Preparation method of N-Fmoc-N '-Boc-alpha-methyl-L-lysine
A n-fmoc-n, -boc- technology, applied in the field of synthetic chemistry, can solve the problems of poor chiral purity of products, and achieve the effect of safety and reliability, simple process conditions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
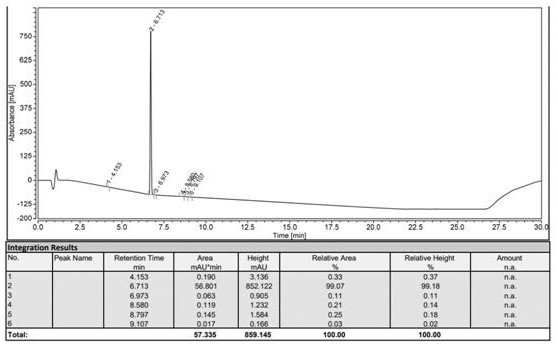
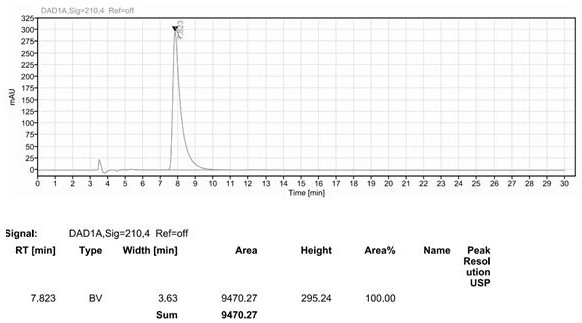
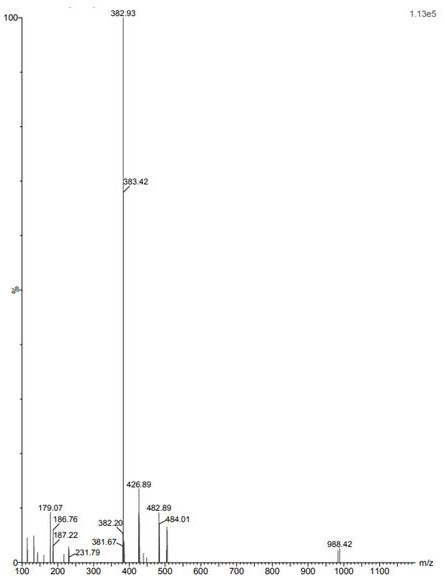
Image
Examples
Embodiment 1
[0044] (1) Synthesis of compound 4
[0045] Under nitrogen protection, 100 g of compound 3 (ie: tert-butylbenzyl carbamate), 448 g of 1,4-diiodobutane were added into 1000 mL of anhydrous tetrahydrofuran, stirred and cooled to 0°C. Control the temperature below 0°C, add 58 g of 60% sodium hydrogen solution in batches, stir for 1 hour after the addition is complete, then warm to room temperature, stir for 6 hours, liquid chromatography detects that the remaining 0.2% of the mass of the raw material compound 3 is 0.2%.
[0046] Control the temperature below 5°C, add the reaction suspension liquid dropwise into 1000mL citric acid aqueous solution with a mass concentration of 15%, add dropwise for 2h, then add 500mL ethyl acetate, stir and separate the layers, wash the organic phase with 500mL water, and concentrate to dry. Use an oil pump to distill under reduced pressure at 150°C and 0.09Mpa to recover 1,4-diiodobutane. The obtained crude concentrate is a product containing co...
Embodiment 2
[0057] (1) Synthesis of compound 4
[0058] Under the protection of helium, 50g of tert-butylbenzyl carbamate and 187g of 1,4-diiodobutane were added into 750mL of anhydrous tetrahydrofuran, stirred and cooled to -10°C. Control the temperature below -10°C, add 24 g of 60% sodium hydrogen solution in batches, stir for 0.5 hours after the addition is complete, then warm up to room temperature, stir for 18 hours, liquid chromatography detects that the remaining 0.5% of the mass of the raw material compound 3 is 0.5%.
[0059] Control the temperature below 10°C, add the reaction suspension liquid dropwise into 500mL citric acid aqueous solution with a mass concentration of 15%, dropwise for 15min, then add 350mL 2-methyltetrahydrofuran, stir and separate the layers, and wash the organic phase with 500mL water, Concentrate to dryness. Use an oil pump to distill under reduced pressure at 120°C and 0.09Mpa to recover 1,4-diiodobutane. The obtained crude concentrate is a product con...
Embodiment 3
[0068] (1) Synthesis of compound 4
[0069] Under nitrogen protection, 50 g of tert-butylbenzyl carbamate and 261 g of 1,4-diiodobutane were added into 1000 mL of anhydrous tetrahydrofuran, stirred and cooled to 5°C. Control the temperature below 5°C, add 34g of 60% sodium hydrogen solution in batches, stir for 0.5 hours after the addition is complete, then warm to room temperature, stir for 5 hours, liquid chromatography detects that the remaining 1.5% of the mass of the raw material compound 3 is 1.5%.
[0070] Control the temperature below 10°C, add the reaction suspension dropwise to 750mL of water for 60min, then add 450mL of methyl tert-butyl ether, stir and separate layers, wash the organic phase with 500mL of water, and concentrate to dryness. Use an oil pump to distill under reduced pressure at 150°C and 0.09Mpa to recover 1,4-diiodobutane. The obtained crude concentrate is a product containing compound 4, and the crude concentrate can be directly used in the next re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com