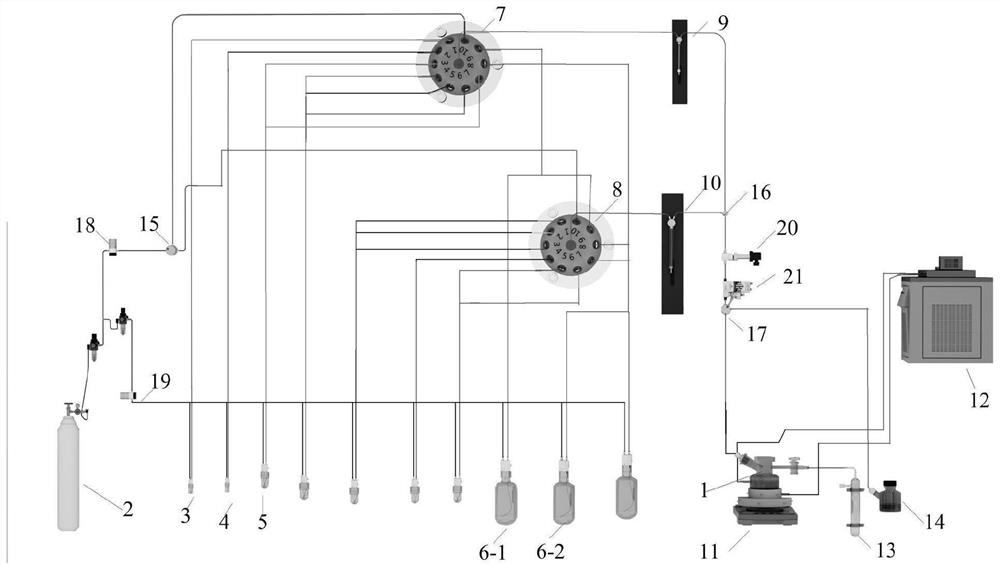
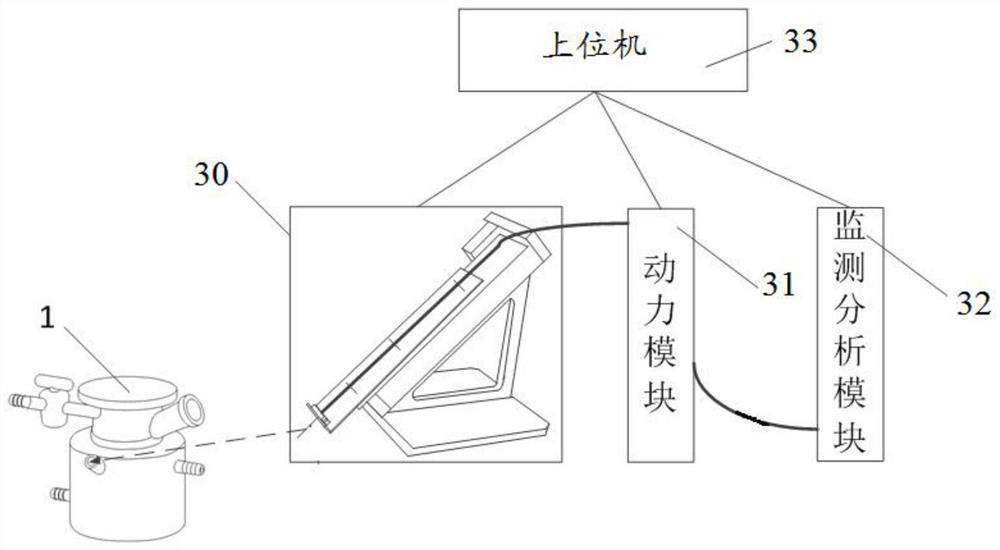
Automatic preparation method of fondaparinux sodium pentasaccharide intermediate
A technology of fondaparinux sodium and intermediates, which is applied in the field of automatic preparation of fondaparinux sodium pentasaccharide intermediates, can solve the problems of time-consuming and laborious, limited wide application, long half-life and the like, and achieves reduction of the influence of human factors, Improve efficiency and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
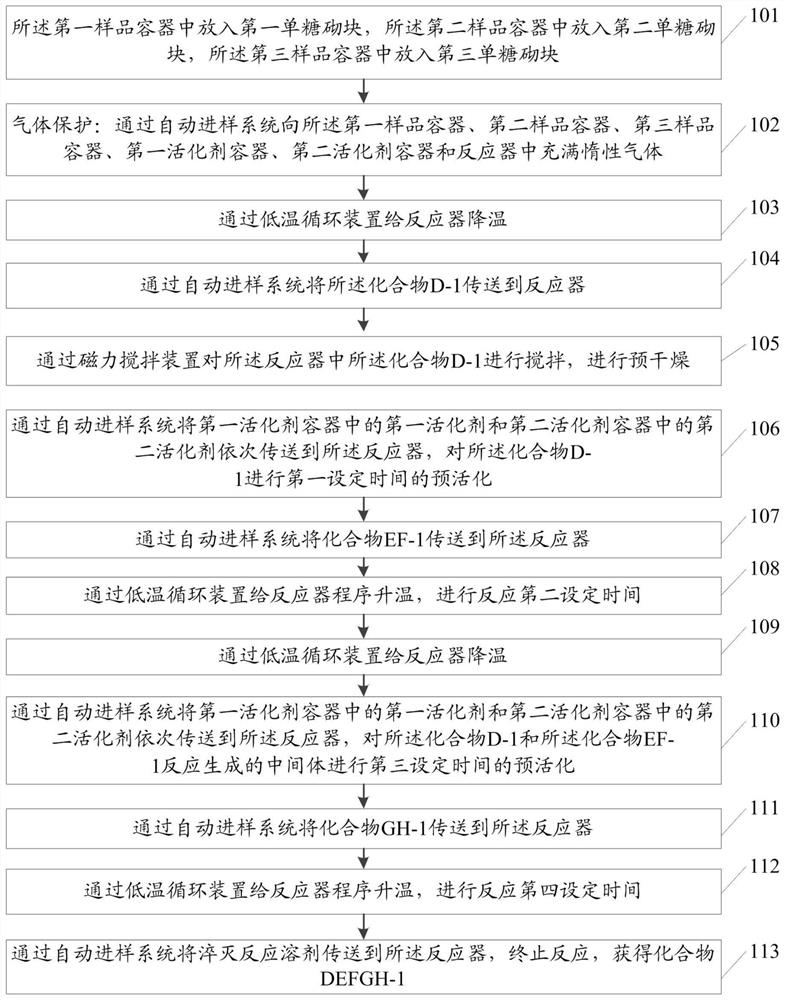
[0073] The raw materials for the preparation of the fondaparinette sodium pentasaccharide intermediate include compound D-1, compound EF-1 and GH-1;
[0074] The methods include:
[0075] Step 101: put compound D-1 into the first sample container 3, and the compound D-1 has the structure shown in formula II:
[0076]
[0077] In formula II, R 1 It is an acyl group or a silicon protecting group, preferably an acetyl group (Ac) or a tert-butyldiphenylsilyl group (TBDPS); SR 8 It is a glucosinolate leaving group, preferably but not limited to phenylthio, p-methylphenylthio, o-methylphenylthio or 2-methyl-5-tert-butylphenylthio; X is N 3 Or various amino groups with protective groups; in the present invention, the compound D-1 specifically preferably has a structure shown in formula II-1, named as compound D-3:
[0078]
[0079] Put compound EF-1 into described second sample container 4; Described compound EF-1 has the structure shown in formula III:
[0080]
[0081]...
Embodiment
[0120] Before automation: phenylthiochloride (p-TolSCl) and silver trifluoromethanesulfonate (AgOTf) are preferred as the "pre-activation" system, and p-TolSCl (diluted in dichloromethane, connected to channel No. 6) and AgOTf[ Dissolved in a mixed solution (V / V=3:1) of toluene and dichloromethane, connected to the stock solution of channel number 5] and connected to the corresponding sampling pipeline, but not limited to other "preactivation" systems; preferably Freshly activated 4A molecular sieves are added to the reactor of the synthesizer in advance and the reactor is sealed; prepare compound D-3 (connection channel number 1), compound EF-4 (connection channel number 2) and GH-2 (connection channel number 3 ) stock solution (dissolved in a certain amount of dichloromethane) and respectively connected with the corresponding sampling pipeline; prepare dry dichloromethane (connection channel number 9) as a solvent for sampling or cleaning pipeline and connect with the corresp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com