Semi-synthetic preparation method of semaglutide
A semaglutide and compound technology, applied in the field of peptide synthesis, can solve the problems of strong steric hindrance effect, increased production cost, low product yield, etc., and achieves the effects of rapid transformation, easy operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The preparation of embodiment 1 compound I crude product
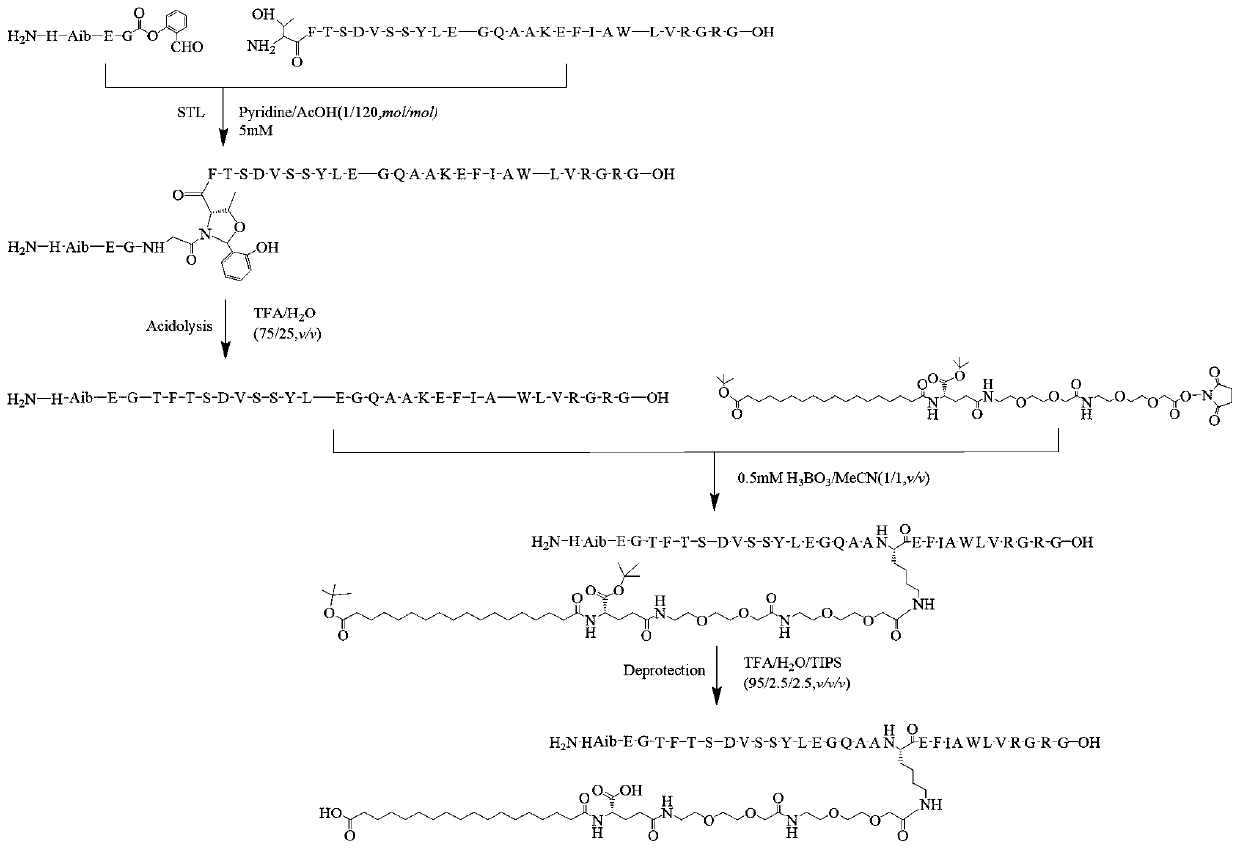
[0075] Weigh respectively Boc-His(Trt)-Aib-Glu(Otbu)-Gly-OH (commercial purchase: purchased from Jill Biochemical (Shanghai), 1g, 1.213mmol), salicylaldehyde dimethyl acetal (407.8mg, , 2.426mmol), PyBOP (946.8mg, 1.8195mmol), dissolved in 50mL DMF, added 600uLDIEA (3.639mmol) in ice-water bath, stirred magnetically at 0°C for 20 hours. The reaction was checked for complete conversion by liquid phase. After the reaction, concentrate the reaction solution under reduced pressure, add DCM to redissolve, wash with saturated brine three times, put the collected DCM solution into a certain amount of anhydrous sodium sulfate for drying, let it stand for 0.5 hours, suction filter, and collect the filtrate After pumping to dryness, 1.89 g of crude compound 0 was obtained, which was set aside.
[0076]
[0077] Take all the samples of the above compound 0, add 50mL cutting reagent (TFA / H 2 0 / TIPS, 95:2.5:2.5, v / v / v)...
Embodiment 2
[0079] Embodiment 2 Purification of compound I crude product
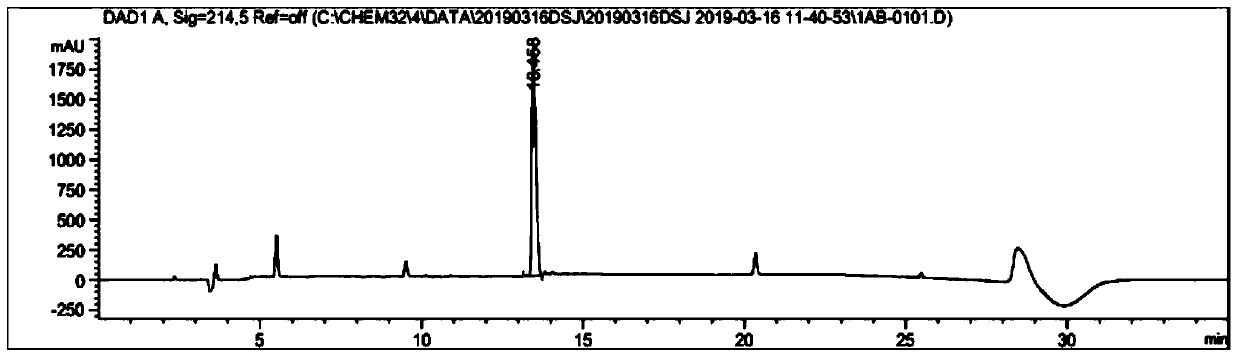
[0080] Weigh 500 mg of the crude compound I prepared in Example 1, add 15 mL of distilled water to dissolve, use Water1525 system for semi-preparative purification, the wavelength is 214 nm, and the chromatographic column is 20 × 250 mm reverse phase C 18 Column, column temperature is 37 ℃, mobile phase is the water (A phase) that contains 0.1% TFA and the acetonitrile (B phase) that contains 0.1% TFA, flow velocity is 8ml / min, gradient: B%: 20%-60%, After 30 min, the target components were collected, and the collected solution was concentrated under reduced pressure.
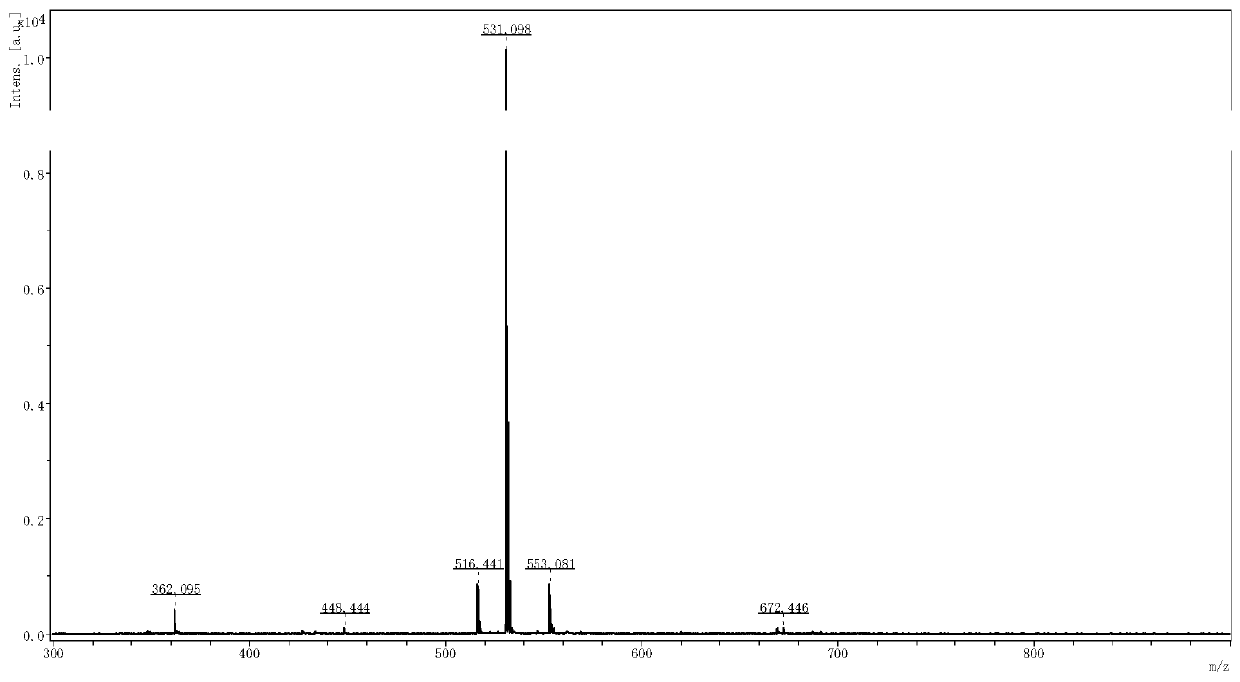
[0081] Using MAIDI-TOFMS for detection, the results are shown in figure 2 , MAIDI-TOFMS calculates C 24 h 30 N 6 o 8 Theoretical molecular weight [M+H] + m / z=531.220,[M+Na] + = 553.202, observed: 531.098, 553.081. The above concentrate was lyophilized to obtain 195 mg of compound I in the form of white solid powder with a yield of 80%. Ana...
Embodiment 3
[0083] The synthesis of embodiment 3 compound II
[0084] Weigh compound Ⅰ (50mg, 0.094mmol), Arg34GLP-1 (11-37) (150mg, 0.05mmol), and dissolve them in 100ul pyridine acetate buffer solution (acetic acid:pyridine, mol:mol, 120:1), 35 ℃ magnetic stirring reaction for 20 hours. The solvent was removed by drying, and then the residue was acidolyzed with 10 mL of acid hydrolysis solution (TFA / water, 75 / 25, v / v), and magnetically stirred at room temperature for 2 hours. The solvent was removed by drying, and lyophilized to obtain 230 mg of the reaction mixture. Analytical liquid phase analysis was carried out to the reaction mixture (using liquid phase conditions: adopt Agilent 1260 system to carry out liquid phase analysis, wavelength 214nm, chromatographic column is the reverse phase C18 column of 4.6 * 250mm, column temperature is 35 ℃, mobile phase is 0.1% Water (phase A) and 0.1% acetonitrile (phase B), the flow rate is 1mL / min, gradient: B%: 30%-45%, 0-20min.), the results...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com