Cysteine functionalized hyaluronic acid conjugate, synthetic method and application in injectable in-situ hydrogel thereof
A technology of hyaluronic acid and cysteine, which is applied in the field of biomedicine, can solve problems such as inability to achieve in situ injection and reduce the ability of cells to repair damage, and achieve good application prospects, good bioadhesion, and the effect of expanding applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] ① Preparation of compound 2: take compound 1 cysteine hydrochloride, add at least 20 times the volume (volume / mass) of compound 1 in dry acetone, reflux for 2-10 hours, and concentrate under reduced pressure to no more than the total reaction solution One-third of the volume, put it in the refrigerator for no less than 0.5h, precipitate crystals, and filter through a Buchner funnel to obtain compound 2, whose chemical structural formula is shown in compound 2 in the synthetic route;
[0069] ② Preparation of Compound 3: Dissolve Compound 2 in acetonitrile, add di-tert-butyl dicarbonate in a molar amount equivalent to 1.1 to 3 times that of Compound 2, mix well and add dropwise in a molar amount equivalent to that of Compound 2 1. 1 to 3 times N, N-diisopropylethylamine, stirred at 15°C to 37°C for 24 to 72 hours, concentrated the solvent under reduced pressure, then added ether or cyclohexane to continue the concentration under reduced pressure, then added ether or cyc...
Embodiment 1
[0083] Example 1: Preparation of cysteine-functionalized hyaluronic acid conjugates
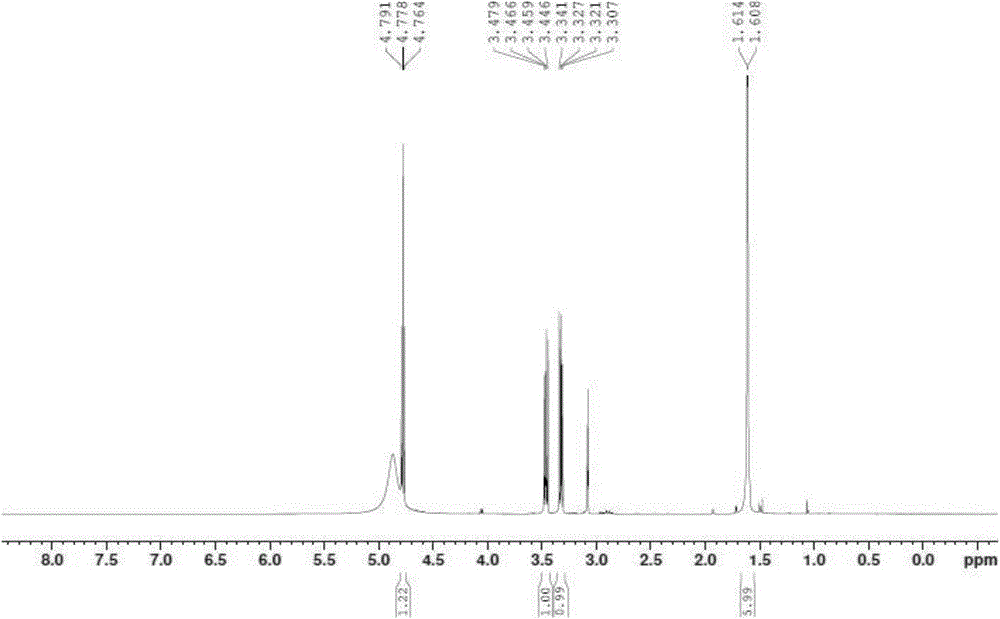
[0084] 1) Preparation of Apc: Take 10g of cysteine hydrochloride, add 1000mL of dry acetone to reflux for 6h, concentrate under reduced pressure to about 200mL, put it in the refrigerator overnight, precipitate crystals, filter through a Buchner funnel, and obtain the intermediate white crystal Apc (compound 2) quality 11g, productive rate is 88%, and its proton nuclear magnetic resonance spectrum is as figure 1 shown.
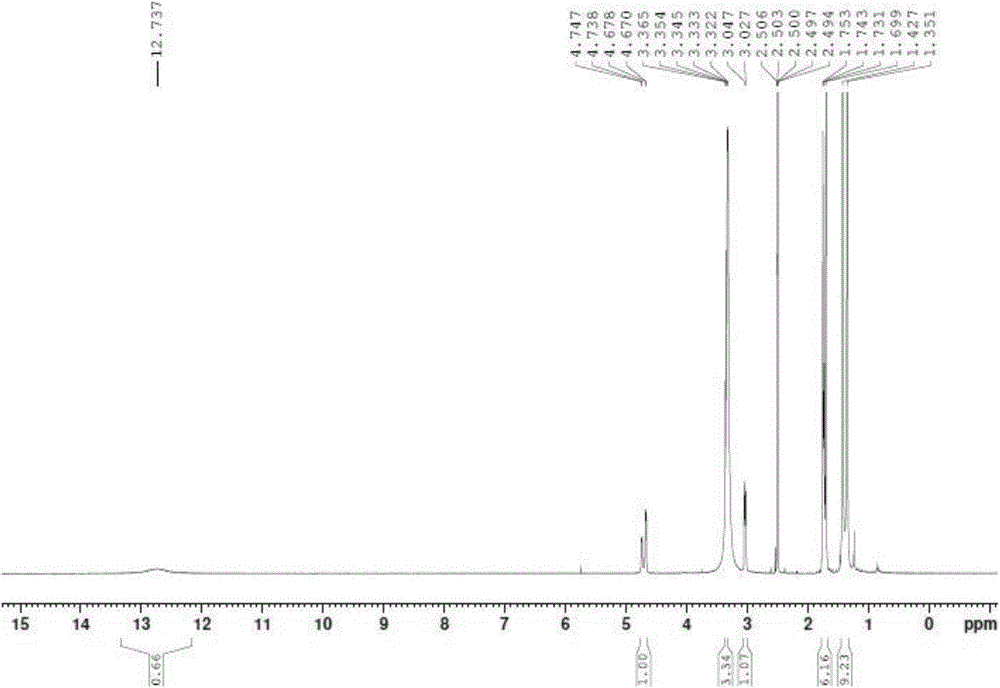
[0085] 2) Preparation of Boc-Apc: Dissolve 10.9g (55mmol) of compound 2 in 250mL of acetonitrile, add 16g (73mmol) of di-tert-butyl dicarbonate, mix well and add 12.8g (99mmol) of N,N-diiso Propylethylamine was stirred at 28°C for 48h. Concentrate the solvent under reduced pressure, then add diethyl ether or cyclohexane to continue concentrating under reduced pressure, then add diethyl ether or cyclohexane, filter with diatomaceous earth, and use 0.01mol / L HCl solution, H...
Embodiment 2
[0092] Example 2: Preparation of cysteine-functionalized hyaluronic acid conjugates
[0093] The preparation method of embodiment 2 is the same as embodiment 1, except that step 6) in embodiment 1 is replaced by:
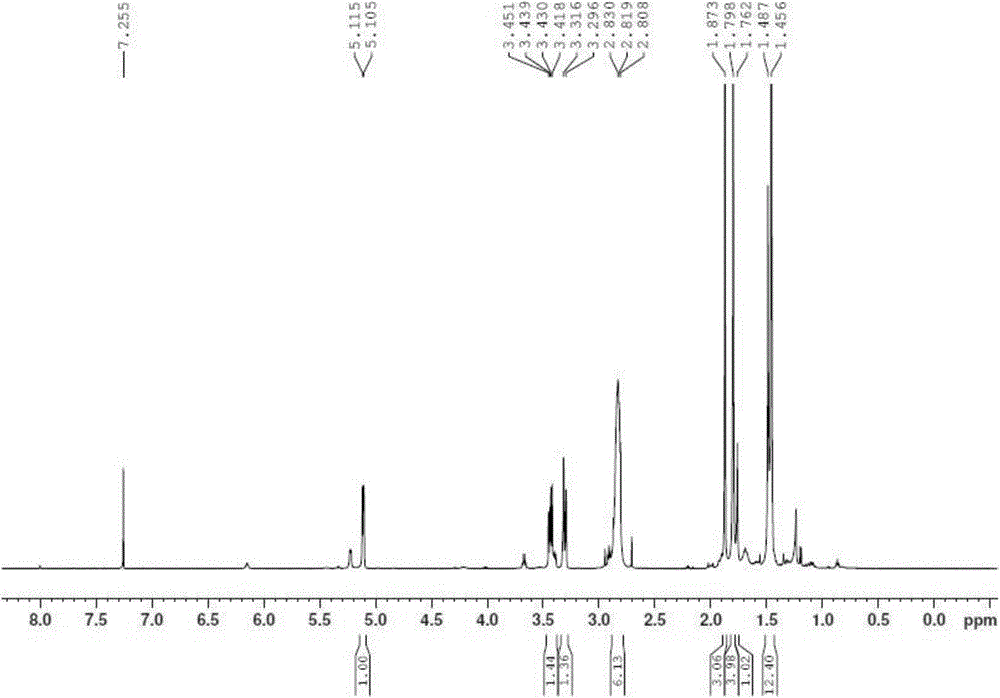
[0094] Preparation of Boc-Apc-PEG-500: Dissolve 5.26g (10mmol) of polyethylene glycol (PEG-500) with a molecular weight of 500 in 5ml of acetonitrile, dissolve 840mg (10mmol) of sodium bicarbonate in 30mL of ultrapure water, and add it. Stir at 28°C, blow argon, dissolve 3.52g (11mmol) of compound 6 in 15mL of acetonitrile, add dropwise to the above reaction solution, pass argon after the dropwise addition, seal, stir and react at 28°C for 12h, TLC detection, Ellamn The color of the reagent does not show yellow, stop the reaction, concentrate the solvent under reduced pressure, extract the water phase with dichloromethane 3 times, wash the combined dichloromethane phase with water 3 times, dry over anhydrous magnesium sulfate for 2 hours, filter, and depressurize C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com