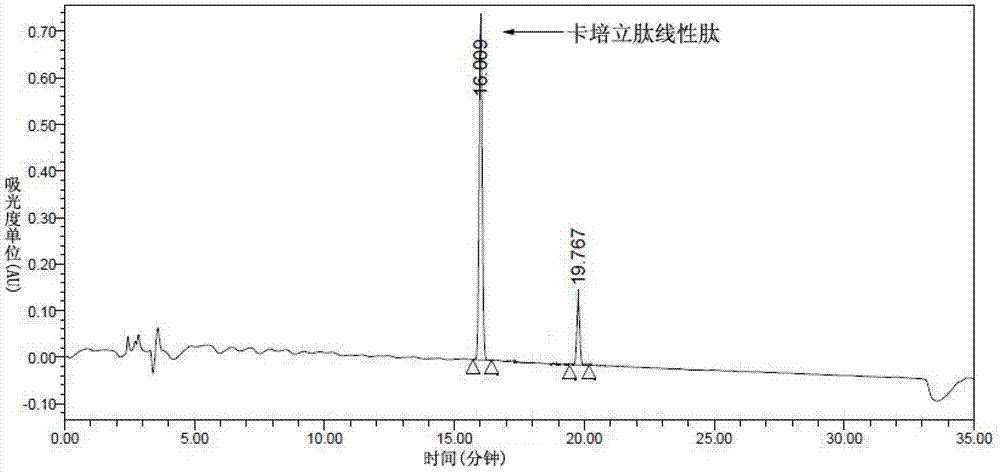
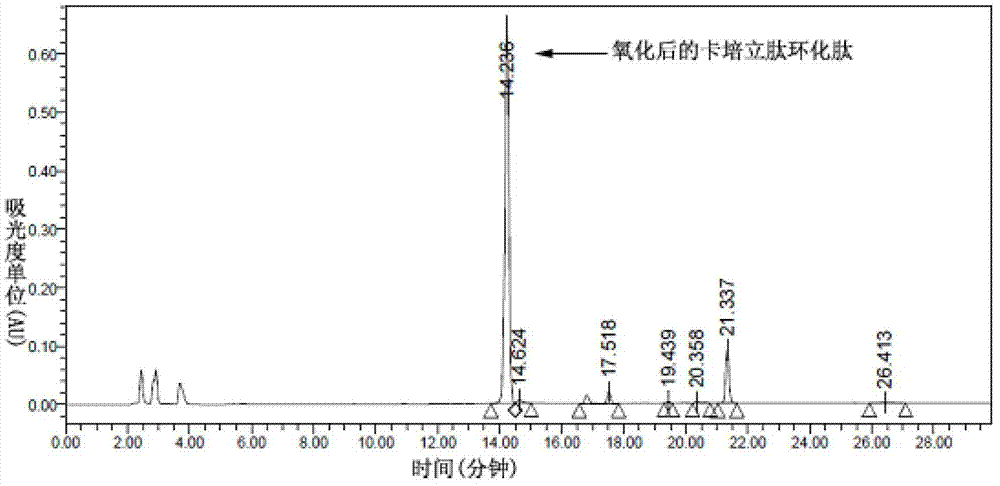
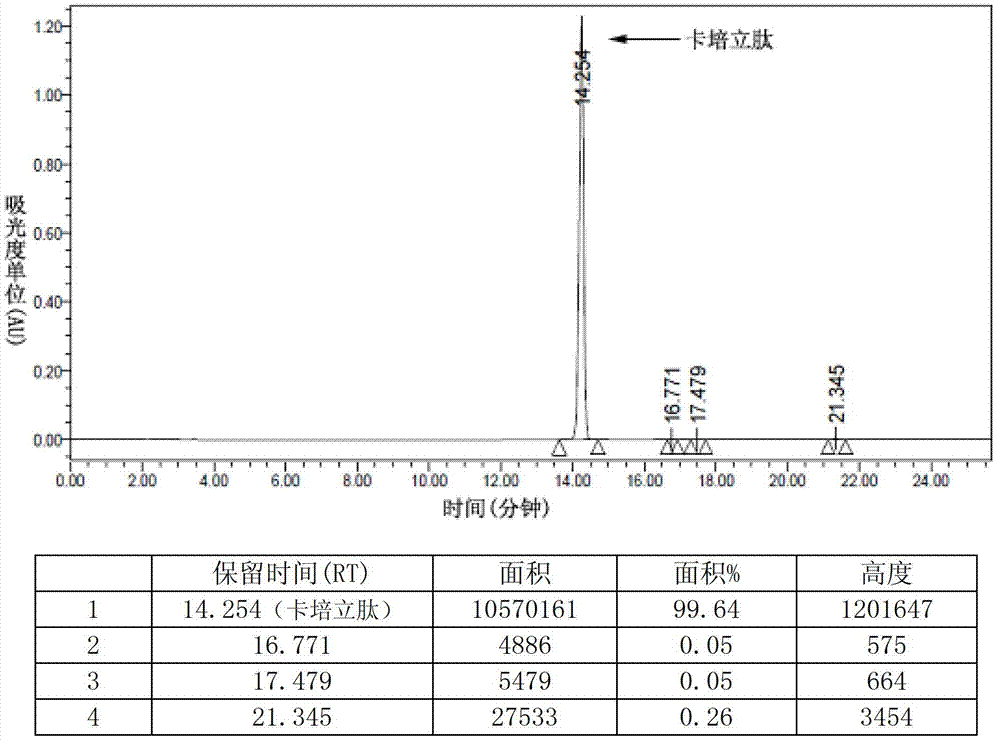
Preparation of carperitide by solid-phase convergence process
A caperitide and fragment technology, which is applied in the preparation field of caperitide, can solve the problems of low drug purity, complicated operation, unfavorable large-scale industrial production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Synthesis of Peptide Resin A'
[0077] Weigh 15.38 g of 2-CTC resin with a substitution degree of 0.65 mmol / g, add it to the solid-phase reaction column, wash it twice with DMF, swell the resin with DMF for 30 minutes, and take 5.94 g of Fmoc-Gly-OH (here and The amino acid used later was purchased from Chengdu Kaitai New Technology Co., Ltd.) was dissolved in DMF, activated by adding 6.97ml of DIEA under an ice-water bath, and then added to the above-mentioned reaction column containing the resin. After 2 hours of reaction, 20ml of anhydrous methanol was added to seal 1 h, washed 6 times with DMF. The Fmoc protection was removed with DBLK for 3 minutes, and the Fmoc protection was continued with new DBLK for 7 minutes, and then washed with DMF for 6 times.
[0078] Dissolve 8.92g Fmoc-Gly-OH, 4.46g HOBT, and 5.13ml DIC in 60ml of DCM and DMF mixed solution with a volume ratio of 1:1, add it to the solid-phase reaction column, and react at room temperature f...
Embodiment 2
[0079] Example 2: Synthesis of Peptide Resin A'
[0080] Weigh 9.17 g of 2-CTC resin with a substitution degree of 1.09 mmol / g, add it to the solid-phase reaction column, wash it twice with DMF, swell the resin with DMF for 30 minutes, take 5.94 g of Fmoc-Gly-OH and dissolve it in DMF, After adding 6.97 ml of DIEA under ice-water bath for activation, it was added to the above reaction column containing resin. After 2 hours of reaction, 20 ml of anhydrous methanol was added to block for 1 hour, and washed 6 times with DMF. The Fmoc protection was removed with DBLK for 3 minutes, and the Fmoc protection was continued with new DBLK for 7 minutes, and then washed with DMF for 6 times.
[0081] Dissolve 8.92g Fmoc-Gly-OH, 4.46g HOBT, 9.63g TBTU, 10.45ml DIEA in 60ml DMF, activate it in an ice-water bath for 3 minutes, add it to the solid-phase reaction column, and react at room temperature for 2h (the reaction end point is detected by the ninhydrin method If the resin is colorless...
Embodiment 3
[0082] Example 3: Synthesis of Peptide Fragment A
[0083] The peptide resin obtained in Example 2 was put into a round bottom flask. The lysis solution was prepared according to the ratio of 1 gram of resin to 10 ml of lysis solution (the ratio of lysis solution: TFE:DCM=1:4, V:V), poured the lysis solution into the flask, and reacted at room temperature for 2.5h. After the reaction was completed, the resin was filtered and the filtrate was collected. After the filtrate was evaporated to dryness on a rotary evaporator, 20 ml of DCM was added, the mixture was added dropwise to 200 ml of ether, a white solid was precipitated, centrifuged, washed with anhydrous ether, and dried in vacuo to obtain a side chain fully protected peptide fragment A (sequence ( 1-10)) 21.27g, purity 94.8%, yield 93.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com