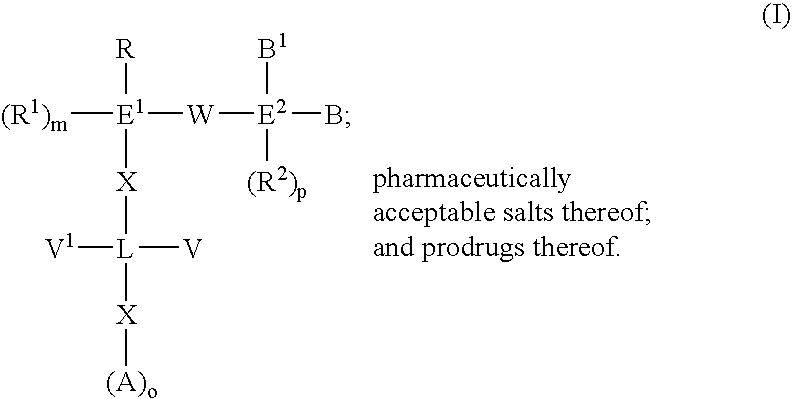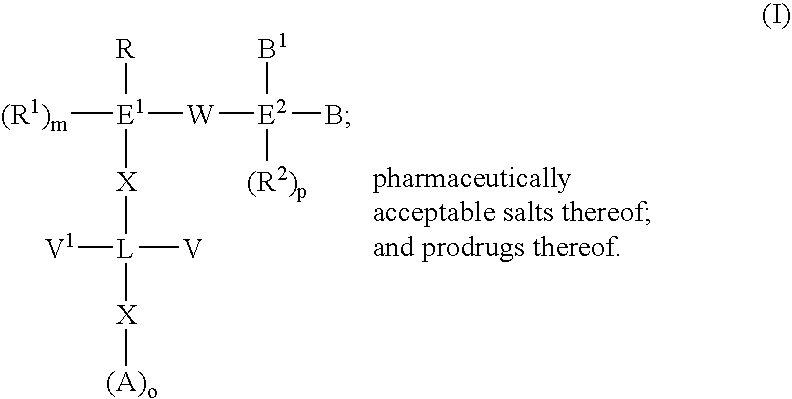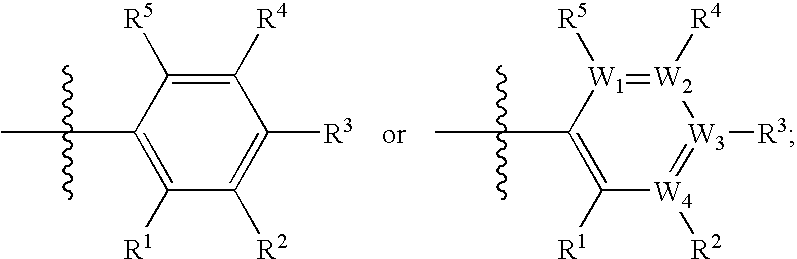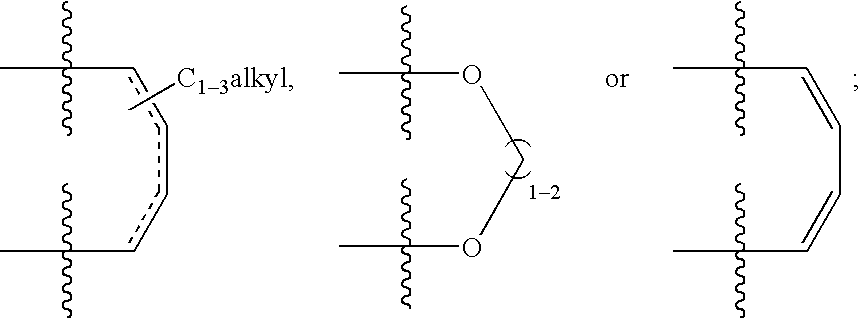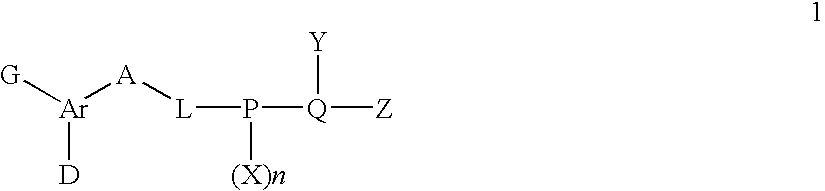Patents
Literature
378 results about "Anticoagulation Agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical Definition of Anticoagulant agent. Anticoagulant agent: A medication used to prevent the formation of blood clots and to maintain open blood vessels. Anticoagulants are called blood "thinners," but they do not thin the blood, they only prevent or reduce clots, or thrombi. Anticoagulants have various uses.
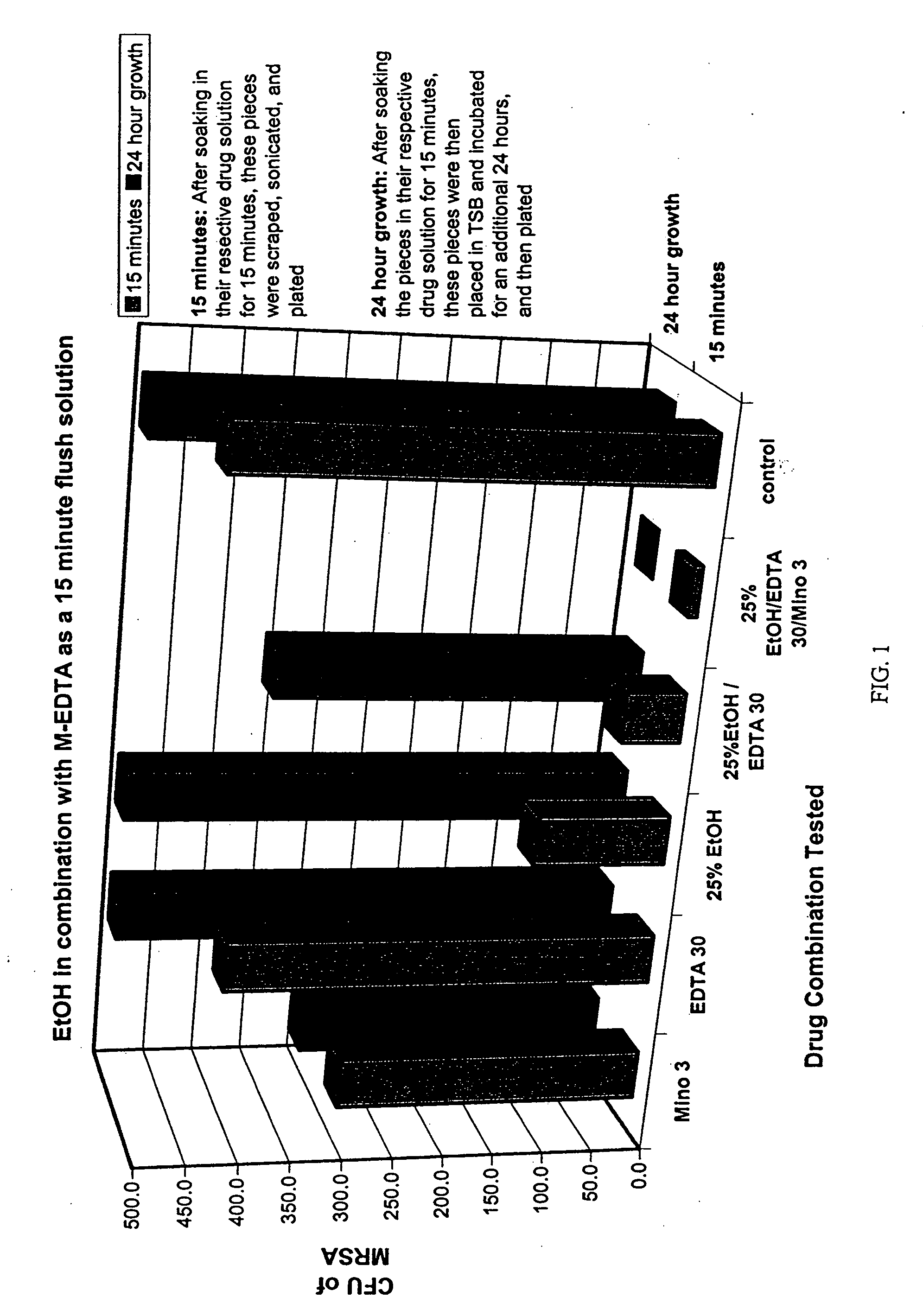
Antimicrobial flush solutions
The present invention provides antimicrobial solutions that comprise at least one alcohol, at least one antimicrobial agent and at least one chelator and / or anticoagulant. Also provided are methods for rapidly reducing a microbe or a virus from surfaces including surfaces of indwelling medical devices and organic surfaces such as skin and sutures, and inorganic surfaces such as hospital equipment, pipelines etc.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
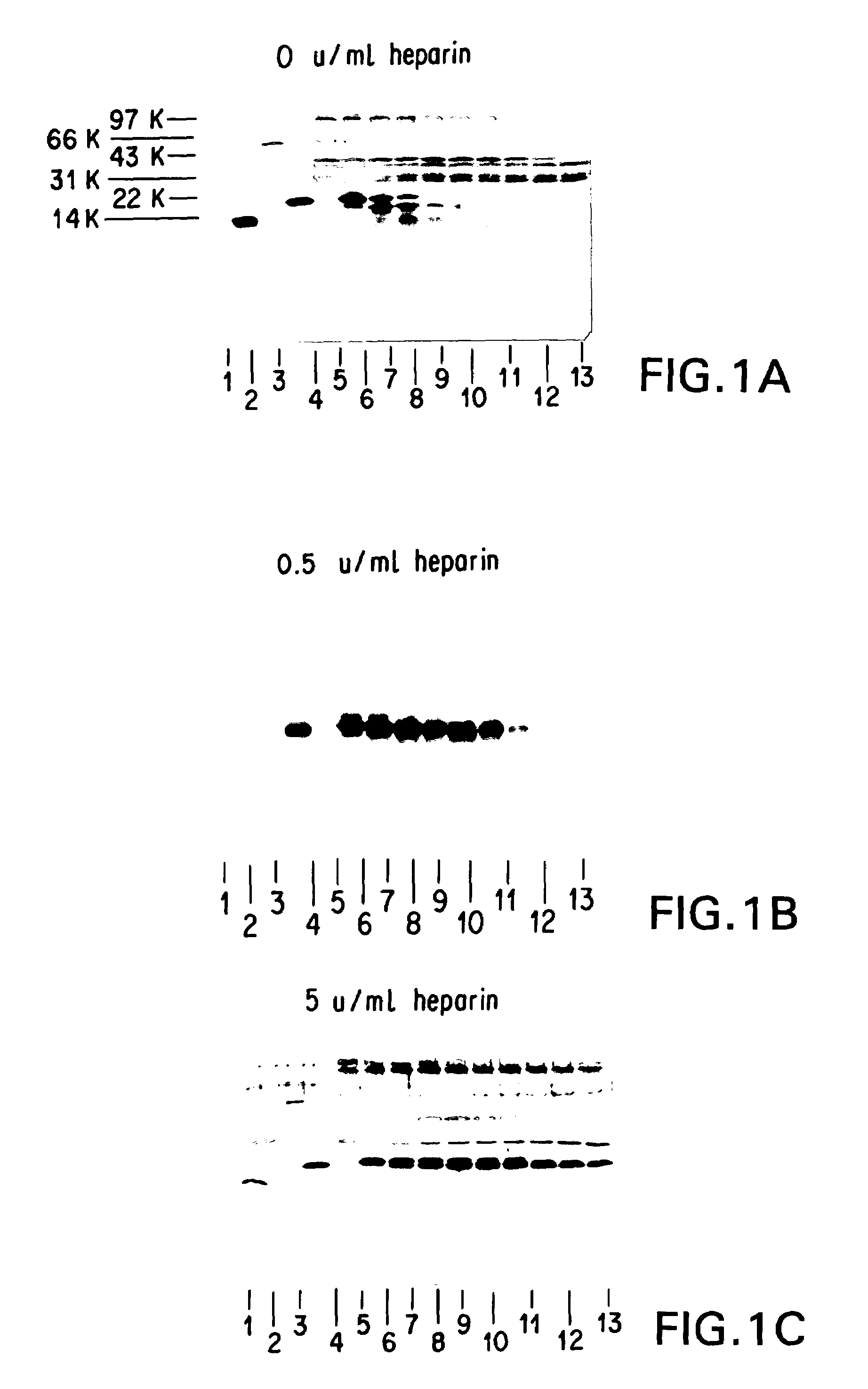
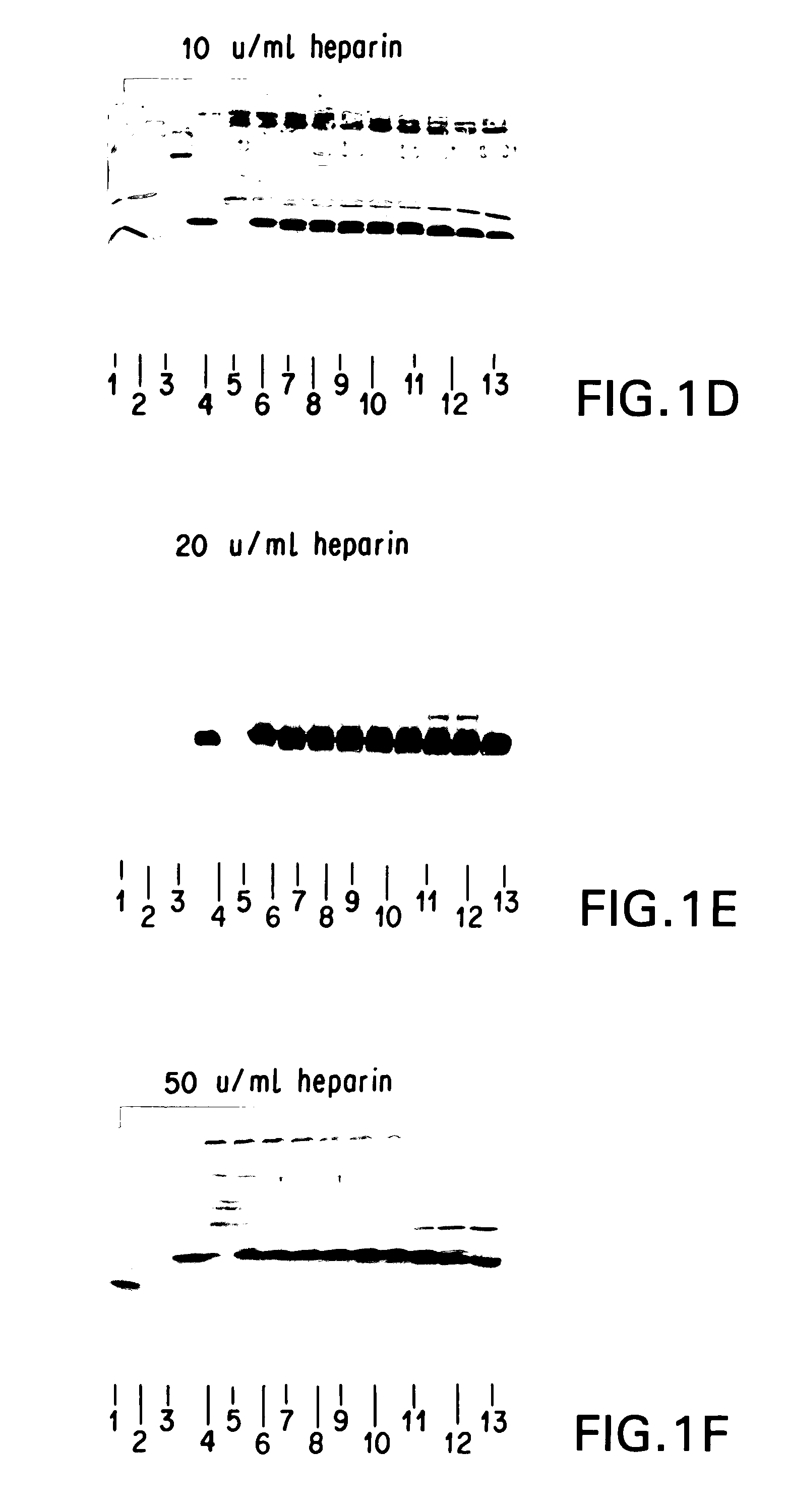
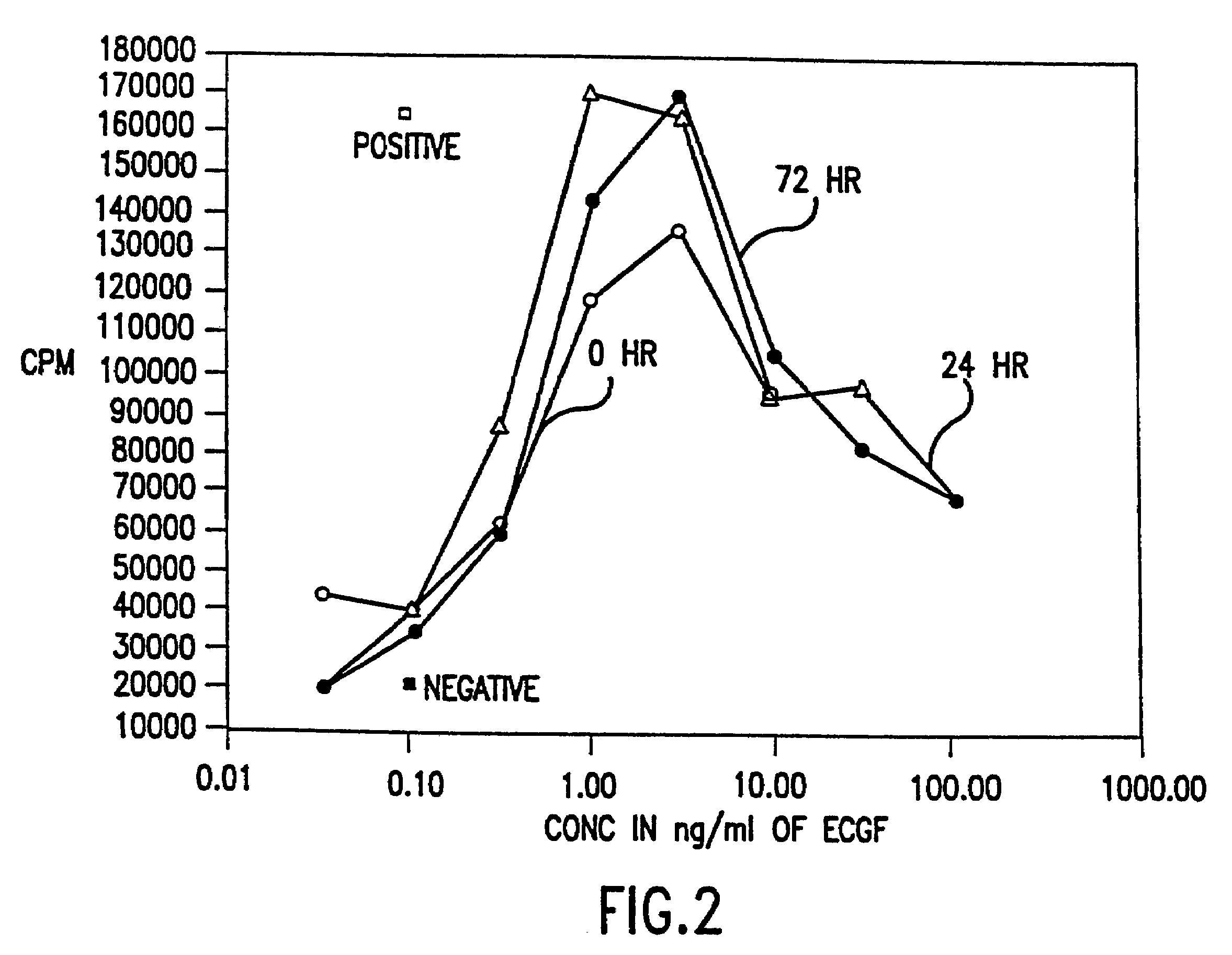
Supplemented and unsupplemented tissue sealants, methods of their production and use
ActiveUS7189410B1Low antigenicityDecreasing thrombogenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant bandage, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, anti-inflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant bandage.
Owner:AMERICAN NAT RED CROSS
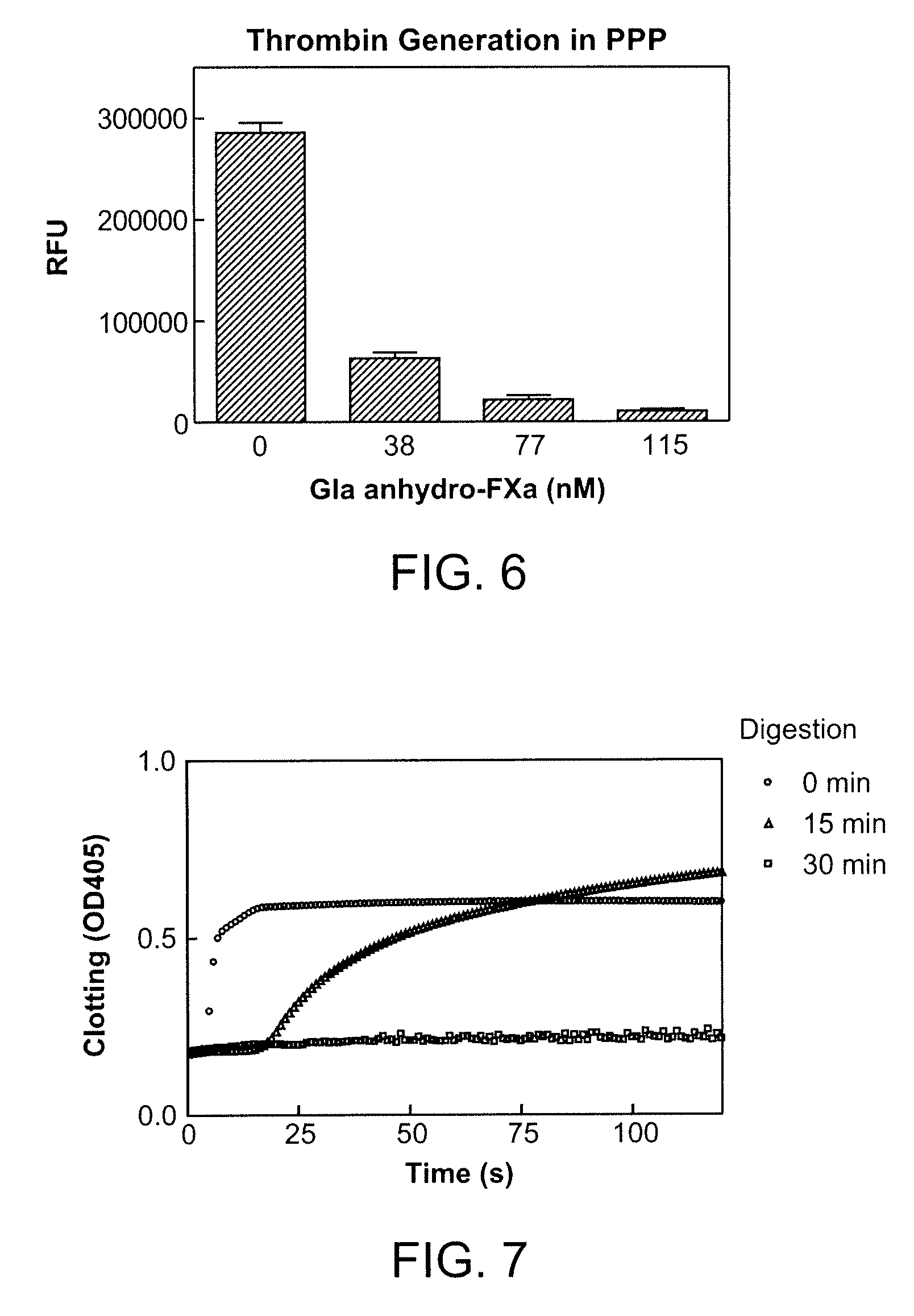
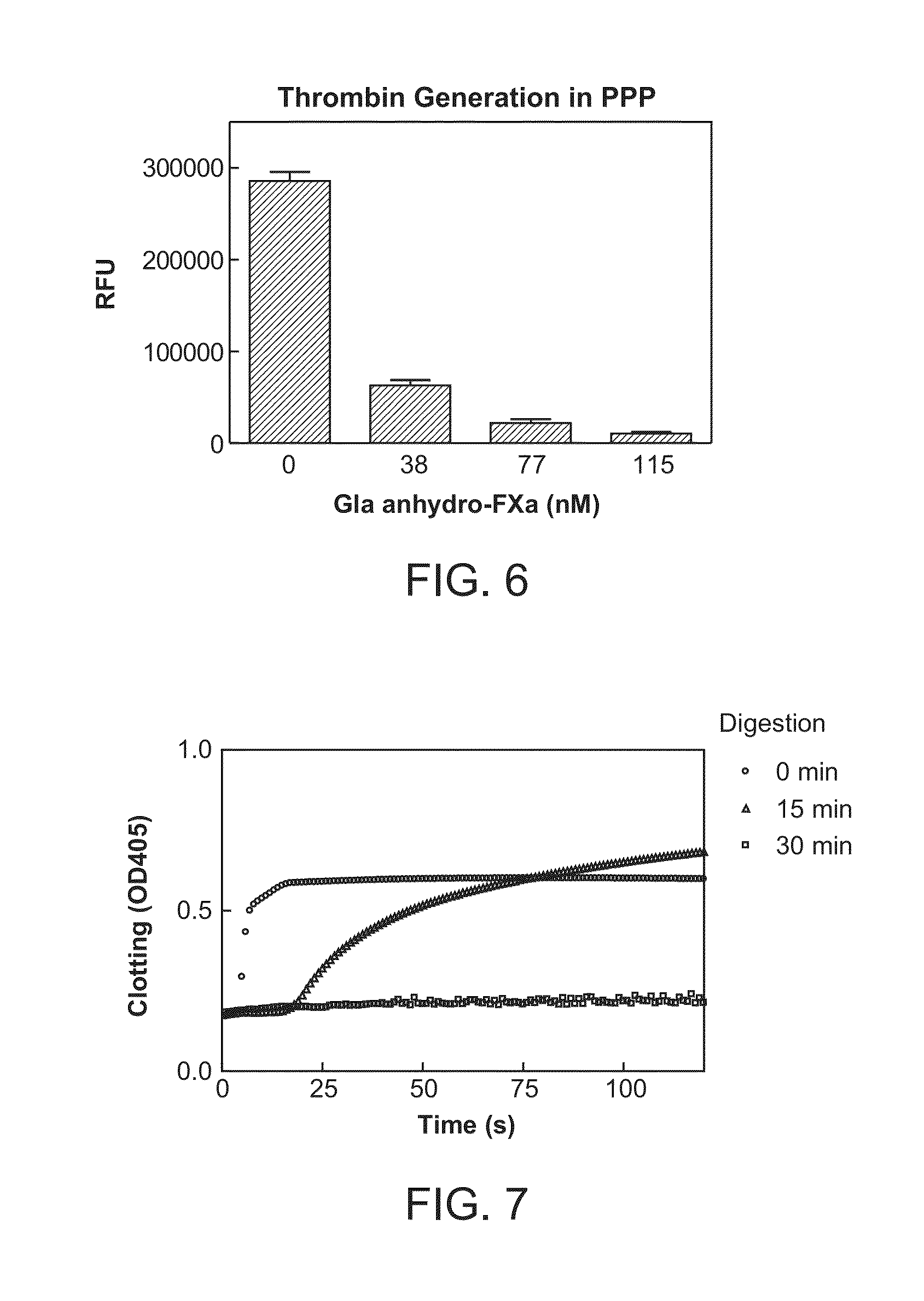
Antidotes for factor xa inhibitors and methods of using the same
ActiveUS20090098119A1Reduce and remove intrinsic procoagulantReduce and remove and anticoagulant activityOrganic active ingredientsHydrolasesMedicineAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Biaryl compounds as serine protease inhibitors
InactiveUS6699994B1Organic chemistryPeptide/protein ingredientsSerine Protease InhibitorsFactor VIIa
Owner:BIOCRYST PHARM INC
Antidotes for factor Xa inhibitors and methods of using the same
ActiveUS8153590B2Reduces and removes anticoagulant effectReduced activityPeptide/protein ingredientsMammal material medical ingredientsAntidoteFactor Xa Inhibitor
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
(Hetero)aryl-bicyclic heteroaryl derivatives, their preparation and their use as protease inhibitors
The present invention provides novel compounds of the Formula (I): A-B, its prodrug forms, or pharmaceutically acceptable salts thereof, wherein A represents a saturated, unsaturated, or a partially unsaturated bicyclic heterocyclic ring structure, and B represents an aryl or a heteroaryl group. Preferred compounds of the present invention comprise a benzimidazole or indole nucleus. The compounds of this invention are inhibitors of serine proteases, Urokinase (uPA), Factor Xa (FXa), and / or Factor VIIa (FVIIa), and have utility as anti cancer agents and / or as anticoagulants for the treatment or prevention of thromboembolic disorders in mammals.
Owner:AXYX PHARMA INC
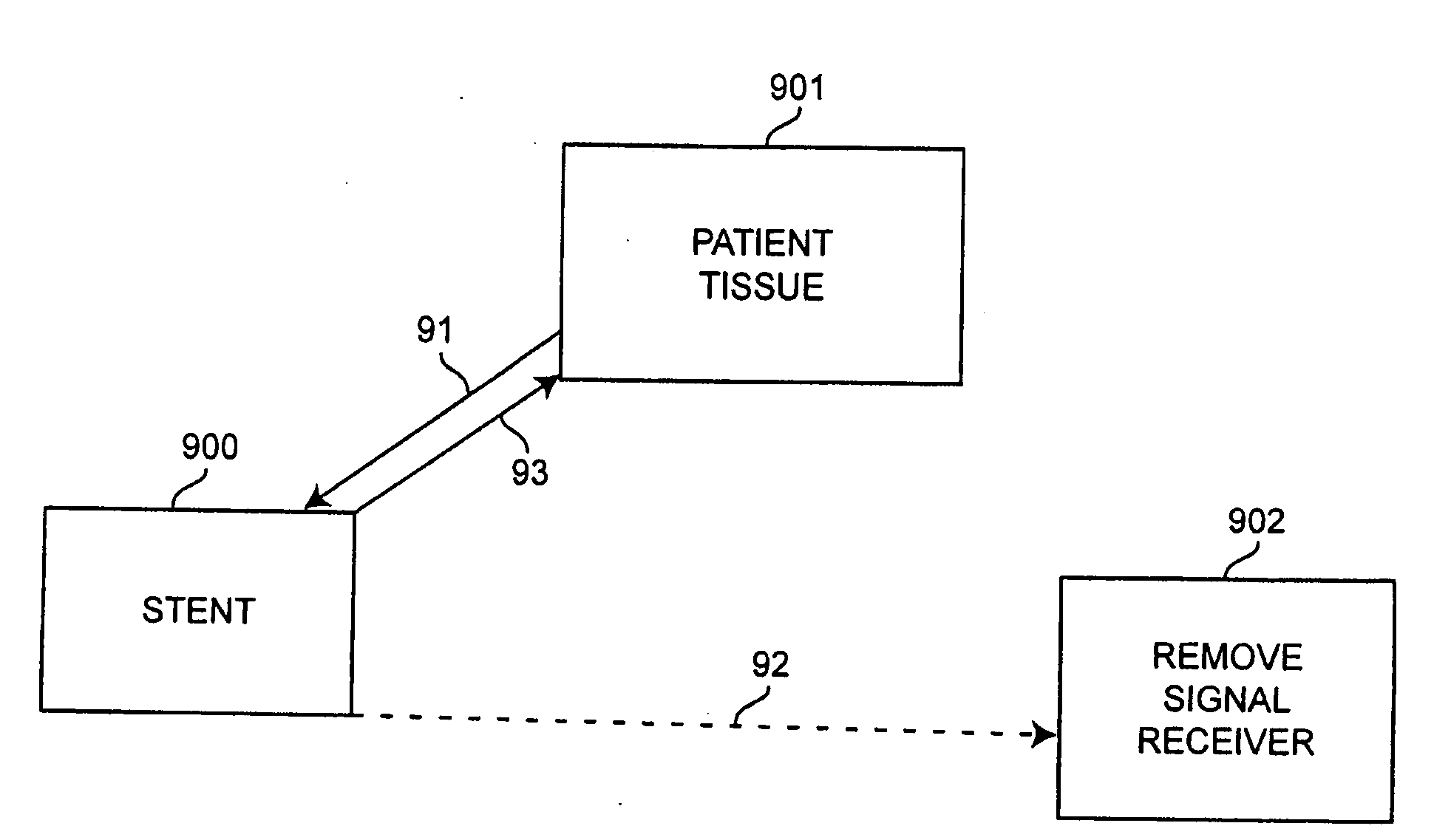
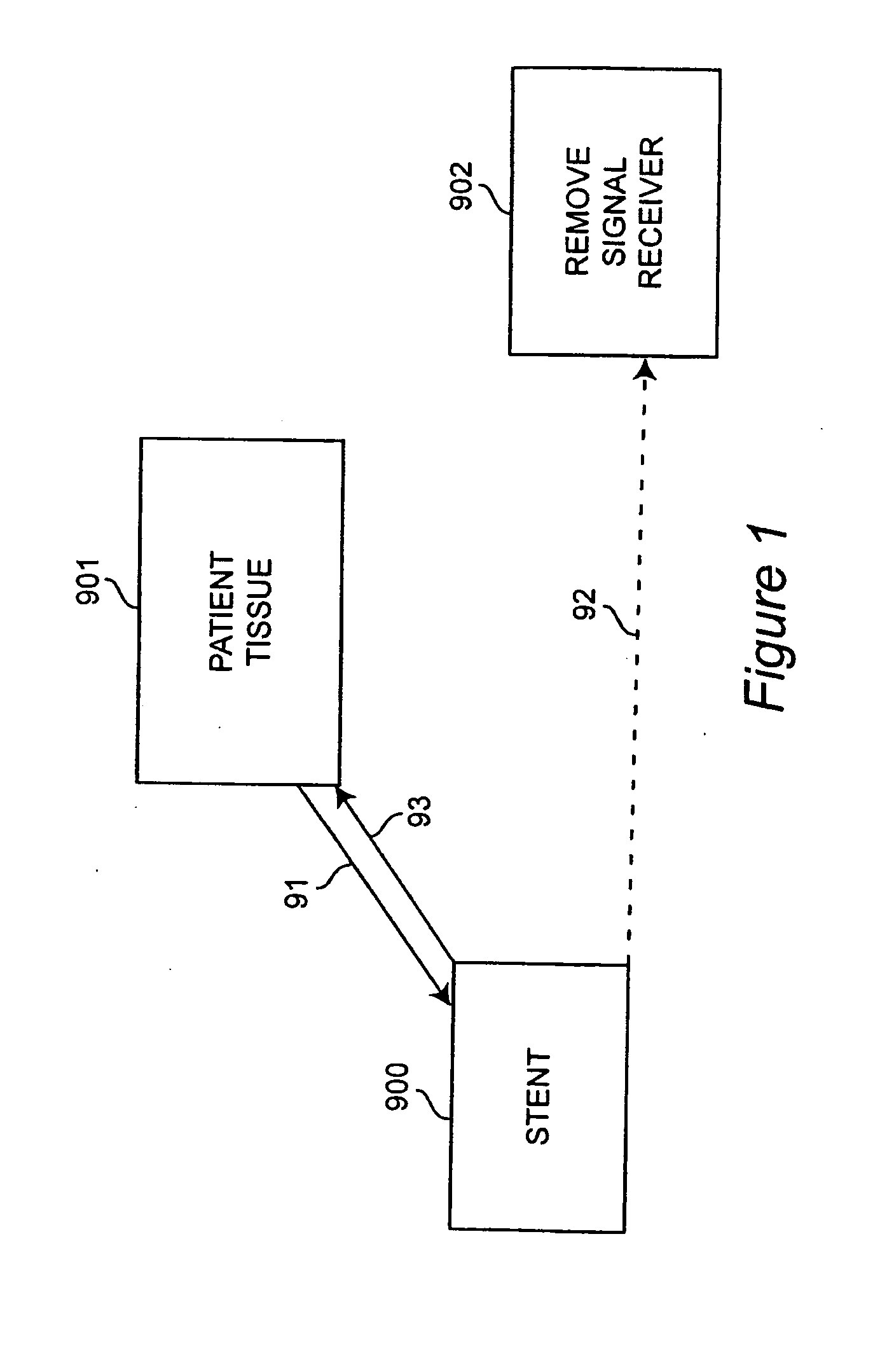
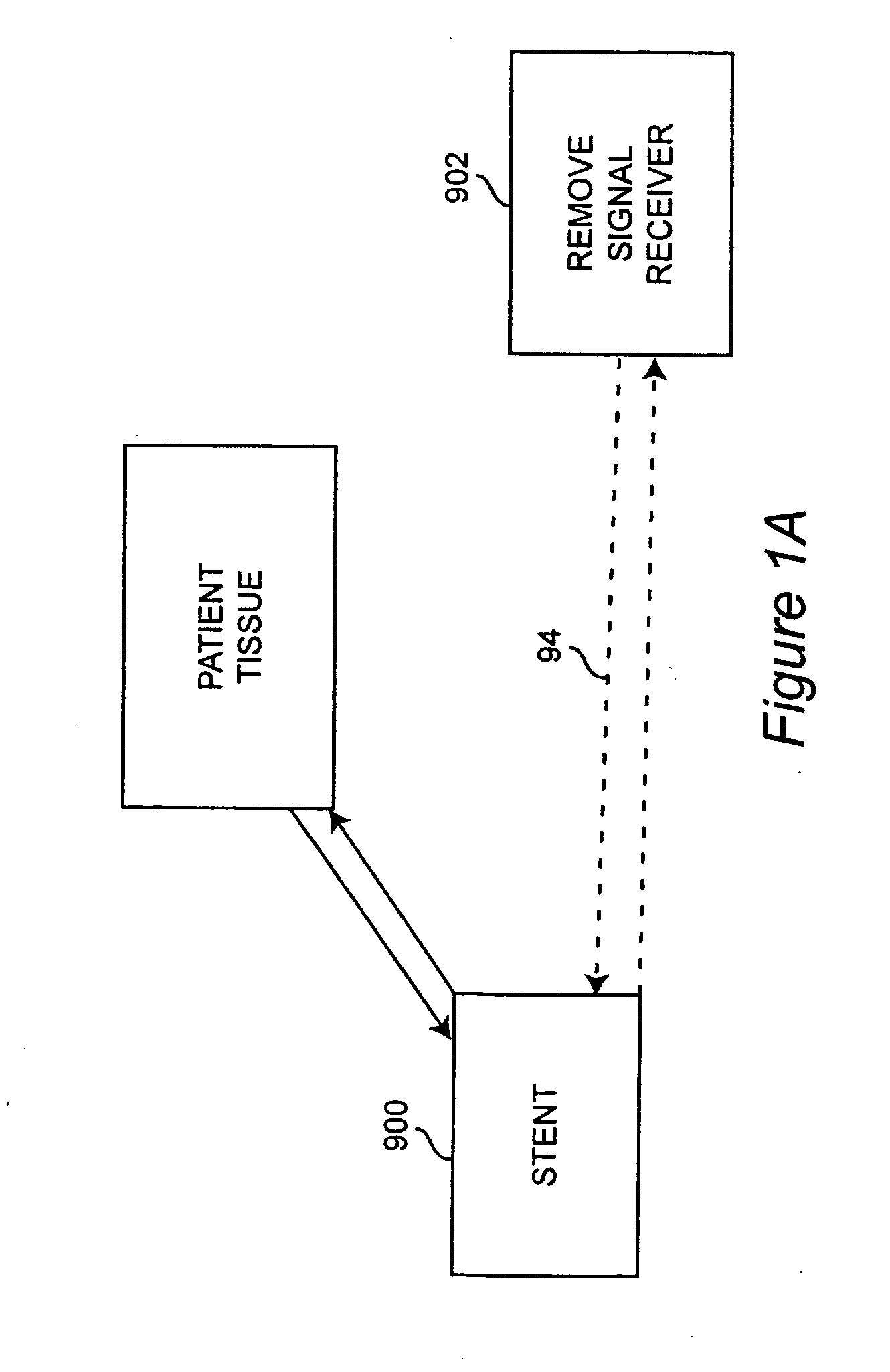
Self-sensing stents, smart materials-based stents, drug delivery systems, other medical devices, and medical uses for piezo-electric materials
InactiveUS20090036975A1Advanced technologyProvide solutionStentsElectrotherapyTreatment effectSelf sensing
A medically implantable stent comprising at least one piezo-electric material may be active, such as by one or more of: delivering an anti-coagulant or other therapeutic effect to a patient in which it is implanted; powering itself; and / or sending an outbound electronic signal to a remote device. When a stent can send such an outbound signal, a physician may non-invasively ascertain the condition of the tissue near the stent.
Owner:VIRGINIA COMMONWEALTH UNIV
Medical device with therapeutic agents
A medical device is adapted for at least partial implantation into a body and includes first and second sections along the length of the device. A first therapeutic agent is associated with the first section and a second therapeutic agent is associated with the second section. The first therapeutic agent can be one or more antiproliferative, such as paclitaxel, a paclitaxel derivative, or a paclitaxel pro-drug, anti-coagulant, antithrombotic, thrombolytic, fibrinolytic, or combination thereof. The second therapeutic agent can be one or more antimicrobials, such as one or more antibiotics. Each of the first and second therapeutic agents can either be posited on one or more surfaces of the respective section, or impregnated within the section. The device can include a separator to space the first and second sections. A method of making a medical device and a method of establishing access to a vessel within a body are also provided.
Owner:COOK MEDICAL TECH LLC
Antidotes for factor Xa inhibitors and methods of using the same
ActiveUS8268783B2Reduces and removes anticoagulant effectReduced activityBiocidePeptide/protein ingredientsFactor XAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor X and factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of reversing anticoagulation, stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
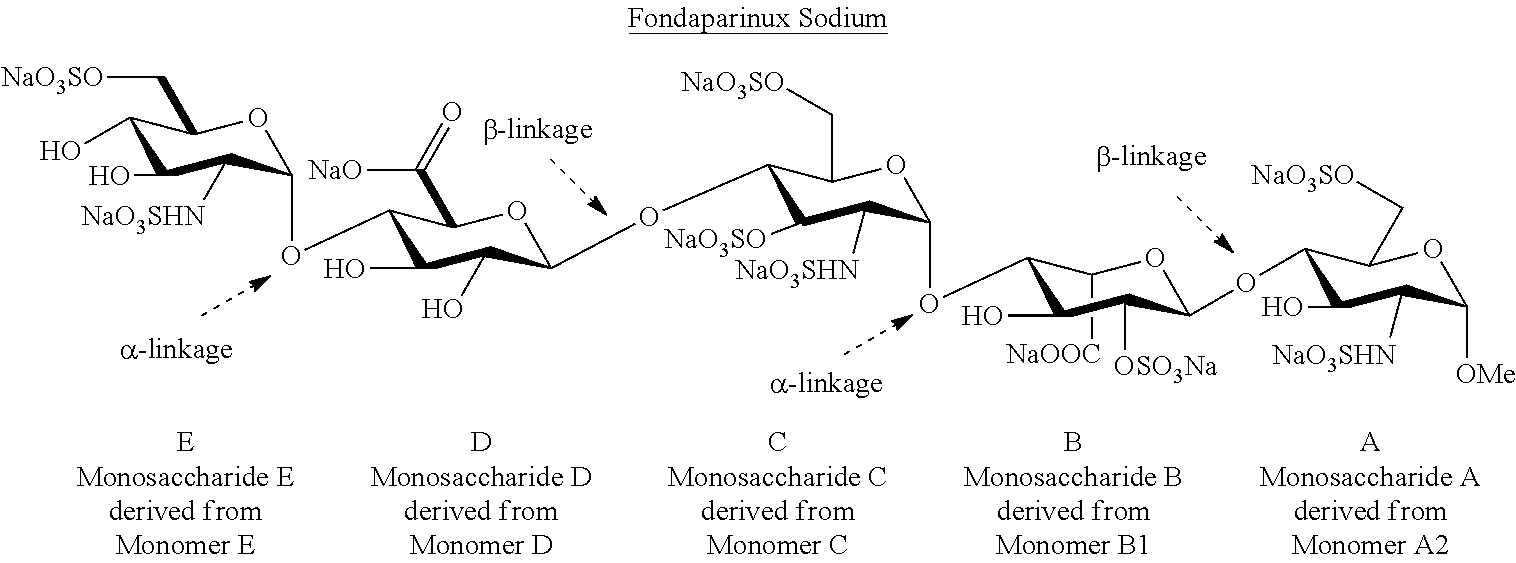
Efficient and scalable process for the manufacture of fondaparinux sodium
ActiveUS20120116066A1Efficient amplificationImprove production yieldSugar derivativesGlycosidesPhotochemistryFondaparinux Sodium
Owner:RELIABLE BIOPHARM LLC
Immobilised biological entities
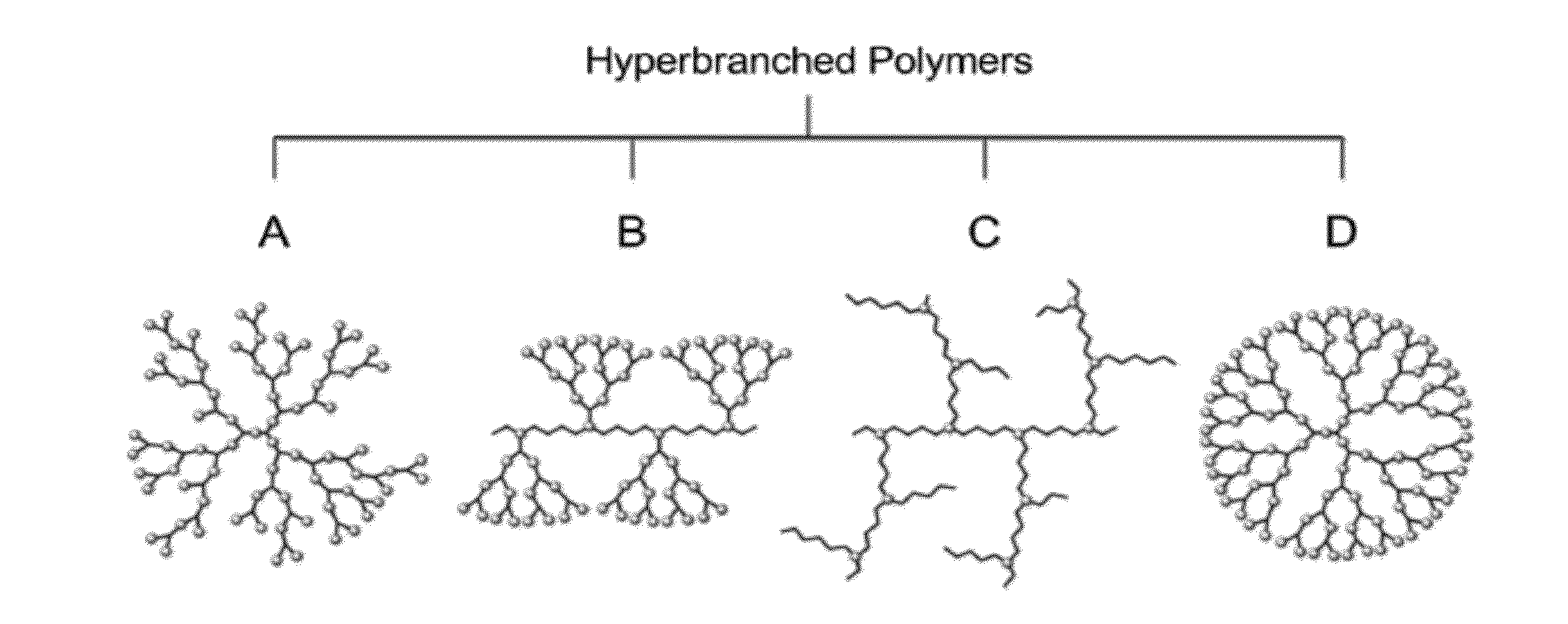
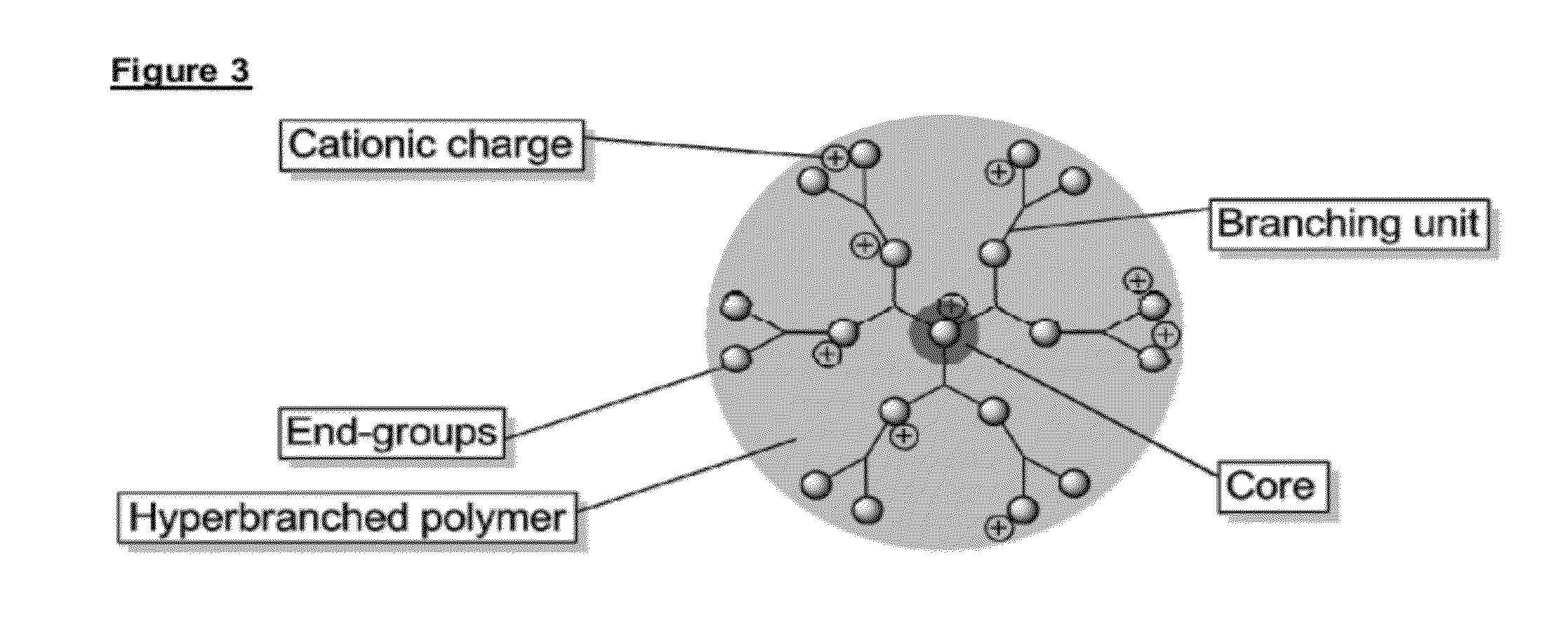
There is described inter alia a device having a surface comprising a layered coating wherein the outer coating layer comprises a plurality of cationic hyperbranched polymer molecules characterized by having (i) a core moiety of molecular weight 14-1,000 Da (ii) a total molecular weight of 1,500 to 1,000,000 Da (iii) a ratio of total molecular weight to core moiety molecular weight of at least 80:1 and (iv) functional end groups, whereby one or more of said functional end groups have an anti-coagulant entity covalently attached thereto.
Owner:WL GORE & ASSOC INC
Antidotes for factor Xa inhibitors and methods of using the same in combination with blood coagulating agents
ActiveUS8455439B2Low effective doseReduce potential side effectsPeptide/protein ingredientsMammal material medical ingredientsAntidoteFactor Xa Inhibitor
The present invention relates to antidotes of anticoagulants targeting factor Xa which antidotes are used in combination with blood coagulating agents or other heparin antidotes to prevent or reduce bleeding in a subject. The antidotes described herein have reduced or no intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is or will be undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
Apheresis methods and devices
InactiveUS20050143684A1Low toxicityAlter metabolismOther blood circulation devicesMedical devicesMedicineAntidote
An apheresis method that includes drawing blood from a mammal, adding an amount of an agent effective in preventing coagulation, wherein the agent is an anticoagulant, extracting one or more constituent components from the blood, wherein an extracted blood and constituent component result therefrom, and diminishing the activity of said anticoagulant by introducing an antidote, wherein the amount of antidote introduced is coupled with the amount of anticoagulant added. The antidote is provided either to the processed blood prior to reintroduction to the donor or directly to the donor. The invention also includes an apheresis machine that includes an antidote delivery conduit, wherein the antidote delivery conduit delivers an amount of antidote that is coupled with an amount of anticoagulant delivered.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39298E1Decreasing thrombogenicityLow antigenicityOrganic active ingredientsPowder deliveryTissue sealantVascular dilatation
This invention provides methods for the localized delivery of supplemented tissue sealants, wherein the supplemented tissue sealants comprise at least one composition which is selected from one or more antibodies, analgesics, anticoagulants, anti-inflammatory compounds, antimicrobial compositions, antiproliferatives, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Further provided are methods of using the site-specific supplemented tissue sealants, including preparation of a biomaterial.
Owner:AMERICAN NAT RED CROSS
Prodrugs of thrombin inhibitors
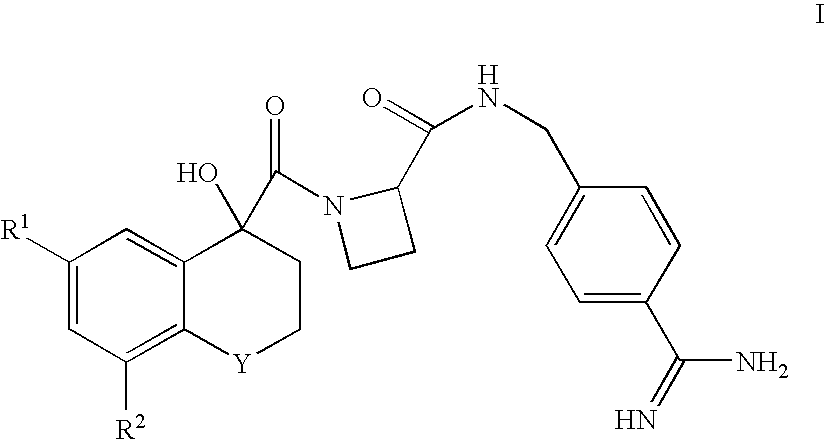
There is provided compounds of formula I,wherein R1 and R2 have meanings given in the description, which are useful as prodrugs of inhibitors of trypsin-like proteases, such as thrombin, and in particular in the treatment of conditions where inhibition of thrombin is required (eg thrombosis) or as anticoagulants.
Owner:ASTRAZENECA AB
Lubricious or/and wettable or/and Anti-thrombin elastomeric gland materials in luer activated devices
InactiveUS20070012893A1Improve wettabilityAvoid clotsMedical devicesCouplingsElastomerClot formation
An elastomeric gland is provided for a luer activating device (LAD). and comprises a unique lubricant and / or wetting agent and / or anti-clotting agent incorporated into the elastomer gland during raw material formulation, calendar blending / molding / curing to deliver the surface lubricity and / or wettability and / or avoid slit plane re-knitting and / or gland induced valve stick down of such devices Functional additive chemistries are selected in terms of generated functional performance level, thermal stability against processing, molecular migratability, molecular weight and elastomer substrate of interest. These additives could include lubricants like chemically modified silicone oils and / or wetting agents like silicone-based surfactant. Elastomer gland with wetting agent would ease fluid path priming and minimize micro air bubble adherence to gland surface. Additives may also include anti-clotting agents intended to reduce potential for clot formation within the fluid path and interstitial space of the valve during blood sampling and infusion.
Owner:BAXTER INT INC +1
Medicinal composition for prevention of or treatment for cerebrovascular disorder and cardiopathy
A pharmaceutical composition comprising at least one of components (a) and at least one of components (b) shown in below: (a) a compound represented by the general formula (I) (wherein R1 represents a hydrogen atom or a hydroxyl group) or an acid addition salt or hydrate thereof; and (b) an ameliorant of cerebral circulation, a vasodilator, a cerebral protecting drug, an brain metabolic stimulants, an anticoagulant, an antiplatelet drug, a thrombolytic drug, an amelirant of psychiatric symptom, a antihypertensive drug, an antianginal drug, a diuretic, a cardiotonic, an antiarrhythmic drug, an antihyperlipidemic drug, an immunosuppressant, or a pharmaceutically acceptable salt (except the components shown in (a)). It is useful as a preventive or remedy for cerebrovascular disorders and cardiac diseases.
Owner:ASAHI KASEI PHARMA
Thiochromane derivatives and their use as thrombin inhibitors
InactiveUS6716834B2Toxic reductionLonger actingOrganic active ingredientsBiocideThrombusPharmaceutical drug
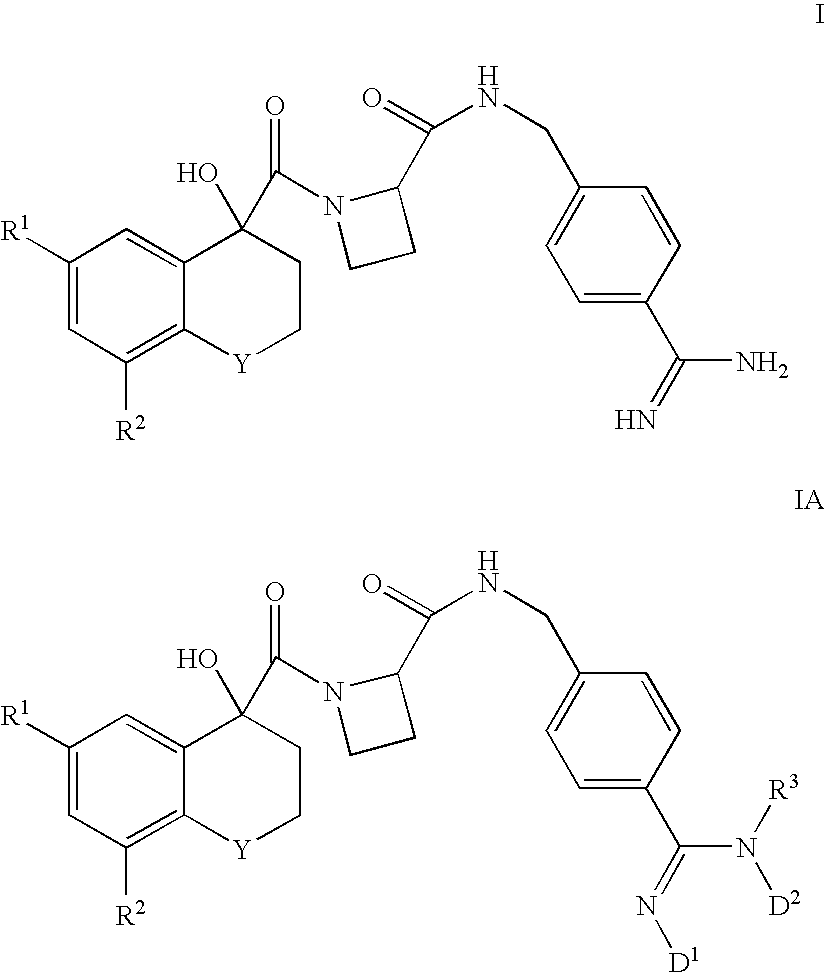
There is provided compounds of formulae I and IAwherein Y, R<1>, R<2>, R<3>, D<1 >and D<2 >have meanings given in the description which are useful as, or as prodrugs of, competitive inhibitors of trypsin-like proteases, such as thrombin, and in particular in the treatment of conditions where inhibition of thrombin is required (e.g. thrombosis) or as anticoagulants.
Owner:ASTRAZENECA AB
Amidino derivatives and their use as thrombin inhibitors
InactiveUS6265397B1More efficaciousToxic reductionBiocideOrganic chemistryThrombusAmidino derivative
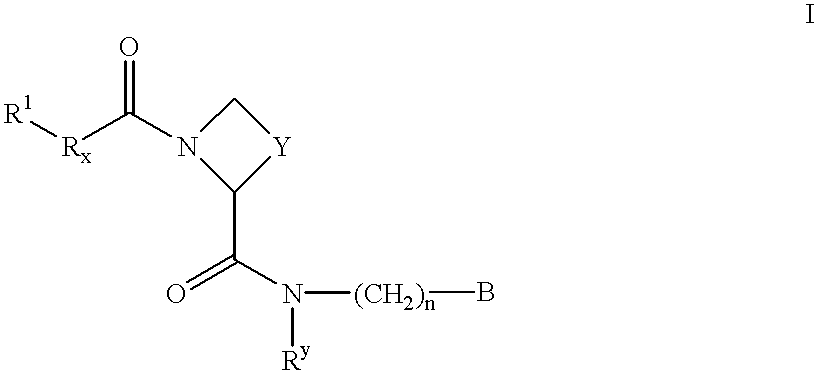
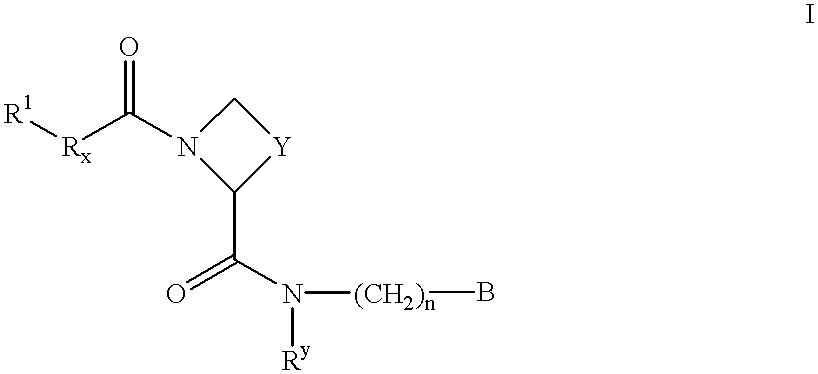
There is provided compounds of formula I,wherein R1, Rx, Y, Ry, n and B have meanings given in the description which are useful as competitive inhibitors of trypsin-like proteases, such as thrombin, and in particular in the treatment of conditions where inhibition of thrombin is required (e.g. thrombosis) or as anticoagulants.
Owner:ASTRAZENECA AB
Antibacterial and antitumor orthopaedic implantation material and preparation method thereof
The invention discloses an antibacterial and antitumor orthopaedic implantation material and a preparation method thereof. The implantation material is a medical pure titanium sheet with the surface modified by chitosan, methotrexate, heparin sodium, polylysine and dopamine. According to the material, dopamine serves as a bridge to allow polylysine and a heparin sodium particle to be bonded on the surface of titanium; heparin, as a good anticoagulant, can improve biocompatibility between the material and blood, and is bonded with chitosan and methotrexate with antibacterial and antitumor effects through an electrostatic effect. Physiological-biochemical characteristics of drugs are utilized ingeniously to form an orderly and uniform antibacterial and antitumor coating with high biocompatibility on the surface of the titanium, and the prepared implantation material has good antibacterial and antitumor activity, and can effectively prevent bacterial adhesion and reproduction, as well as recurrence and transfer of a cancer.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
Three-Line Apheresis System and Method
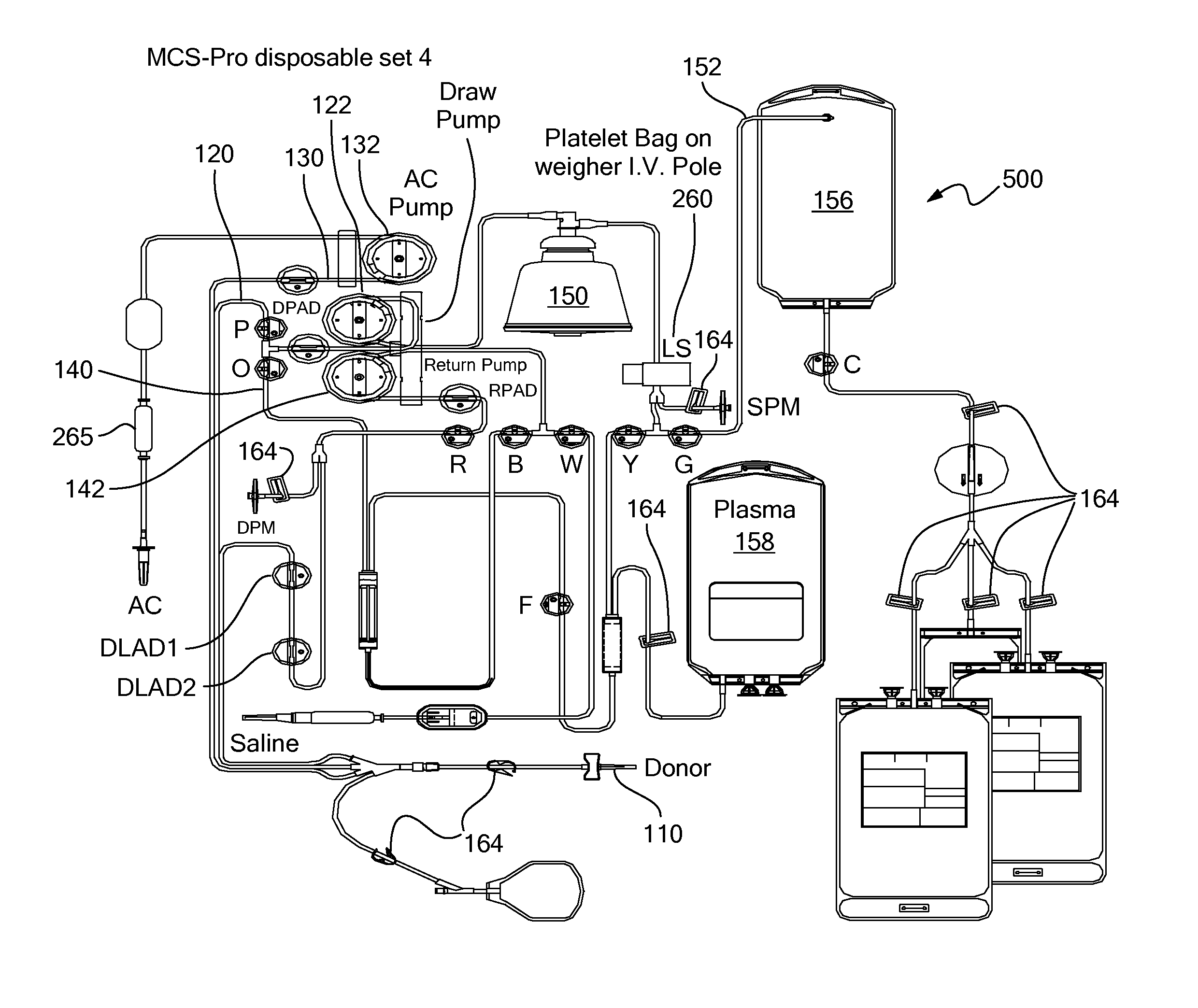
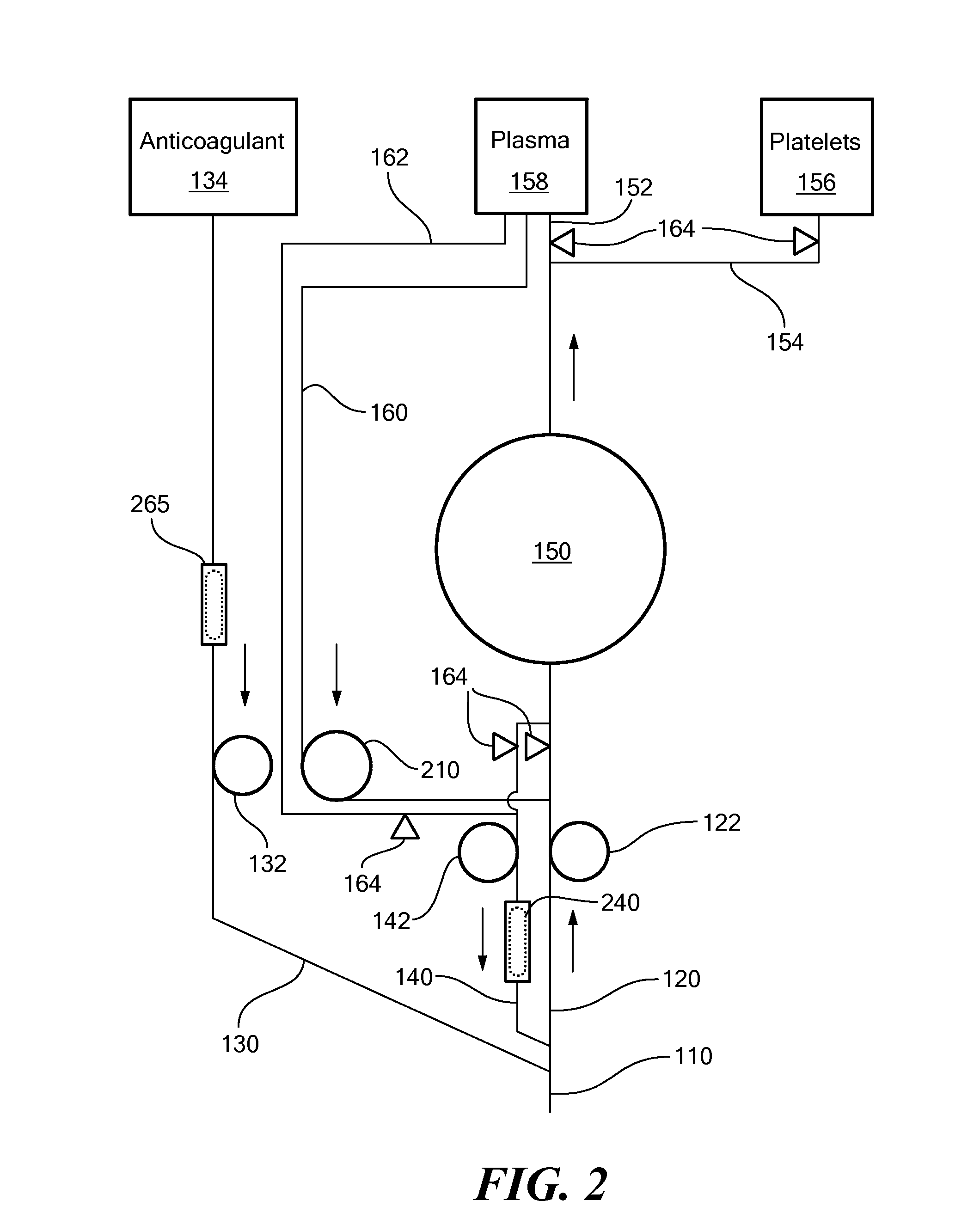
A blood processing system for collecting and exchanging blood components includes a venous-access device for drawing whole blood from a subject and returning blood components to the subject. The system may also include three lines connecting the venous access device to a blood component separation device and an anticoagulant source. A blood draw line fluidly connects to the venous-access device to the blood component separation device. An anticoagulant line connected to an anticoagulant source, introduces anticoagulant into the drawn whole blood. A return line, fluidly connected to the venous-access device and the blood component separation device, and returns uncollected blood component to the subject. A draw pump, an anticoagulant pump, and a return pump, respectively control the flows through the draw line, anticoagulant line, and the return line. The blood component separation device separates the drawn blood into a first blood component and a second blood component. The blood component separation device also may be configured to send the first blood component to a first blood component bag.
Owner:HAEMONETICS
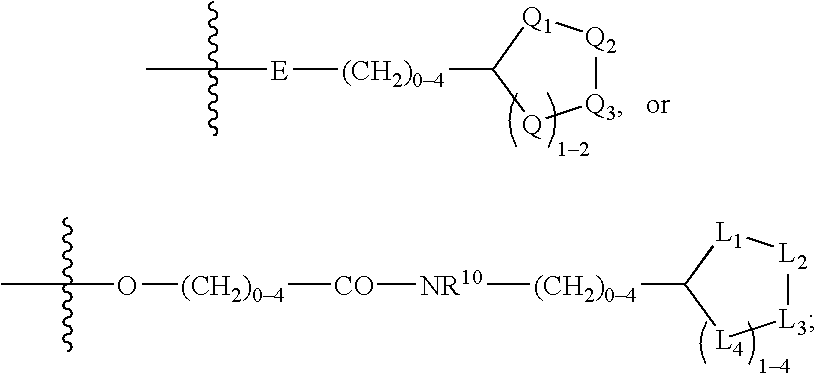
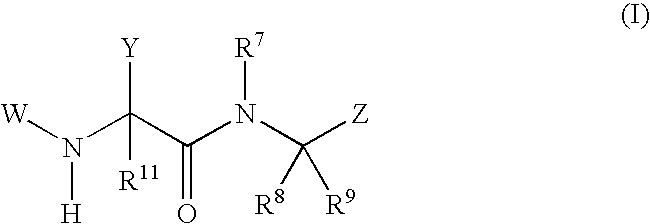
Phenylglycinamide and pyridylglycinamide derivatives useful as anticoagulants
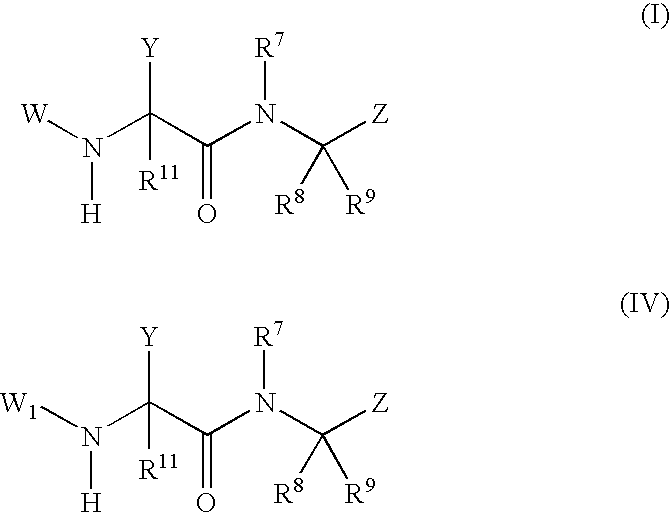
The present invention provides novel phenylglycinamide derivatives of Formula (I) or (IV): or a stereoisomer, tautomer, pharmaceutically acceptable salt, solvate, or prodrug thereof, wherein the variables W, W1, Y, Z, R7, R8, R9, and R11 are as defined herein. These compounds are selective inhibitors of factor VIIa which can be used as medicaments.
Owner:BRISTOL MYERS SQUIBB CO
Microfluidic chip-based, universal coagulation assay
ActiveUS20160069913A1Easy doseEnsures comparabilityBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careShear flow
A microfluidic, chip-based assay device has been developed for measuring physical properties of an analyte (particularly, whole blood or whole blood derivatives). The technologies can be applied to measure clotting times of whole blood or blood derivatives, determine the effects of anticoagulant drugs on the kinetics of clotting / coagulation, as well as evaluate the effect of anticoagulant reversal agents. These technologies can additionally be used to optimize the dosage of anticoagulation drugs and / or their reversal agents. The assay is independent of the presence of anticoagulant; clotting is activated by exposure of the blood sample in the device to a glass (or other negatively charged material such as oxidized silicon) surface, which activates the intrinsic pathway and can be further hastened by the application of shear flow across the activating materials surface. The absence of chemical activating agents and highly controlled and reproducible micro-environment yields a point of care universal clotting assay.
Owner:PEROSPHERE TECH INC
Extraction method of platelet rich plasma (PRP) and extracted PRP
ActiveCN102755770ACentrifugal force sediment separationMammal material medical ingredientsCentrifugationMedicine
The invention relates to an extraction method of platelet rich plasma (PRP) and extracted PRP and particularly relates to a method for extracting PRP from blood. The method comprises the following steps of: (a) putting the collected whole blood in a container containing an anticoagulant, and fully and uniformly mixing the blood and the anticoagulant; (b) putting the blood mixed with the anticoagulant in a centrifuge tube, carrying out primary centrifugation, and enabling the blood to be divided into three layers; (c) extracting the uppermost layer and most of the middle layer, transferring the uppermost layer and most of the middle layer into a new centrifuge tube, mixing uniformly and carrying out secondary centrifugation; and (d) discarding the plasma at the upper layer of the centrifuge tube, and resuspending the precipitated plasma by utilizing the remaining plasma, thus obtaining the PRP. The method provided by the invention has the advantages that the operation process is simple, the yield is high and the concentration of platelet in rich plasma is high.
Owner:FIVE DIMENSION BY INCOSC HEALTH MANAGEMENT JIANGSU
Methods for Treating Bleeding Disorders
A method of factor XI-dependent blood coagulation enhancement in a subject in need of enhanced blood coagulation comprising administering a therapeutically effective amount of a composition comprising a non-anticoagulant sulfated polysaccharide (NASP) to the subject. A method of factor XI-dependent blood coagulation enhancement in a subject in need of enhanced blood coagulation comprising: (i) selecting a subject that is not deficient for factor XI; and (ii) administering a therapeutically effective amount of a composition comprising a non-anticoagulant sulfated polysaccharide (NASP) to the subject, wherein the NASP enhances blood coagulation in a factor XI-dependent manner. A method of identifying a non-anticoagulant sulfated polysaccharide (NASP) which is capable of enhancing blood coagulation in dependence on FXI, the method comprising: a) combining a blood or plasma sample comprising activation competent FXI with a composition comprising a sulfated polysaccharide and measuring the clotting or thrombin generation parameters of the blood or plasma sample; b) combining a corresponding blood or plasma sample deficient in activation competent FXI with a composition comprising the sulfated polysaccharide and measuring the clotting or thrombin generation parameters of the blood or plasma sample; and c) comparing the clotting or thrombin generation parameters of the blood or plasma samples as determined in steps (a) and (b) with each other, wherein a decrease in the clotting time of the blood sample or an increase in peak thrombin or decrease in peak time of the plasma sample comprising activation competent FXI compared to the clotting time of the blood sample or peak thrombin or peak time of the plasma sample deficient in activation competent FXI is indicative of a NASP which is capable of enhancing blood coagulation in dependence on FXI.
Owner:TAKEDA PHARMA CO LTD
Factor xa inhibitors with aryl-amidines and derivatives, and prodrugs thereof
InactiveUS20030065176A1Good choicePrevent coagulationOrganic compound preparationOrganic chemistry methodsPharmaceutical medicinePyrrole
The present invention relates to a compound with aryl-amidines, particularly amidinoaryl-cyclopropanes, amidinoarylmethyl-pyrroles, amidinoaryl-benzenes, amidinoaryl-pyridines, or amindonoaryl-alanines, represented by formula (1), a pharmaceutically acceptable salt, a prodrug, a hydrate, a solvate or an isomer thereof, which are inhibitors of coagulation enzyme, factor Xa (FXa). The present invention also relates to a pharmaceutical composition containing the compound, and a method of using the same as an anticoagulant agent for treatment and prevention of thrombosis disorders.
Owner:LG CHEM INVESTMENT LTD
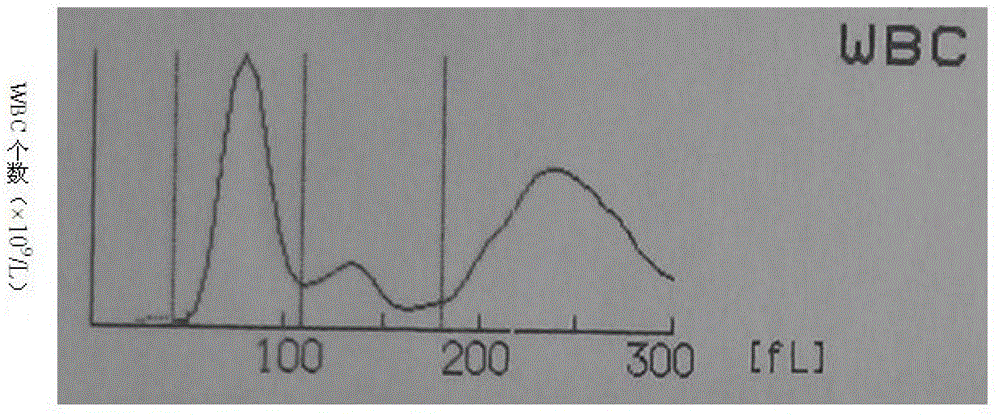
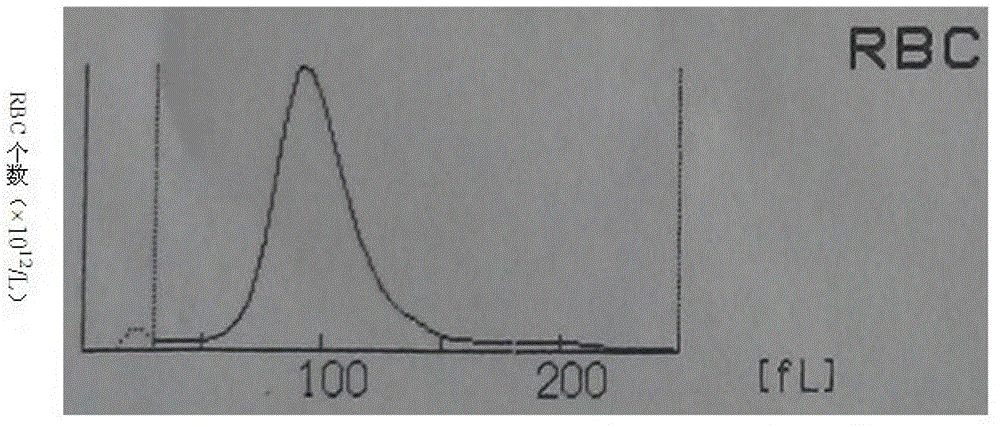
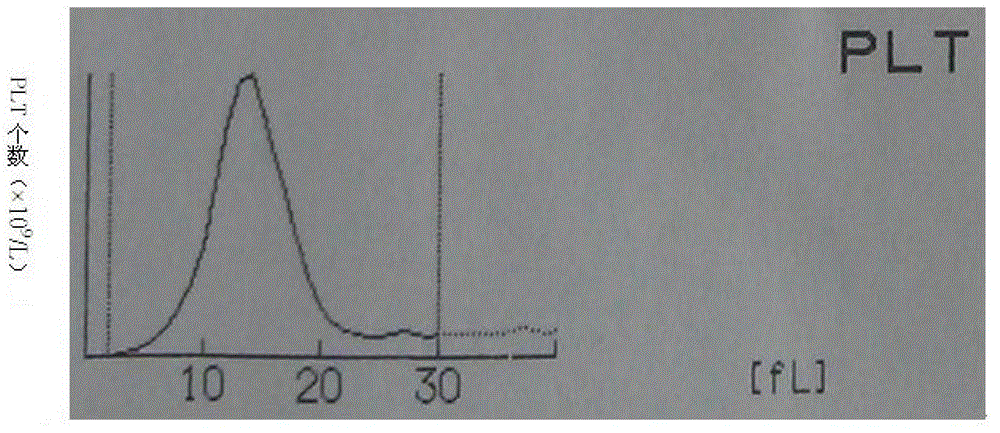
Whole blood quality control material and preparation method thereof
ActiveCN103336110AWide variety of sourcesSimple preparation processPreparing sample for investigationBiological testingSize differenceQuality control
The invention discloses a whole blood quality control material. The whole blood quality control material comprises three cell simulating materials, an anticoagulation agent, white cell stationary liquid, and red cell and blood platelet stationary liquid. The invention further discloses a preparation method of the whole blood quality control material. The whole blood quality control material is wide in raw material resources and simple in preparation process; three classes of white cells can be clearly identified on a three-class blood cell instrument; the size difference of red cells and blood platelets is obvious, and the red cells and the blood platelets are easy to identify by the instrument; interferences of the small red cells on the blood platelets are small and the storage life can reach 6 months; the use requirements of clinical quality control are met.
Owner:NANJING PERLONG MEDICAL EQUIP
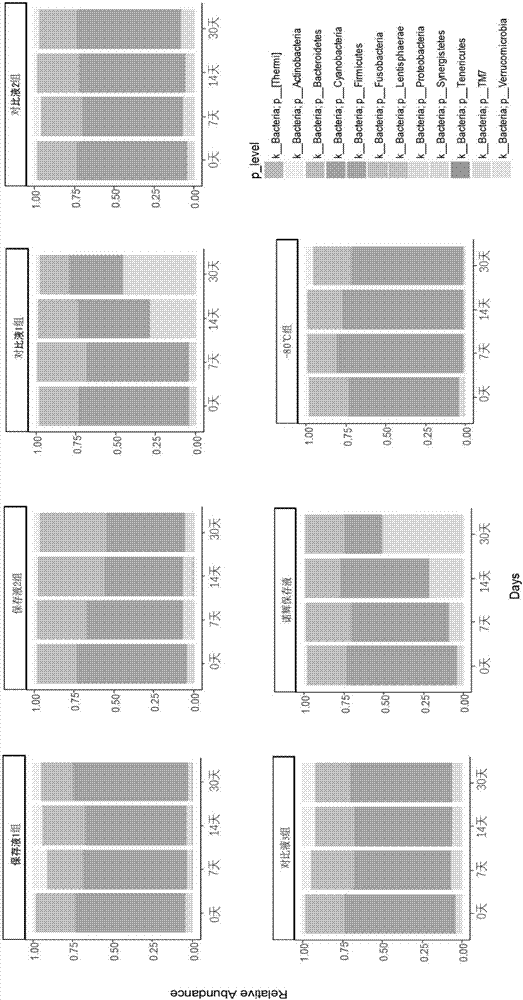
Excrement preserving fluid and applications thereof
ActiveCN107988076AStable quantityComposition is stableMicroorganism preservationSequence analysisFeces
The invention discloses an excrement preserving fluid and applications thereof. Ethanol is taken as a fixing agent, sodium citrate is taken as an auxiliary fixing agent, EDTA-disodium is taken as an anti-coagulant, Tris-HCl is taken as a buffer solution, NaCl is taken as an ion strength maintaining agent, and the preserving fluid also comprises lauryl sodium sulfate. Through a reasonable formula,the number, composition, and abundance of microbes in an excrement sample are stabilized; the accuracy of sequencing analysis on human intestinal flora is guaranteed; the preserving fluid can preservean excrement sample at a room temperature; compared with a conventional preserving fluid, the preserving effect is better, moreover, provided preserving fluid does not contain any toxic component such as guanidinium thiocyanate, and the operation is safer.
Owner:CAPITALBIO GENOMICS
Blood sample collecting device
ActiveCN104673623AImprove integrityFor long-term storageBioreactor/fermenter combinationsBiological substance pretreatmentsBlood plasmaSodium hydroxide
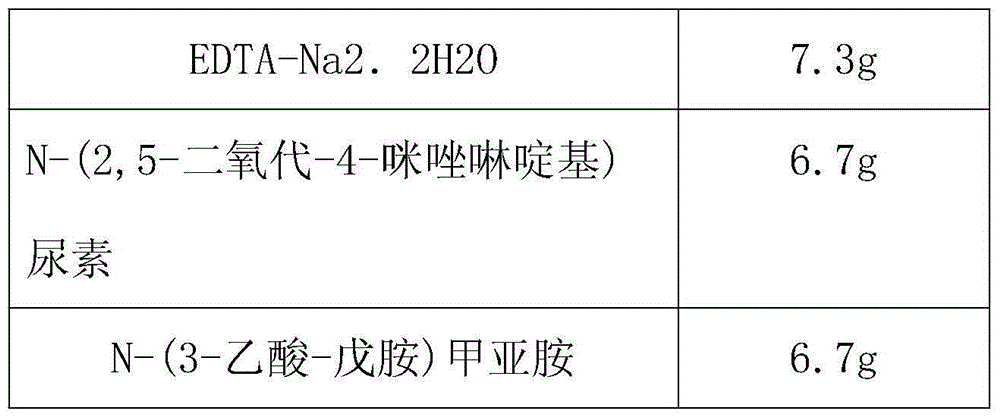
The invention discloses a blood sample collecting device. The collecting device comprises a collecting tube, wherein an orifice of the collecting tube is provided with a sealing rubber plug, the outside of the rubber plug is provided with a protective cap, the collecting tube is filled with additives, the additives are composed of a blood anticoagulation, a hemocyte nucleic acid stabilizing agent, a blood plasma nucleic acid stabilizing agent, and a pH buffer solution; the blood anticoagulation is one or more of oxalate, heparinate and citrate, the pH buffer solution is one of glycine-sodium hydroxide-hydrochloric acid buffer solution; the hemocyte nucleic acid stabilizing agent is one or more of allantoin, 5,5-dimethyl hydantoin and oxazolidine; the blood plasma nucleic acid stabilizing agent is N-acetic acid azomethine, N-(3-acetic acid-amylamine)azomethine. The blood sample collecting device simultaneously contains the hemocyte nucleic acid stabilizing agent and the blood plasma nucleic acid stabilizing agent, and does not contain free aldehyde material, so that the blood sample can be stored in long term at normal temperature, and the free nucleic acid level of the blood sample can be stabilized, and the completeness of the free nucleic acid can be maintained.
Owner:广州维帝医疗技术有限公司
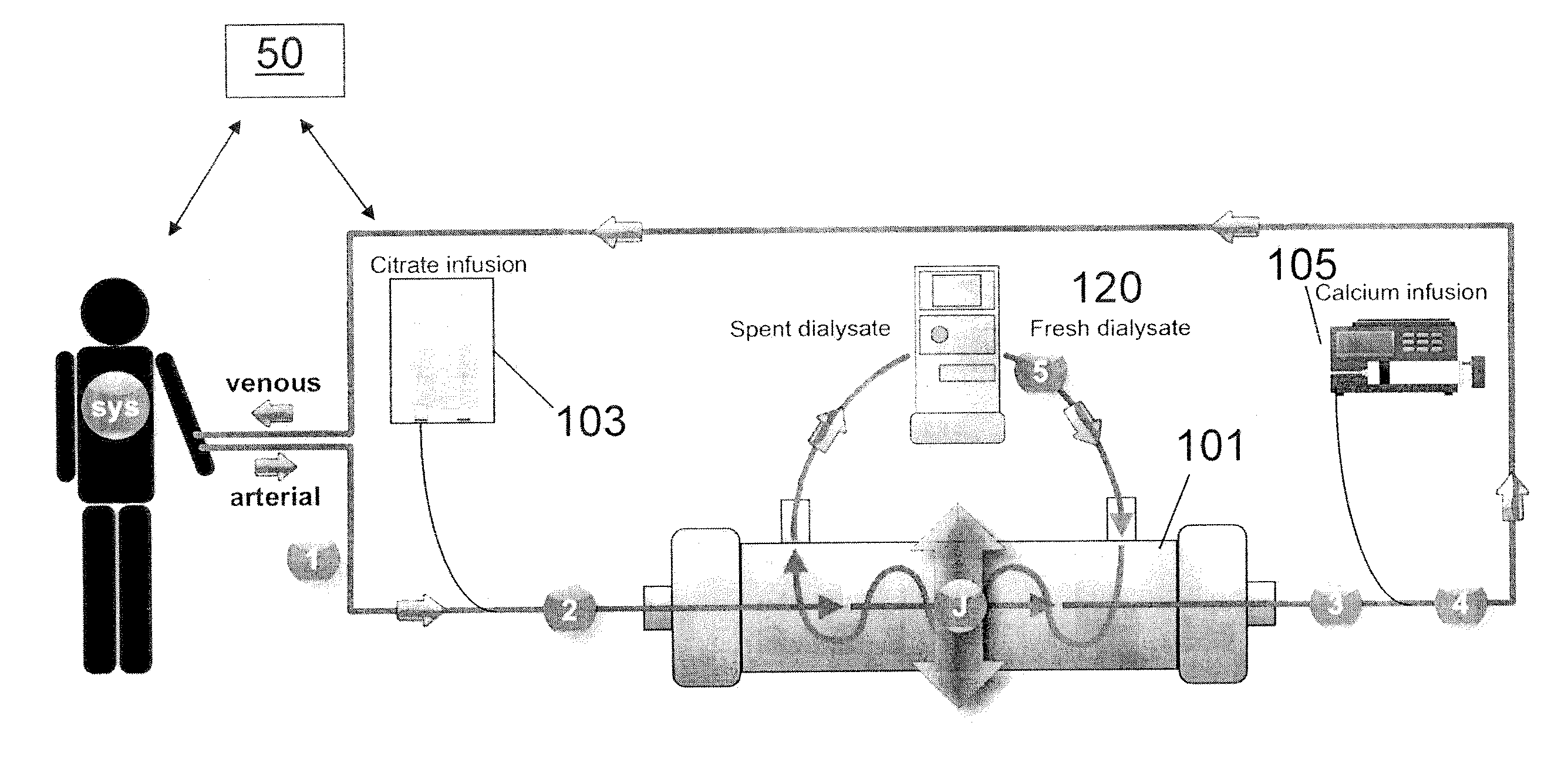
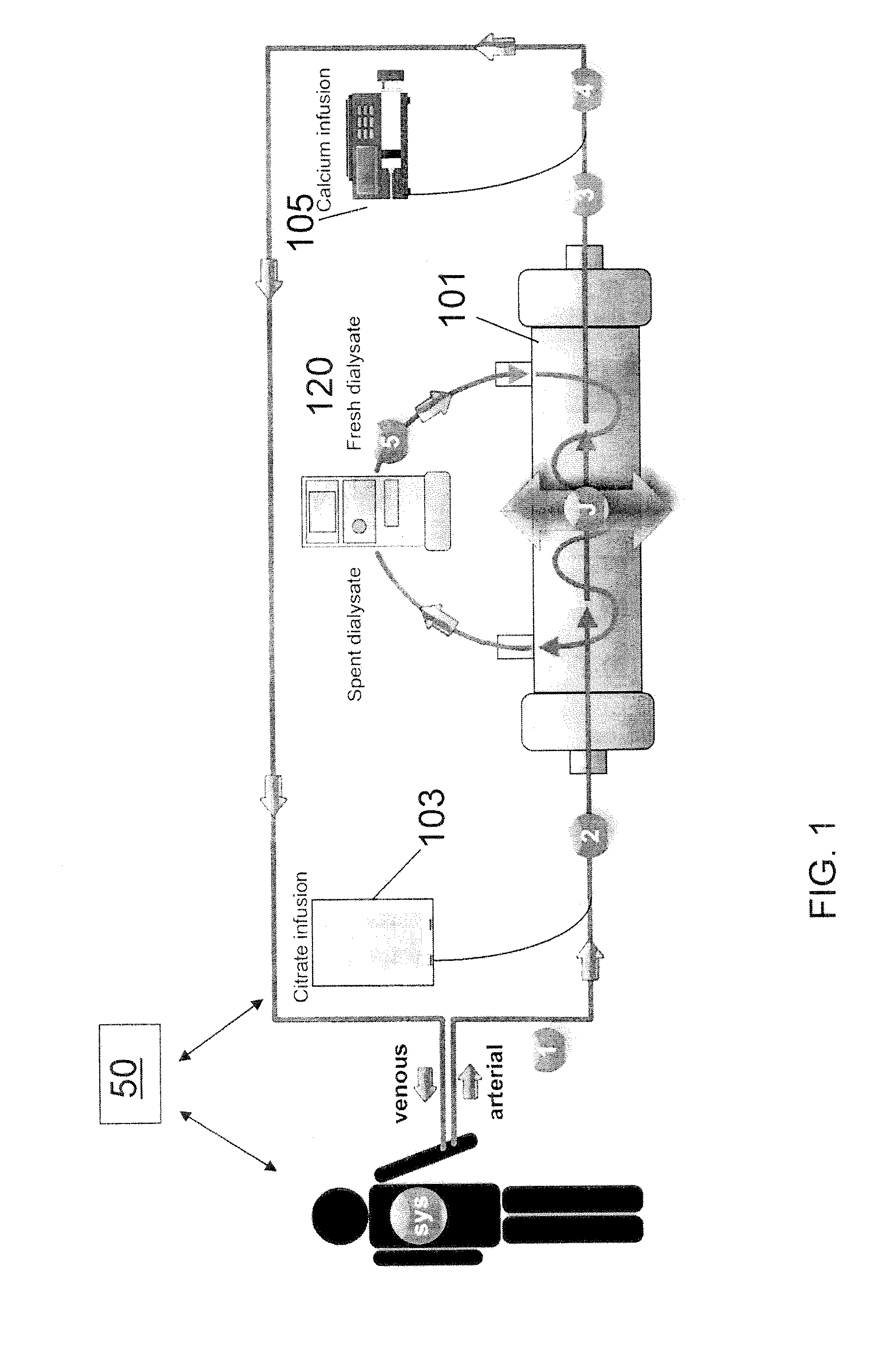
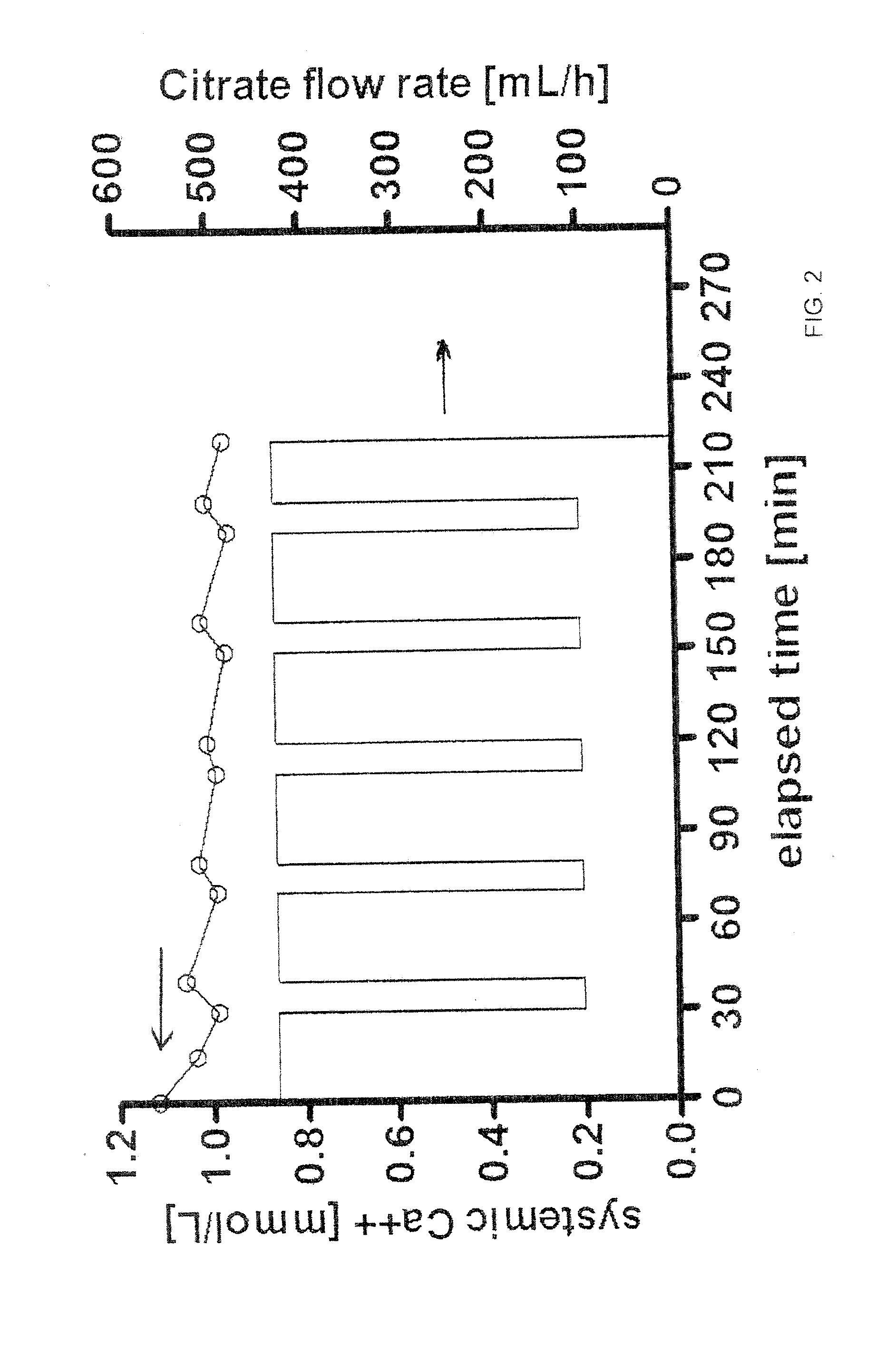
Methods of Regional Citrate Anticoagulation Dialysis
ActiveUS20110237996A1Eliminating well-known downsideEliminates potentialDialysis systemsMedical devicesDialysis membranesCITRATE ESTER
A method of performing regional citrate anticoagulant dialysis of a patient's blood includes flowing blood from and back to the patient through an extracorporeal circuit including a dialyzer having semi-permeable dialysis membranes and a dialysate chamber surrounding the membranes. The method further includes flowing a dialysate containing calcium and citrate through the dialysate chamber of the dialyzer and introducing citrate into the patient's blood upstream of the dialyzer, whereby the patient's blood is dialyzed. The method can further include predicting the concentration of systemic ionized calcium in the blood of the patient at any point in the dialysis treatment or post-dialysis, such as by a mathematical model. The method can further include statistically correcting the preliminary predicted post-dialysis concentration of systemic ionized calcium in the patient's blood to provide a final predicted post-dialysis systemic ionized calcium concentration. The method can further include statistically correcting the preliminary predicted systemic ionized calcium concentration for any time point during the dialysis treatment to provide a final predicted systemic ionized calcium concentration for that time point.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com