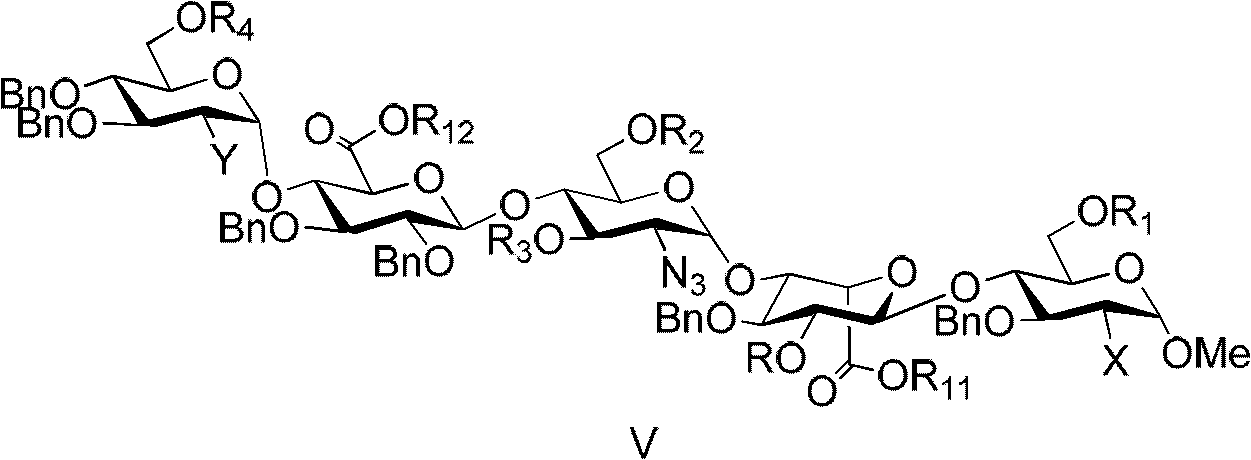
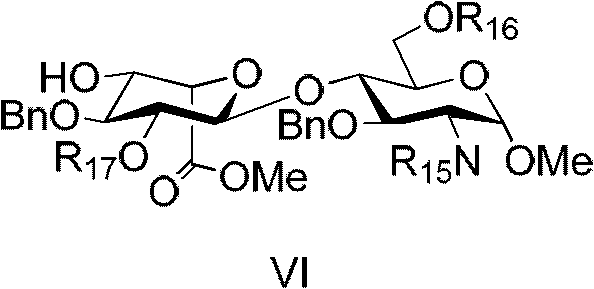
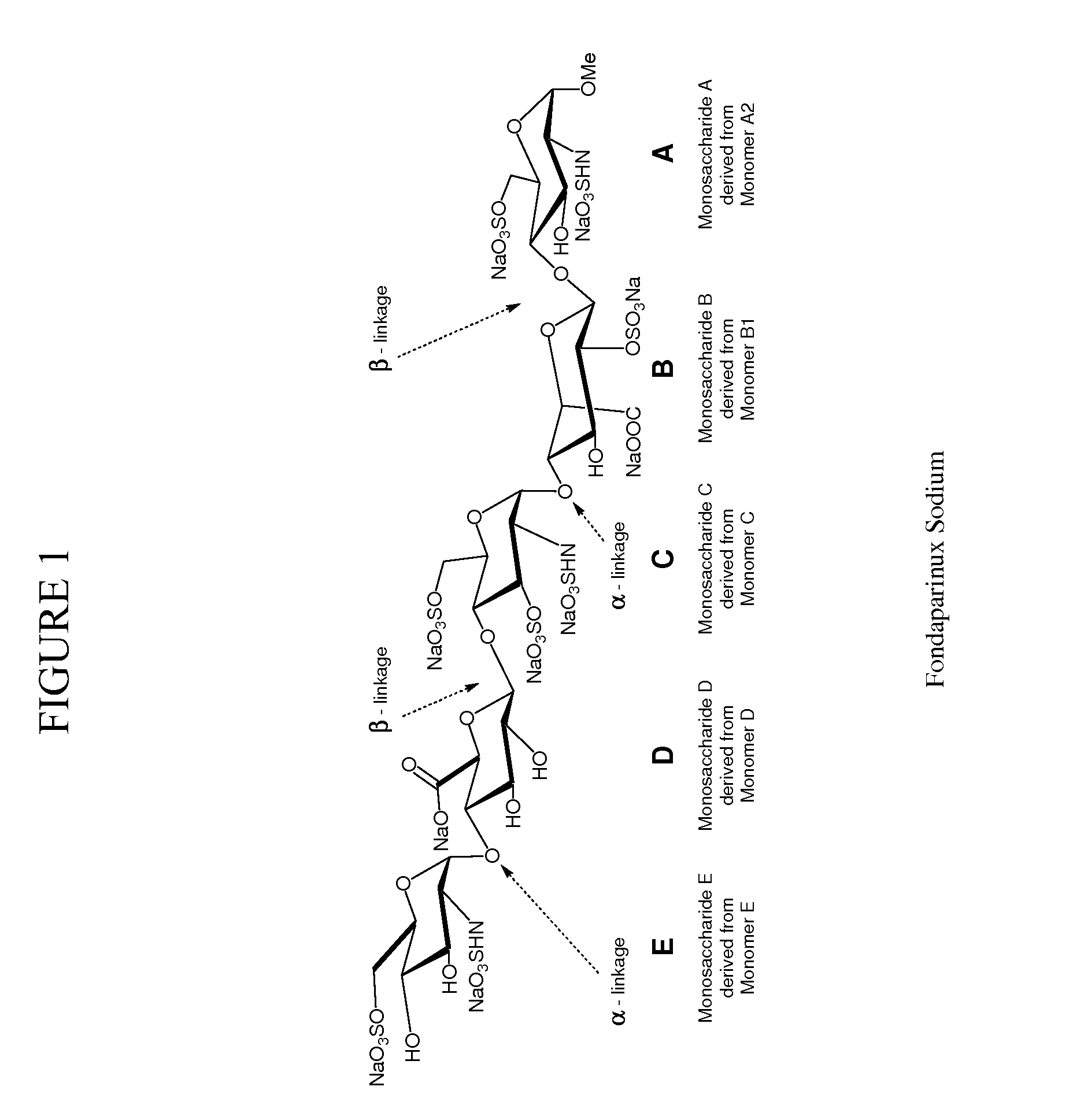
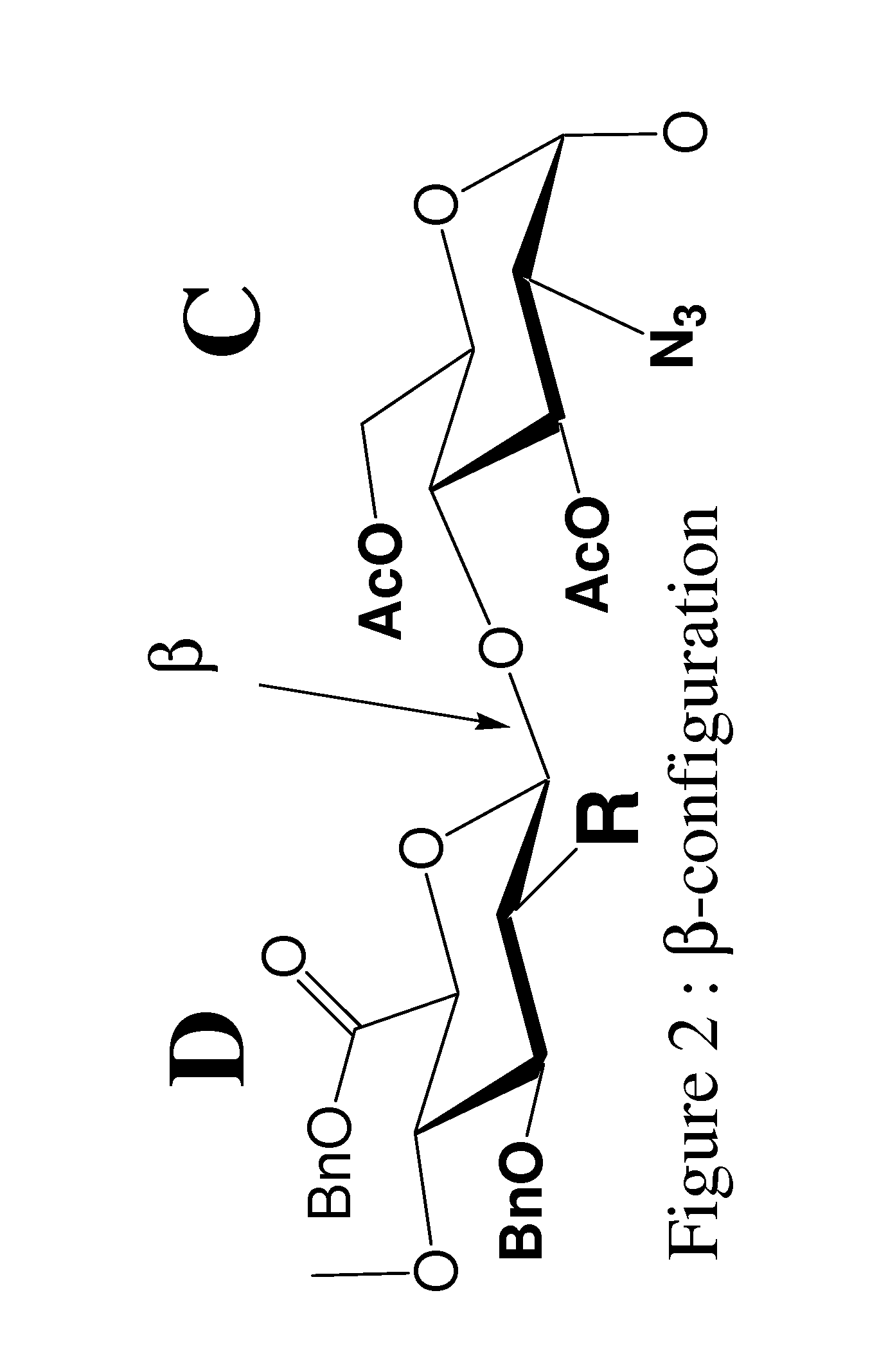
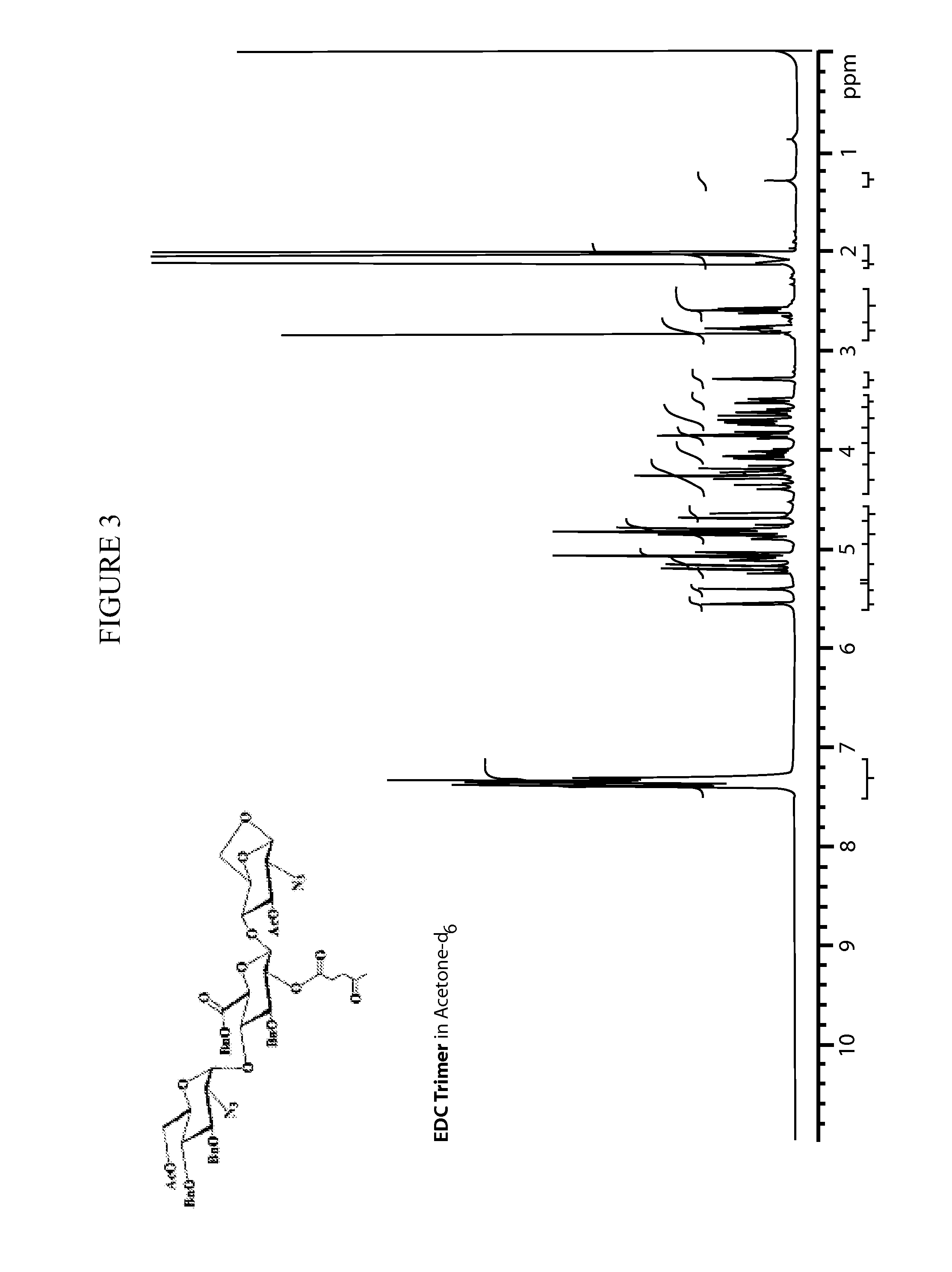
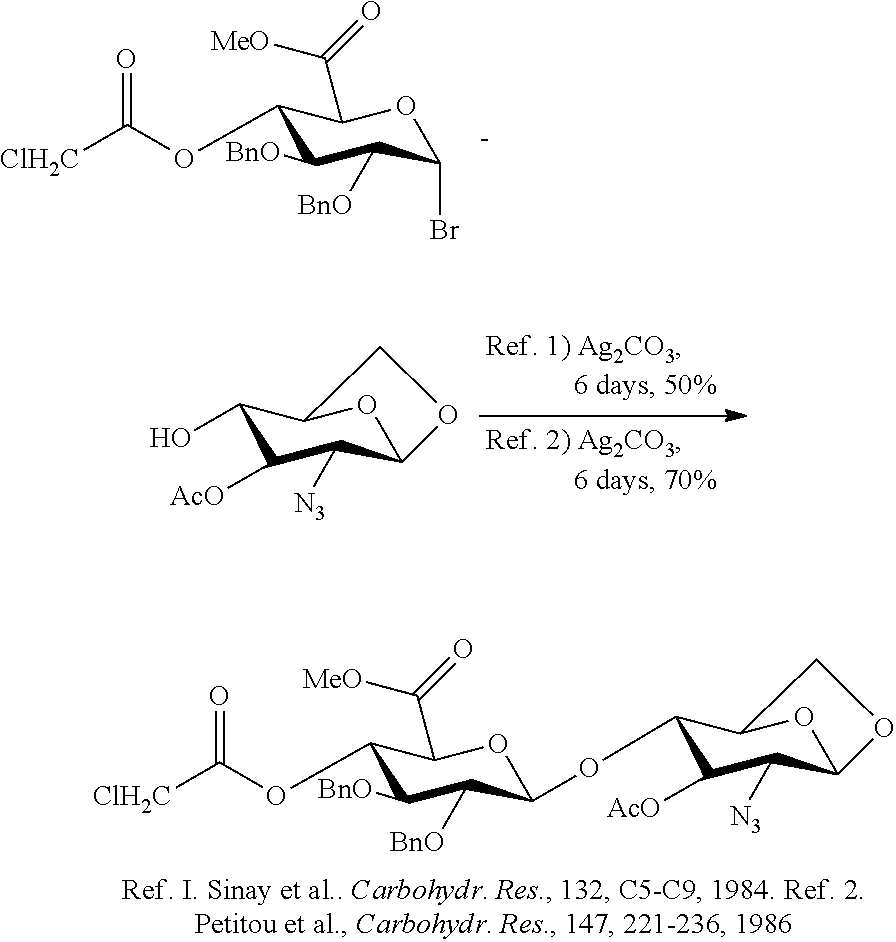
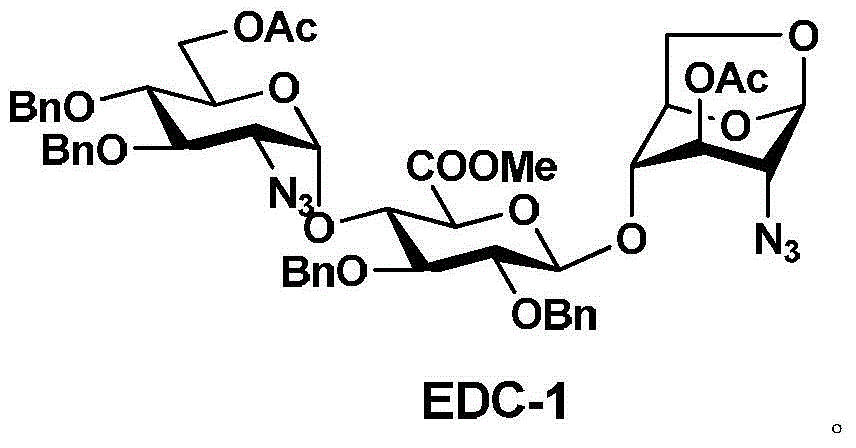
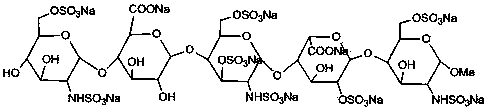
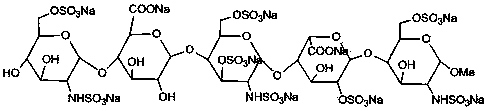
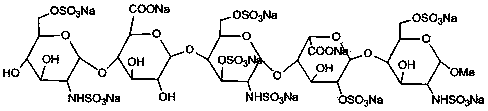
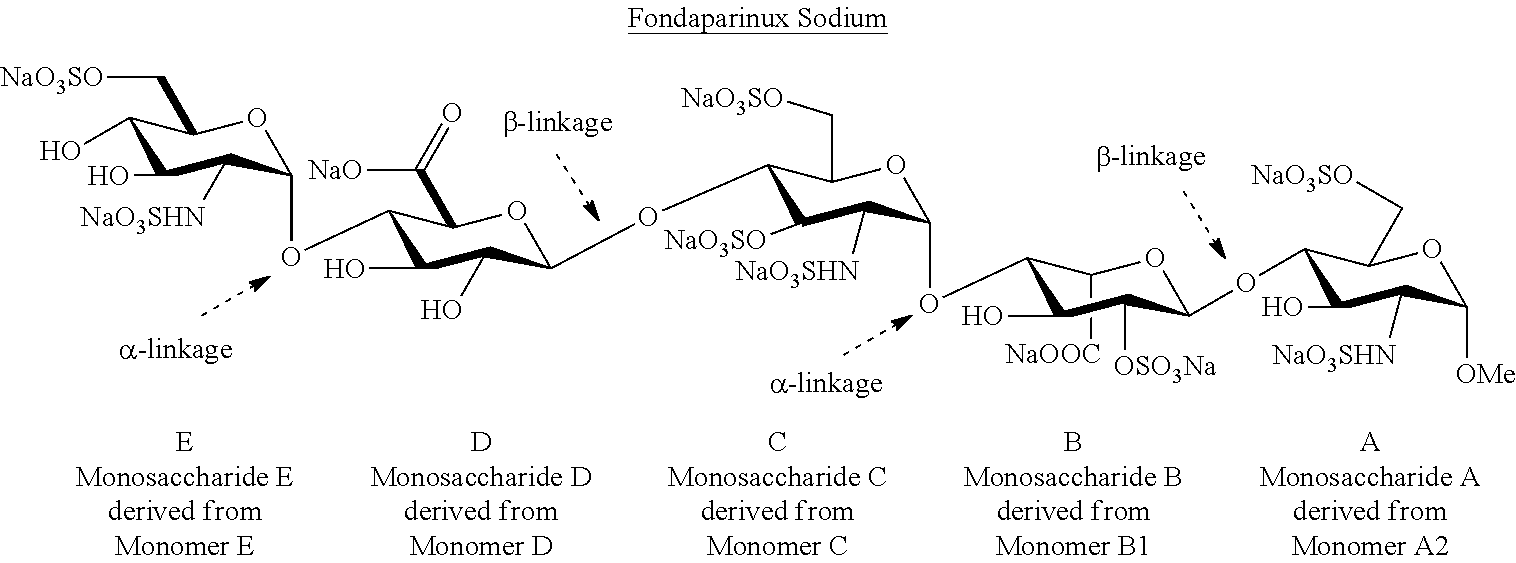
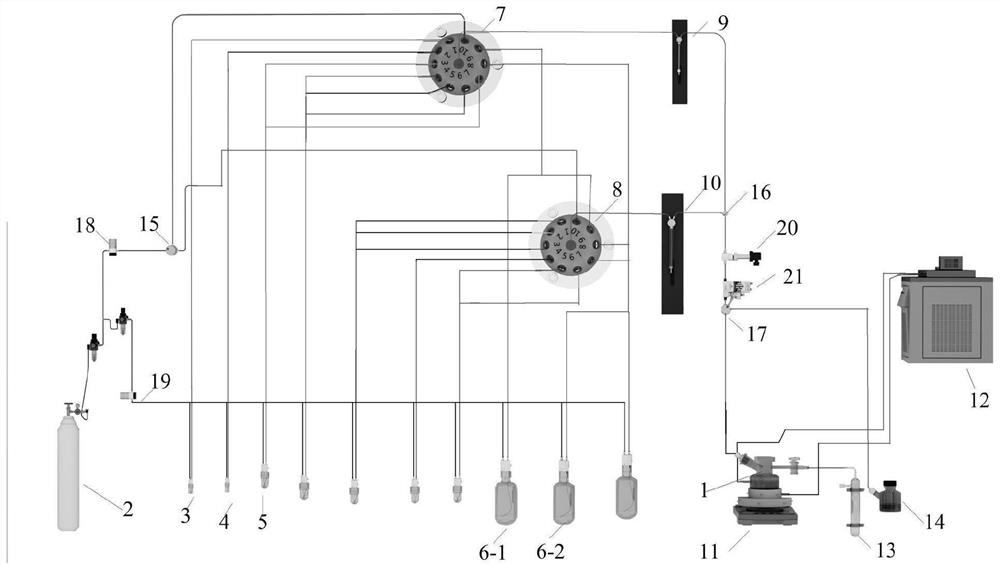
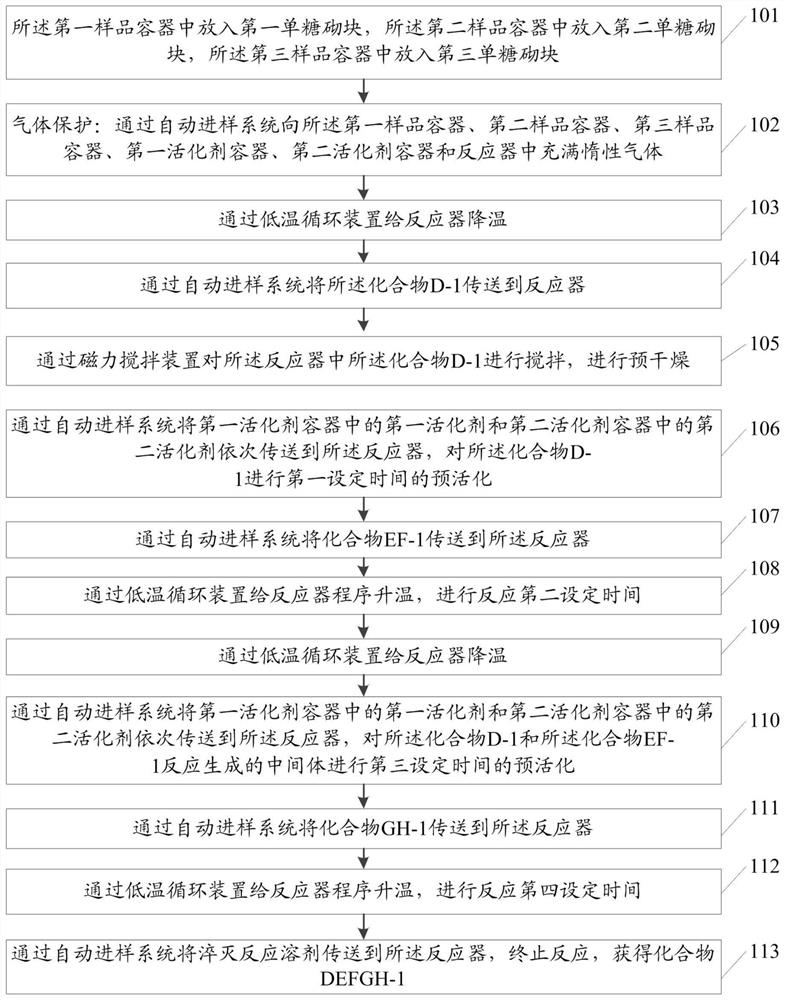
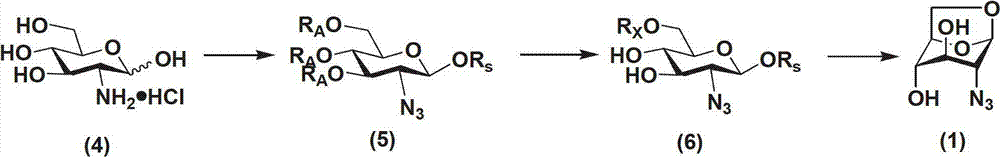Patents
Literature
80 results about "Fondaparinux Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
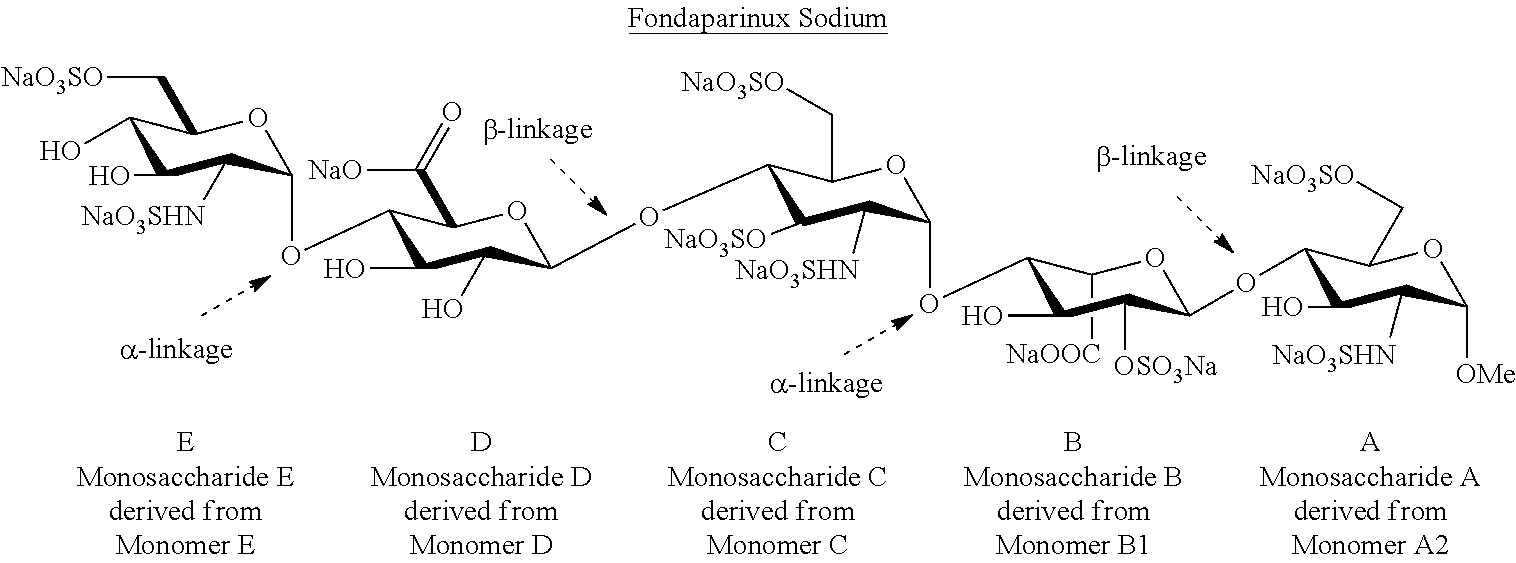
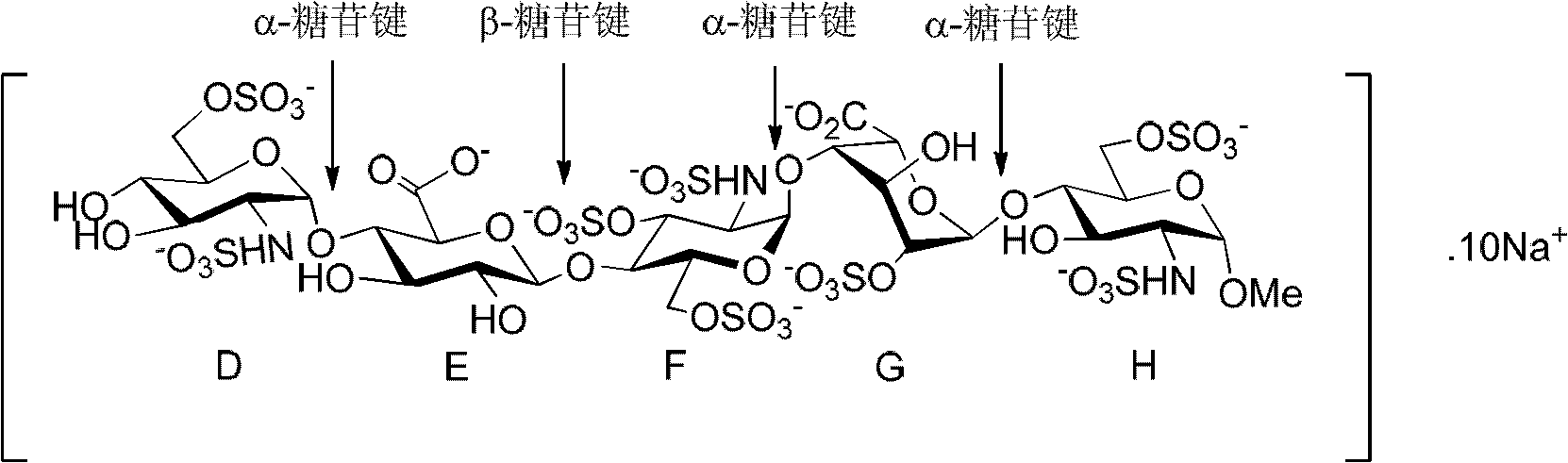
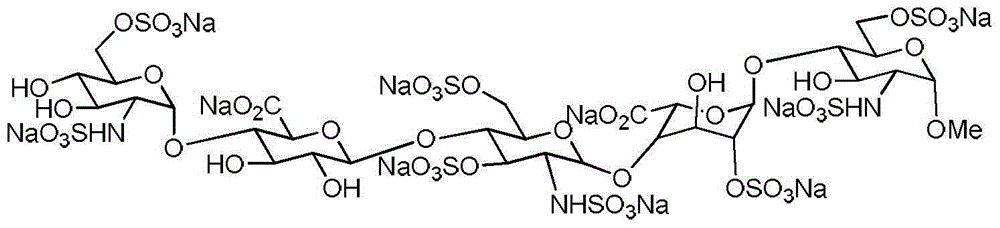
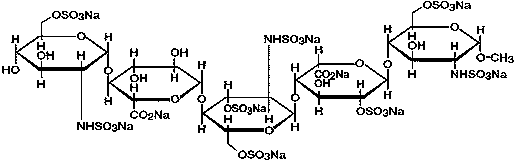
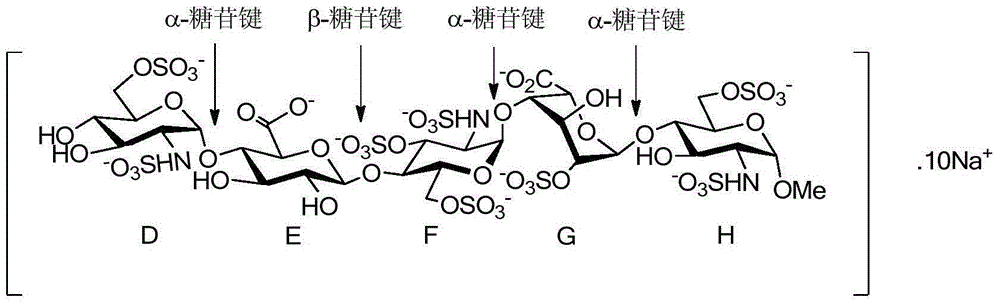
The sodium salt form of fondaparinux, a synthetic glucopyranoside with antithrombotic activity. Fondaparinux sodium selectively binds to antithrombin III, thereby potentiating the innate neutralization of activated factor X (Factor Xa) by antithrombin. Neutralization of Factor Xa inhibits its activity and interrupts the blood coagulation cascade, thereby preventing thrombin formation and thrombus development. (NCI05)
Efficient and scalable process for the manufacture of fondaparinux sodium
ActiveUS20120116066A1Efficient amplificationImprove production yieldSugar derivativesGlycosidesPhotochemistryFondaparinux Sodium
Owner:RELIABLE BIOPHARM LLC
Compound for preparing fondaparinux sodium, preparation method thereof and preparation method of fondaparinux sodium
ActiveCN103122012AHigh yieldEasy to synthesizeSugar derivativesBulk chemical productionCombinatorial chemistryHigh selectivity
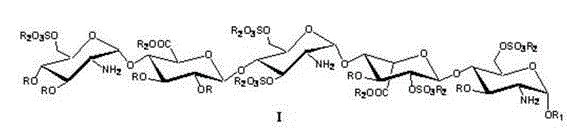
The invention relates to a compound as shown in formula I for preparing fondaparinux sodium, a preparation method thereof and a preparation method of the fondaparinux sodium. The compound disclosed by the invention can be prepared from raw materials which are easy to obtain at higher selectivity and yield, thereby greatly simplifying the preparation process of the fondaparinux sodium. The definition of various substitutional groups contained in the formula I is the same as the definition in a specification.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Process for preparing Fondaparinux sodium and intermediates useful in the synthesis thereof
ActiveUS8288515B2Highly efficient glycosylation reactionGood yieldEsterified saccharide compoundsOrganic active ingredientsAnticoagulant AgentCombinatorial chemistry
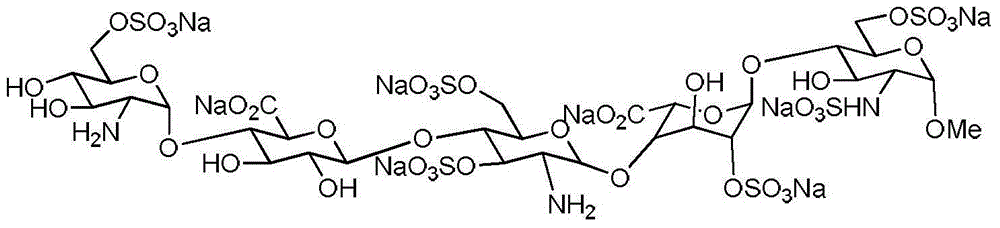
Processes for the synthesis of the Factor Xa anticoagulent Fondaparinux, and related compounds are described. Also described are protected pentasaccharide intermediates as well as efficient and scalable processes for the industrial scale production of Fondaparinux sodium by conversion of the protected pentasaccharide intermediates via a sequence of deprotection and sulfonation reactions.
Owner:RELIABLE BIOPHARM LLC
Process for preparing heparinoids and intermediates useful in the synthesis thereof
InactiveUS20130005954A1Highly efficient glycosylation reactionSmooth and feasible processEsterified saccharide compoundsSugar derivativesIndustrial scaleFondaparinux Sodium
Processes are disclosed for the synthesis of the Factor Xa anticoagulant fondaparinux and related compounds. Protected pentasaccharide intermediates and efficient and scalable processes for the industrial scale production of fondaparinux sodium by conversion of the protected pentasaccharide intermediates via a sequence of deprotection and sulfonation reactions are provided.
Owner:MYLAN PHARMA INC
Fondaparinux sodium, intermediates thereof and preparation methods
ActiveCN103601765AMild responseGood reaction selectivitySugar derivativesSugar derivatives preparationChemical compoundChemical preparation
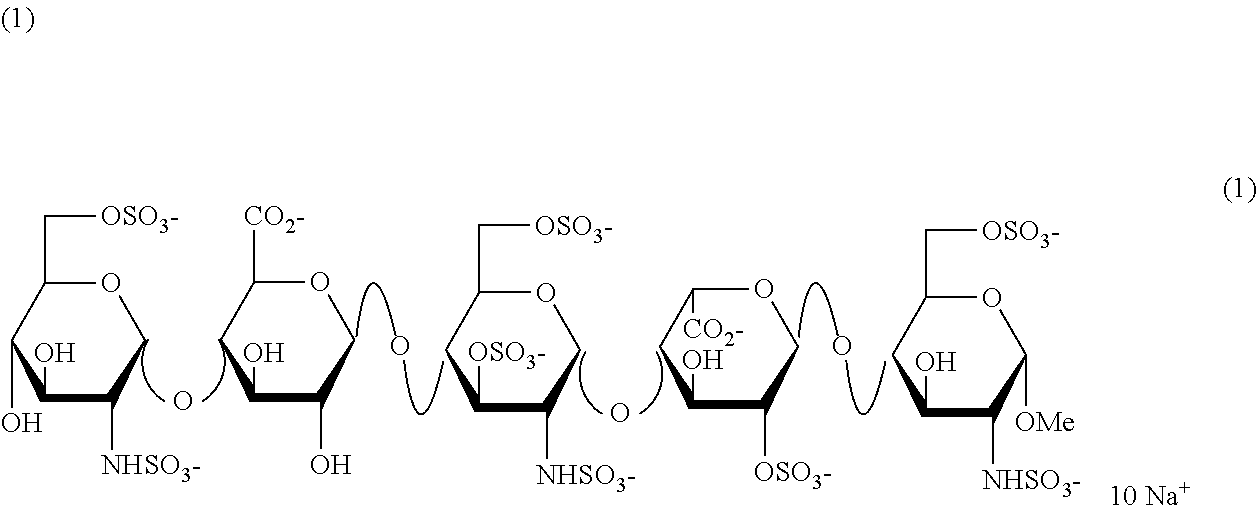
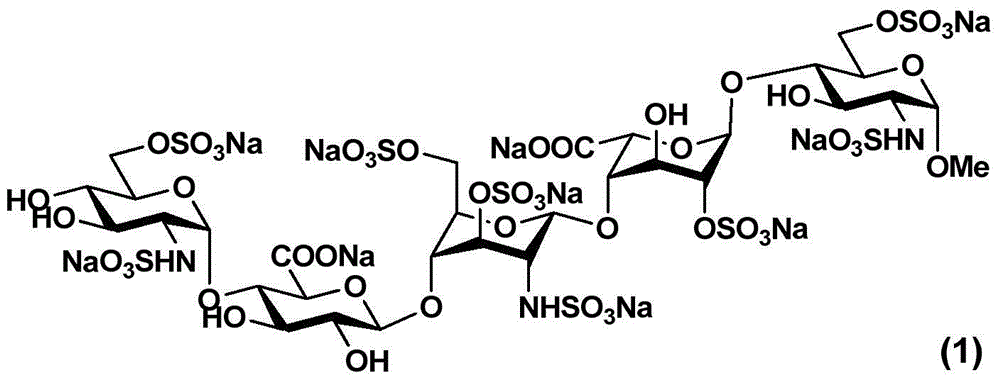
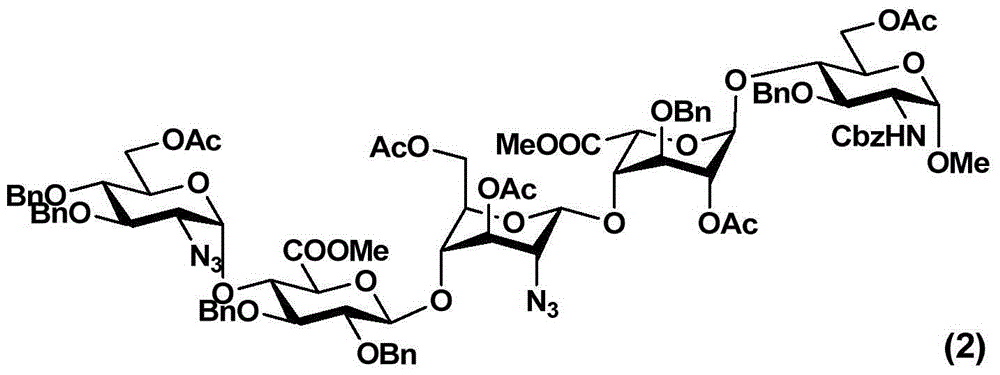
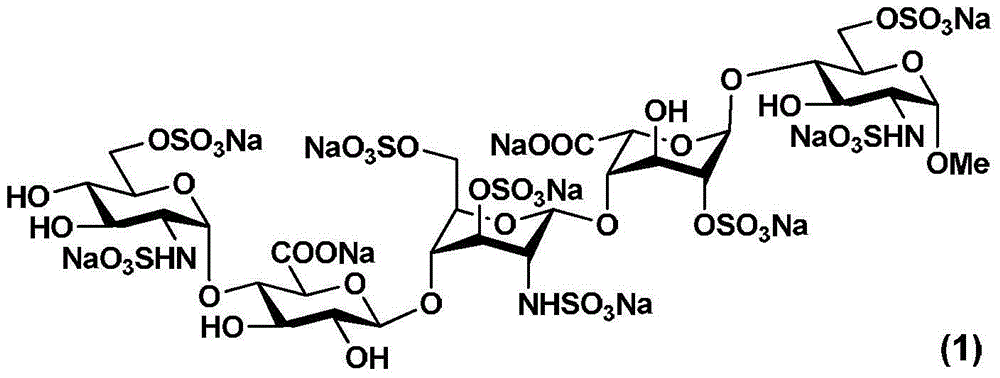
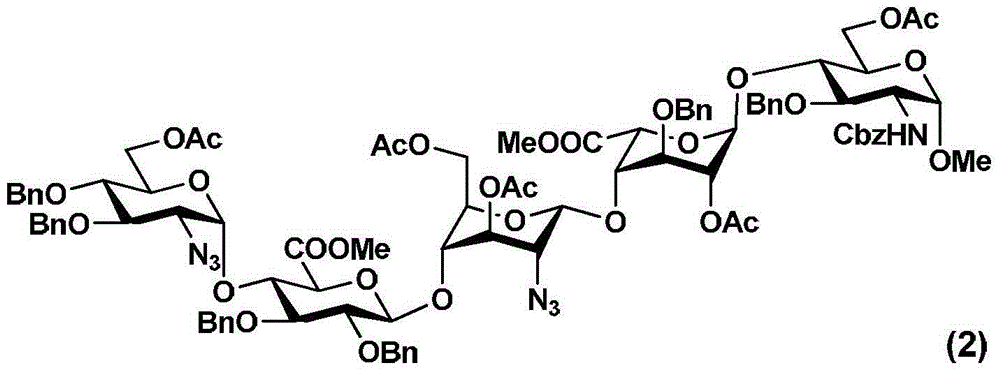
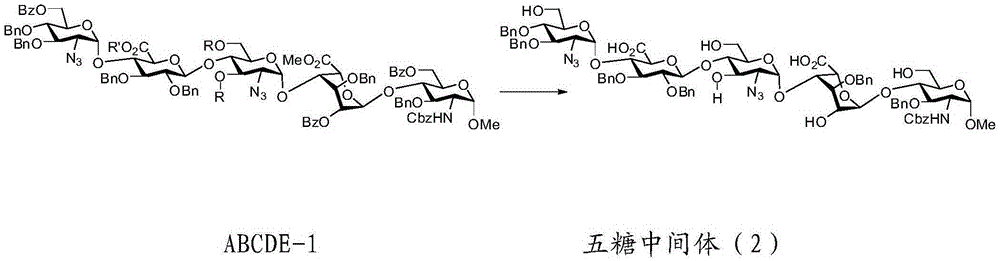
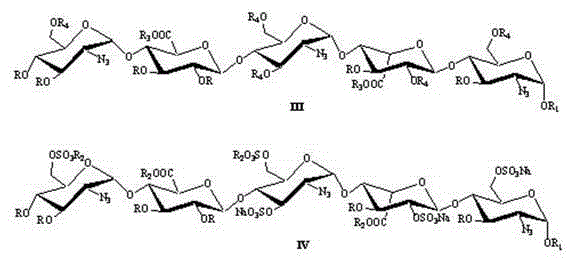
The invention relates to fondaparinux sodium, intermediates thereof and preparation methods in the technical field of chemical preparation, particularly relates to the preparation methods of the fondaparinux sodium and the intermediates of the fondaparinux sodium, comprising the fondaparinux sodium, a disaccharide intermediate of the fondaparinux sodium, a tetrasccharide intermediate of the fondaparinux sodium, a pentasaccharide intermediate of the fondaparinux sodium and the corresponding preparation methods. The structure of the fondaparinux sodium is shown as the chemical compound (1). The invention also relates to the disaccharide intermediate, the tetrasccharide intermediate and the pentasaccharide intermediate which are used for preparing the fondaparinux sodium. The invention designs a novel synthetic route to prepare the fondaparinux sodium. The novel synthetic route has mild reactions, good reaction selectivity, strong controllability and low operation difficulty, and is prone to achievement of industrial production.
Owner:SHANGHAI ACANA PHARMTECH
Method for preparing fondaparinux sodium intermediate
ActiveCN102942601AThe synthesis process is simpleReduce manufacturing costSugar derivativesSugar derivatives preparationRaw materialN acetyl glucosamine
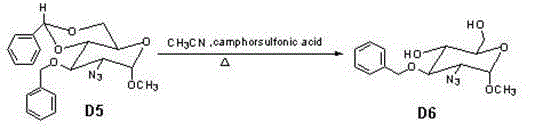
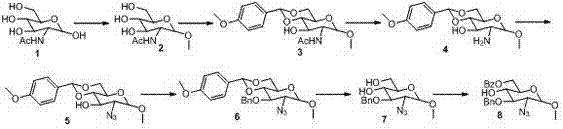
The invention provides a method for preparing a fondaparinux sodium intermediate. N-acetyl glucosamine is taken as a raw material, and through a plurality of steps of reaction, a compound D7 with the formula shown in the specification is synthesized, therefore, a technology for synthesizing fondaparinux sodium is simplified, and the production cost is lowered.
Owner:CUREGEN JIANGSU PHARMA
Application of monodispersed polymethacrylate ion exchange chromatography medium in column chromatography purification of fondaparinux sodium
ActiveCN102659859AHigh purityHigh recovery rateSugar derivativesSugar derivatives preparationIon exchangeHigh pressure
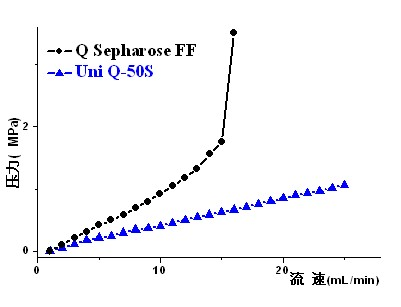
The invention discloses an application of a monodispersed polymethacrylate ion exchange chromatography medium in the column chromatography purification of fondaparinux sodium, wherein the column chromatography is carried out through adopting the monodispersed polymethacrylate ion exchange chromatography medium as the filler of a chromatographic column. The application is in favor of the separation and the purification of crude fondaparinux sodium with an aqueous solution as a mobile phase, and the reaching of high purity and high recovery rate, the column chromatography does not change with the change of the salt concentration and the salt flow velocity, the chromatography filler can resist high pressures and high flow velocities, and simultaneously the fondaparinux sodium adsorbed in the chromatography column can be eluted through the salt with a low concentration.
Owner:SUZHOU NANOMICRO TECH CO LTD
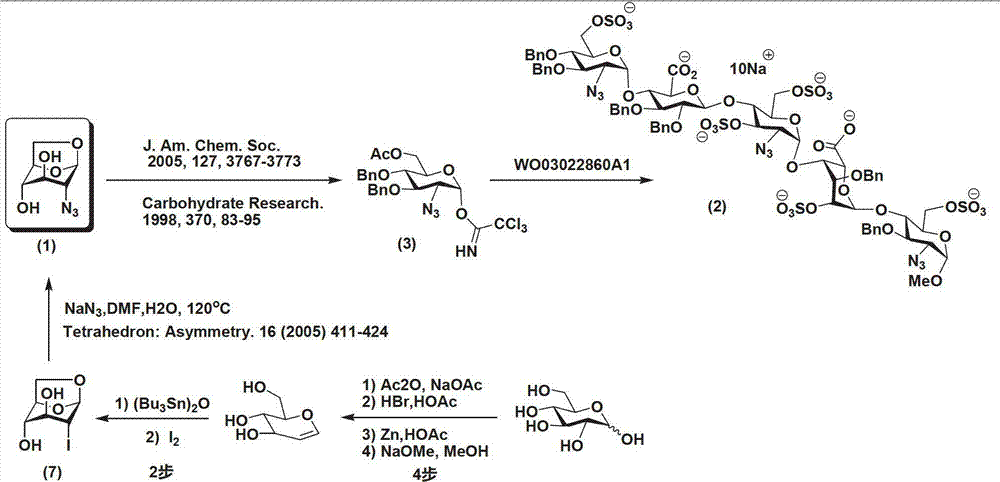
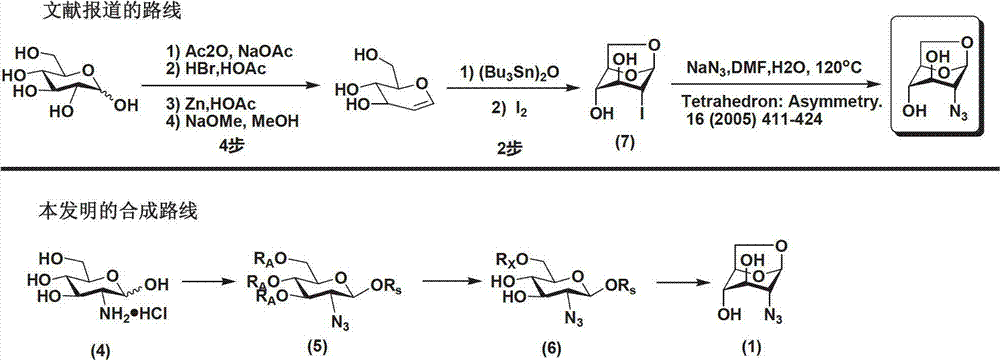
Method for preparing 1,6-Anhydro-2-azido-2-deoxy-beta-D-glucopyranose
ActiveCN102775450AEasy to operateNo risk of explosionSugar derivativesSugar derivatives preparationD-GlucopyranoseImidazole-1-sulfonyl azide hydrochloride
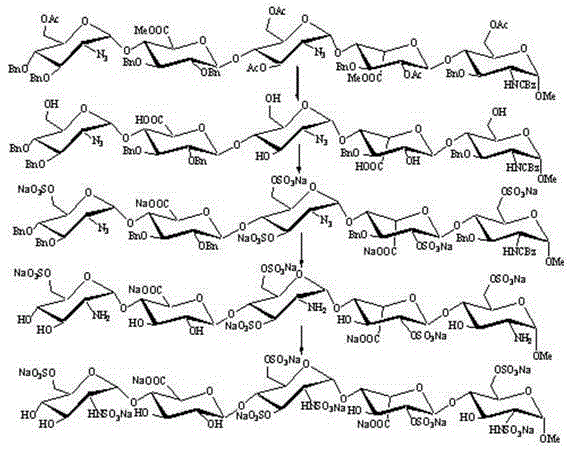
The invention provides a method for preparing the 1,6-Anhydro-2-azido-2-deoxy-beta-D-glucopyranose. At a room temperature, 2-Amido-2-deoxy-D-glucopyranose hydrochloride serves as a raw material, imidazole-1-sulfonyl azide hydrochloride serves as a nitrine reagent, a dowex1X8 resin serves as a acid-binding agent for acylation, silylation and sulfonylation, 1,8-Diazabicyclo-(5,4,0) undec-7-ene or the dowex1X8 resin serves as an alkali reagent for ring closing reaction, and the 1,6-Anhydro-2-azido-2-deoxy-beta-D-glucopyranose is synthesized through nitrine introduction, acylation reaction, silylation reaction, sulfonylation reaction and ring closing reaction in sequence; and the reaction process is represented as formula (I). The 1,-Anhydro-2-azido-2-deoxy-beta-D-glucopyranose can serve as an intermediate for synthesizing anticoagulant drug fondaparinux sodium. The method for preparing the 1,6-Anhydro-2-azido-2-deoxy-beta-D-glucopyranose has the advantages of being mild in reaction, simple in synthetic route, low in cost, safe and reliable, and suitable for large scale production.
Owner:EAST CHINA NORMAL UNIV +2
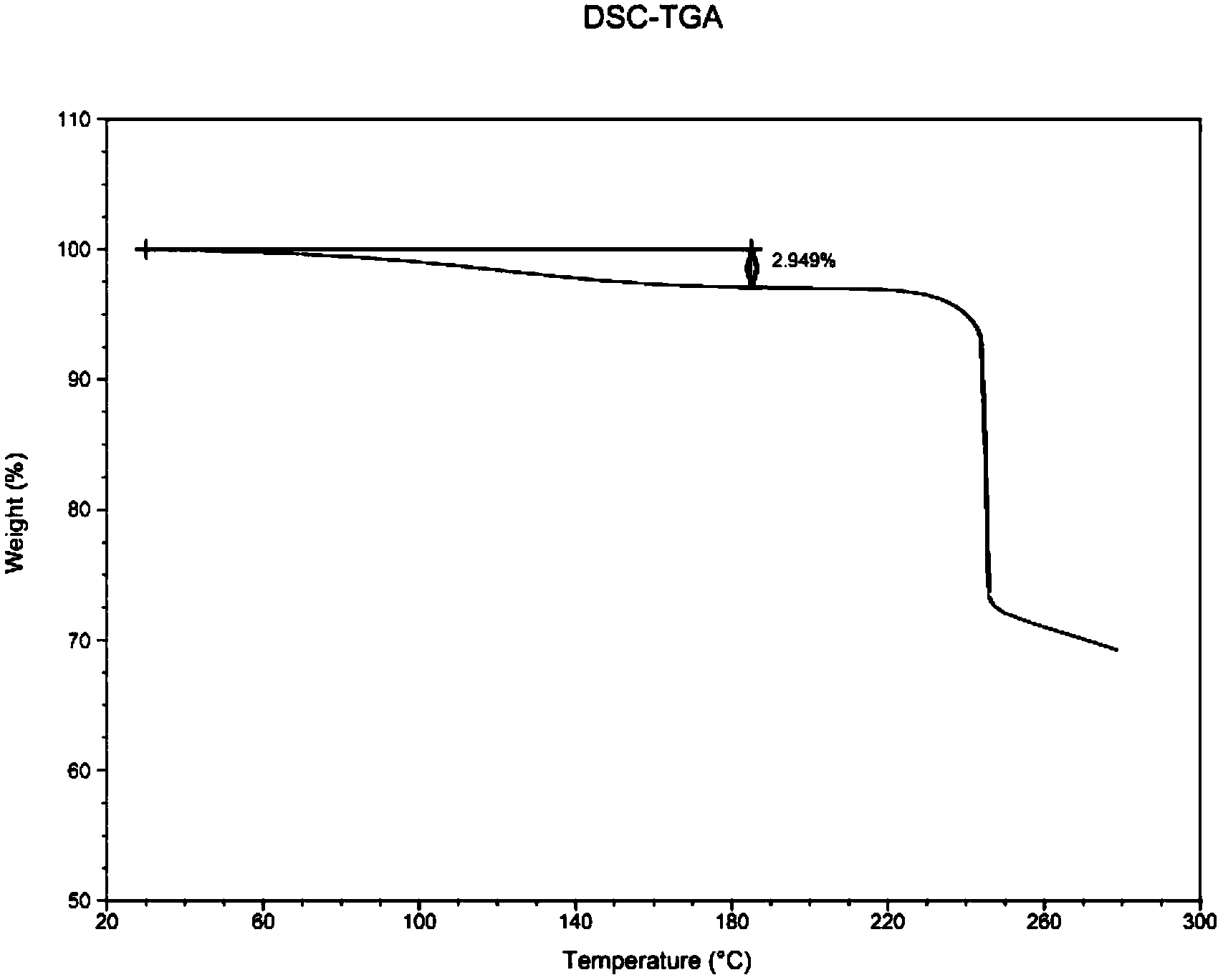
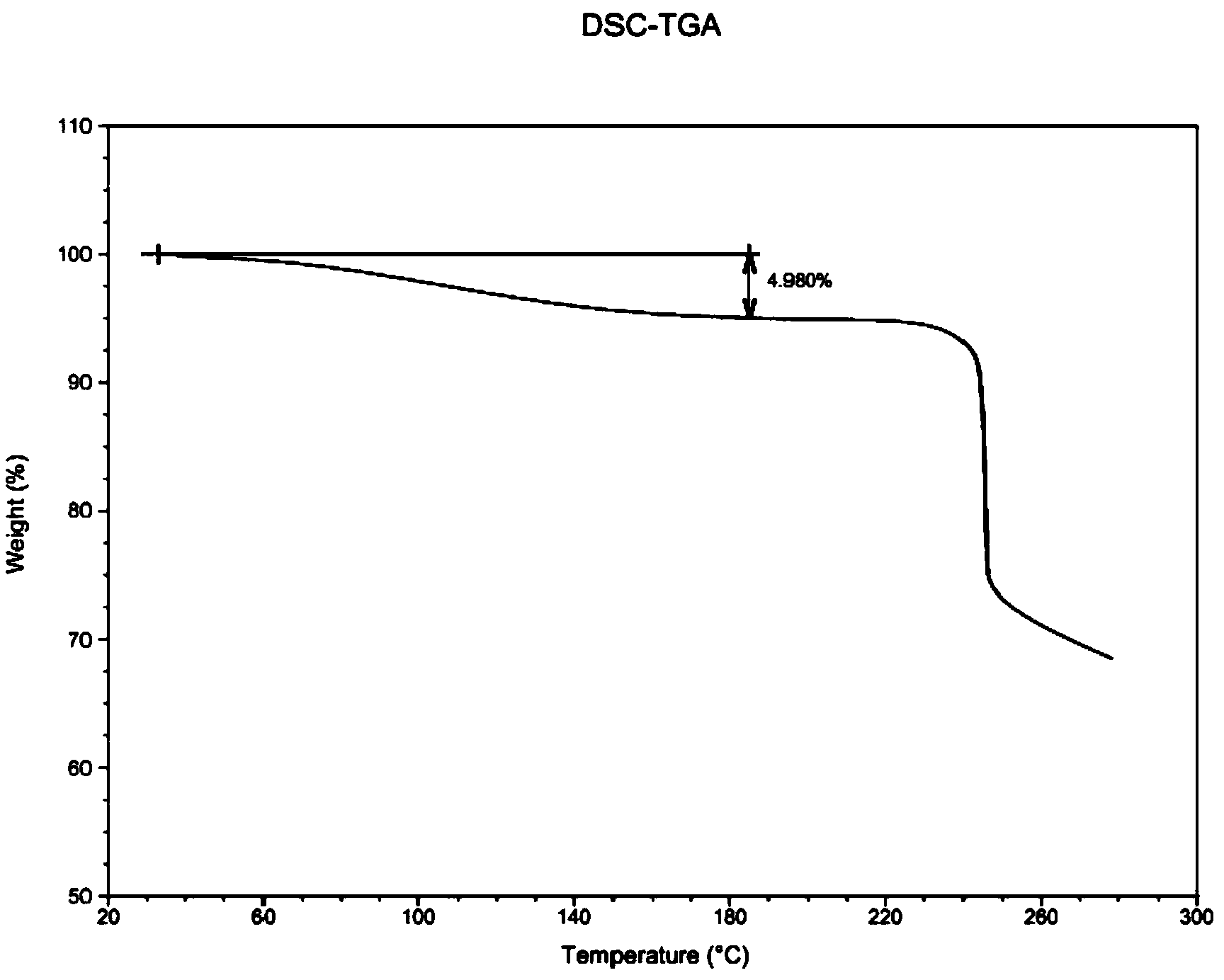
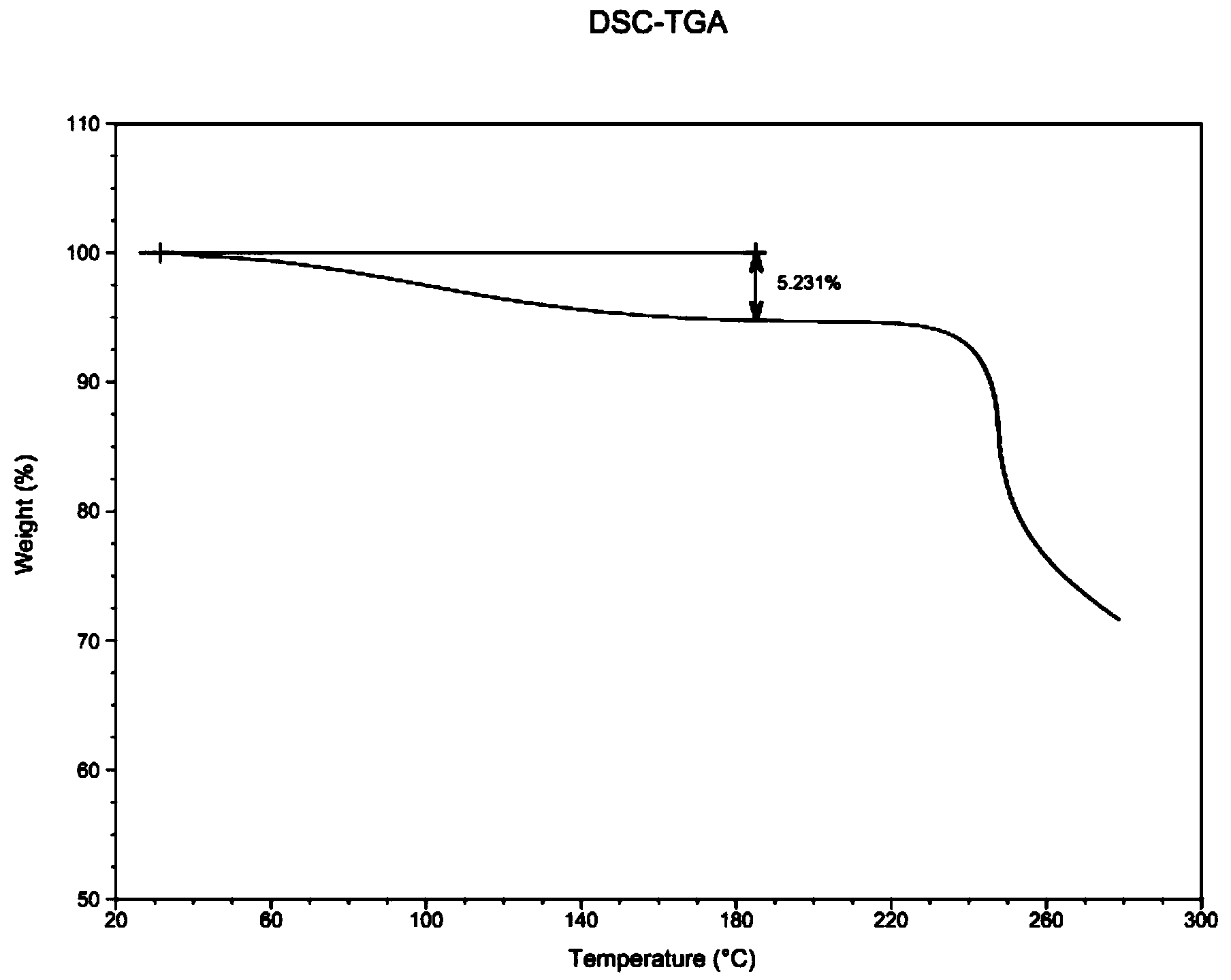
Low-moisture high-purity fondaparinux sodium and preparation method thereof
ActiveCN103965269AReduced bound water contentReduces the chance of contaminating raw materialsSugar derivativesSugar derivatives preparationMoistureImpurity
The invention discloses low-moisture high-purity fondaparinux sodium and a preparation method thereof. During a freezing process, by adjusting a heating rate, heat preservation time and vacuum degree in sublimation and secondary drying stages, the problems that the fondaparinux sodium is higher in moisture content, low in purity, and difficult to store, and the raw material of the fondaparinux sodium is easy to pollute by bacteria are solved. Meanwhile, an impurity in front of a main peak, which cannot be removed, is removed in a column chromatography stage, and the fondaparinux sodium obtained with the preparation method is low in impurity content and high in purity.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
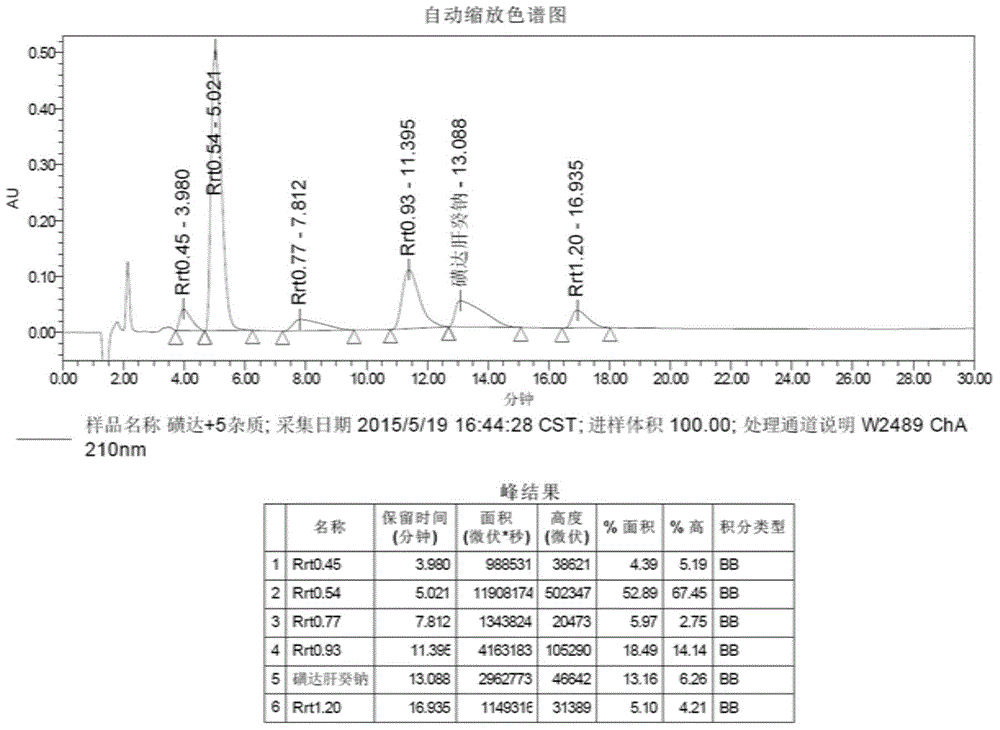
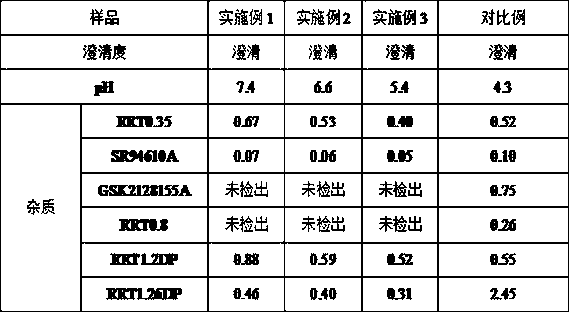
Reference compound for controlling quality of fondaparinux sodium
ActiveCN104910217ARaise quality standardsSugar derivativesComponent separationCompound aOrganic chemistry
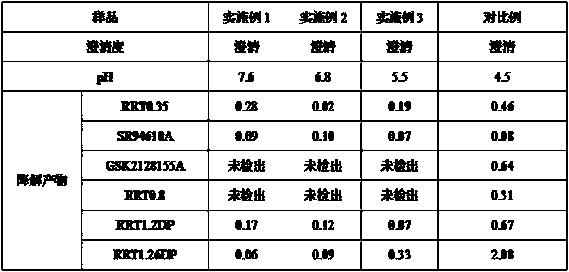
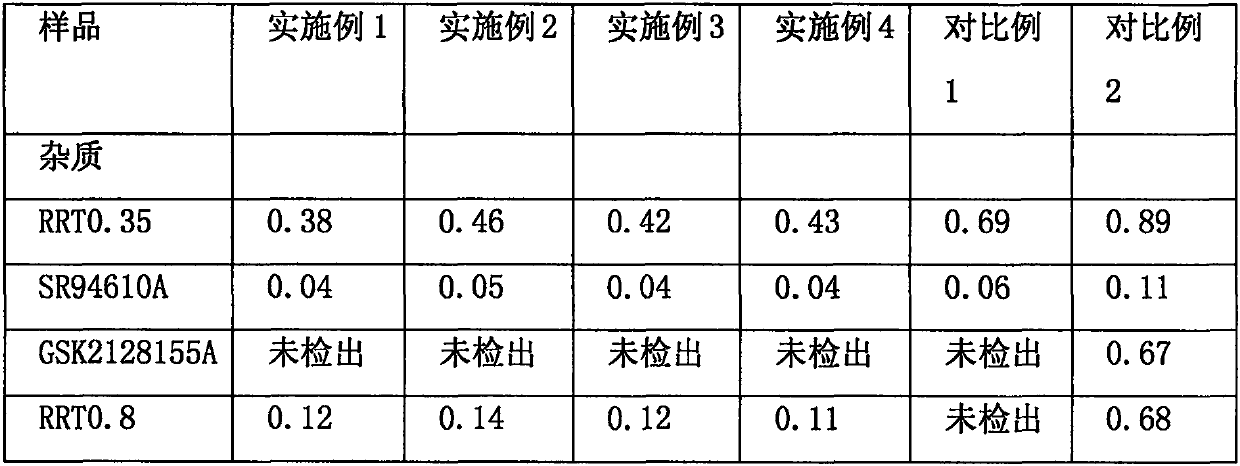
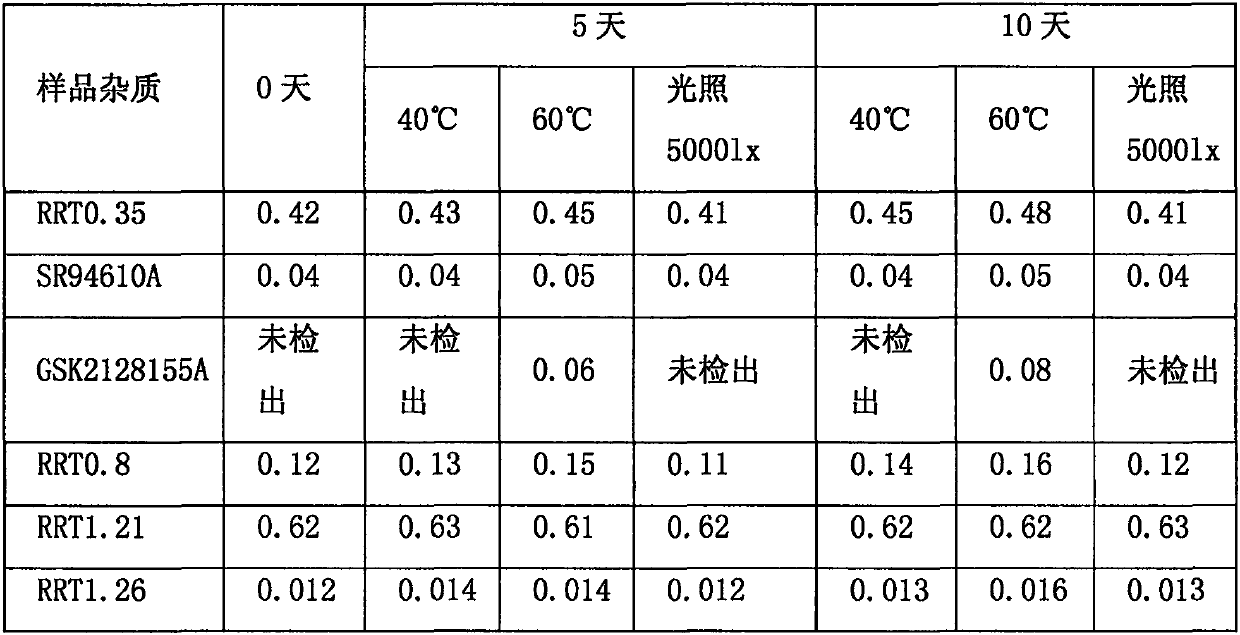
The invention relates to the technical field of medicines and relates to a reference compound for controlling quality of fondaparinux sodium. Relevant reference compounds of fondaparinux sodium include a reference compound A (Rrt0.45), a reference compound B (Rrt0.54), a reference compound C (Rrt0.77), a reference compound D (Rrt0.93) and a reference compound E (Rrt1.20).
Owner:TIANJIN CHASE SUN PHARM CO LTD
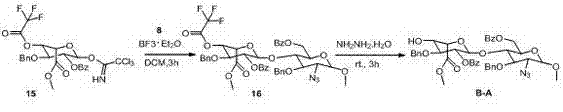
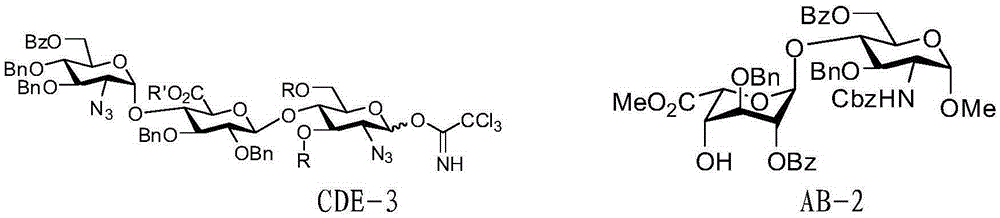
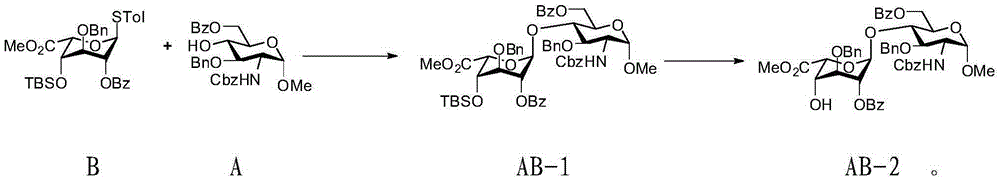
Fondaparinux sodium disaccharide intermediate fragment BA and synthetic method thereof
InactiveCN102898487AEasy to purifyOptimize the synthetic routeSugar derivativesSugar derivatives preparationCombinatorial chemistryReaction step
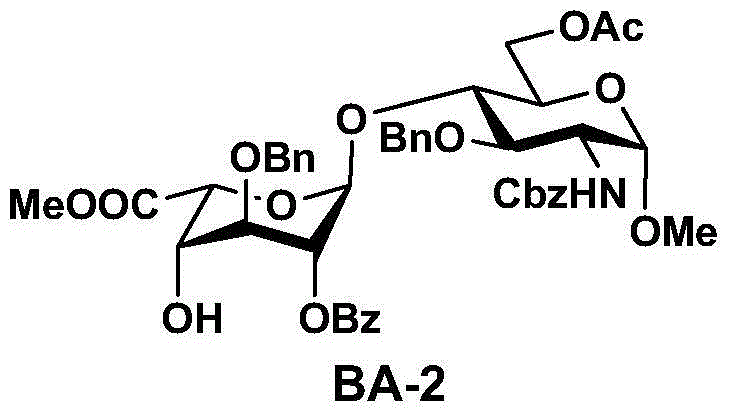
The invention discloses a fondaparinux sodium disaccharide intermediate fragment BA and a synthetic method thereof. The synthetic method is novel in synthesis strategy, available in raw materials, relatively fewer in reaction steps, mild in reaction conditions, simple in post-treatment conditions, easy to operate, low in production cost, high in yield and suitable for large-scale industrial production.
Owner:麦科罗夫(南通)生物制药有限公司
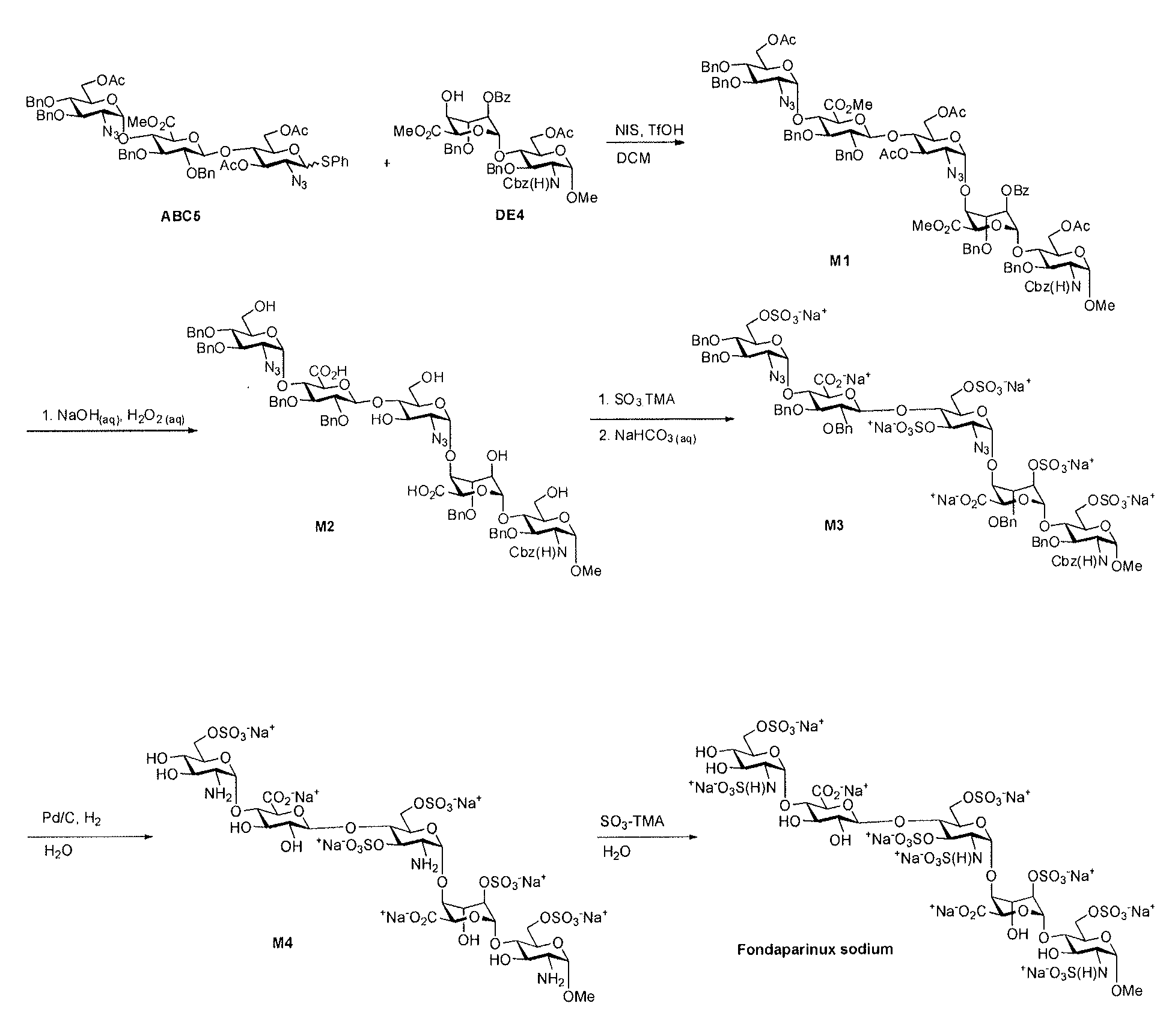
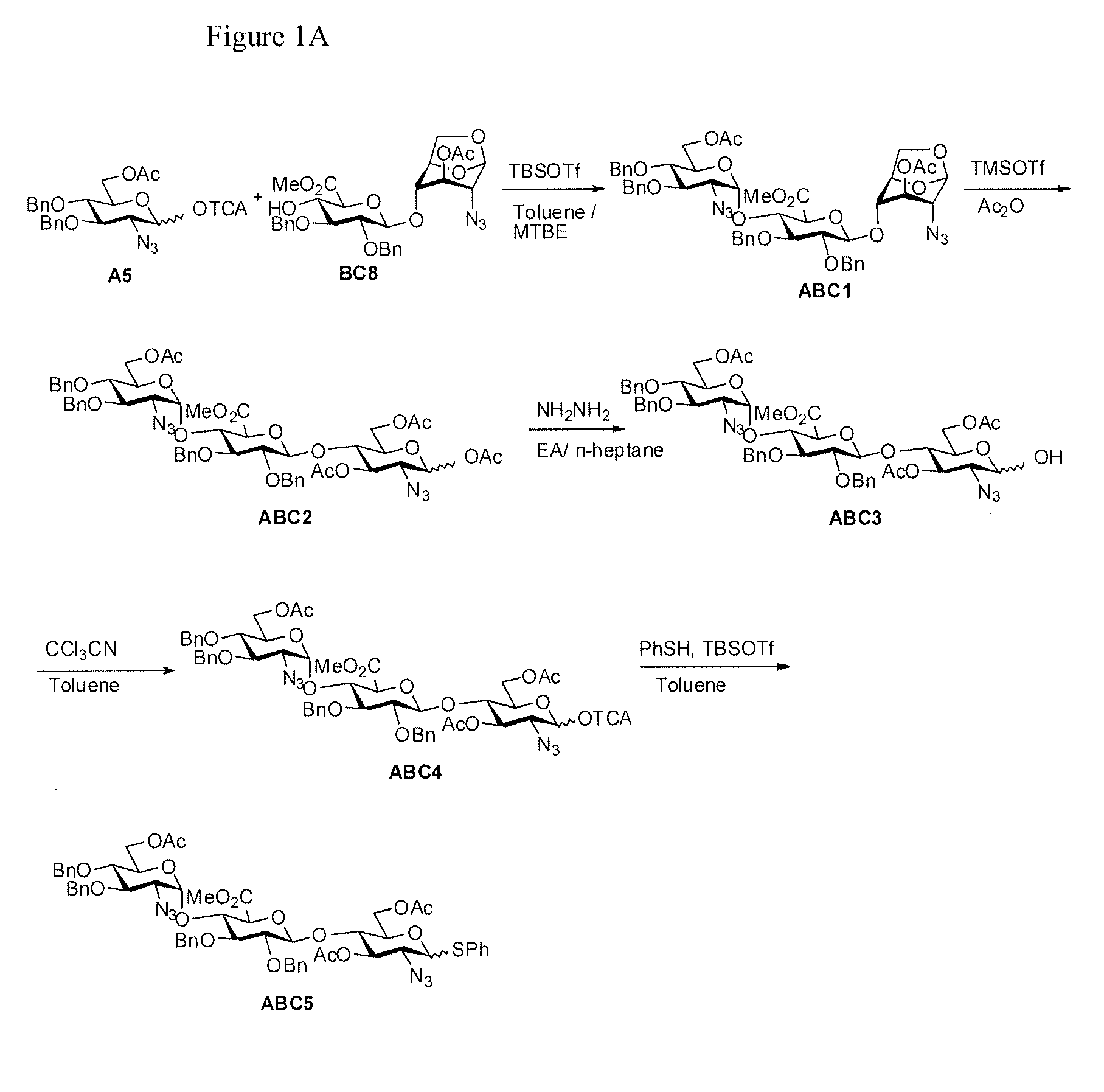
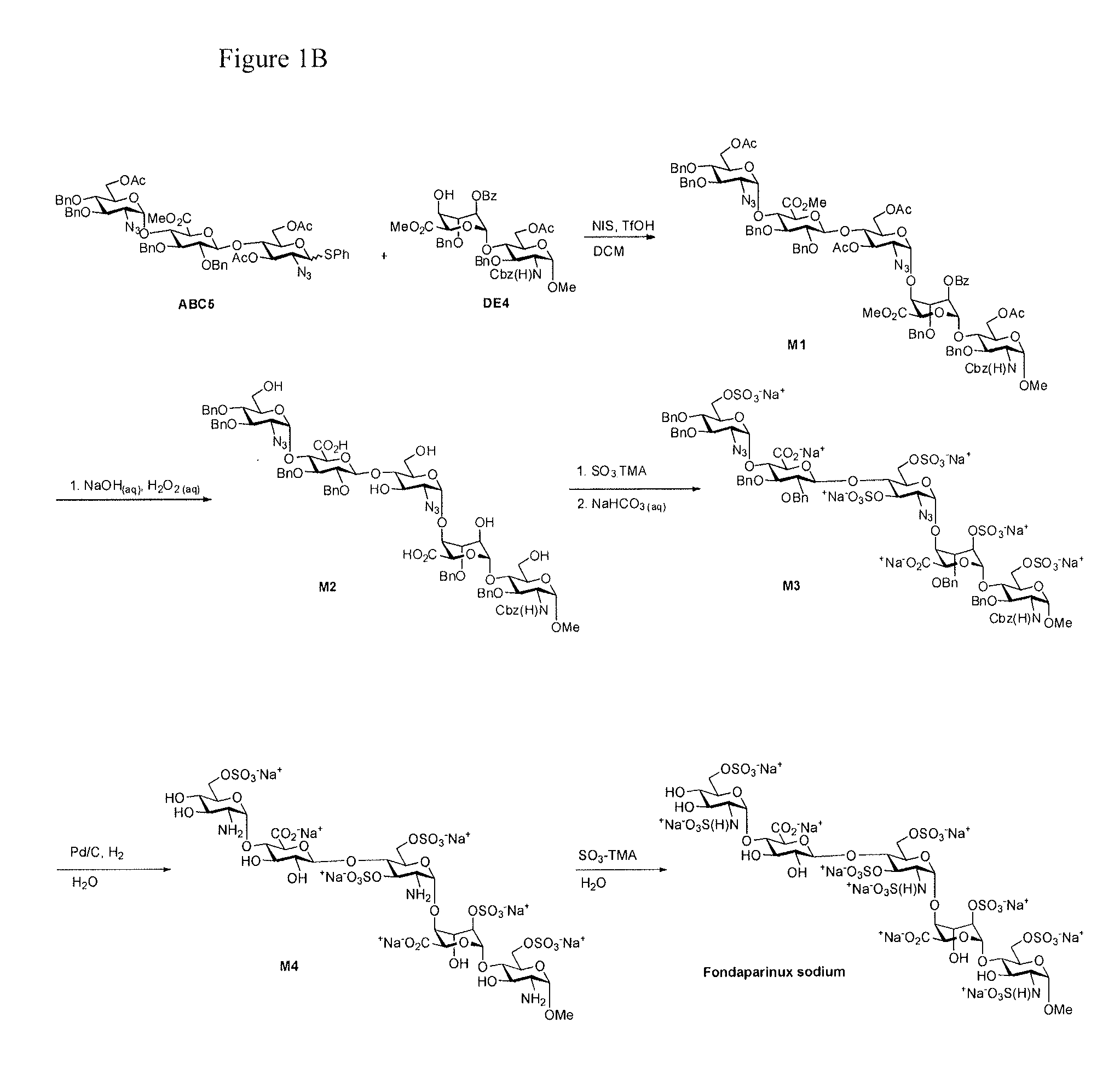
Process for the production of fondaparinux sodium
InactiveUS9346844B2Reduce yieldRapid responseSugar derivativesSugar derivatives preparationDalteparin sodiumPhotochemistry
Owner:SCINOPHARM TAIWAN LTD
Process for the production of fondaparinux sodium
InactiveUS20150031865A1Reduce yieldRapid responseSugar derivativesSugar derivatives preparationPhotochemistryDalteparin sodium
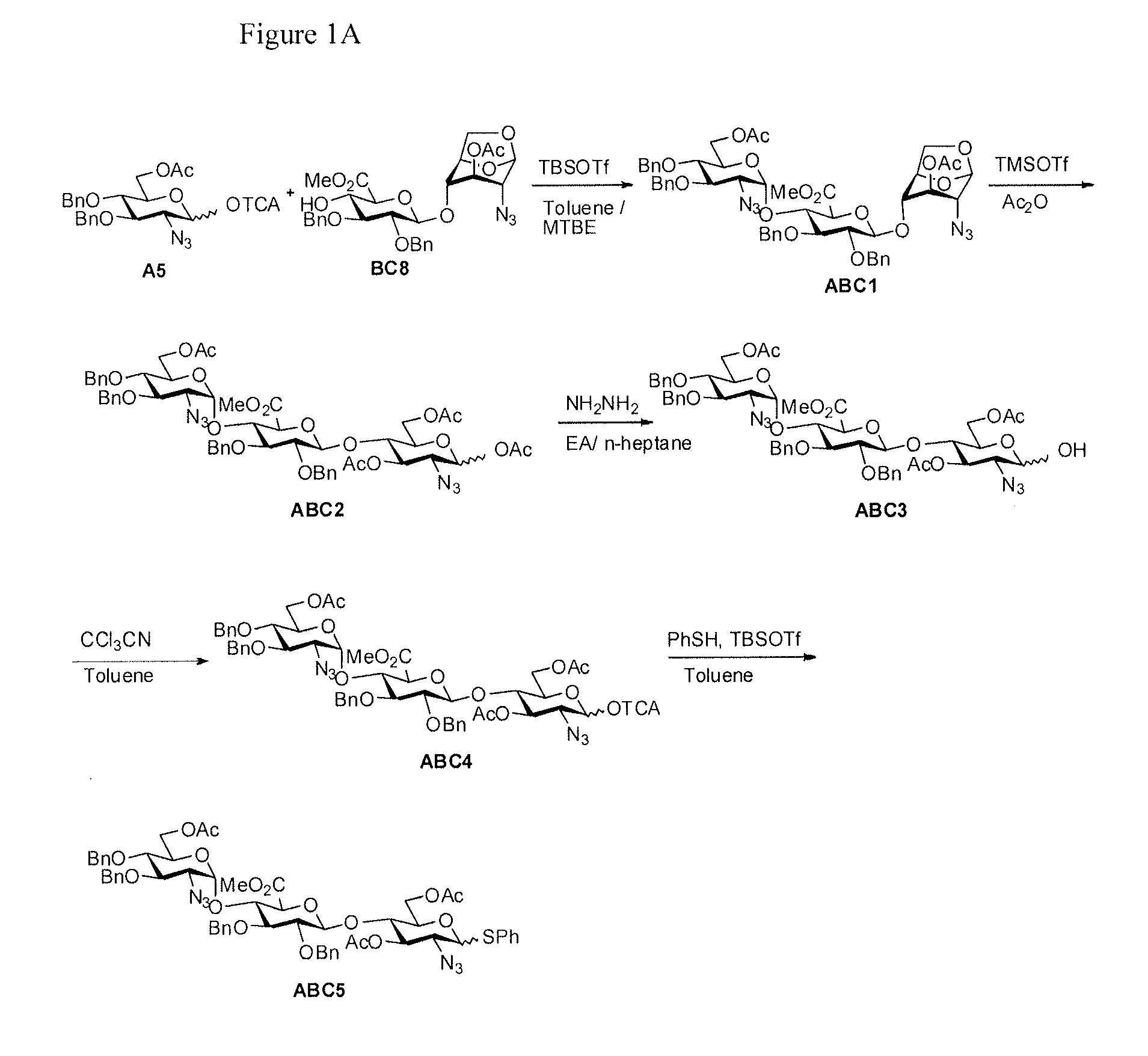
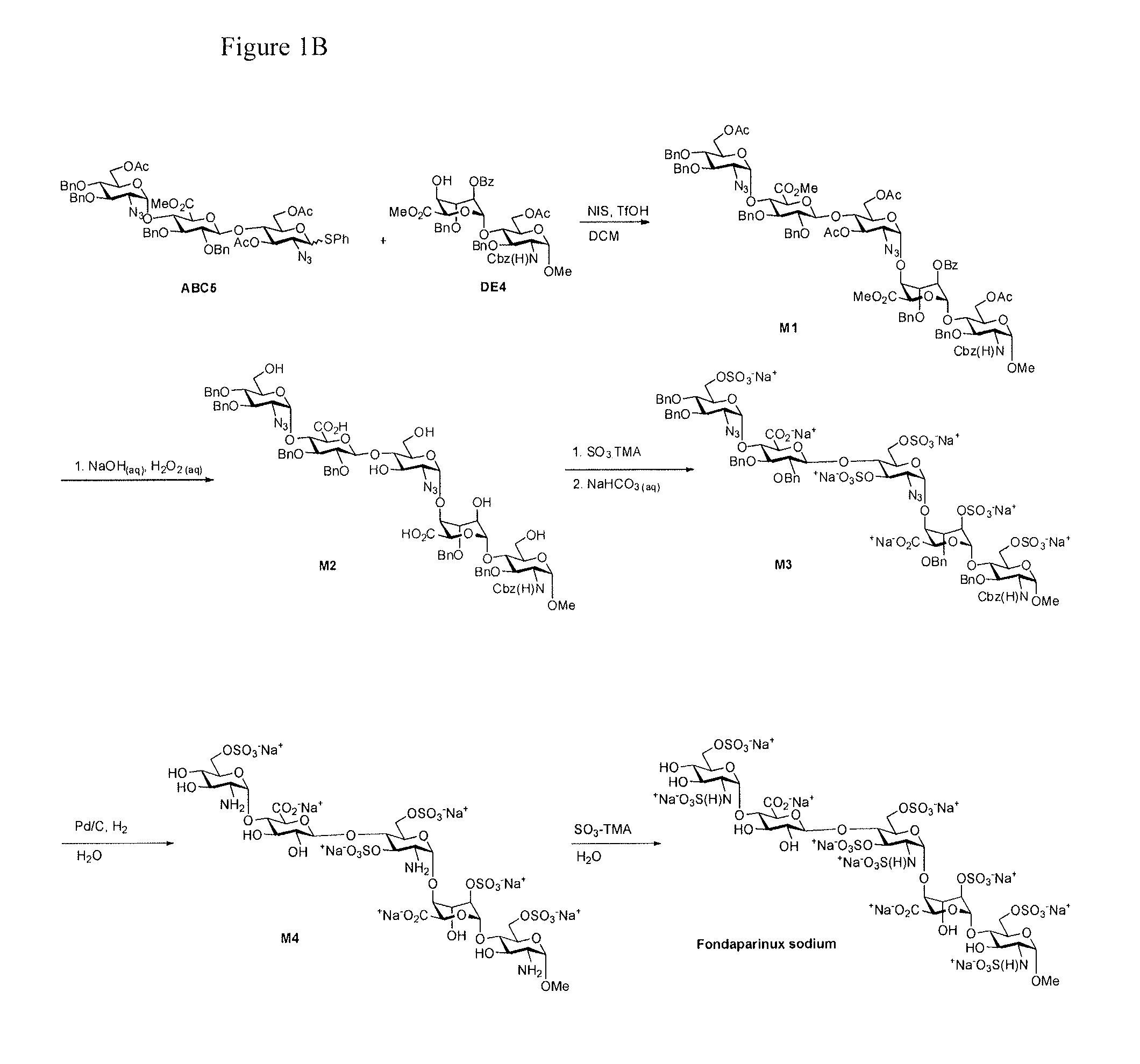
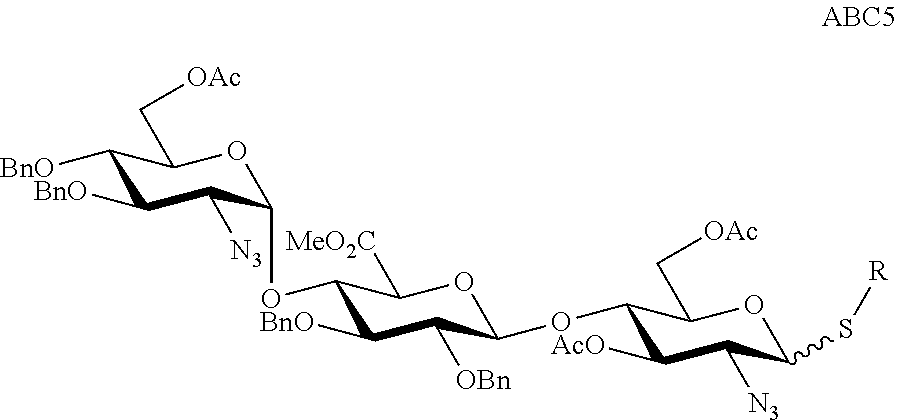
The present invention provides novel processes for the preparation of Fondaparinux sodium by using the compound of formula ABC5In some embodiments, the intermediates for the synthesis of Fondaparinux sodium, are also provided.
Owner:SCINOPHARM TAIWAN LTD
Novel technology for preparing disaccharide fragment of fondaparinux sodium intermediate
InactiveCN105622678ASugar derivativesSugar derivatives preparationAnticoagulant drugPharmaceutical Substances
The invention relates to a novel technology for preparing disaccharide fragment of an anticoagulant drug fondaparinux sodium intermediate. The technology has the advantages of short reaction route, high efficiency, and easy purifying of the intermediate, and is suitable for commercial production of the full protection heparin pentasaccharide.
Owner:WISDOM PHARM CO LTD
Fondaparinux sodium composition injection and preparation method thereof
InactiveCN106727289AEffective pH adjustmentHigh clarityOrganic active ingredientsInorganic non-active ingredientsSodium hydroxideChemistry
The invention relates to a Fondaparinux sodium composition injection and a preparation method thereof. The injection is prepared from raw materials as follows: 5-10 parts by mass of Fondaparinux sodium, 6-12 parts by mass of sodium chloride, 2.6-3 parts by mass of a buffer solution and 3-6 parts by volume of water for injection, wherein the buffer solution is a mixture of citric acid, sodium hydroxide and hydrochloric acid, and a mass ratio of citric acid, sodium hydroxide and hydrochloric acid is (2.4-2.6):1:(1.3-1.9); the raw materials are subjected to orderly feeding, stirring, filtering, reflowing, filling, nitrogen charge, fused sealing, high-temperature sterilization, light inspection after cooling, packaging and checking, and the Fondaparinux sodium composition injection is obtained. The Fondaparinux sodium composition injection prepared with the method cannot produce crystals, is good in clarity, greatly improves the stability and is better applied to clinical treatment.
Owner:ABA CHEM SHANGHAI
Fondaparinux sodium pentasaccharide intermediate and preparation method thereof
ActiveCN103601766AMild responseGood reaction selectivitySugar derivativesSugar derivatives preparationA-trisaccharideChemical preparation
The invention relates to a fondaparinux sodium pentasaccharide intermediate in the technical field of chemical preparation and a preparation method thereof, particularly to a preparation method of the fondaparinux sodium pentasaccharide intermediate, and comprises a trisaccharide intermediate of fondaparinux sodium and preparation method thereof. The invention designs a novel synthetic route to prepare the fondaparinux sodium pentasaccharide intermediate. The route has mild reactions, good reaction selectivity, strong controllability and low operation difficulty, and is beneficial to achievement of industrial production. The route has a low cost so that the method can meet requirements of large-scale industrial production.
Owner:SHANGHAI ACANA PHARMTECH
Separation extraction method of fondaparinux sodium
ActiveCN103992364AEasy post-processingEasy to operateSugar derivativesSugar derivatives preparationIon exchangeIon-exchange resin
The invention discloses a separation extraction method of fondaparinux sodium. The method includes the following steps: adsorbing a dilute salt solution of a fondaparinux sodium compound by ion exchange resin, then eluting with concentrated brine solution to achieve the purpose of salt removal and concentration, so as to obtain concentrated liquid of fondaparinux sodium. The invention uses ion exchange resin before fondaparinux sodium adsorption, to separate most of the salt and water, and then a small amount of concentrated brine solution is employed to elute the product, and the subsequent precipitation is carried out directly to obtain the product. The method is simple and practical in operation and low in cost and can control processing batch arbitrarily, so as to greatly facilitate the posttreatment process of the fondaparinux sodium product.
Owner:NANJING TIANXIANG PHARMA TECH
Synthetic method of fondaparinux sodium intermediate
ActiveCN104151370AThe synthetic route is simpleReduce manufacturing costEsterified saccharide compoundsSugar derivativesCerium nitrateAcetylation
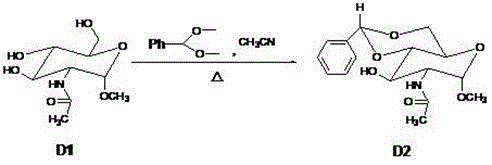
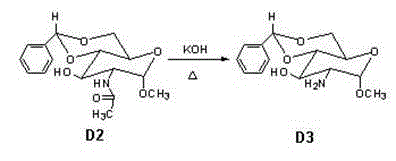
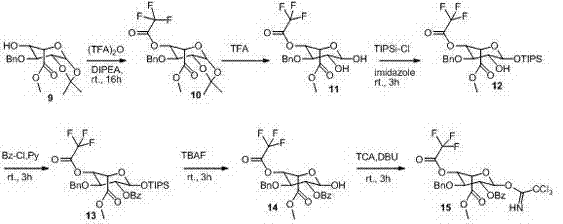
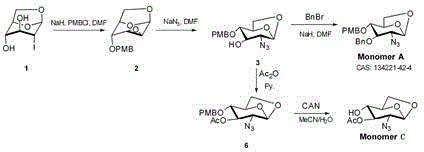
The invention discloses a synthetic method of a fondaparinux sodium intermediate. The synthetic method comprises the following steps: by using a compound 3 as a raw material, performing hydroxyl acetylation to obtain a compound 6, and removing p-methoxybenzyl (PMB) of the compound 6 by using ammonium cerium nitrate to obtain a Monomer C ring. By virtue of the mode, compared with a conventional five-step method for synthesizing the Monomer C ring, the synthetic method of the fondaparinux sodium intermediate, disclosed by the invention, can be used for simplifying the synthetic route of the Monomer C ring and reducing the production cost, and is suitable for large-scale industrial production.
Owner:PHARMA SHANGHAI
Process for the production of fondaparinux sodium
InactiveUS20150031866A1High purityReduce contentSugar derivativesSugar derivatives preparationPhotochemistryFondaparinux Sodium
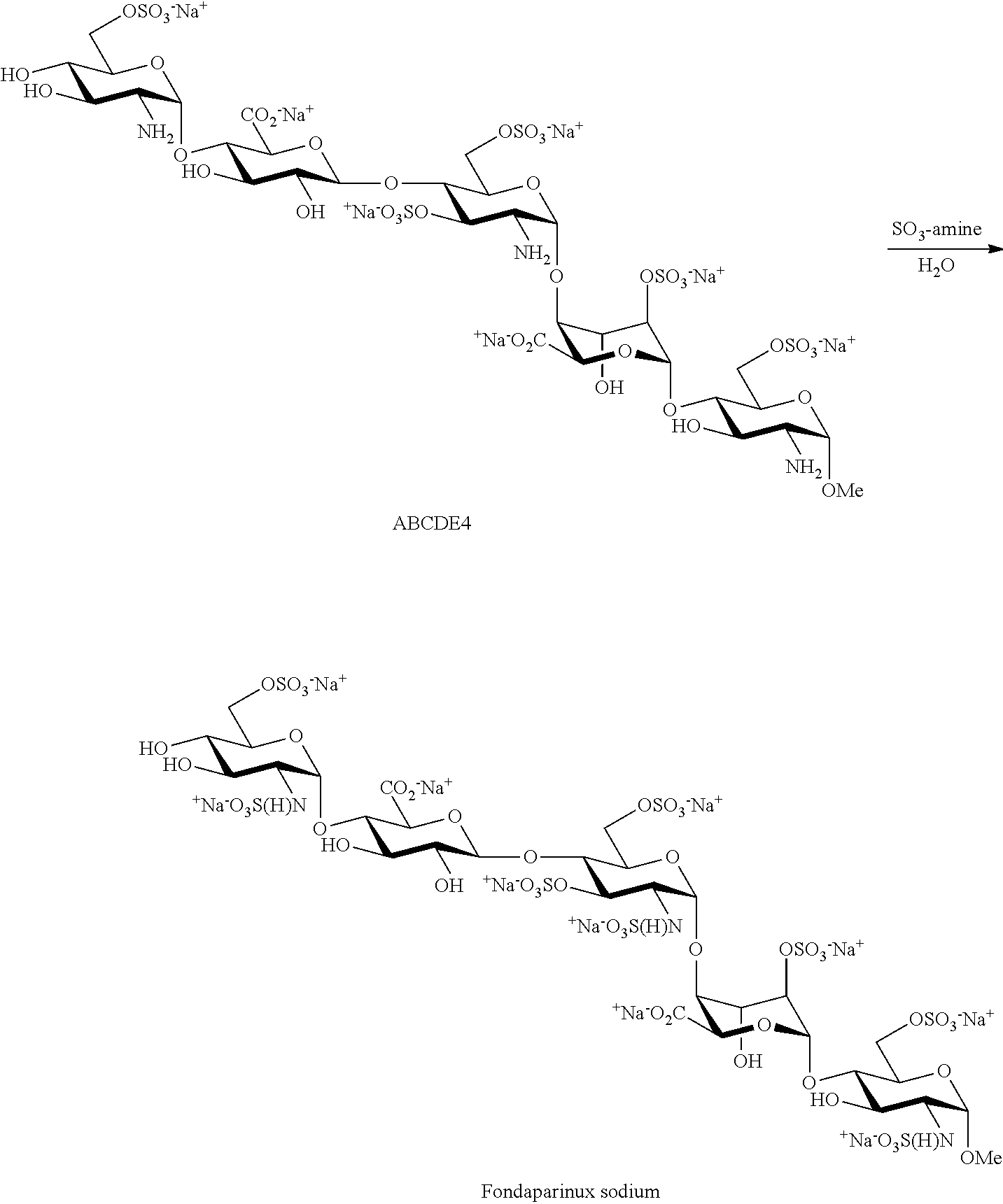
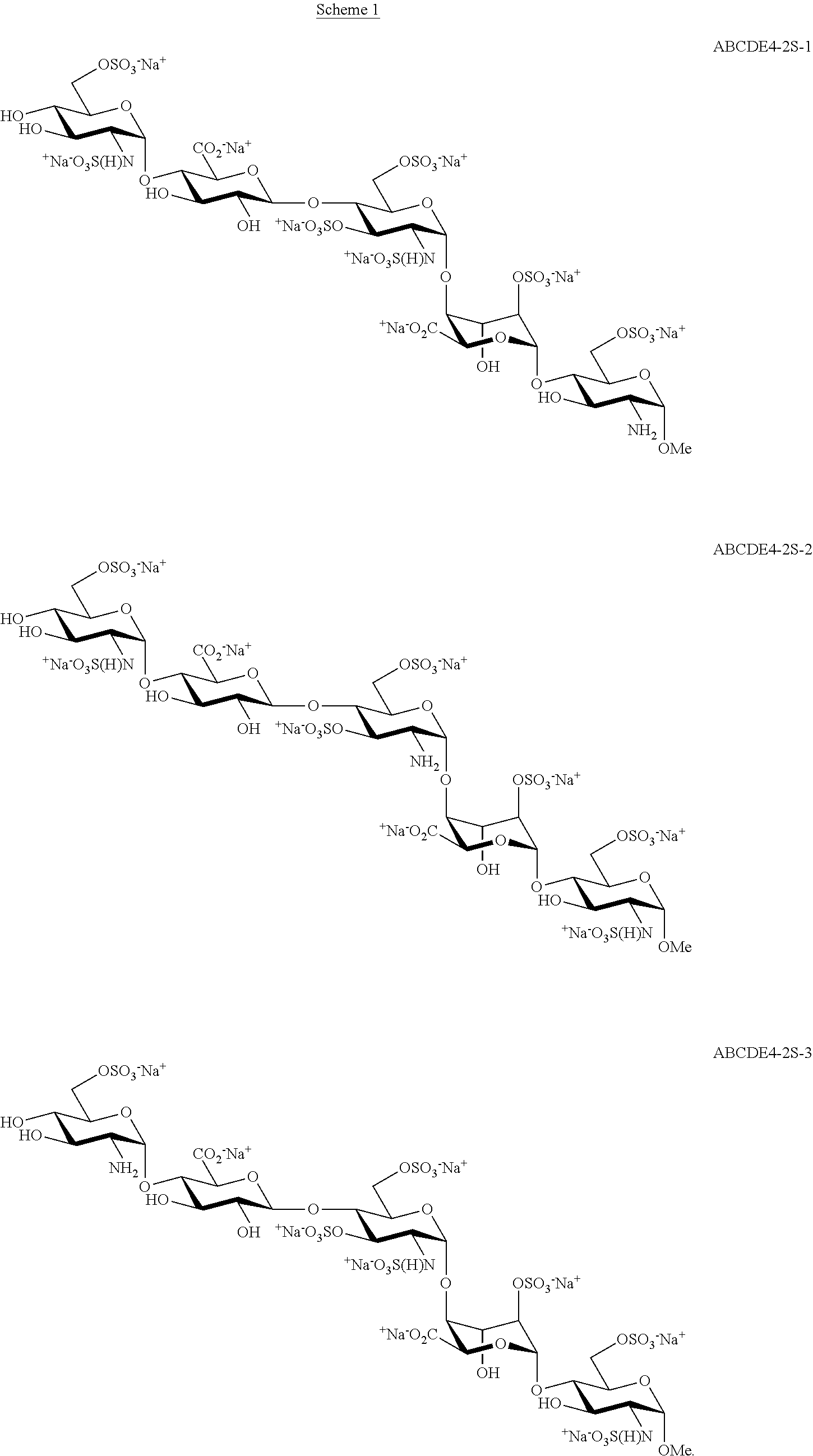
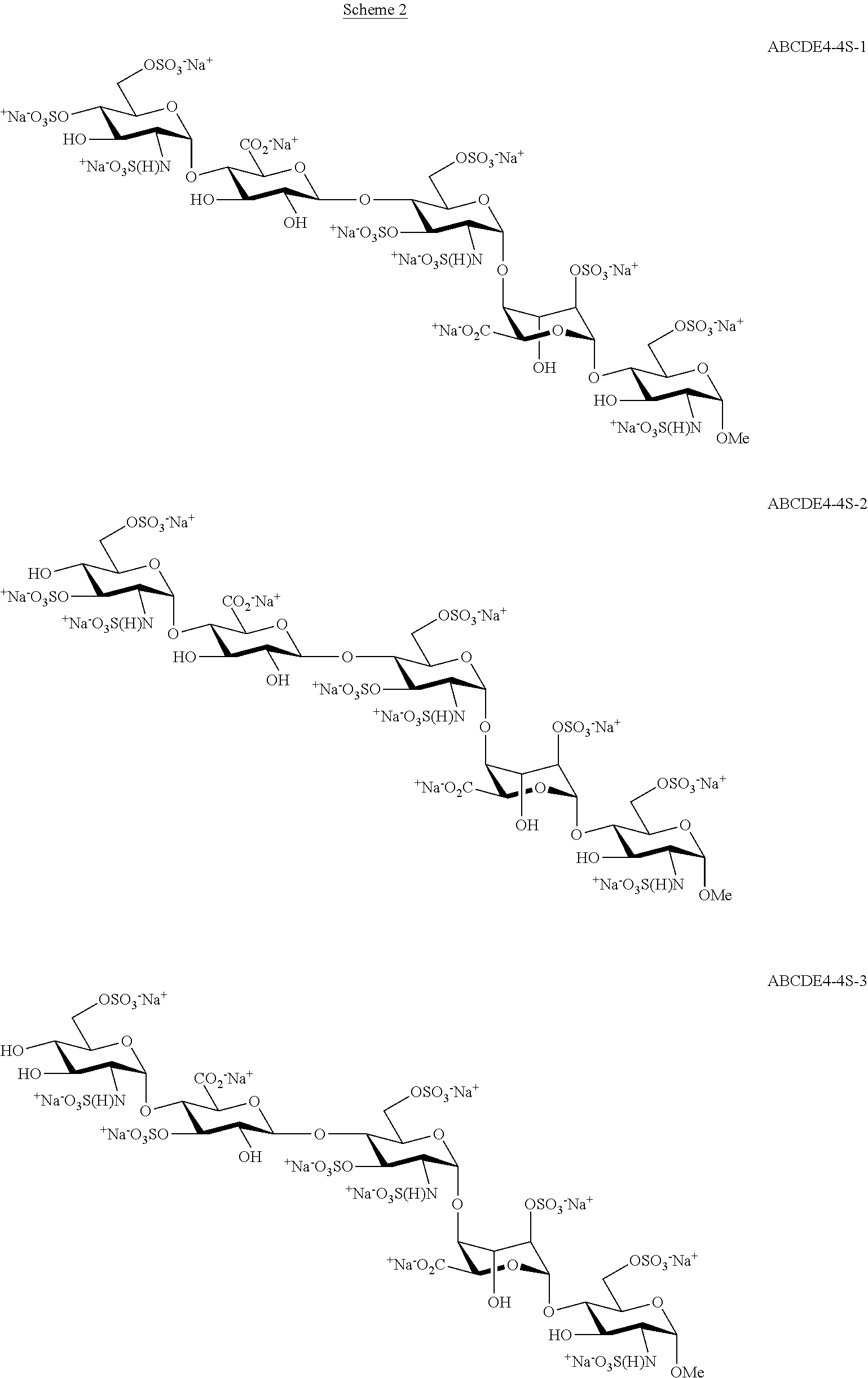
The present invention provides improved processes of preparing Fondaparinux sodium comprising converting a compound of formula ABCDE4 to Fondaparinux sodium at a reaction pH of no more than about 9.0. In some embodiments, the intermediates for the synthesis of Fondaparinux sodium, are also provided.
Owner:SCINOPHARM TAIWAN LTD
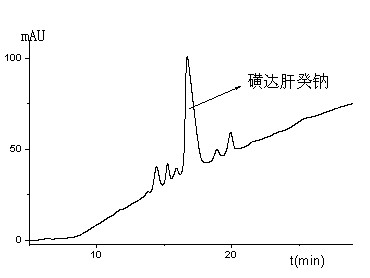
Analytical method for detecting sulfated oligosaccharides
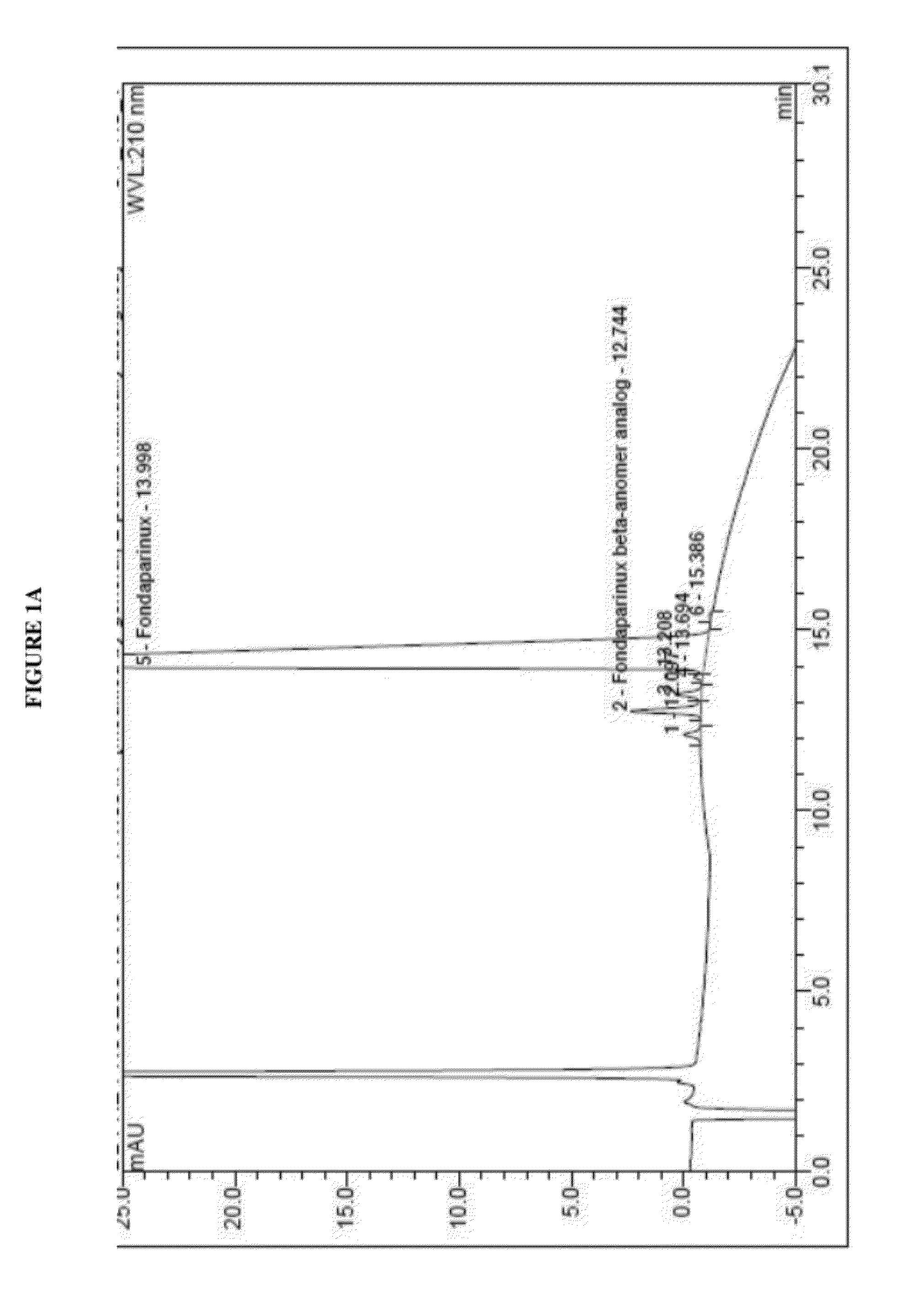
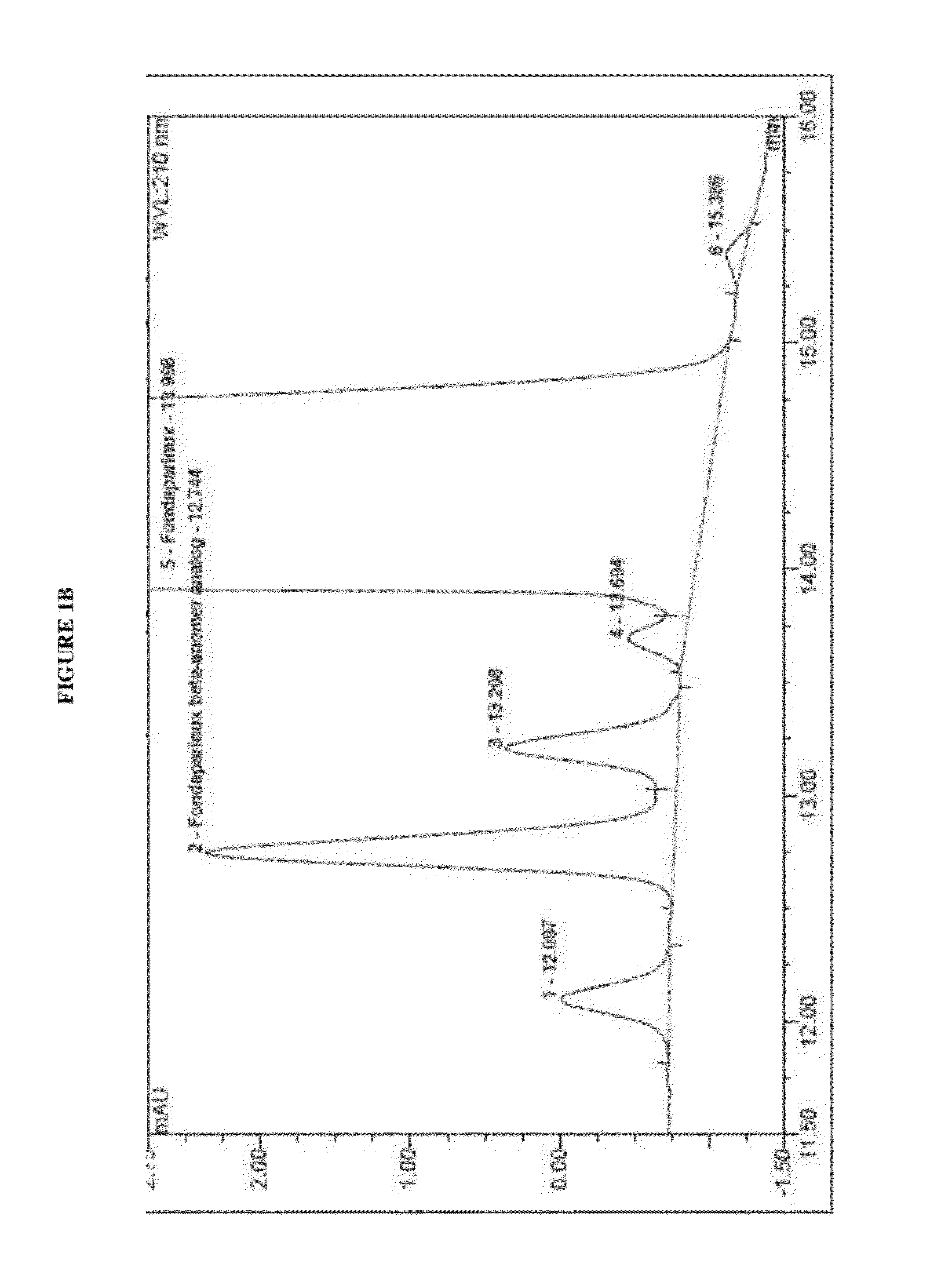
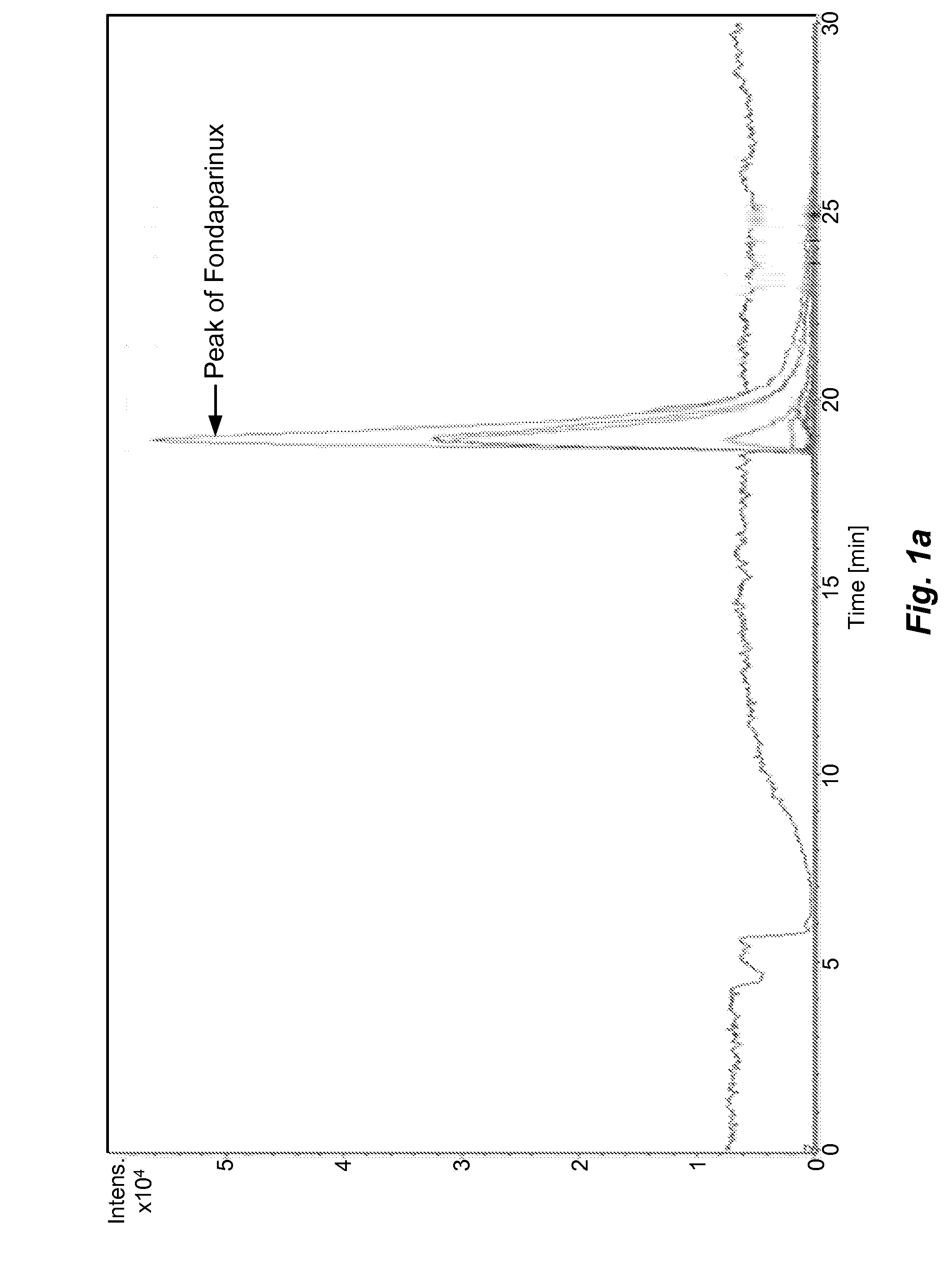
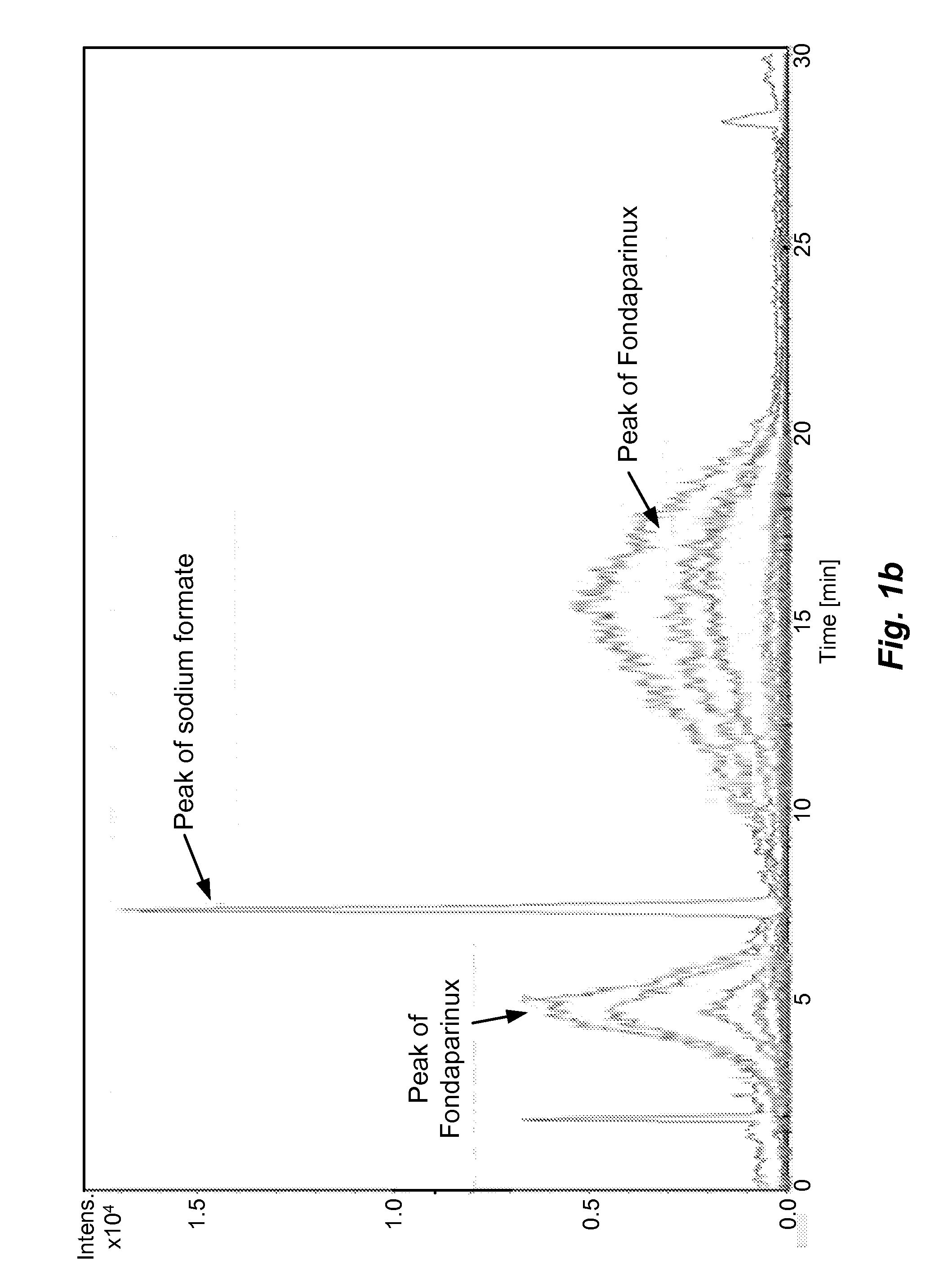
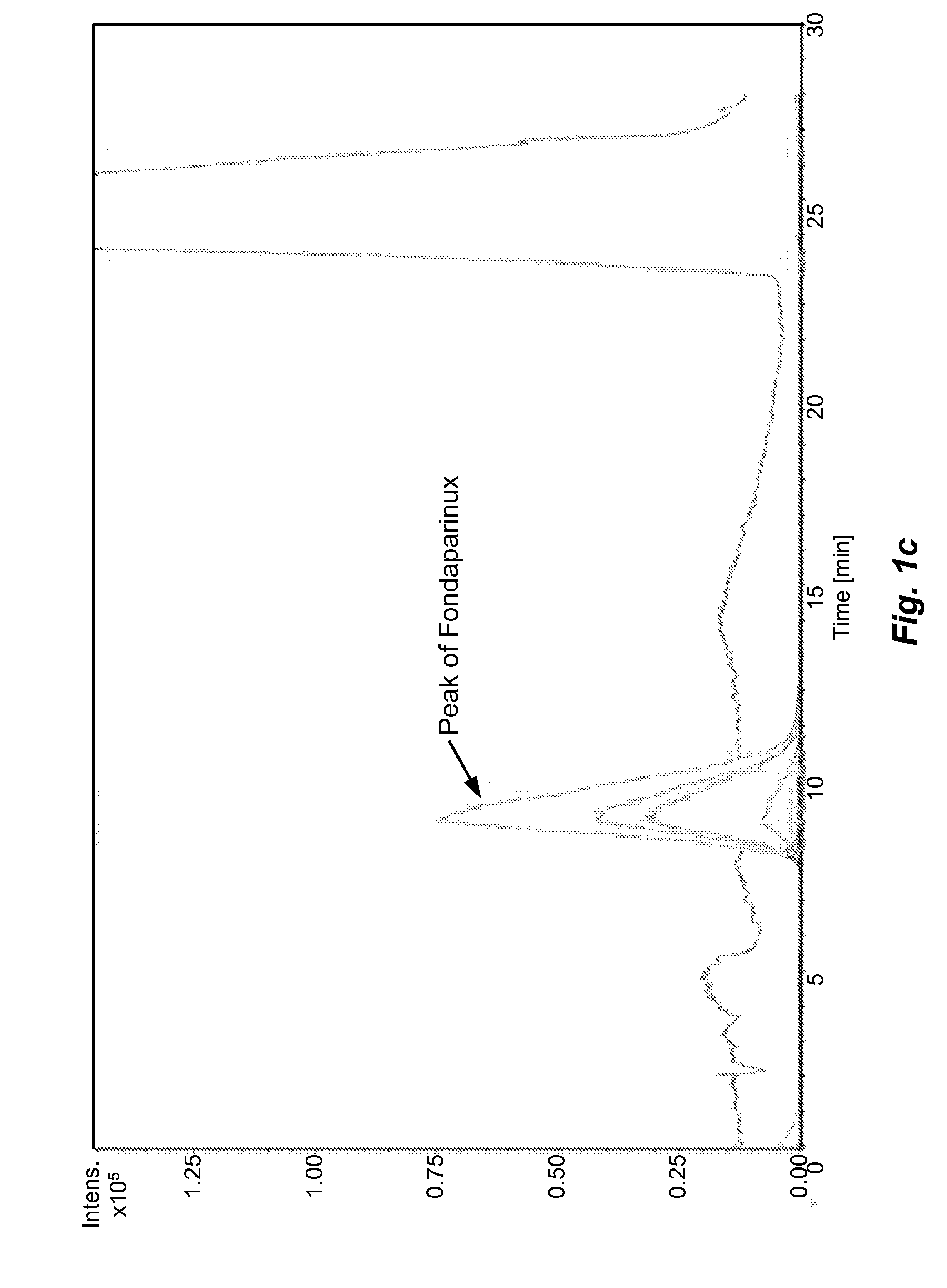
The present invention describes an analytical method for detecting and quantitating poly-sulfated oligosaccharides, including Fondaparinux sodium, using hydrophilic interaction ultra-performance liquid chromatography (HILIC-UPLC) coupled with a charged aerosol detector (CAD) or a mass spectrometer (MS). This analytical method provides in-process control in a total synthesis of highly sulfated oligosaccharides by separation, quantification and mass identification. Systems and conditions utilizing such methods are also provided.
Owner:SCINOPHARM TAIWAN LTD
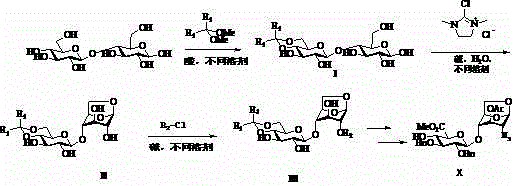
Preparation method for pentosaccharide intermediate of anticoagulant drug fondaparinux sodium
ActiveCN105315315AFew synthetic stepsAchieve glycosidationGroup 4/14 element organic compoundsSugar derivativesGlycosidic bondFondaparinux Sodium
The invention discloses a preparation method for a pentosaccharide intermediate of anticoagulant drug fondaparinux sodium. Monosaccharide segments A, B, C and D are prepared through a novel synthesizing method, and the synthesizing steps are simplified; meanwhile, appropriate protecting groups are mainly selected to protect hydroxyl groups and carboxylic acid, the stereospecific glycosylation achievement in the later period is facilitated, and then the needed glycosidic bond types are obtained; in addition, the protecting groups can be removed in one pot, and therefore the synthesizing steps are shortened. The preparation route is practical, convenient to operate and feasible.
Owner:四川奥邦古得药业有限公司
Preparation method of fondaparinux sodium injection composition
InactiveCN107595769AEffective pH adjustmentHigh clarityOrganic active ingredientsInorganic non-active ingredientsTurbidityMedical prescription
The invention discloses a preparation method of a fondaparinux sodium injection composition. The preparation method comprises the following steps of 1) according to a prescription of the fondaparinuxsodium injection composition, weighing all materials by prescription dosage; 2) taking fondaparinux sodium and sodium chloride, combining, then adding injection water with the weight being 75-85% of the total weight, after stirring and dissolving, adopting a pH regulator to regulate the pH to 5-8, then adding remaining injection water, stirring and mixing uniformly to obtain liquid medicine; 3) filling prepared liquid medicine, and carrying out high-temperature sterilization, cooling, lamp inspection, package and inspection to obtain the fondaparinux sodium injection composition. The fondaparinux sodium injection composition prepared by the invention has the advantages that by optimizing the pH and sterilization process of the fondaparinux sodium injection composition, the obtained solution is good in clarity and conforms to all detection requirements of the injection liquid item in Chinese pharmacopoeia, so that the quality stability of products is greatly improved, the crystallization and the turbidity of the solution are reduced, and the generation of impurities is reduced.
Owner:SHANGHAI SCIENPHARM CO LTD
Intermediate for preparing fondaparinux sodium, preparation method thereof, and fondaparinux sodium preparation method
ActiveCN105131054AHigh yieldEasy to synthesizeSugar derivativesSugar derivatives preparationCombinatorial chemistryHigh selectivity
The invention relates to a compound as shown in formula I for preparing fondaparinux sodium, a preparation method thereof and a preparation method of the fondaparinux sodium. The compound disclosed by the invention can be prepared from raw materials which are easy to obtain at higher selectivity and yield, thereby greatly simplifying the preparation process of the fondaparinux sodium. The definition of various substitutional groups contained in the formula I is the same as the definition in a specification.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Intermediate of fondaparinux sodium and preparation method for intermediate and fondaparinux sodium
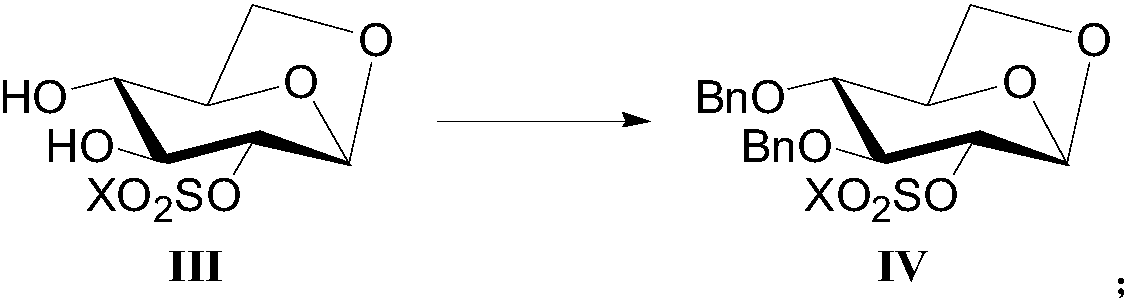
InactiveCN104098618AHigh purityHigh yieldSugar derivativesSugar derivatives preparationPalladium on carbonPtru catalyst
The invention discloses an intermediate of fondaparinux sodium and a preparation method for the intermediate and the fondaparinux sodium. The preparation method is as follows: a protective pentasaccharide compound III is subjected to esterolysis reaction at presence of sodium hydroxide, the hydrolyzed compound is sulfated to obtain a compound IV, an intermediate compound I reacts with SO3 in pyridine at the temperature of 50 DEG C for 18 h and sulfated to obtain a compound II, the compound II is subjected to catalytic hydrogenation in methyl alcohol and water with palladium hydroxide or a palladium carbon catalyst to enable the compound II to get rid of all R groups to obtain the fondaparinux sodium. According to the invention, the reactions are high in selectivity and specificity and the productive rate of each reaction can reach about 90%; the obtained crude product of the intermediate is relatively high in purity, easy to supervise and trace, and relatively high in productive rate without need for purification; the operation is simple and the reproducibility is good; final products are obtained through the catalytic hydrogenation with benzyl groups and protecting groups removed, the final products are high in purity and suitable for large-scale industrial production, the production cost is greatly reduced, and the products are competitive in markets.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Multiple dose pharmaceutical compositions containing heparin and/or heparin-like compounds and devices and methods for delivering the same
ActiveUS20160361501A1Convenient treatmentLess cumbersomeOrganic active ingredientsAmpoule syringesMultiple doseLow molecular weight heparin
The present invention discloses novel devices, methods, and formulations for multiple dose delivery of an appropriate formulation of Heparin or low molecular weight heparin or a heparin-like compound. Multiple dose formulations of fondaparinux sodium, and preparation methods thereof, are also disclosed
Owner:VIRCHOW BIOTECH PVT +1
Fondaparinux sodium disaccharide intermediate preparation method
InactiveCN104098619AEasy to prepareLow costSugar derivativesSugar derivatives preparationCombinatorial chemistryMedicinal chemistry
The invention discloses a fondaparinux sodium disaccharide intermediate preparation method which comprises the following steps: firstly, using a formula (II) compound and a formula (III) compound for preparation of a formula (IV) compound; then using the prepared formula (IV) compound for the preparation of a formula (I) compound. The technical scheme of the fondaparinux sodium disaccharide intermediate preparation method is low in cost and high in yield, raw materials are easy to prepare, and by use of the intermediate as a raw material for preparation of fondaparinux, the cost can be greatly reduced.
Owner:李正海
Efficient and scalable process for the manufacture of Fondaparinux sodium
ActiveUS8420790B2High product yieldEfficient amplificationSugar derivativesGlycosidesPhotochemistryFondaparinux Sodium
Owner:RELIABLE BIOPHARM LLC
Automatic preparation method of fondaparinux sodium pentasaccharide intermediate
ActiveCN112552359ARealize automated manufacturingImprove acquisition efficiencySugar derivativesSugar derivatives preparationTemperature controlProcess engineering
The invention relates to an automatic preparation method of a fondaparinux sodium pentasaccharide intermediate. The method is based on an automatic preparation device. According to the method, automatic preparation of three components (D+EF+GH) is realized through automatic sample injection and automatic sampling and monitoring, the fully-protected fondaparinux sodium pentasaccharide intermediate(formula I) is obtained, and automatic synthesis of the fondaparinux sodium pentasaccharide intermediate is realized, so that the manpower can be saved, the efficiency and productivity are improved, and relatively high safety and reproducibility are achieved; direct online monitoring can be realized, the real-time state of a reaction is convenient to optimize and monitor, and meanwhile, the automatic temperature control can better meet the requirements of the reaction on heating and cooling; a pre-activation one-kettle mode is adopted, so that the separation frequency is reduced, and the operation is convenient; and a common ester protecting group is selected, relatively high stereoselectivity and yield are achieved, a universal deprotection method can be used, and the method has importantsignificance in reducing the production cost of fondaparinux sodium and achieving large-scale production. The definition of each substituent in the formula I is the same as the definition in the specification.
Owner:PEKING UNIV
Preparation method of Fondaparinux sodium injection
InactiveCN111249227ANot affected by qualityReduce oxygen contentOrganic active ingredientsInorganic non-active ingredientsDrugs solutionBULK ACTIVE INGREDIENT
The invention discloses a preparation method of a Fondaparinux sodium injection. The method comprises the following steps: (1) weighing each raw material and subsidiary material according to a prescription amount according to a prescription of the Fondaparinux sodium injection; (2) adding part of water for injection into a liquid preparing container, performing heating to 100 DEG C, performing cooling to room temperature, then adding an active ingredient and an isotonic regulator, and performing stirring and dissolving; (3) adjusting a pH value of a solution obtained in the step (2) to 6.5-7.5with a pH regulator, and adding the remaining water for injection to make a constant volume; (4) filtering a drug solution through a 0.22 micron micro-porous filter membrane or filter element; (5) filling a filtered drug solution into a pre-filled syringe, and performing moist heat sterilization at 121-125 DEG C for 12-15 min; and (6) performing lamp inspection, packaging and inspection after sterilization to obtain the Fondaparinux sodium injection. The preparation method of the invention meets requirements of drug registration regulations, has few impurities, has good stability, has high sterility assurance level, and is suitable for industrial production.
Owner:YANTAI DONGCHENG PHARMA GRP
Preparation method of fondaparinux sodium monosaccharide intermediate
ActiveCN109096348AHigh yieldNothing producedEsterified saccharide compoundsSugar derivativesAcetic anhydrideTrifluoroacetic acid
The invention discloses a preparation method of a fondaparinux sodium monosaccharide intermediate. The preparation method comprises the following steps: (1) in the presence of an acid-binding agent, carrying out esterification reaction on a compound as shown in a formula (I) and a substituent sulfonic anhydride and / or a substituent sulfonyl halogen to generate a compound as shown in a formula (II); (2) carrying out a ring opening reaction on the compound as shown in the formula (II) in an acidic condition to generate a compound as shown in a formula (III); (3) carrying out benzylation reactionon the compound as shown in the formula (III) and benzyl monohalide in an alkaline condition to generate a compound as shown in a formula (IV); and (4) carrying out an azido reaction on the compoundas shown in the formula (IV) and alkali metal azide salt to generate a compound as shown in a formula (V) and caring out inner ether ring opening and acetylation reaction on the compound as shown in the formula (V) in a mixed solution of acetic anhydride and trifluoroacetic acid to generate a compound as shown in a formula (VI). The method can be used for obtaining an ideal product yield, and theraw materials are cheap, easily available and relatively few in three wastes. The formulae are as shown in the description.
Owner:江苏美迪克化学品有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com