Process for the manufacture of glp-1 analogues
一种GLP-1、类似物的技术,应用在肽合成领域,能够解决无法获得目标肽、降低氨基酸偶联和去保护的效率等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
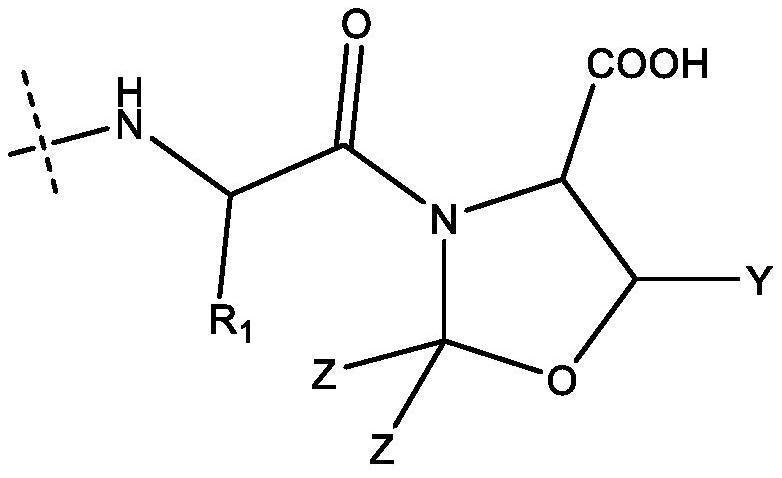
[0136] In the preparation of semaglutide, a protected lysine derivative as shown below was used:
[0137]
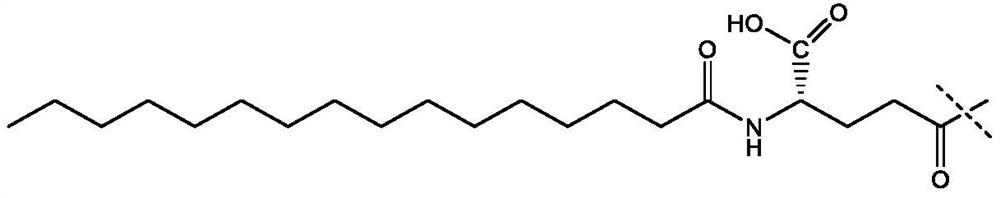
[0138] This derivative is commercially available and its preparation is also described eg in CN104356224. This derivative is also represented as Fmoc-Lys[(tBuOOC-(CH 2 ) 16-CO-γGlu-OtBu)-AEEA-AEEA]-OH, wherein AEEA represents 2-[2-(2-aminoethoxy)ethoxy]acetyl.
[0139] The X 2 The tBu ester protecting groups of the building blocks are all removable under acidic conditions and thus cleaved at the end of the preparation process.
[0140] In a preferred aspect of the invention, said coupling step is performed in the presence of a coupling agent.
[0141] Preferably, the coupling agent is selected from N-hydroxysuccinimide (NHS), N,N'-diisopropylcarbodiimide (DIC), N,N'-dicyclohexylcarbodiimide (DCC), (benzotriazol-1-yloxy)tripyrrolidinylphosphonium hexafluorophosphate (PyBOP), 2-(7-aza-1H-benzotriazol-1-yl)-1, 1,3,3-tetramethylurea hexafluorophosphate (HATU), 2-(1H...
Embodiment 1
[0330] Preparation of liraglutide
[0331] Step 1. Preparation of Fragment 1, Fragment D (17-31)
[0332] H-Gln(Trt) 17 -Ala-Ala-Lys(Pal-Glu-OtBu)-Glu(OtBu)-Phe-Ile-Ala-Trp(N in Boc)-Leu-Val-Arg(Pbf)-Gly-Arg(Pbf)-Gly 31 -Wang Resin
[0333]
[0334] The synthesis of the peptide fragment 1 was performed by stepwise Fmoc SPPS using 0.25 g of Wang resin (0.75 mmol / g) at room temperature. After the resin was swollen in 2ml DMF, Fmoc-Gly-OH, DIC and DMAP in DMF were added (4 eq, 2 eq and 0.1 eq relative to the resin loading, respectively). After 1h, by using DMF / DIPEA / Ac 2 The O mixture (v / v / v 5 / 2 / 1) was treated for 30 minutes to cap the unreacted hydroxyl groups of the resin. Fmoc deprotection was performed using 20% piperidine in DMF (2 x 2 ml, 5 min and 15 min) and the resin was washed with DMF (4 x 2 ml). The degree of substitution was checked by UV absorbance measurement of the solution collected after Fmoc deprotection and was 0.53 mmol / g. The Fmoc-protected ami...
Embodiment 2
[0359] Preparation of semaglutide
[0360] Step 1. Preparation of Fragment 5, Fragment D (17-31)
[0361] H-Gln(Trt) 17 -Ala-Ala-Lys[(tBuOOC-(CH 2 ) 16 -CO-γGlu-OtBu)-AEEA-AEEA]-Glu(OtBu)-Phe-Ile-Ala-Trp(N in Boc)-Leu-Val-Arg(Pbf)-Gly-Arg(Pbf)-Gly 31 -O-MBH resin
[0362]
[0363] The synthesis of peptide fragment 5 was performed at room temperature using 0.5 g of H-Gly-O-MBH resin (loading 0.60 mmol / g). The resin was swollen in 3 ml DMF and used for stepwise Fmoc SPPS. Fmoc-protected amino acids (in two-fold excess relative to the resin loading) were preactivated with a mixture of DIC (2eq) and OxymaPure (2eq) for 3 min and sequentially coupled to the resin within 90 min. Arg, Val, Trp, Ala 24 The coupling of , Phe and Gln was repeated for 60 min using 1 eq coupling mixture. Using 1.5eqFmoc-Lys[(tBuOOC-(CH 2 ) 16 -CO-γGlu-OtBu)-AEEA-AEEA]-OH for the introduction of lipidated side chains in the peptide sequence, which was preactivated with 1.5eq DIC and 1.5eq O...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com