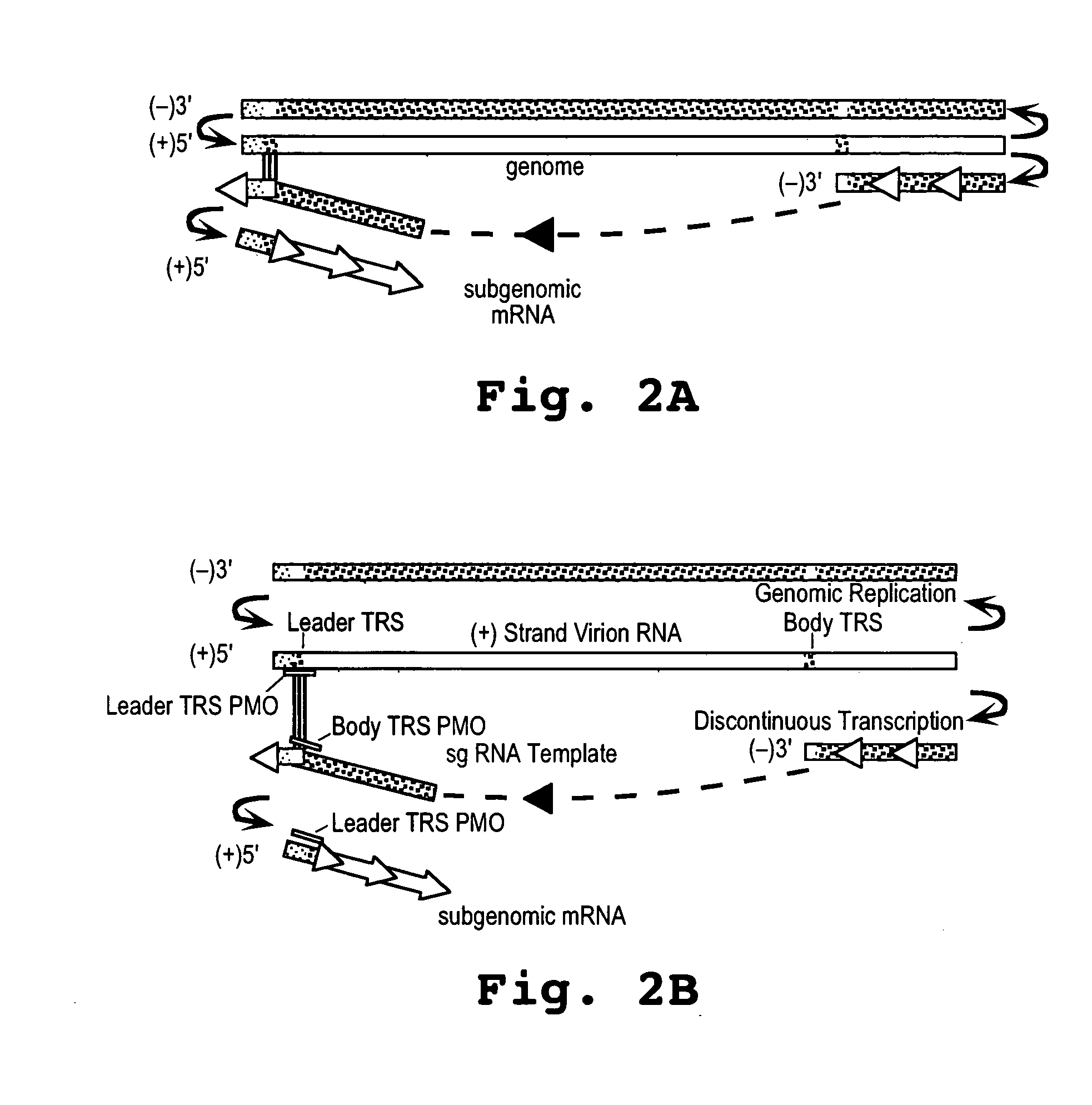
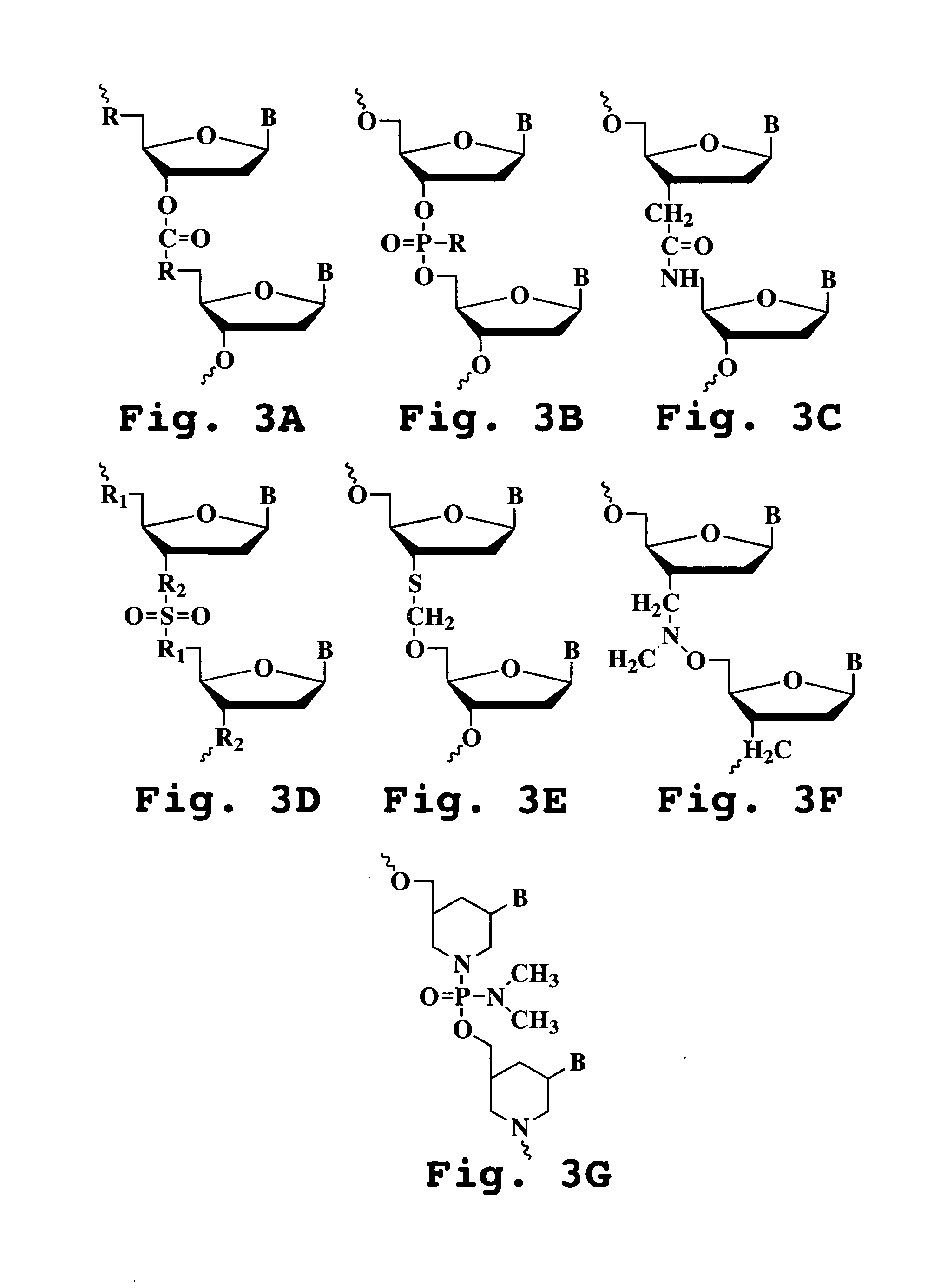
Oligonucleotide compound and method for treating nidovirus infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Antisense PMO Reduction of SARS Virus and MHV Titer in vitro
[0162] The capability of an antiviral drug to reduce the production of viable virus is a classic measure of antiviral drug activity. The reduction of SARS virus titer produced from SARS-infected Vero-E6 cells cultured in the presence of anti-leader TRS PMO (SEQ ID NOS:26 and 27) was measured and the results shown in FIG. 5. The reduction of MHV titer when MHV-infected Vero-E6 cells were cultured in the presence of a PMO that targets the leader TRS (SEQ ID NO:29) was also determined and is shown in FIG. 9.
[0163] Vero-E6 cells were cultured in DMEM with 10% fetal bovine serum. Vero-E6 cells were plated at approximately 75% confluence in replicate 25 cm2 culture flasks. Cells were rinsed and incubated in 1 ml of complete VP-SFM (virus production serum-free medium, Invitrogen) containing the specified concentration of antisense PMO-P003 conjugate (SEQ ID NOS: 26 or 27) or a PMO-P003 conjugate with an irrelevant sequence (DSsc...
example 2
Antisense PMO Reduction of SARS Plaque Size
[0165] As a separate measure of the antiviral activity of antisense PMO drugs, the anti-leader TRS PMOs were used to measure the reduction of viral replication in a plaque size assay. As viral replication is inhibited a corresponding reduction in the spread of cytopathic effects is observed as a reduction in plaque size. Furthermore, since virus entry and spread are separable phenomena with different criteria, and since some reports indicate that the antisense PMO drugs may inhibit initial virus entry, the plaque size reduction assay is performed. This assay also tests the non-toxicity of drug over longer treatment periods.
[0166] As described above in Example 1, 75% confluent Vero-E6 cells were prepared in plates but not pretreated. Cells were transported to the BSL-3 facility, inoculated with serial dilutions of SARS virus calculated to produce 1, 10 and 100 plaques per plate. The same serial dilution stocks were used for all control and...
example 3
Antisense PMO Reduction of SARS CytoPathic Effects In Vitro
[0167] This assay is a byproduct of the virus titer reduction assay. The observation of cytopathic effects (CPE) is a visual measure of antiviral drug activity. Vero-E6 cells were pretreated, inoculated with SARS virus and cultured as above in the presence of anti-leader TRS PMOs (SEQ ID NOS: 26 & 27). After 24 h, the medium was replaced by fresh complete VP-SFM and cells were incubated a further 24 h at 37 C in the presence of 5% CO2. 48 h after inoculation, the cells were fixed, decontaminated and stained with crystal violet as described in Example 1. CPE is visualized by phase contrast microscopy and recorded with a digital camera as shown in FIG. 6. The data for TRS-1-P003 and TRS-2-P003 correspond to SEQ ID NOS: 26 and 27, respectively. The other treatments presented in FIG. 7 are not relevant to the present invention. From the data presented in FIG. 7, it is clear that anti-leader TRS PMO prevented SARS-induced CPE at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com