequences of the antimicrobial peptides are shown in Table 1.
[00...
Embodiment 2
[0025] Synthesis of RLL Antimicrobial Peptides by Solid Phase Chemical Synthesis
[0026] 1. The preparation of antimicrobial peptides is carried out one by one from the C-terminal to the N-terminal, and is completed by a peptide synthesizer. First, Fmoc-X (X is the first amino acid at the C-terminal of each antimicrobial peptide) is inserted into Wang resin, and then the Fmoc group is removed to obtain X-Wang resin; then Fmoc-Y-Trt-OH (9 -Fmoxy-trimethyl-Y, Y is the second amino acid at the C-terminus of each antimicrobial peptide); according to this procedure, it is synthesized from the C-terminus to the N-terminus until the synthesis is completed, and the side of the Fmoc group is removed chain protection resin;
[0027] 2. Add a cleavage reagent to the peptide resin obtained above, react for 2 hours at 20°C in the dark, filter; wash the precipitate with TFA (trifluoroacetic acid), mix the washing liquid with the above filtrate, concentrate with a rotary evaporator, and th...
Embodiment 3
[0031] Determination of antimicrobial activity of antimicrobial peptides
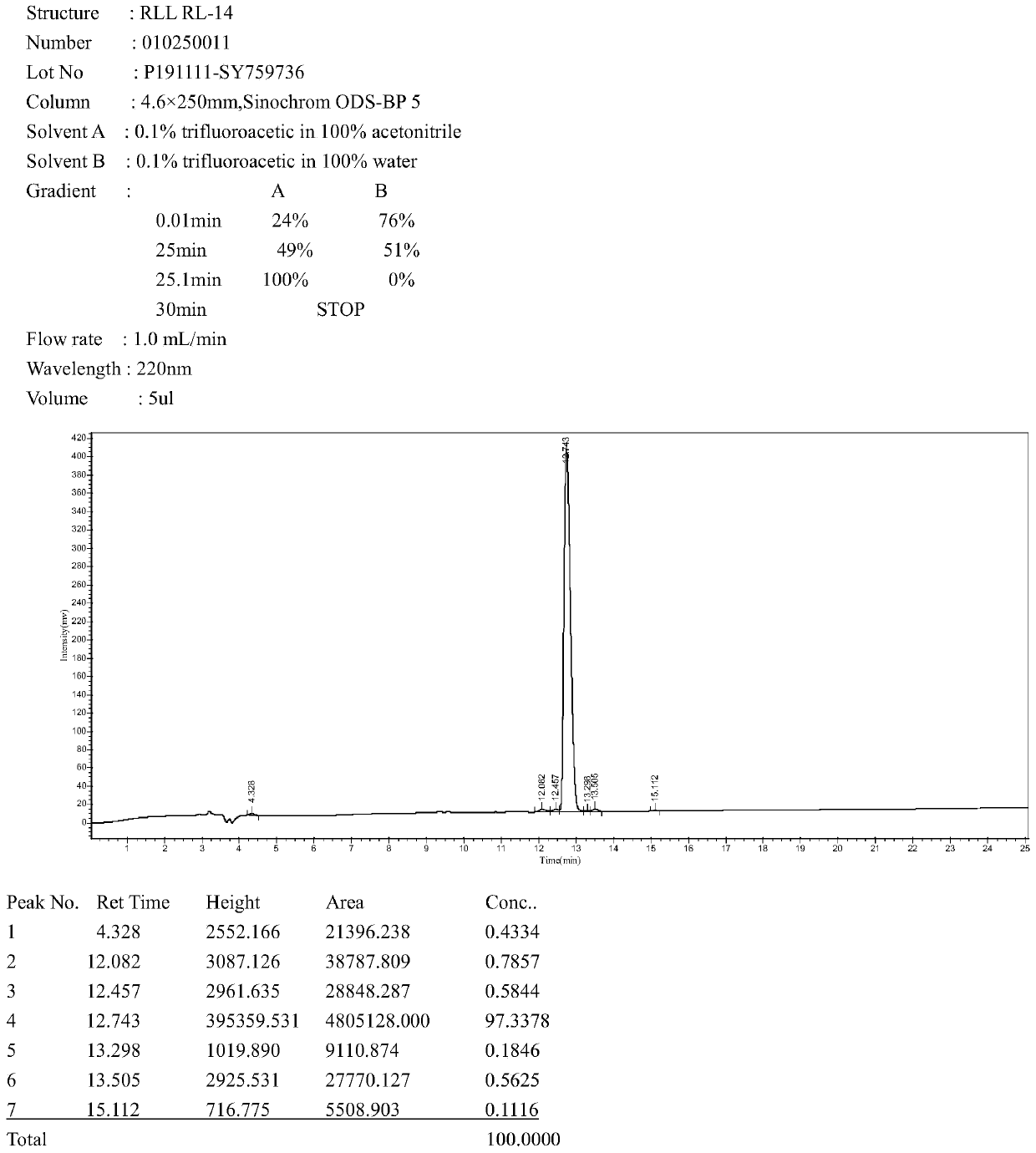
[0032] 1. Determination of antibacterial activity: The minimum inhibitory concentration of several antibacterial peptides was determined by the micro broth dilution method. Using 0.01% acetic acid (containing 0.2% BSA) as the diluent, a series of gradient antimicrobial peptide solutions were sequentially prepared using the double dilution method. Take 100 μL of the above solution and place it in a 96-well cell culture plate, then add an equal volume of the bacteria solution to be tested (~10 5 individual / mL) in each well. Positive controls (containing bacterial fluid but not antimicrobial peptides) and negative controls (neither bacterial fluid nor peptides) were set up. Cultivate at a constant temperature of 37°C for 14-18h, measure the light absorption value at 492nm (OD492nm) with a microplate reader, and determine the minimum inhibitory concentration. The test results are shown in Table 2.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com