Antitumor compound, synthesis method and applications thereof
A synthetic method and compound technology, applied in the field of anti-tumor compounds and their synthesis, can solve the problems of poor activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
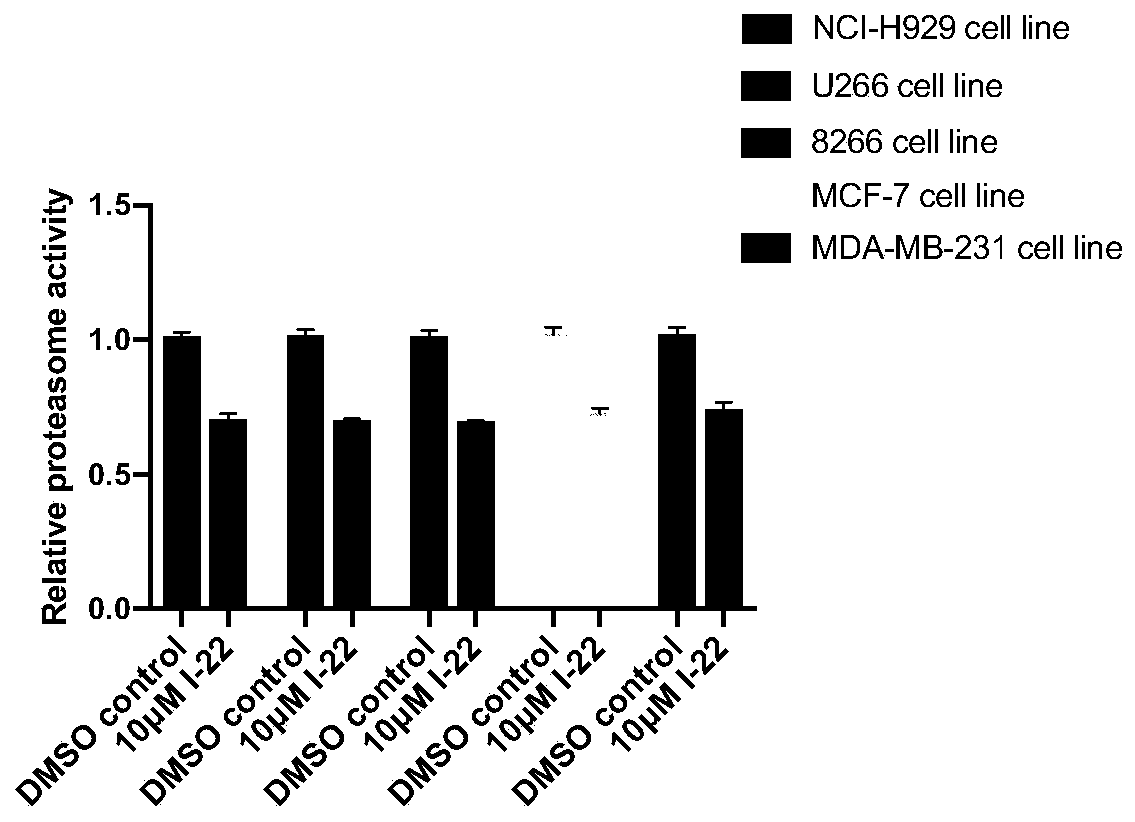
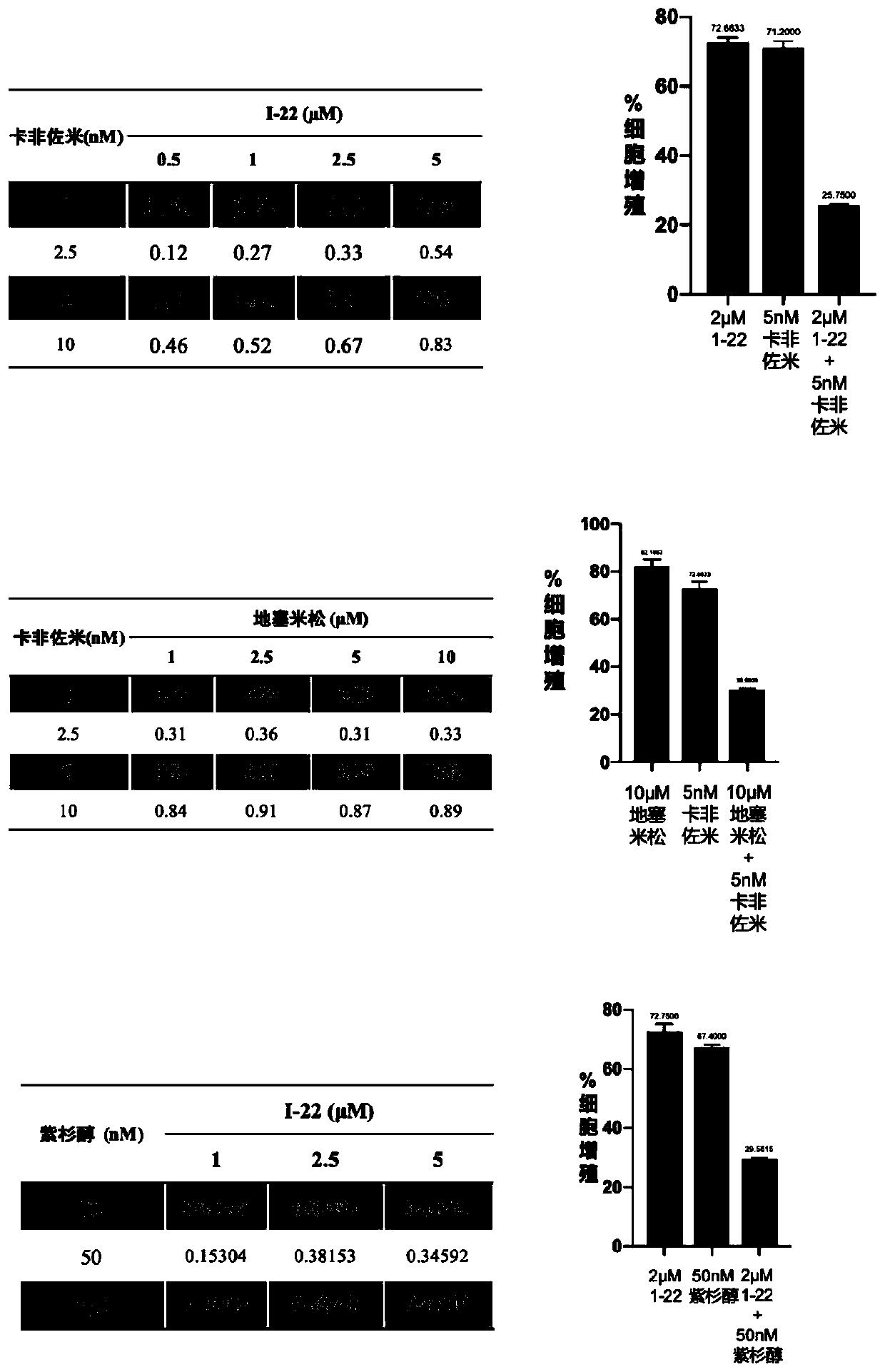
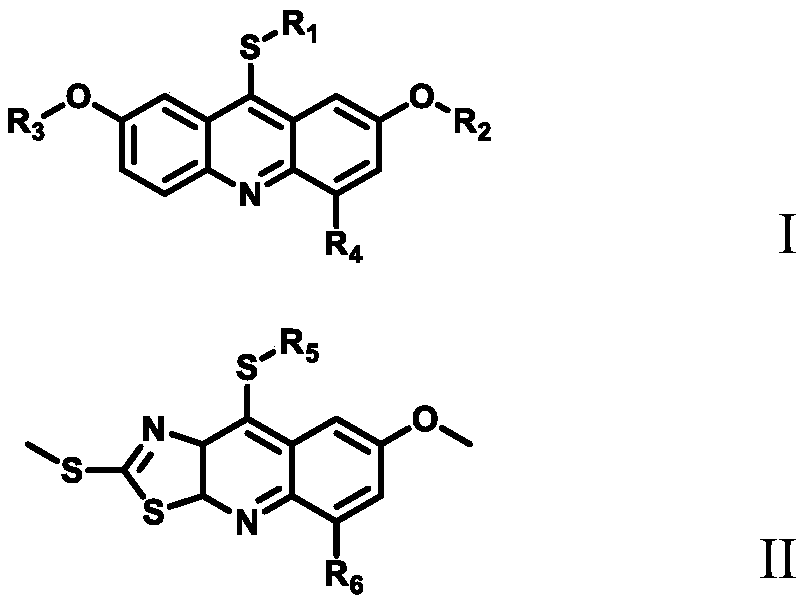
Image
Examples
Embodiment 1
[0136] The synthesis of embodiment 1 compound I-1, I-2, I-3, I-4
[0137] Synthesize I-1 according to the following steps:
[0138]
[0139] (1) Synthesis of compound 3:
[0140] To commercially available starting materials 1 (9.26 g, 40 mmol), 2 (6.90 g, 56 mmol) were added Cu (0..73 g, 11 mmol), Cu 2 O (0.82g, 5.7mmol), potassium carbonate (7.74g, 56mmol), DMF 100ml, stirred and reacted at 80°C overnight. The reaction solution was cooled to room temperature, and 2M dilute hydrochloric acid was added thereto until the system became acidic, and a large amount of solids precipitated out. The precipitated solid was suction-filtered, washed with water, and dried to obtain compound 3 (4.02 g, 37%), which was directly used in subsequent reactions without purification.
[0141] (2) Synthesis of compound 4:
[0142] Compound 3 (2.58 g, 9.45 mmol) was placed in a sealed tube, 30 ml of phosphorus oxychloride was added under the protection of argon, and reacted at 130° C. for 8 h...
Embodiment 2
[0156] The synthesis of embodiment 2 compound I-5, I-6
[0157] Synthesize I-5 according to the following steps:
[0158]
[0159] Under argon protection, 5ml of dry DMF was added to compound 4 (50mg, 0.183mmol) and 67% sodium hydrosulfide hydrate (22.9mg, 0.274mmol), and reacted at 50°C for 2h. Then tert-butyl 4-bromopiperidine-1-carboxylate (72.4 mg, 0.274 mmol) and potassium carbonate (50.5 mg, 0.365 mmol) were added thereto, and reacted at room temperature overnight. The reaction solution was spin-dried under reduced pressure, separated into water / dichloromethane, the organic phase was separated, dried over anhydrous sodium sulfate, and the solvent was spin-dried under reduced pressure. The obtained crude product was placed in 2 ml of dichloromethane solution containing 5% trifluoroacetic acid, reacted at room temperature for 2 h until no raw material remained, and the solvent was spin-dried under reduced pressure. Purified by HPLC / MS on Waters automatic purification ...
Embodiment 3
[0165] The synthesis of embodiment 3 compound I-7, I-8, I-9, I-10, I-11
[0166] Follow the steps below to synthesize I-10:
[0167]
[0168] (1) Synthesis of compounds 5 and 7:
[0169] Slowly add 30ml of boron tribromide to the suspension of compound 5 (1.65g, 6.05mmol) in 200ml of dry dichloromethane, and react at 0°C for 2h. Methanol was added to the reaction system to quench the reaction, and the solvent was spin-dried under reduced pressure. The crude product was separated and purified by silica gel column (dichloromethane / methanol=92 / 8) to obtain product 5 (0.22g, 14%) and reported compound 7 (0.80g, 56%), and recovered 0.70g of raw material.
[0170] The characterization information of compound 5 is specifically:
[0171] 1 H NMR (400MHz, MeOD) δ8.08(dd, J=9.4, 4.8Hz, 2H), 7.64(dd, J=7.6, 4.4Hz, 4H), 4.07(s, 3H).
[0172] (2) Synthesis of Compound 6:
[0173] Under the protection of argon, add dry DMF 10ml to compound 2 (94.5mg, 0.364mmol), potassium carbonate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com