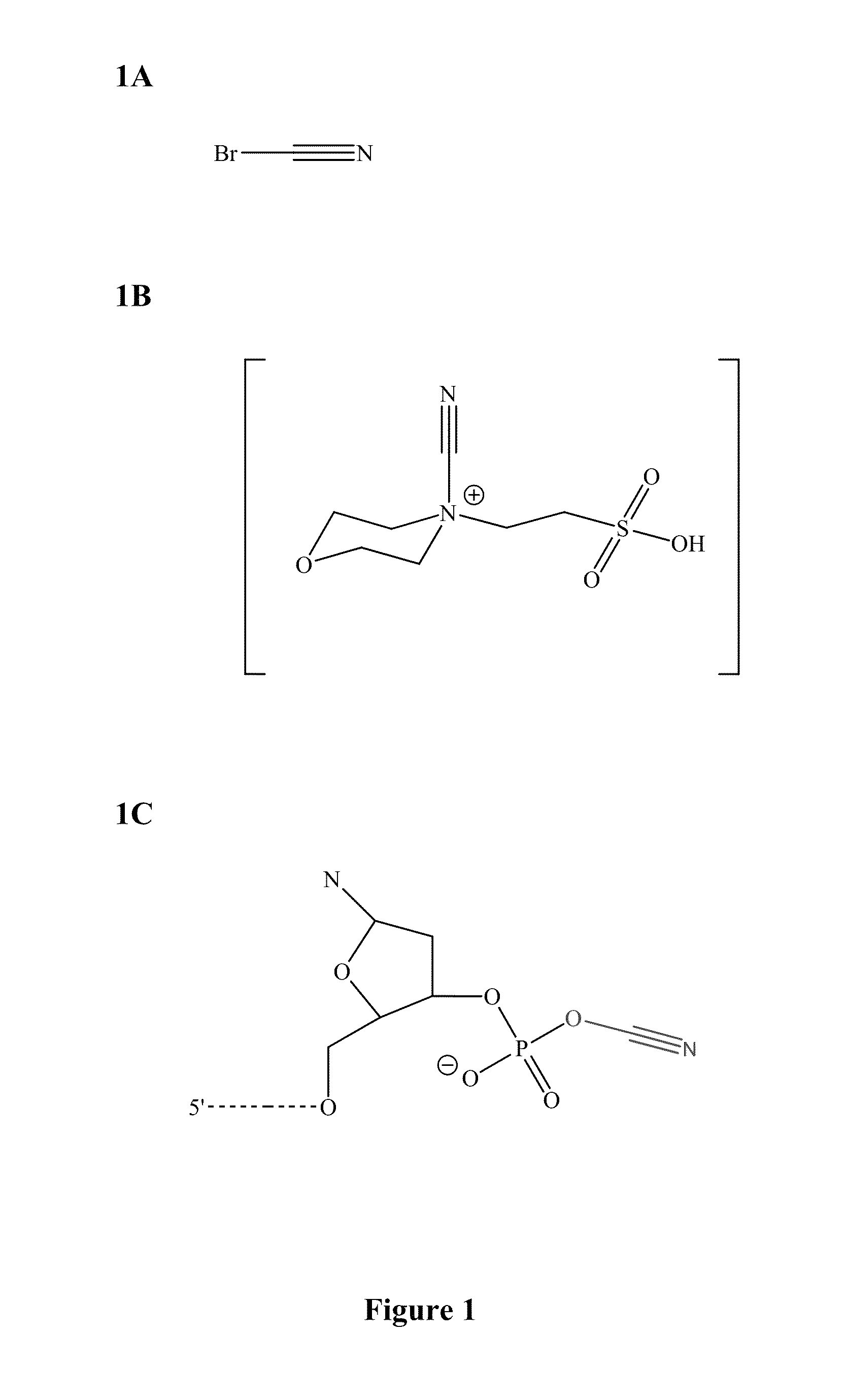
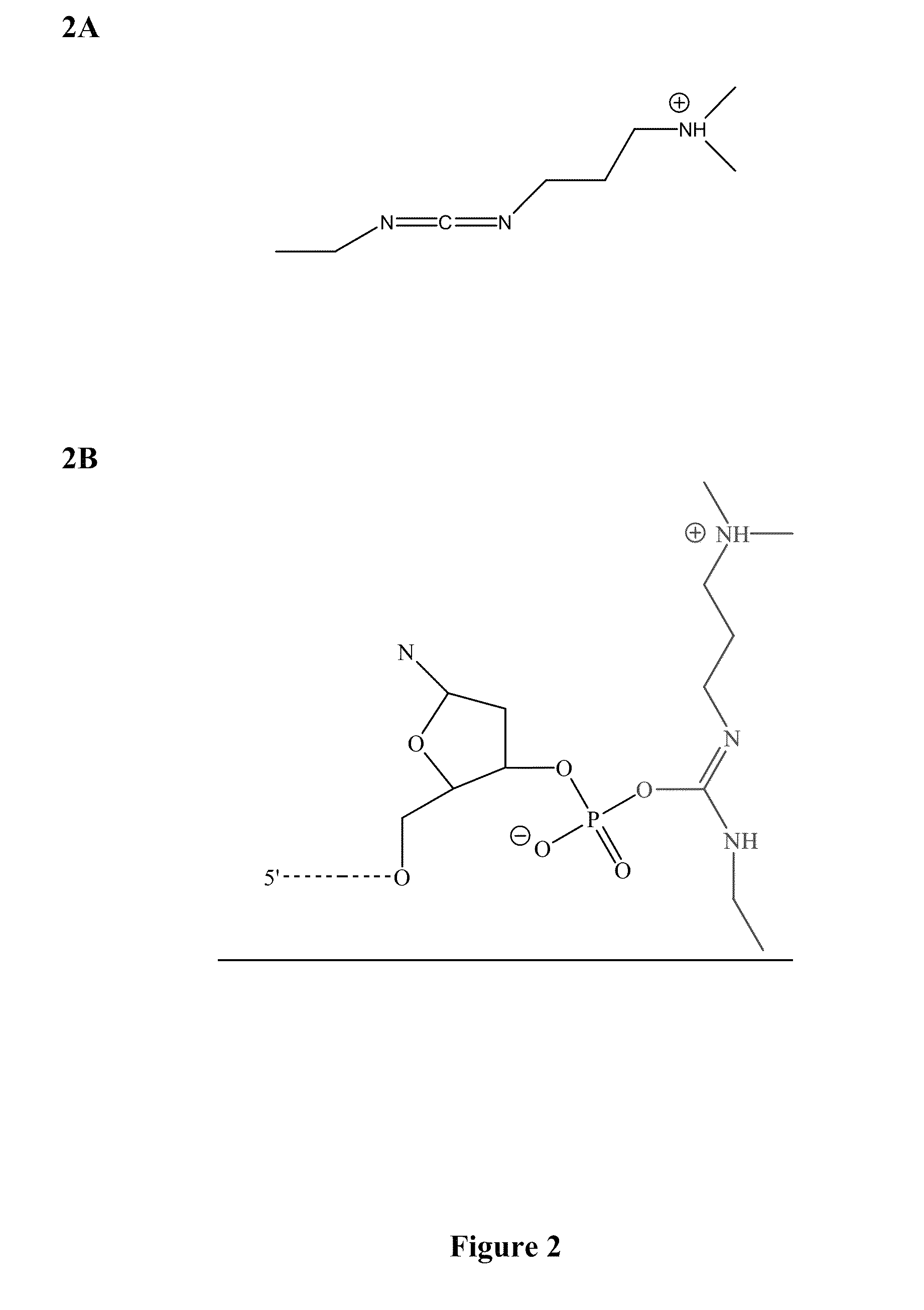
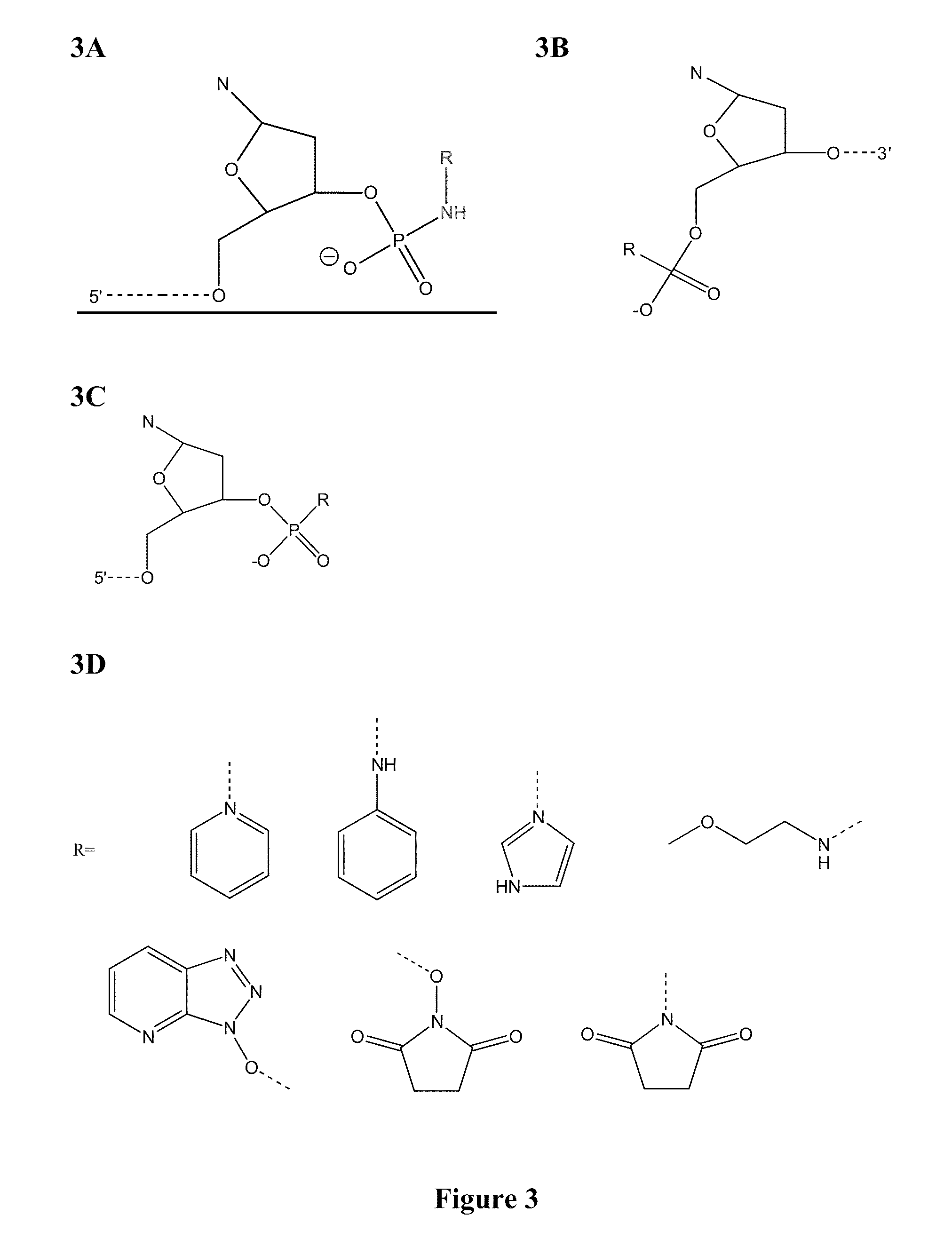
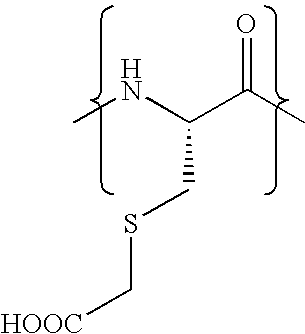
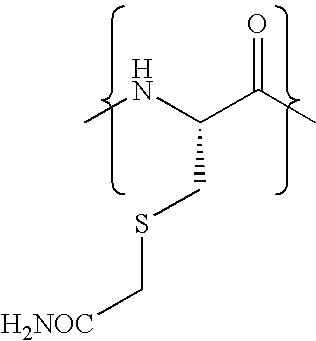
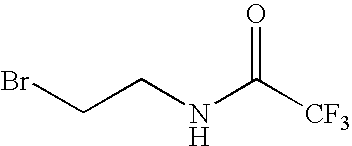
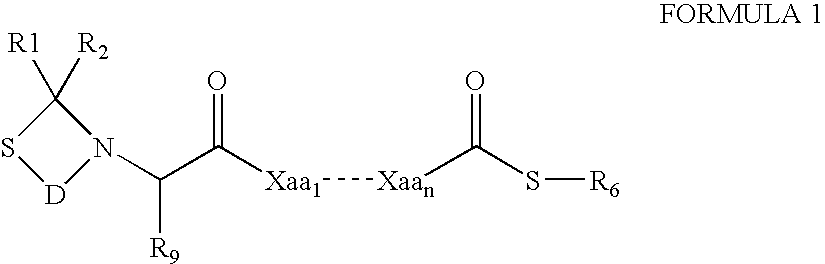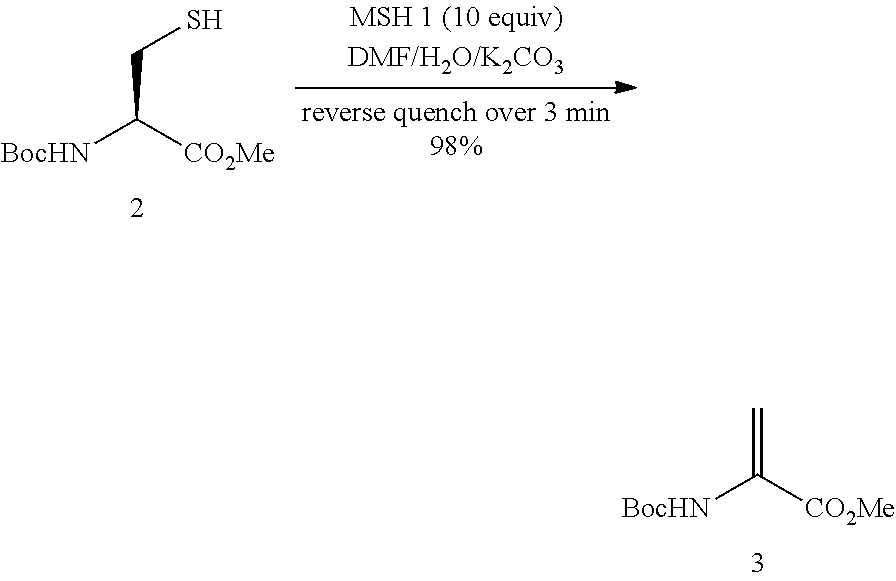Patents
Literature
33 results about "Native chemical ligation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
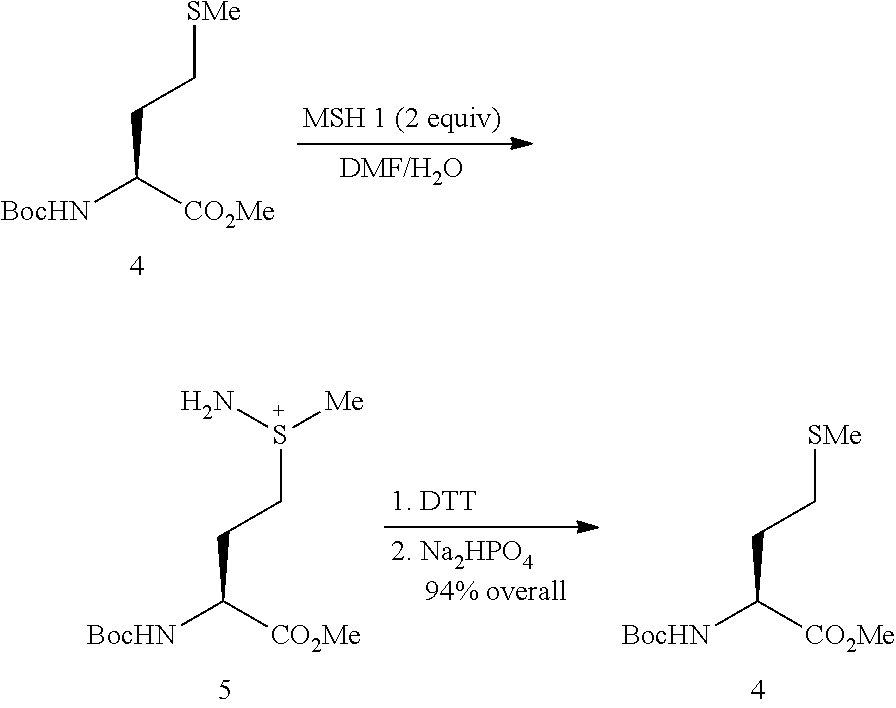
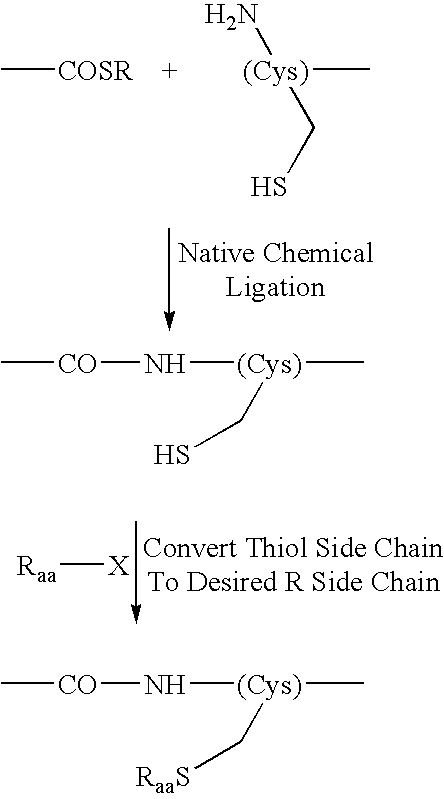
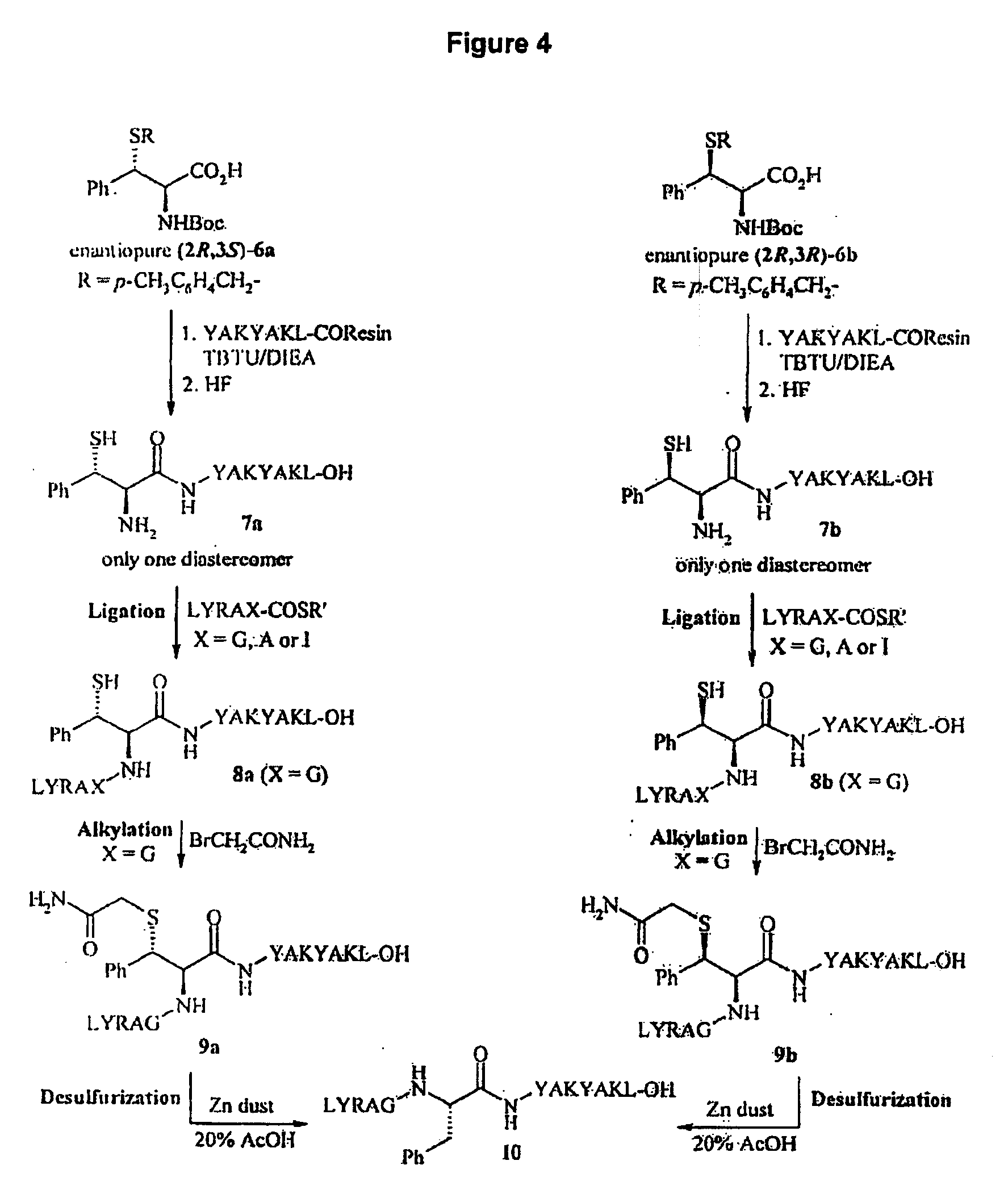
Native chemical ligation or NCL is an important extension of the chemical ligation field, a concept for constructing a large polypeptide formed by the assembling of two or more unprotected peptides segments. Especially, NCL is the most powerful ligation method for synthesizing native backbone proteins or modified proteins of moderate size (i.e., small proteins< 200 AA).
Methods and compounds for chemical ligation
ActiveUS8481258B2Eliminating risk of UV damageSugar derivativesMicrobiological testing/measurementChemical ligationNucleic acid sequencing
Compositions and methods for chemical ligation are provided. Methods for nucleic acid sequencing, nucleic acid assembly and nucleic acid synthesis are also provided.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Pseudo-native chemical ligation
InactiveUS20060149039A1Easily employedProduct is unsuitablePeptide/protein ingredientsAntipyreticPolymer modifiedChemical ligation
The present invention concerns methods and compositions for extending the technique of native chemical ligation to permit the ligation of a wider range of peptides, polypeptides, other polymers and other molecules via an amide bond. The invention further provides methods and uses for such proteins and derivatized proteins. The invention is particularly suitable for use in the synthesis of optionally polymer-modified, synthetic bioactive proteins, and of pharmaceutical compositions that contain such proteins.
Owner:AMYLIN PHARMA INC
Cysteine functionalized hyaluronic acid conjugate, synthetic method and application in injectable in-situ hydrogel thereof
The invention discloses a cysteine functionalized hyaluronic acid conjugate, a synthetic method and an application in preparation of injectable in-situ hydrogel thereof. According to the method, a hydroxy of hyaluronic acid is modified to obtain the cysteine functionalized hyaluronic acid conjugate with a stable ether bond, and tetrabutyl ammonium hydroxide is introduced in the reaction process. The new method improves the solubility of hyaluronic acid and increases the reaction efficiency. The obtained cysteine functionalized hyaluronic acid conjugate has a sulfhydryl active group which not only improves the adhesion of hyaluronic acid, but also can be further functionalized. According to the invention, injectable in-situ hydrogel is generated by a native chemical ligation reaction or a Michael addition reaction of the prepared cysteine functionalized hyaluronic acid conjugate with a polyethylene glycol conjugate; rheological research shows that the generated hydrogel has good rheological properties, the gel performance is controllable, and the hydrogel has wide application prospects in the biological medicine field.
Owner:HAINAN UNIVERSITY
Chemical peptide ligation with three or more components
InactiveUS20050113563A1Improve efficiencyMethod can be usedPeptide/protein ingredientsPeptide preparation methodsCompound (substance)Protecting group
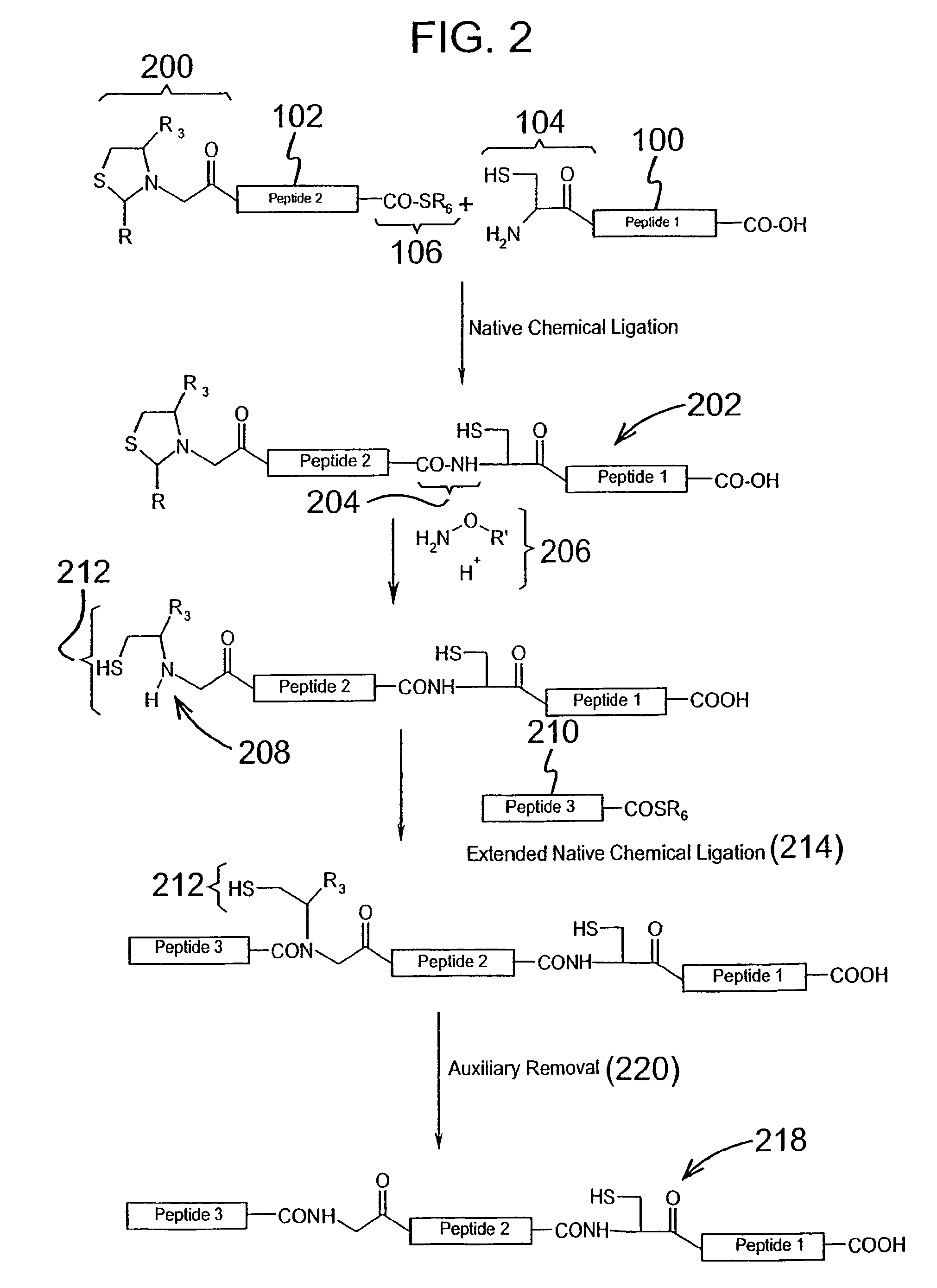
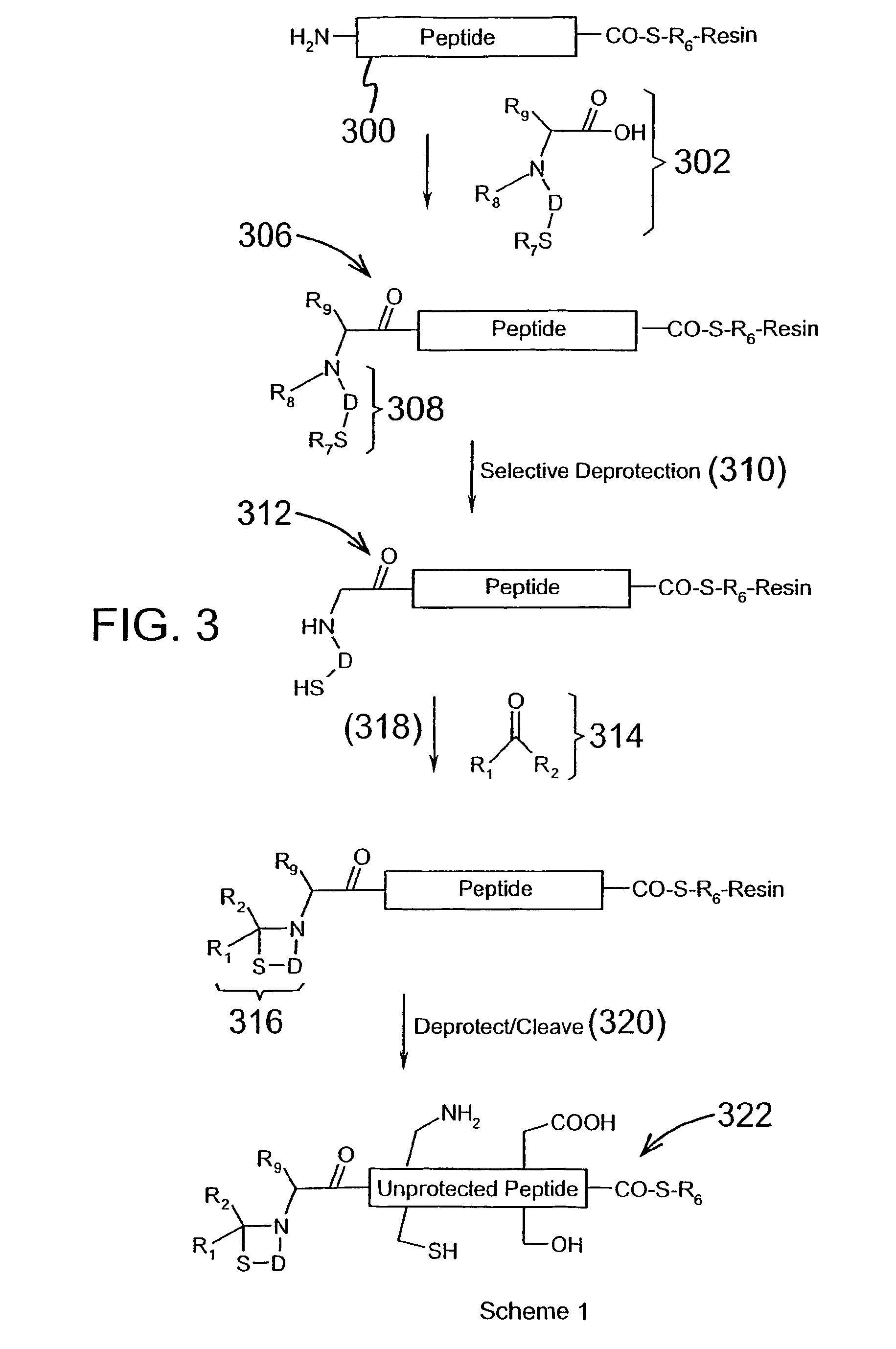
The invention provides a method of assembling oligopeptide intermediates in a native chemical ligation reaction that eliminates self-ligation of bi-functional intermediates. An important aspect of the invention is a bi-functional intermediate with an N-terminal heterocyclic protecting group which effectively prevents self-ligation in the chemical assembly process. The present invention is useful in methods for convergent synthesis of polypeptides and proteins and improve,s; the efficiency of native chemical ligation reactions, particularly where three or more peptide fragments are used to assemble a polypeptide or protein product.
Owner:ASTRAZENECA PHARMA LP
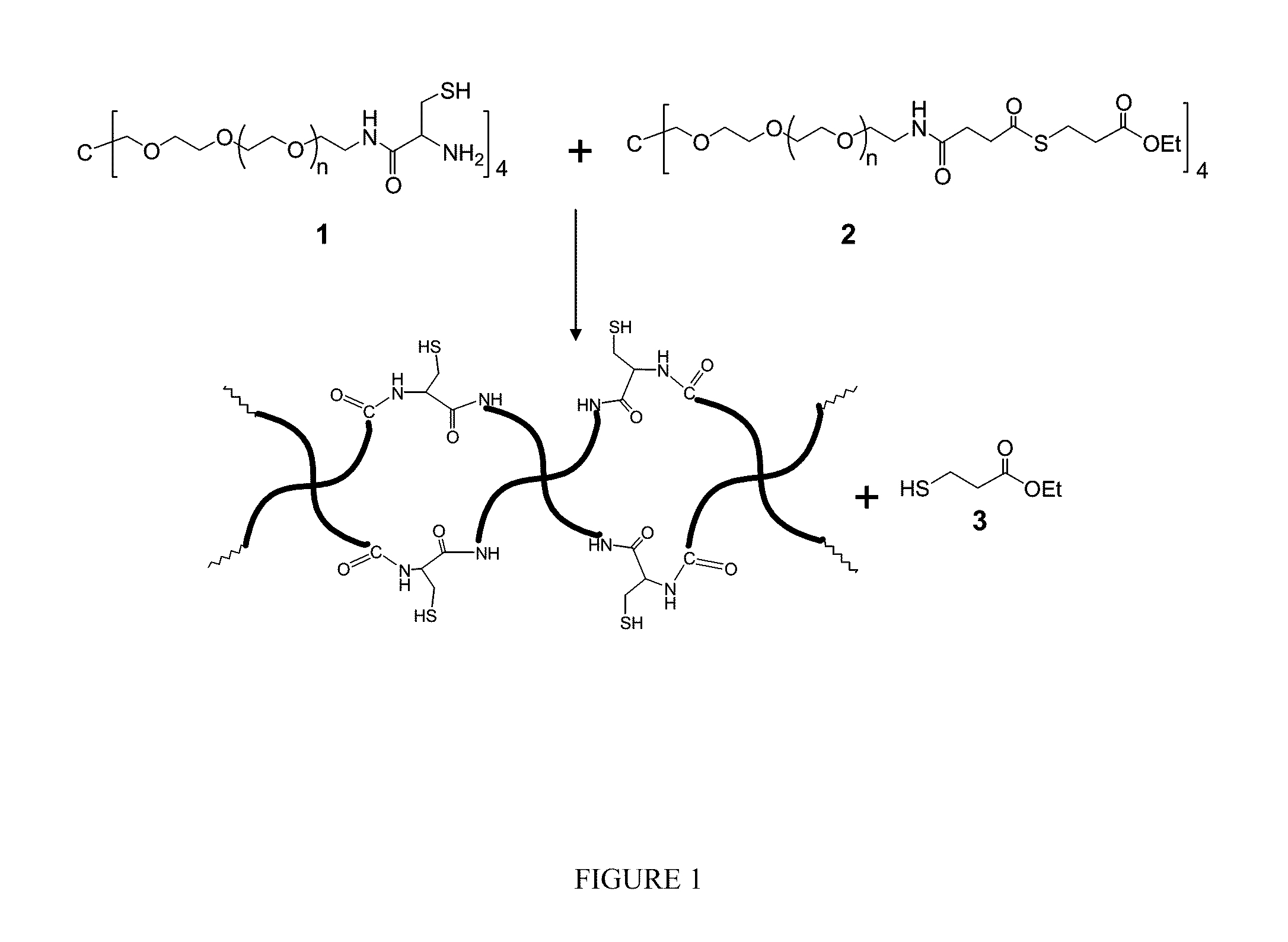
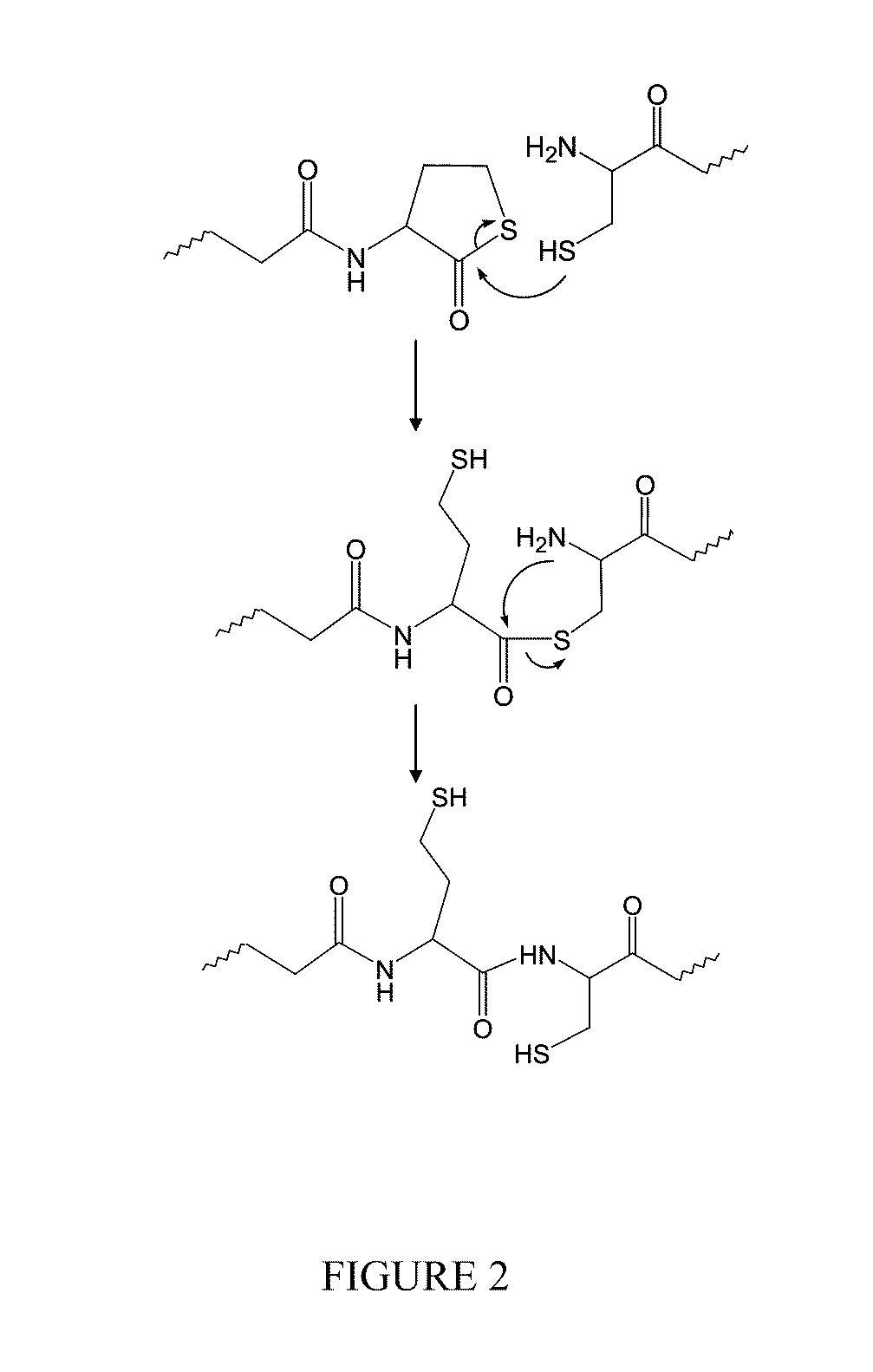
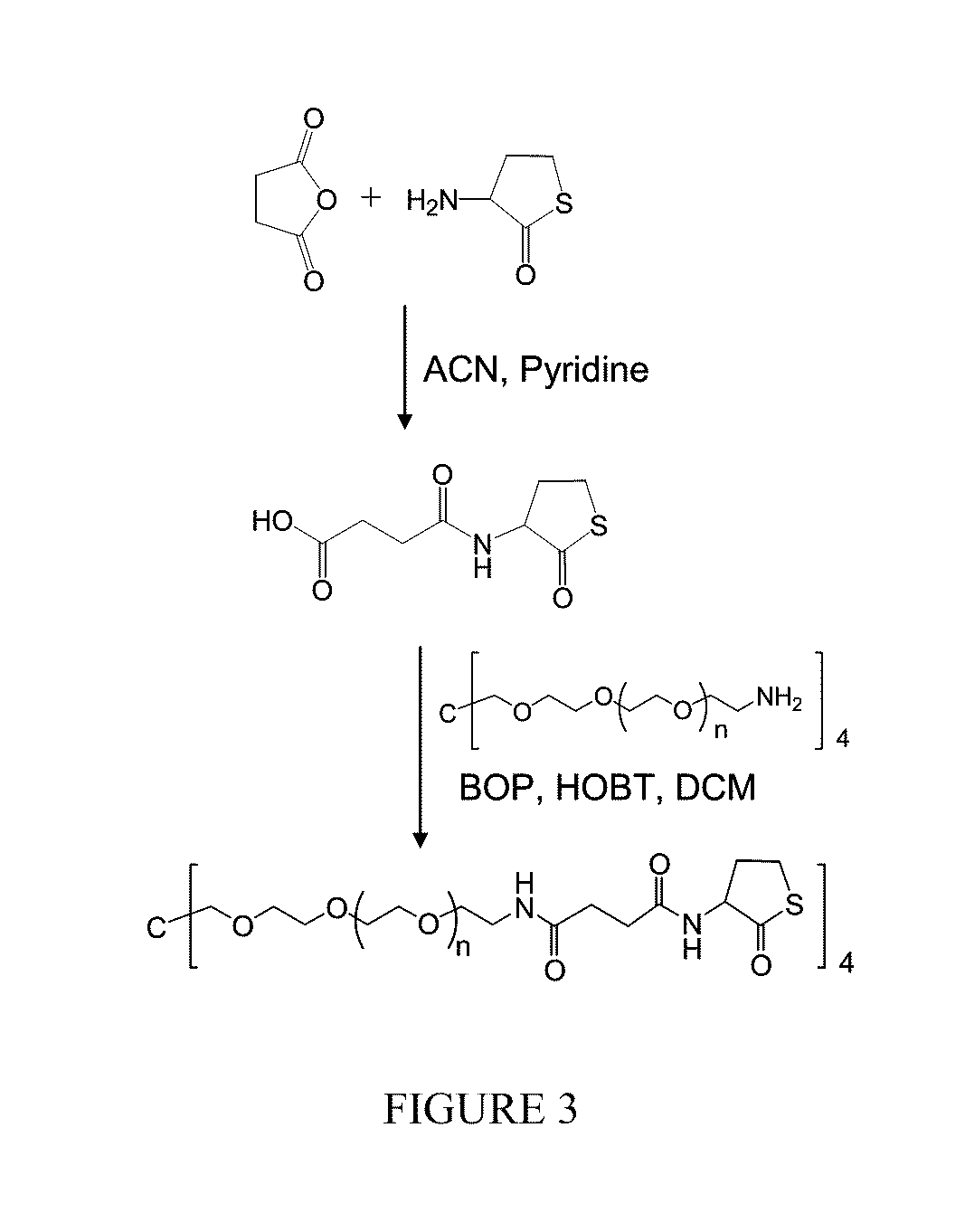
Catalyst and byproduct-free native chemical ligation using cyclic thioester precursors
ActiveUS20110262492A1Good biocompatibilityCross-linking is sufficiently rapidOrganic chemistryPeptide/protein ingredientsPolymer sciencePharmaceutical drug
A method of synthesizing a biocompatible hydrogel by covalently cross-linking an effective amount of a first macromonomer including a cyclic thioester group with an effective amount of a second macromonomer including a terminal cysteine group is disclosed. In addition, the synthesis and use of the following specific cyclic thioester macromonomer that can be used in the method, as well as specific hydrogels made using this macromonomer are disclosed. The disclosed method produces a biocompatible hydrogel, while producing substantially no toxic free thiol by-product. Accordingly, the method can be used in making biomedical products, such as sutures and tissue replacement biomaterials, and for encapsulating therapeutic cells and pharmaceuticals.
Owner:NORTHWESTERN UNIV
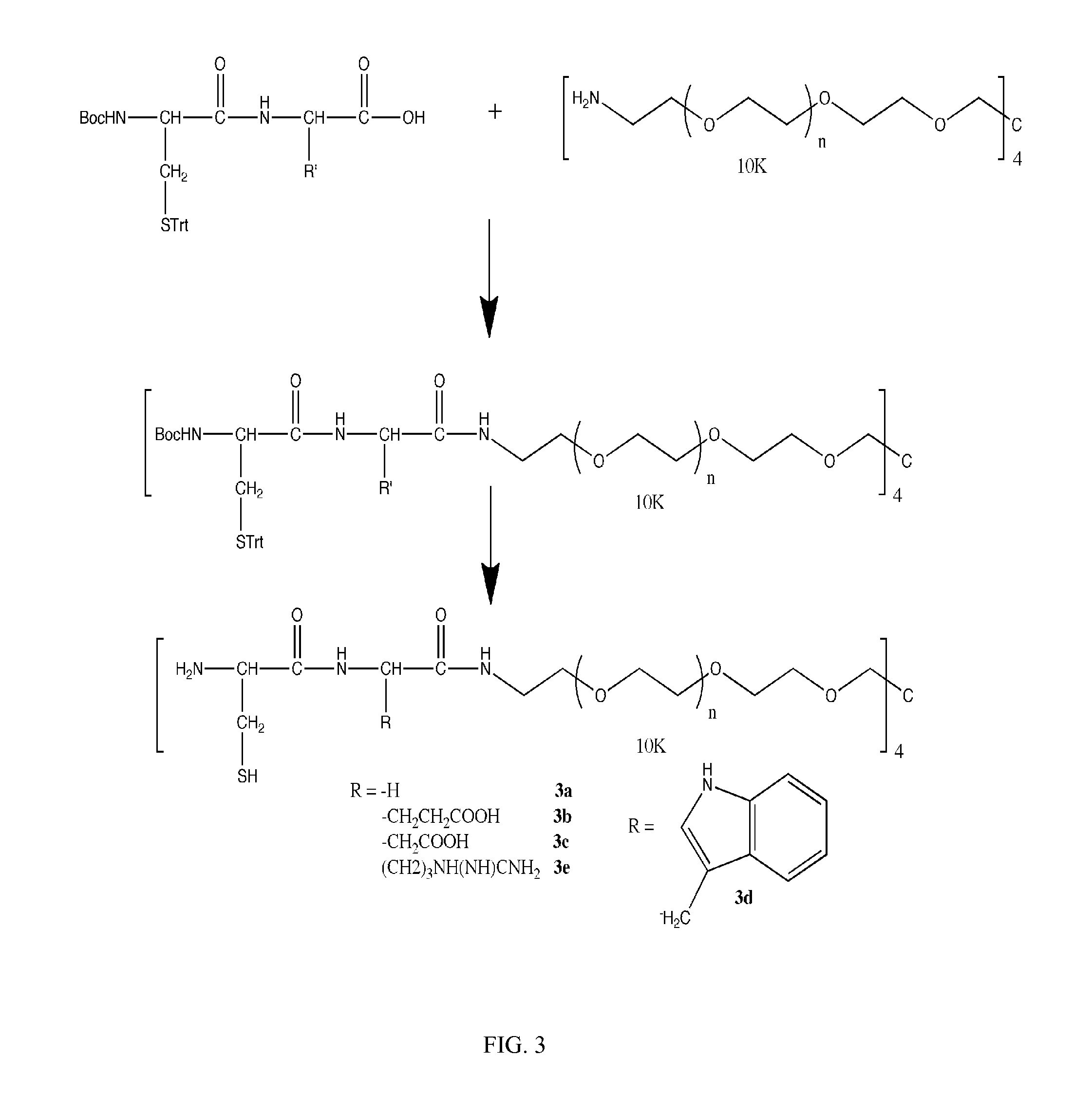
Method for preparing polyfunctionalized peptides and/or proteins via native chemical ligation
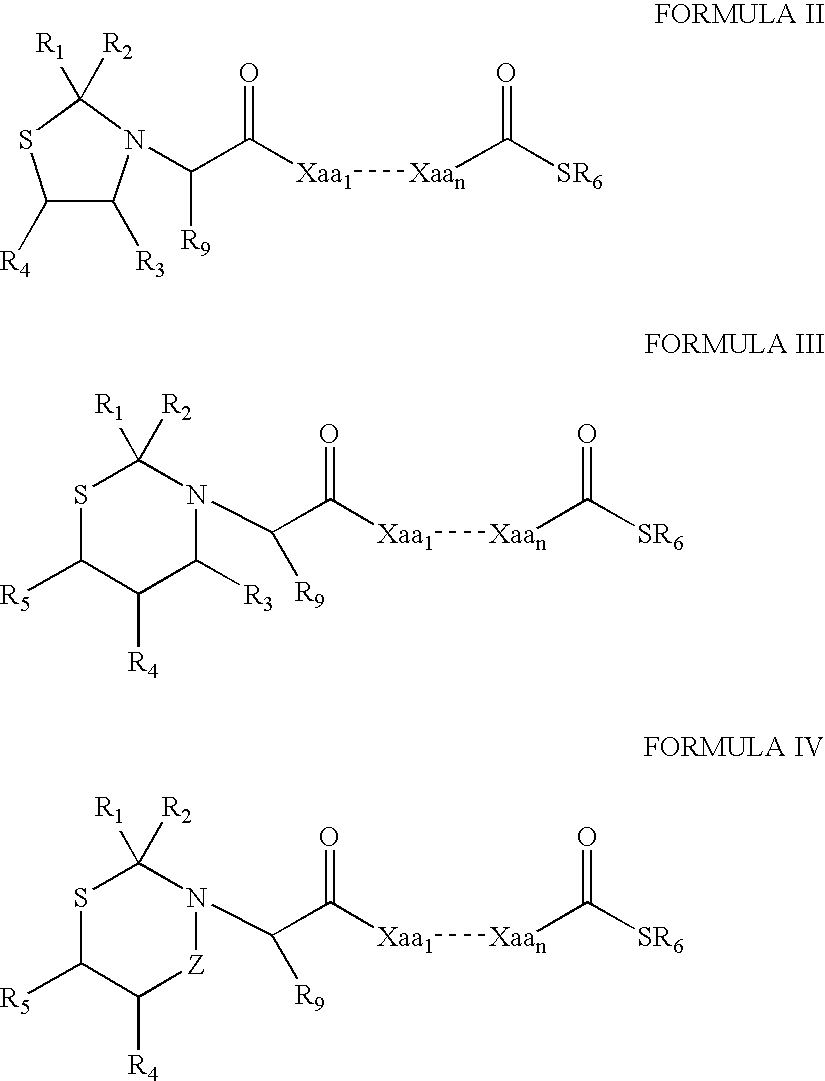
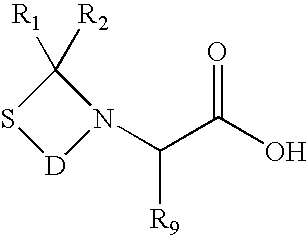
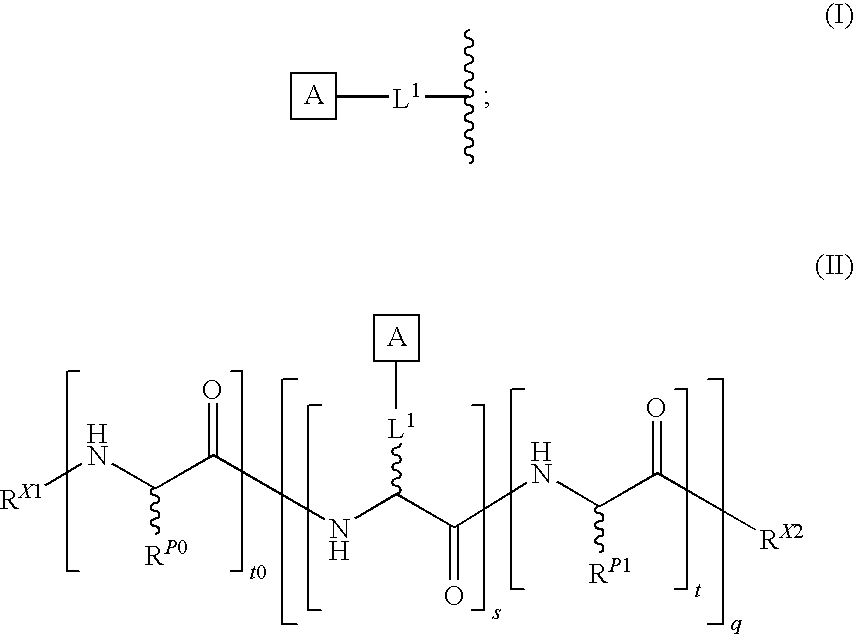
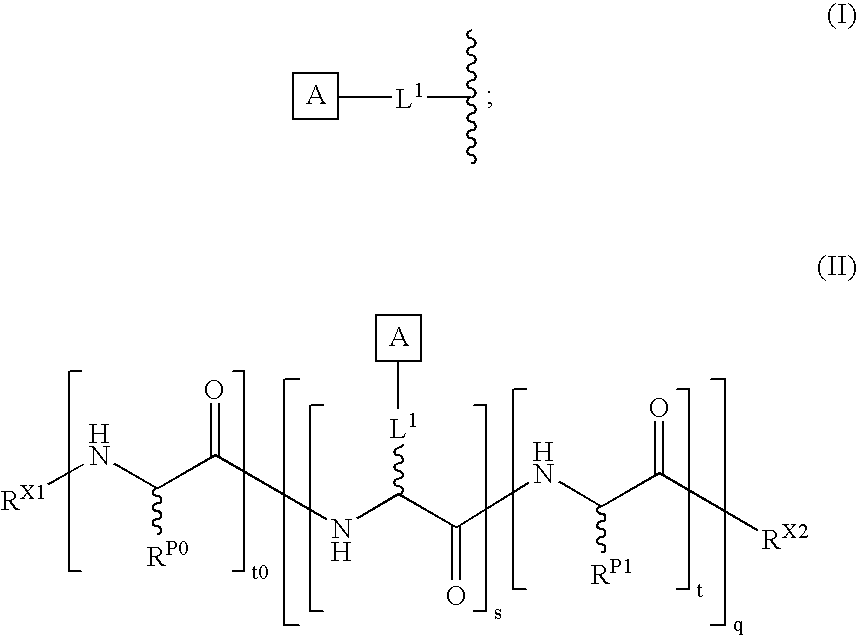
The present invention provides a method for preparing polyfunctionalized peptides and / or proteins at non-adjacent designated sites via native chemical ligation. In certain embodiments, the inventive method is a method for preparing a polyfunctionalized peptide comprising a peptidic backbone made up of four or more amino acids, wherein two or more non-adjacent amino acids are independently subsituted with a moiety having the structure: wherein A and L1 are as defined herein. In certain other embodiments, the inventive method allows the preparation of polyfunctionalized peptides having the general structure: wherein A, RP0, RP1, PX1, RX2, L1, to, s, t and q are as defined herein.
Owner:SLOAN KETTERING INST FOR CANCER RES
Method for preparing polyfunctionalized peptides and/or proteins via native chemical ligation
InactiveUS20070173636A1Reduce molecular weightSuitable for useVirus peptidesSaccharide peptide ingredientsChemical ligationAmino acid composition
The present invention provides a method for preparing polyfunctionalized peptides and / or proteins at non-adjacent designated sites via native chemical ligation. In certain embodiments, the inventive method is a method for preparing a polyfunctionalized peptide comprising a peptidic backbone made up of four or more amino acids, wherein two or more non-adjacent amino acids are independently subsituted with a moiety having the structure: wherein A and L1 are as defined herein. In certain other embodiments, the inventive method allows the preparation of polyfunctionalized peptides having the general structure: wherein A, RP0, RP1, PX1, RX2, L1, to, s, tand q are as defined herein.
Owner:SLOAN KETTERING INST FOR CANCER RES
Solid phase native chemical ligation of unprotected or N-terminal cysteine protected peptides in aqueous solution
InactiveUS7030217B2Easy to purifySynthesis fastBioreactor/fermenter combinationsBiological substance pretreatmentsESI mass spectrometryChemical ligation
Owner:AMYLIN PHARMA INC
Chemical peptide ligation with three or more components
InactiveUS7884182B2Improve efficiencyMethod can be usedPeptide/protein ingredientsDepsipeptidesChemical ligationPeptide fragment
The invention provides a method of assembling oligopeptide intermediates in a native chemical ligation reaction that eliminates self-ligation of bi-functional intermediates. An important aspect of the invention is a bi-functional intermediate with an N-terminal heterocyclic protecting group which effectively prevents self-ligation in the chemical assembly process. The present invention is useful in methods for convergent synthesis of polypeptides and proteins and improves the efficiency of native chemical ligation reactions, particularly where three or more peptide fragments are used to assemble a polypeptide or protein product.
Owner:ASTRAZENECA PHARMA LP
Chemical Modification of Proteins
InactiveUS20110144304A1Easy to useReadily crystalisesPeptide/protein ingredientsPeptide sourcesSelenocysteineDehydroalanine
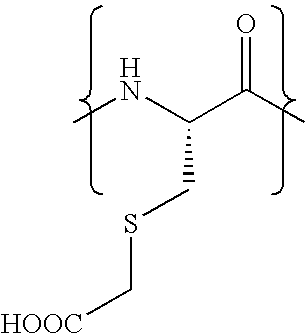
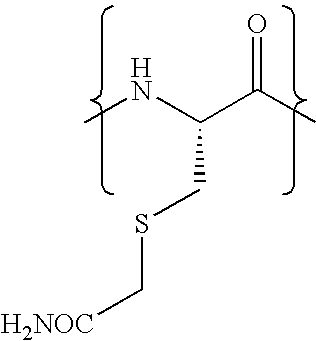
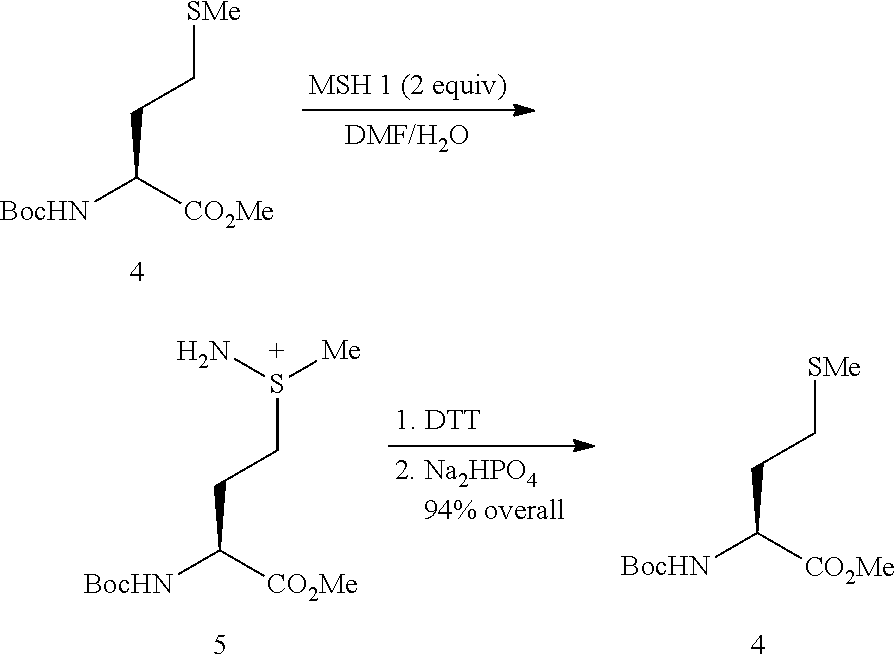
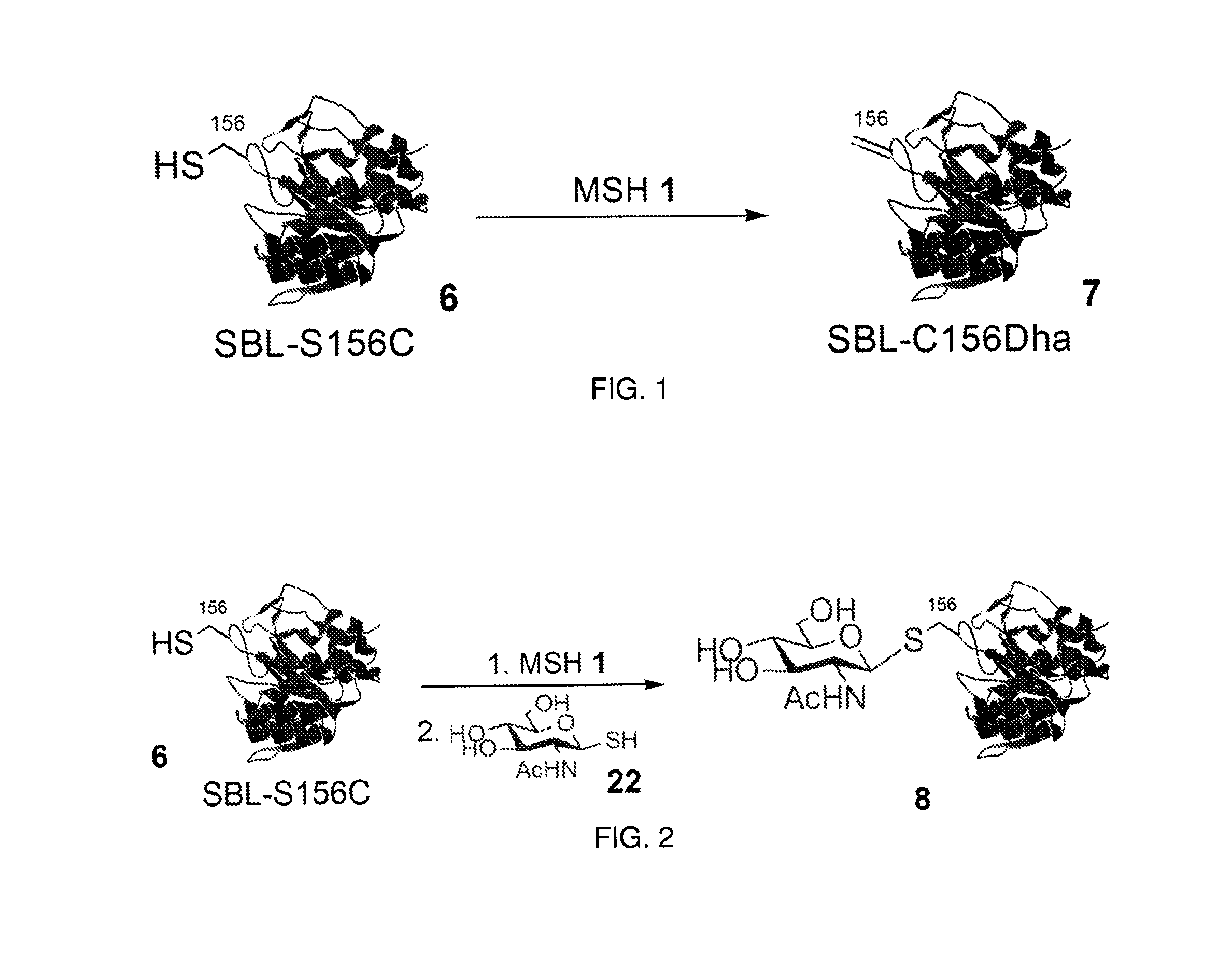
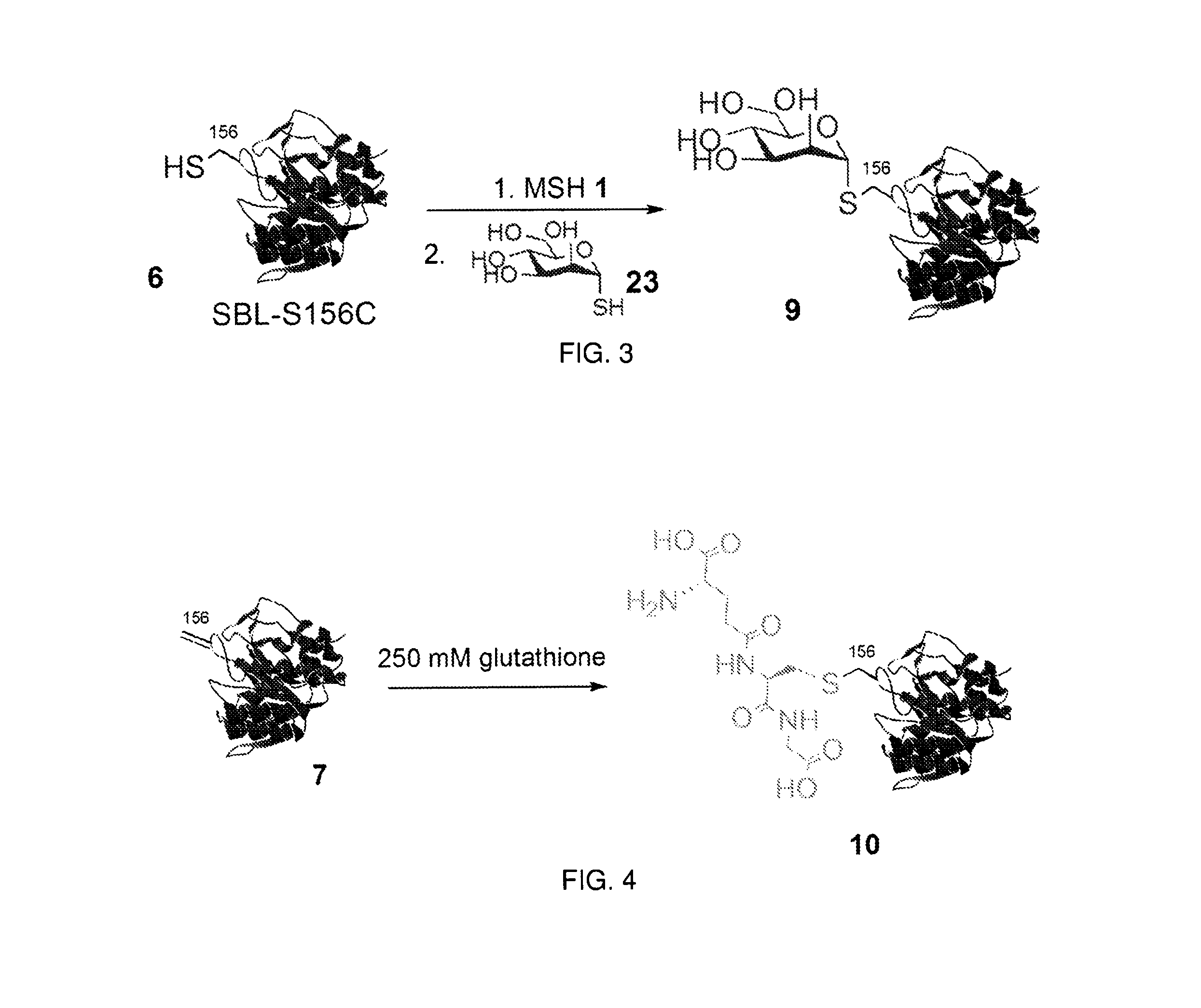
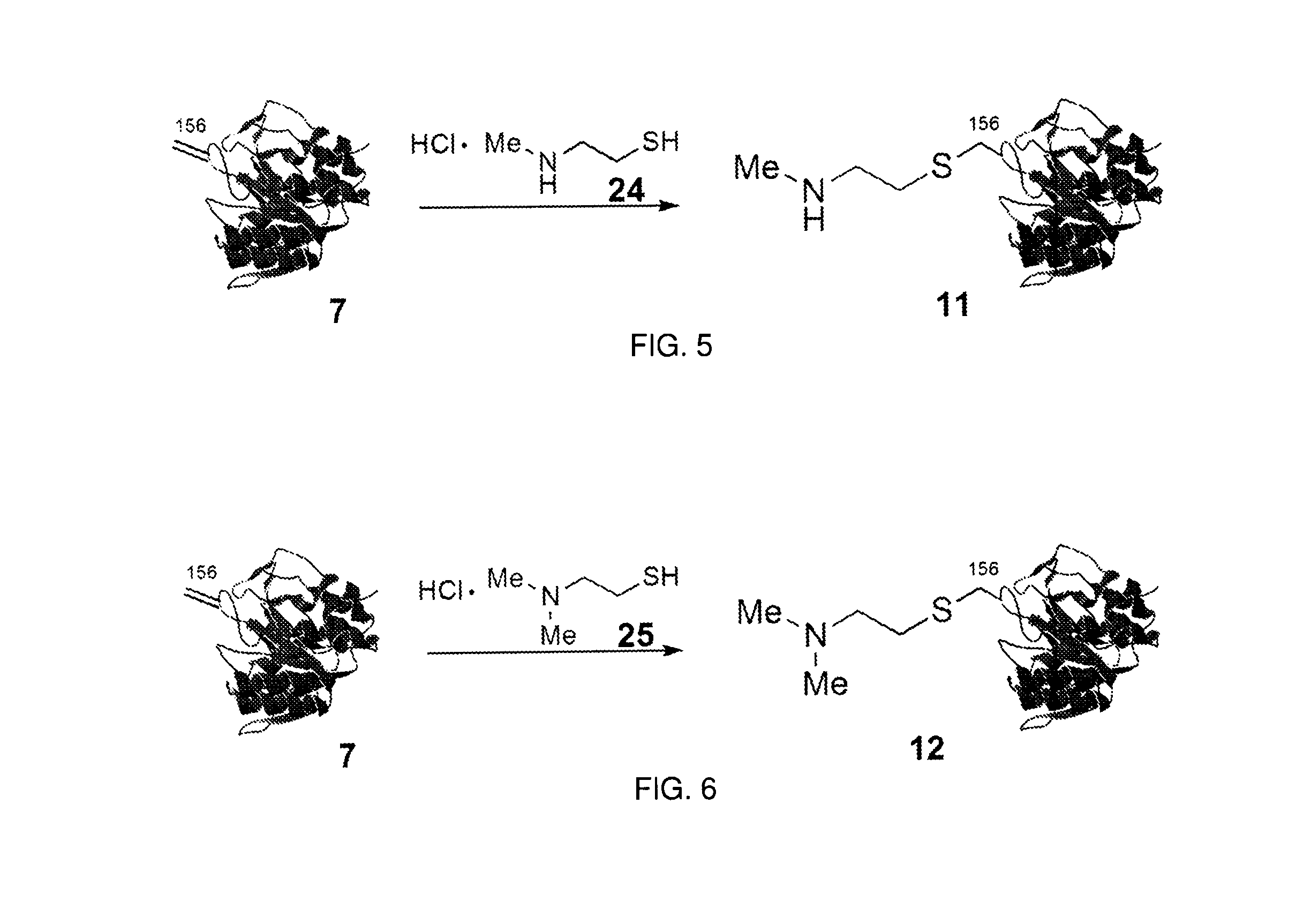
The invention relates to methods for selectively converting a cysteine residue in a peptide or protein to the dehydroalanine (Dha) residue. The method also works on selenocysteine and substituted cysteine and selenocysteine residues, resulting in the Dha residue which may be converted to any natural or unnatural amino acid residue desired without the alteration of the remainder of the peptide or protein. The invention also allows ligation of a desired peptide at any point rather than at a point where there should be a naturally occurring cysteine, thereby allowing native chemical ligation to be used in the synthesis of peptides that do not contain cysteine. The methodology allows for the synthesis of very large peptides.
Owner:DAVIS BENJAMIN G +1
Synthetic method of polypeptide hydrazide containing cysteine residues and application of synthetic method
ActiveCN112457372AShorten the lengthImprove solubilityAntibody mimetics/scaffoldsPeptide preparation methodsSide chainCombinatorial chemistry
The invention provides a synthetic method of polypeptide hydrazide containing cysteine residues and an application of the synthetic method. According to the synthetic method, a tetracysteine label isinserted into the C end of a target polypeptide, and a double-arsenic fluorescent dye labeling reagent is used for specific recognition and binding of the tetracysteine label sequence. Cysteine sulfydryl side chains in the target polypeptide are protected by Pac groups, then the double-arsenic fluorescent dye labeling reagent is removed, and cyano hydrazinolysis on the tetracysteine label sequenceis conducted to obtain the polypeptide hydrazide containing cysteine. The synthetic method is helpful for perfecting native chemical ligation reactions and more effectively synthesizing proteins.
Owner:SOUTH CHINA UNIV OF TECH
Detection of chemical ligation using fluorescence quenching leaving groups
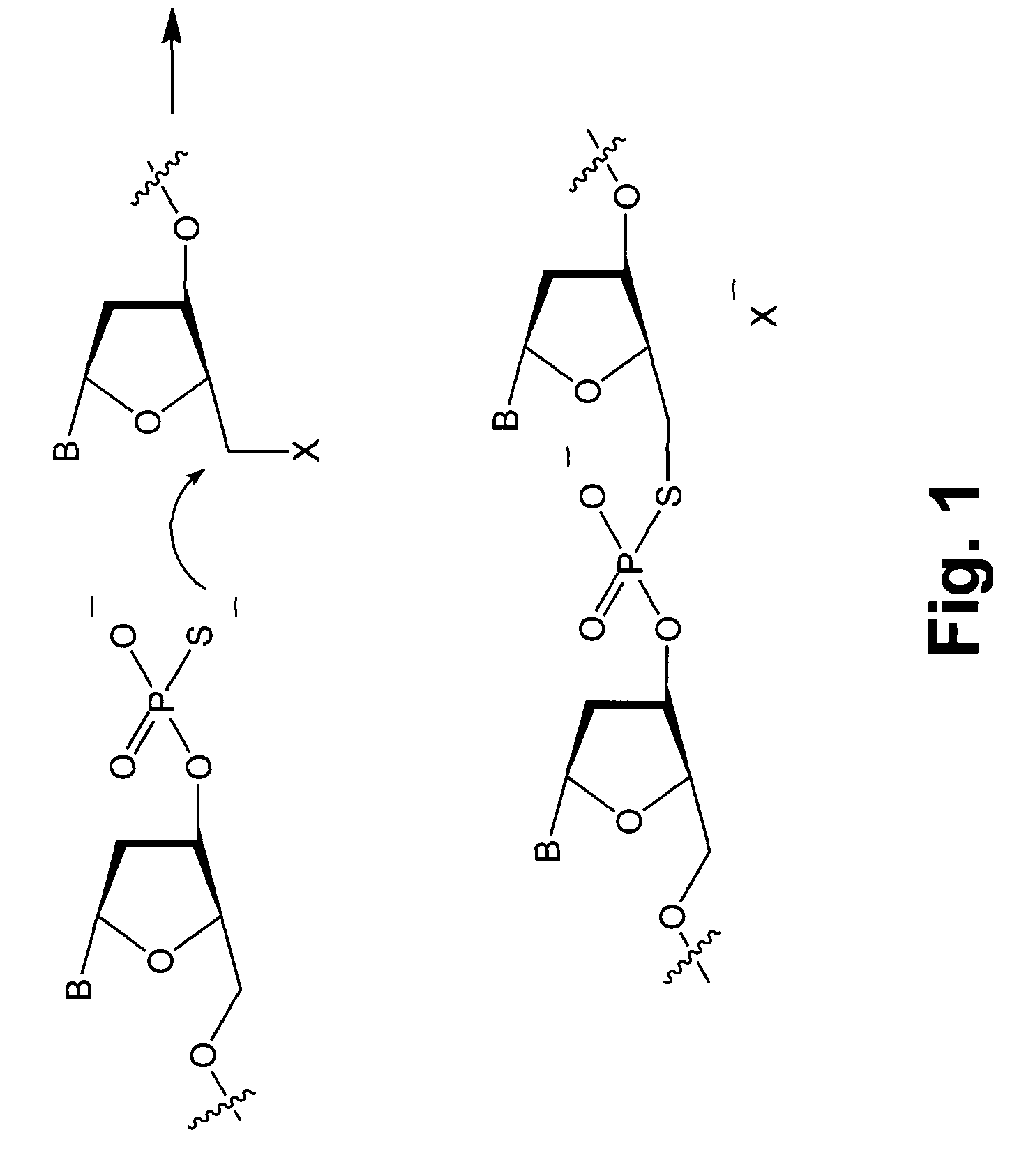
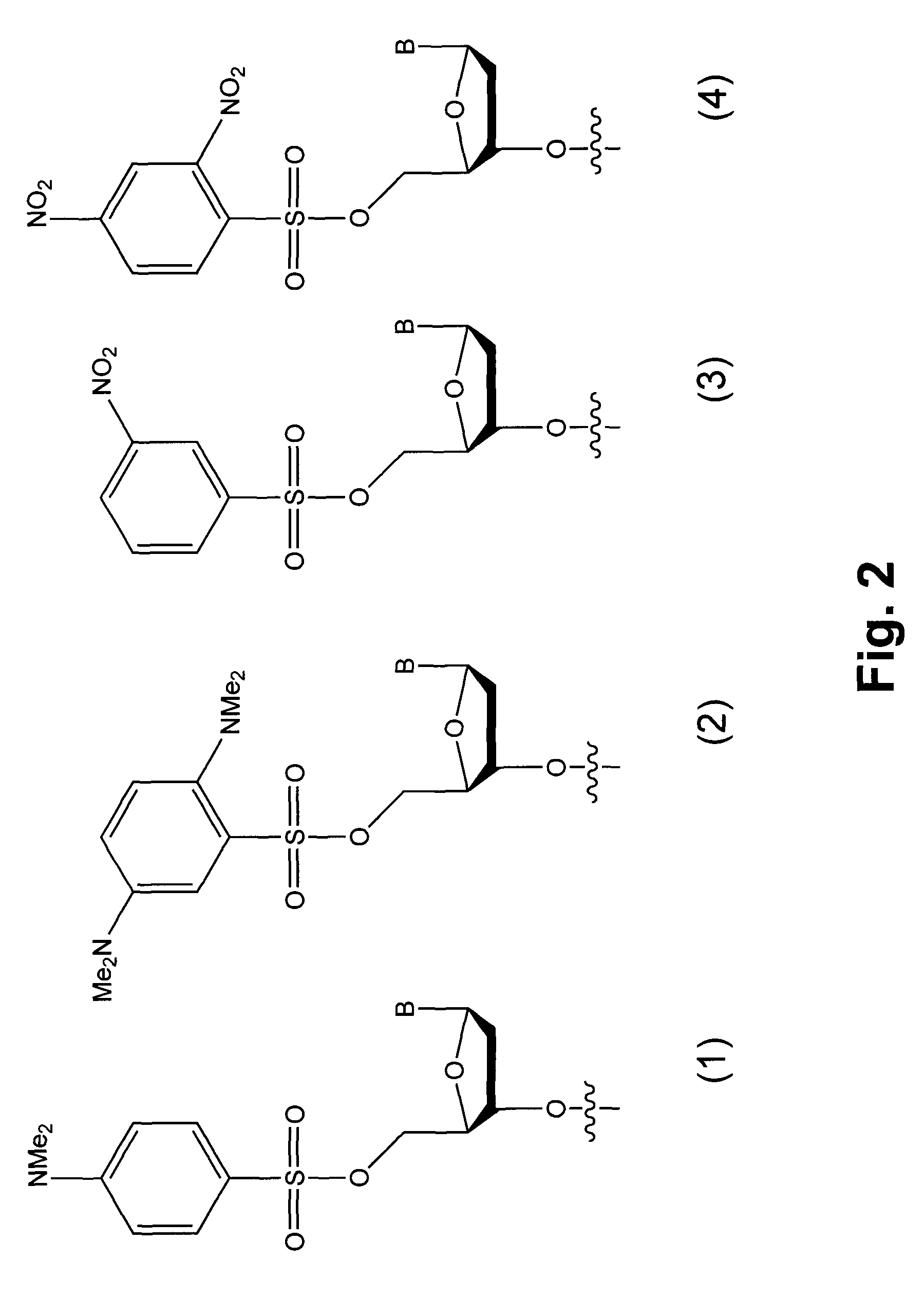
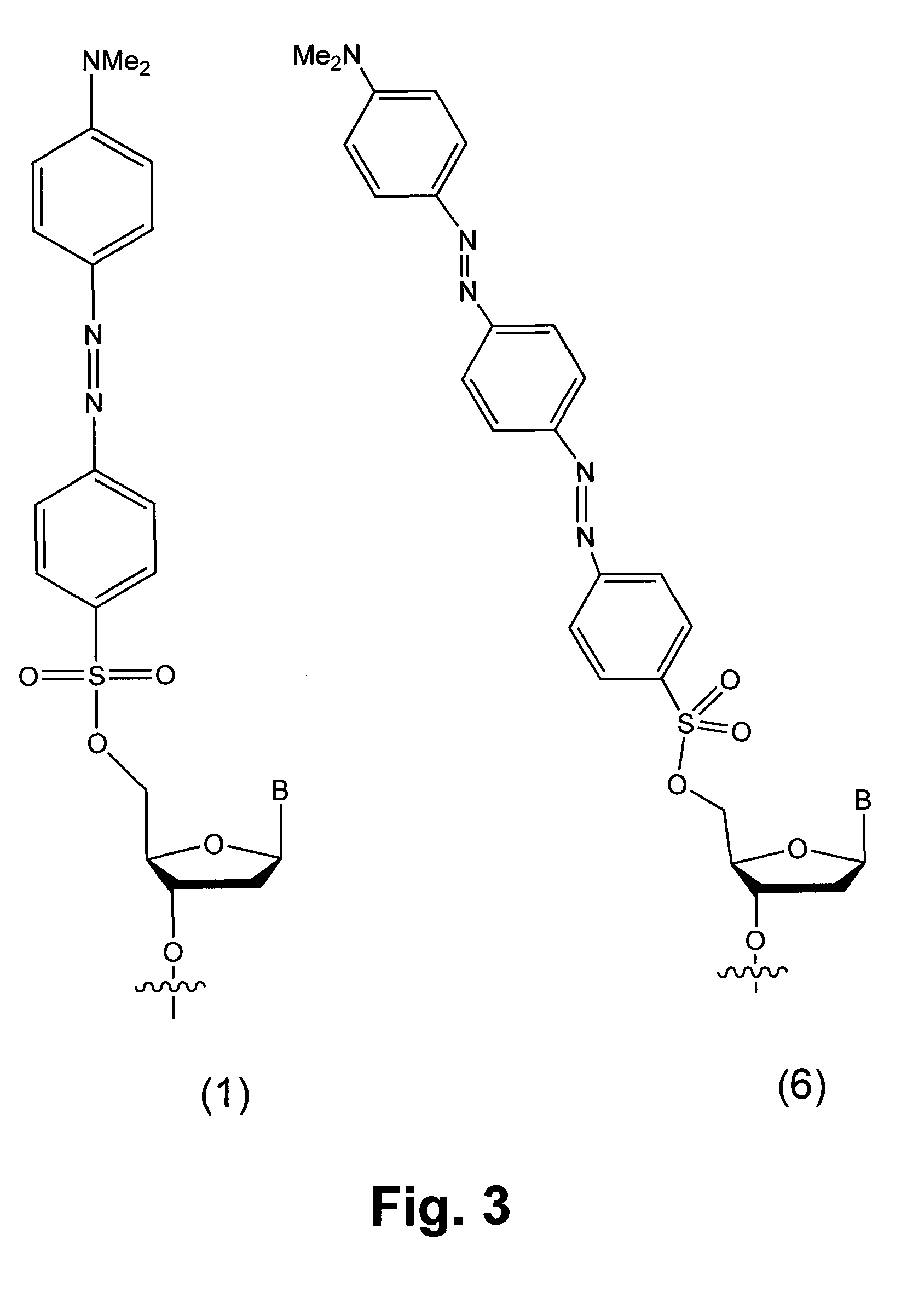
InactiveUS7749699B2Sugar derivativesMicrobiological testing/measurementChemical ligationLeaving group
Novel compounds having a fluorescence quencher as a leaving group are disclosed. Nucleic acids and other molecules containing a fluorophore and a fluorescence quencher are disclosed as an embodiment of this invention. The use of the oligonucleotides in enzyme-free oligonucleotide ligation reactions results in an increase in fluorescence when the oligonucleotide also contains a nearby fluorophore. The ligation reactions can be performed in solution, on surfaces, or in cells.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
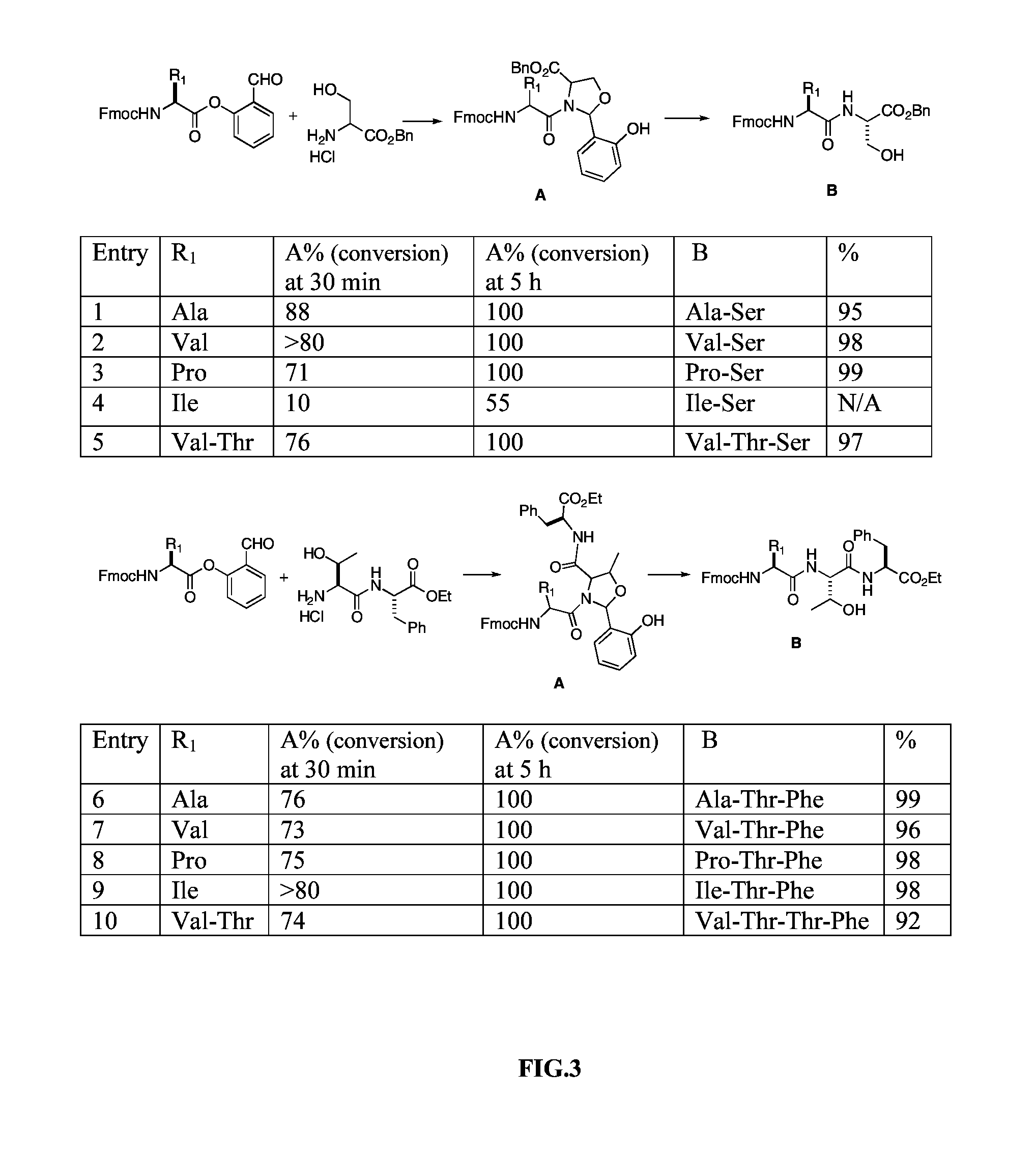
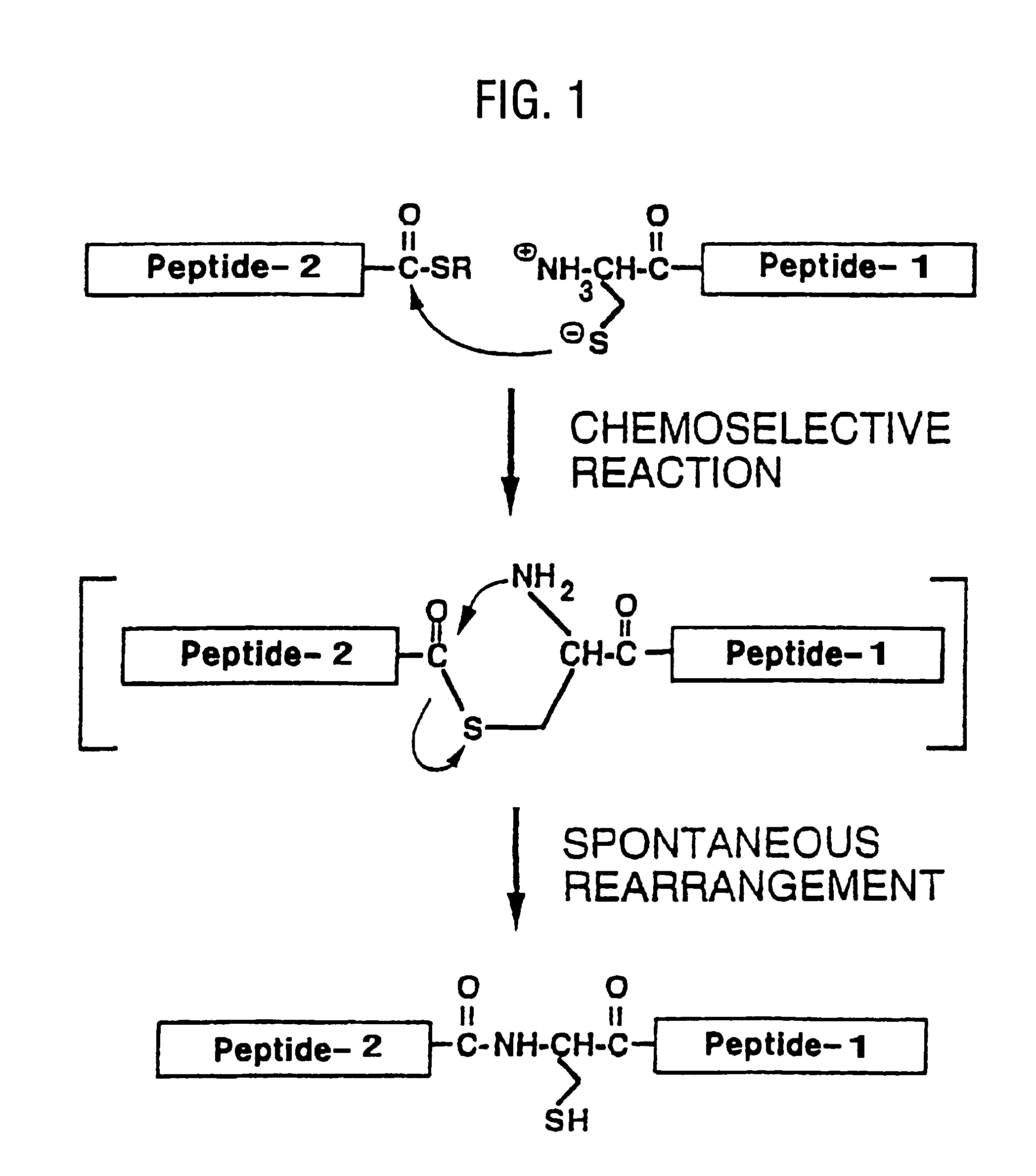
Native chemical ligation at serine and threonine sites
ActiveUS8859730B2Easy to operateQuick conversionPeptide-nucleic acidsPeptide/protein ingredientsChemical ligationThreonine
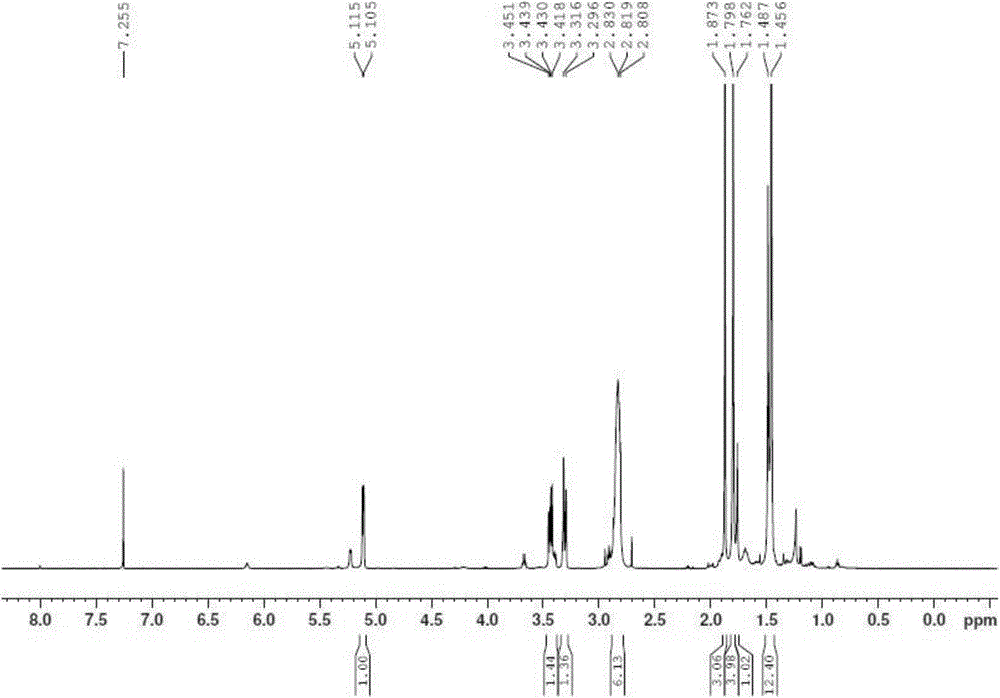
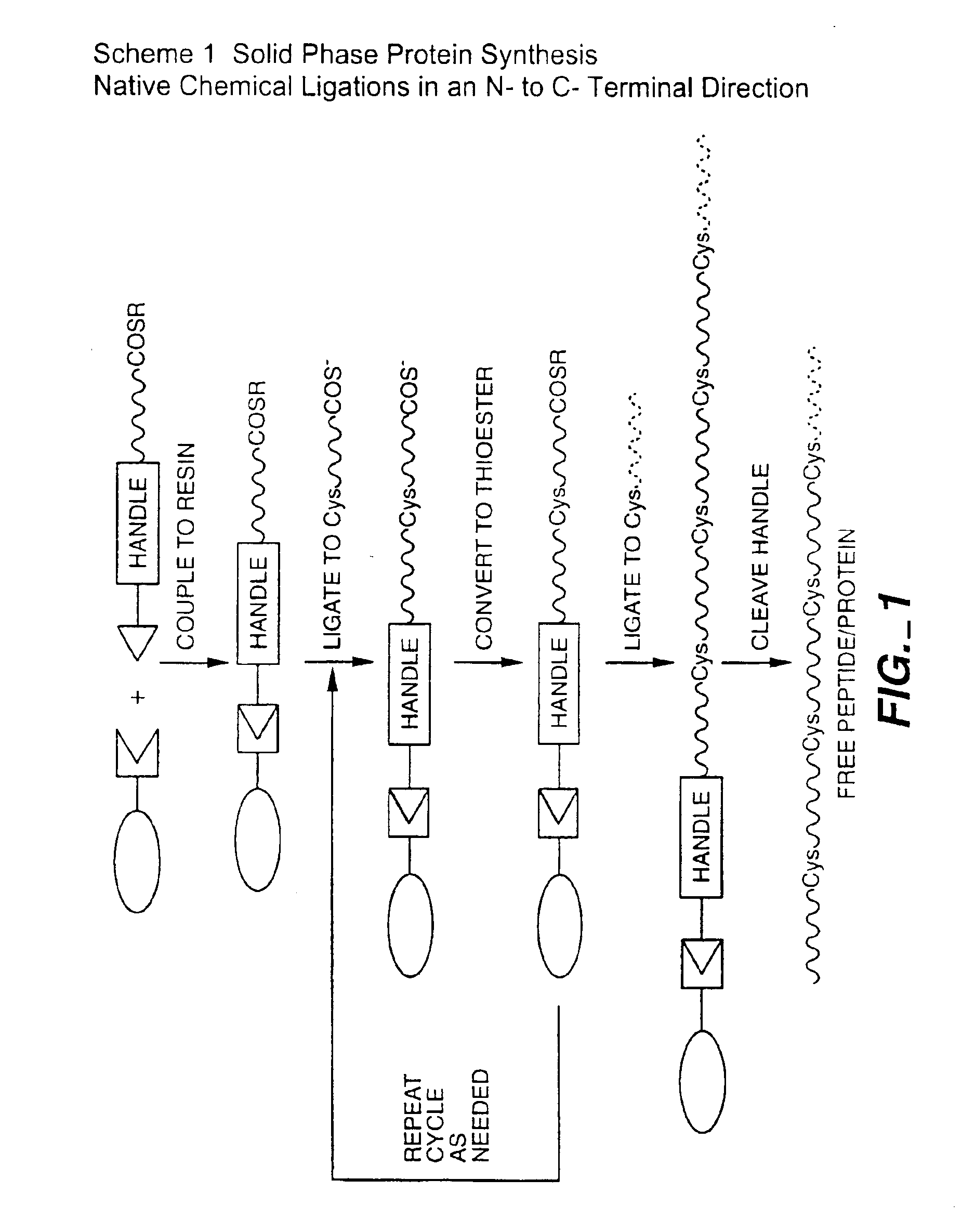
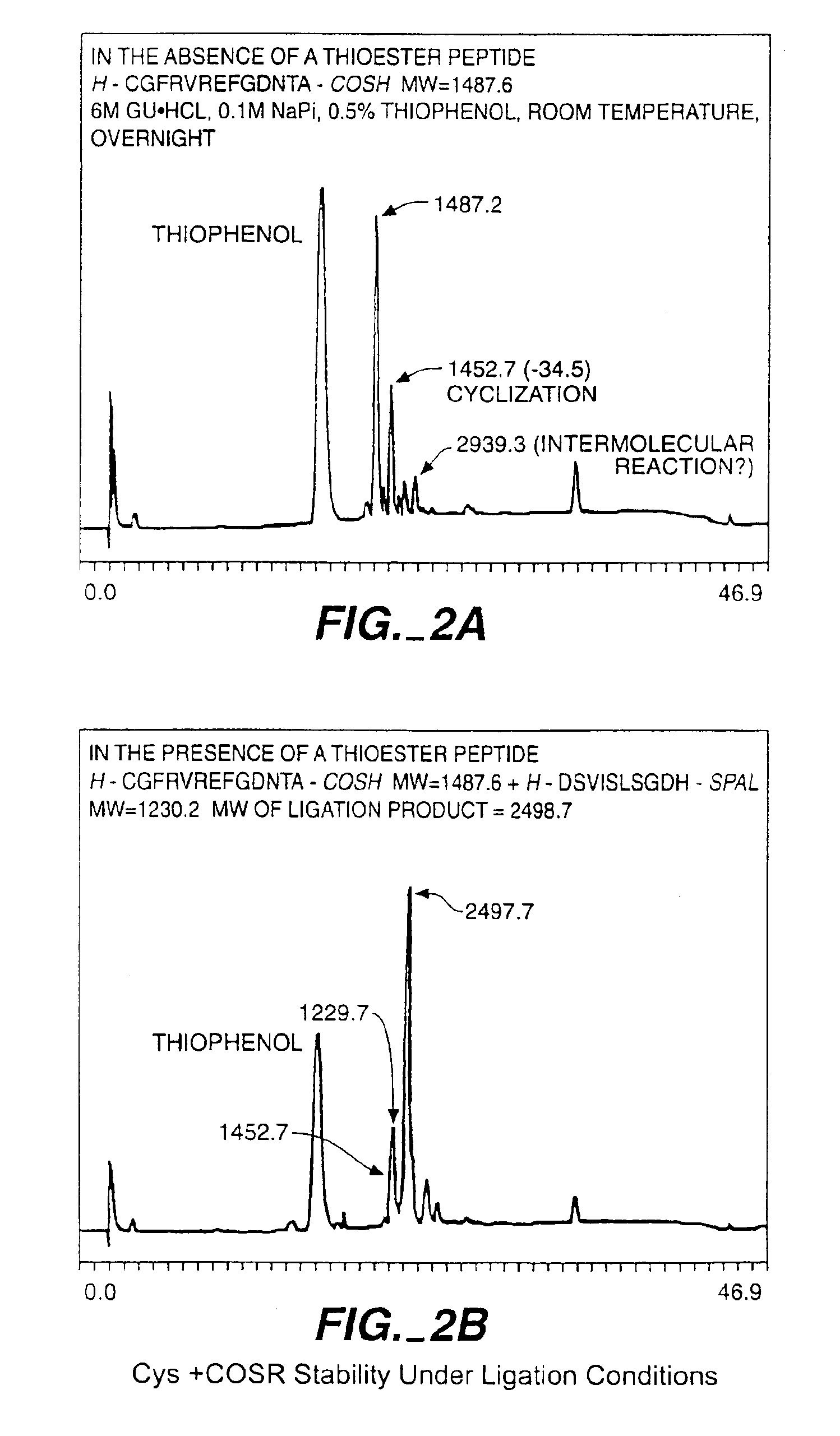
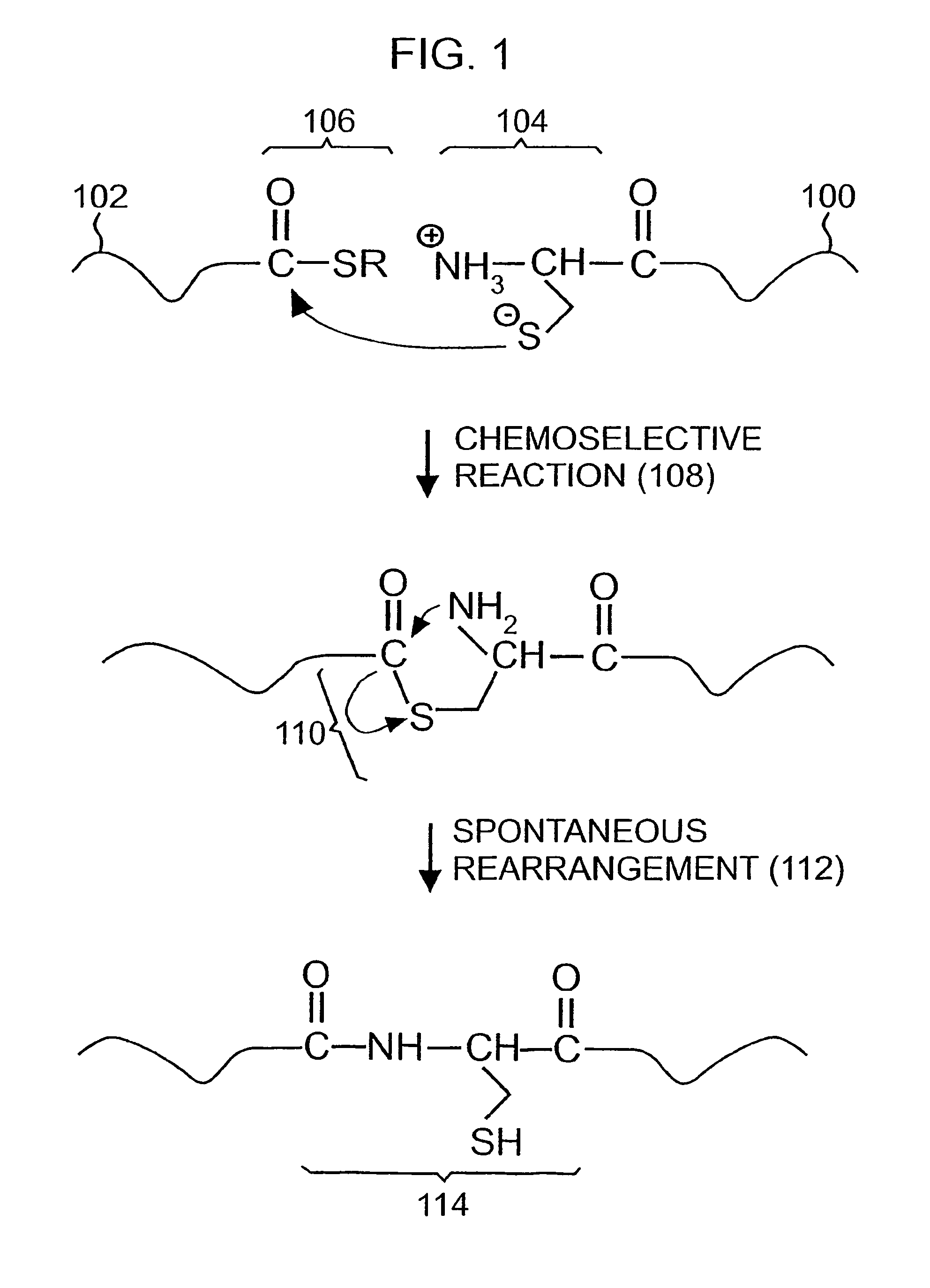
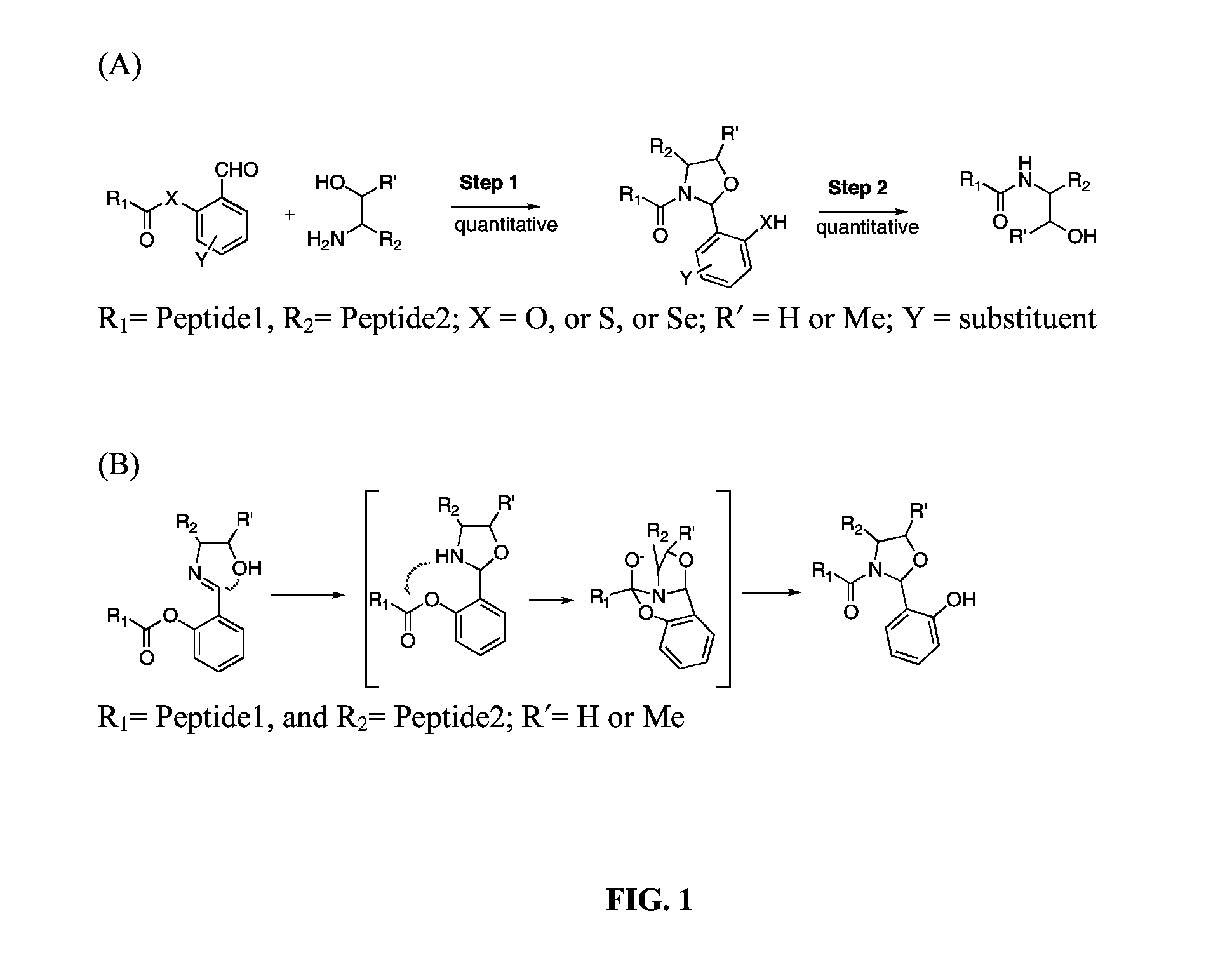
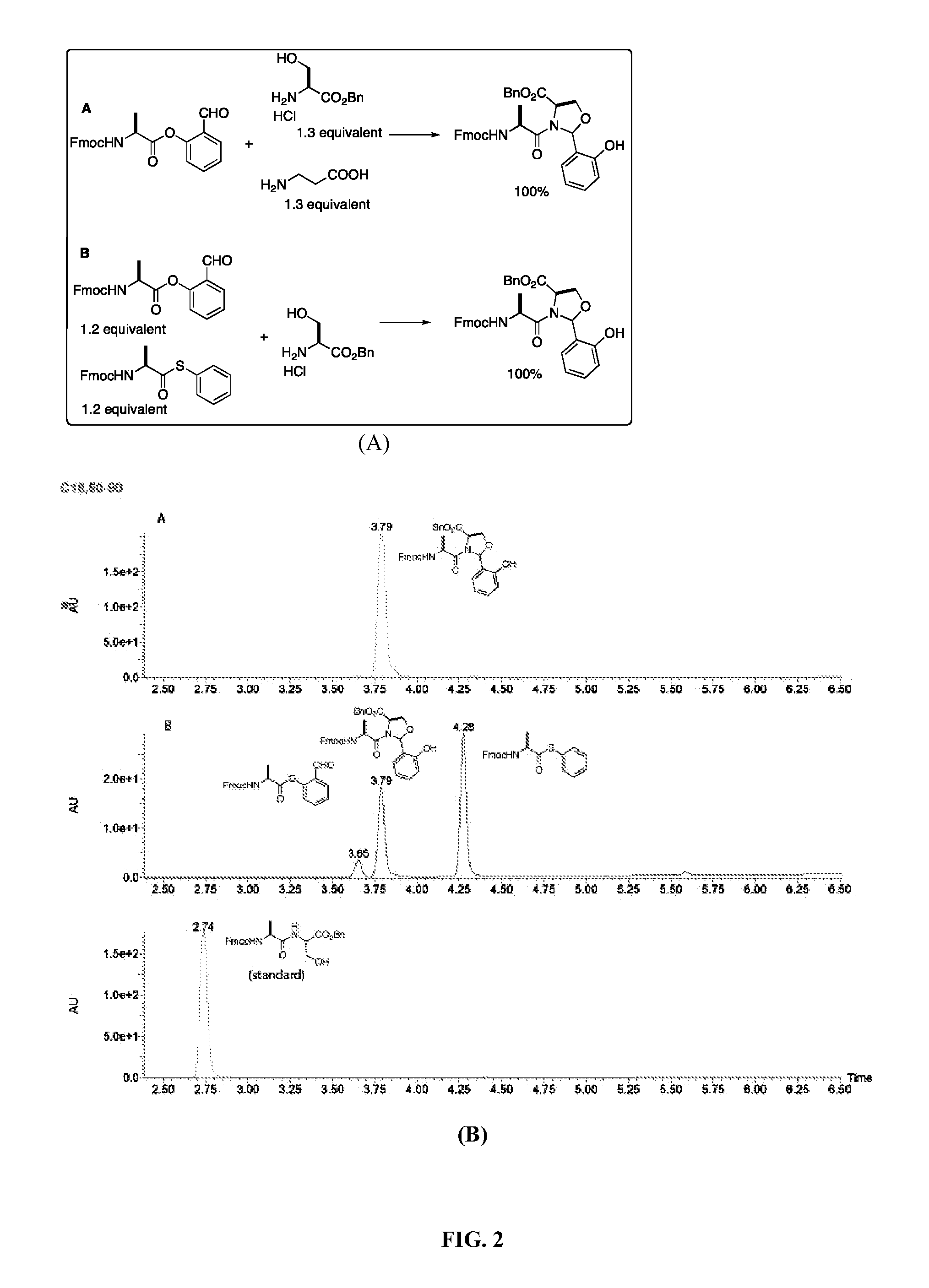
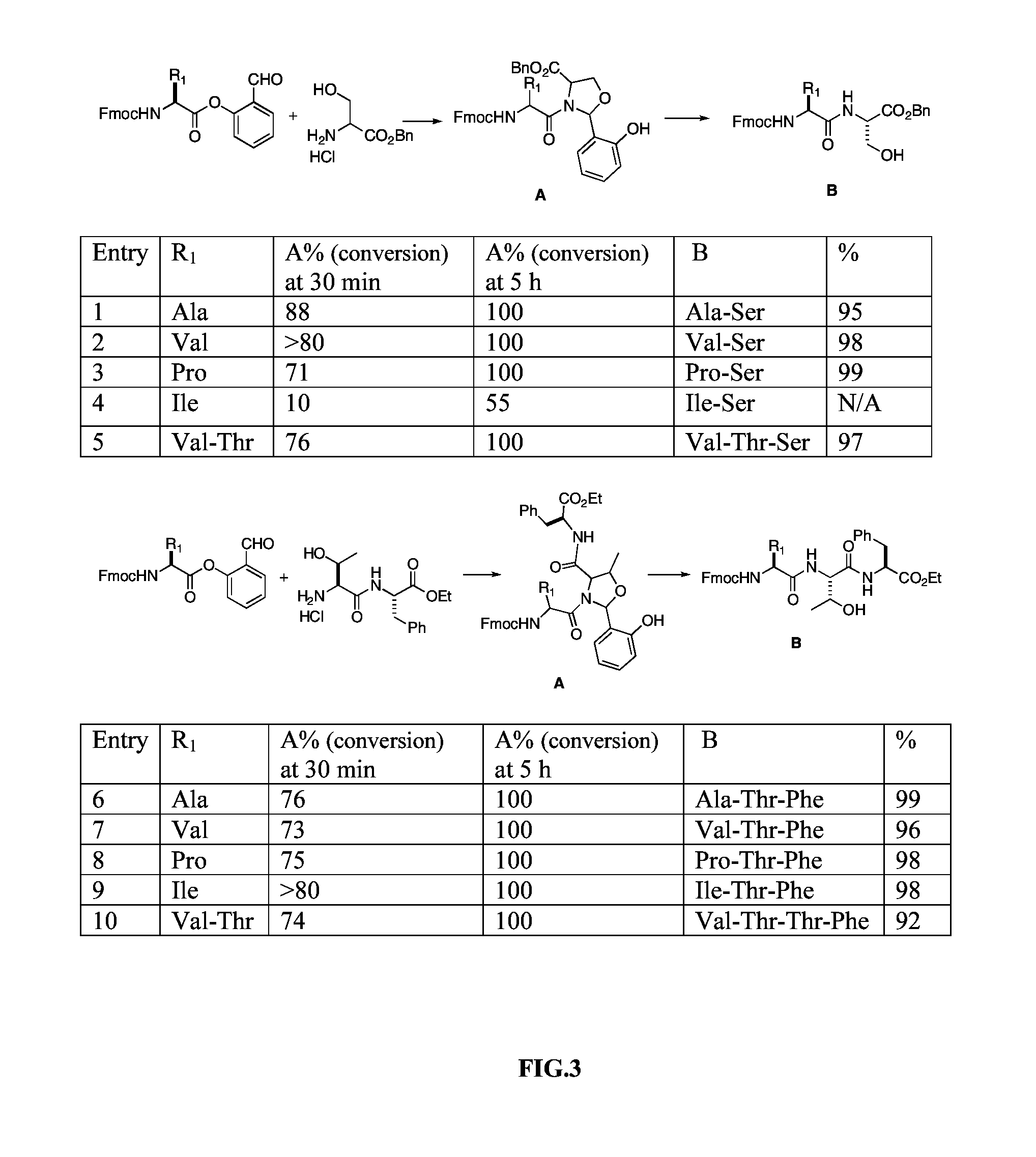
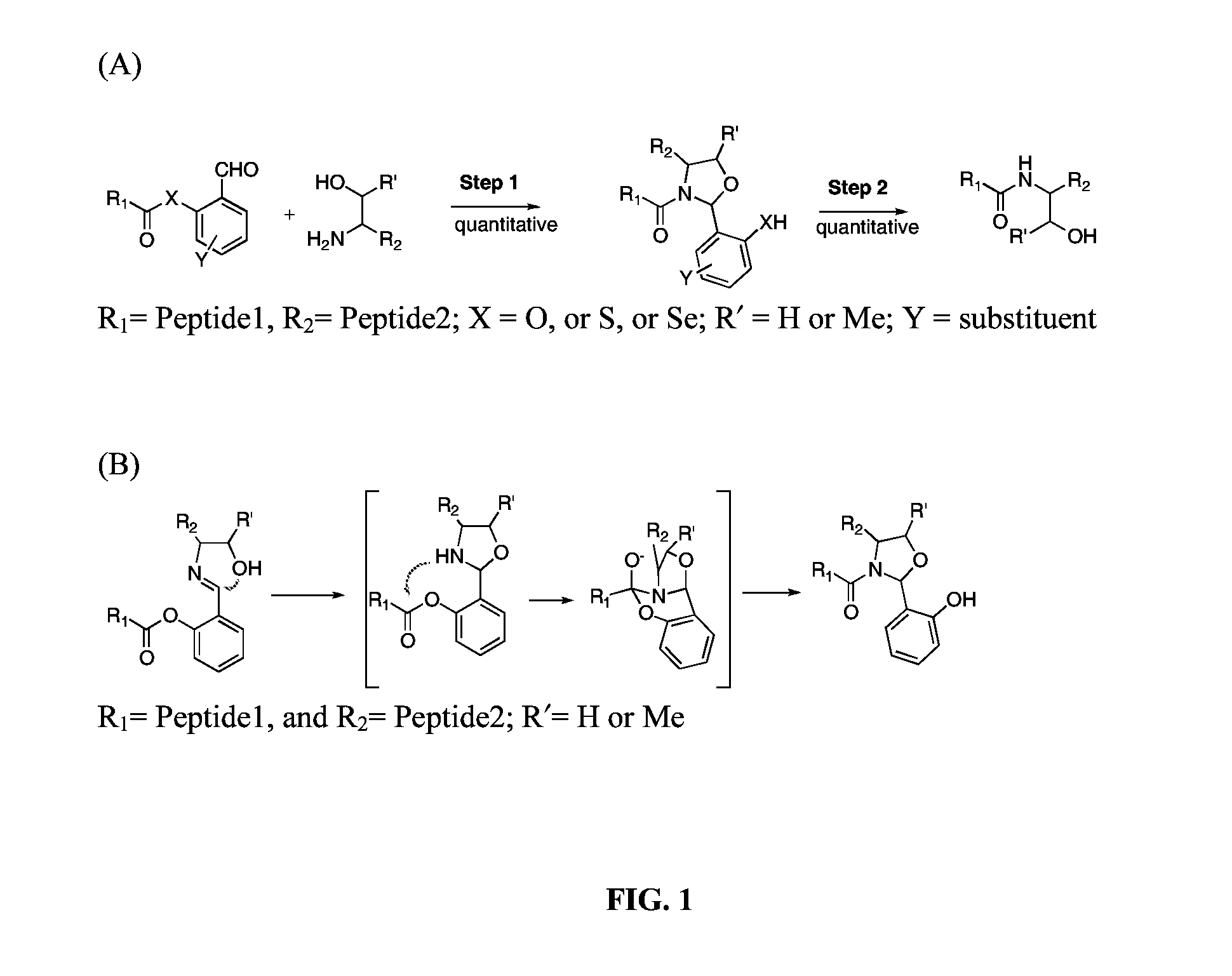
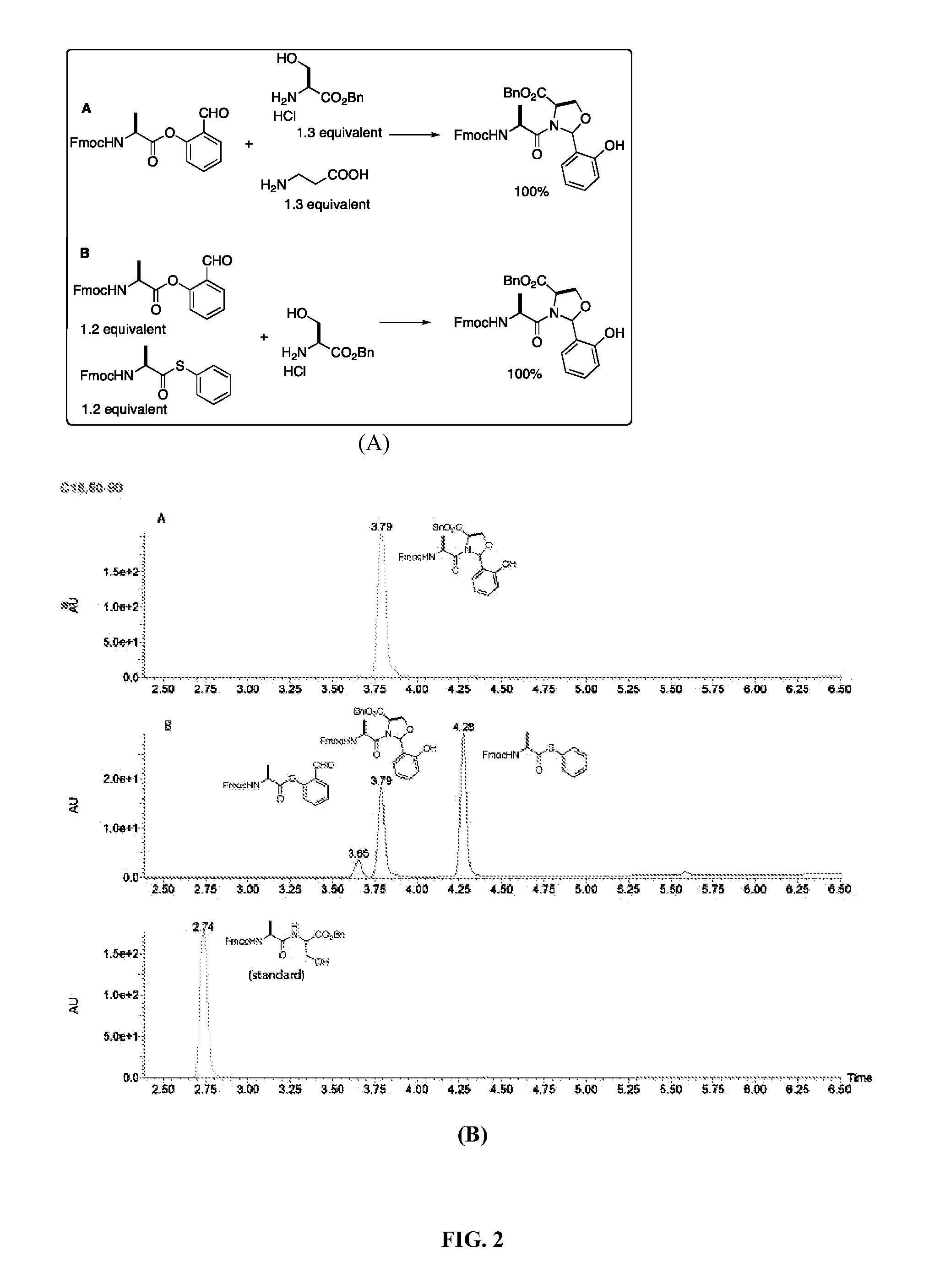
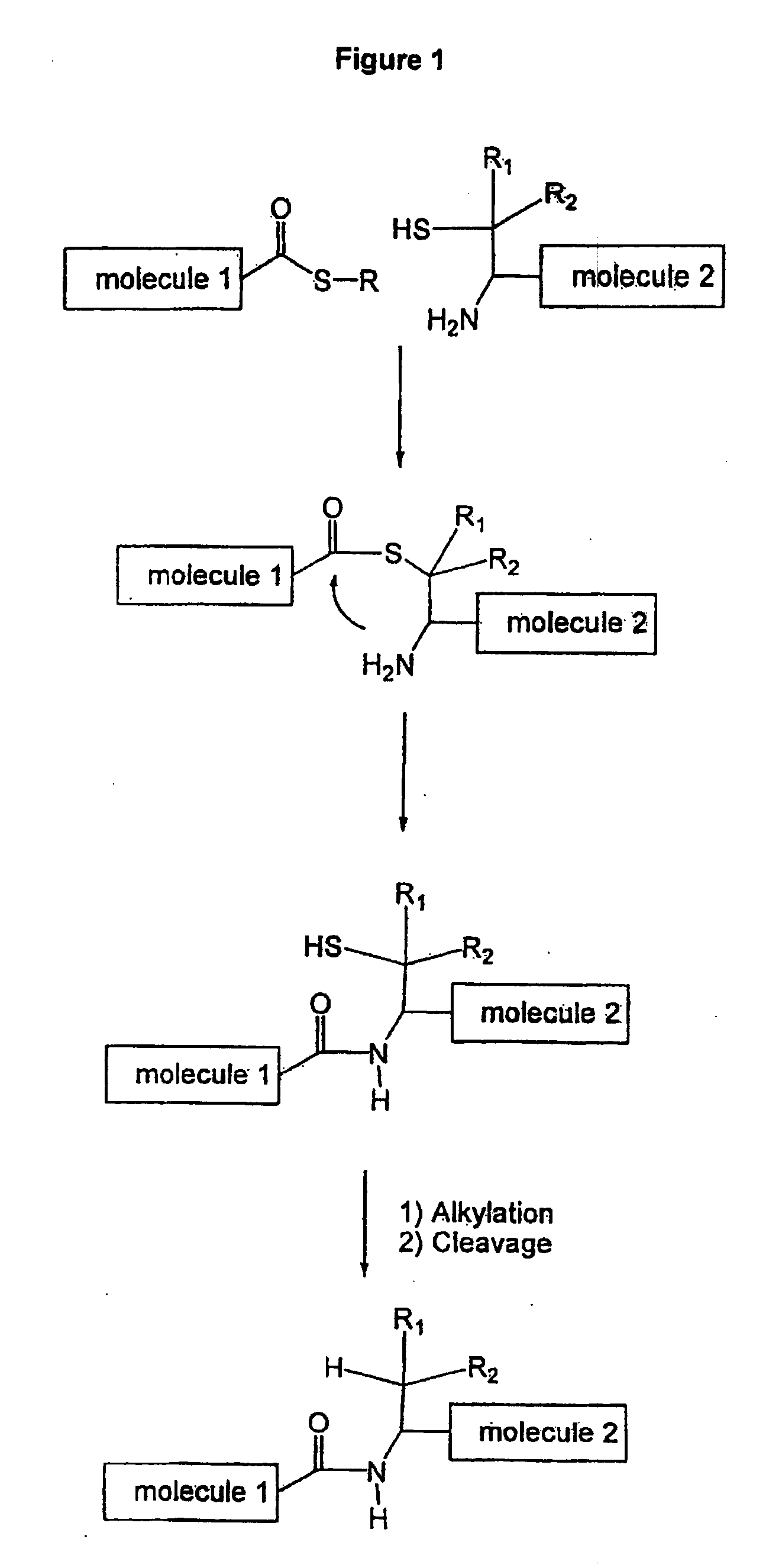
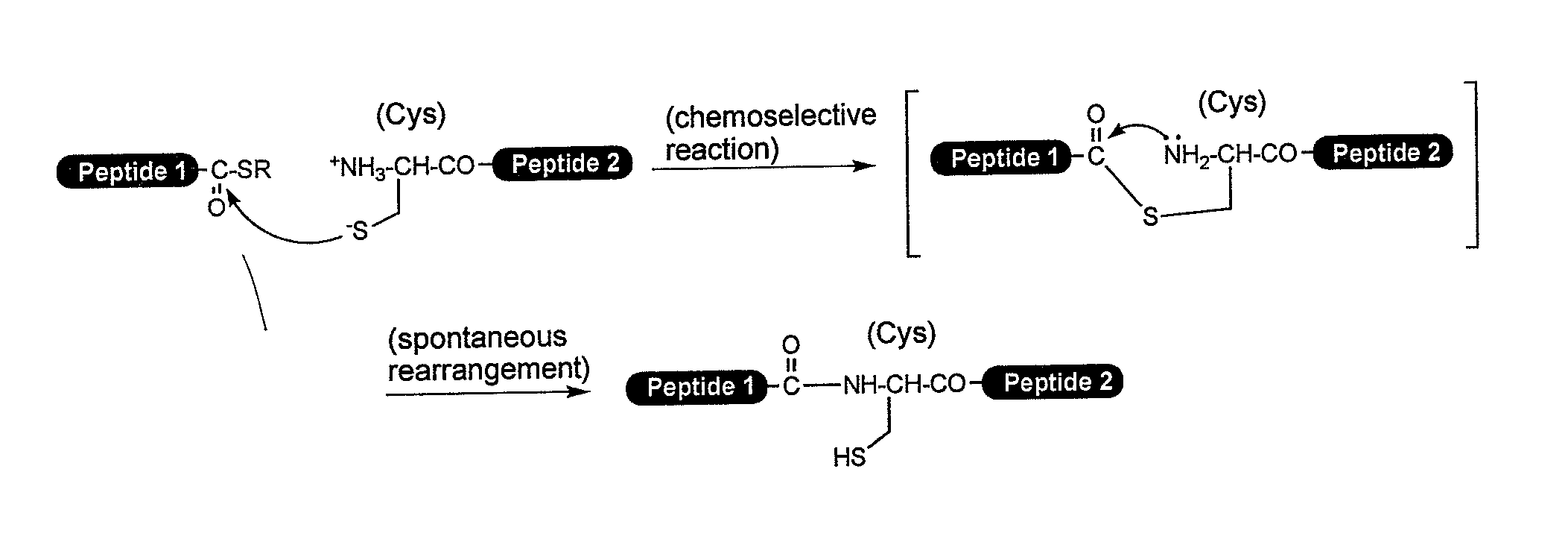
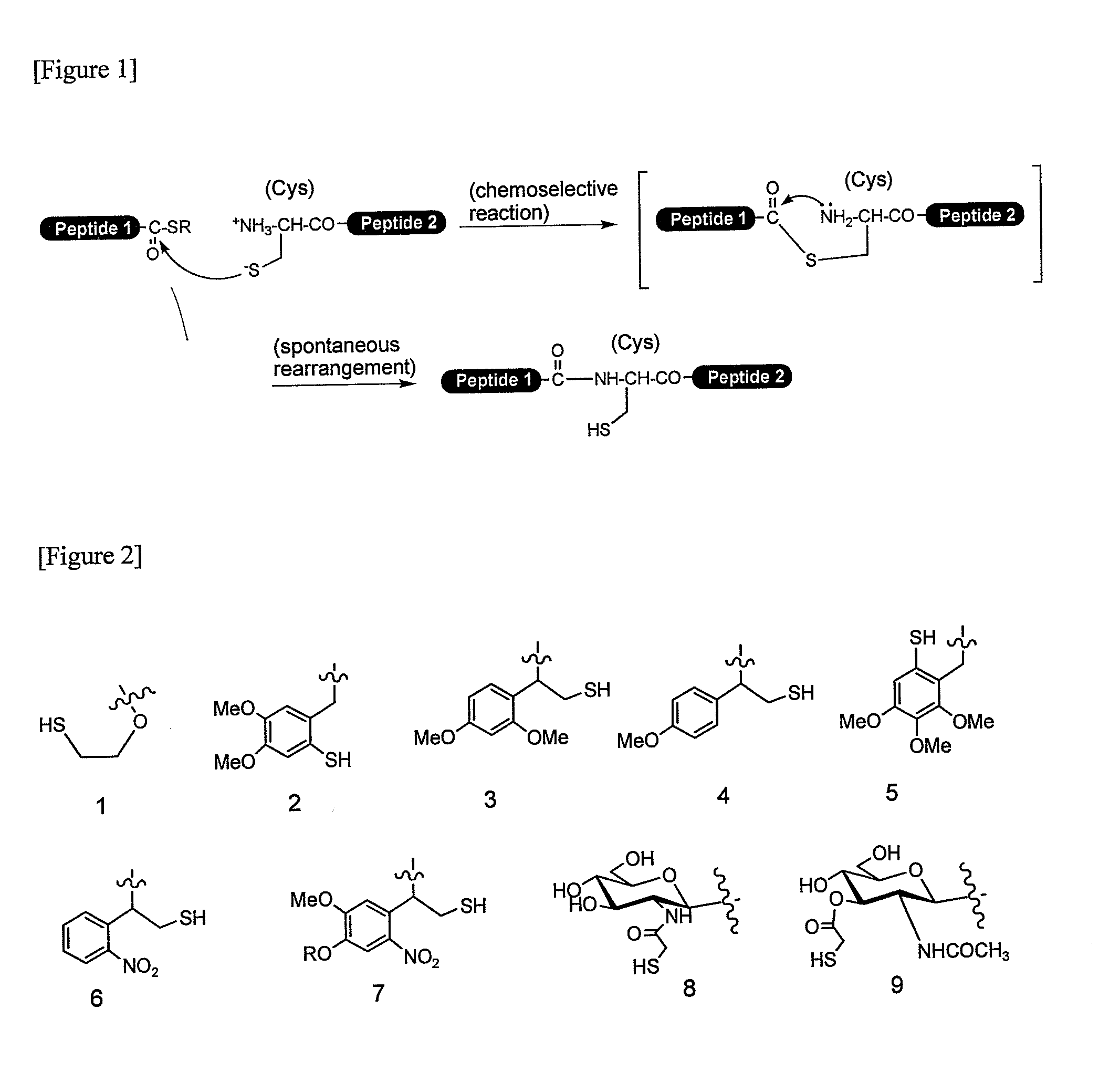
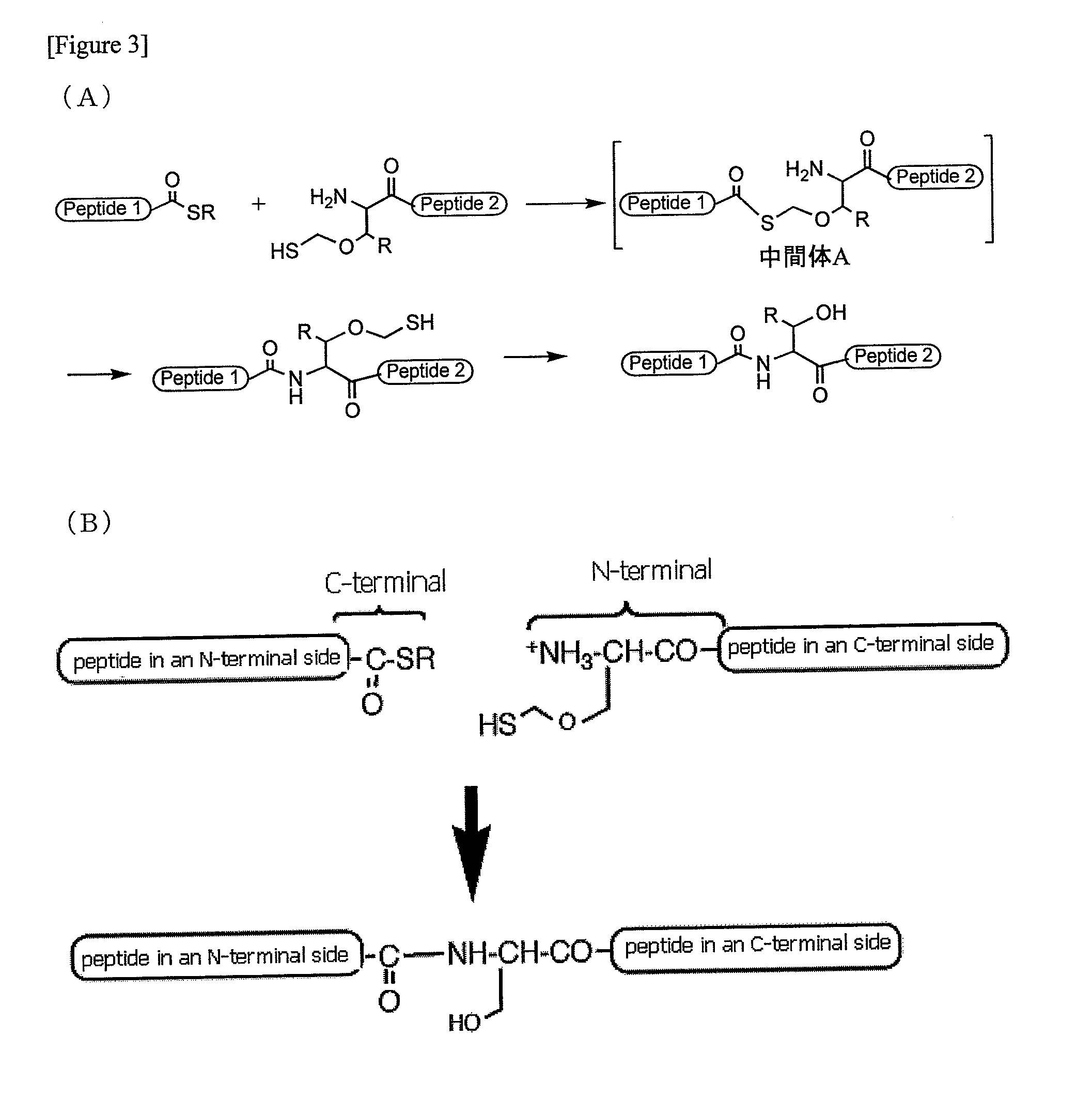
A chemoselective chemical ligation method is disclosed. The method joins two peptide segments efficiently to produce a larger peptide or protein, by generating a natural peptide bond (Xaa-Ser and Xaa-Thr) at the ligation site (Xaa represents any 5 amino acid). The method requires two steps (FIG. 1 (a)): a) reacting the starting peptide(s) to form an acetal intermediate with an acetal group at the ligation site; b) converting said acetal intermediate to a desired peptide or protein with said natural peptide bond.
Owner:SHENZHEN PEPBIOTIC CO LTD
Native Chemical Ligation at Serine and Threonine Sites
ActiveUS20120253011A1Easy to operateQuick conversionPeptide-nucleic acidsPeptide/protein ingredientsChemical ligationThreonine
A chemoselective chemical ligation method is disclosed. The method joins two peptide segments efficiently to produce a larger peptide or protein, by generating a natural peptide bond (Xaa-Ser and Xaa-Thr) at the ligation site (Xaa represents any 5 amino acid). The method requires two steps (FIG. 1 (a)): a) reacting the starting peptide(s) to form an acetal intermediate with an acetal group at the ligation site; b) converting said acetal intermediate to a desired peptide or protein with said natural peptide bond.
Owner:SHENZHEN PEPBIOTIC CO LTD
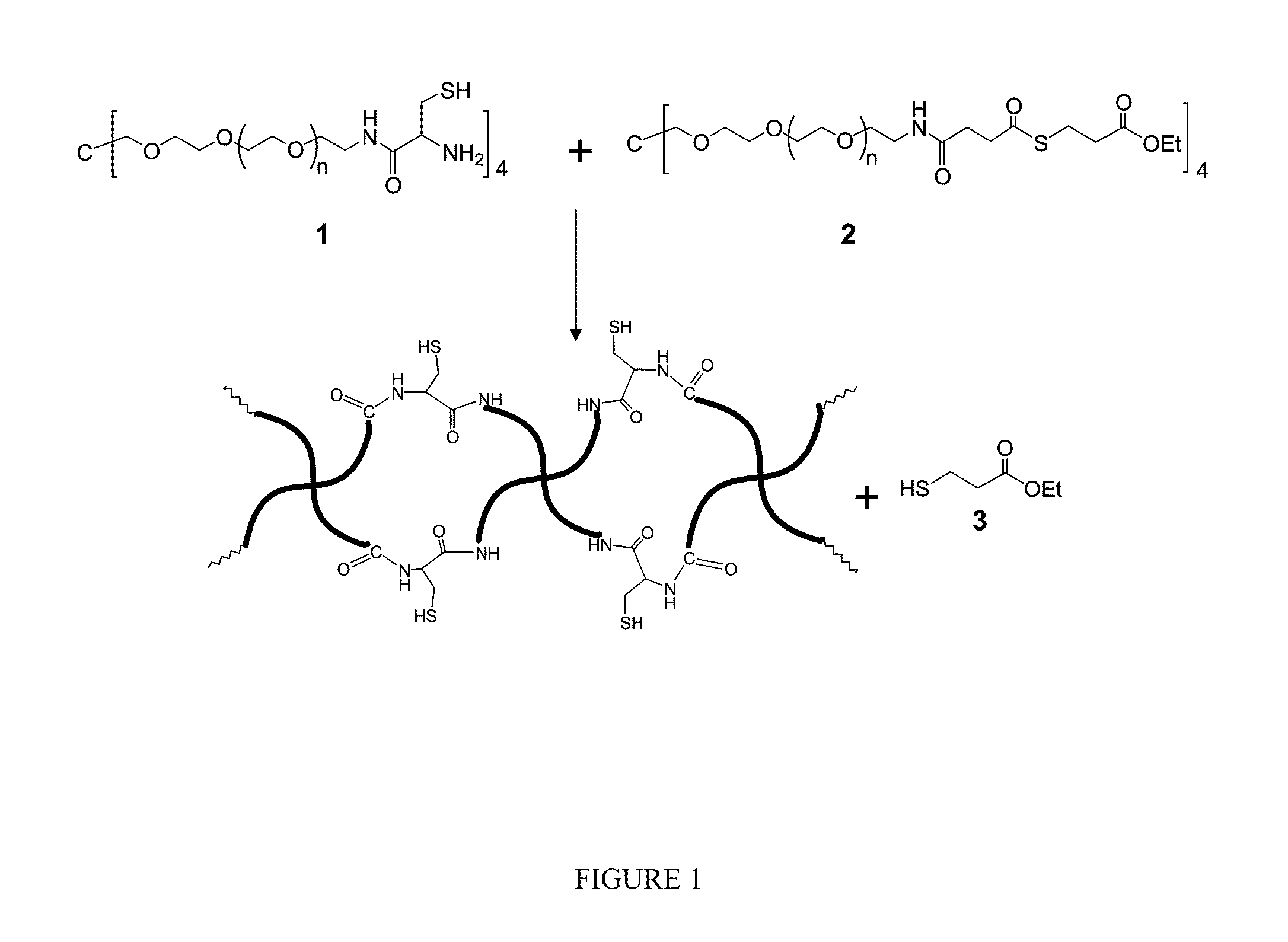
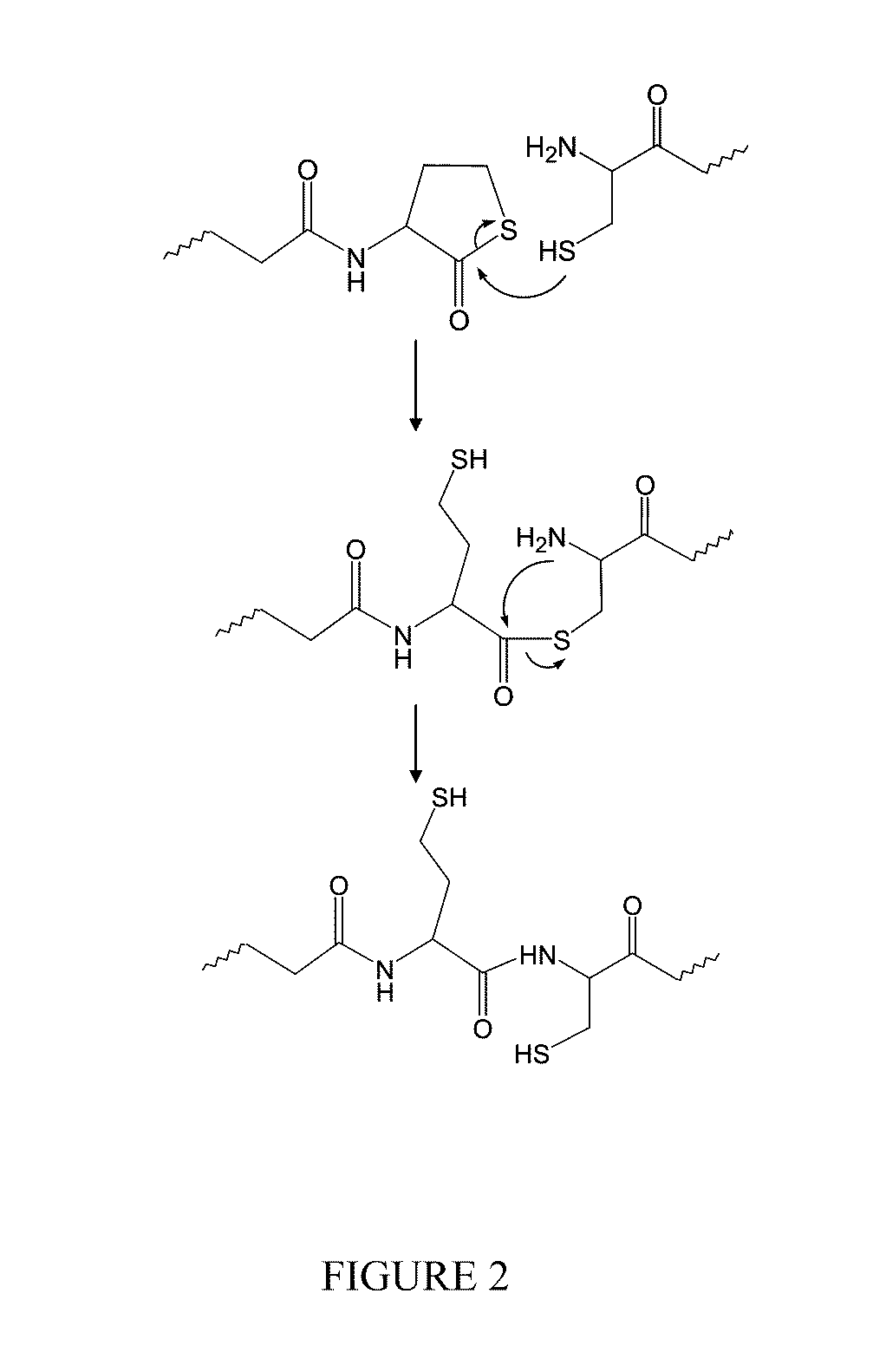
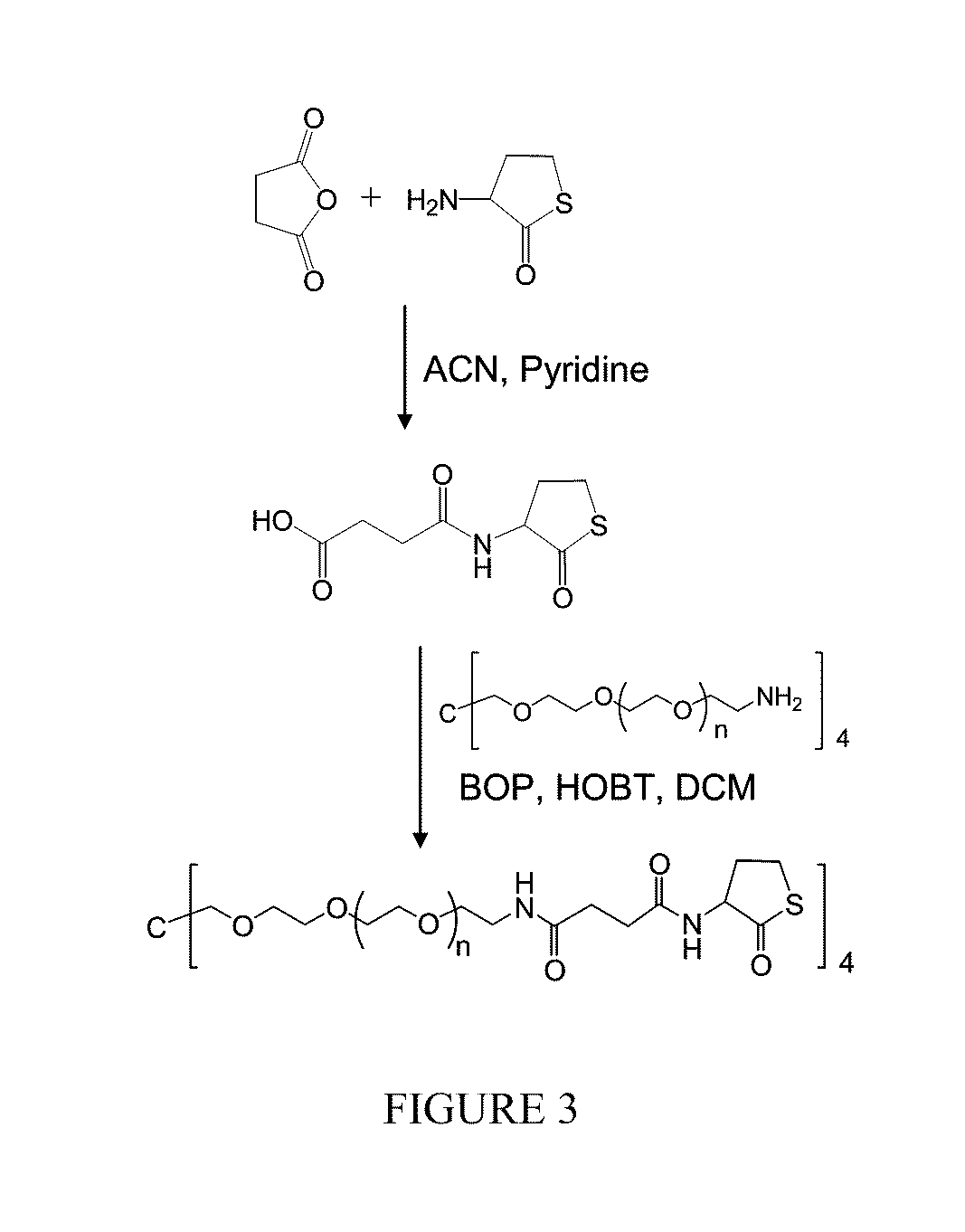
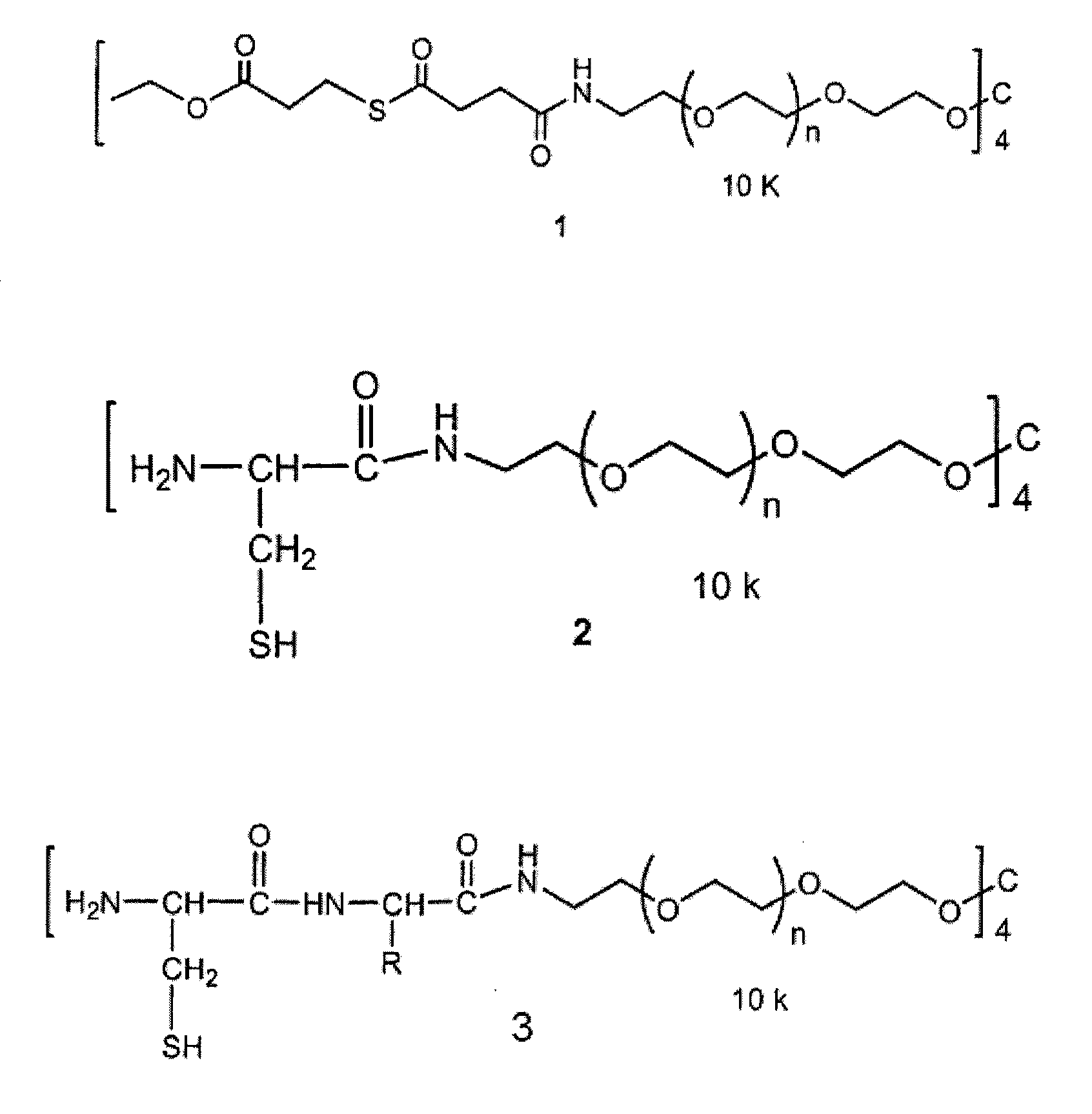
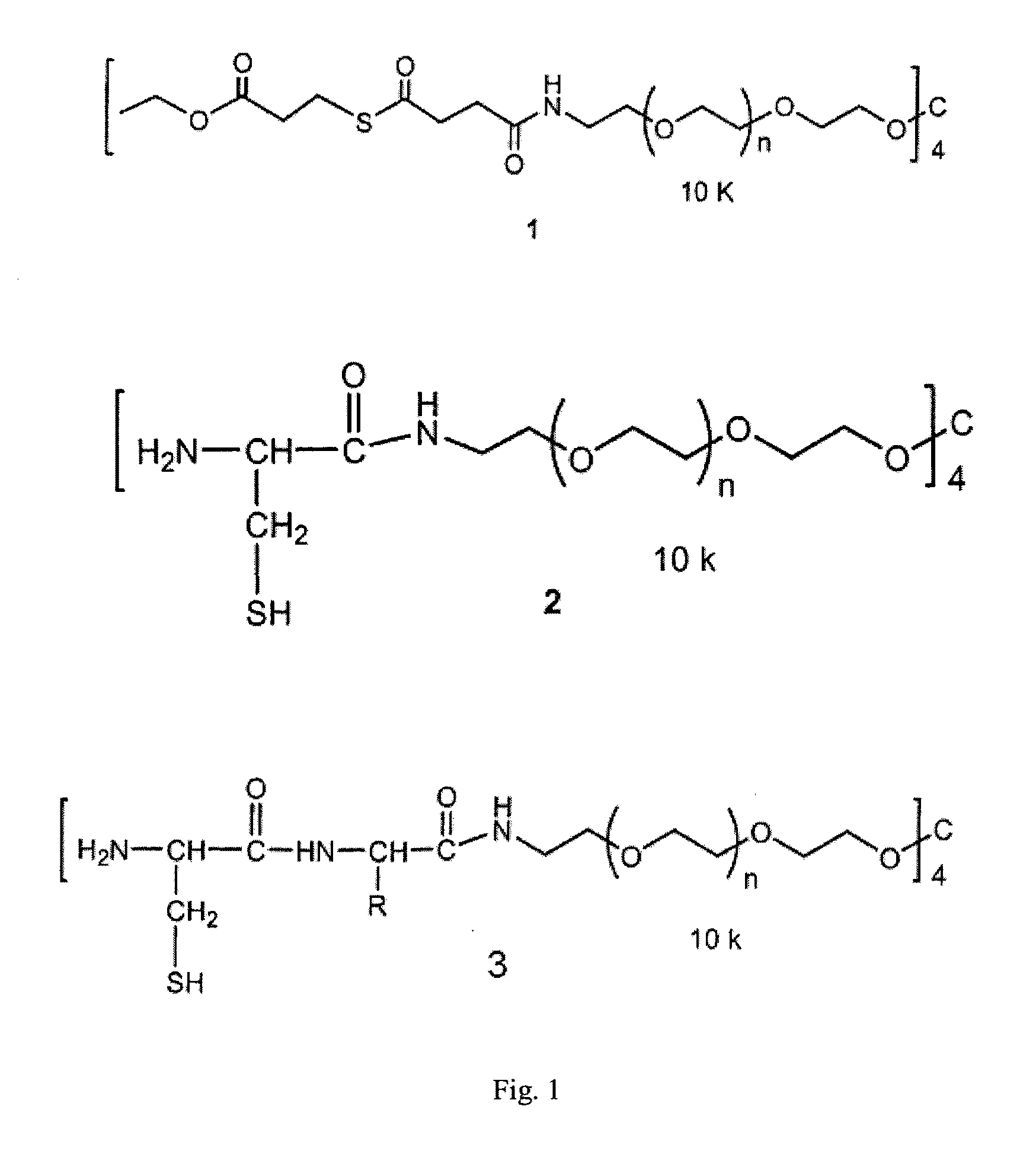
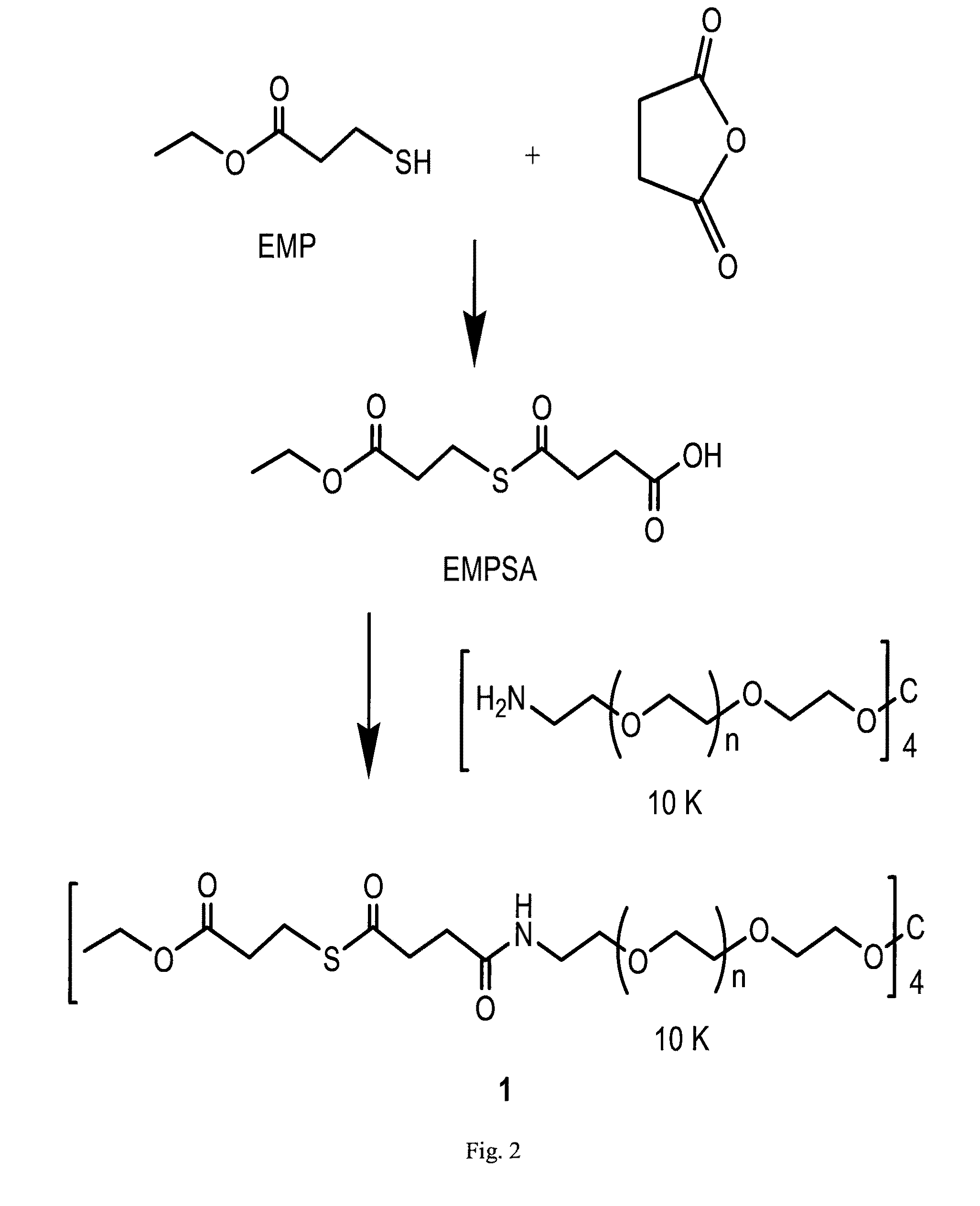
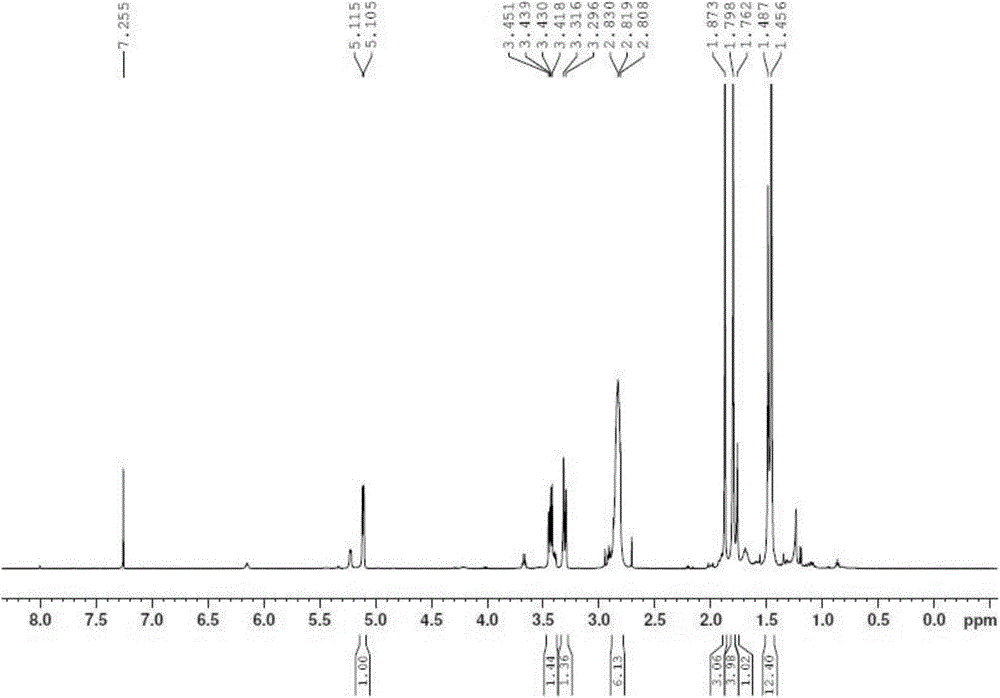
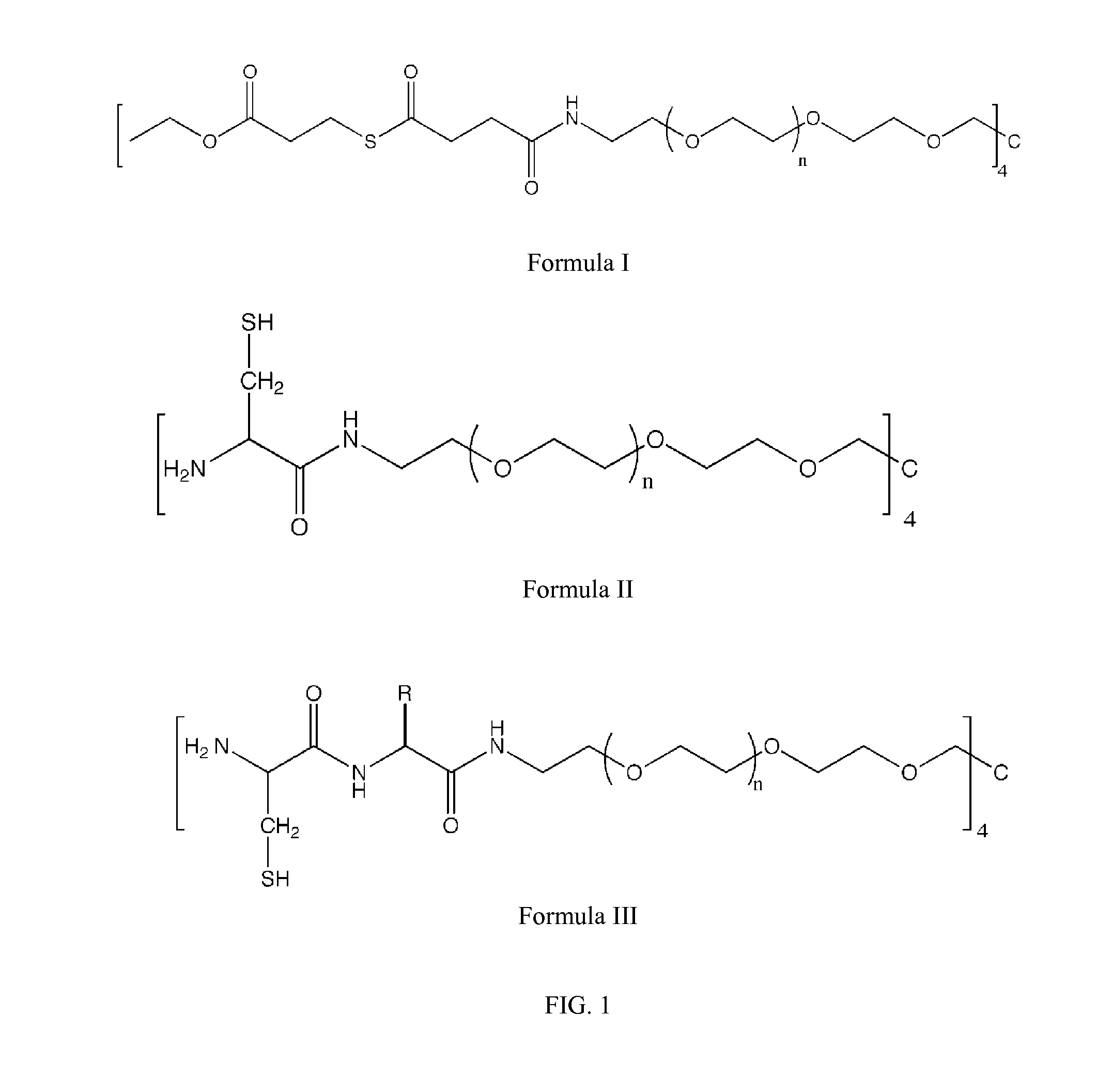
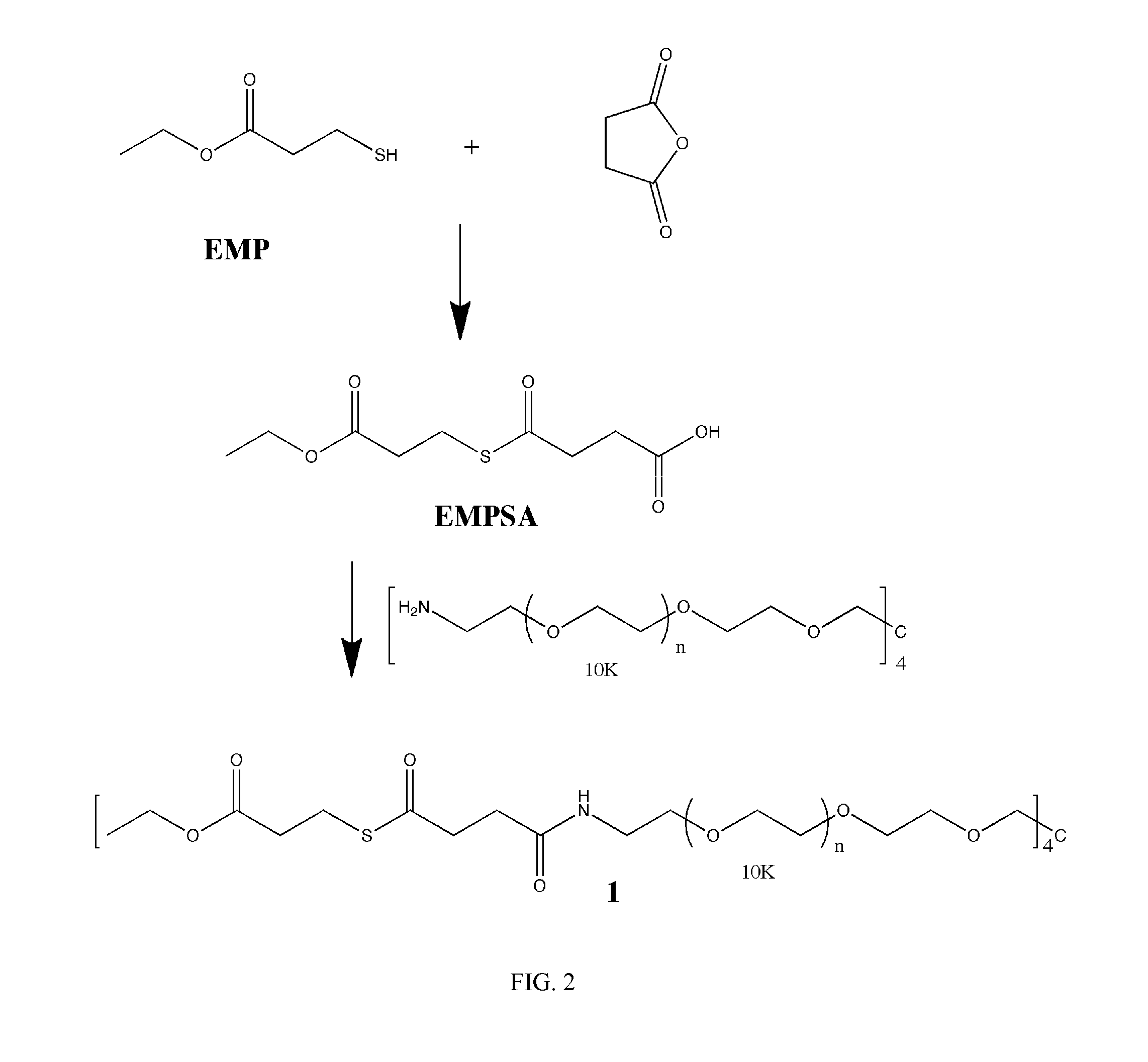
Macromonomers and hydrogel systems using native chemical ligation, and their methods of preparation
InactiveUS20080274980A1Good bioadhesionImprove permeabilityPeptide/protein ingredientsMetabolism disorderChemical ligationThiol
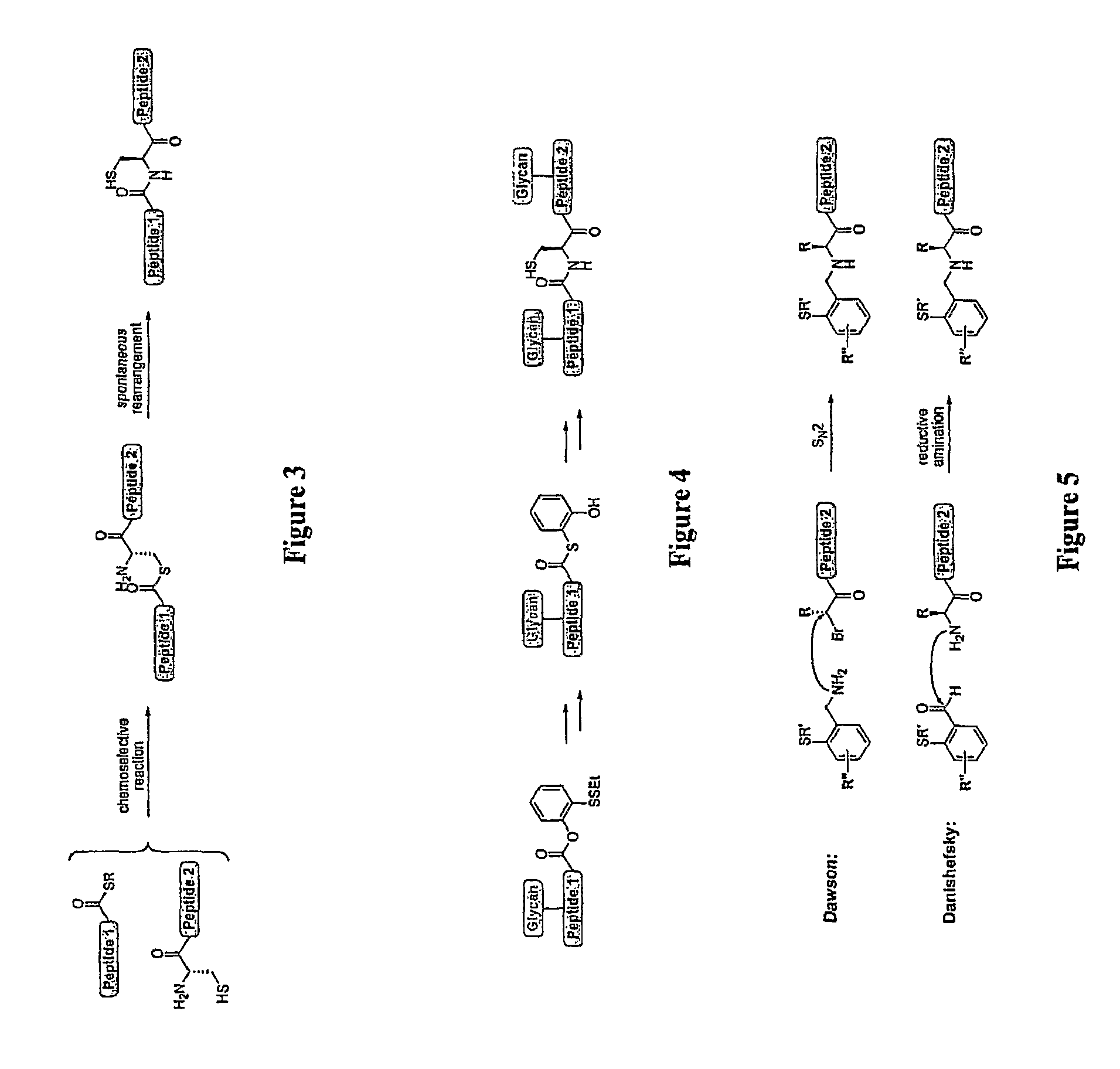
The present invention provides novel biocompatible macromonomers, hydrogels, methods of synthesis and methods of use thereof. The biocompatible hydrogels of the present invention are prepared using native chemical ligation (NCL), in which a thioester readily reacts with a N-terminal thiol (cysteine) through transesterification and rearrangement to form an amide bond through a five-member ring intermediate.
Owner:NORTHWESTERN UNIV
Semi-synthetic preparation method of semaglutide
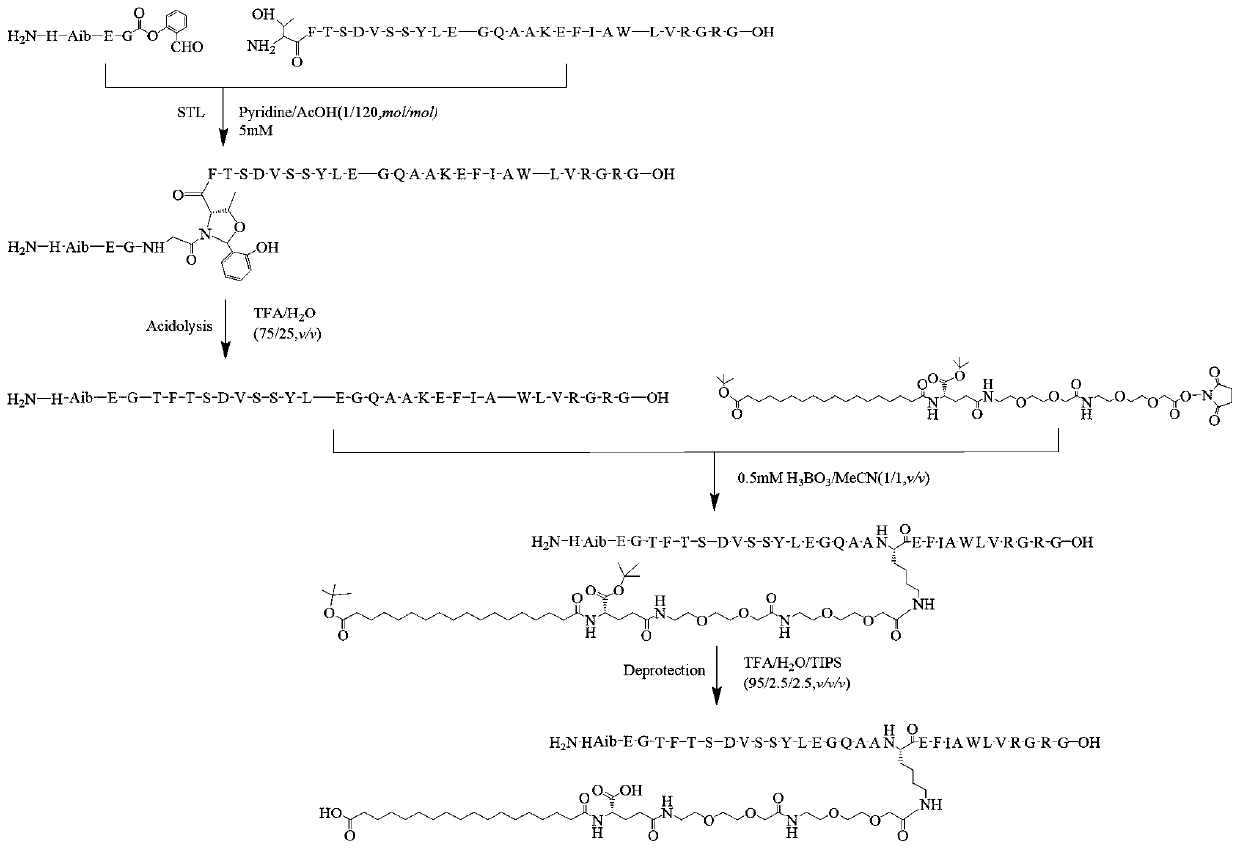
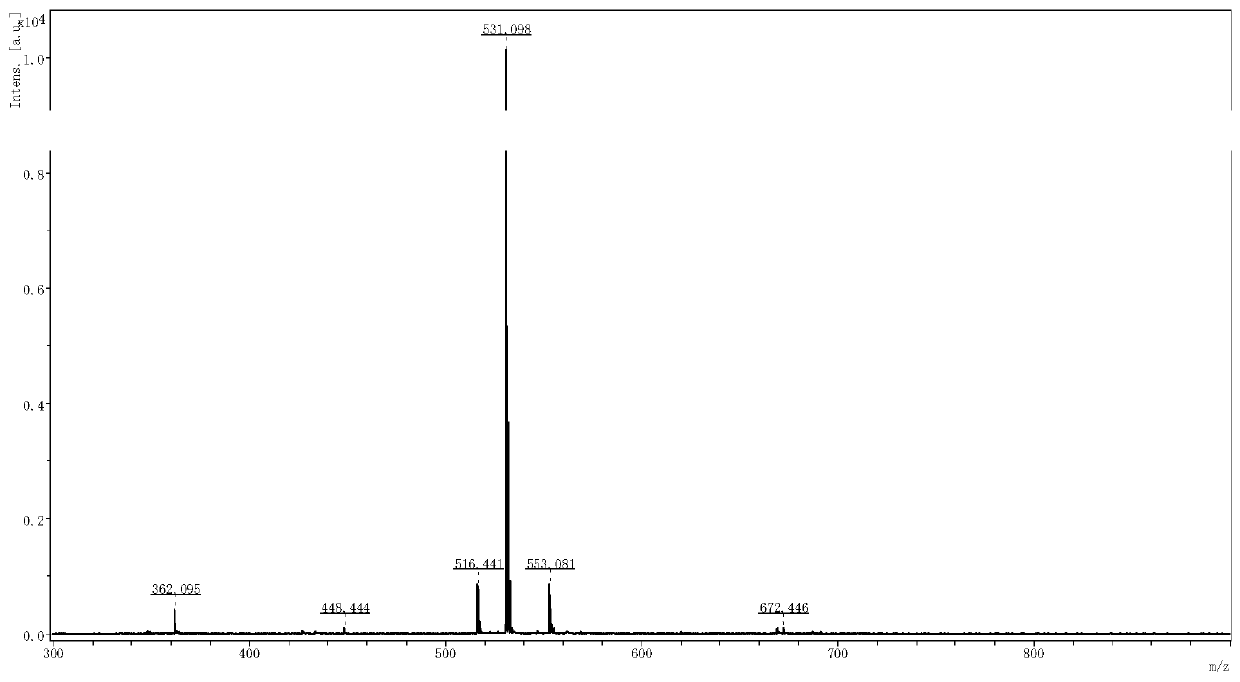
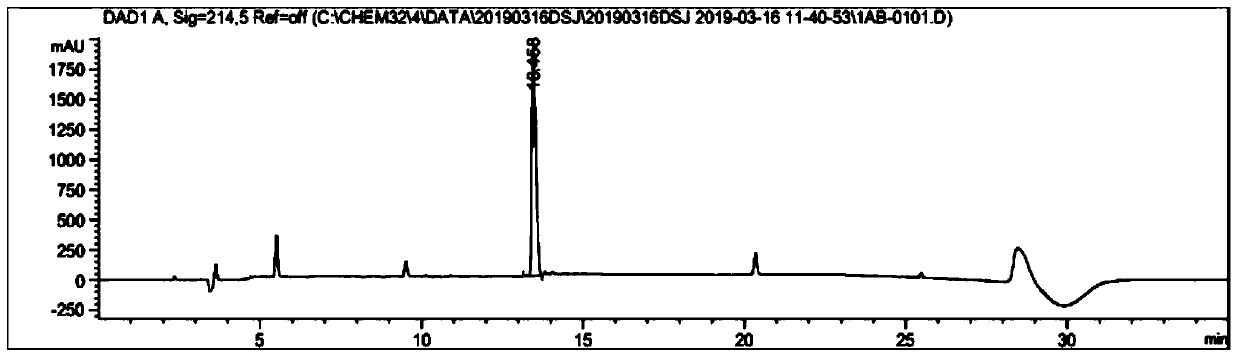
ActiveCN111153983AHigh purityHigh yieldPeptide preparation methodsGlucagonsBiochemical engineeringThreonine
The invention relates to a technology of polypeptide synthesis, and belongs to the technical field of pharmaceutical chemistry. A preparation method of semaglutide is provided. Based on a method (STL)of natural chemical connection of serine and threonine, the semaglutide can be high in purity, high in yield, less in reaction step and simple in operation, so that large-scale preparation of the semaglutide can be realized.
Owner:JIANGNAN UNIV
Solid phase native chemical ligation of unprotected or N-terminal Cysteine protected peptides in aqueous solution
InactiveUS20050209440A1Easy to purifyFast ligation reactionPeptide/protein ingredientsPeptide preparation methodsESI mass spectrometryCysteine thiolate
The present invention provides methods, apparatus and kits for synthesizing assembled peptides and proteins on a solid phase with sequential ligation of three or more unprotected peptide segments using chemoselective and mild ligation chemistries in aqueous solution. Also provided are methods of monitoring solid phase sequential ligation reactions using MALDI or electrospray ionization mass spectrometry of reaction products.
Owner:AMYLIN PHARMA INC
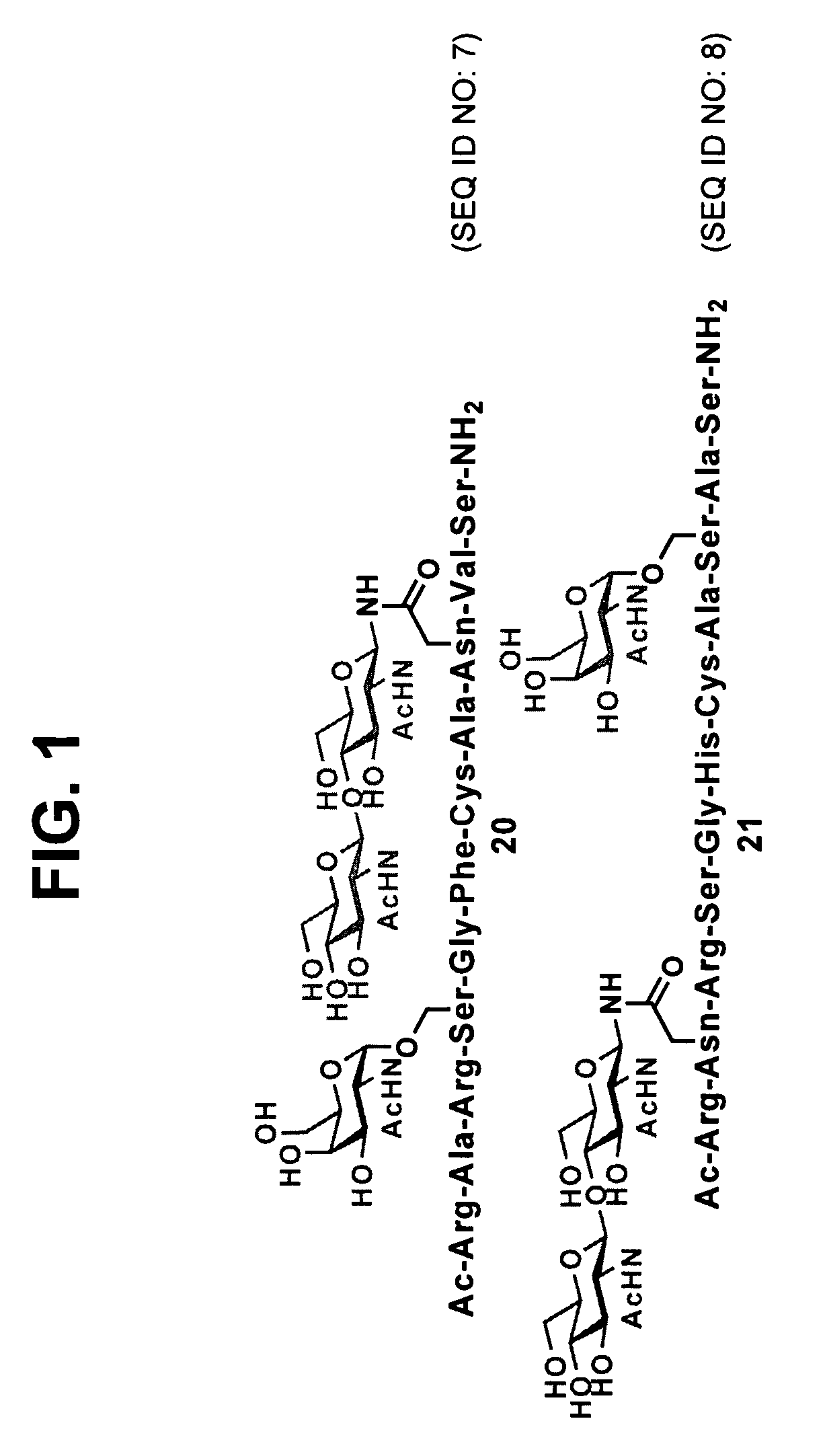
Compositions comprising homogeneously glycosylated erythropoietin
InactiveUS8754192B2Saccharide peptide ingredientsPeptide preparation methodsCrystallographyGlycopeptide
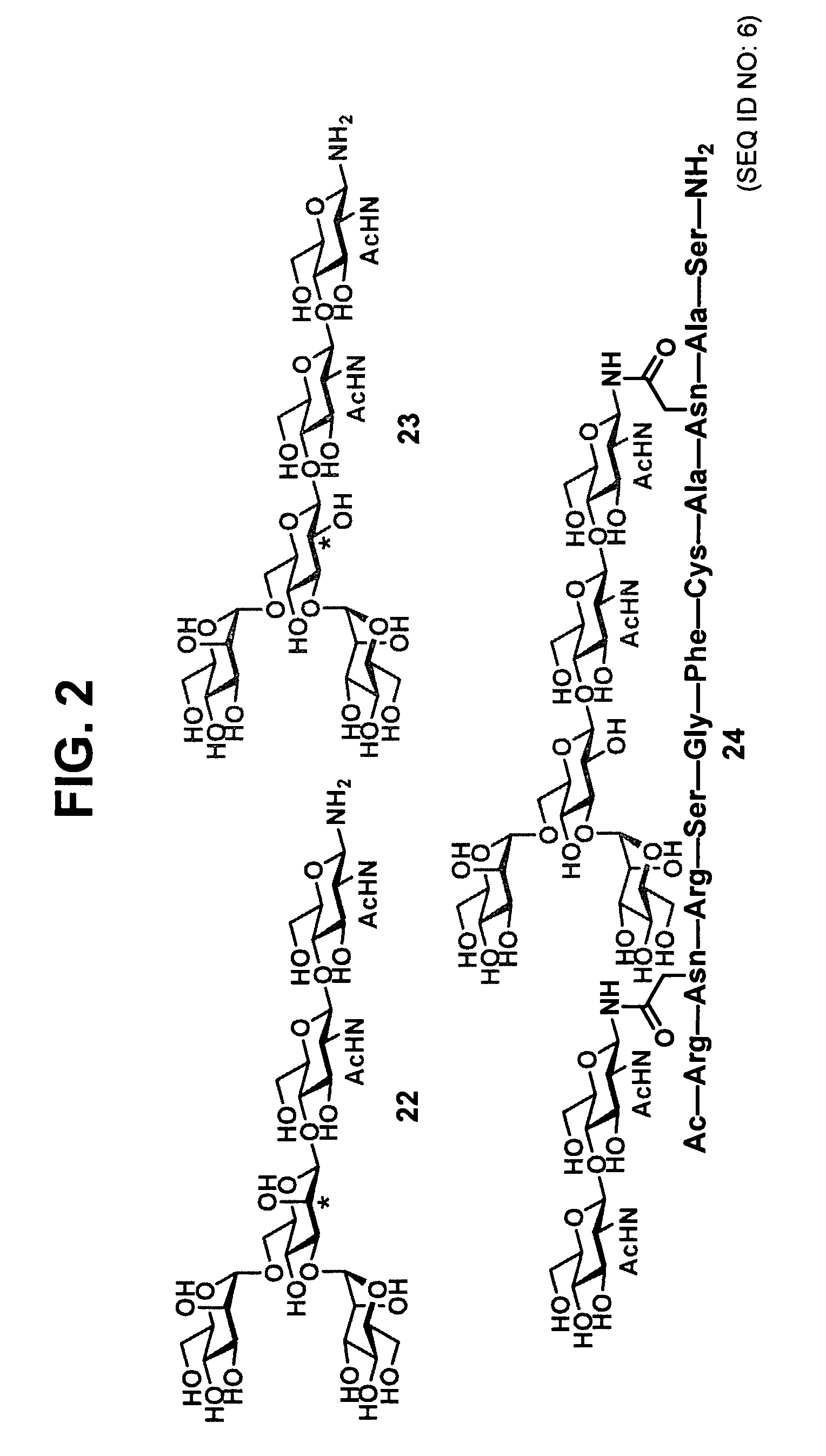
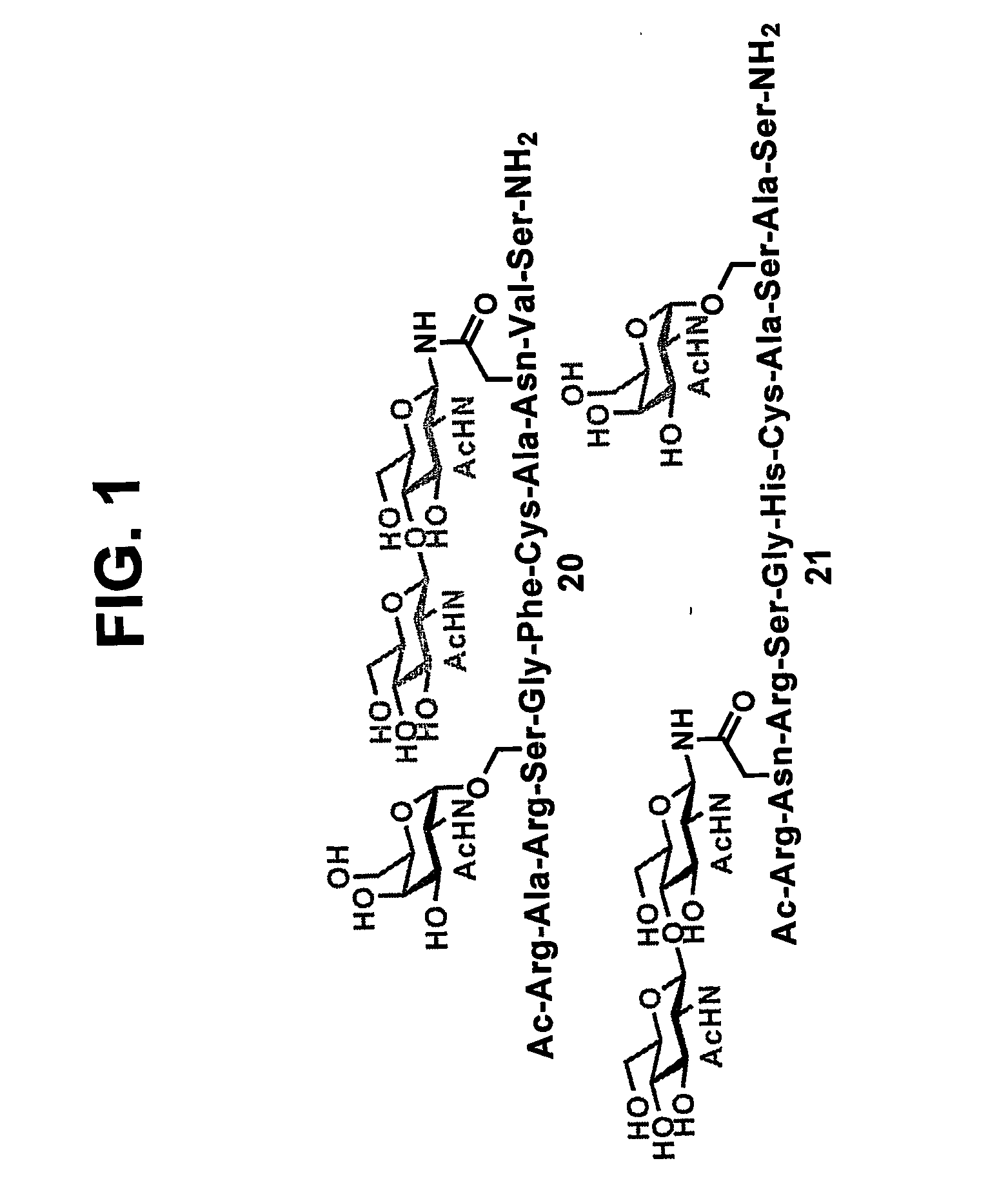
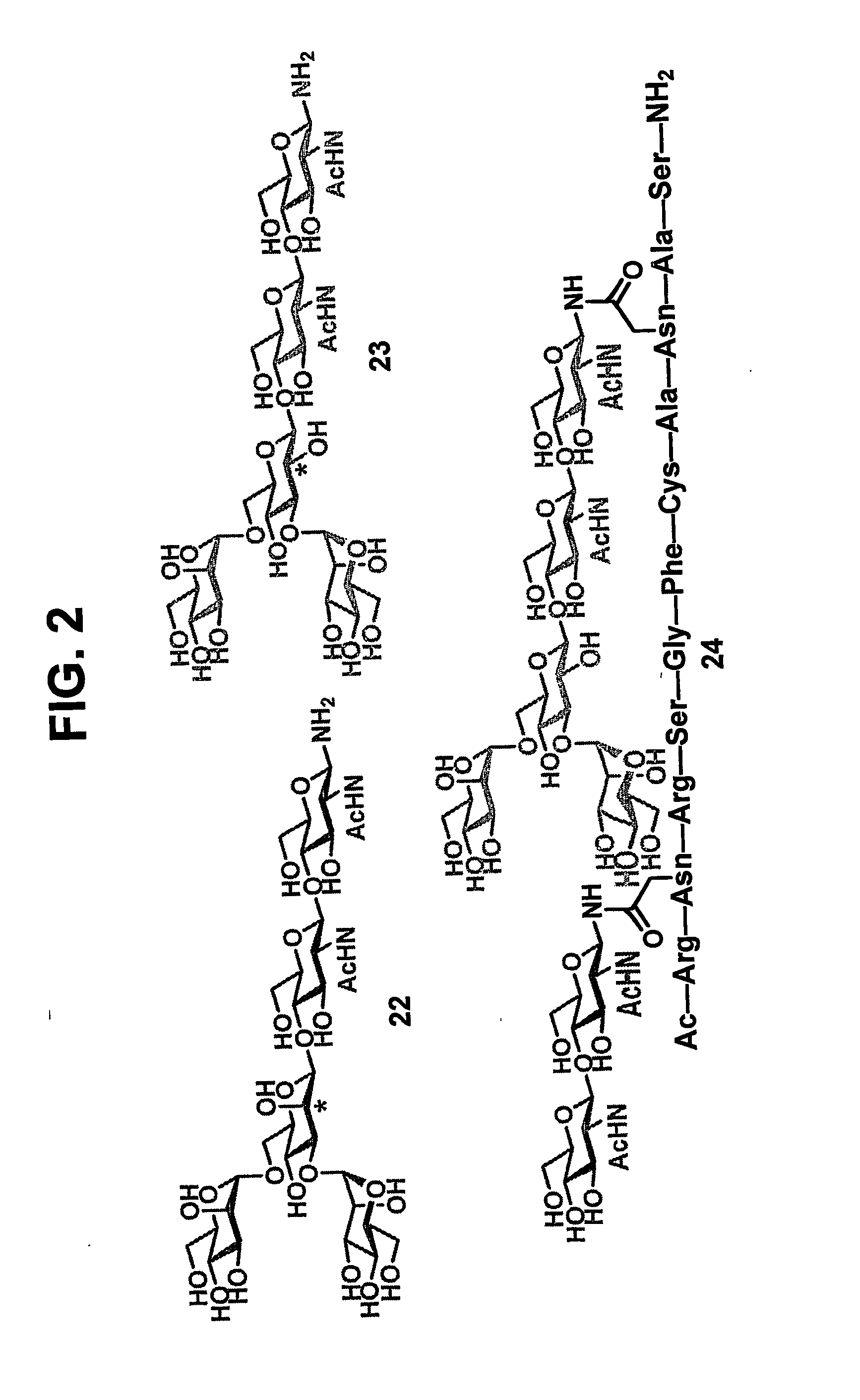
The present invention provides isolated homogeneous polyfunctionalized proteins (e.g., erythropoietin), isolated glycopeptides, and a method for preparing polyfunctionalized peptides and / or proteins via cysteine-free native chemical ligation. In certain embodiments, the invention provides an isolated homogeneous polyfunctionalized protein having the structure (I). In certain other embodiments, the invention provides an isolated glycopeptide having Formula (II). In certain other embodiments, the inventive method is a method for preparing a polyfunctionalized peptide comprising a peptidic backbone made up of four or more amino acids, wherein two or more non-adjacent amino acids are independently substituted with a moiety having the structure (III) -LH. wherein A and L1 are as defined herein.
Owner:SLOAN KETTERING INST FOR CANCER RES
Versatile native chemical ligation technologies
InactiveUS20140213758A1Avoid restrictionsLevelEsterified saccharide compoundsSugar derivativesChemical ligationCoupling
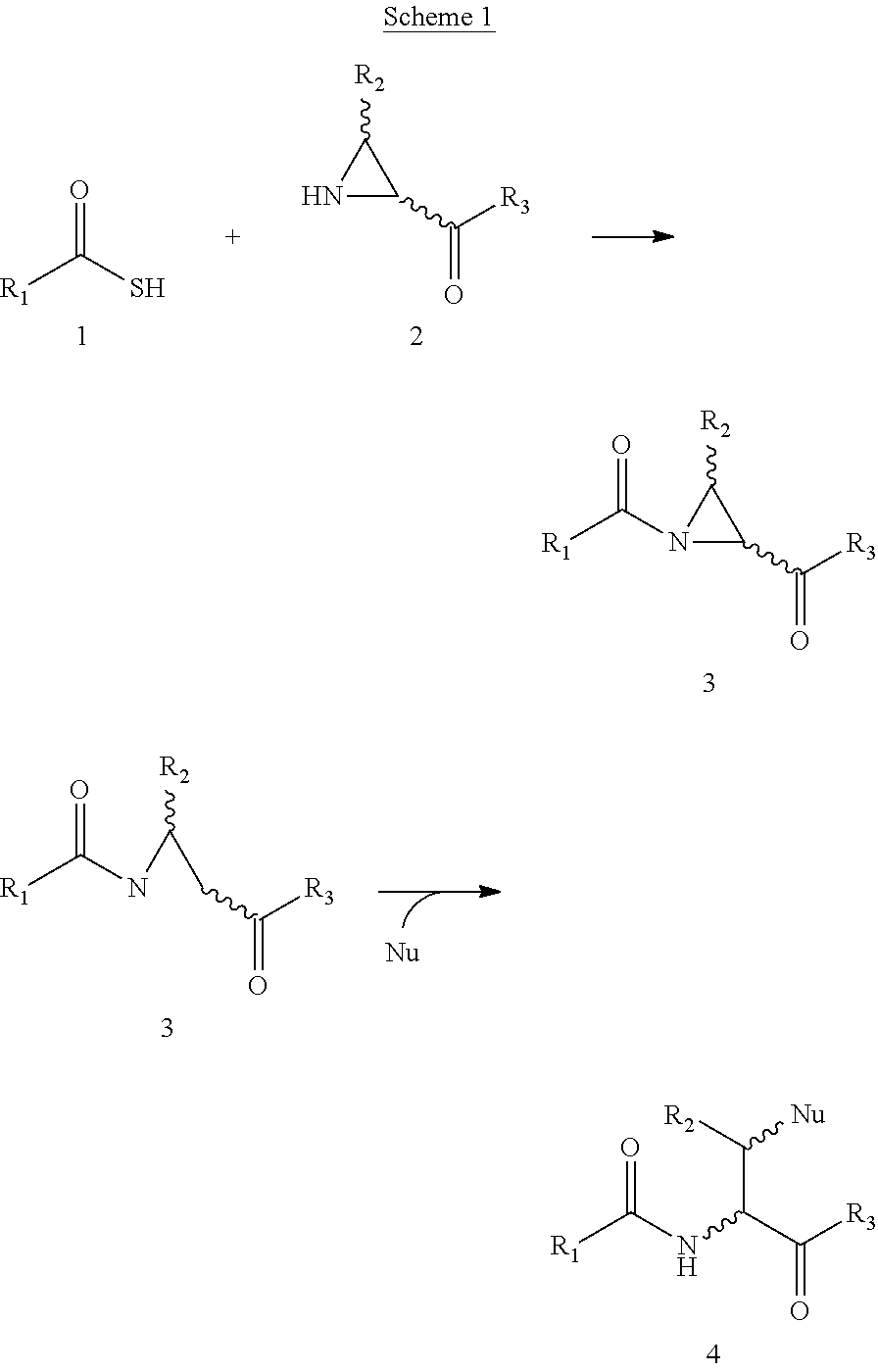
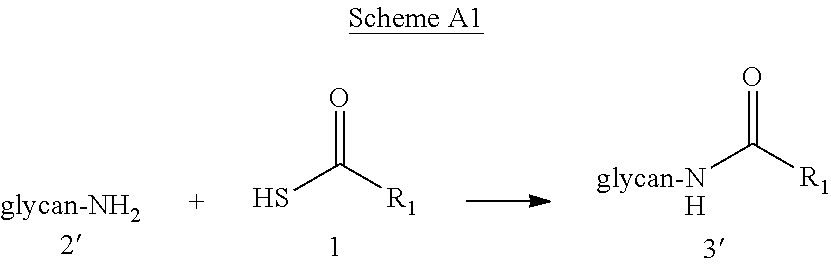
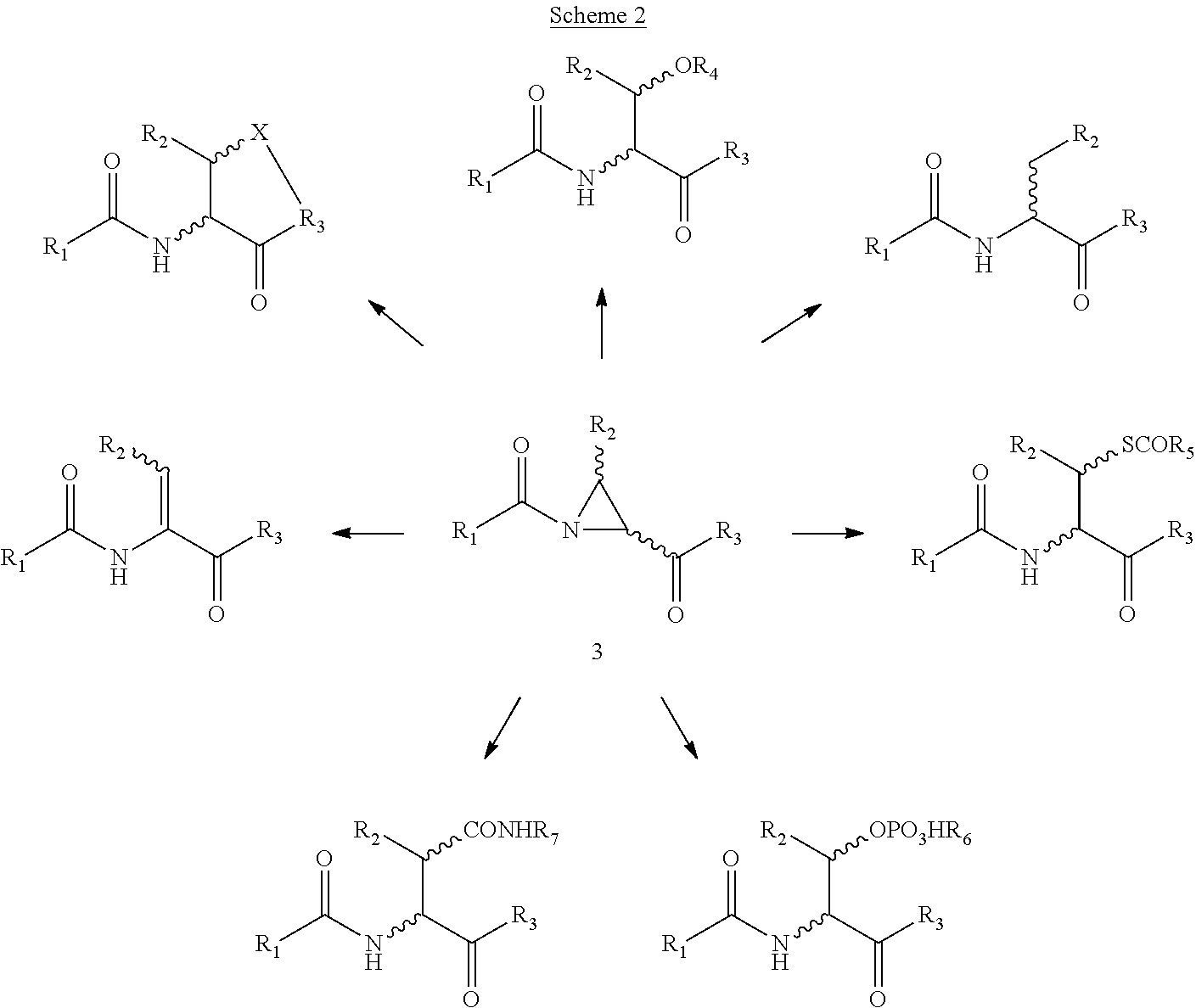
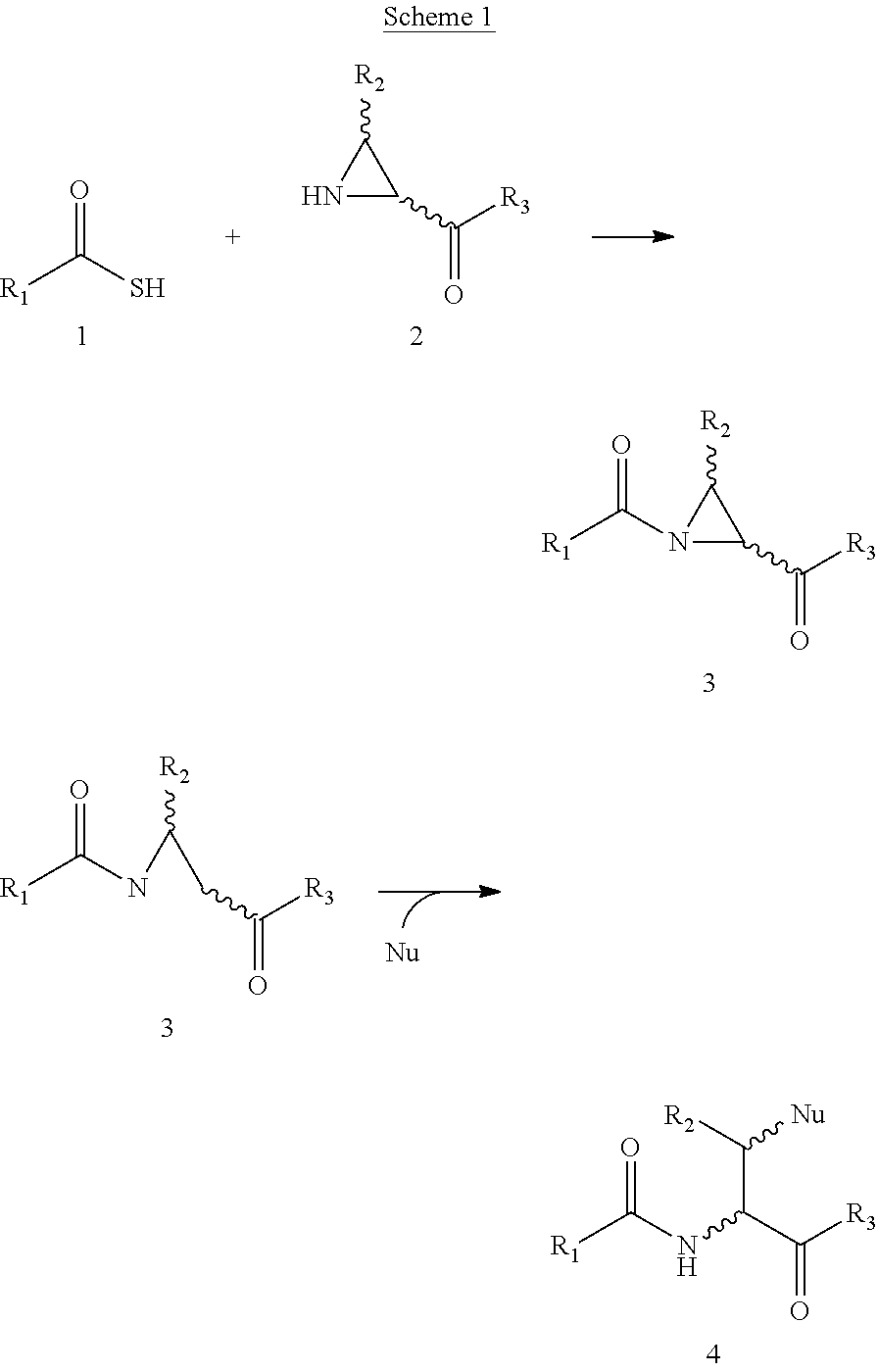
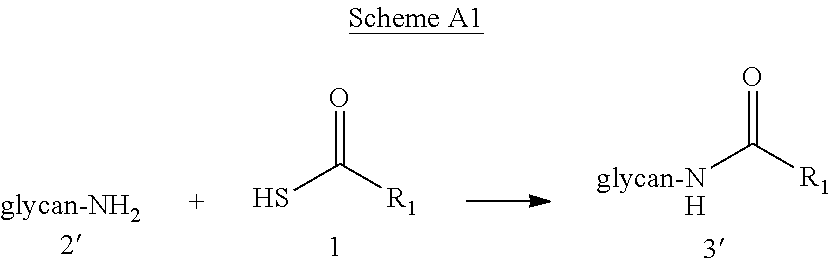
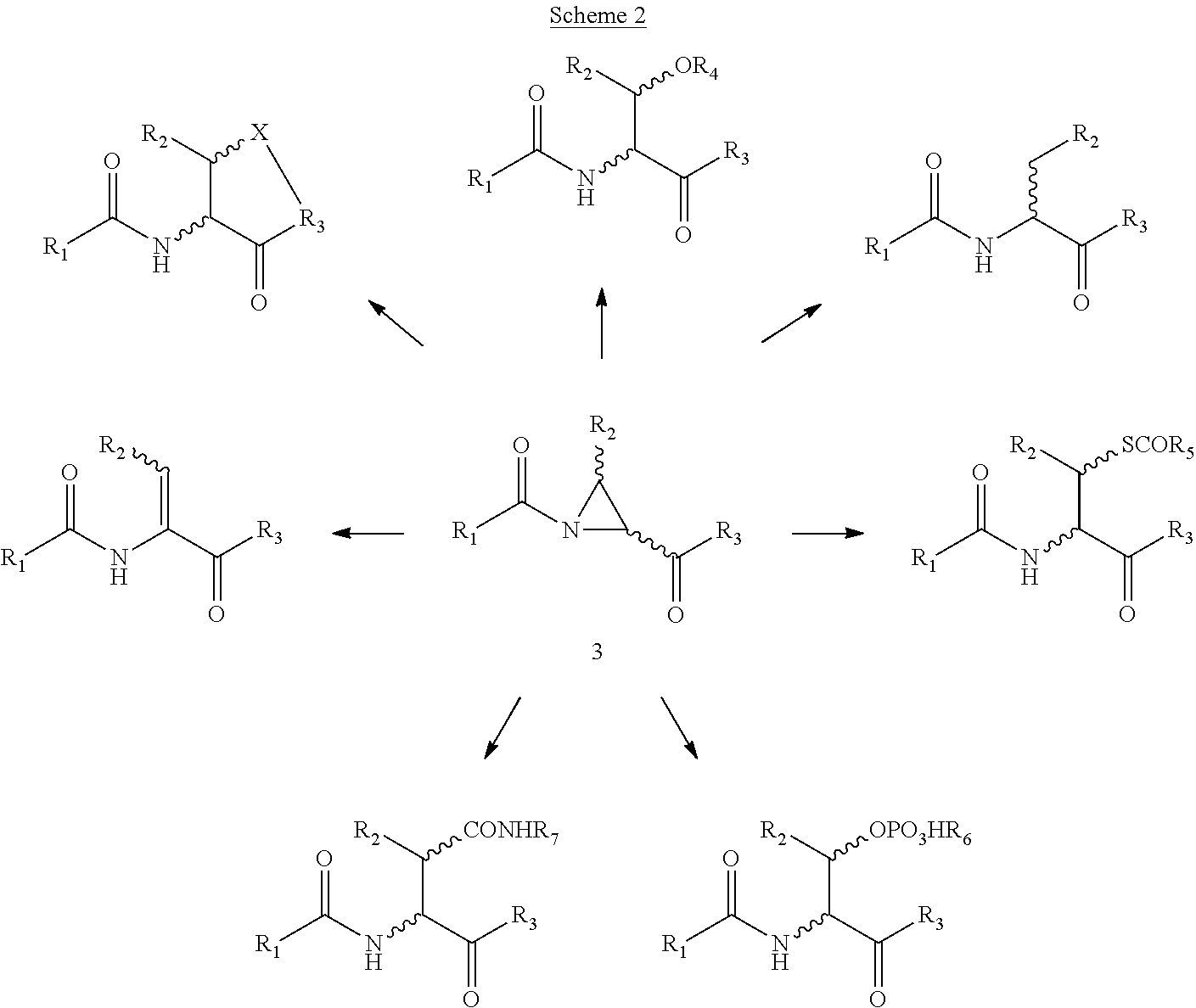
Novel methods of native chemical ligation are provided. The methods involve reacting a thioacid (e.g. a peptide thioacid) with an aziridinyl compound (e.g. an aziridinyl peptide) or glycosylamine under mild conditions without the use of protecting groups, and without requiring that a cysteine residue be present in the ligation product. Initial coupling of the thioacid and the aziridinyl compound yields a ligation product containing an aziridinyl ring. Optional subsequent opening of the aziridinyl ring (e.g. via a nucleophilic attack) produces a linearized and modified ligation product. Coupling of a peptide thioacid and glycosylamine yields a glycosylated peptide.
Owner:WASHINGTON STATE UNIVERSITY
Pseudo native chemical ligation
InactiveUS7030218B2Sufficient formEasily employedPeptide/protein ingredientsAntipyreticChemical ligationPolymer modified
The present invention concerns methods and compositions for extending the technique of native chemical ligation of a wider range of peptides, polypeptides, other polymers and other molecules via an amide bond (see FIG. 1). The invention further provides methods and uses for such proteins and derivatized proteins. The invention is particularly suitable for use in the synthesis of optionally polymer-modified, synthetic bioactive proteins, and of pharmaceutical compositions that contain such proteins.
Owner:ASTRAZENECA PHARMA LP
Versatile native chemical ligation technologies
InactiveUS9145440B2Avoid restrictionsLower Level RequirementsEsterified saccharide compoundsSugar derivativesChemical ligationCoupling
Owner:WASHINGTON STATE UNIVERSITY
Cysteine-functionalized hyaluronic acid conjugates and their synthesis and application in injectable in situ formed hydrogels
The invention discloses a cysteine functionalized hyaluronic acid conjugate, a synthetic method and an application in preparation of injectable in-situ hydrogel thereof. According to the method, a hydroxy of hyaluronic acid is modified to obtain the cysteine functionalized hyaluronic acid conjugate with a stable ether bond, and tetrabutyl ammonium hydroxide is introduced in the reaction process. The new method improves the solubility of hyaluronic acid and increases the reaction efficiency. The obtained cysteine functionalized hyaluronic acid conjugate has a sulfhydryl active group which not only improves the adhesion of hyaluronic acid, but also can be further functionalized. According to the invention, injectable in-situ hydrogel is generated by a native chemical ligation reaction or a Michael addition reaction of the prepared cysteine functionalized hyaluronic acid conjugate with a polyethylene glycol conjugate; rheological research shows that the generated hydrogel has good rheological properties, the gel performance is controllable, and the hydrogel has wide application prospects in the biological medicine field.
Owner:HAINAN UNIV
Catalyst and byproduct-free native chemical ligation using cyclic thioester precursors
ActiveUS9012594B2Good biocompatibilityCross-linking is sufficiently rapidPowder deliveryOrganic chemistryCross-linkCombinatorial chemistry
A method of synthesizing a biocompatible hydrogel by covalently cross-linking an effective amount of a first macromonomer including a cyclic thioester group with an effective amount of a second macromonomer including a terminal cysteine group is disclosed. In addition, the synthesis and use of the following specific cyclic thioester macromonomer that can be used in the method, as well as specific hydrogels made using this macromonomer are disclosed. The disclosed method produces a biocompatible hydrogel, while producing substantially no toxic free thiol by-product. Accordingly, the method can be used in making biomedical products, such as sutures and tissue replacement biomaterials, and for encapsulating therapeutic cells and pharmaceuticals.
Owner:NORTHWESTERN UNIV
Macromonomers and hydrogel systems using native chemical ligation, and their methods of preparation
InactiveUS8841408B2Improve permeabilityGood bioadhesionPowder deliveryOintment deliveryThiolTransesterification
Biocompatible macromonomers, hydrogels, methods of synthesis and methods of use thereof are provided. The biocompatible hydrogels of the present invention are prepared using native chemical ligation (NCL), in which a thioester readily reacts with a N-terminal thiol (cysteine) through transesterification and rearrangement to form an amide bond through a five-member ring intermediate.
Owner:NORTHWESTERN UNIV
Native chemical ligation with three or more components
InactiveUS7662914B2Improve efficiencyMethod can be usedPeptide/protein ingredientsPeptide preparation methodsThiazoleLigation
The invention provides a method of assembling oligopeptide intermediates in a native chemical ligation reaction that eliminates self-ligation of bi-functional intermediates. An important aspect of the invention is a bi-functional intermediate with an N-terminal cyclic thiazolidine protecting group which effectively prevents self-ligation in the chemical assembly process. The present invention is useful in methods for convergent synthesis of polypeptides and proteins and improves the efficiency of native chemical ligation reactions, particularly where three or more peptide fragments are used to assemble a polypeptide or protein product.
Owner:ASTRAZENECA PHARMA LP
Side-Chain Extended Ligation
InactiveUS20090192300A1Quick responseHigh yieldDepsipeptidesPeptide preparation methodsThiolChemical ligation
Owner:AMYLIN PHARMA INC
Conjugates of hydroxyalkyl starch and a protein
InactiveCN102302787APeptide preparation methodsPharmaceutical non-active ingredientsHydroxyethyl starchThioester
The present invention relates to an active substance and hydroxyalklyl starch, preferably hydroxyalkyl starch, wherein the conjugates are prepared by native chemical ligation via covalently linking the hydroxyalkyl stach and the active substance by reacting a thioester group with an alpha-X beta-amino group, wherein X is selected from the group consisting of -SH and NH-N. The present invention also relates to the method of producing these conjugates and the use of the conjugates.
Owner:FRESENIUS KABI DEUT GMBH
Method for producing peptide
InactiveUS20120004457A1Easy to connectEasy to produceOrganic compound preparationOrganic chemistry methodsThiolThreonine
An object of the present invention is to provide a novel method for producing a peptide utilizing a ligation reaction in which ligation efficiency is excellent and side reactions to other functional groups in the peptide are hard to occur, in comparison with the conventional native chemical ligation methods utilizing the thiol auxiliary group. The present invention provides a method for producing a peptide which comprises a step of causing a first peptide and a second peptide to react in the presence of a reducing agent to obtain a ligated product of the first peptide and the second peptide, wherein the first peptide contains, at the C-terminal end, an amino acid derivative having a thioester group, and the second peptide contains, at the N-terminal end, a serine or threonine derivative having a thiol auxiliary group.
Owner:TOKAI UNIV
Chemical Modification of Proteins
InactiveUS20160326212A1Easy to useReadily crystalisesPeptide preparation methodsSelenocysteineDehydroalanine
Owner:R P SCHERER TECH INC
Chemical modification of proteins
The invention relates to methods for selectively converting a cysteine residue in a peptide or protein to the dehydroalanine (Dha) residue. The method also works on selenocysteine and substituted cysteine and selenocysteine residues, resulting in the Dha residue which may be converted to any natural or unnatural amino acid residue desired without the alteration of the remainder of the peptide or protein. The invention also allows ligation of a desired peptide at any point rather than at a point where there should be a naturally occurring cysteine, thereby allowing native chemical ligation to be used in the synthesis of peptides that do not contain cysteine. The methodology allows for the synthesis of very large peptides.
Owner:DAVIS BENJAMIN G +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com