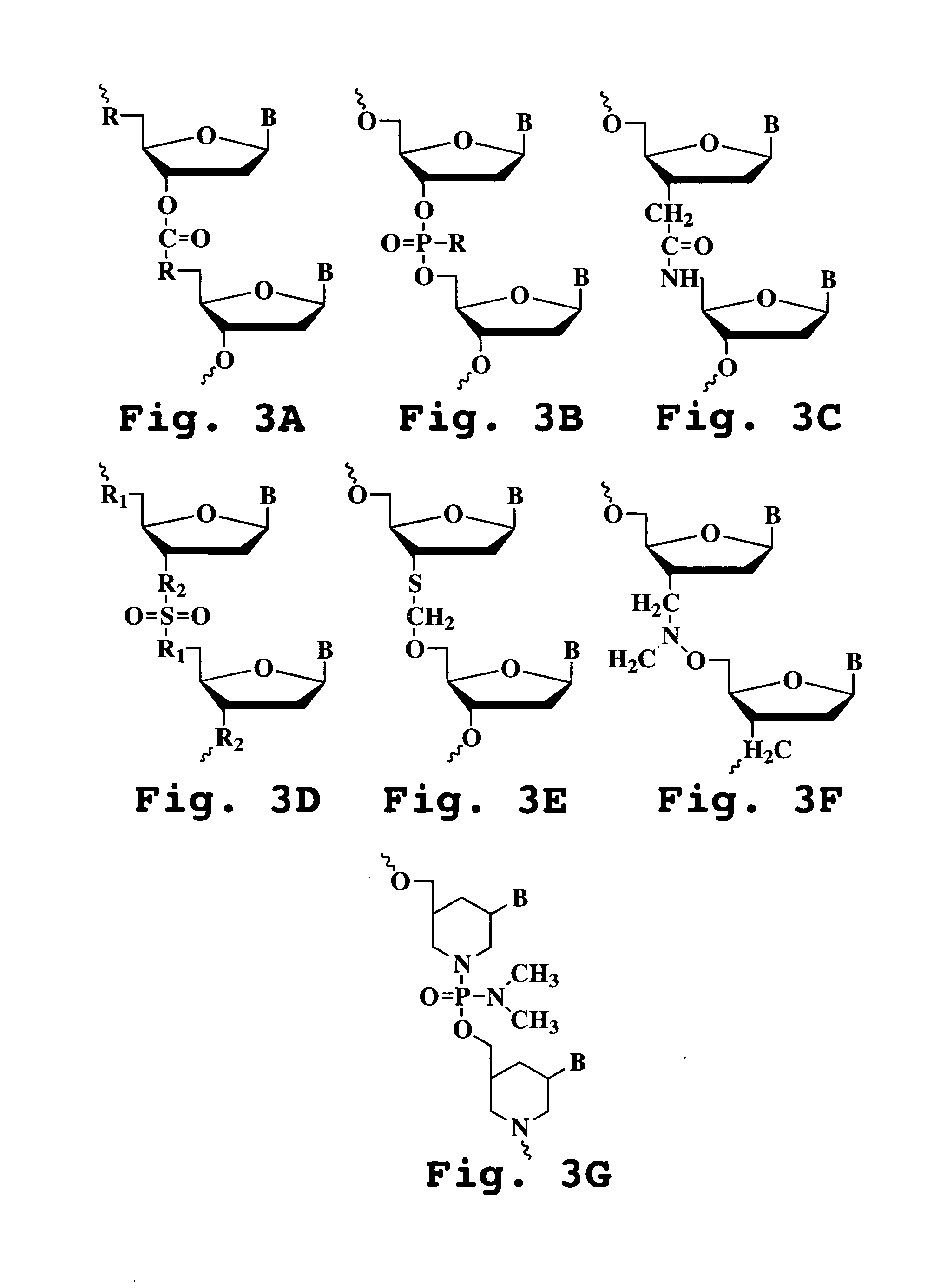
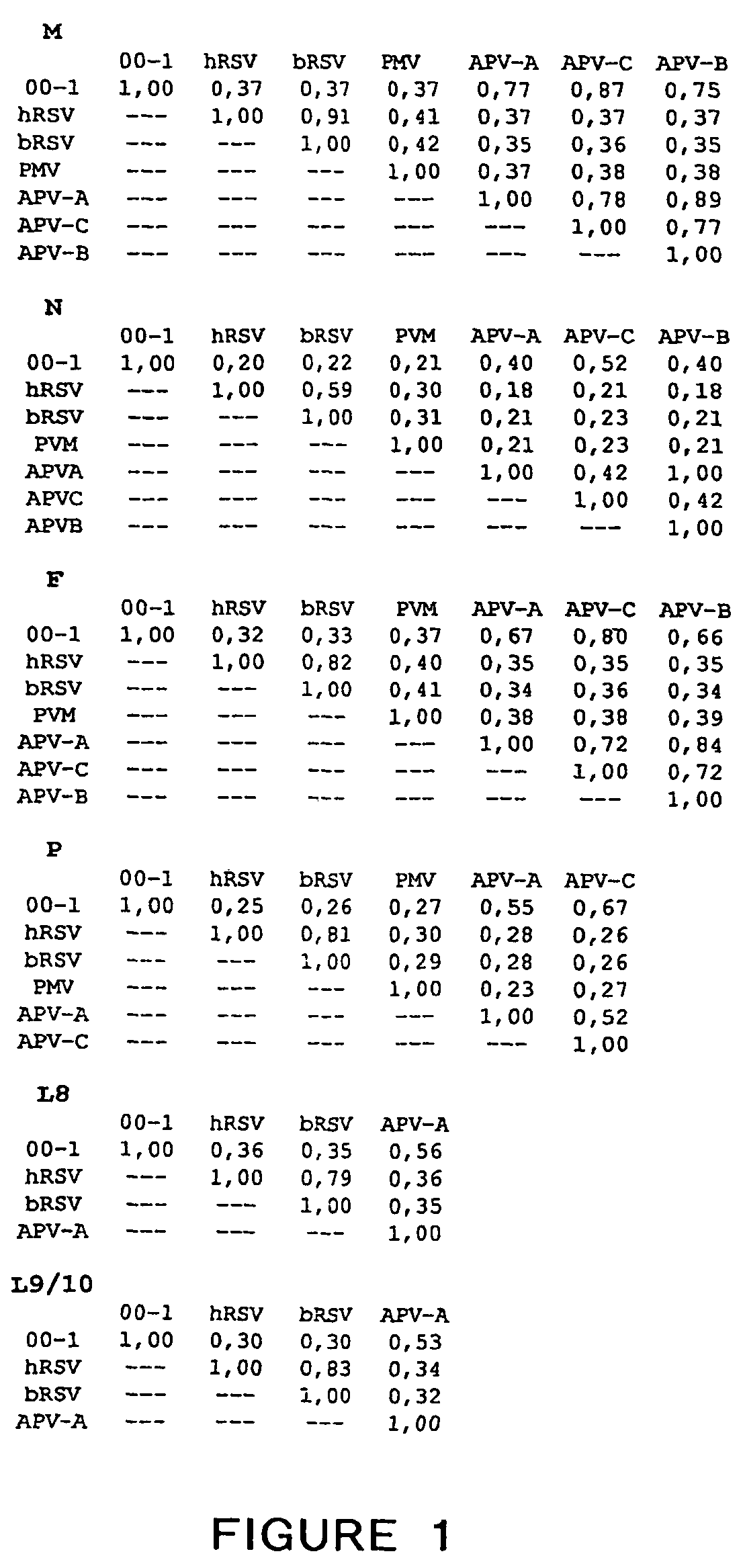
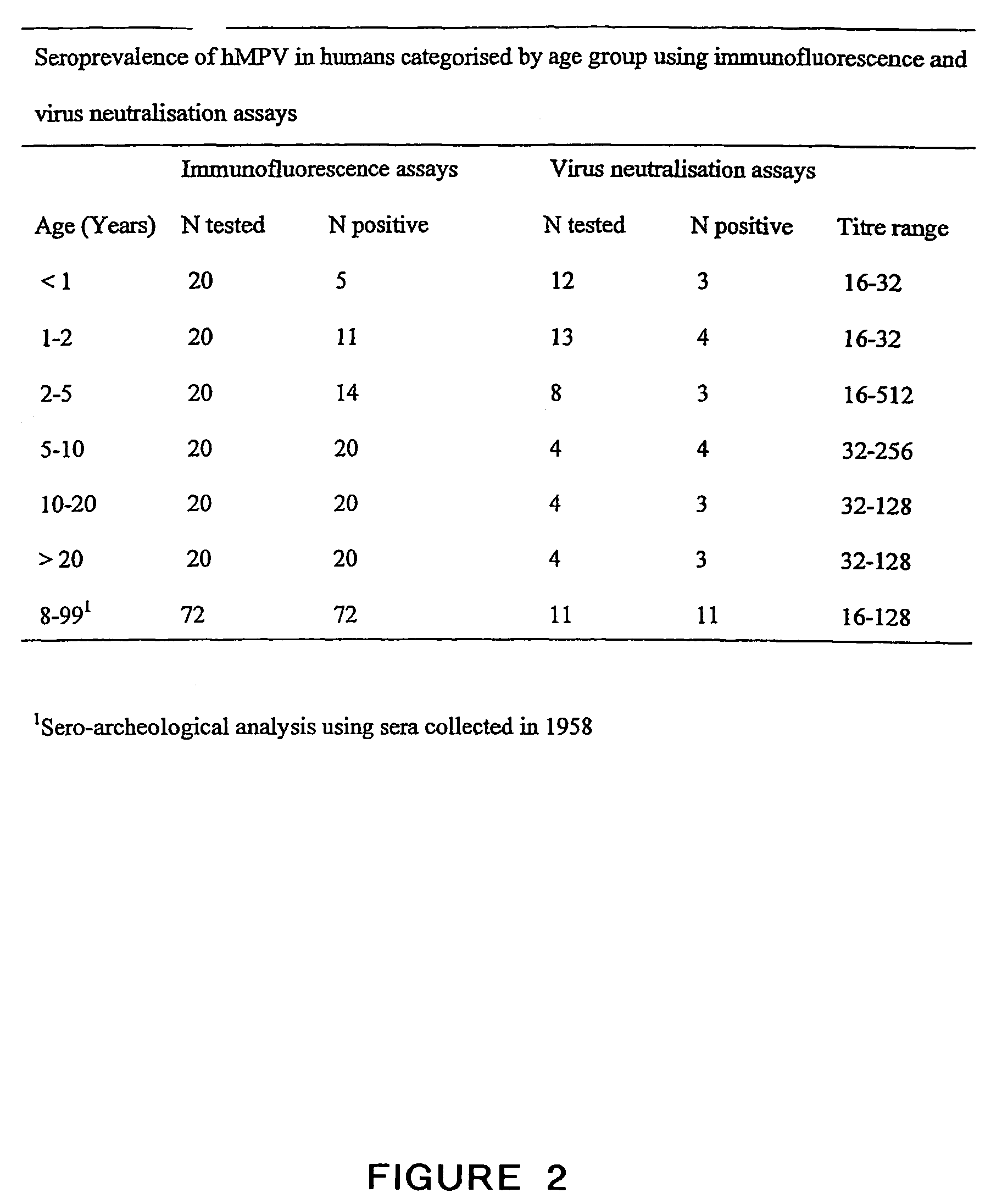
Patents
Literature
117 results about "Negative strand" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The positive strand of DNA is the one whose sequence contains the instructions for building a protein. The negative strand merely contains the complementary sequence, according to the base-pairing rules (A pairs with T, C pairs with G); the negative strand is not normally transcribed into RNA nor translated into protein.
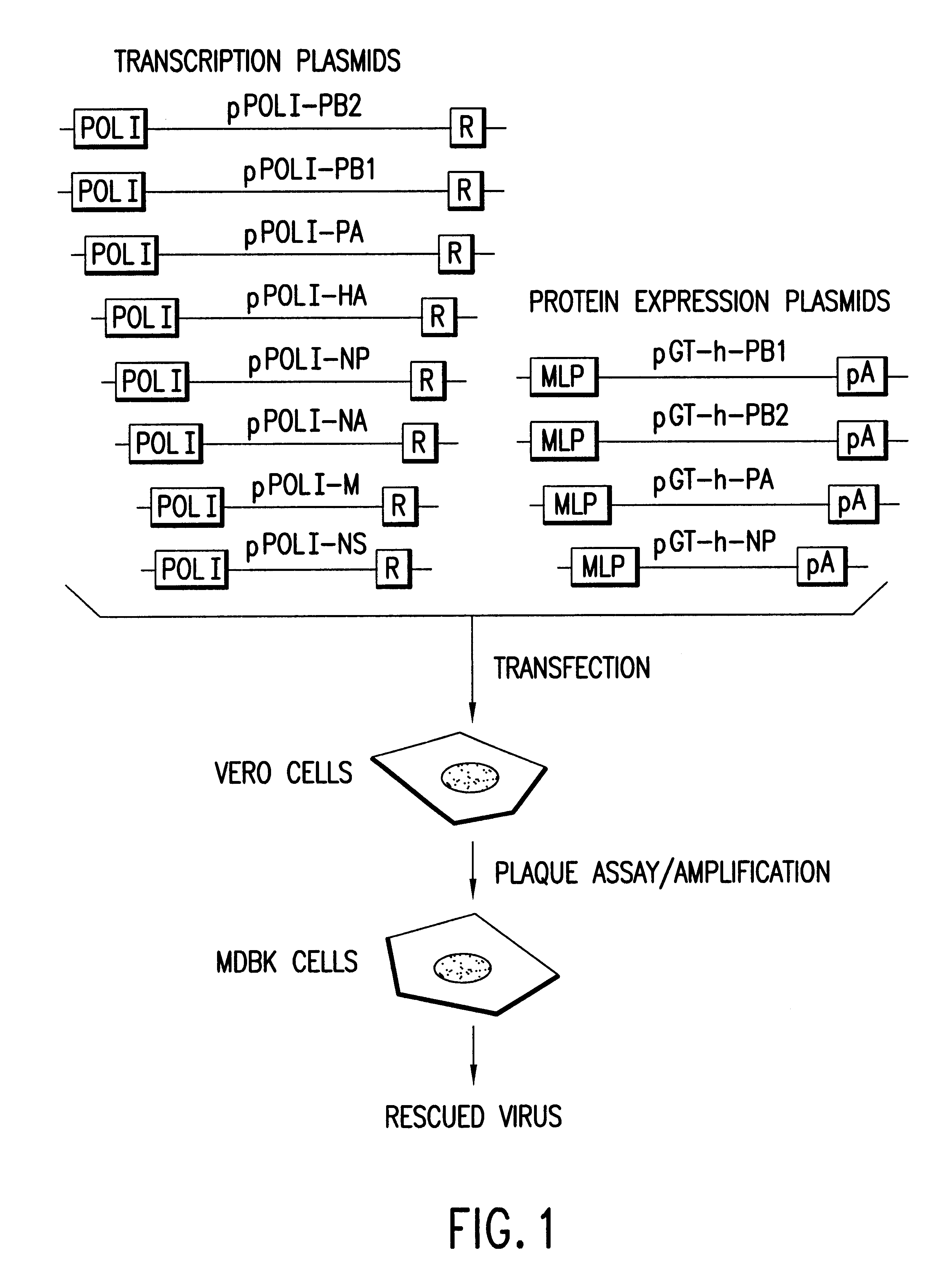
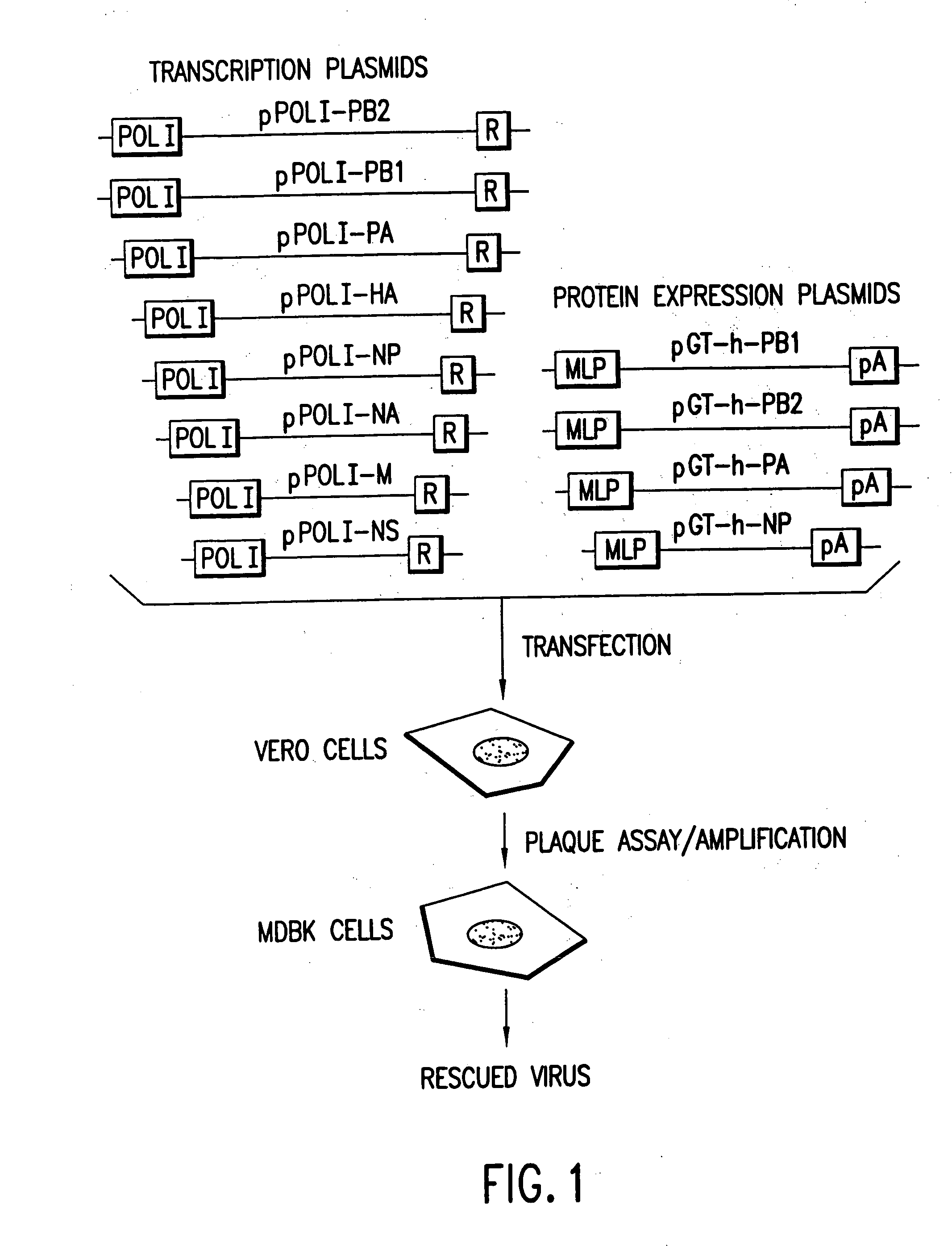
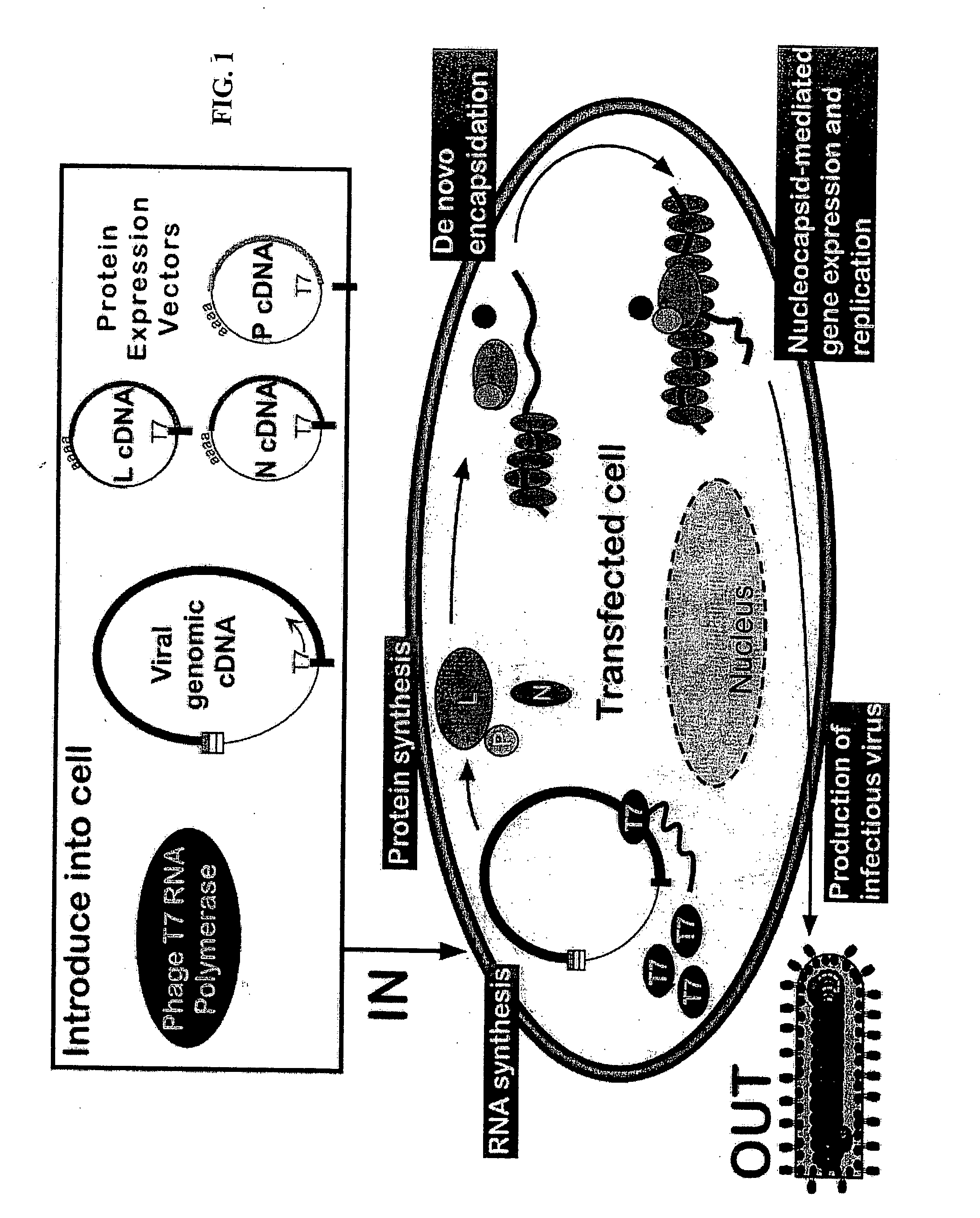
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
Helper-free rescue of recombinant negative strand RNA viruses
InactiveUS6544785B1SsRNA viruses negative-senseGenetic material ingredientsNegative strandNucleic acid sequence
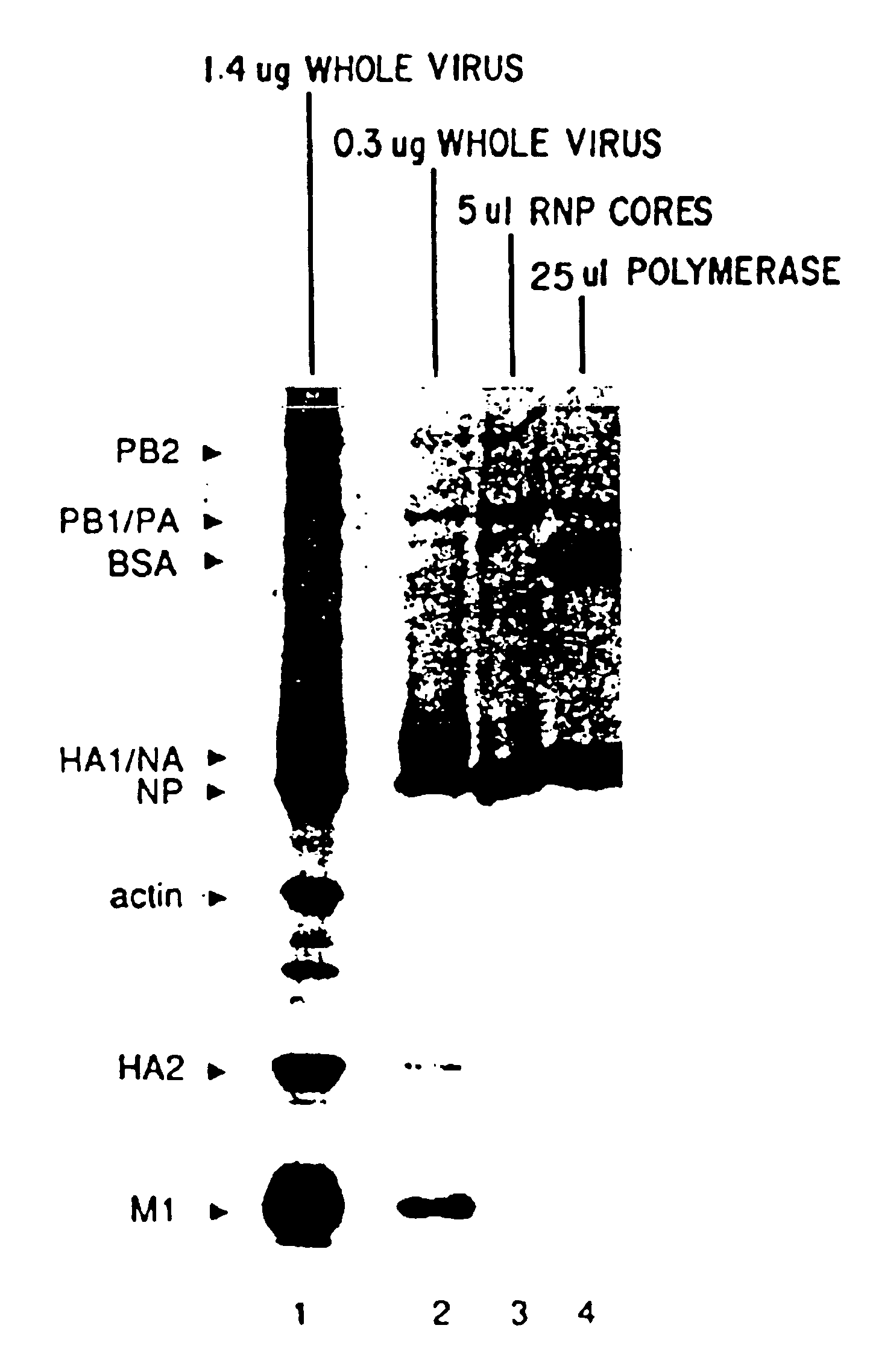
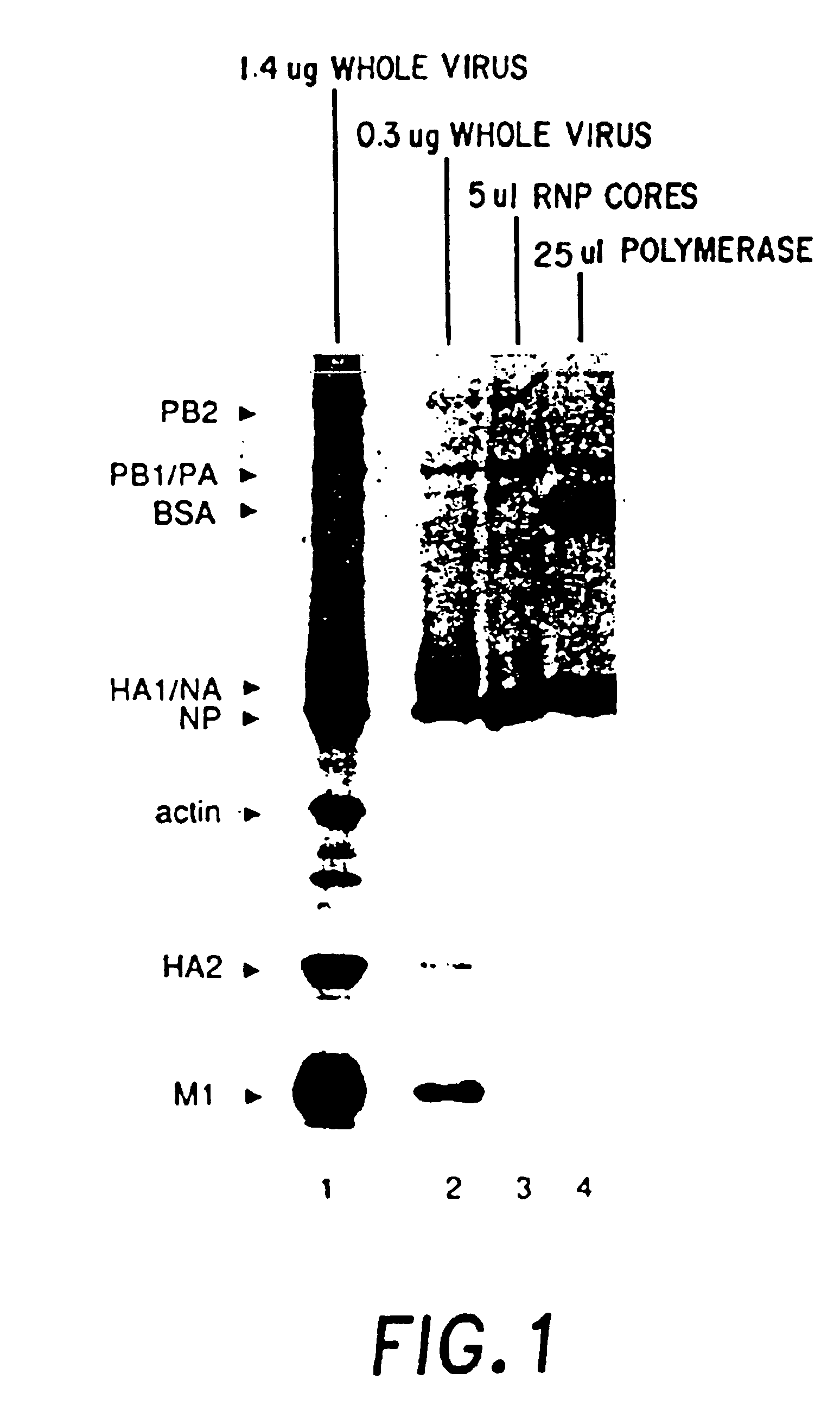
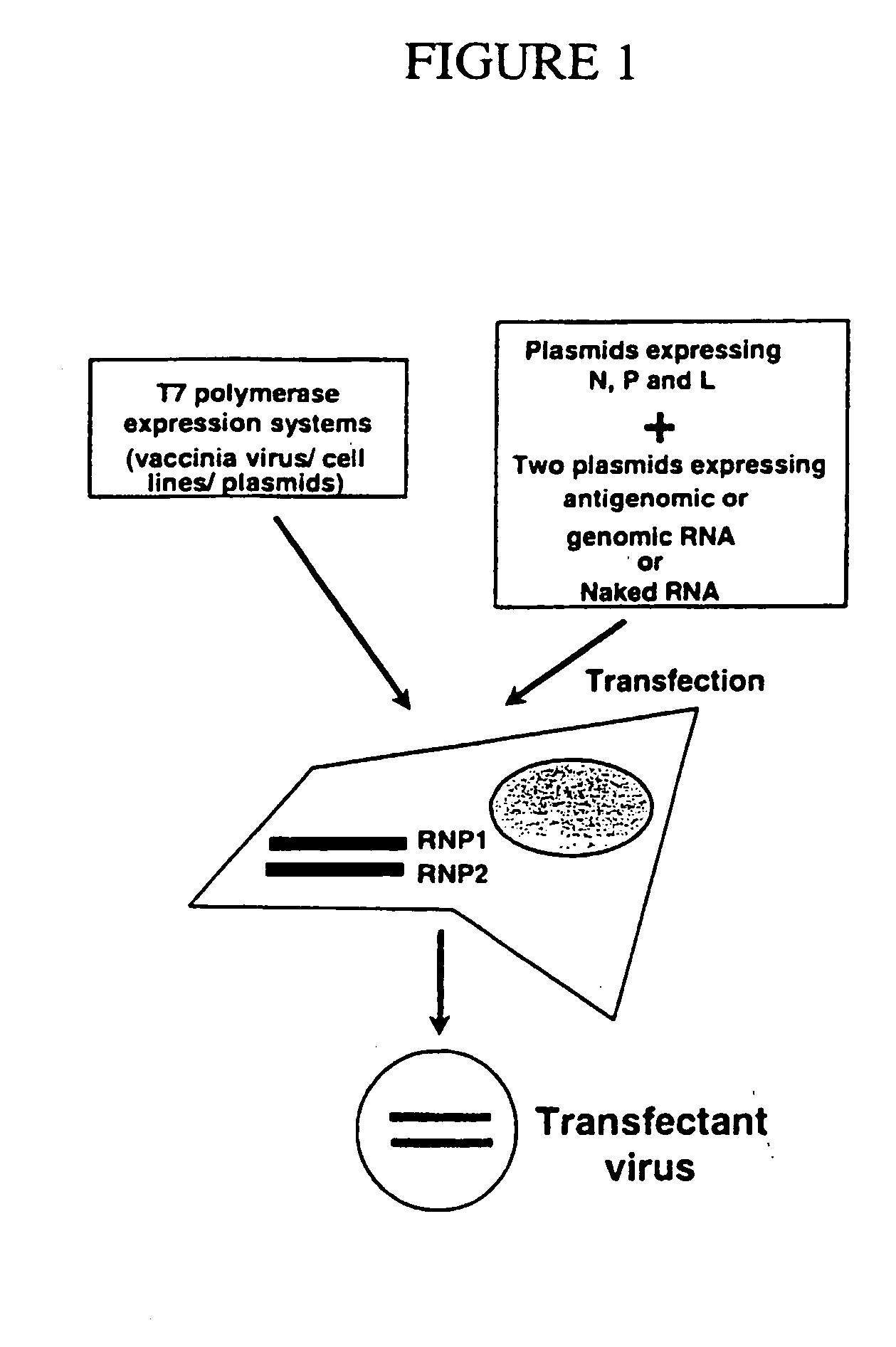
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
Effecting virus resistance in plants through the use of negative strand RNAs
InactiveUS6617496B1Sugar derivativesOther foreign material introduction processesNegative strandPlant cell
A strategy for effecting virus resistance in plants causes the transcription in the plant cells of negative RNA strands which are substantially complementary to a target RNA strand. The target RNA strand can be an mRNA transcript created in gene expression, a viral RNA, or other RNA present in the plant cells. The negative RNA strand is complementary to at least a portion of the target RNA strand to inhibit its activity in vivo.
Owner:AGRACETUS MIDDLETON WISCONSIN A GENERAL PARTNERSHIP OF NEW YORK +1
Chimeric viruses presenting non-native surface proteins and uses thereof
ActiveUS20120122185A1Readily immunizeEffective immune responseSsRNA viruses negative-sensePolypeptide with localisation/targeting motifEctodomainVirosome
The present invention provides chimeric negative-stand RNA viruses that allow a subject, e.g., an avian, to be immunized against two infectious agents by using a single chimeric virus of the invention. In particular, the present invention provides chimeric influenza viruses engineered to express and incorporate into their virions a fusion protein comprising an ectodomain of a protein of an infectious agent and the transmembrane and cytoplasmic domain of an influenza virus protein. Such chimeric viruses induce an immune response against influenza virus and the infectious agent. The present invention also provides chimeric Newcastle Disease viruses (NDV) engineered to express and incorporate into their virions a fusion protein comprising the ectodomain of a protein of an infectious agent and the transmembrane and cytoplasmic domain of an NDV protein. Such chimeric viruses induce an immune response against NDV and the infectious agent.
Owner:MT SINAI SCHOOL OF MEDICINE
Recombinant negative strand RNA virus expression systems and vaccines
Owner:MEDIMMUNE VACCINES
Attenuated negative strand viruses with altered interferon antagonist activity for use as vaccines and pharmaceuticals
InactiveUS6669943B1Impaired ability to antagonizeAvoiding and minimizing side effectSsRNA viruses negative-senseVirus peptidesNegative strandPharmaceutical drug
Owner:AVIR GREEN HILLS BIOTECH RES DEVMENT TRADE +1
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences and methods for propagating virus
ActiveUS20050019891A1Narrow downSymptoms improvedSsRNA viruses negative-senseVirus peptidesNegative strandHeterologous
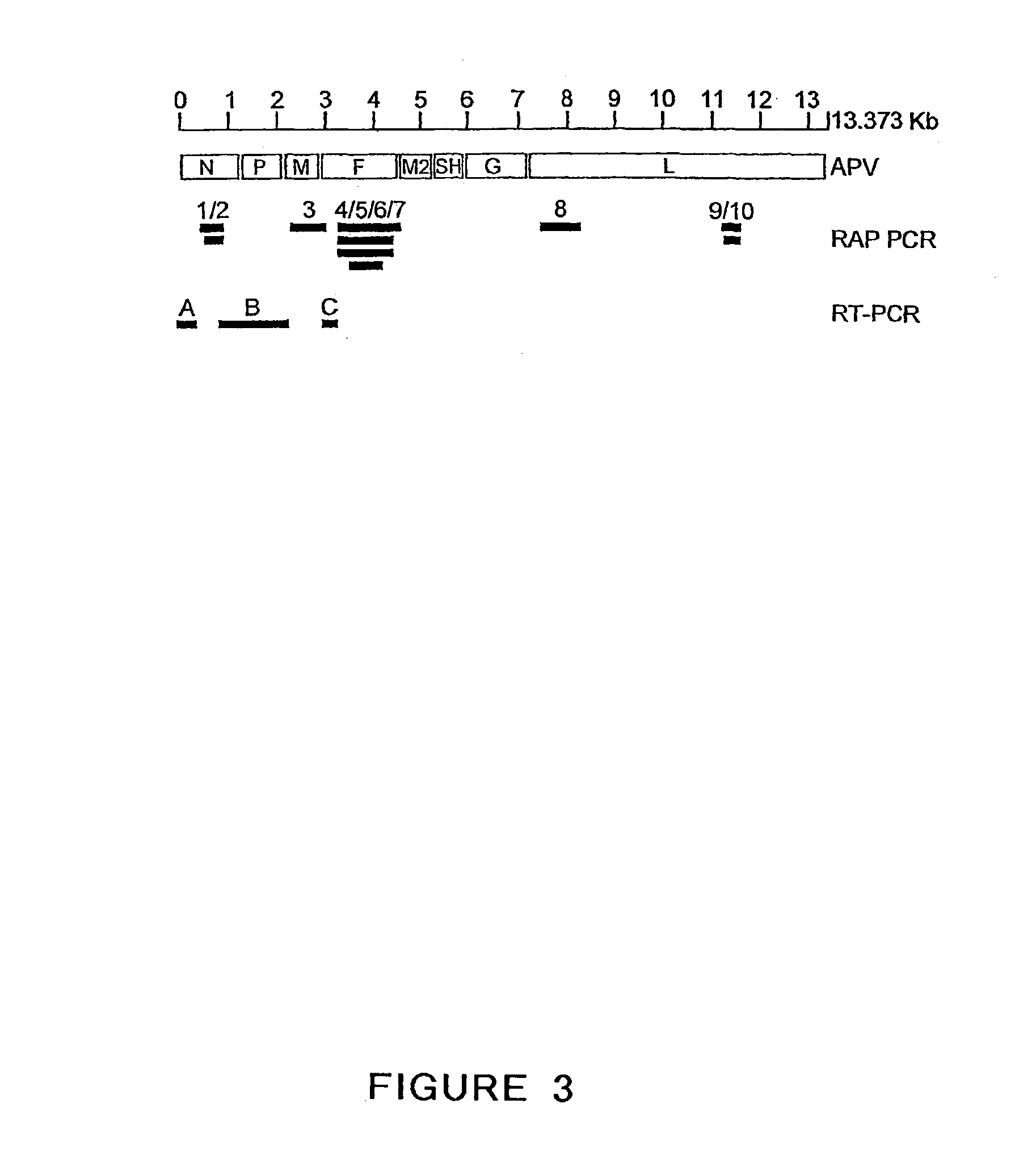
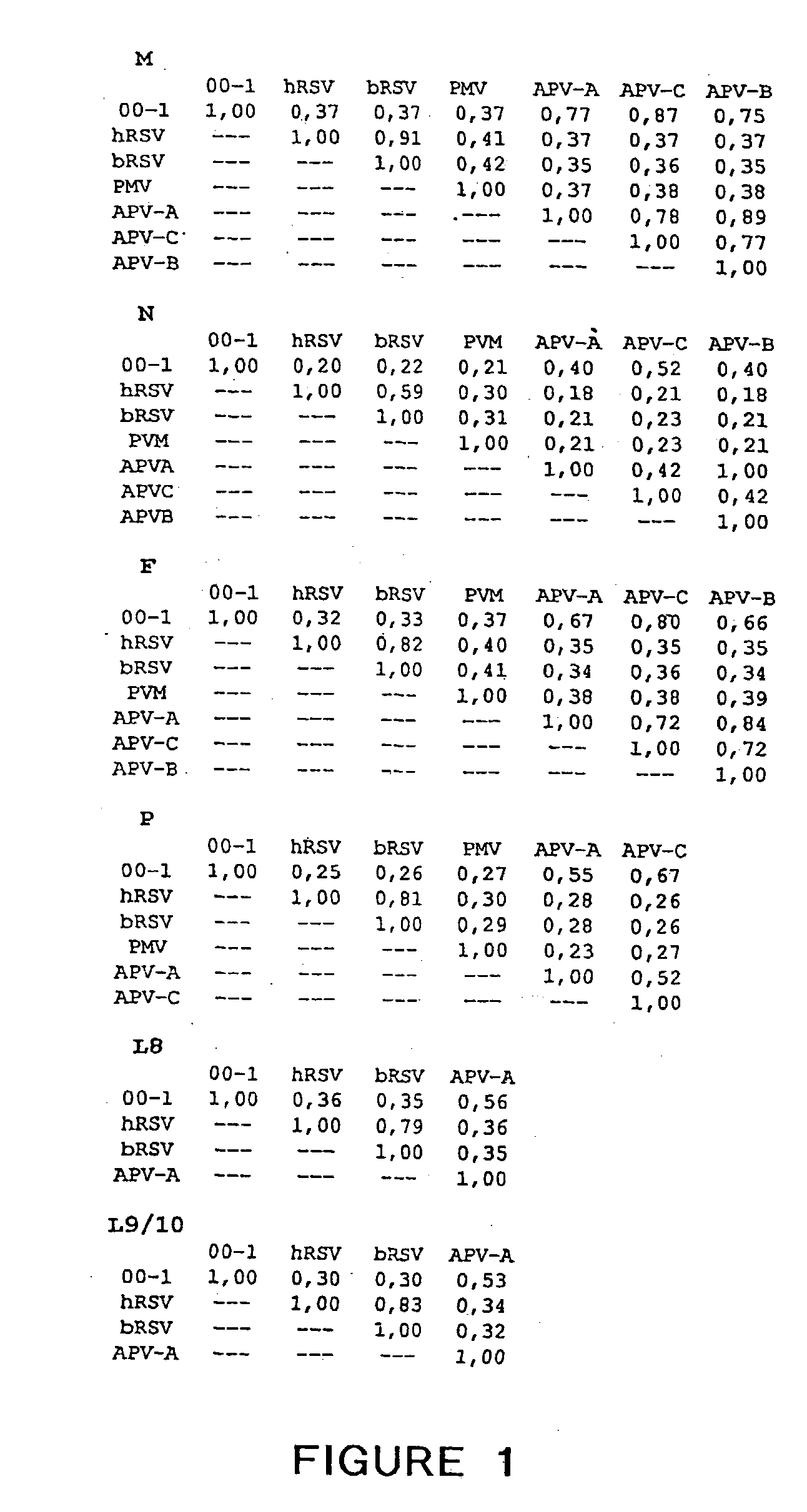
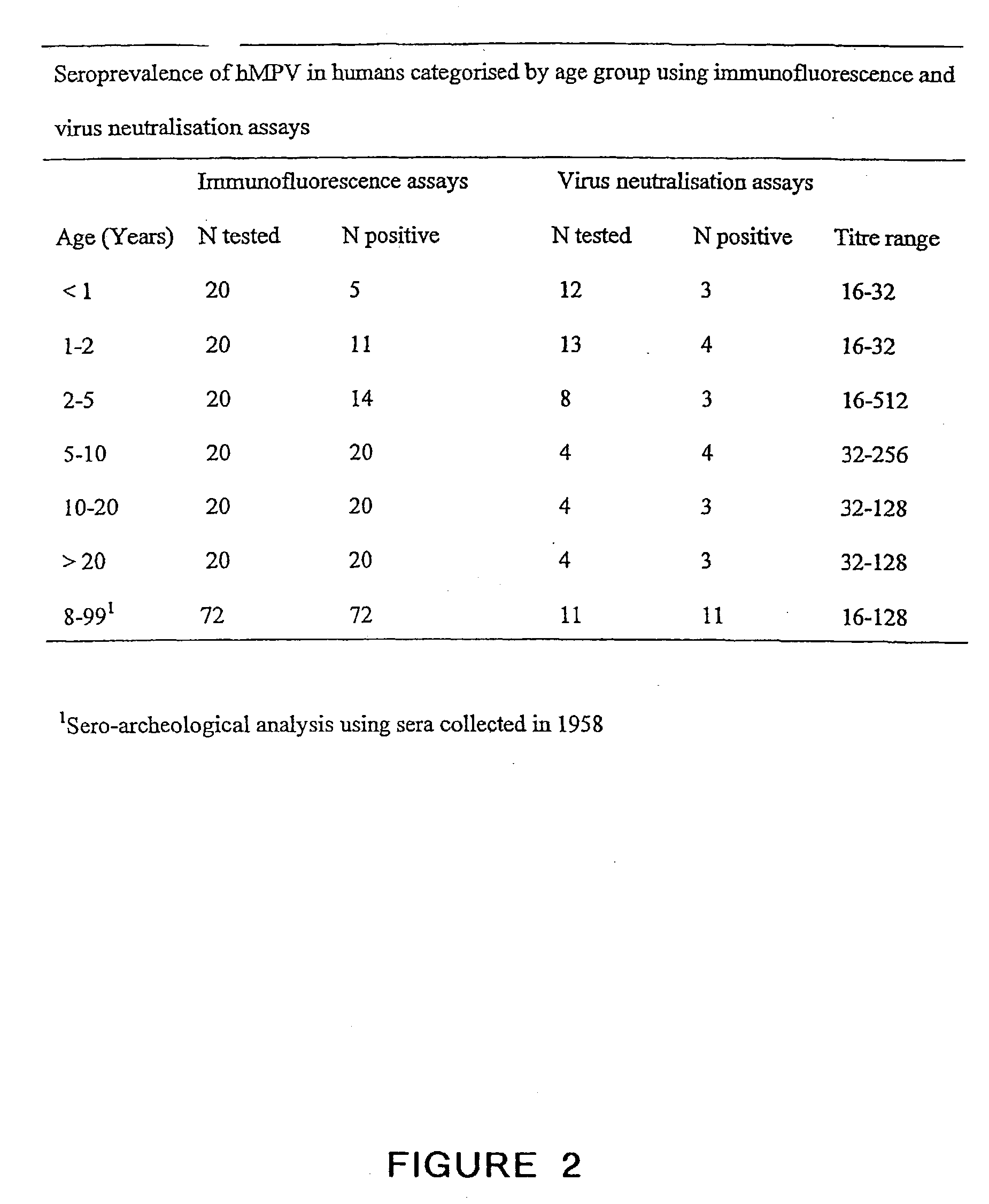
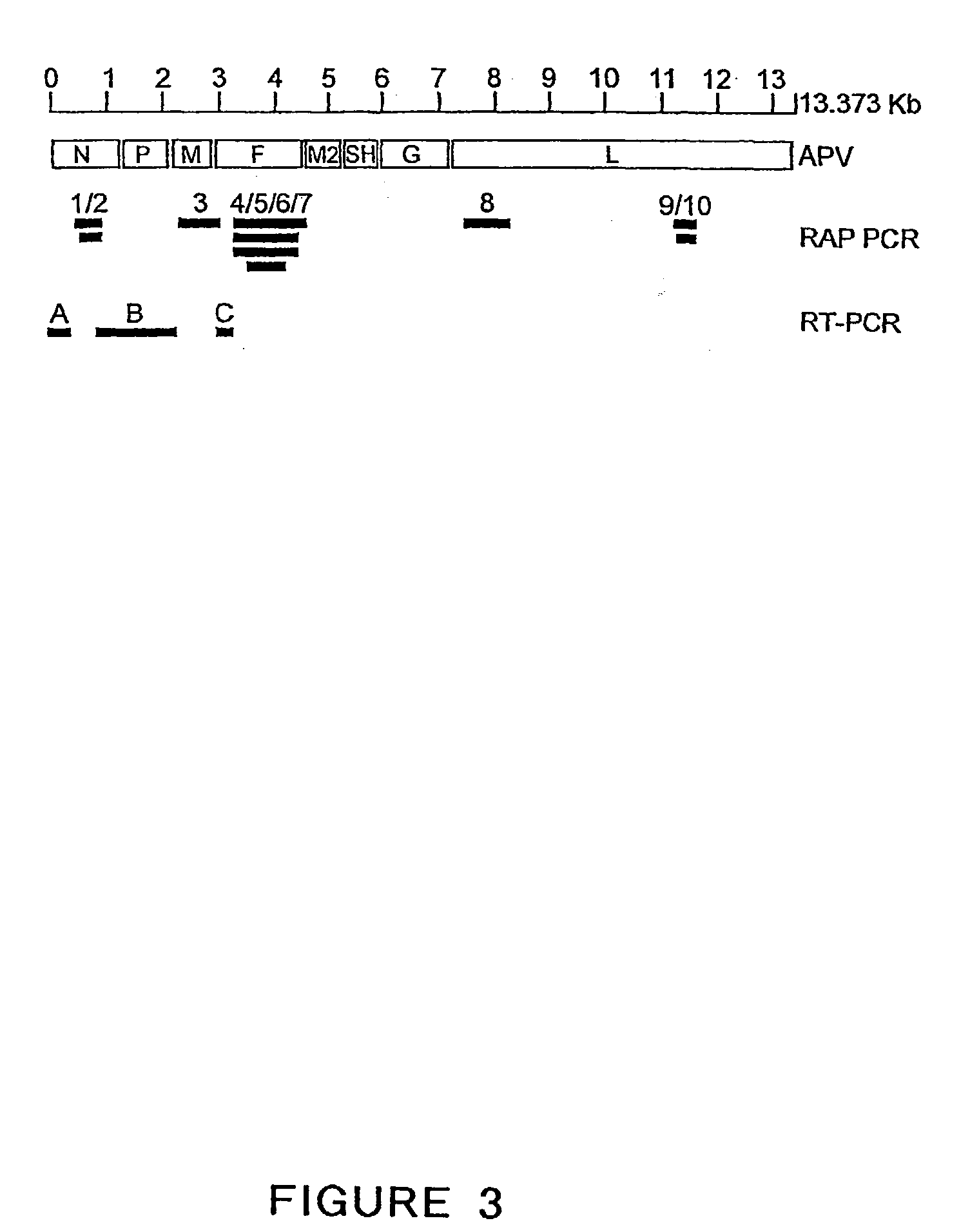
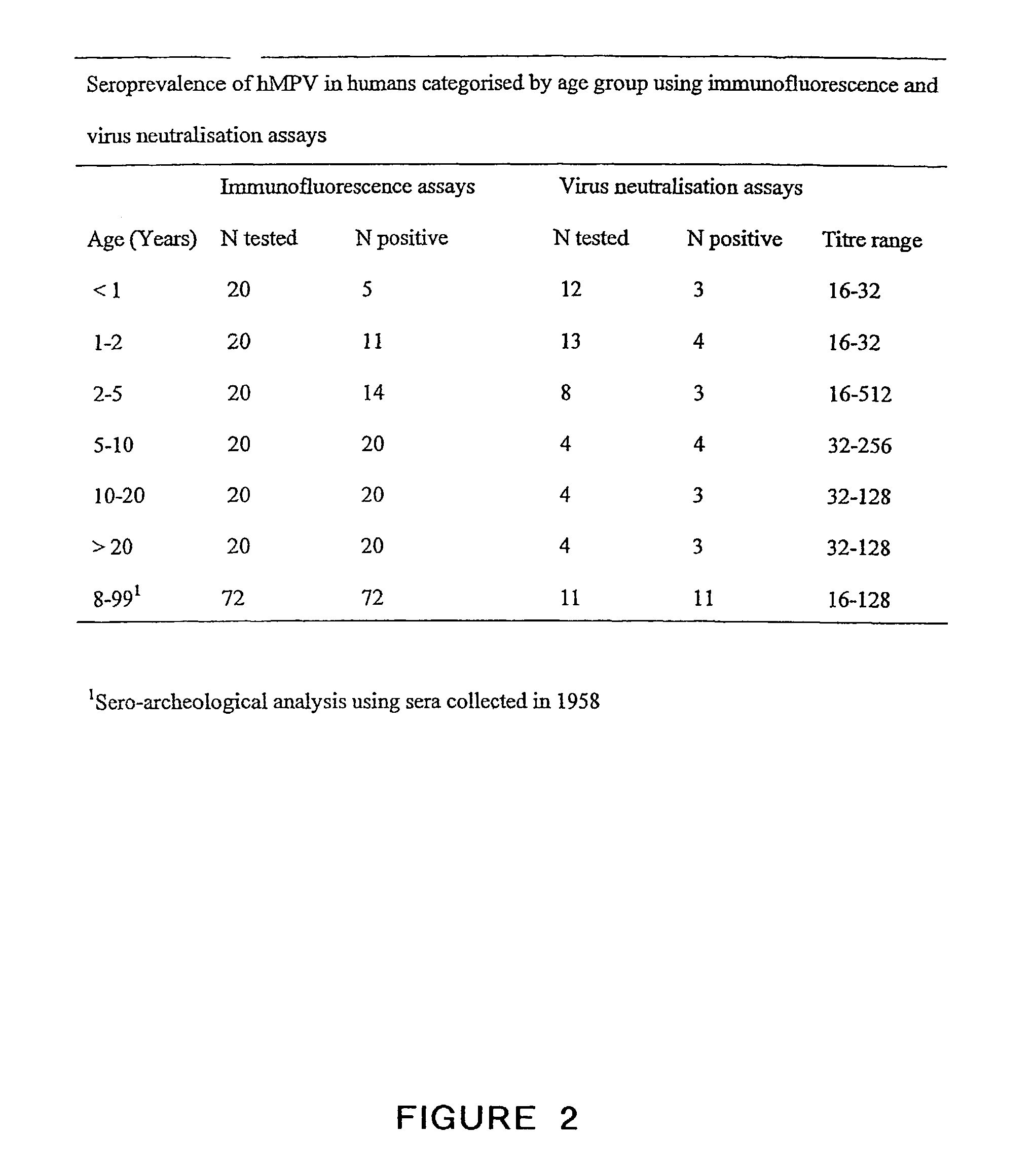
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations. The invention also provide methods for propagating virus.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
Owner:VIRONOVATIVE
Recombinant parainfluenza virus expression systems and vaccines comprising heterologous antigens derived from metapneumovirus
InactiveUS20030232061A1Optimization orderImprove scalabilitySsRNA viruses negative-senseVectorsNegative strandAntigen
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. In particular, the heterologous gene products include gene product of another species of PIV or from another negative strand RNA virus, including but not limited to, influenza virus, respiratory syncytial virus, human metapneumovirus and avian pneumovirus. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:VIRONOVATIVE
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences
ActiveUS20040005544A1Narrow downSymptoms improvedOrganic active ingredientsFungiHeterologousNegative strand
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
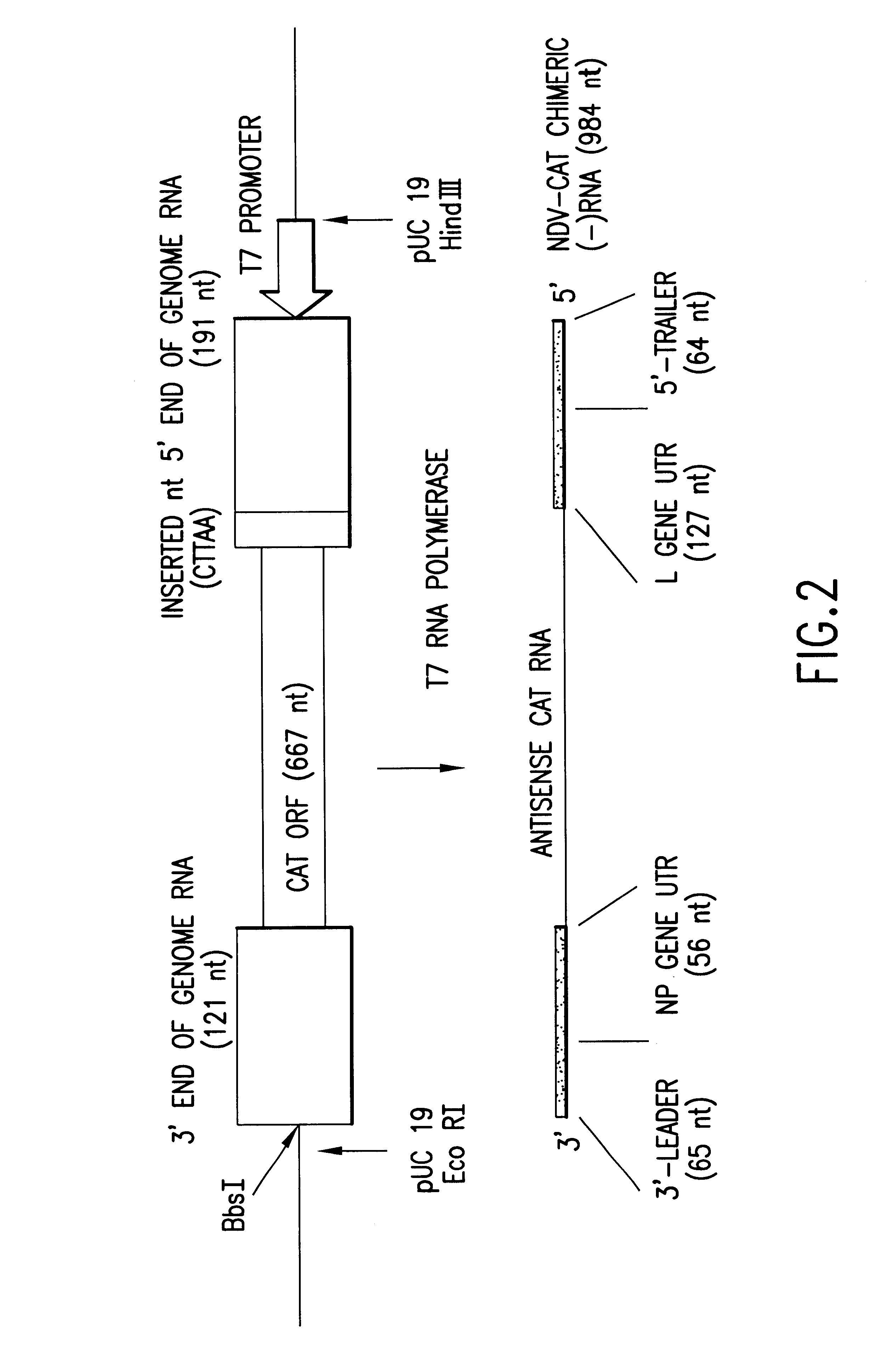
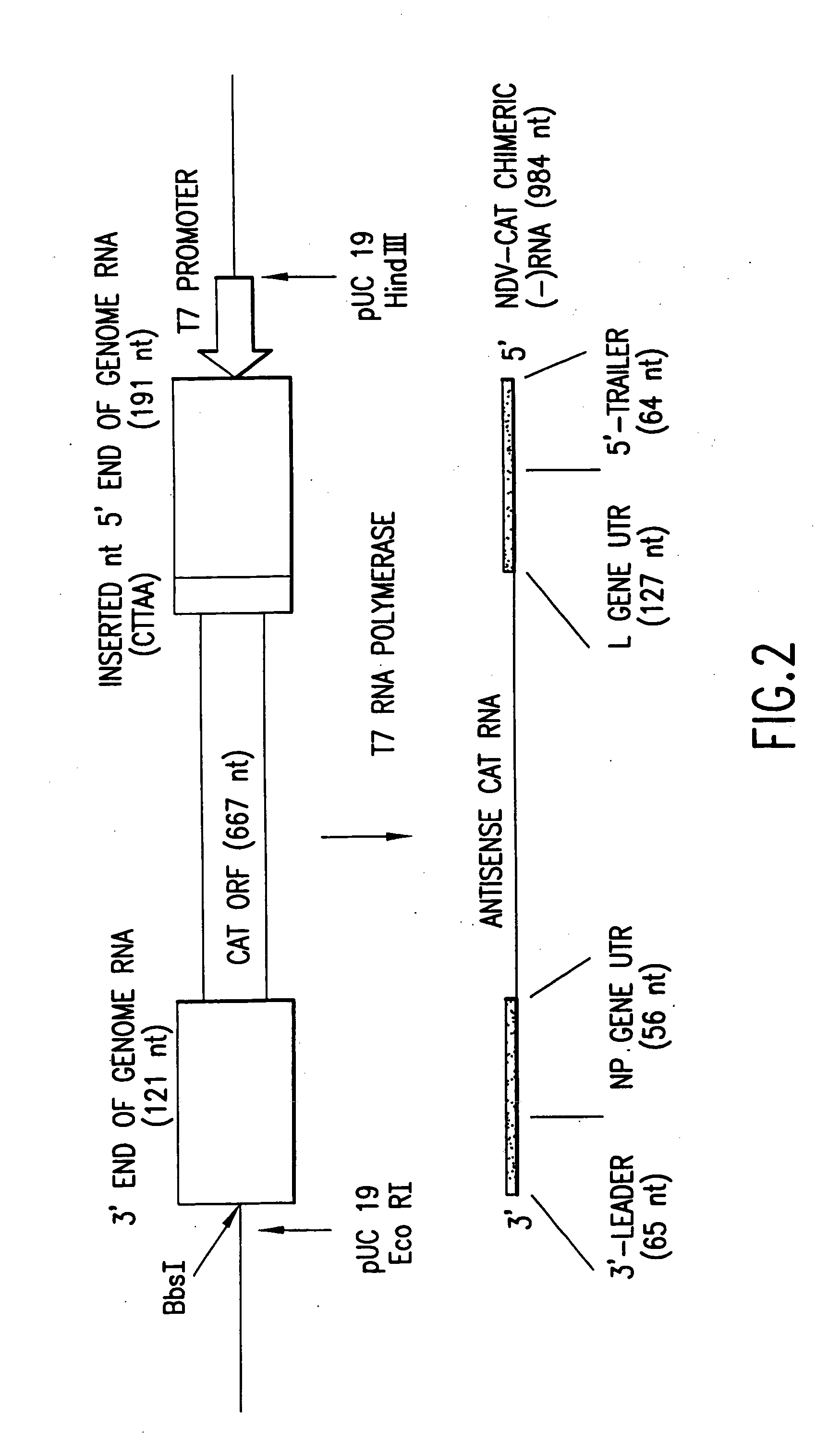
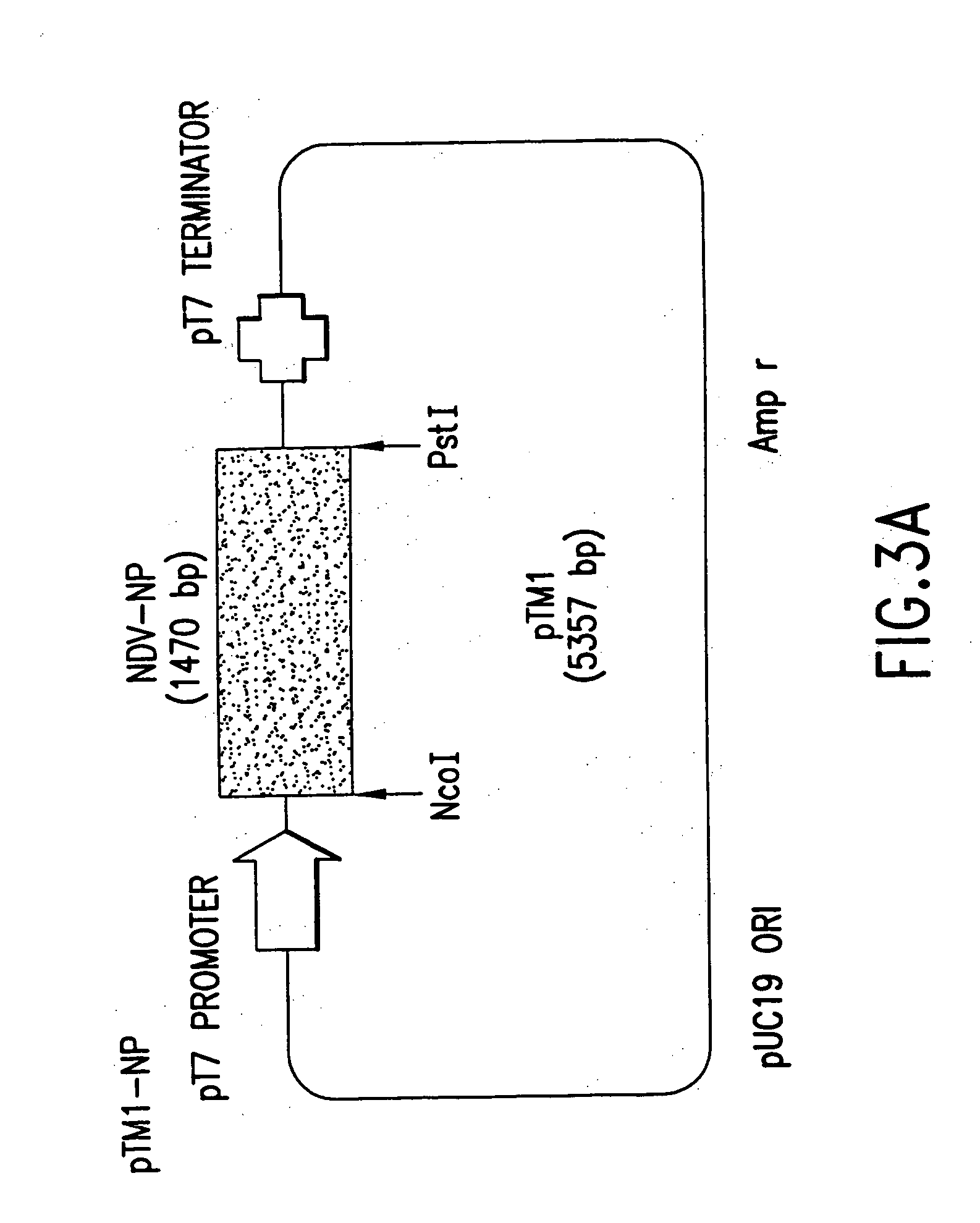
Recombinant Newcastle disease virus RNA expression systems and vaccines
This invention relates to genetically engineered Newcastle disease viruses and viral vectors which express heterologous genes or mutated Newcastle disease viral genes or a combination of viral genes derived from different strains of Newcastle disease virus. The invention relates to the construction and use of recombinant negative strand NDV viral RNA templates which may be used with viral RNA-directed RNA polymerase to express heterologous gene products in appropriate host cells and / or to rescue the heterologous gene in virus particles. In a specific embodiment of the invention, the heterologous gene product is a peptide or protein derived from the genome of a human immunodeficiency virus. The RNA templates of the present invention may be prepared by transcription of appropriate DNA sequences using any DNA-directed RNA polymerase such as bacteriophage T7, T3, SP6 polymerase, or eukaryotic polymerase I.
Owner:MT SINAI SCHOOL OF MEDICINE
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
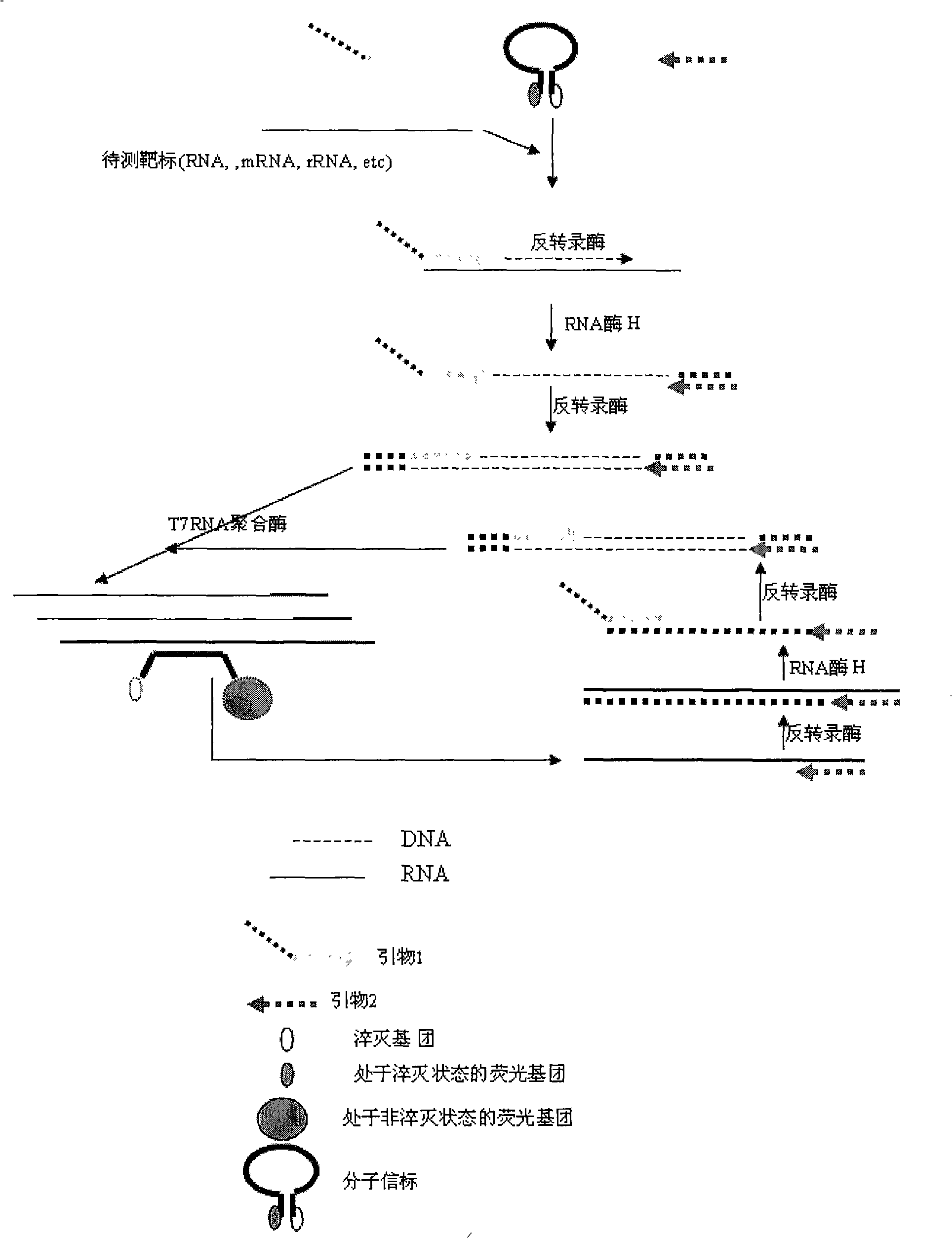
Constant temperature synchronous amplification detecting process for nucleic acid and use thereof
ActiveCN101333565AAvoid pollutionShorten the timeMicrobiological testing/measurementFluorescence/phosphorescenceNegative strandFluorescence
Owner:SHANGHAI RENDU BIOTECH
Attenuated negative strand viruses with altered interferon antagonist activity for use as vaccines and pharmaceuticals
InactiveUS20040109877A1Inhibition of replicationReduce the numberSsRNA viruses negative-senseViral antigen ingredientsNegative strandPharmaceutical drug
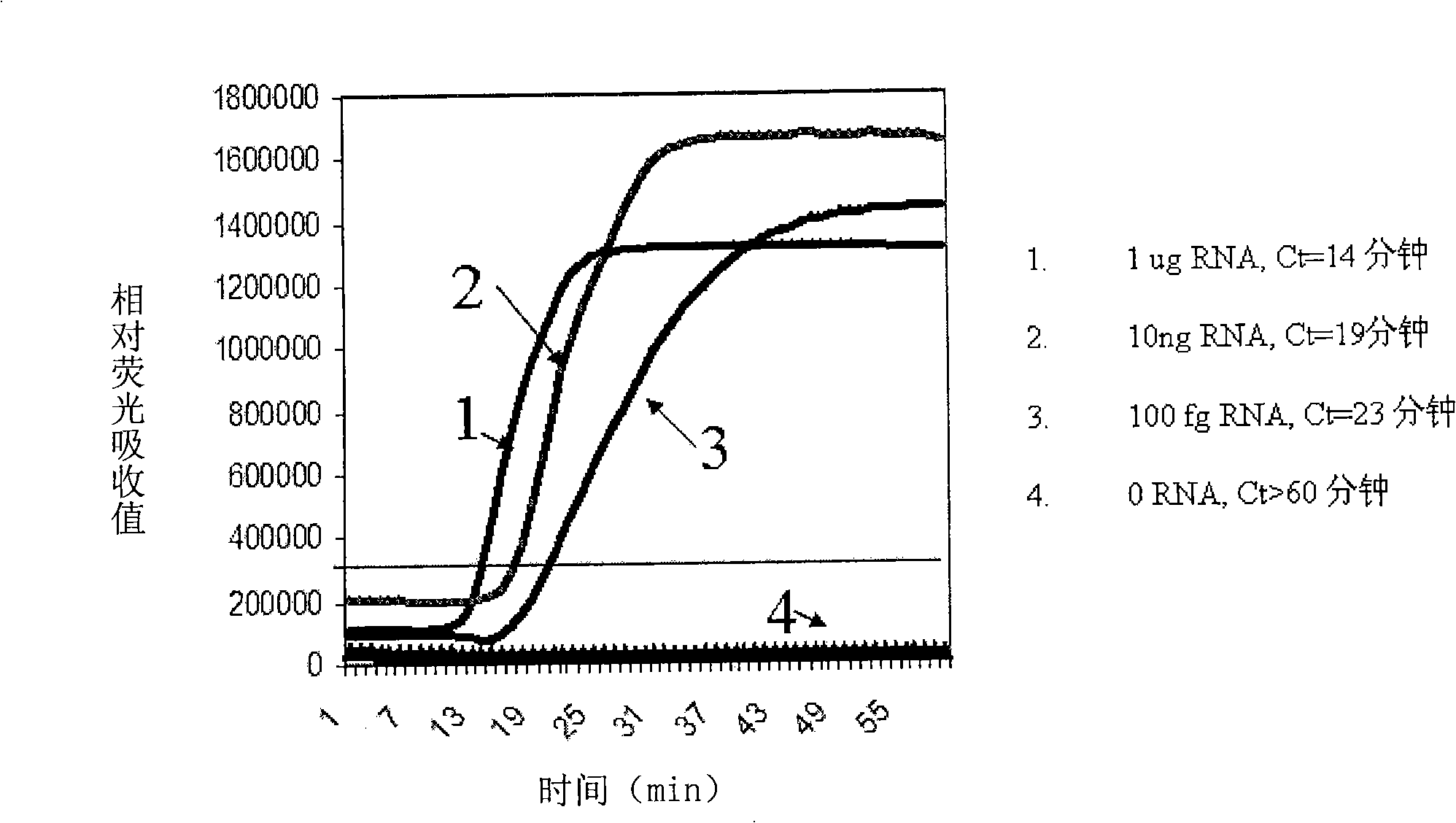
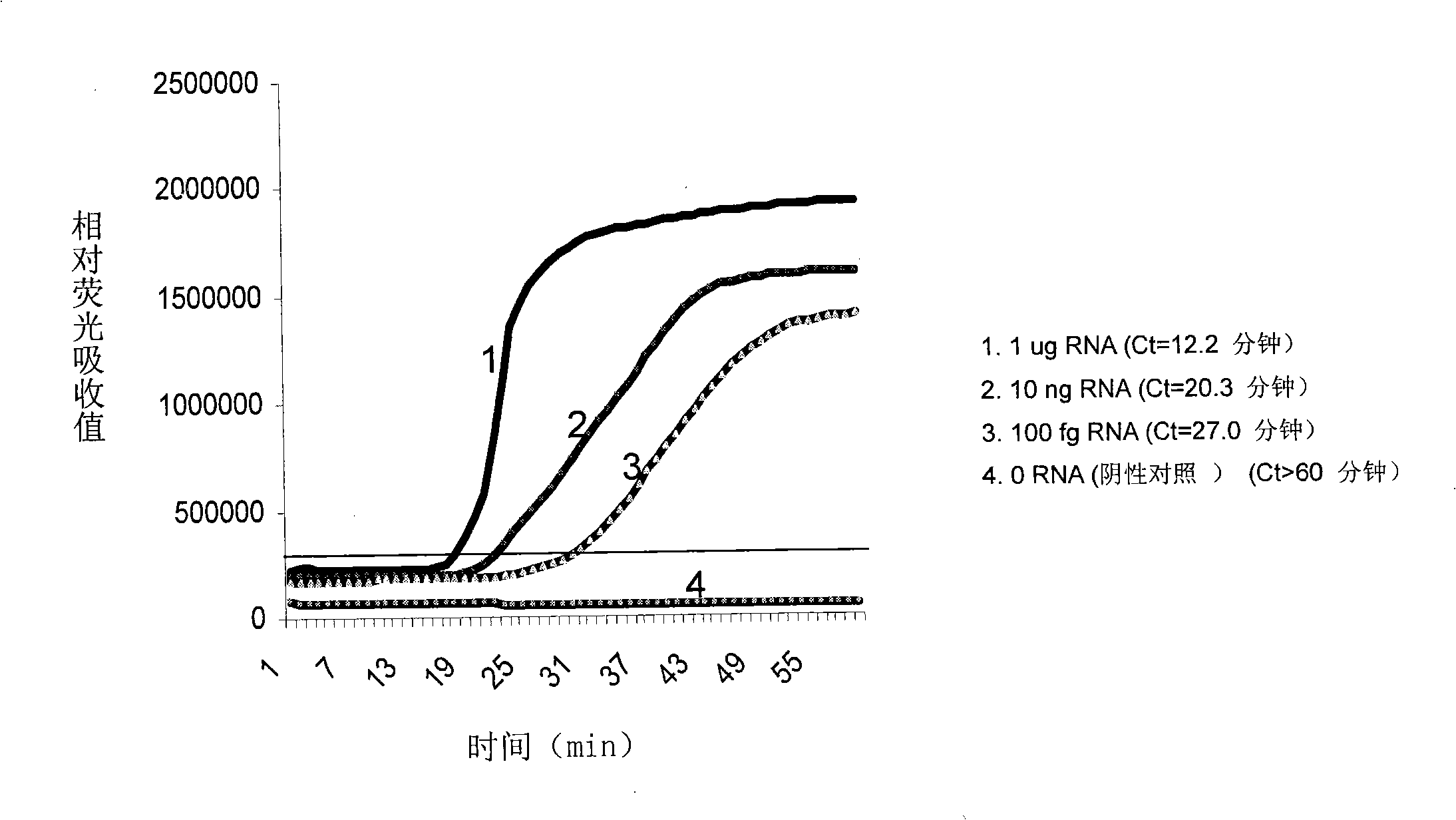
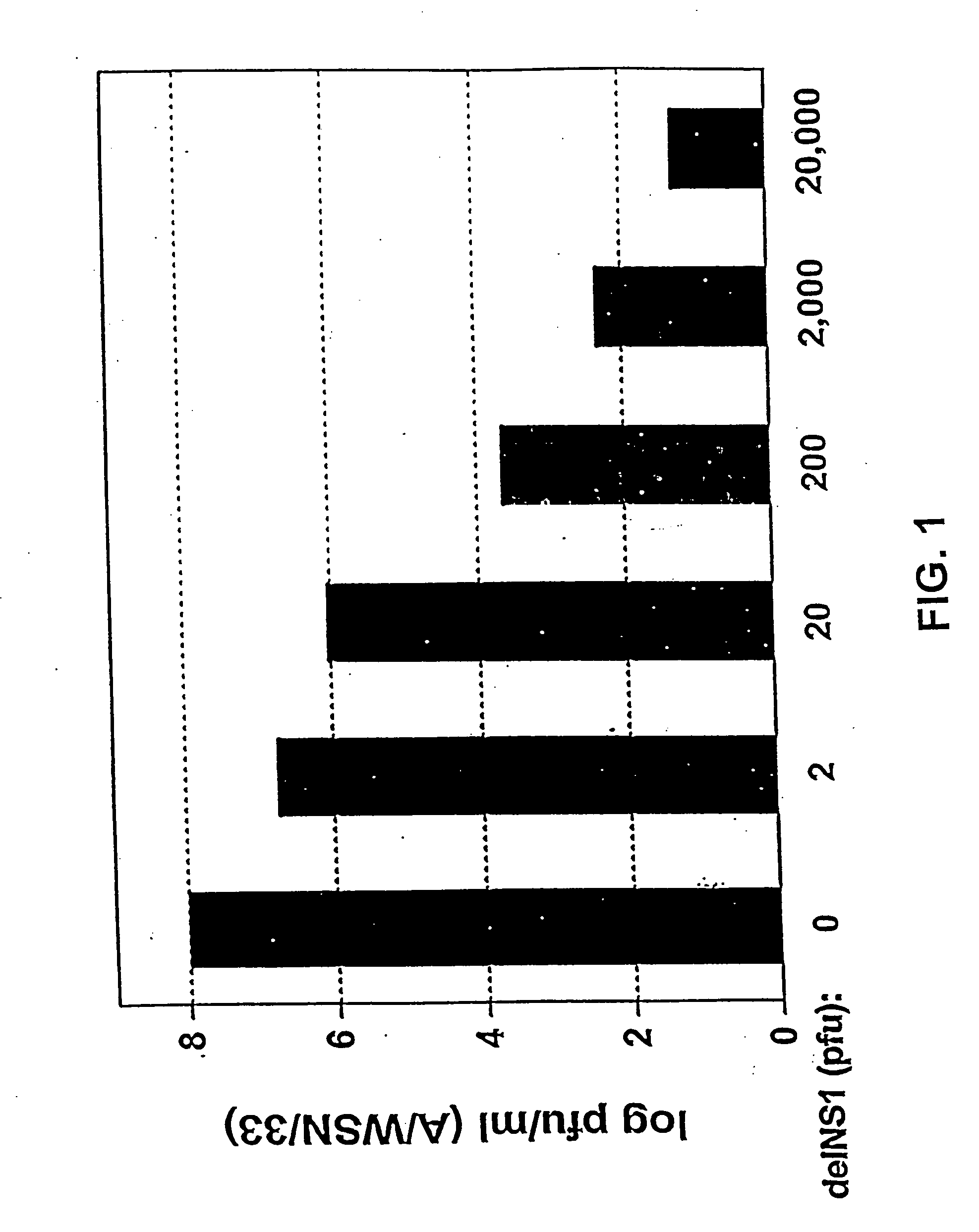
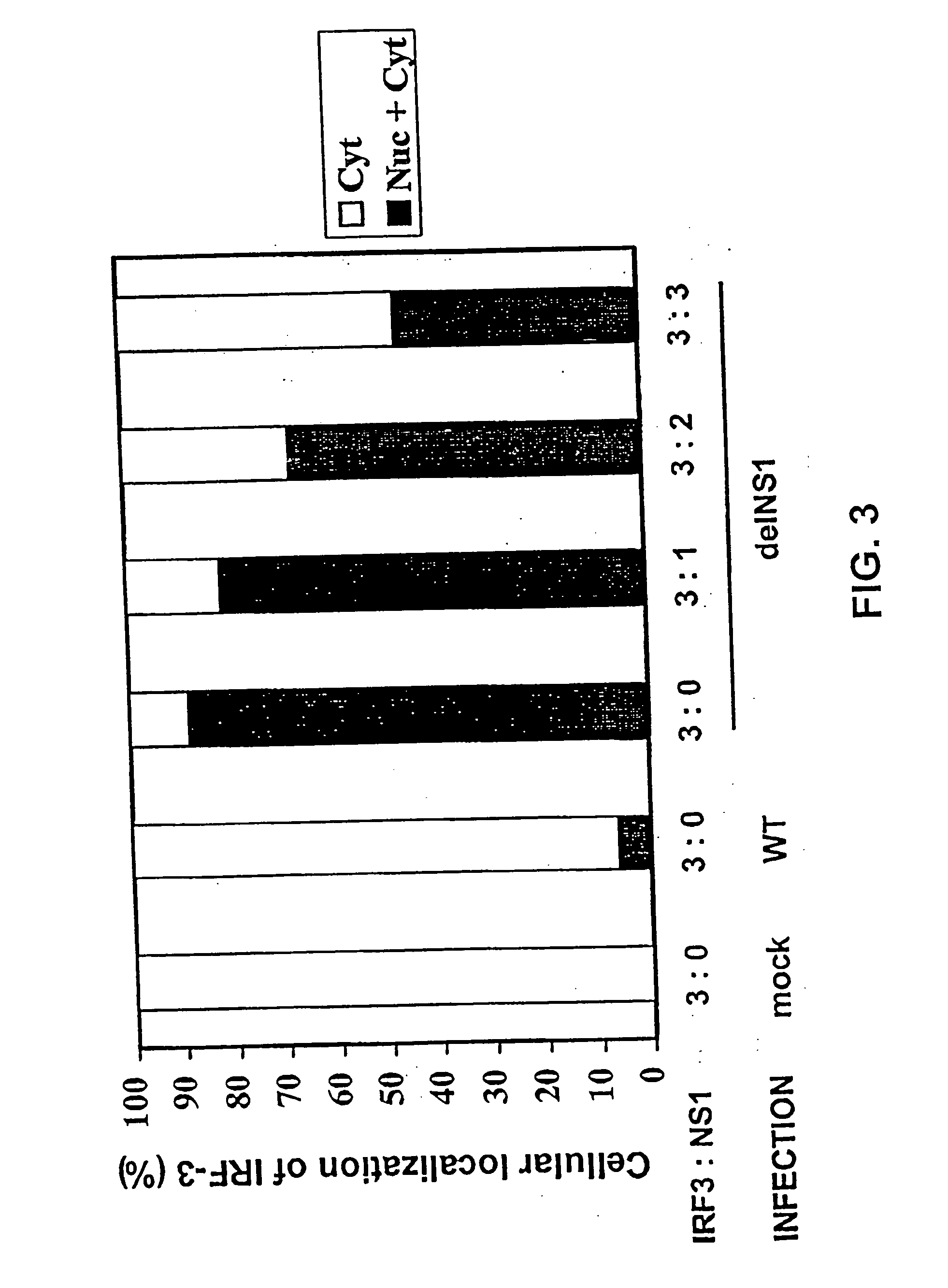
The present invention relates, in general, to attenuated negative-strand RNA viruses having an impaired ability to antagonize the cellular interferon (IFN) response, and the use of such attenuated viruses in vaccine and pharmaceutical formulations. The invention also relates to the development and use of IFN-deficient systems for selection of such attenuated viruses. In particular, the invention relates to attenuated influenza viruses having modifications to the NS1 gene that diminish or eliminate the ability of the NS1 gene product to antagonize the cellular IFN response. The mutant viruses replicate in vivo but demonstrate reduced pathogenicity, and therefore are well suited for live virus vaccines, and pharmaceutical formulations.
Owner:AVIR GREEN HILLS BIOTECH RES DEVMENT TRADE +1
Recombinant negative strand virus rna expression systems and vaccines
ActiveUS20050221489A1Improving immunogenicityAttenuated phenotypeSsRNA viruses negative-senseAntibacterial agentsHeterologousNegative strand
The present invention relates to recombinant RNA virus templates derived from and applicable to negative strand naturally non-segmented viruses, including the families Bornaviridae, Filoviridae, and Paramyxoviridae, and methods for generating such recombinant RNA virus templates, wherein the templates are RNA generated from two or more recombinant RNA molecules. The invention relates to the use of segmented recombinant RNA virus templates for naturally non-segmented RNA viruses to express heterologous gene products in appropriate host cell systems and / or to construct recombinant viruses taken from that family and that express, package, and / or present the heterologous gene product. The invention includes the expression products and recombinant and chimeric viruses thus prepared and vaccine and therapeutic formulations comprising the recombinant RNA viruses.
Owner:MT SINAI SCHOOL OF MEDICINE
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae , of the family Paramyxoviridae . The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Oligonucleotide compound and method for treating nidovirus infections
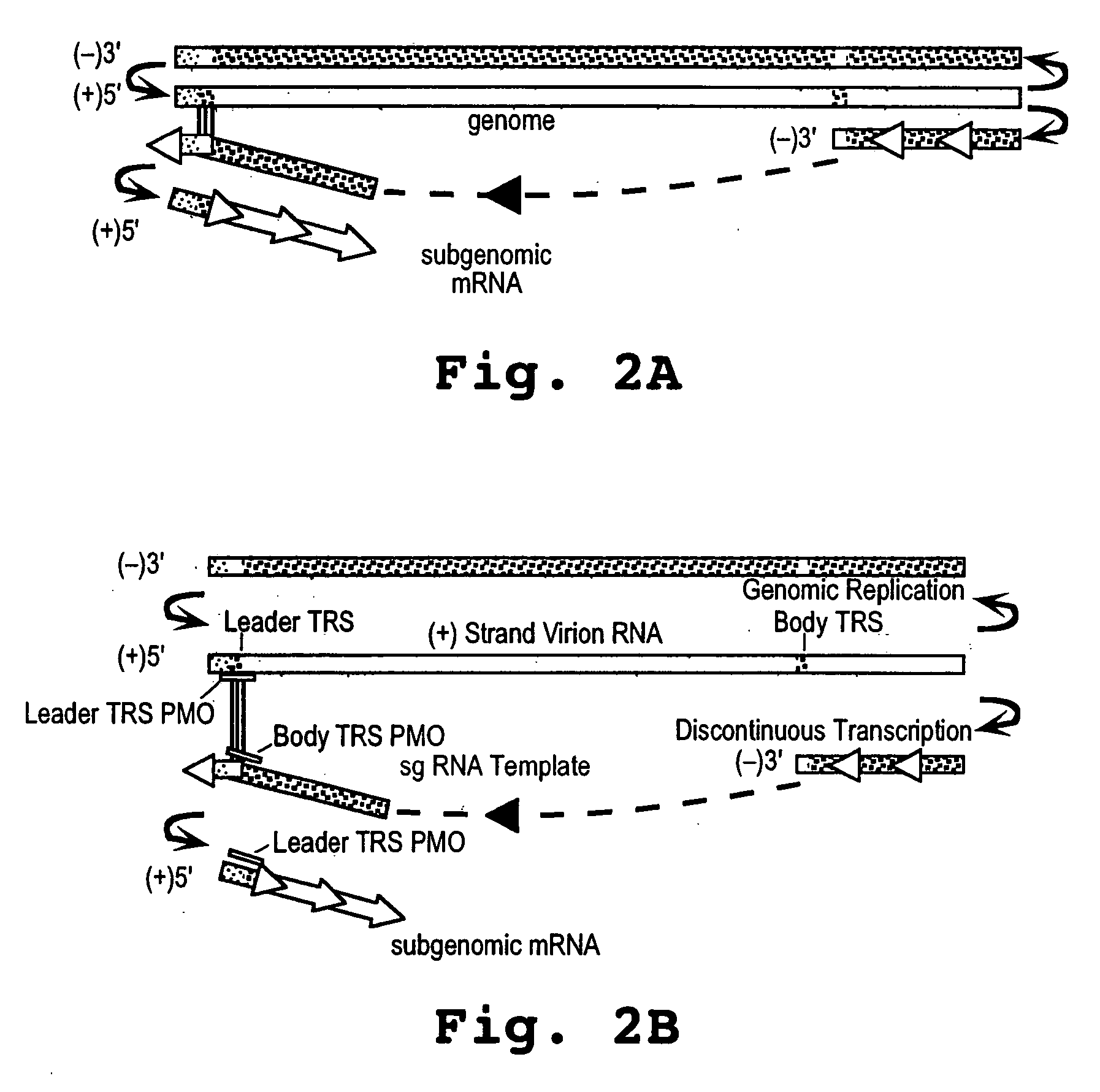
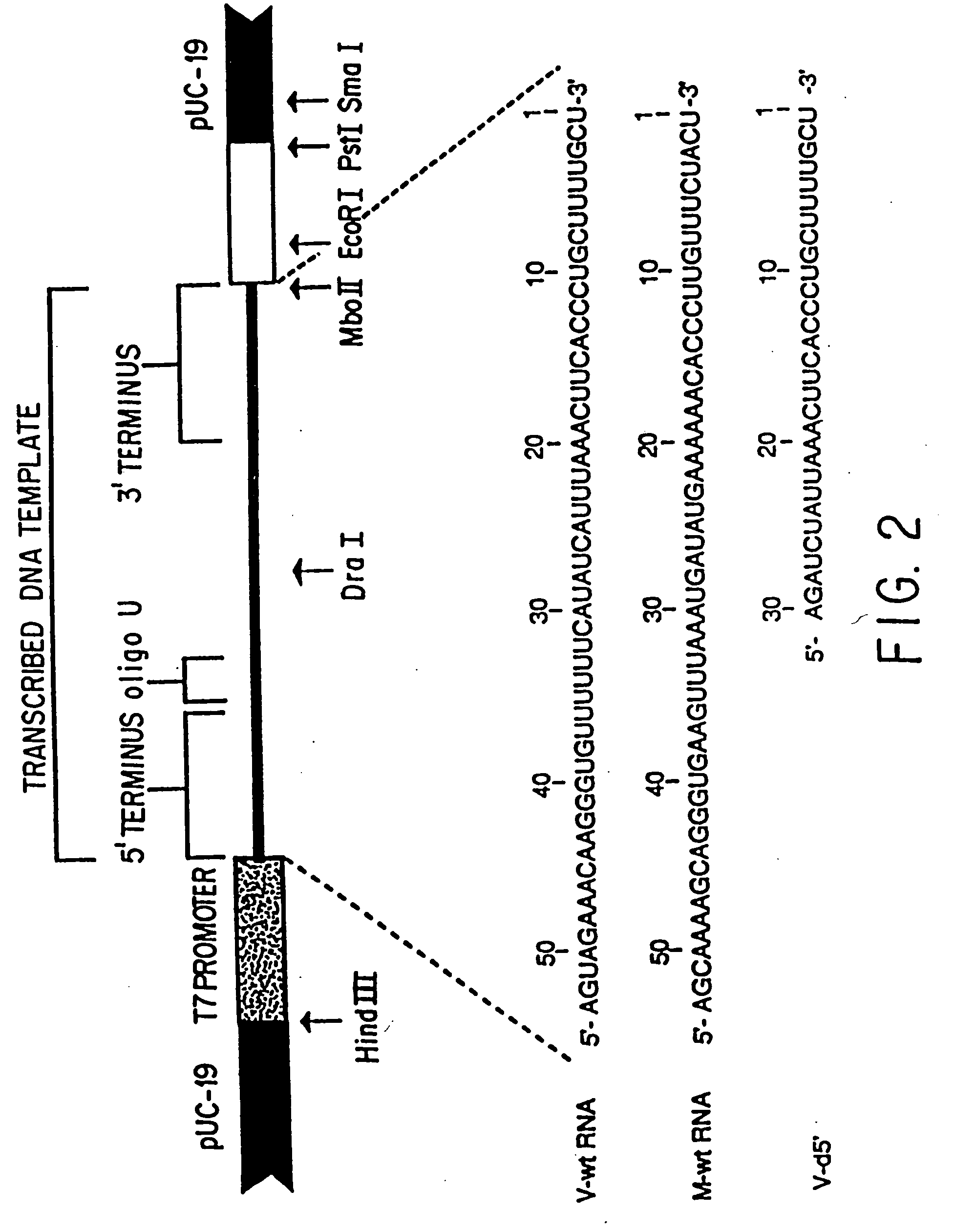
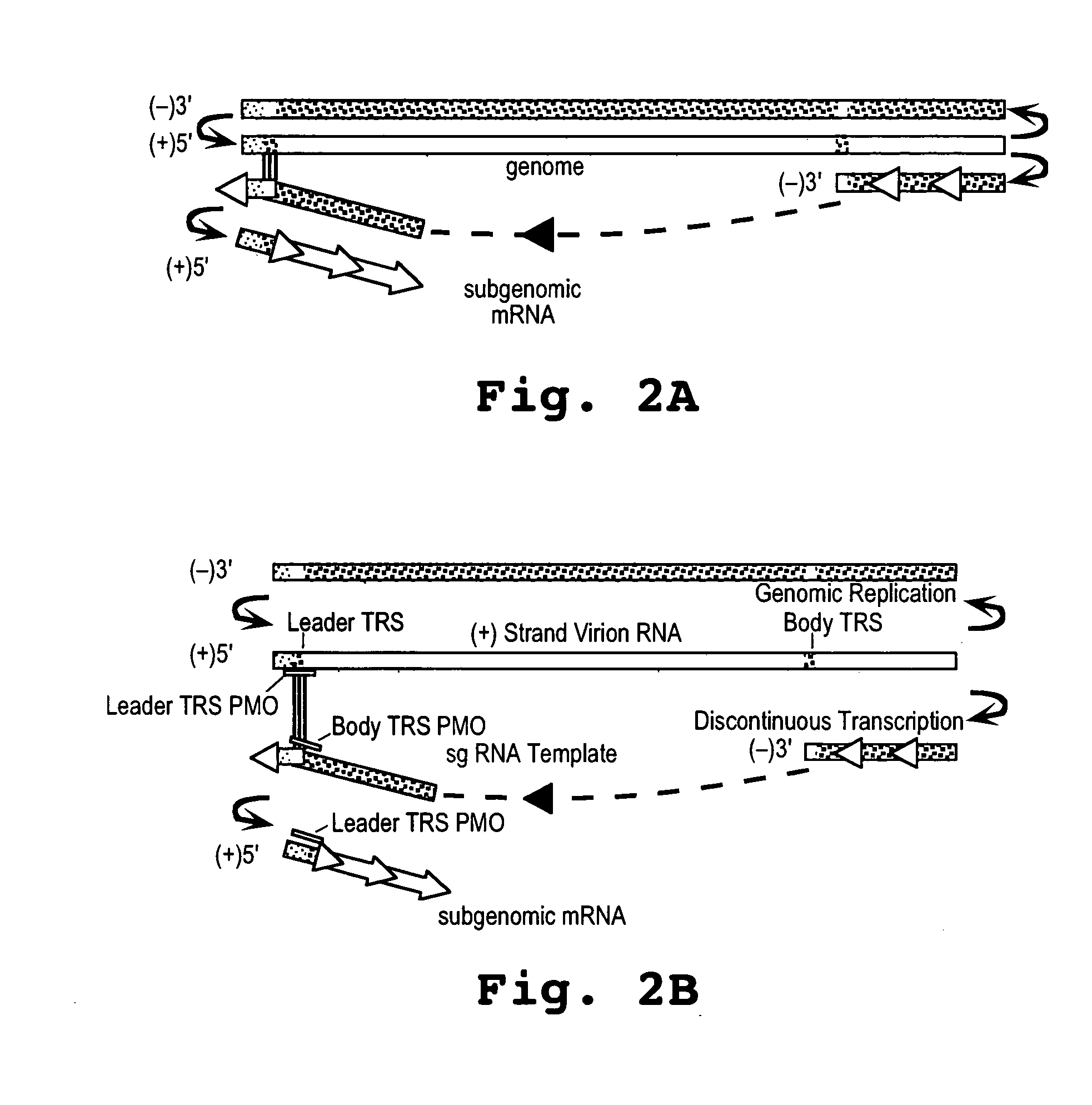
A method and oligonucleotide compound for inhibiting replication of a nidovirus in virus-infected animal cells are disclosed. The compound (i) has a nuclease-resistant backbone, (ii) is capable of uptake by the infected cells, (iii) contains between 8-25 nucleotide bases, and (iv) has a sequence capable of disrupting base pairing between the transcriptional regulatory sequences in the 5′ leader region of the positive-strand viral genome and negative-strand 3′ subgenomic region. In practicing the method, infected cells are exposed to the compound in an amount effective to inhibit viral replication.
Owner:THE SCRIPPS RES INST +1
Paramyxovirus-derived RNP
InactiveUS20070009949A1Small genome sizeLow costSsRNA viruses negative-senseMicrobiological testing/measurementNegative strandLipofectamine
A functional RNP containing negative-strand single-stranded RNA derived from Sendai virus, which has been modified so as not to express at least one envelope protein, has been successfully prepared. An RNP comprising a foreign gene is prepared and inserted into a cell with the use of a cationic liposome, thereby successfully expressing the foreign gene.
Owner:KITAZATO KAIO +9
Helper-free rescue of recombinant negative strand RNA virus
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
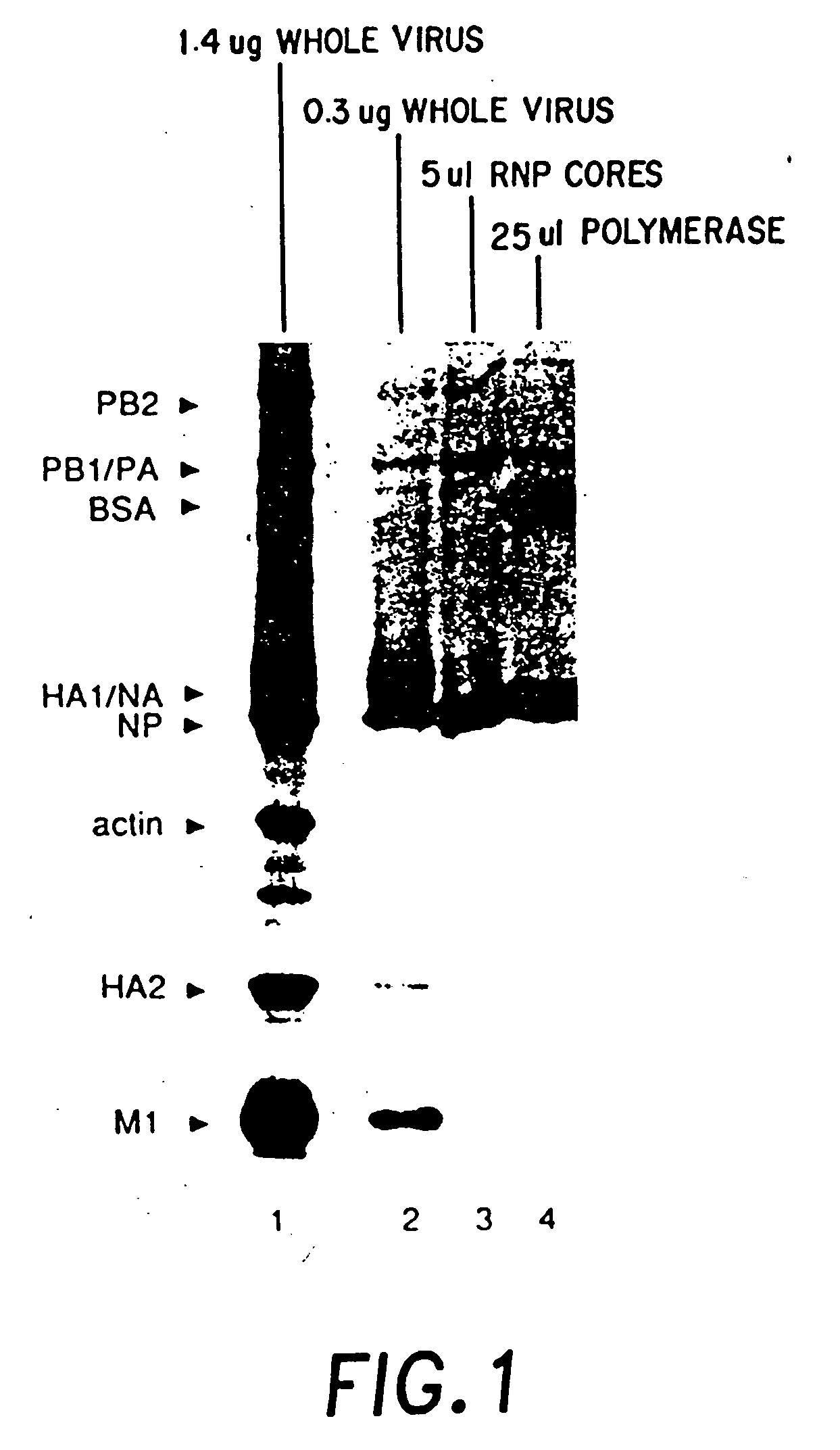
Recombinant negative strand RNA virus expression systems and vaccines
InactiveUS20050032043A1Equal efficiencySsRNA viruses negative-senseVirus peptidesNegative strandHeterologous
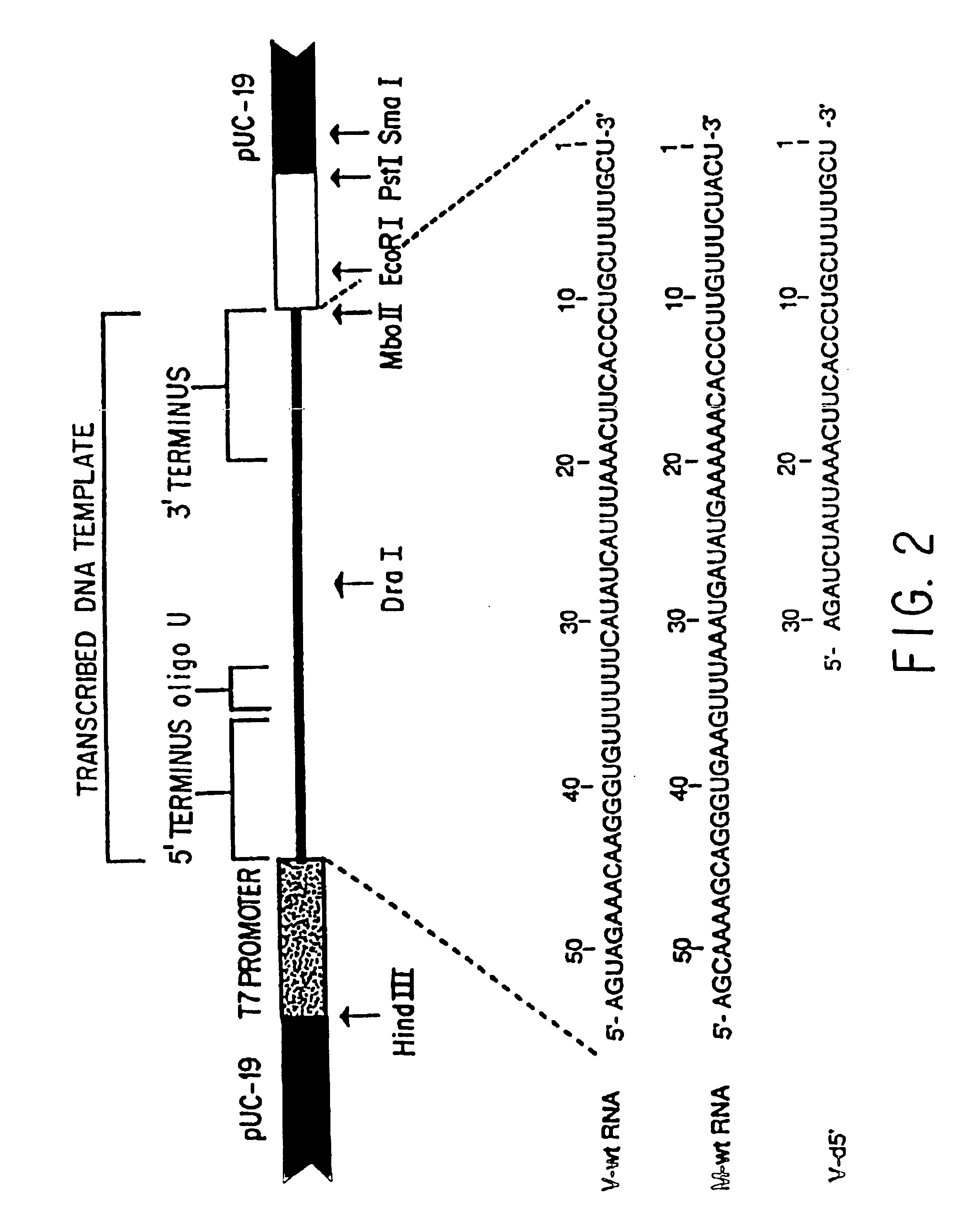
Recombinant negative-strand viral RNA templates are described which may be used with purified RNA-directed RNA polymerase complex to express heterologous gene products in appropriate host cells and / or to rescue the heterologous gene in virus particles. The RNA templates are prepared by transcription of appropriate DNA sequences with a DNA-directed RNA polymerase. The resulting RNA templates are of the negative-polarity and contain appropriate terminal sequences which enable the viral RNA-synthesizing apparatus to recognize the template. Bicistronic mRNAs can be constructed to permit internal initiation of translation of viral sequences and allow for the expression of foreign protein coding sequences from the regular terminal initiation site, or vice versa.
Oligonucleotide compound and method for treating nidovirus infections
InactiveUS20050171044A1Inhibition of replicationOrganic active ingredientsBiocideNegative strandInfected cell
A method and oligonucleotide compound for inhibiting replication of a nidovirus in virus-infected animal cells are disclosed. The compound (i) has a nuclease-resistant backbone, (ii) is capable of uptake by the infected cells, (iii) contains between 8-25 nucleotide bases, and (iv) has a sequence capable of disrupting base pairing between the transcriptional regulatory sequences in the 5′ leader region of the positive-strand viral genome and negative-strand 3′ subgenomic region. In practicing the method, infected cells are exposed to the compound in an amount effective to inhibit viral replication.
Owner:AVI BIOPHARMA +1
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences and methods for propagating virus
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations. The invention also provide methods for propagating virus.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Method for the recovery of non-segmented, nagative-stranded RNA viruses from cDNA
InactiveUS20060153870A1SsRNA viruses negative-senseGenetic material ingredientsNegative strandSubviral particle
Methods for producing infectious, non-segmented, negative-stranded RNA viruses of the Order Mononegavirales are provided that involve coexpression of a viral cDNA along with essential viral proteins, N, P, and L in a host cell transiently transfected with an expression vector encoding an RNA polymerase. In alternate methods, after the host cell is transfected with a viral cDNA expression vector and one or more vectors encoding the RNA polymerase, N protein, P protein, and L protein, the host cell is exposed to an effective heat shock under conditions sufficient to increase recovery of the recombinant virus. In other alternate embodiments, the host cells are transferred after viral rescue begins into co-culture with a plaque expansion cell, typically a monolayer of expansion cells, and the assembled infectious, non-segmented, negative-stranded RNA virus is recovered from the co-culture. Also provided within the invention are compositions for producing infectious, non-segmented, negative-stranded RNA virus of the Order Mononegavirales, recombinant viruses produced using the foregoing methods and compositions, and immunogenic compositions and methods employing the recombinant viruses. In additional embodiments, the methods and compositions of the invention are employed to produce growth- or replication-defective non-segmented negative-stranded RNA viruses and subviral particles.
Owner:WYETH LLC
Use of substances that act as cascade inhibitors of the raf/mek/erk signal cascade, for producing a medicament to treat dna and rna viruses
The invention consists in that substances acting as cascade inhibitors of the Raf / MEK / ERK signaling path-way, in particular MEK inhibitors, are used for the production of a drug for the preventive and antiviral therapy against DNA and RNA viruses, in particular against intranuclear-replicating negative strand RNA viruses, for instance influenza or Borna disease viruses.
Owner:MEDINNOVA FUR MEDIZINISCHE INNOVATIONEN AUS AKADSCHER FORSCHUNG MBH
Caspase inhibitors, especially caspase 3 inhibitors, for the treatment of influenza
The invention relates to the use of at least one caspase inhibitor, in particular a caspase-3 inhibitor, for preparing a pharmaceutical composition for the prophylaxis and / or therapy of a viral infection, in particular an infection with an RNA negative-strand virus, preferably an influenza infection, and to a test system for identifying suitable active substances.
Owner:ACTIVAERO
Small interference RNA molecule (siRNA) of attacking SARS virus mRNA melecule and application
A small-interference ribonucleic acid (SiRNA) molecule able to attack the mRNA molecular of SARS virus and its application in preparing the medicines for treating SARS and its virus infection associated diseases are disclosed. Said SiRNA is a double-stranded RNA molecule.
Owner:杭州新瑞佳生物医药技术开发有限公司
Highly safe intranasally administrable gene vaccines for treating alzheimer's disease
InactiveUS20090170798A1Large scaleLow costSsRNA viruses negative-senseOrganic active ingredientsDiseaseNose
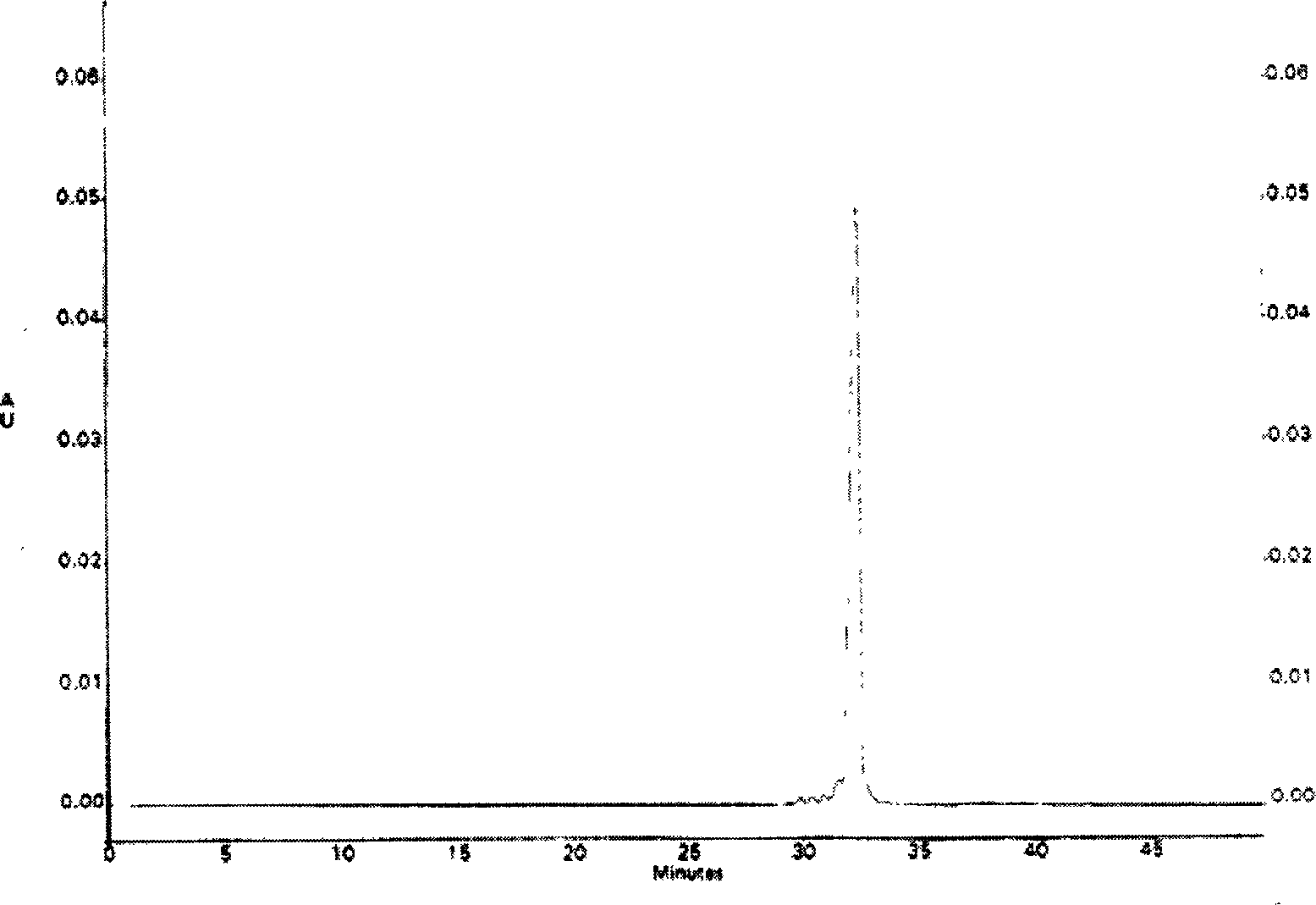
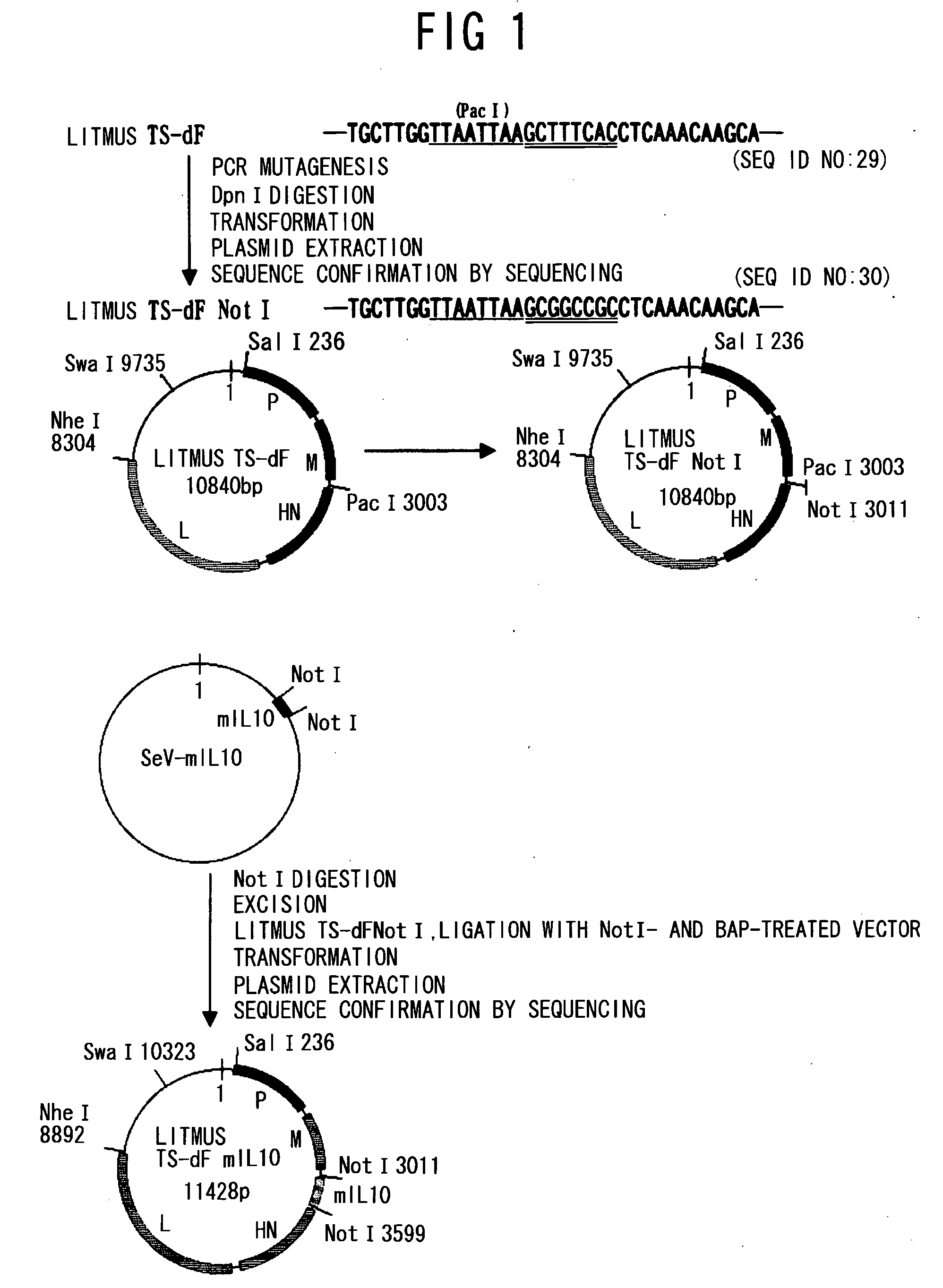
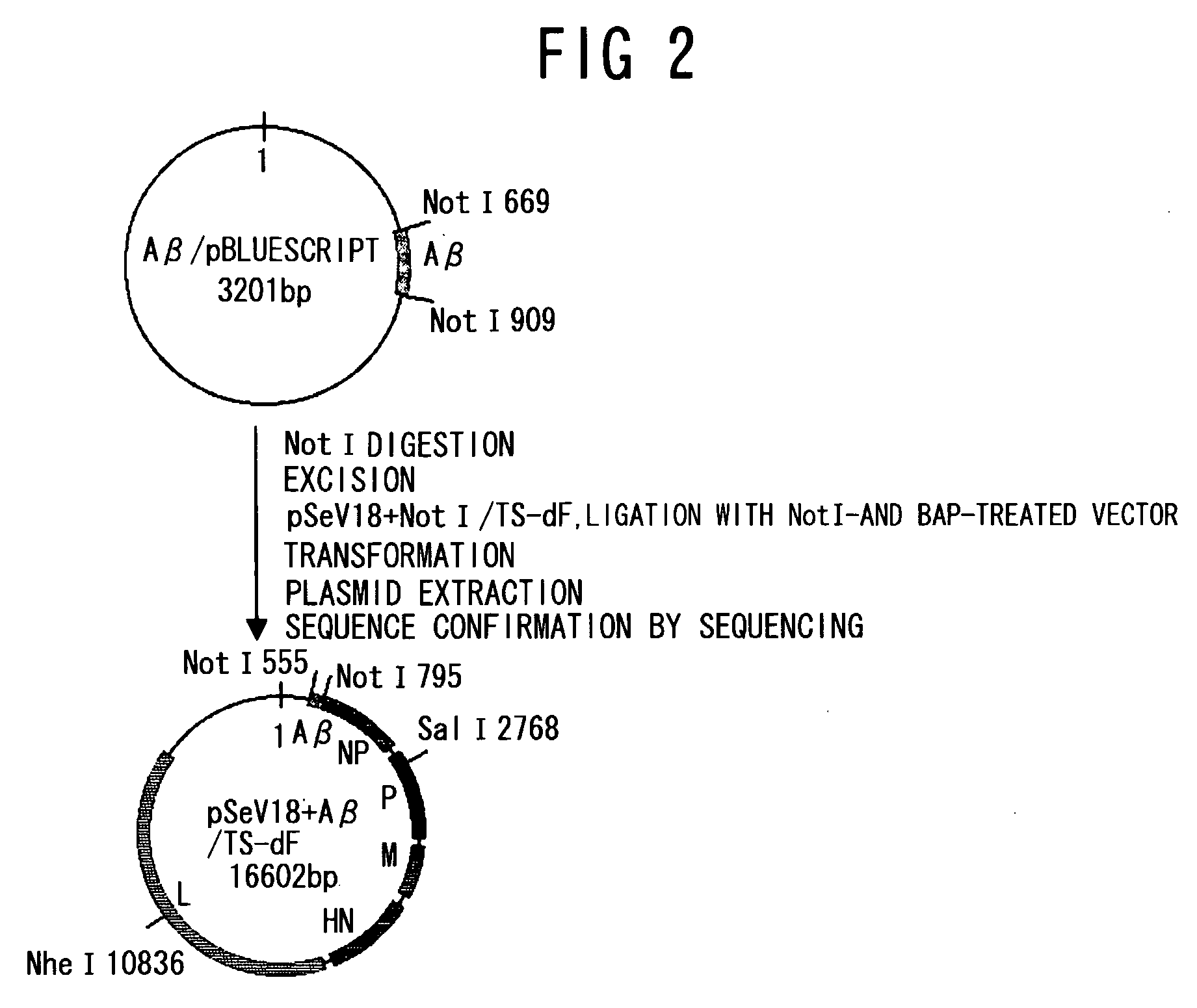
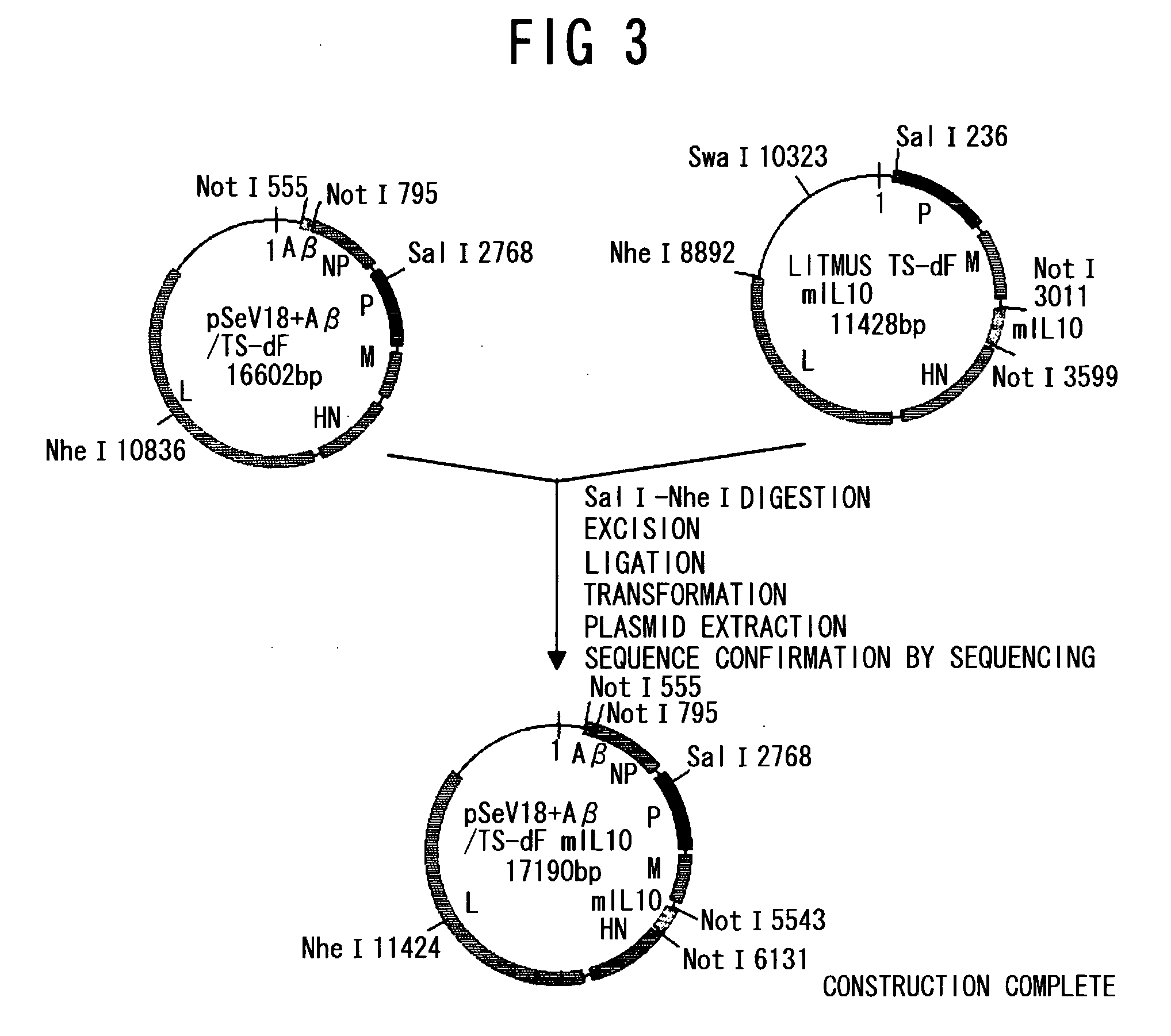
An objective of the present invention is to provide a safe and effective vaccine therapy for Alzheimer's disease. A minus strand RNA viral vector carrying amyloid gene was constructed, and administered intranasally to 24- to 25-months-old APP transgenic mice. The level of serum anti-A 42 antibody was determined and showed to be markedly higher than the control. The results of histological investigation showed that the administration of a vector of the present invention markedly reduced senile plaques in all of the frontal lobe, parietal lobe, and hippocampus. The brain A level was also markedly reduced. Furthermore, the administration of a vector of the present invention did not result in lymphocyte infiltration in the central nervous system.
Owner:DNAVEC CORP +1
Recombinant parainfluenza virus expression systems and vaccines
The present invention relates to recombinant bovine parainfluenza virus (bPIV) cDNA or RNA which may be used to express heterologous gene products in appropriate host cell systems and / or to rescue negative strand RNA recombinant viruses that express, package, and / or present the heterologous gene product. The chimeric viruses and expression products may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE LLC
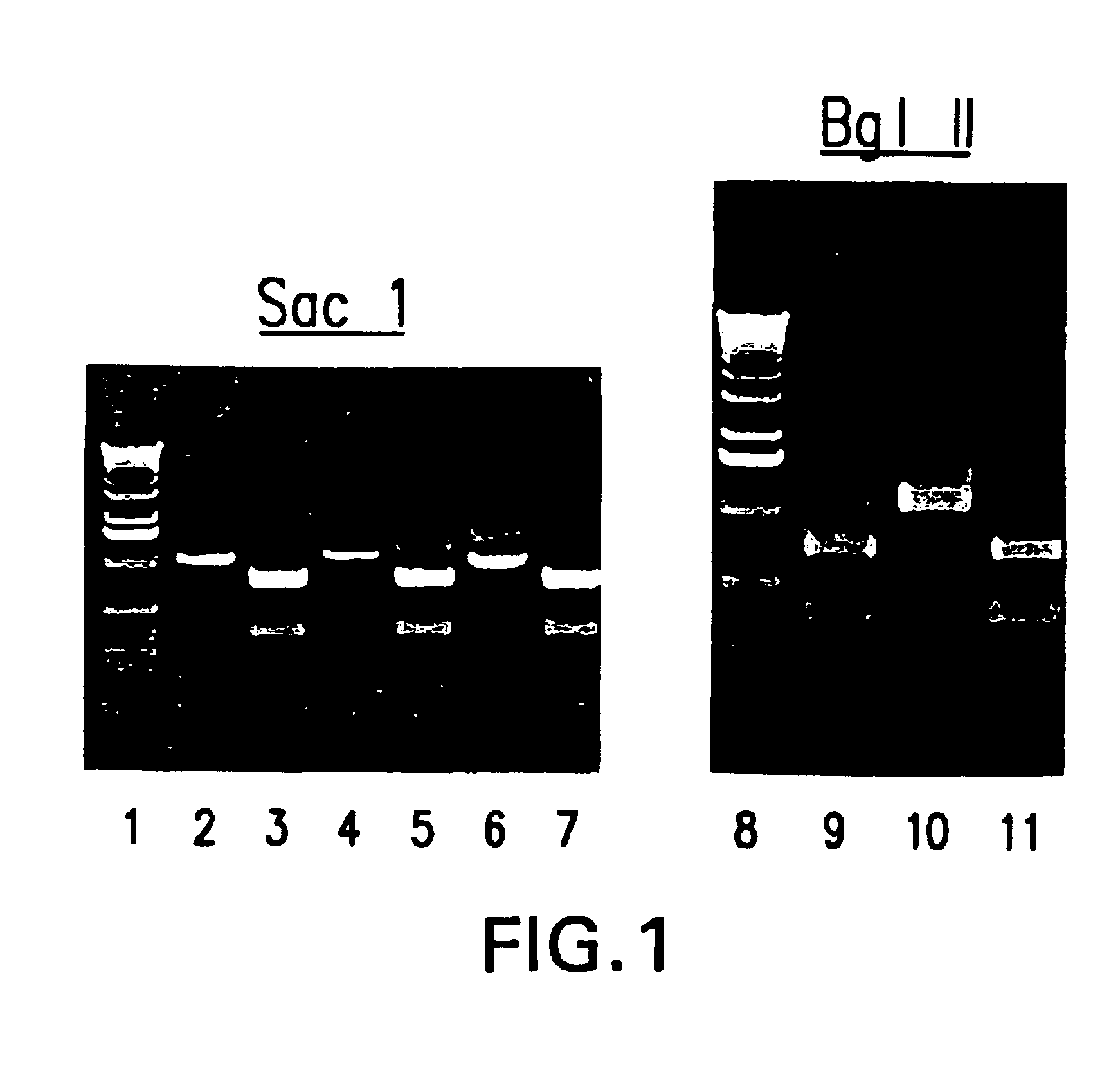
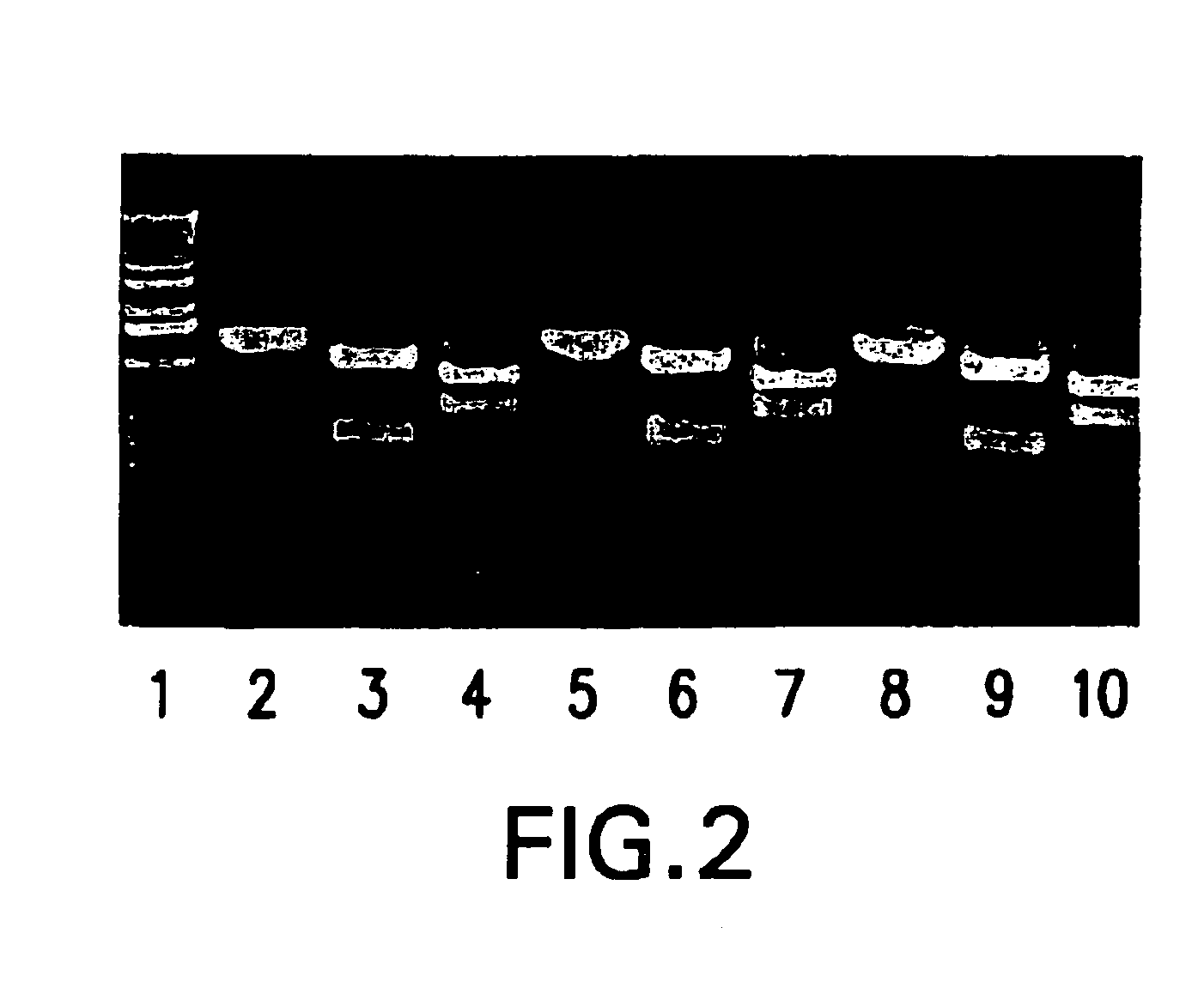
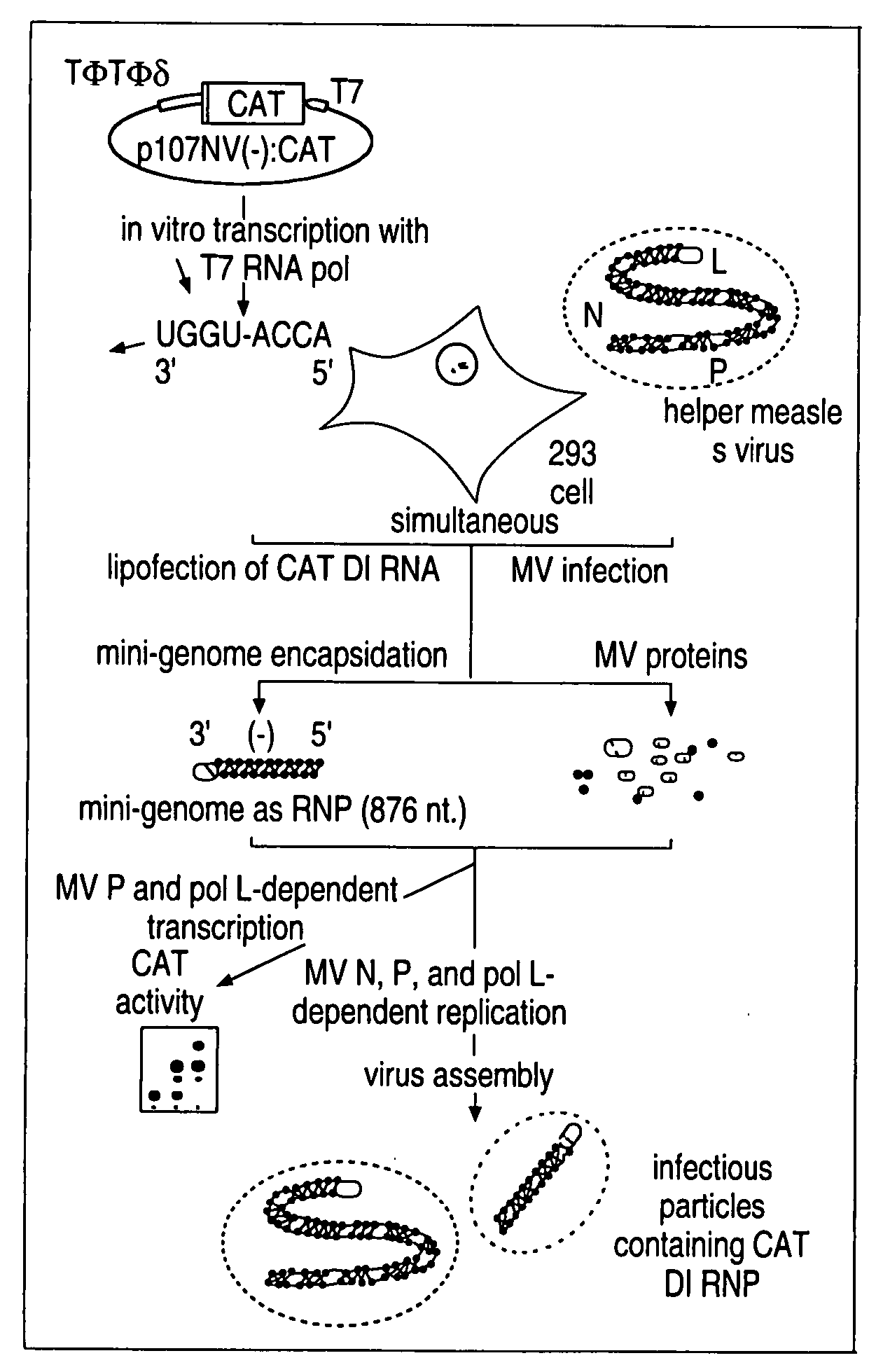
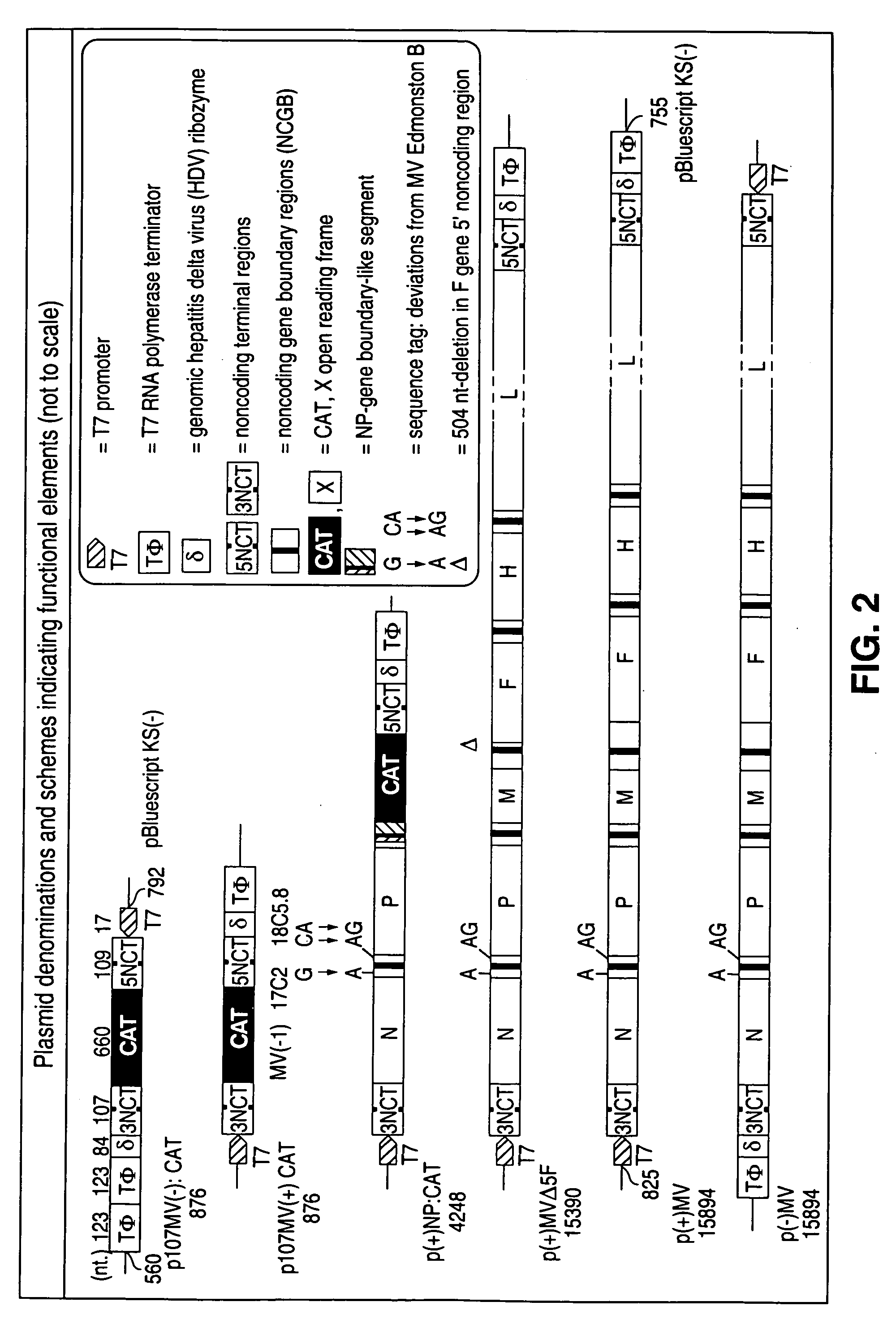
cDNA corresponding to the antigenome of nonsegmented negative strand RNA viruses and process for the production of such viruses
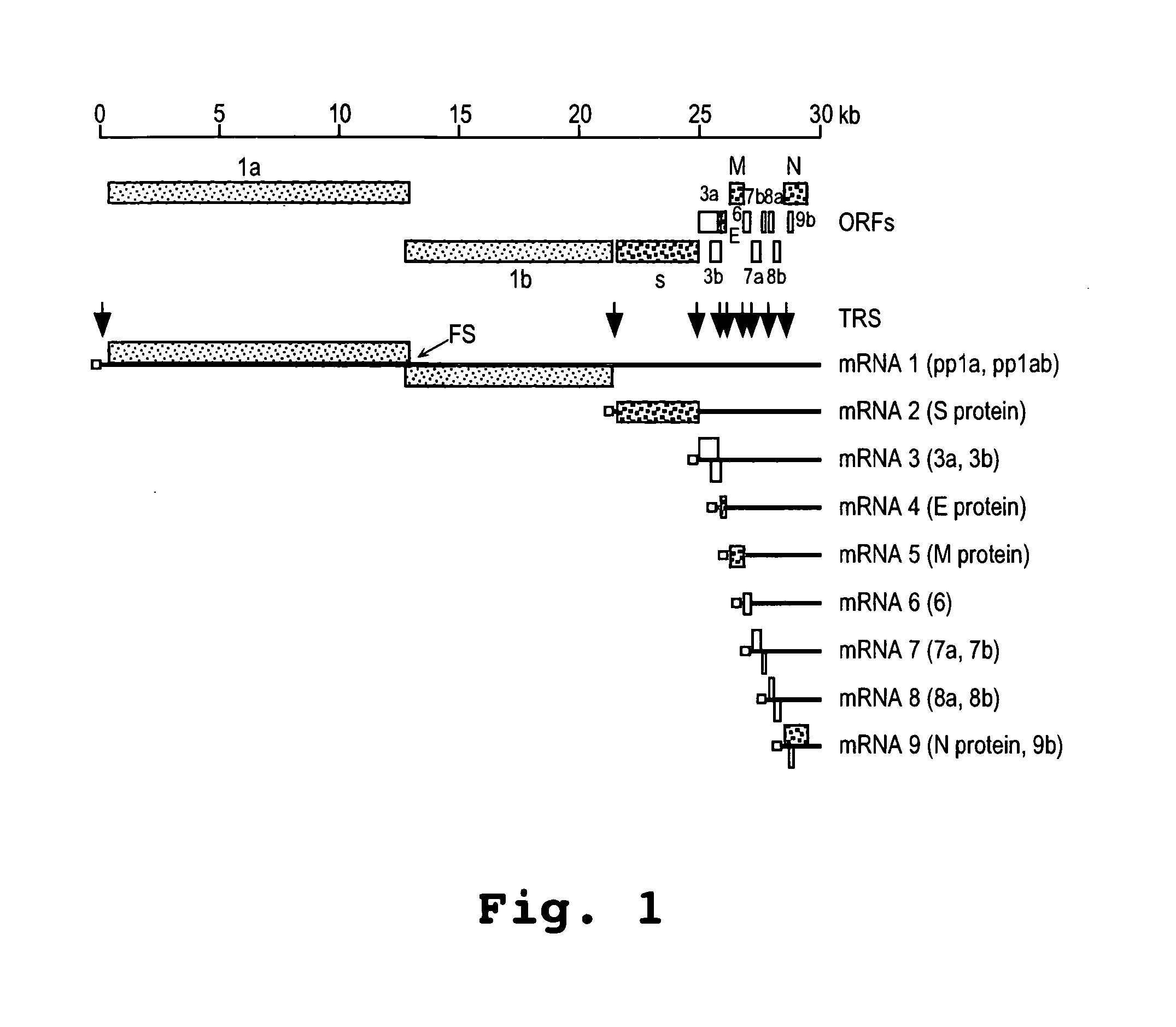
The present invention relates, in general, to a methodology or the generation of nonsegmented negative-strand RNA viruses (Pringle, 1991) from cloned deoxyribonucleic acid (cDNA). Such rescued viruses are suitable for use as vaccines, or alternatively, as plasmids in somatic gene therapy applications. The invention also relates to cDNA molecules suitable as tools in this methodology and to helper cell lines allowing the direct rescue of such viruses. Measles virus (MV) is used as a model for other representatives of the Mononegavirales, in particular the family Paramyxoviridae.
Owner:CRUCELL SWITZERLAND AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com