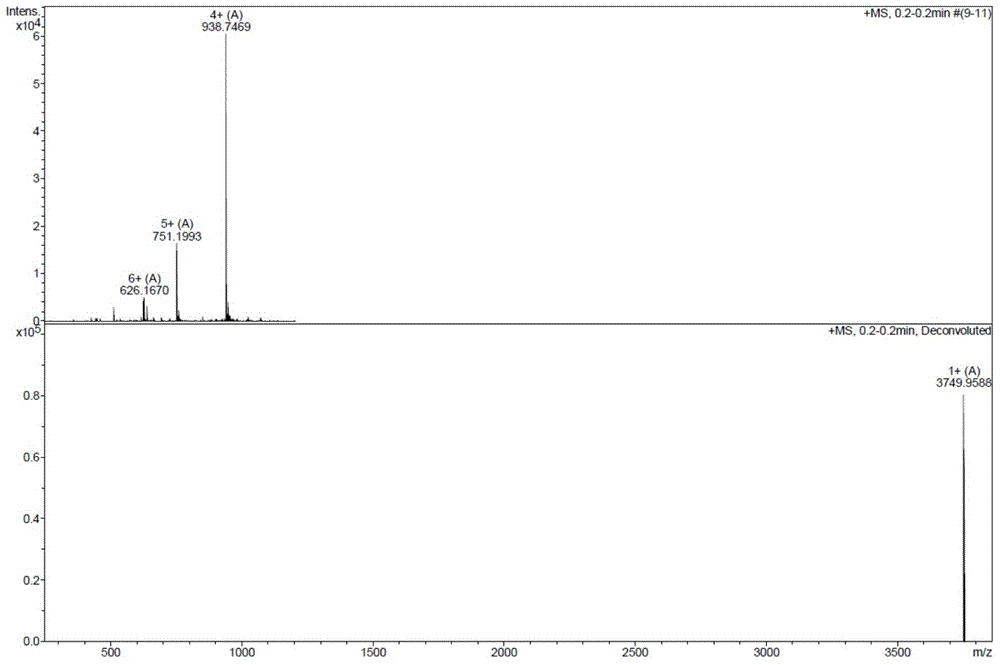
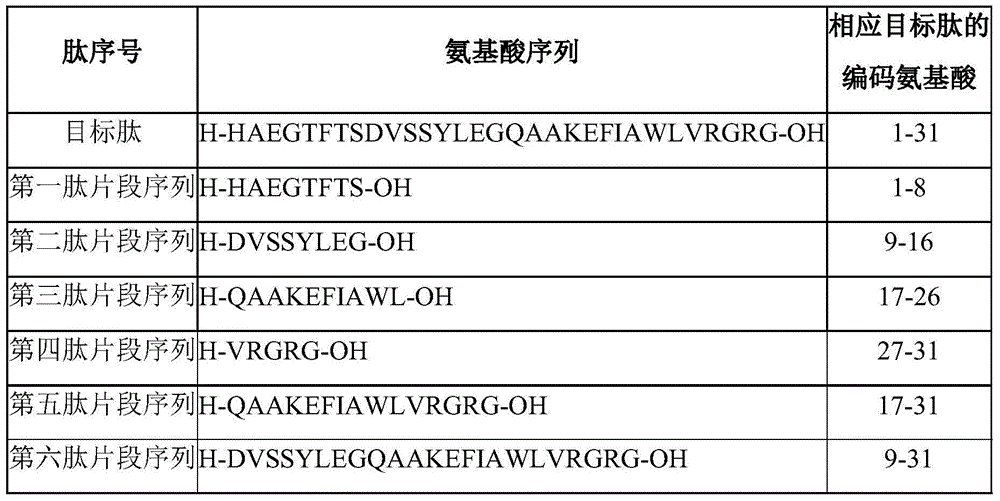
Method for preparing liraglutide by convergent synthesis
A technology for liraglutide and fragments, which is applied in the field of preparing liraglutide by fragment condensation, can solve the problems of low cost and high efficiency, generating a lot of waste liquid, and difficulty in product purification, so as to shorten the synthesis cycle and reduce the generation of waste liquid. , The effect of low material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1. Resin Preparation
[0054] 1.1 Preparation of Fmoc-Gly-2-chloro-trityl resin: add 2-chloro-trityl chloride resin (10g, substitution value 0.84mmol / g resin, 1eq) into the polypeptide synthesizer, wash the resin with 100mL DCM . The solvent was drained and a solution of Fmoc-Gly-OH (1.3 eq) and DIEA (2.5 eq) in 50 mL of DCM was added. The mixture was mechanically stirred under an argon atmosphere for 1 hour. Add 20 mL of chromatographic methanol (2 ml / g resin) to block the active part on the resin for 30 minutes. The solvent was drained, washed with 3×80mL DMF, 3×80mL DCM, 3×80mL MeOH, and vacuum-dried to constant weight to obtain 11.44g of Fmoc-Gly-2-chloro-trityl resin. The amount of Fmoc in the piperidine deprotection solution was measured by ultraviolet spectrophotometry, and the loading amount of the resin was 0.55 mmol / g.
[0055] 1.2 Preparation of Fmoc-Leu-2-chloro-trityl resin: add 2-chloro-trityl chloride resin (5 g, substitution value 0.84 mmol / g resin, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com