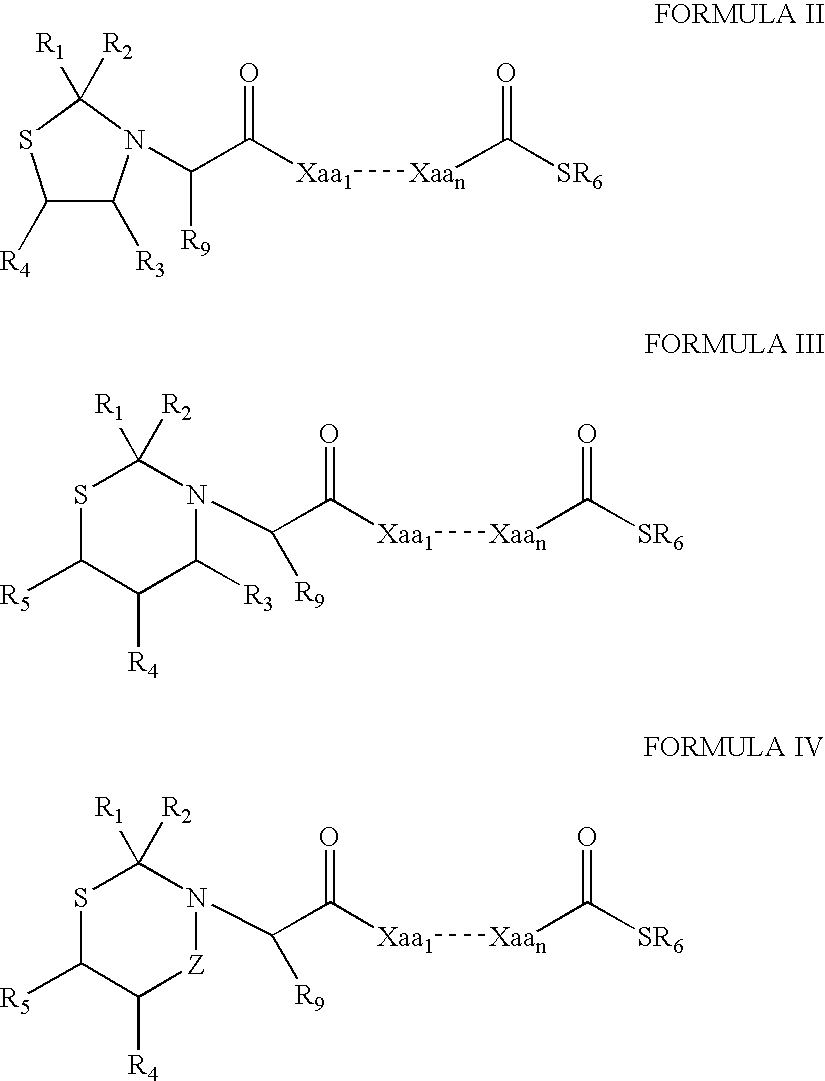
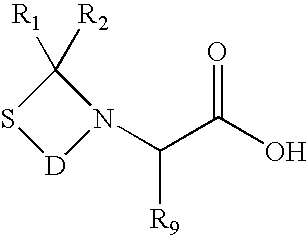
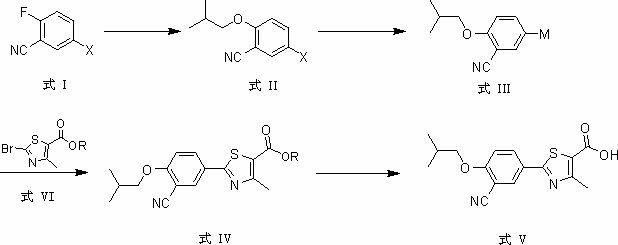
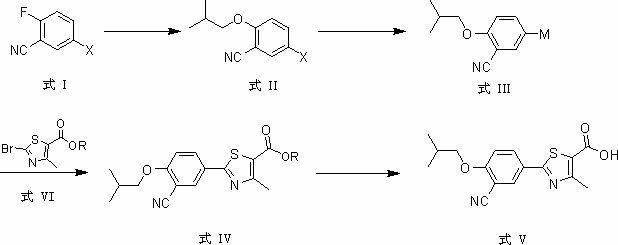
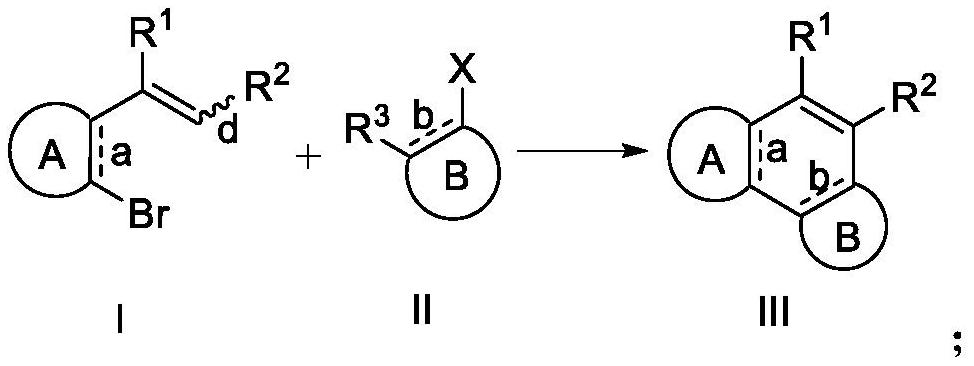
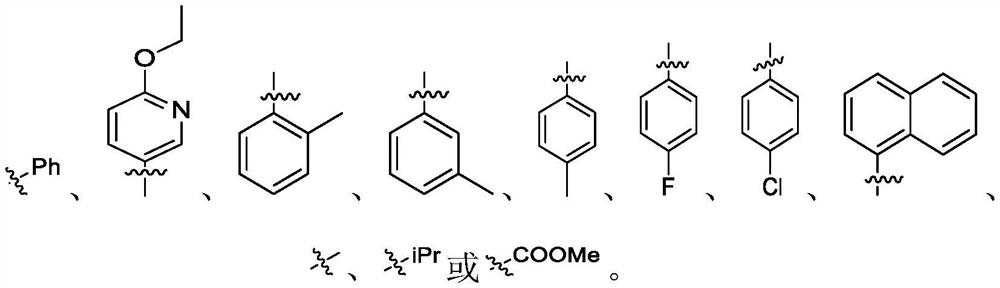
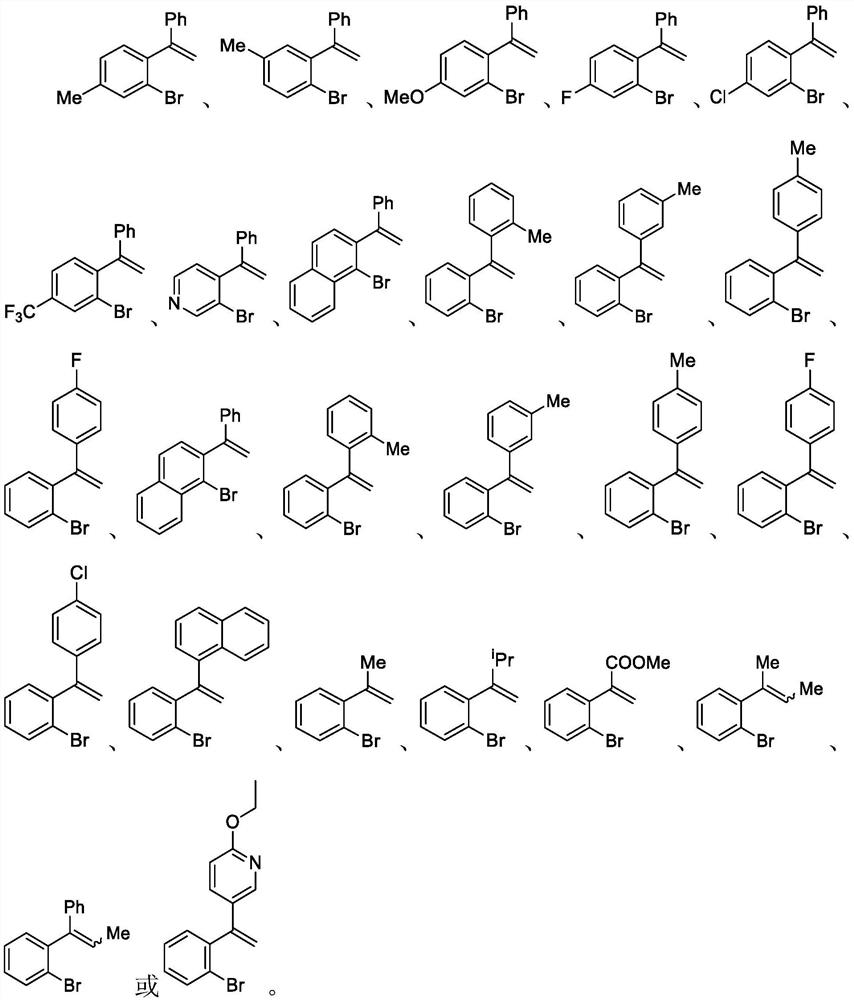
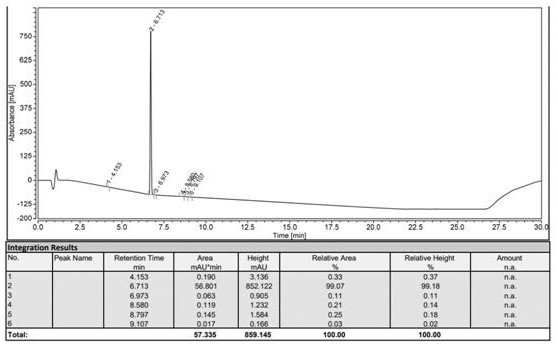
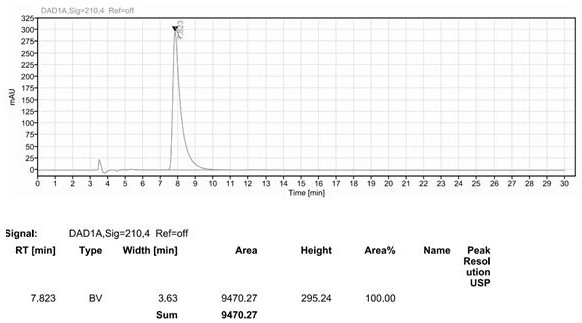
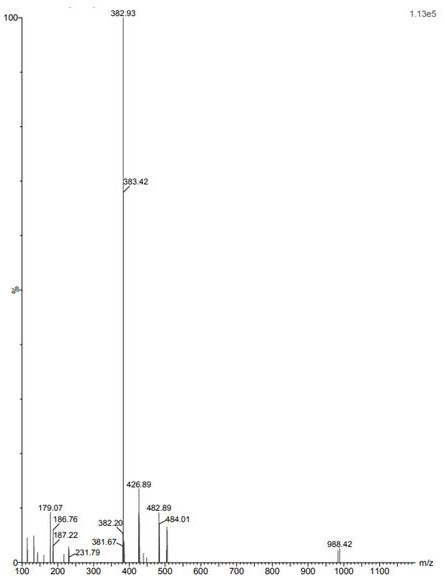
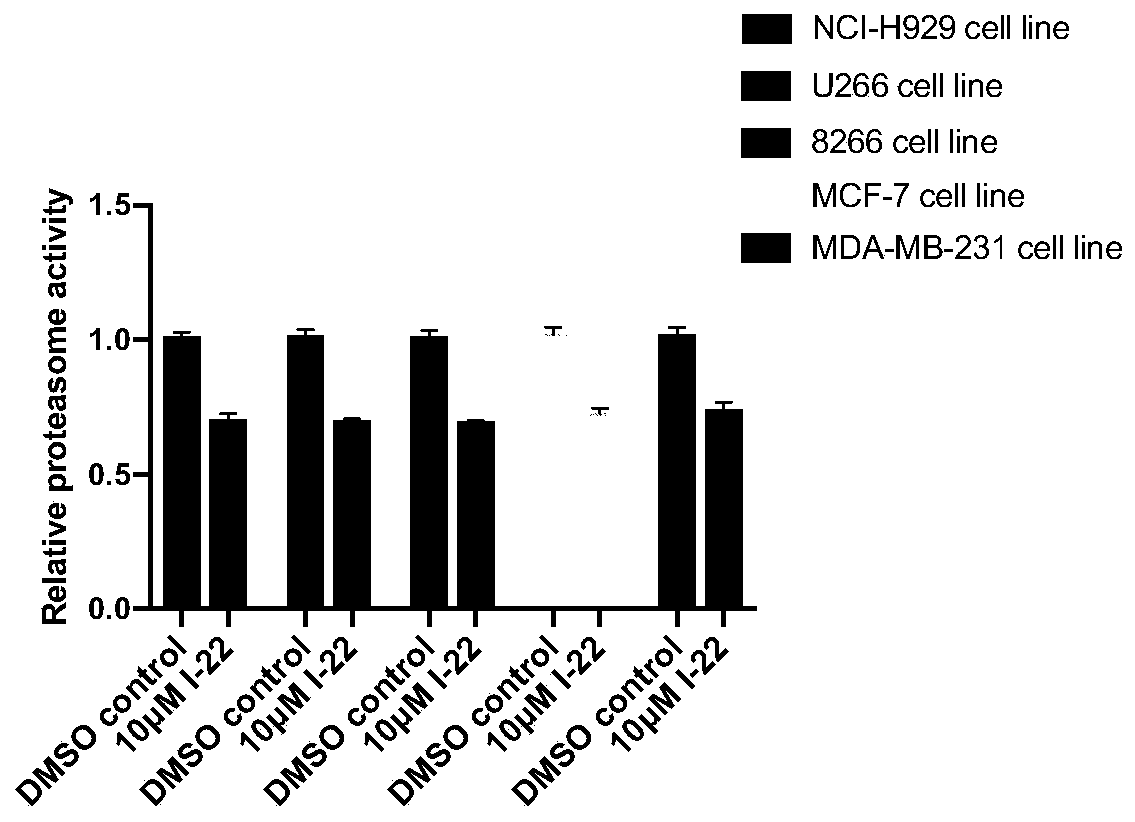
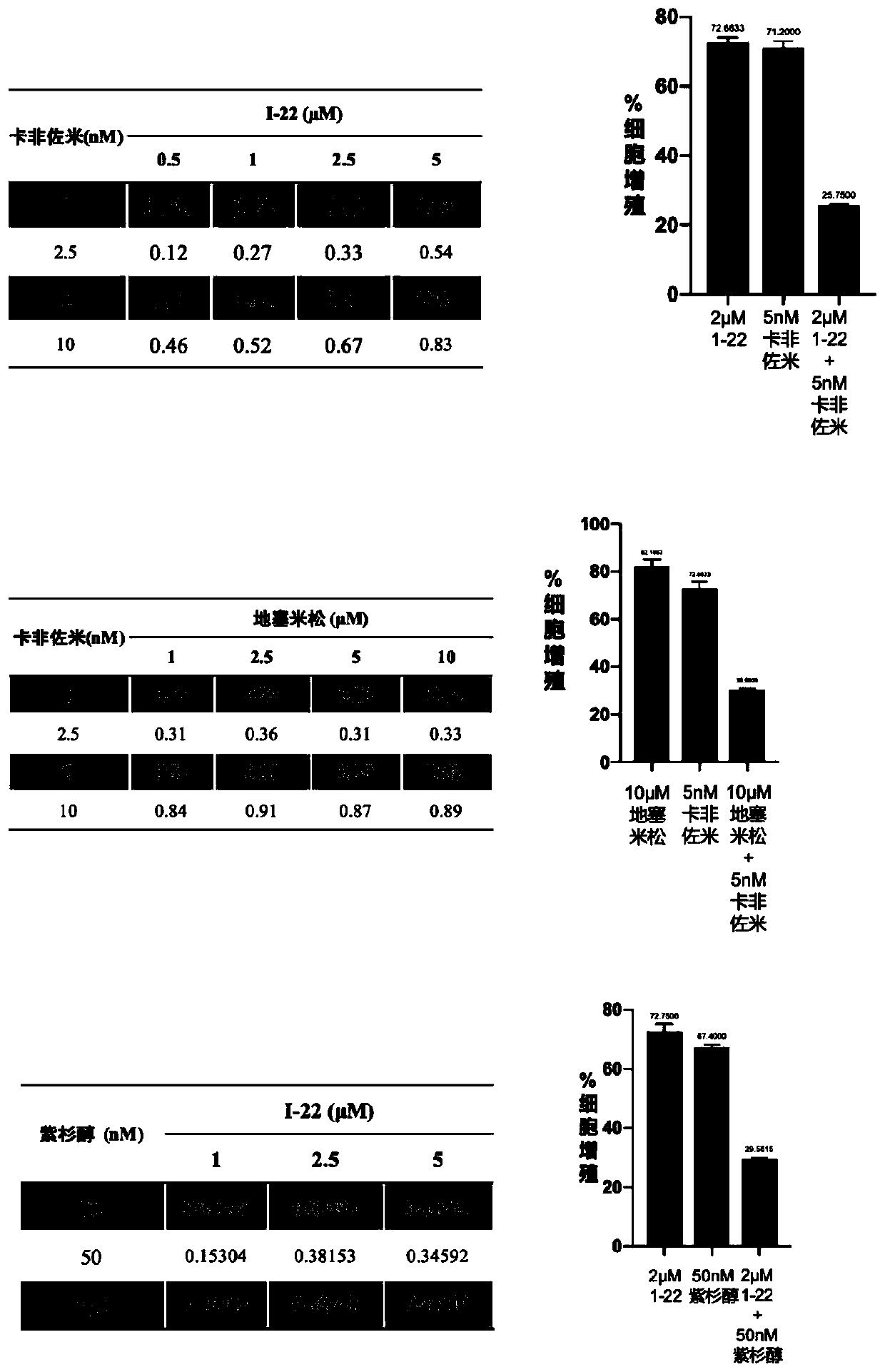
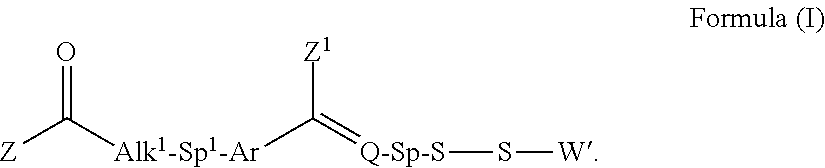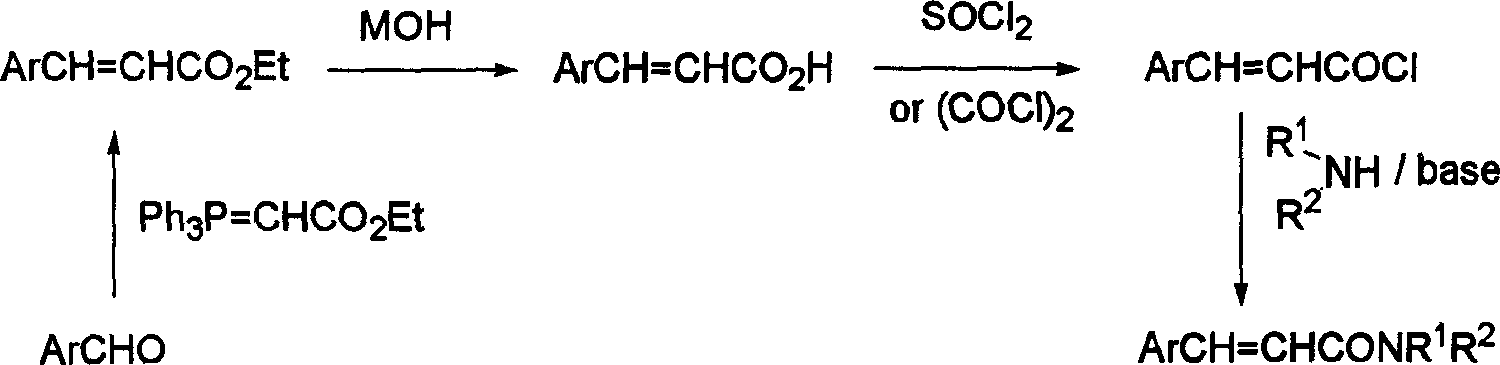Patents
Literature
60 results about "Convergent synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
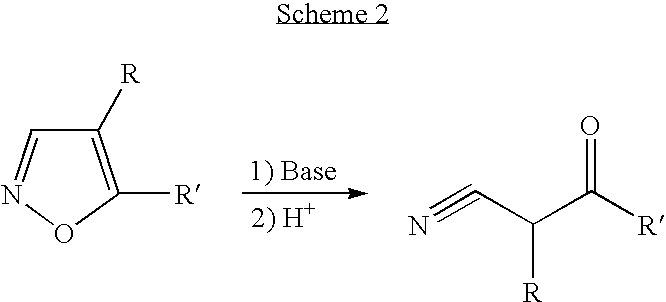
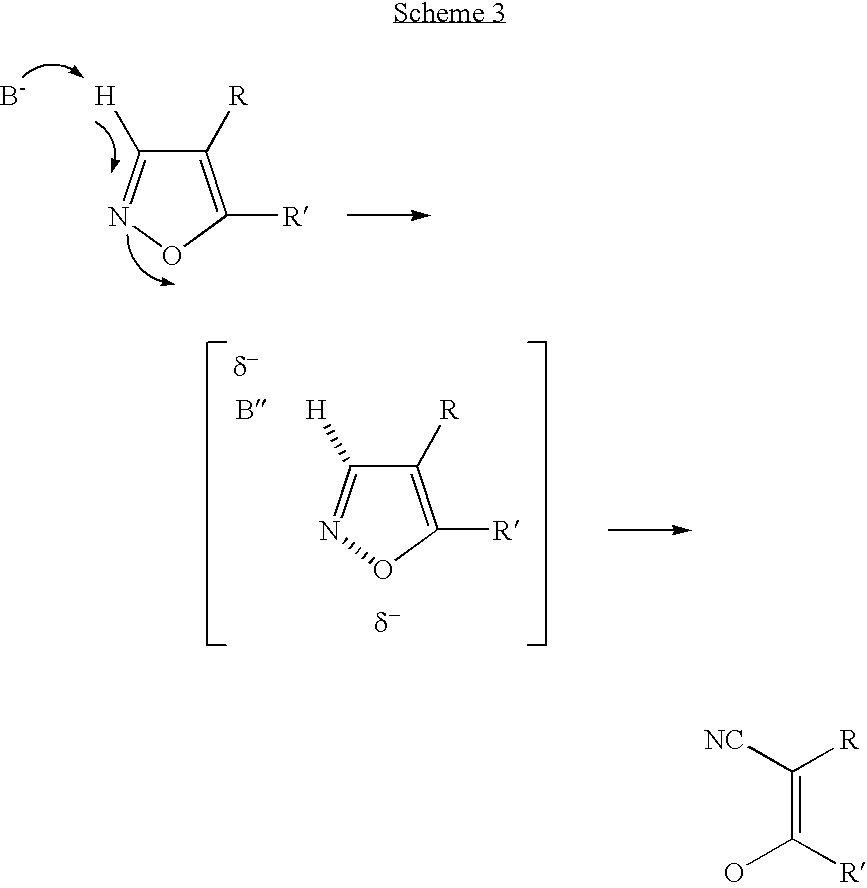
In chemistry a convergent synthesis is a strategy that aims to improve the efficiency of multistep synthesis, most often in organic synthesis. In this type of synthesis several individual pieces of a complex molecule are synthesized in stage one, and then in stage two these pieces are combined to form the final product.
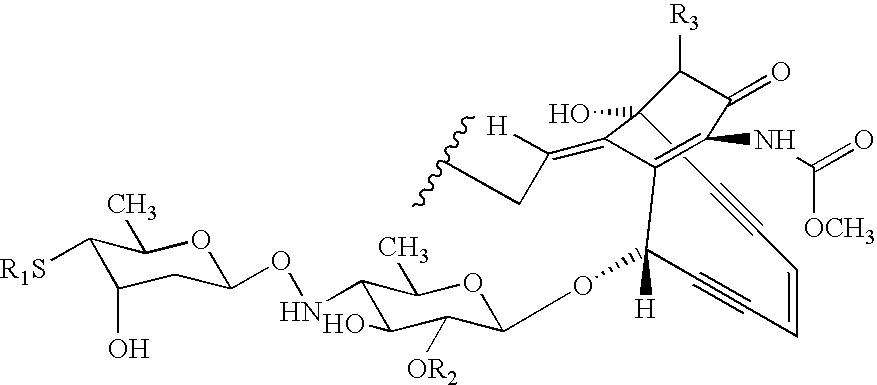
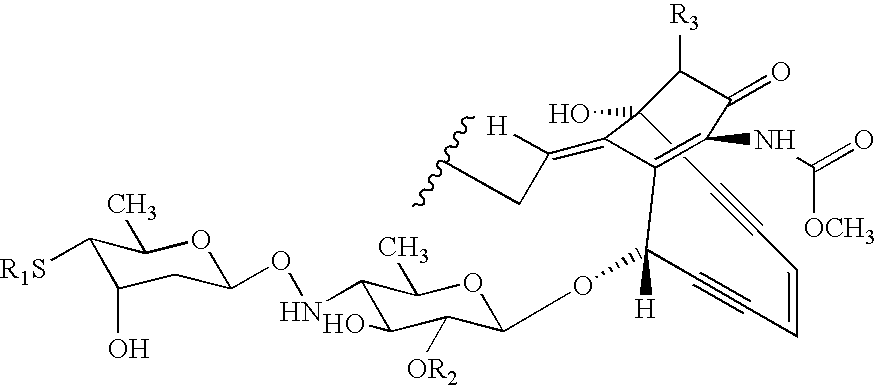
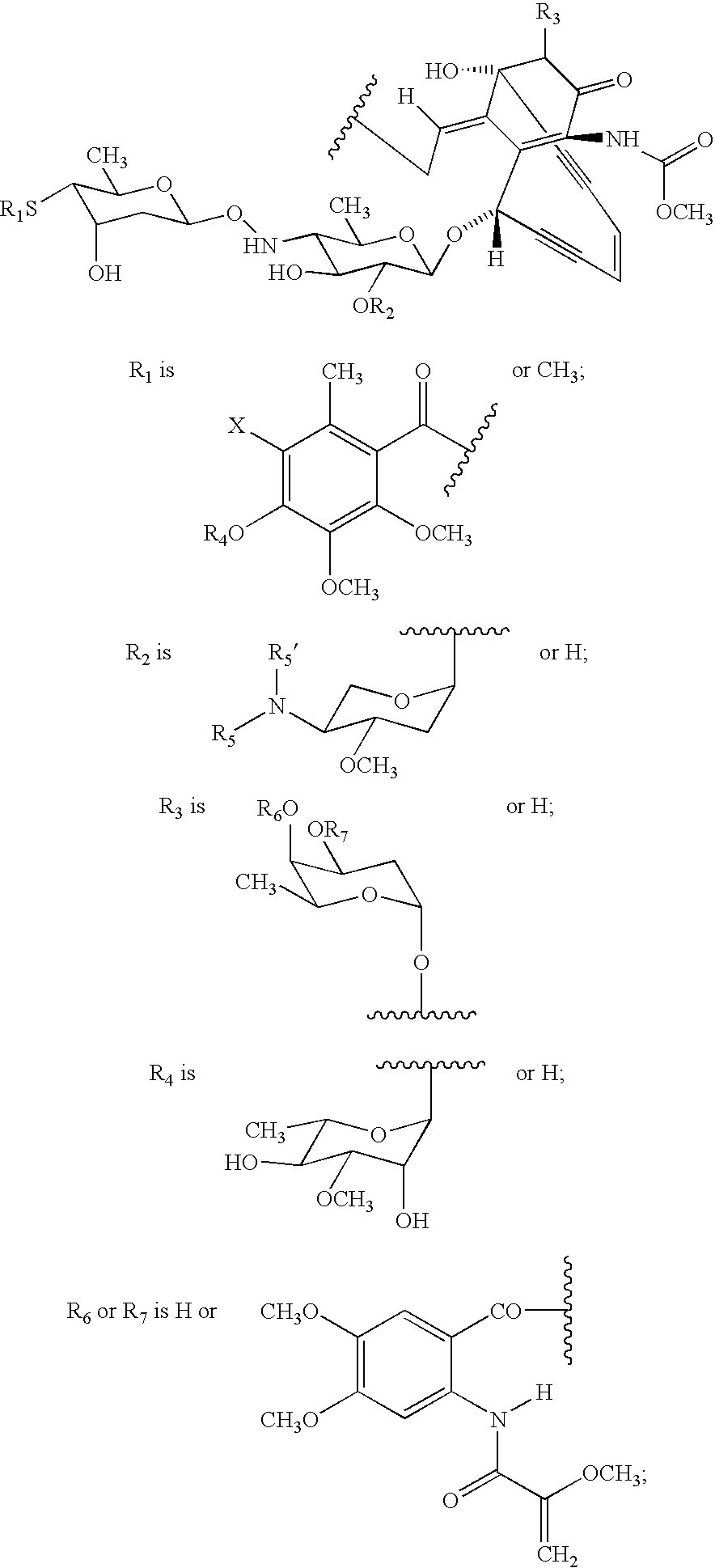
Processes for the convergent synthesis of calicheamicin derivatives
This invention describes processes for the convergent synthesis of calicheamicin derivatives, and similar analogs using bifunctional and trifunctional linker intermediates.
Owner:WYETH LLC
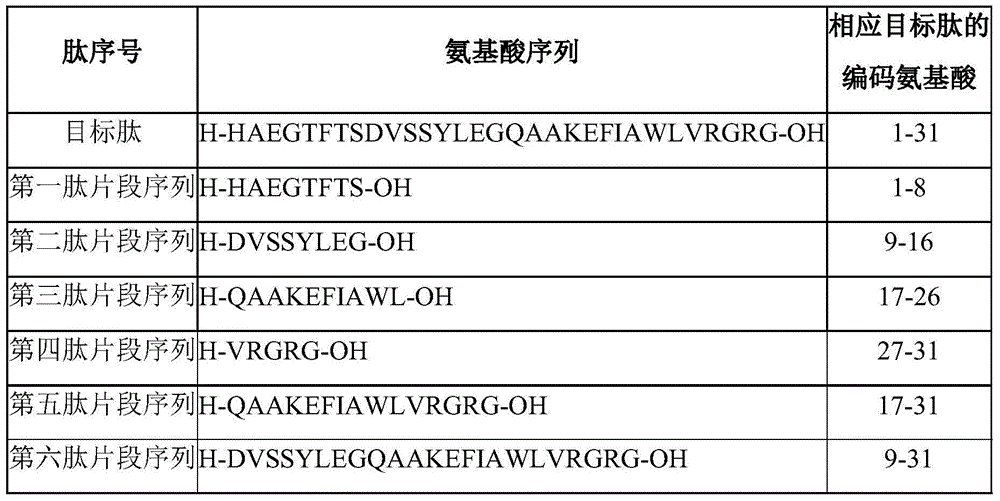
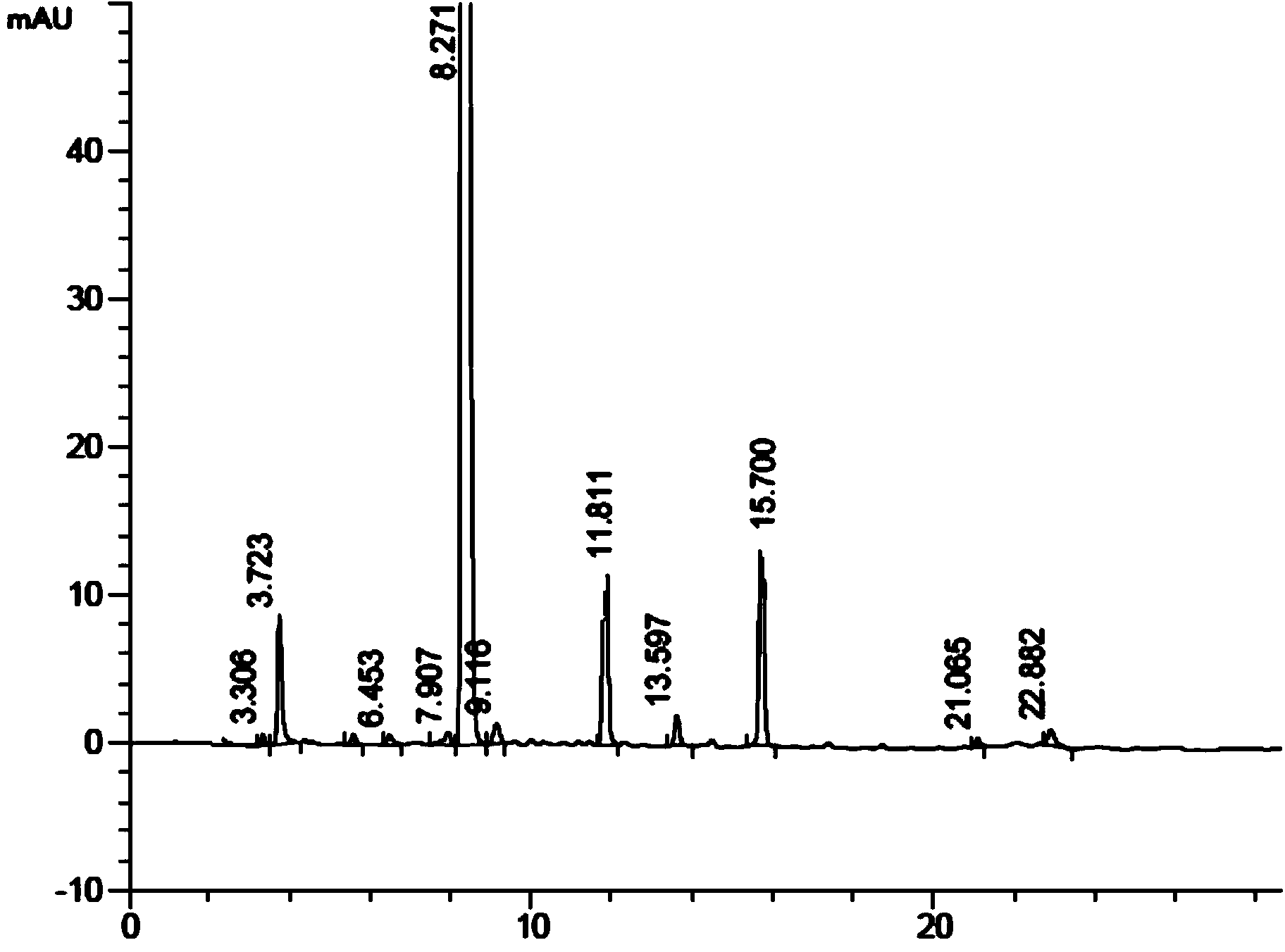
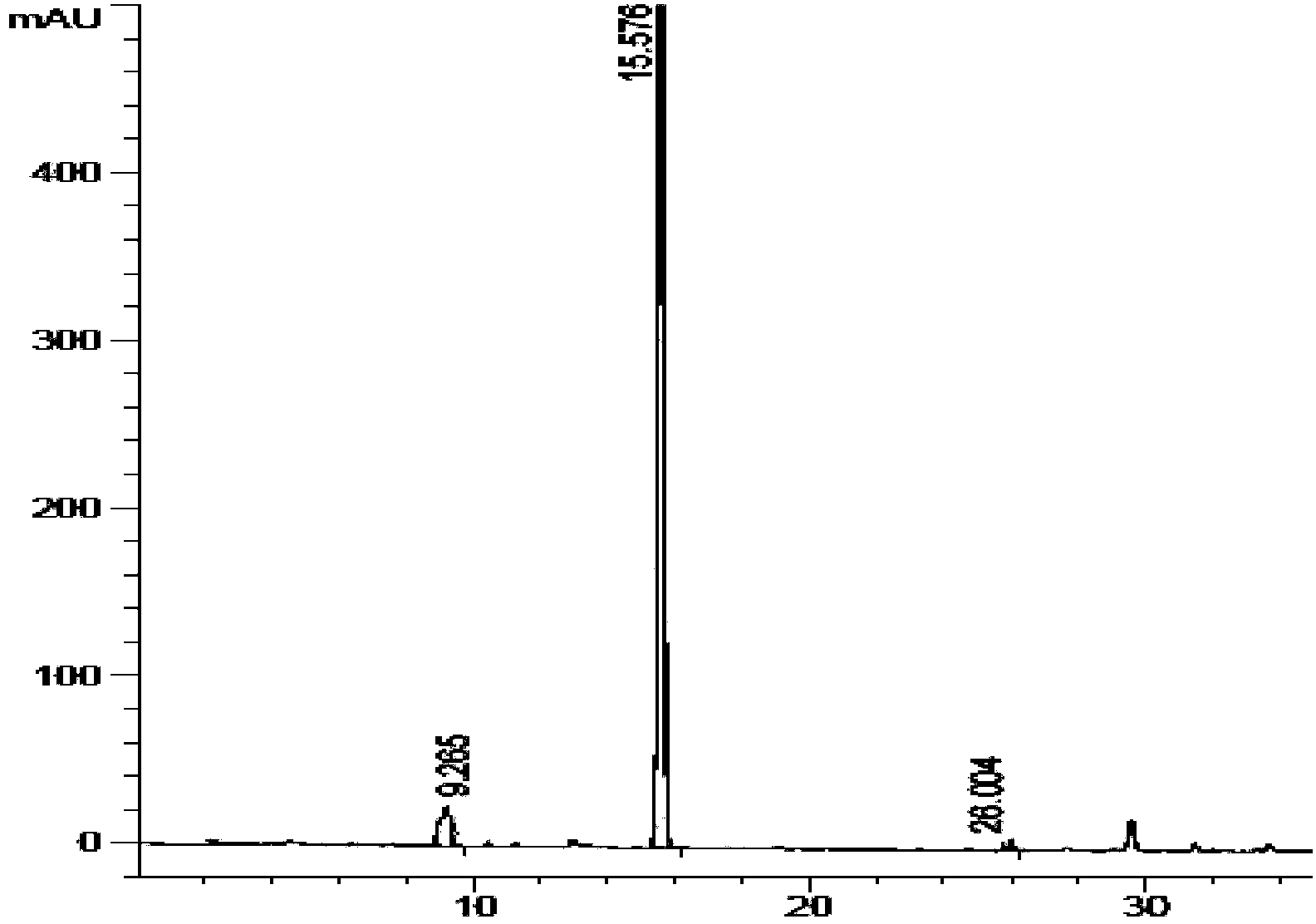
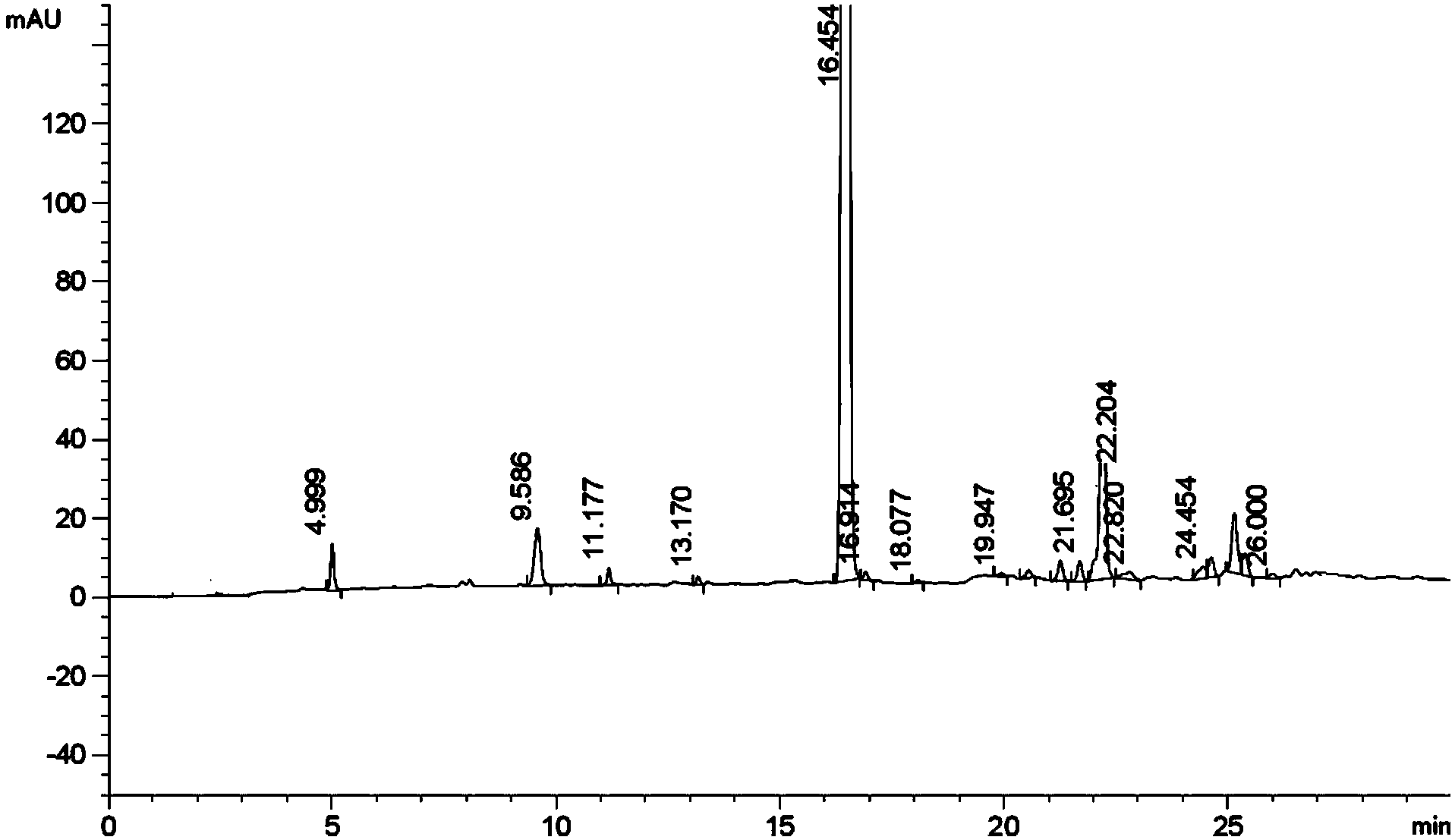
Method for preparing liraglutide by convergent synthesis
ActiveCN104650219AHigh purityHigh yieldPeptide preparation methodsBulk chemical productionSide chainPeptide fragment
The invention discloses a method for preparing liraglutide by convergent synthesis. The method comprises the steps of performing solid phase synthesis to obtain four side chain protected peptide fragment sequences, gradually coupling the peptide fragments in a solution system to obtain an all-protected liraglutide straight chain polypeptide, removing the side chain protection of the 20th Lys, performing modification to form all-protected liraglutide, then cracking to remove protecting groups to obtain a crude liraglutide peptide, purifying and exchanging salt to obtain liraglutide; wherein in the four peptide fragment sequences, the first peptide fragment sequence is 1st to 8th amino acids in the liraglutide sequence, the second peptide fragment sequence is 9th to 16th amino acids in the liraglutide sequence, the third peptide fragment sequence is 17th to 26th amino acids in the liraglutide sequence, and the fourth peptide fragment sequence is 27th to 31st amino acids in the liraglutide sequence. By adopting the method, the yield is improved, and the synthesis cost is greatly reduced; and the method is favorable for large-scale and industrialized production.
Owner:LANZHOU UNIVERSITY
Preparation method of kyprolis
The invention provides a preparation method of kyprolis, which comprises the steps of carrying out a condensation reaction among N-Boc-L-homophenylalanine shown in a formula (I), L-leucine ester shown in a formula (III) and salt, and carrying out deamination protection so as to obtain a compound shown in a formula (V); carrying out a condensation reaction between the compound shown in the formula (V) and morpholin-4-yl-acetic acid shown in a formula (VI), and carrying out decarboxylation protection so as to obtain a compound shown in a formula (IX); carrying out a condensation reaction between the compound shown in the formula (IX) and a compound shown in a formula (VIII), so that kyprolis is directly generated. According to the invention, a convergent synthesis method is adopted, and condensation and deprotection among amino acids are only involved, so that the reaction steps are less, reaction conditions are mild and controllable, and the yield of kyprolis is increased, therefore, the method is suitable for industrial production. Experimental results show that by using the method provided by the invention, the yield of kyprolis can reach 19.3-30.8%.
Owner:CHONGQING TAIHAO PHARM CO LTD
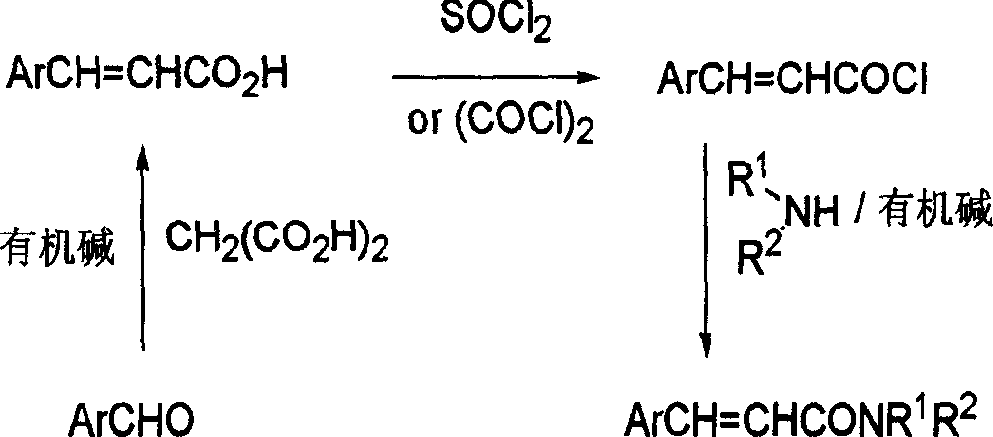
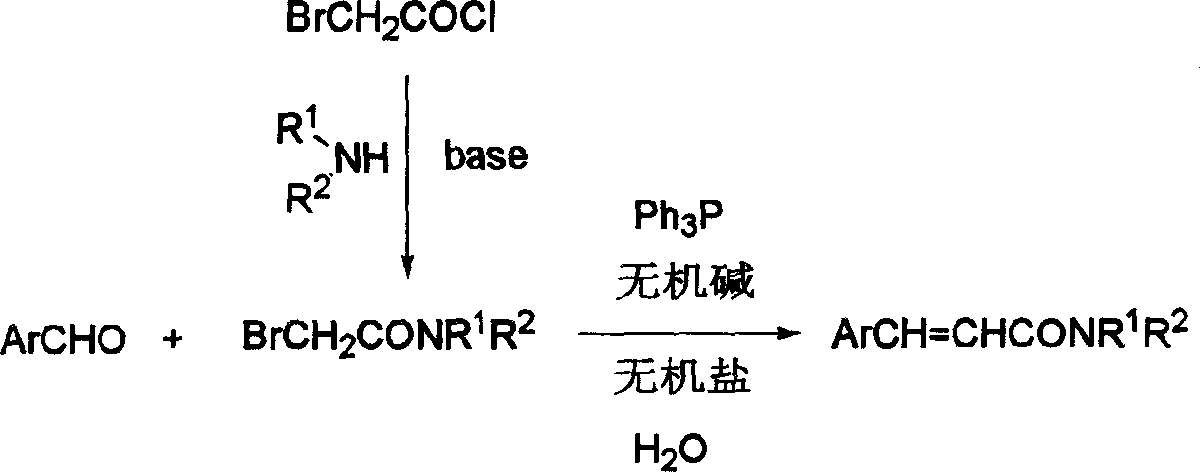
Cinnamide compound synthesizing process
InactiveCN1740145AEmission reductionReduce reaction processOrganic compound preparationCarboxylic acid amides preparationOrganic synthesisReaction step
The present invention discloses cinnamide compound synthesizing process. Inside water phase and in the presence of triphenyl phosphine, inorganic alkali and inorganic salt, bromoacetylamide and aromatic aldehyde react to synthesize cinnamide compound. During the convergent synthesis process, bromoacetylamide and triphenyl phosphine first react to produce quarternary ammonium salt as intermediate, the quarternary ammonium salt then reacts with alkali to produce corresponding ylide, and the ylide finally reacts with aromatic aldehyde to produce the cinnamide compound. The intermediate needs no separation, and this results in less reaction steps, short reaction period, low power consumption, high yield and reduced effluent. In addition, the present invention performs reaction in water phase to avoid use harmful expensive organic solvent.
Owner:ZHEJIANG UNIV
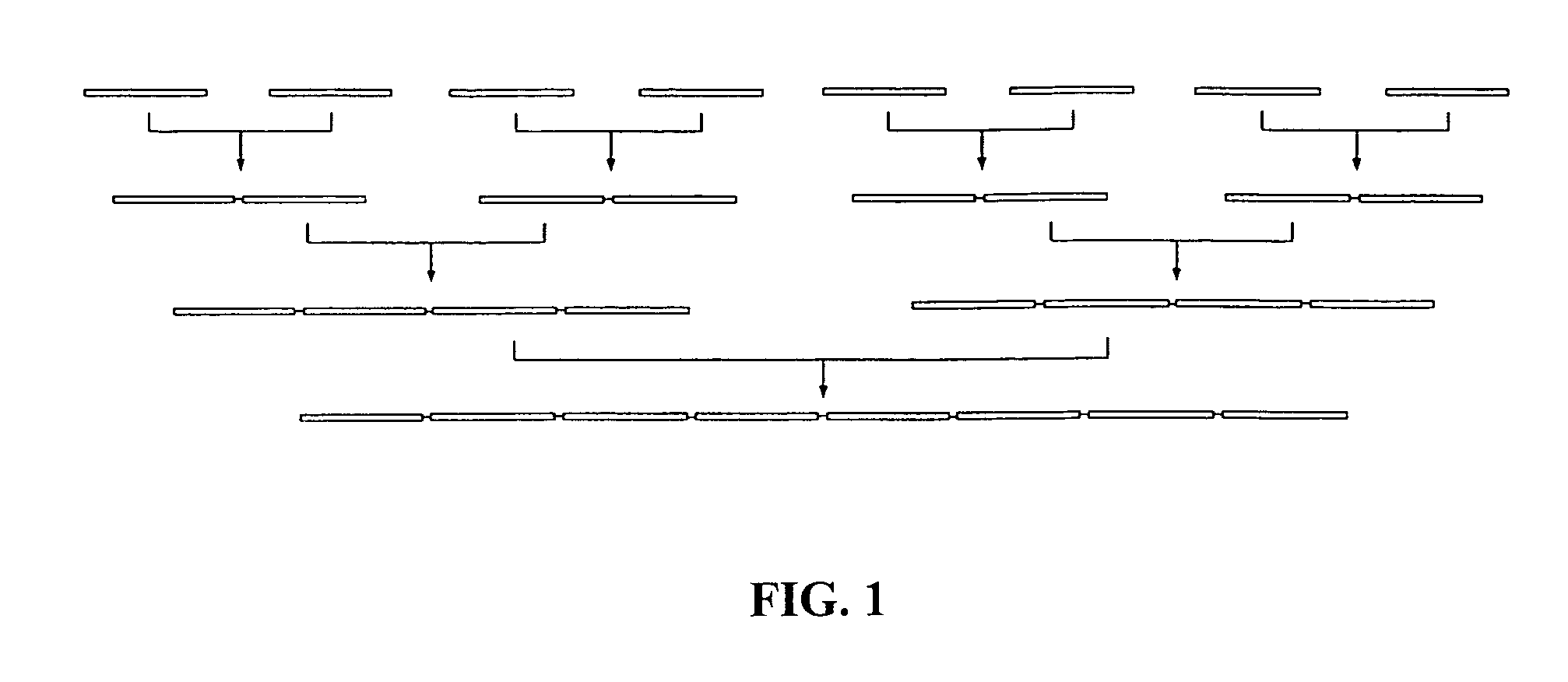
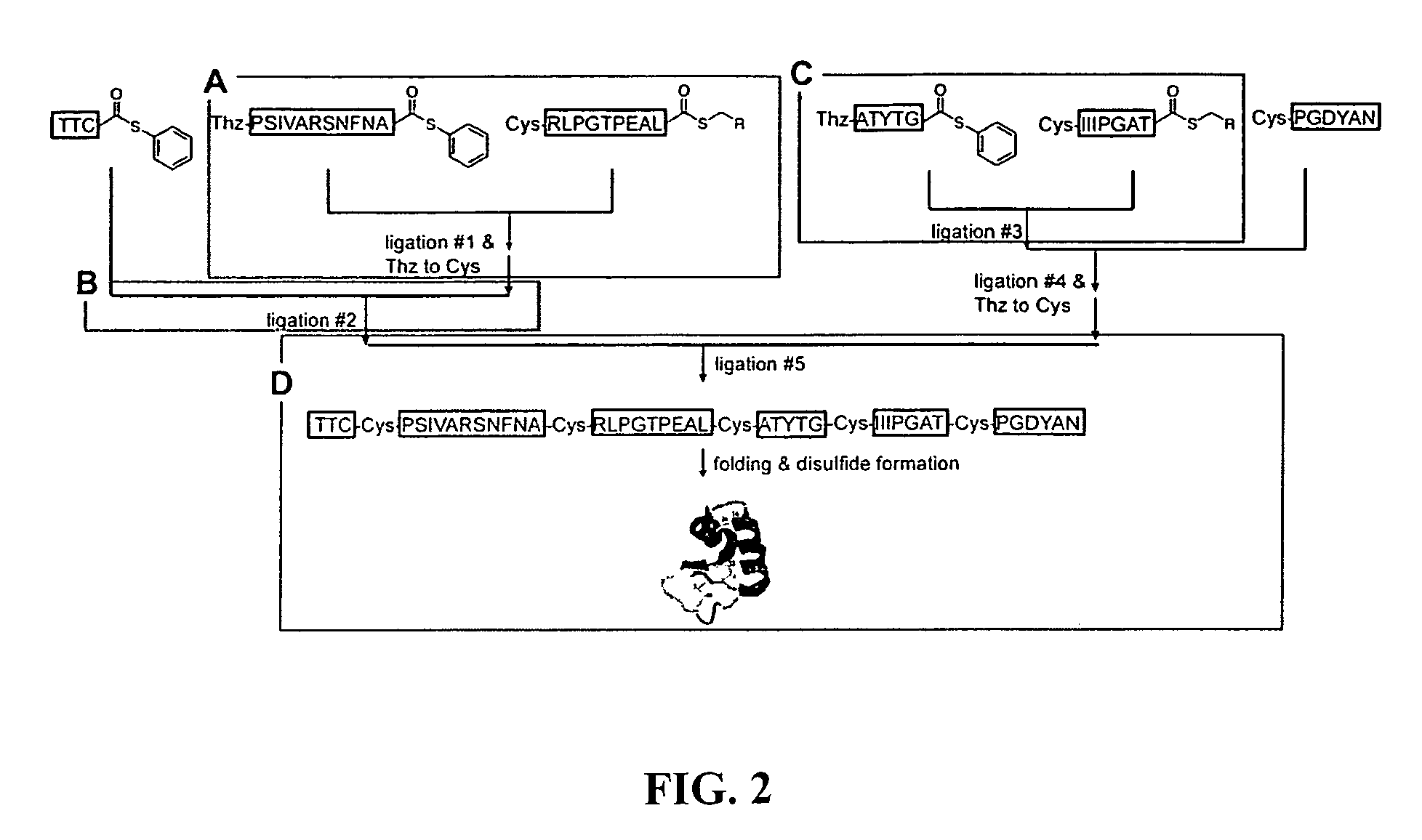
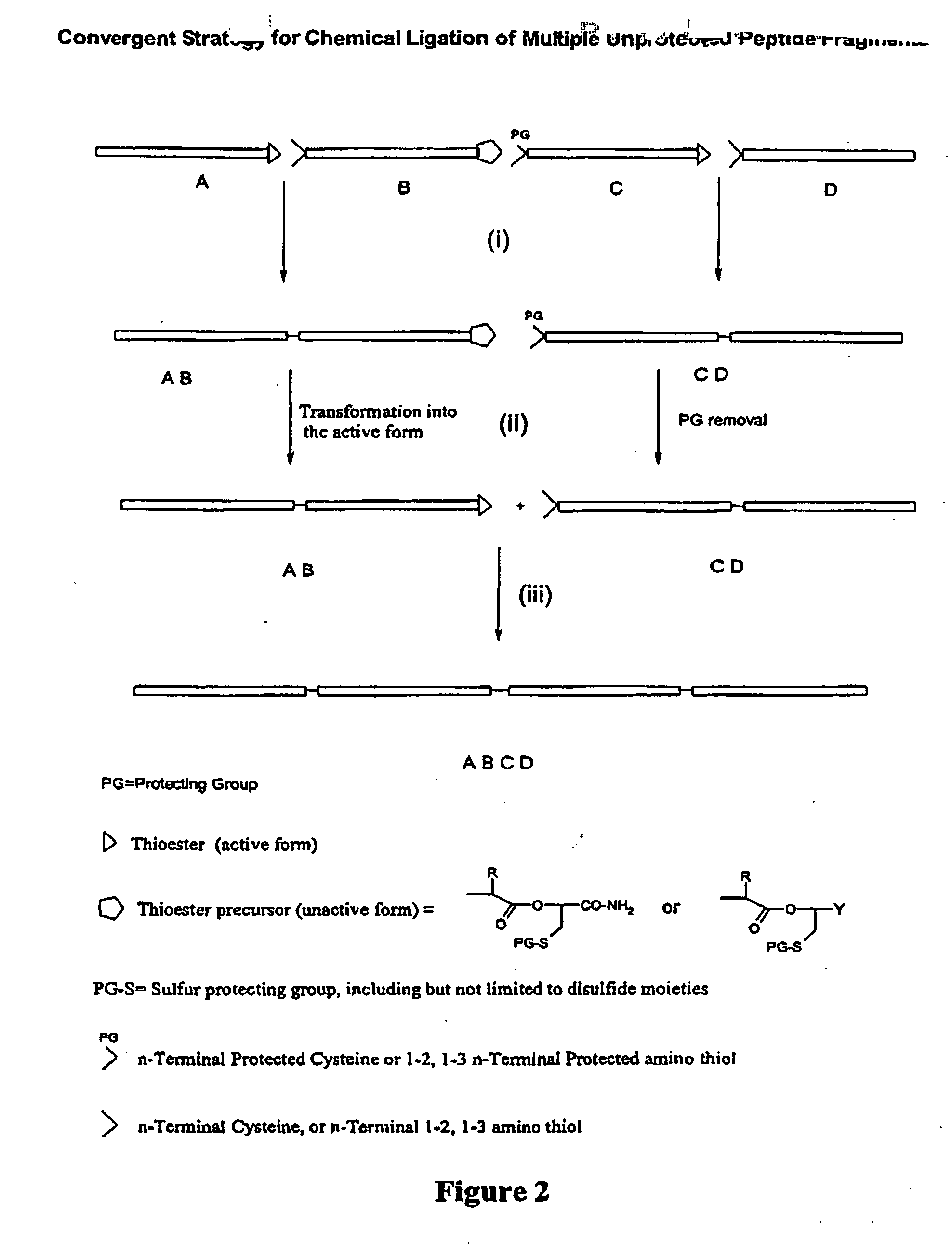
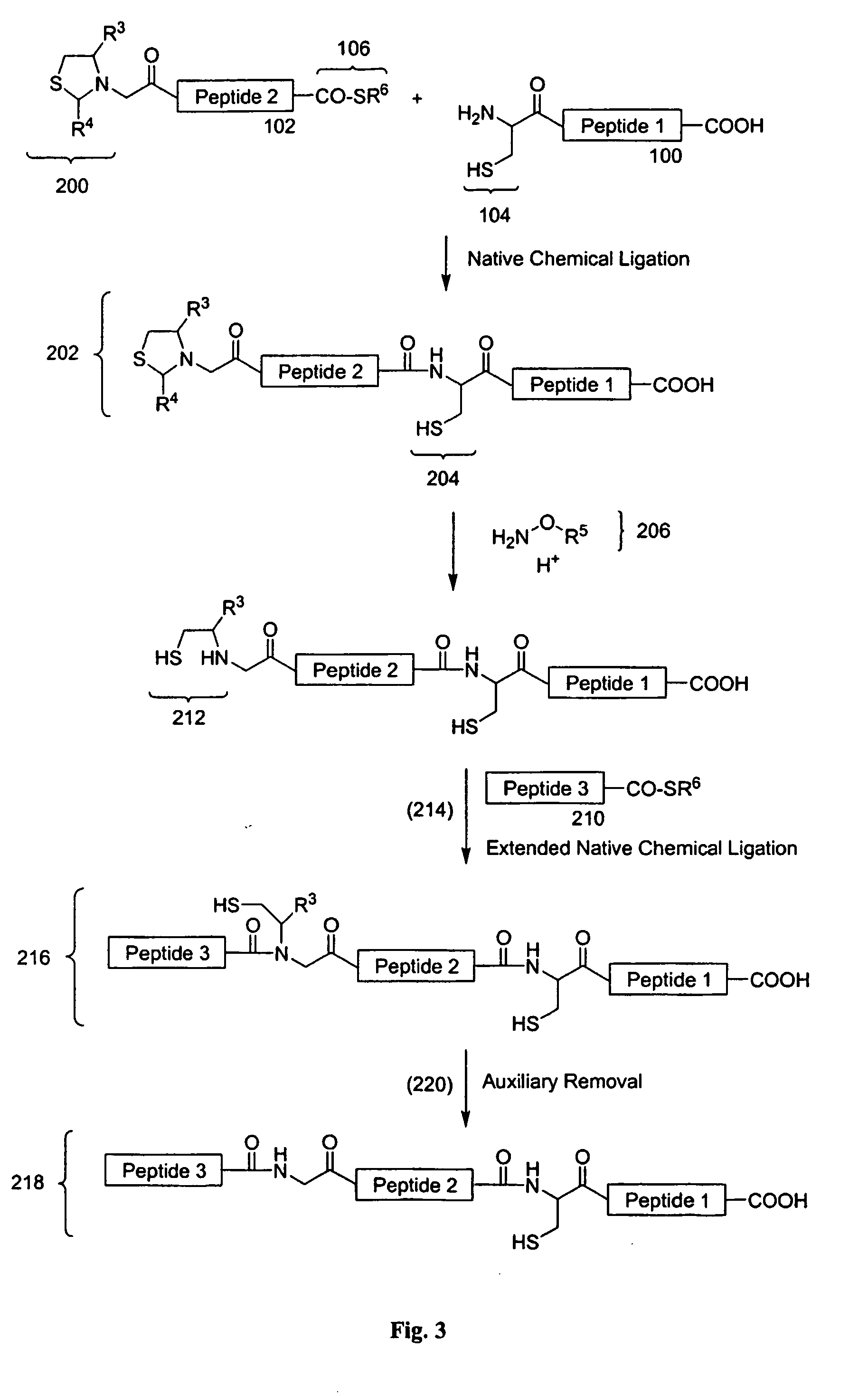
Chemical peptide ligation with three or more components
InactiveUS20050113563A1Improve efficiencyMethod can be usedPeptide/protein ingredientsPeptide preparation methodsCompound (substance)Protecting group
The invention provides a method of assembling oligopeptide intermediates in a native chemical ligation reaction that eliminates self-ligation of bi-functional intermediates. An important aspect of the invention is a bi-functional intermediate with an N-terminal heterocyclic protecting group which effectively prevents self-ligation in the chemical assembly process. The present invention is useful in methods for convergent synthesis of polypeptides and proteins and improve,s; the efficiency of native chemical ligation reactions, particularly where three or more peptide fragments are used to assemble a polypeptide or protein product.
Owner:ASTRAZENECA PHARMA LP
The synthetic method of febuxostat
The invention provides a method for synthesizing febuxostat. The method comprises the following steps of: (a) performing aromatic ring substitution reaction on a compound of a formula I to obtain a compound of a formula II; (b) performing halogen-metal exchange reaction on the compound of the formula II to obtain a compound of a formula III; (c) performing coupling reaction on the compound of the formula III and the compound of a formula VI under the action of a metal catalyst to obtain a compound of a formula IV; and (d) performing hydrolysis reaction on the compound of the formula IV to obtain the compound of a formula V, namely febuxostat, wherein X is I or Br; M is selected from boric acid ester or SnBu3; and R is H or an alkyl group. In the method, a convergent synthesis strategy is adopted, and a carbon-carbon bond is formed by applying metal catalyzed aromatic ring coupling reaction in a key step, so that a system in which a benzene ring is coupled with a thiazole ring is established. The method has the advantages of simple and short steps, high yield and low environmental pollution and can be suitable for industrial production.
Owner:ARROMAX PHARMATECH
Method for synthesizing glucagon-like peptide (GLP)-1 analogue in solid-phase mode
InactiveCN102977204AReduce generationReduce the difficulty of purificationHormone peptidesPeptide preparation methodsSolid-phase synthesisGLP-1 Analogue
The invention relates to the field of polypeptide solid-phase synthesis and provides a method for synthesizing glucagon-like peptide (GLP)-1 analogue in a solid-phase mode. The method for synthesizing the GLP-1 analogue in the solid-phase mode solves the problems that in the process of synthesizing the GLP-1 analogue in the solid-phase mode, peptide deficiency products and by-products are generated because of difficult or incomplete amino acid sequence connection, and the by-products are resulted to be difficulty to separate in subsequent purification. The peptide deficiency products and by-products are quite same in properties. According to the method for synthesizing the GLP-1 analogue in the solid-phase mode, a segment convergent synthesis method and a substituent-introduced method are adopted in difficult-connection points and polypeptide synthesis yield coefficient is improved.
Owner:JILIN AOTENG BIOTECH
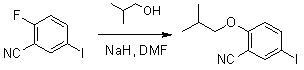
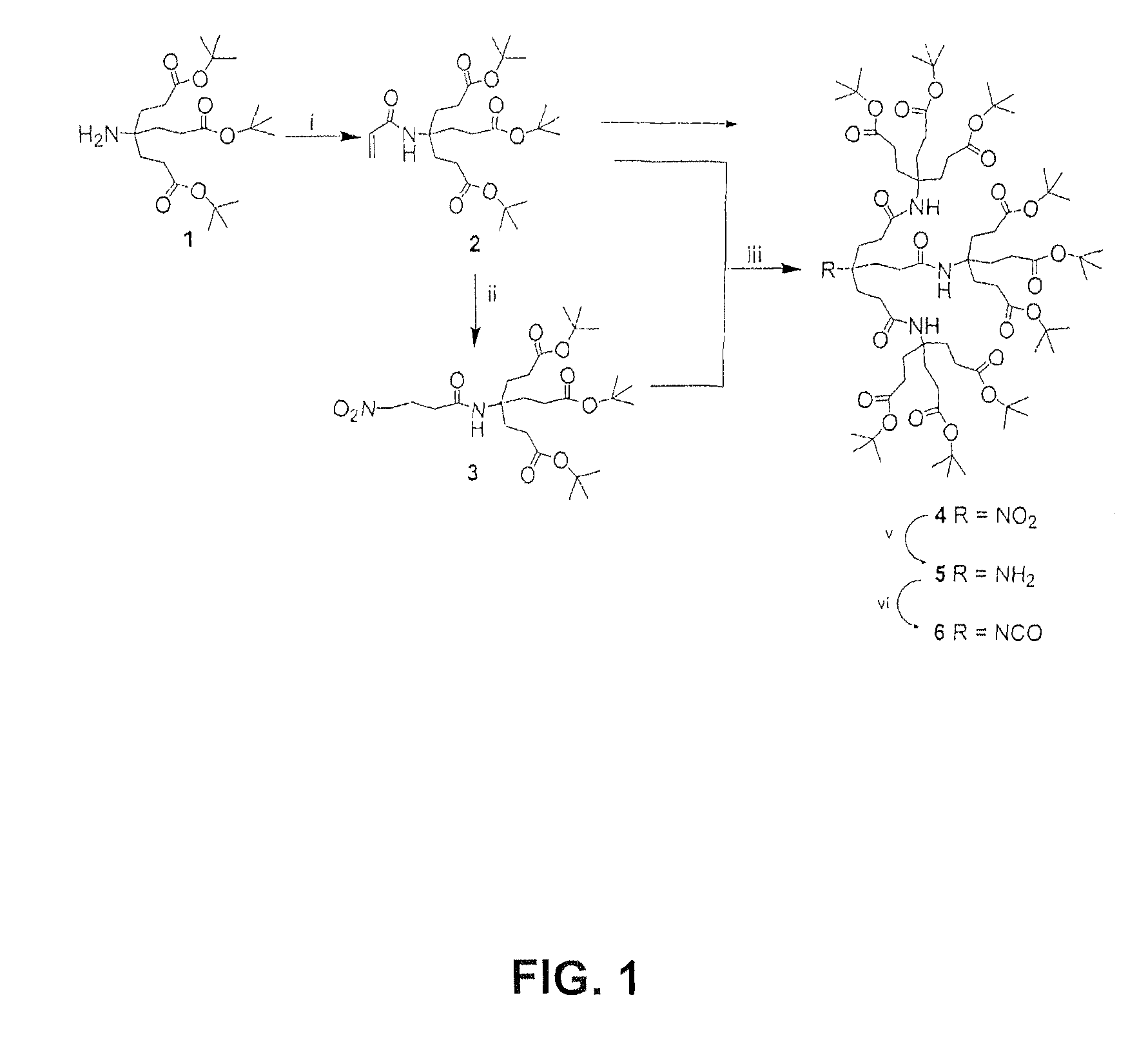
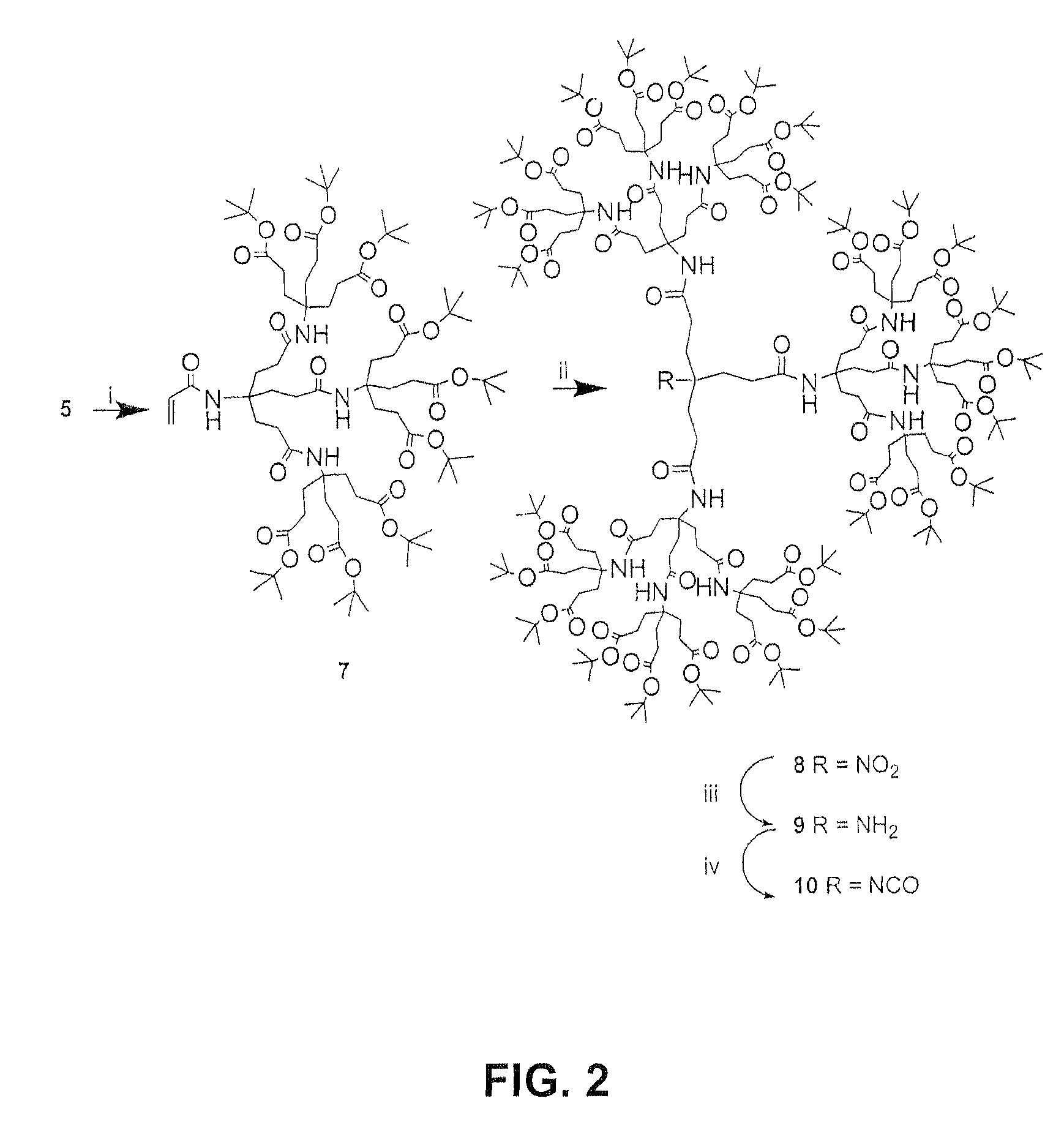
Convenient synthesis of 1→3 C-branched dendrons
In accordance with the present invention, there is provided a method of preparing higher generation 1→3 C-branched polyamide dendrons. The combination of commercially available acryloyl chloride with 1→3 C-branched amines, e.g., di-tert-butyl 4-[2-(tert-butoxycarbonyl)ethyl]-4-aminoheptanedioate, resulted in generally high yields of acryl amides, which upon treatment with other reagents, generated the desired higher generation dendrons. These second and third generation dendrons were fully characterized and compared to the samples prepared from a convergent synthesis.
Owner:THE UNIVERSITY OF AKRON
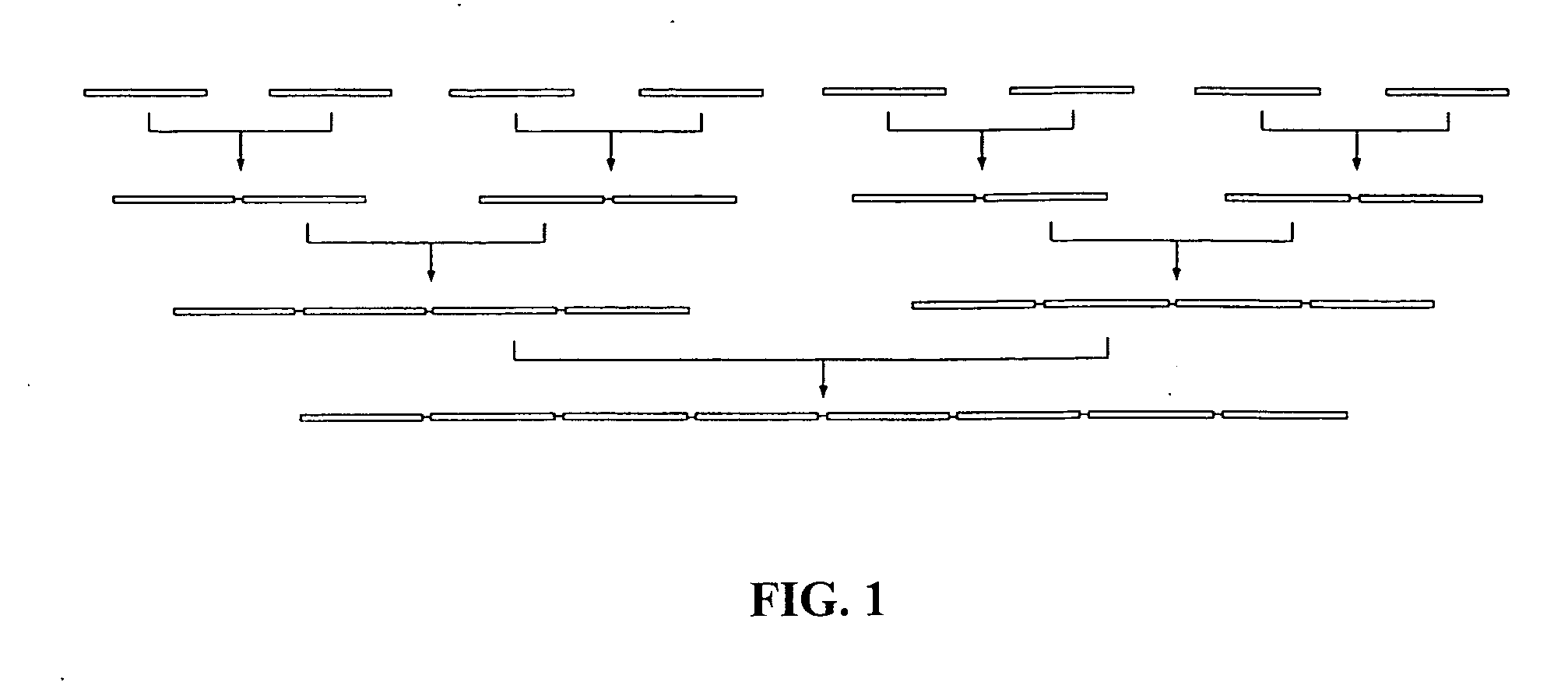
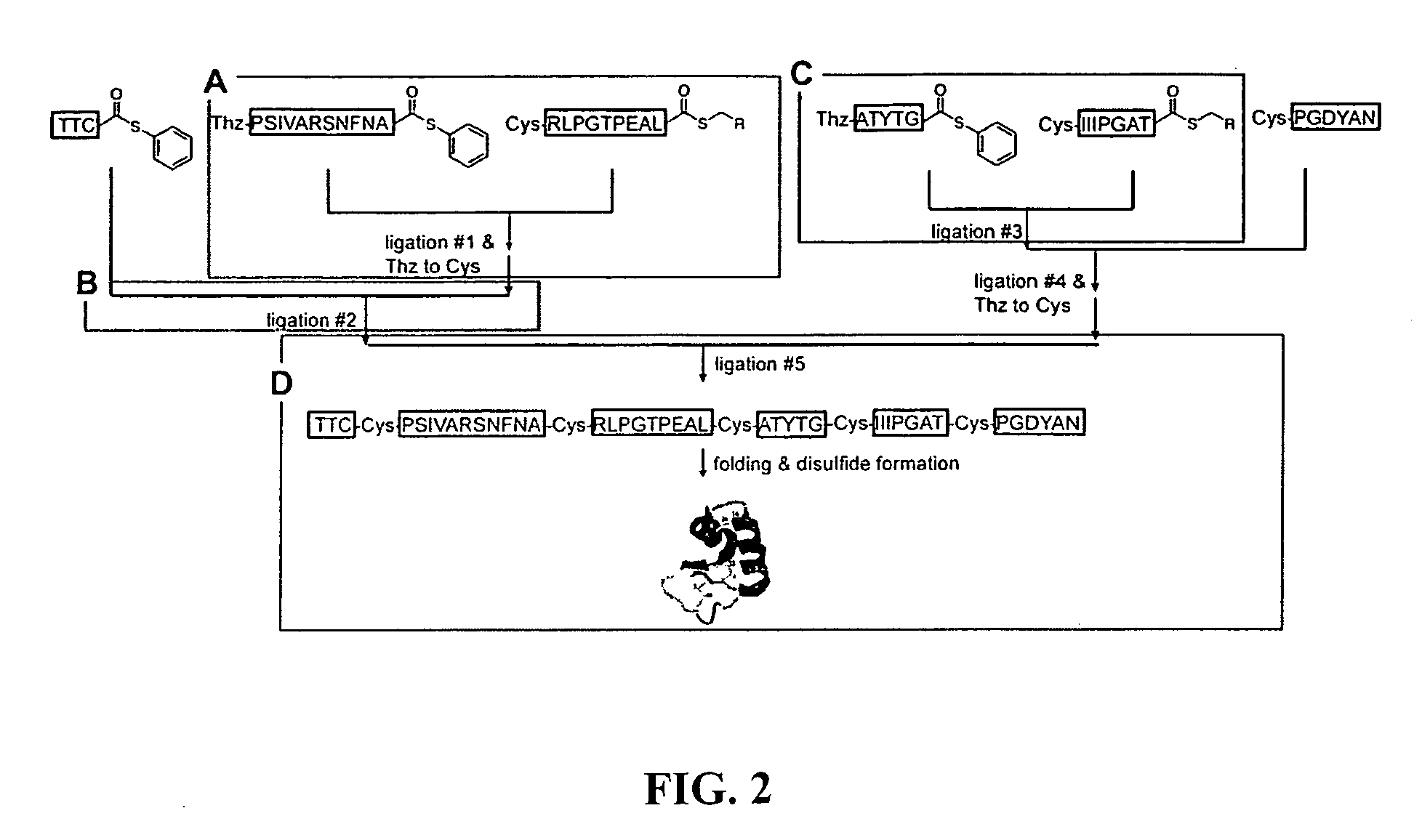
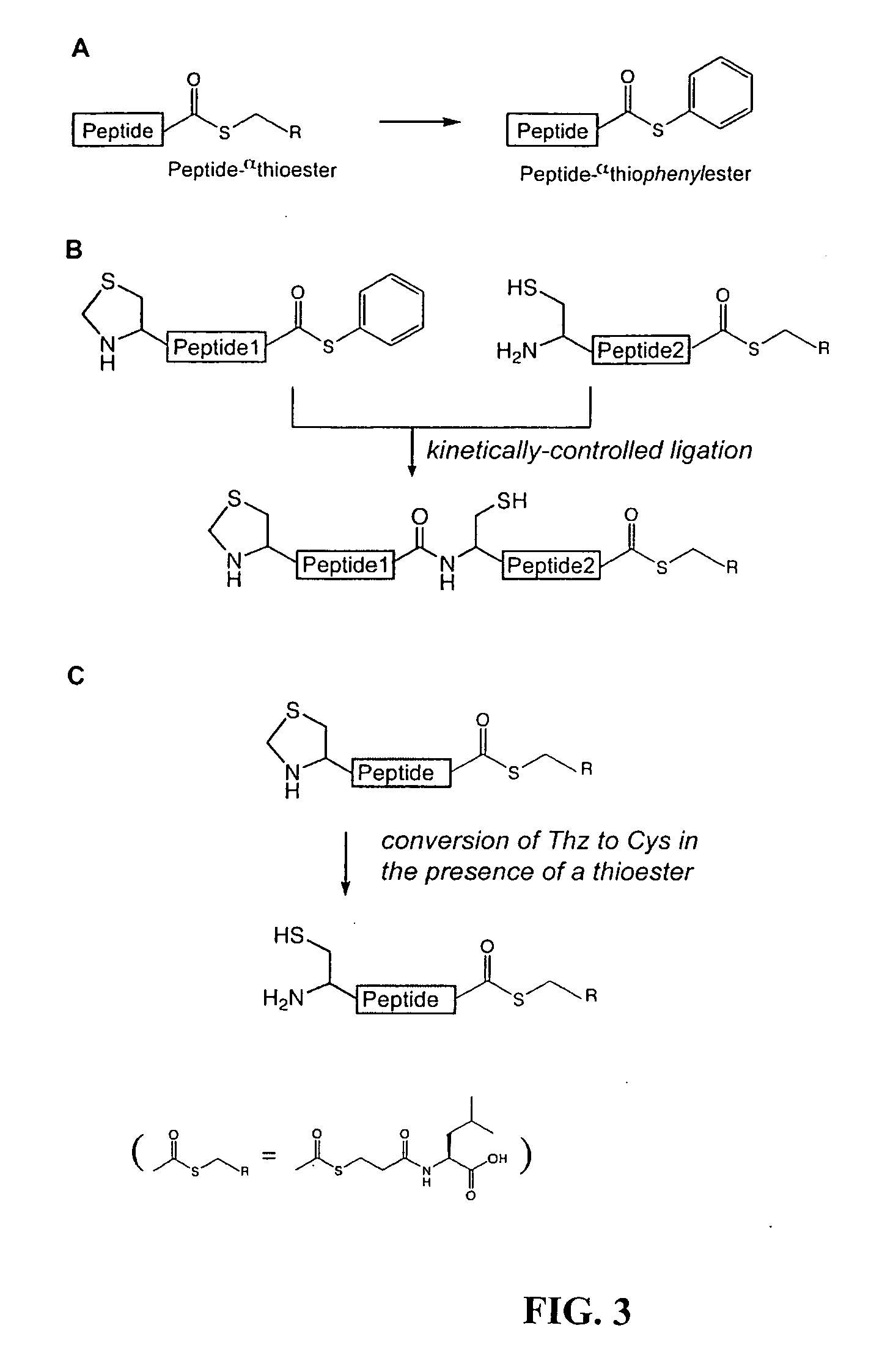
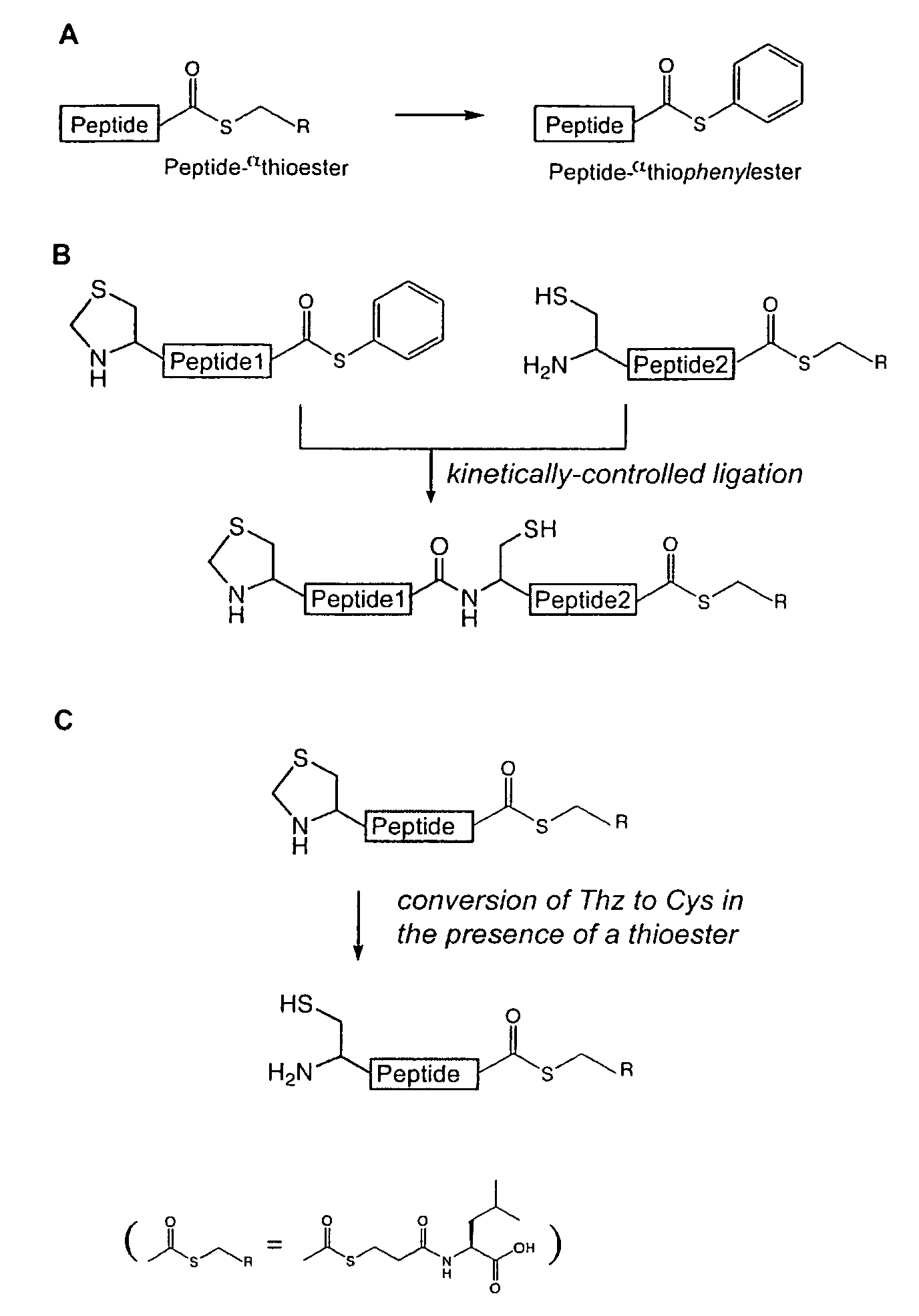
Convergent synthesis of proteins by kinetically controlled ligation
InactiveUS20070082378A1Easily substitutedPeptide/protein ingredientsPeptide preparation methodsThioester synthesisCysteine thiolate
The present invention concerns methods and compositions for synthesizing a polypeptide using kinetically controlled reactions involving fragments of the polypeptide for a fully convergent process. In more specific embodiments, a ligation involves reacting a first peptide having a protected cysteyl group at its N-terminal and a phenylthioester at its C-terminal with a second peptide having a cysteine residue at its N-termini and a thioester at its C-termini to form a ligation product. Subsequent reactions may involve deprotecting the cysteyl group of the resulting ligation product and / or converting the thioester into a thiophenylester.
Owner:UNIVERSITY OF CHICAGO
Convergent synthesis of proteins by kinetically controlled ligation
Owner:UNIVERSITY OF CHICAGO
Chemical peptide ligation with three or more components
InactiveUS7884182B2Improve efficiencyMethod can be usedPeptide/protein ingredientsDepsipeptidesChemical ligationPeptide fragment
The invention provides a method of assembling oligopeptide intermediates in a native chemical ligation reaction that eliminates self-ligation of bi-functional intermediates. An important aspect of the invention is a bi-functional intermediate with an N-terminal heterocyclic protecting group which effectively prevents self-ligation in the chemical assembly process. The present invention is useful in methods for convergent synthesis of polypeptides and proteins and improves the efficiency of native chemical ligation reactions, particularly where three or more peptide fragments are used to assemble a polypeptide or protein product.
Owner:ASTRAZENECA PHARMA LP
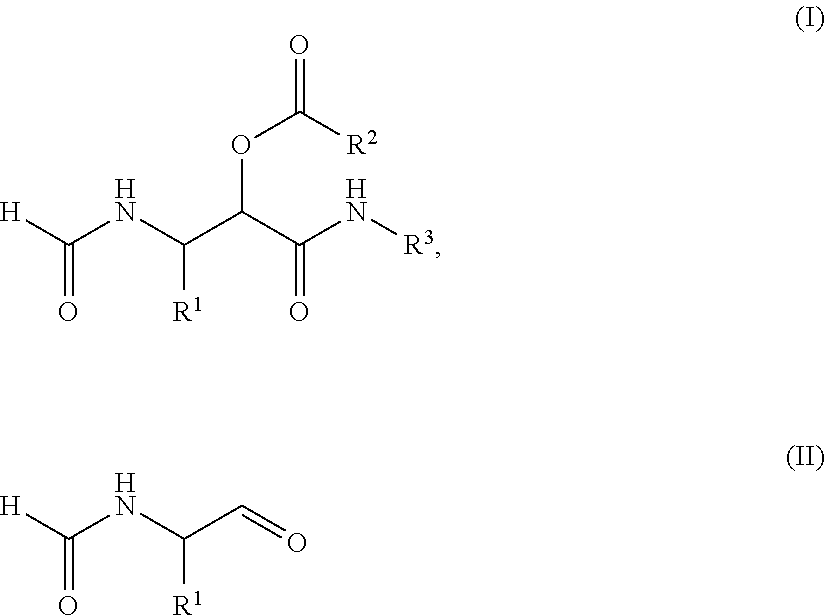
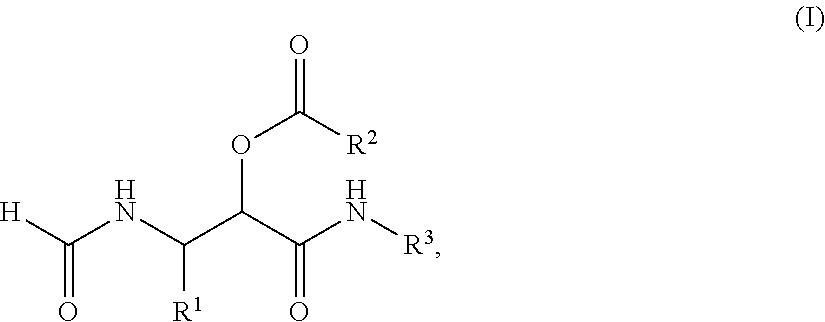
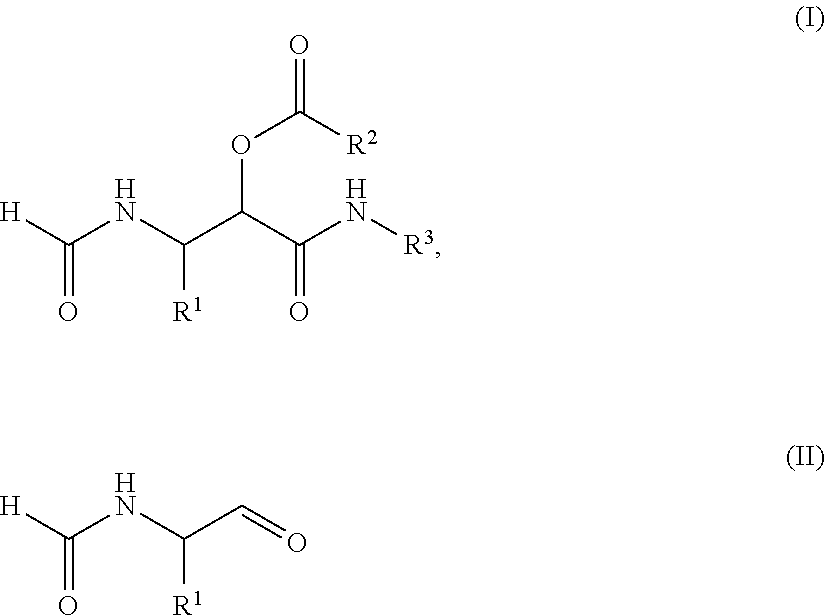
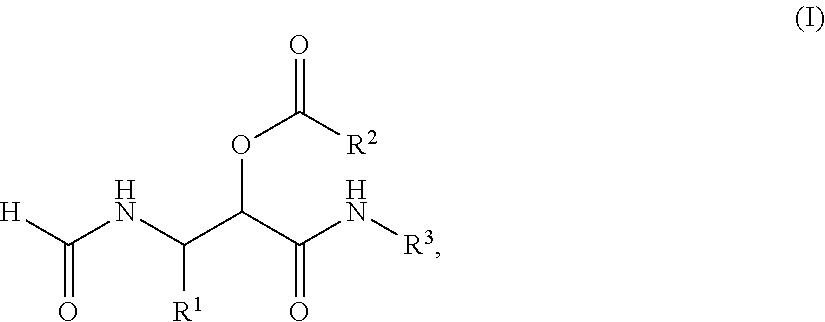
PROCESS FOR THE PREPARATION OF alpha-ACYLOXY beta-FORMAMIDO AMIDES
InactiveUS20120330015A1High yieldImprove efficiencyIsocyanic acid derivatives preparationOrganic compound preparationDipeptideTricyclic
The present invention relates to a process for the preparation of a compound of the general Formula (I), comprising: a) reacting a compound of the general Formula (II) with a compound of the Formula III R2COOH and a compound of the general Formula IV R3NC under such conditions that compound I is formed, wherein R1 represents a substituted or unsubstituted alkyl, alkenyl, alkynyl, aromatic or non-aromatic, mono-, di- or tricyclic, or heterocyclic structure, and R2 represents a substituted or unsubstituted alkyl, alkenyl, alkynyl, aromatic or non-aromatic, mono-, di- or tricyclic, or heterocyclic structure, and R3 represents a substituted or unsubstituted alkyl, alkenyl, or alkynyl structure. In further aspect the subject invention relates to the use of the obtained products as intermediates for various peptidomimetics, and preferably as a building block in a convergent synthesis of prolyl dipeptide structures.
Owner:VER VOOR CHRISTELIJK HOGER ONDERWIJS WETENSCHAPPELIJK ONDERZOEK & PATIENTENZORG TECH TRANSFER OFFICE VU & VUMC
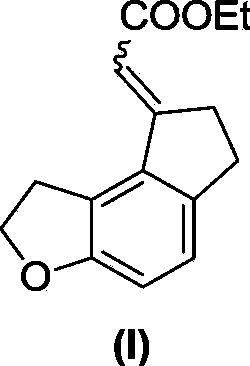
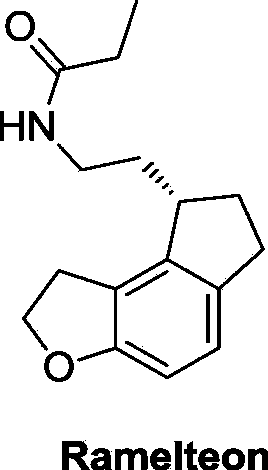
Preparation method for key intermediate of ramelteon
The invention discloses a preparation method for a key intermediate of ramelteon (a compound represented by formula (I)). The preparation method includes the steps as follows: dissolving a compound represented by formula 1, a compound represented by formula 2, a metal catalyst, an alkali, norbornene and a ligand in an organic solvent for carrying out a reaction to obtain the compound represented by formula (I). The synthetic route of the invention is a convergent synthesis which is different from a linear synthesis in a conventional synthetic route. The synthetic route of the invention is short in linearity and is high in yield. The reaction equation of the invention is represented by a formula as follows.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
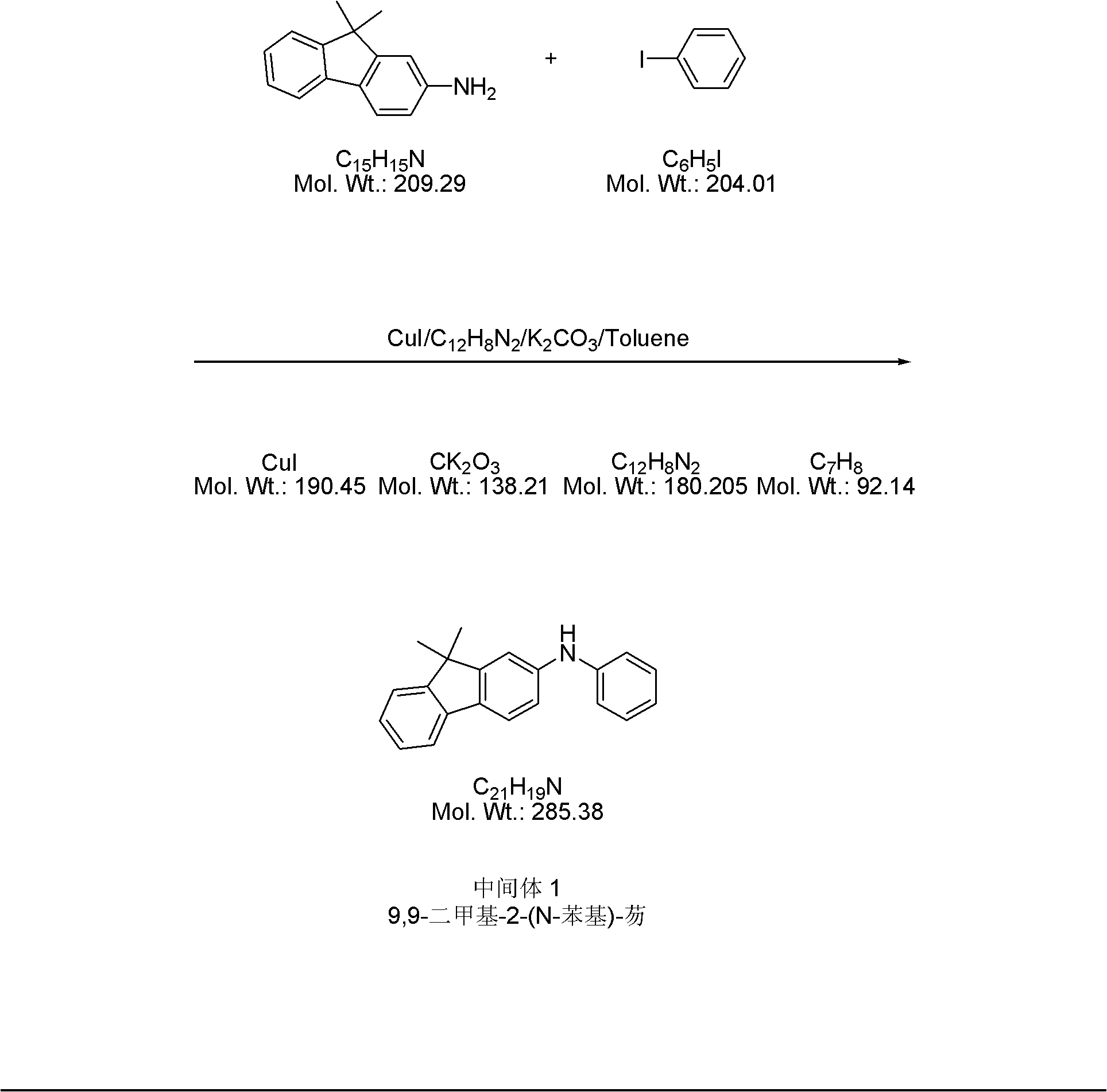
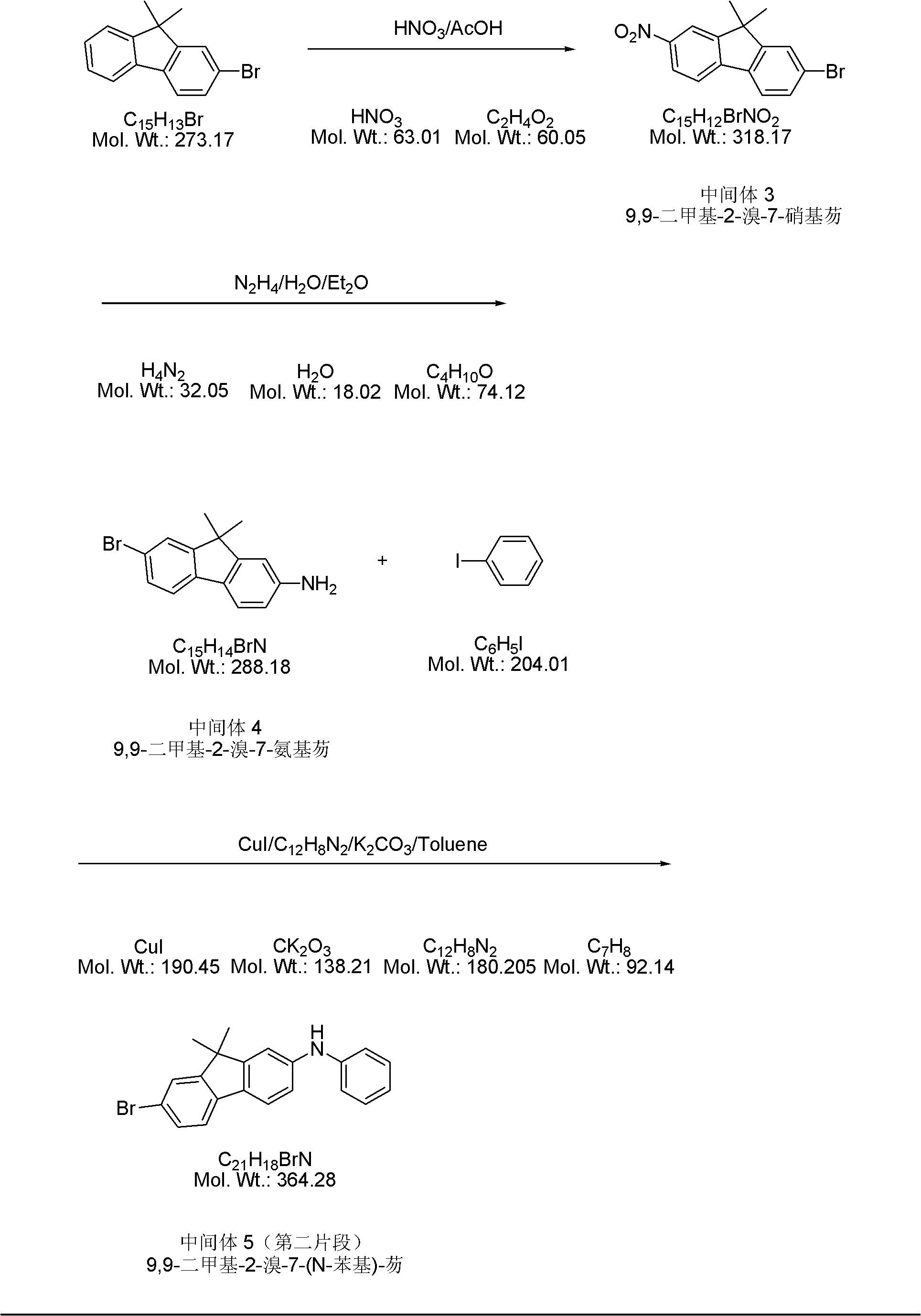
Method for synthesizing N,N'-diphenyl-N-(9,9-dimethyl-2-fluorenyl)-N'-(9',9'-dimethyl-7'-bromo-2'-fluorenyl)-benzidine
InactiveCN101973895ALow costImprove production safetyOrganic compound preparationAmino compound preparationSynthesis methodsBromine
The invention relates to a method for synthesizing N,N'-diphenyl-N-(9,9-dimethyl-2-fluorenyl)-N'-(9',9'-dimethyl-7'-bromine-2'-fluorenyl)-benzidine. In the method, a novel convergent synthesis route is adopted, and 9,9-dimethyl-2-aminofluorene and 9,9-dimethyl-2-bromofluorene are respectively used as starting raw materials for respectively preparing two fragments for synthesizing a final product;and finally, the two fragments are condensed and purified to obtain the final product N,N'-di-phenyl-N-(9,9-dimethyl-2-fluorenyl)-N'-(9',9'-dimethyl-7'-bromine-2'-fluorenyl)-benzidine. Because the price of the raw materials used by the synthesis method is low, the consumption of a solvent is small and the solvent is easy to recover, the production cost can be greatly reduced on the premise of guaranteeing the product purity and the productivity, thus the method is suitable for industrial production. In addition, the synthesis method has the advantages of simple operation, convenience, safety,and the like.
Owner:天津市佰斯康科技有限公司
Process for the preparation of α-acyloxy β-formamido amides
InactiveUS8686145B2Improve efficiencyHigh yieldIsocyanic acid derivatives preparationOrganic compound preparationDipeptideMedicinal chemistry
The present invention relates to a process for the preparation of a compound of the general Formula (I), comprising: a) reacting a compound of the general Formula (II) with a compound of the Formula III R2COOH and a compound of the general Formula IV R3NC under such conditions that compound I is formed, wherein R1 represents a substituted or unsubstituted alkyl, alkenyl, alkynyl, aromatic or non-aromatic, mono-, di- or tricyclic, or heterocyclic structure, and R2 represents a substituted or unsubstituted alkyl, alkenyl, alkynyl, aromatic or non-aromatic, mono-, di- or tricyclic, or heterocyclic structure, and R3 represents a substituted or unsubstituted alkyl, alkenyl, or alkynyl structure. In further aspect the subject invention relates to the use of the obtained products as intermediates for various peptidomimetics, and preferably as a building block in a convergent synthesis of prolyl dipeptide structures.
Owner:VER VOOR CHRISTELIJK HOGER ONDERWIJS WETENSCHAPPELIJK ONDERZOEK & PATIENTENZORG TECH TRANSFER OFFICE VU & VUMC
Processes for the convergent synthesis of calicheamicin derivatives
This invention describes processes for the convergent synthesis of calicheamicin derivatives, and similar analogs using bifunctional and trifunctional linker intermediates.
Owner:WYETH LLC
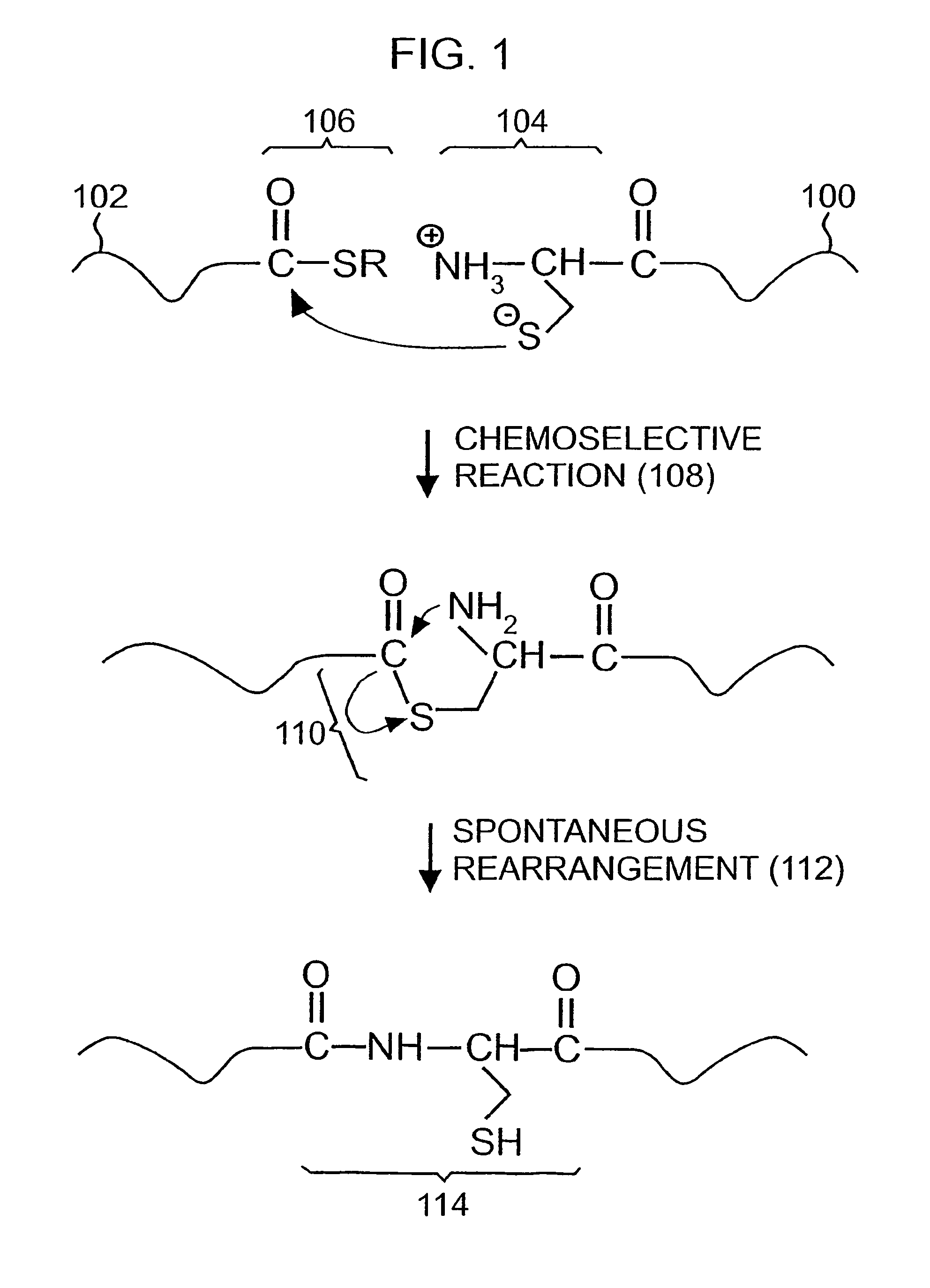
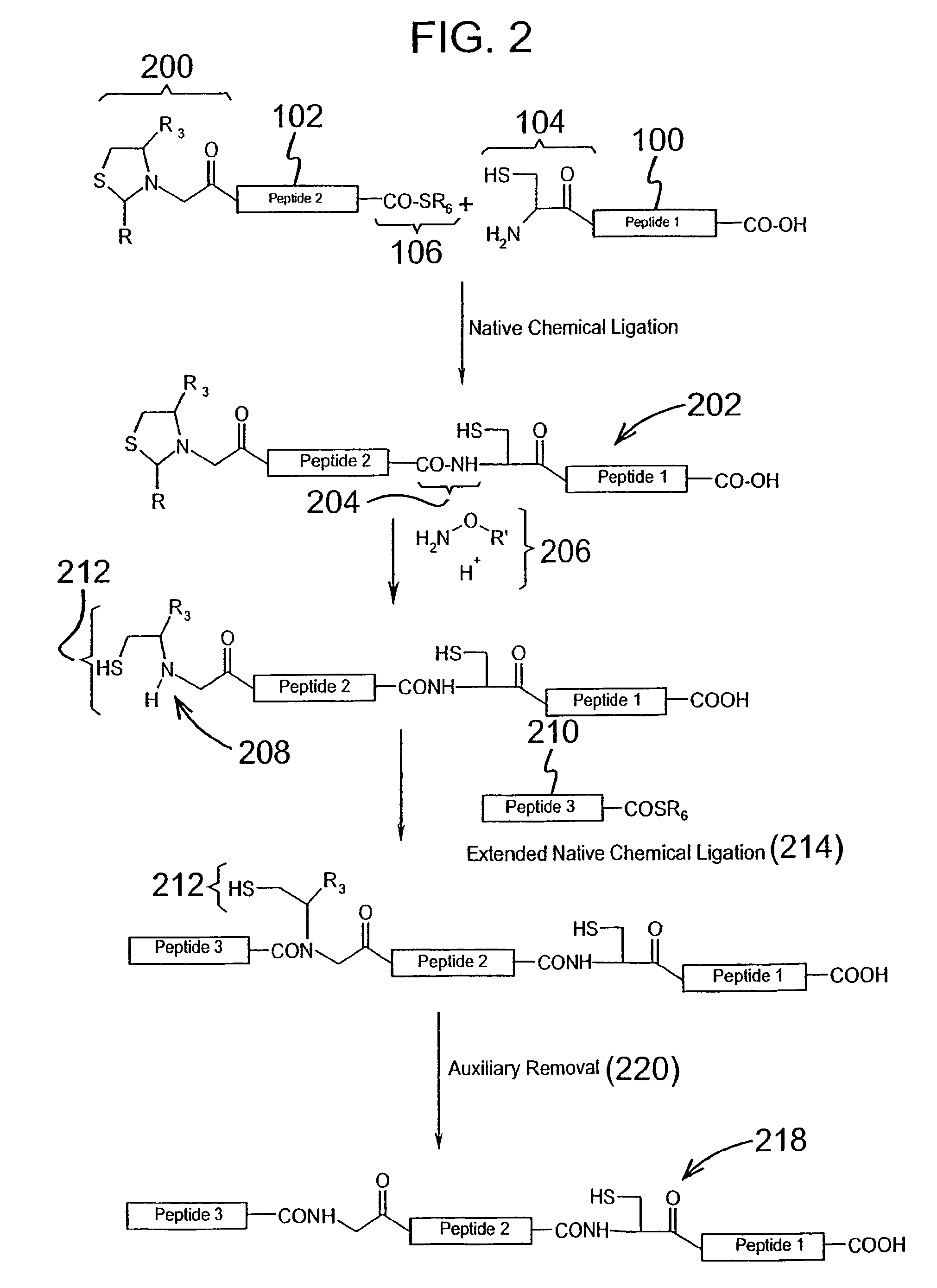
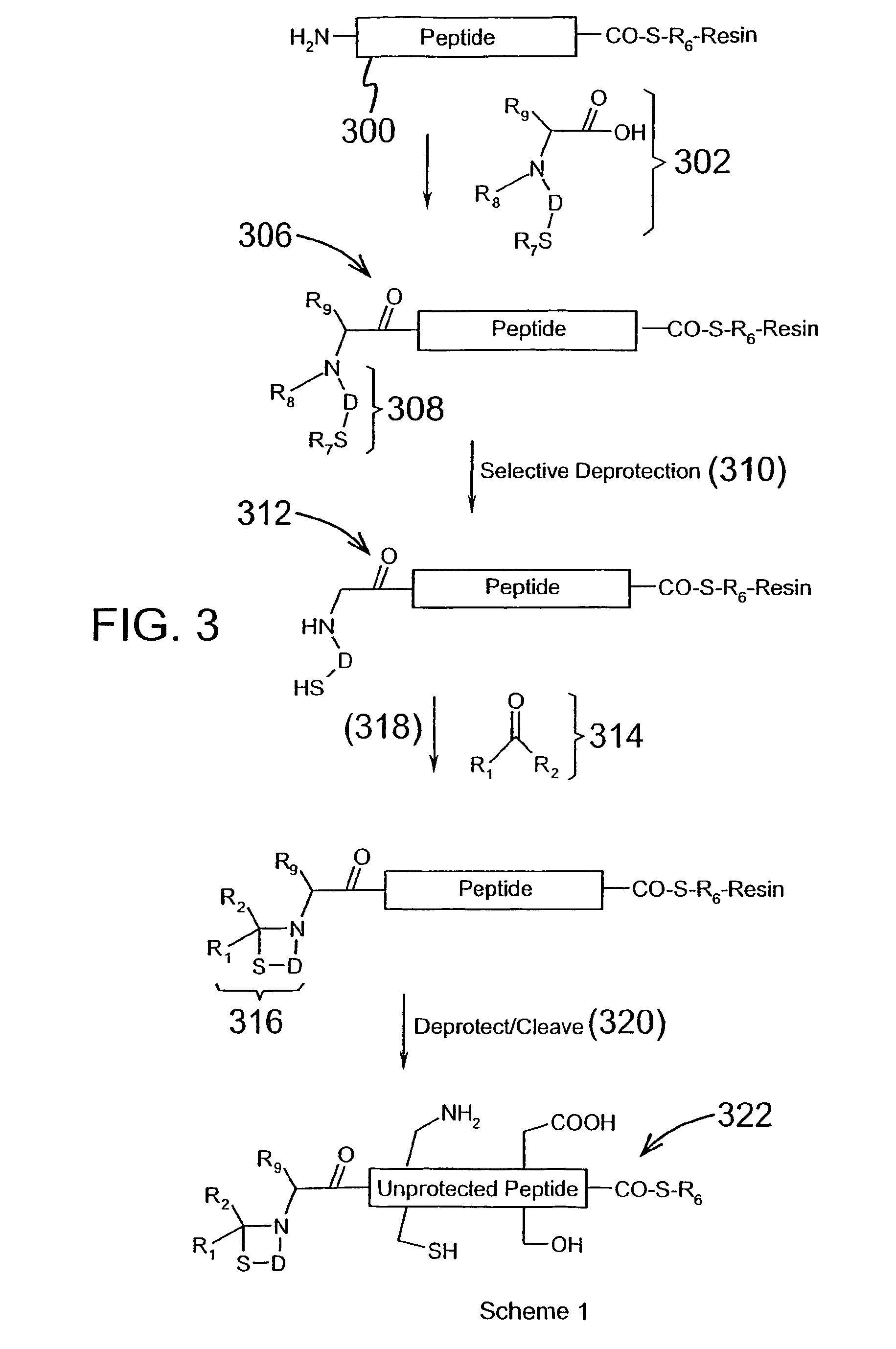
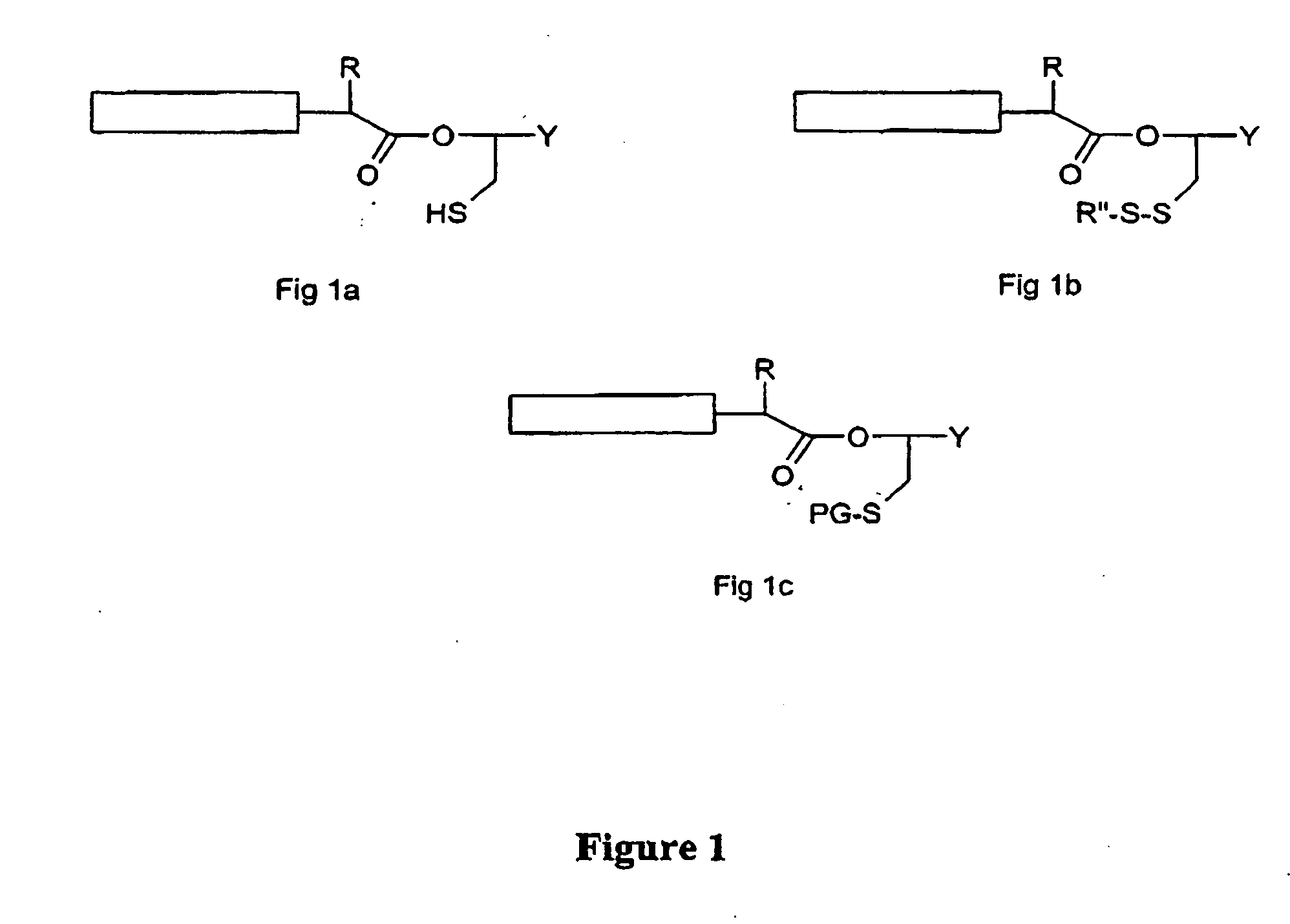
Post-cleavage sulfur deprotection for convergent protein synthesis by chemical ligation
The present invention provides a method and compositions for synthesizing an oligopeptide or polypeptide by convergent assembly of a plurality of pairs of oligopeptides in chemical ligation reactions. An important aspect of the present invention is an oligopeptide having a C-terminal disulfide-protected carboxythioester group that can be deprotected to spontaneously generate a free C-terminal thioester moiety. This allows a single precursor to participate in a succession of chemical ligation reactions, thereby making the convergent synthesis approach possible. The present invention is useful in methods for chemical synthesis of oligopeptides, polypeptides and proteins, and improves the efficiency of native chemical ligation reactions, particularly where four or more peptide fragments are used to assemble an oligopeptide, polypeptide or protein product.
Owner:ASTRAZENECA PHARMA LP
Process for the manufacture of glp-1 analogues
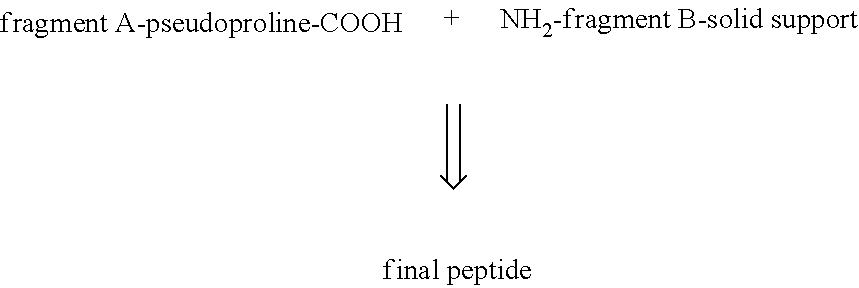
The present invention provides a process for the manufacture of GLP-1 analogues with high yield and purity by fragment condensation on the solid phase. In particular, it describes a convergent synthesis by condensation of a C-terminal pseudoproline fragment A with a fragment B bound to a solid support, followed by deprotection and cleavage from the support and final purification to yield the desired peptide. The invention further provides intermediates useful in the manufacturing process.
Owner:BEIJING FRESENIUS KABI PHARM CO LTD
Processes for the convergent synthesis of calicheamicin derivatives
This invention describes processes for the convergent synthesis of calicheamicin derivatives, and similar analogs using bifunctional and trifunctional linker intermediates.
Owner:WYETH LLC
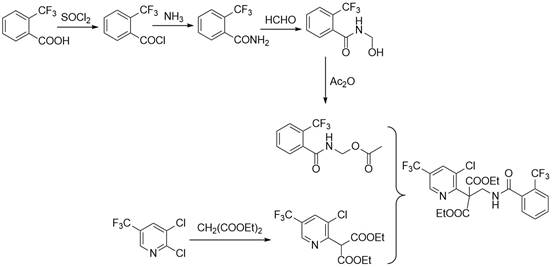
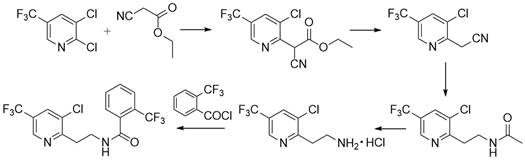
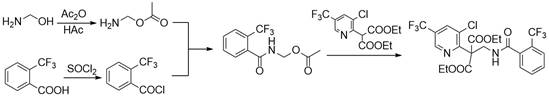
Synthesis method of fluopyram intermediate
The invention discloses a synthesis method of a fluopyram intermediate, and belongs to the field of organic synthesis of pesticides. The process comprises the following steps: by taking methanol amine as a starting raw material, carrying out esterification reaction on methanol amine and acetic anhydride in the presence of acetic acid to obtain methyl aminoacetate, and carrying out amidation reaction on methyl aminoacetate and o-trifluoromethyl benzoyl chloride to obtain N-acetoxymethyl-2-trifluoromethyl benzamide; and finally, carrying out a splicing reaction with diethyl 3-chloro-5-trifluoromethyl-2-pyridylmalonate, so as to obtain the 3-chloro-5-trifluoromethyl-2-pyridylethyl (diethyl ester)-2-trifluoromethyl benzamide. The method adopts a convergent synthesis route, has the characteristics of low raw material cost, high atom economy, high yield, simple process operation and the like, and is suitable for large-scale production.
Owner:湖南速博生物技术有限公司
Total synthesis method of optically pure tetrandrine
ActiveCN111518108AFew reaction stepsLow costOrganic chemistry methodsChemical recyclingPtru catalystTetrandrine
The invention discloses a total synthesis method of optically pure tetrandrine, and belongs to the technical field of drug synthesis. The method comprises the following steps: (1) under the action ofa catalyst (1), carrying out intermolecular Ullmann reaction on a compound (1) and a compound (2) under alkaline and high-temperature conditions to synthesize a compound 3; (2) removing a hydroxyl protecting group from the compound (3) under an acidic condition to synthesize a compound (4); (3) carrying out intramolecular Ullmann reaction on the compound (4) under the action of a catalyst (2) under alkaline and high-temperature conditions to synthesize a compound (5), namely the optically pure tetrandrine. A convergent synthesis strategy is adopted, and only three steps are needed from the compound (1) to the synthesis of optically pure tetrandrine so that the reaction steps are greatly reduced, and the time and the material cost are saved; the yield can be as high as 28.7%-38.9%, and theyield is increased by dozens of times; the target product can be obtained on the gram scale, 1.3 g-1. 5 g of final optically pure product is synthesized, and the method has better industrialization potential.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD +1
Preparation method of fluorocalciferol
PendingCN114276284AReduce usageUse silyl-containing hydroxyl protection to avoidOrganic chemistryOrganic solventChemical compound
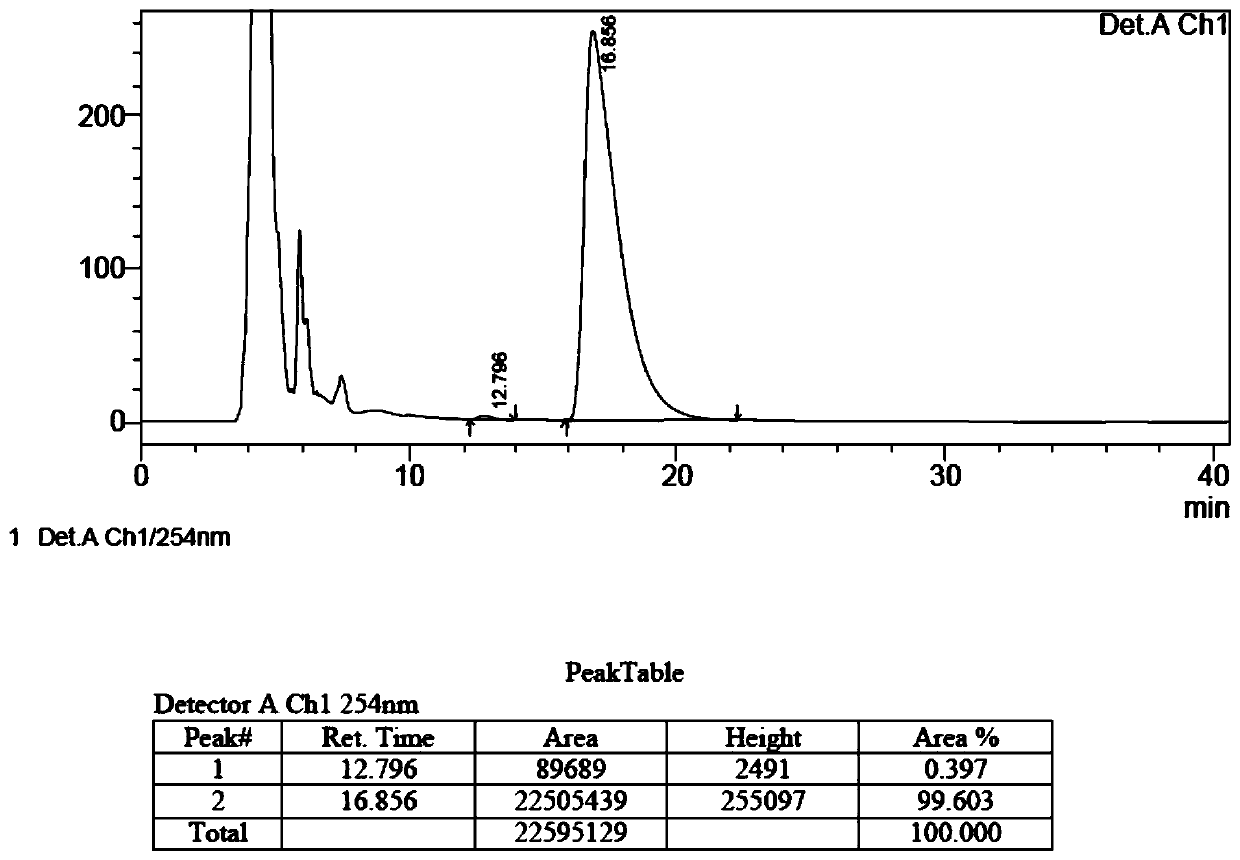
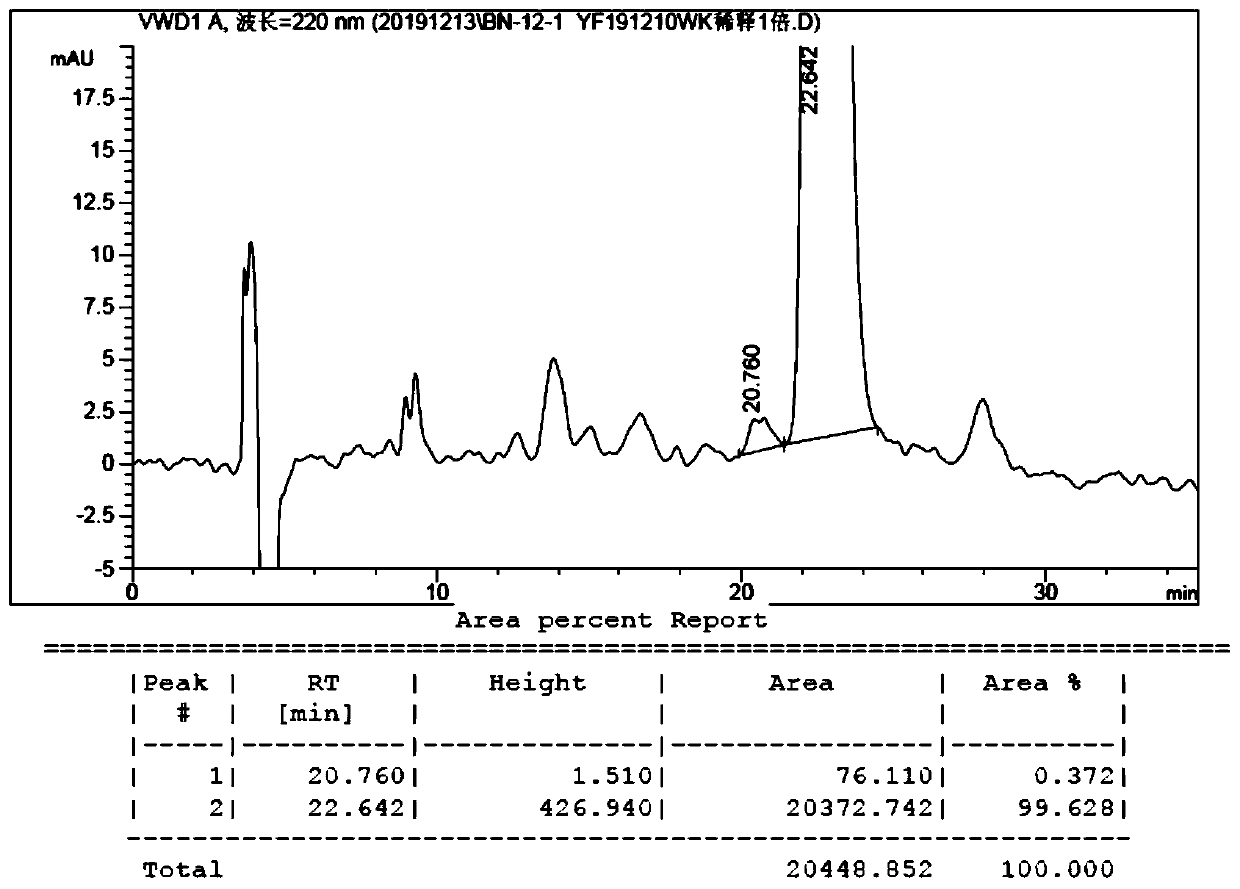
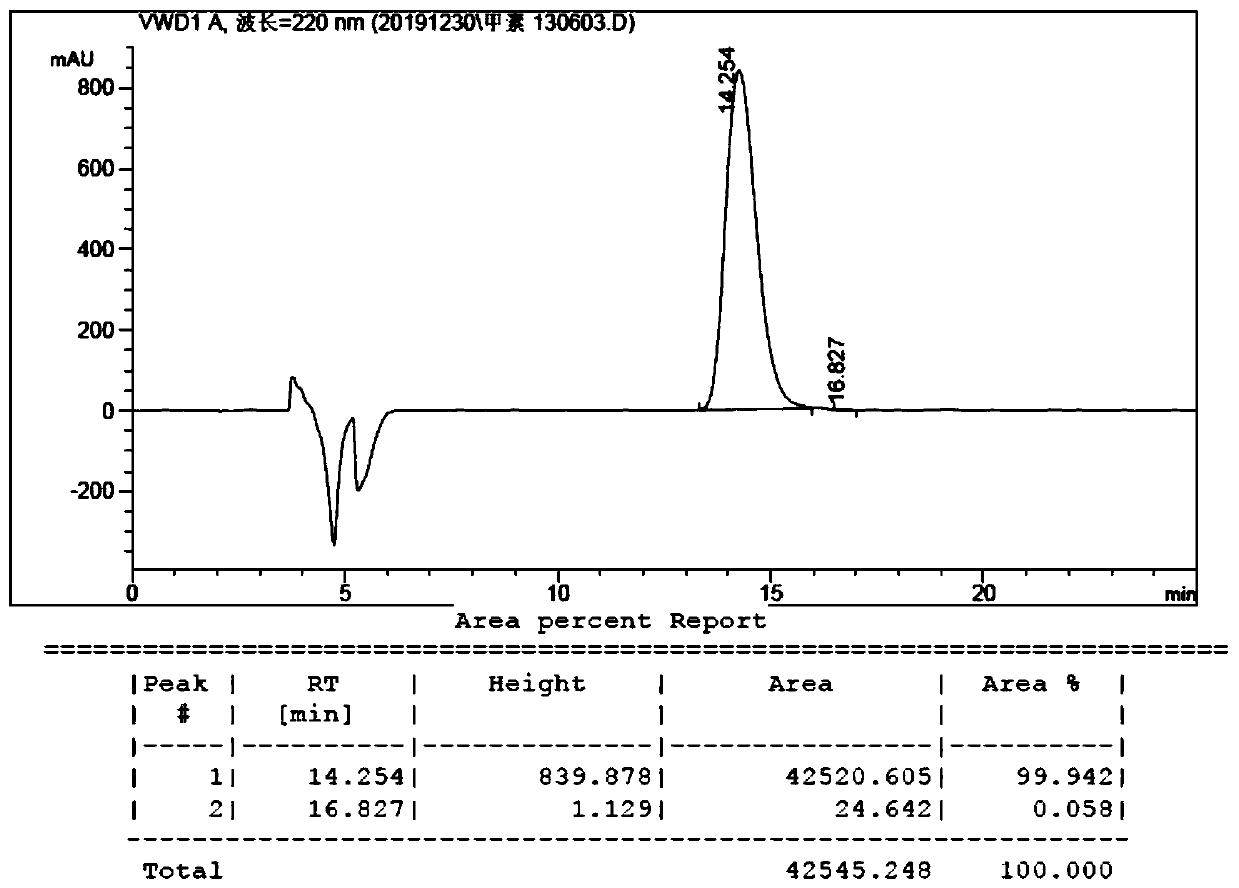
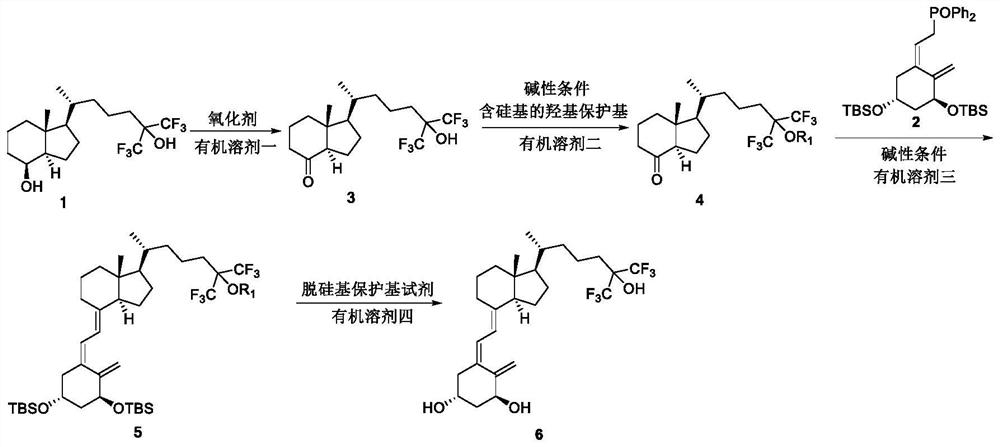
The invention belongs to the field of organic chemistry, and discloses a method for preparing fluorocalciferol by adopting a convergent synthesis route, which comprises the following steps: reacting a compound 1 with an oxidant in an organic solvent 1 to obtain a compound 3; reacting the compound 3 with a hydroxyl protecting group reagent containing a silicon group in an organic solvent 2 under an alkaline condition to obtain a compound 4; carrying out coupling reaction on the compound 4 and a compound 2 in an organic solvent 3 under an alkaline condition to obtain a compound 5; and reacting the compound 5 with a desilicication protecting group reagent in an organic solvent IV to obtain the fluorocalciferol. Compared with the traditional synthesis route, the method has the advantages of short synthesis route, few byproducts, simplicity in purification, high yield and suitability for industrial production, avoids the use of highly toxic reagents MOMCl and methanesulfonic acid, is environment-friendly, improves the yield, and improves the total yield to 70% or above.
Owner:CP PHARMA QINGDAO CO LTD
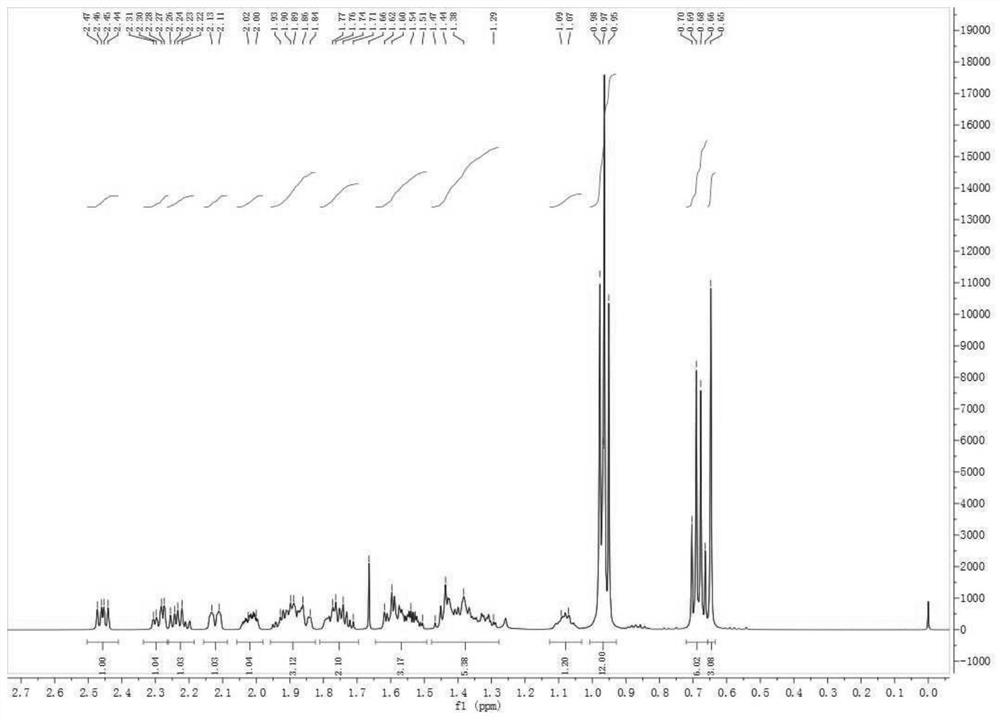
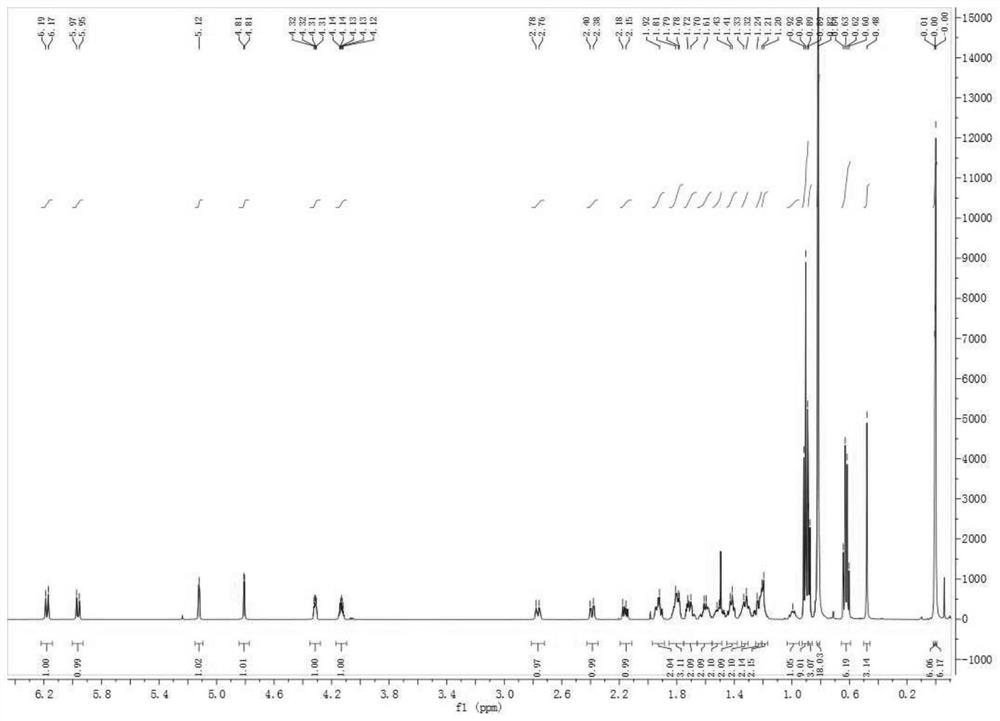
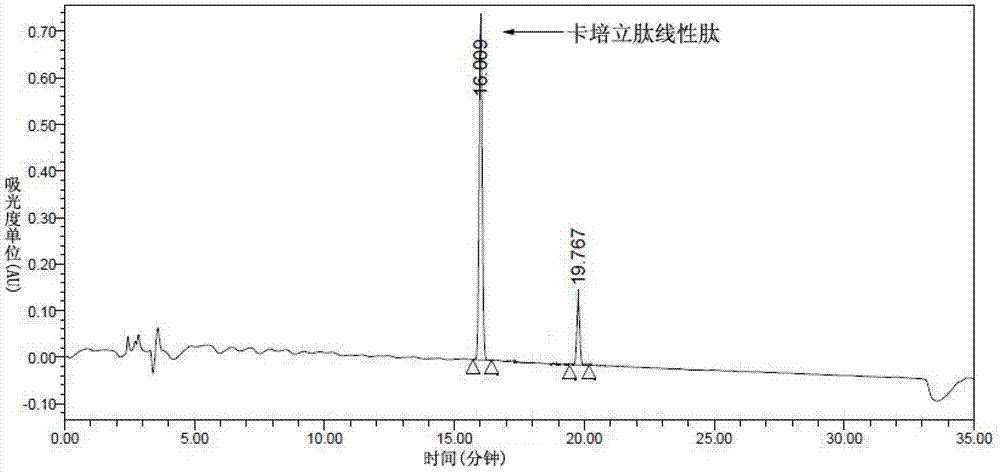
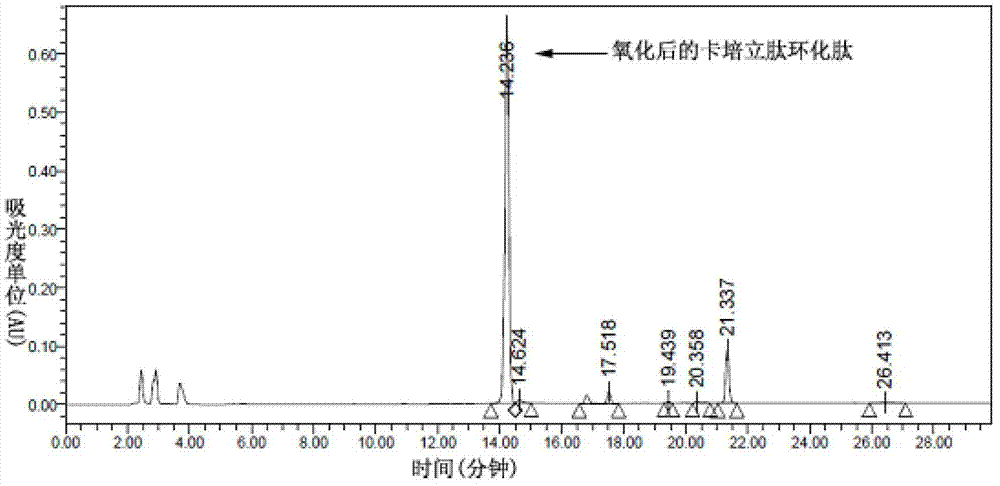
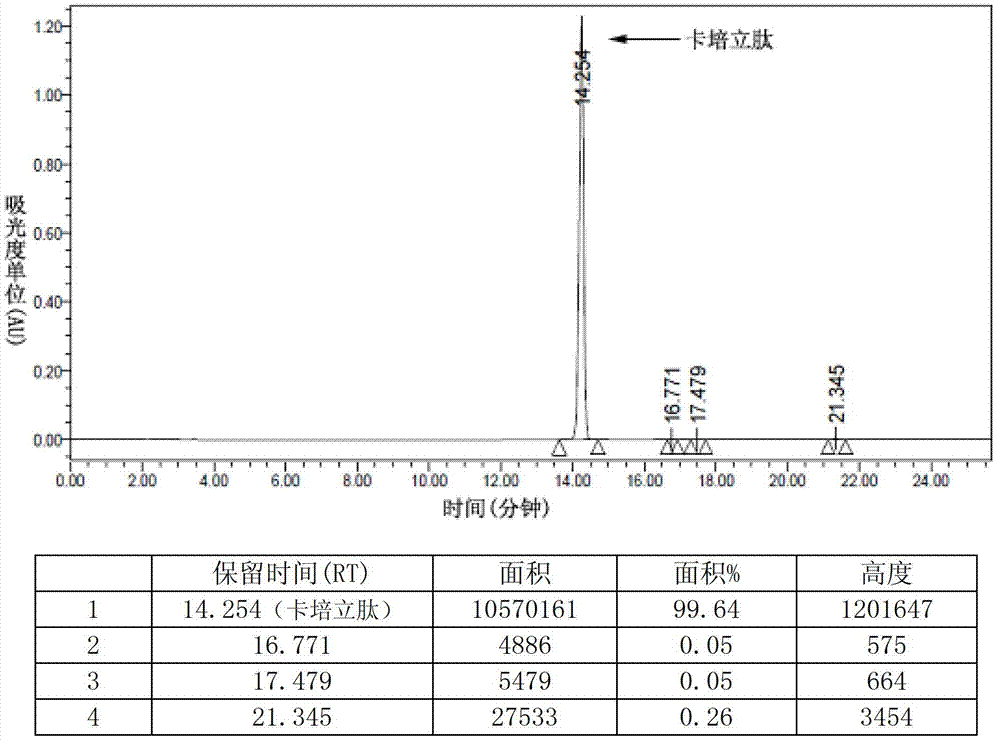
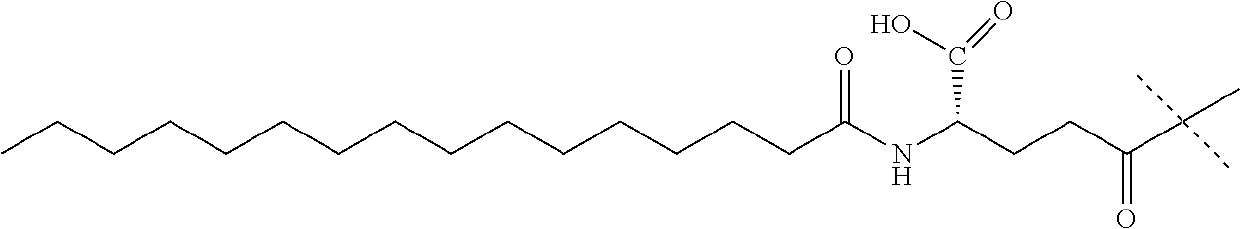
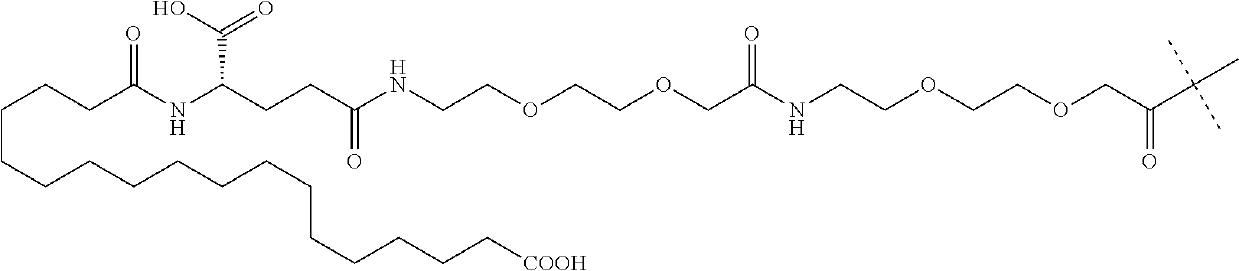
Preparation of carperitide by solid-phase convergence process
The invention discloses a synthetic method of carperitide by Fmoc (fluorenylmethyloxycarbonyl) route solid-liquid convergent synthesis. Multiple segments are synthesized simultaneously. The synthetic cycle is decreased by two thirds. Intermediate is easy to purify. The cost is low. The finished product is highly pure. Side products are few. The product yield is high. Large-scale production of carperitide is facilitated. The carperitide prepared by the method is above 75% in crude peptide purity and above 25% in total yield.
Owner:HYBIO PHARMA
Process for the manufacture of glp-1 analogues
ActiveUS20220041680A1High yieldGood impurity profilePeptide preparation methodsGlucagonsEngineeringPseudoproline
The present invention provides a process for the manufacture of GLP-1 analogues with high yield and purity by fragment condensation on the solid phase. In particular, it describes a convergent synthesis by condensation of a C-terminal pseudoproline fragment A with a fragment B bound to a solid support, followed by deprotection and cleavage from the support and final purification to yield the desired peptide. The invention further provides intermediates useful in the manufacturing process.
Owner:FRESENIUS KABI IPSUM SRL
A kind of preparation method of fused ring compound
ActiveCN111995488BImprove compatibilityHigh regional selectivitySilicon organic compoundsOrganic compound preparationRegioselectivityHeteroatom
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Preparation method of N-Fmoc-N '-Boc-alpha-methyl-L-lysine
PendingCN114276278ASimple process conditionsHigh yieldCarbamic acid derivatives preparationOrganic compound preparationMethyllysineBiochemical engineering
The invention belongs to the field of medical chemistry, and particularly relates to a preparation method of N-Fmoc-N '-Boc-alpha-methyl-L-lysine (hereinafter referred to as a compound 1). Aiming at the defects of tedious steps, high byproduct content, low yield and poor production safety in the existing preparation method of N-Fmoc-N '-Boc-alpha-methyl-L-lysine, the invention provides a new synthesis route and preparation method: 1, 4-diiodobutane is used as a starting material and is subjected to nucleophilic substitution with a compound 3 to generate a compound 4; the method comprises the following steps: reacting a compound 1 with a compound 2 to generate a compound 5 under the action of KHMDS, removing chiral prothetic groups and protecting groups through hydrogenation reduction, and protecting with Fmoc to obtain a compound 1, and the total yield is 52.6%. According to the route, N-Fmoc-N '-Boc-alpha-methyl-L-lysine is obtained through four-step convergent synthesis, and the production period is greatly shortened. The method has the advantages of simple production process steps, high yield, few byproducts, high process safety, good stability and environmental protection.
Owner:上海药坦药物研究开发有限公司
Antitumor compound, synthesis method and applications thereof
ActiveCN110981803AEffective treatmentStrong inhibitory activityOrganic active ingredientsOrganic chemistryChemical synthesisPerylene derivatives
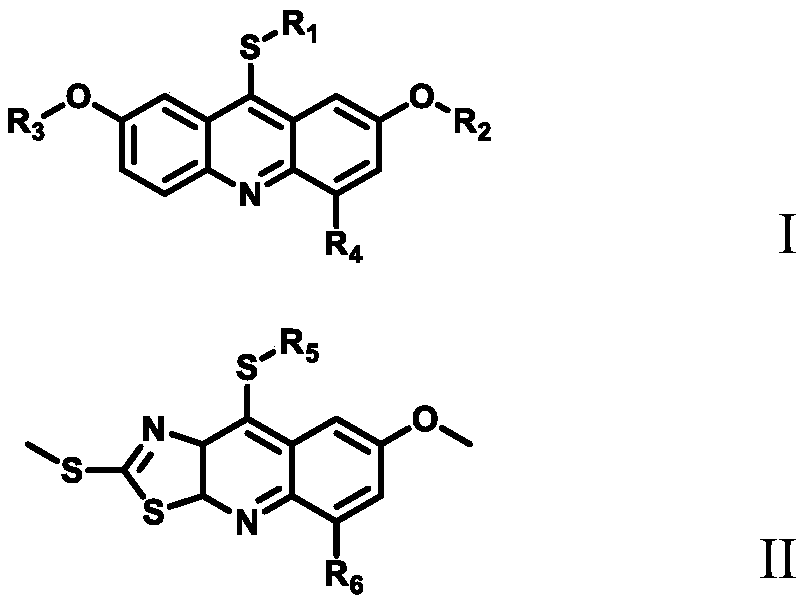
The invention belongs to the field of medicinal chemistry, and particularly relates to an antitumor compound, a synthesis method and applications thereof, wherein the compound has a structure represented by a general formula I or a general formula II, and shows good inhibitory activity on proteasome regulatory kinase DYRK2, so that antitumor activity can be achieved by inhibiting proteasome. The invention also relates to a synthesis method of the compound, wherein the method adopts a chemical total synthesis route, and the convergent synthesis route can be applied to chemical synthesis of compounds with similar structures and related derivatives, and develops a wide development space for novel antitumor drugs.
Owner:PEKING UNIV
The preparation method of Prosymod
ActiveCN111087358BHigh yieldHigh purityGroup 3/13 element organic compoundsBiochemical engineeringBromobenzenes
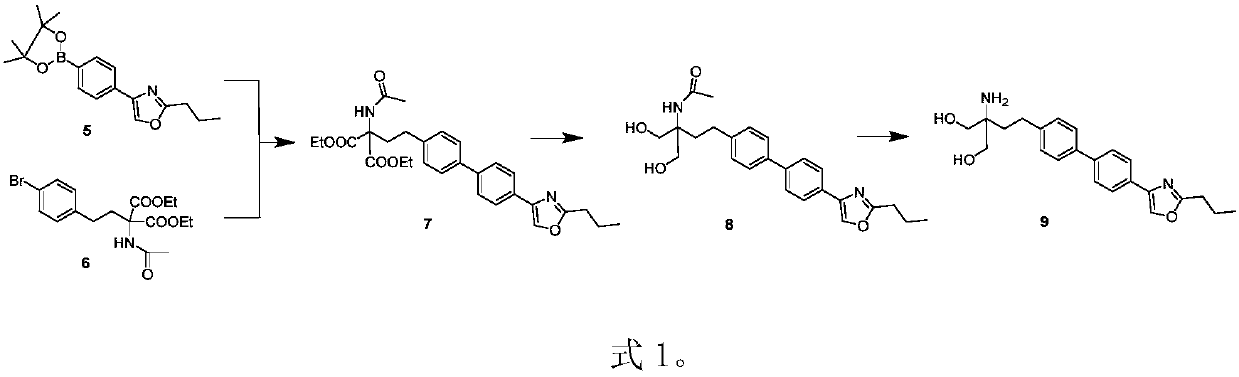
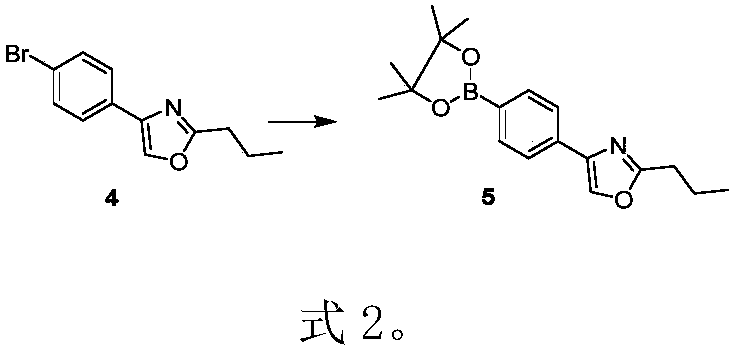
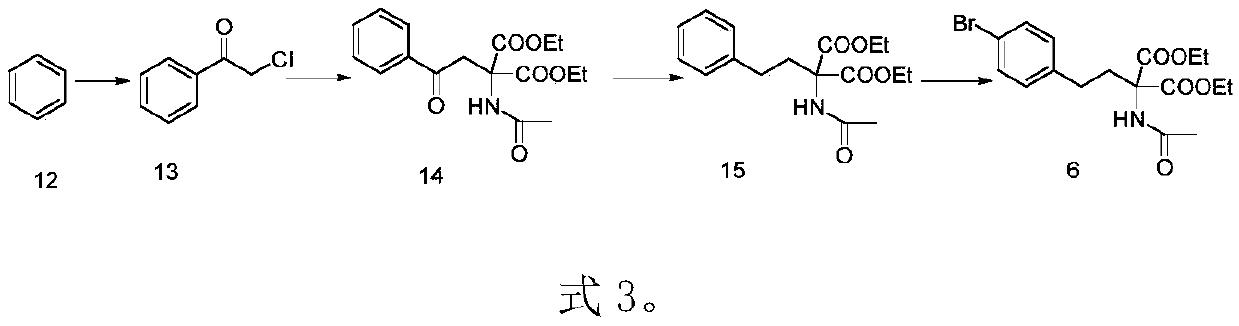
The invention discloses a preparation method of prosamod, which uses bromobenzene and benzene as starting materials to prepare prosamod by adopting a convergent synthesis route. The method has high yield, low cost, less pollution of three wastes, and is easy to operate. It has high application value.
Owner:渐宽(苏州)生物科技有限公司 +1
Preparation method of pusaimode (Chinese name)
ActiveCN111087358AHigh yieldHigh purityGroup 3/13 element organic compoundsBenzeneEnvironmental engineering
The invention discloses a preparation method of pusaimode. Bromo-benzene and benzene are used as initial raw materials to prepare pusaimode by adopting a convergent synthesis route. The yield of the preparation method is high, the cost is low, the amount of generated waste water, waste gas, and waste solids is low, the operation is convenient, and the application value is high.
Owner:渐宽(苏州)生物科技有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com