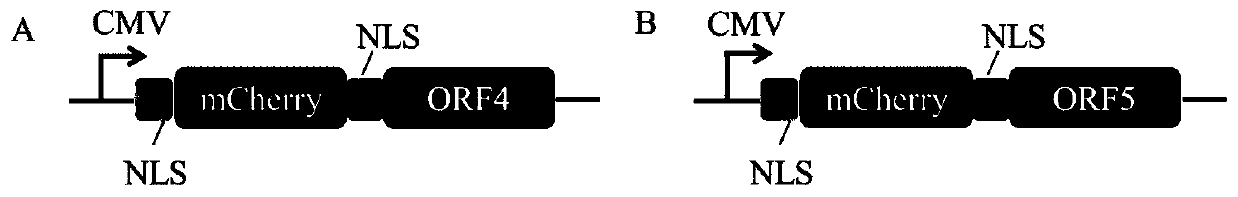Patents
Literature
391 results about "RNA virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An RNA virus is a virus that has RNA (ribonucleic acid) as its genetic material. This nucleic acid is usually single-stranded RNA (ssRNA) but may be double-stranded RNA (dsRNA). Notable human diseases caused by RNA viruses include Ebola virus disease, SARS, rabies, common cold, influenza, hepatitis C, hepatitis E, West Nile fever, polio and measles.
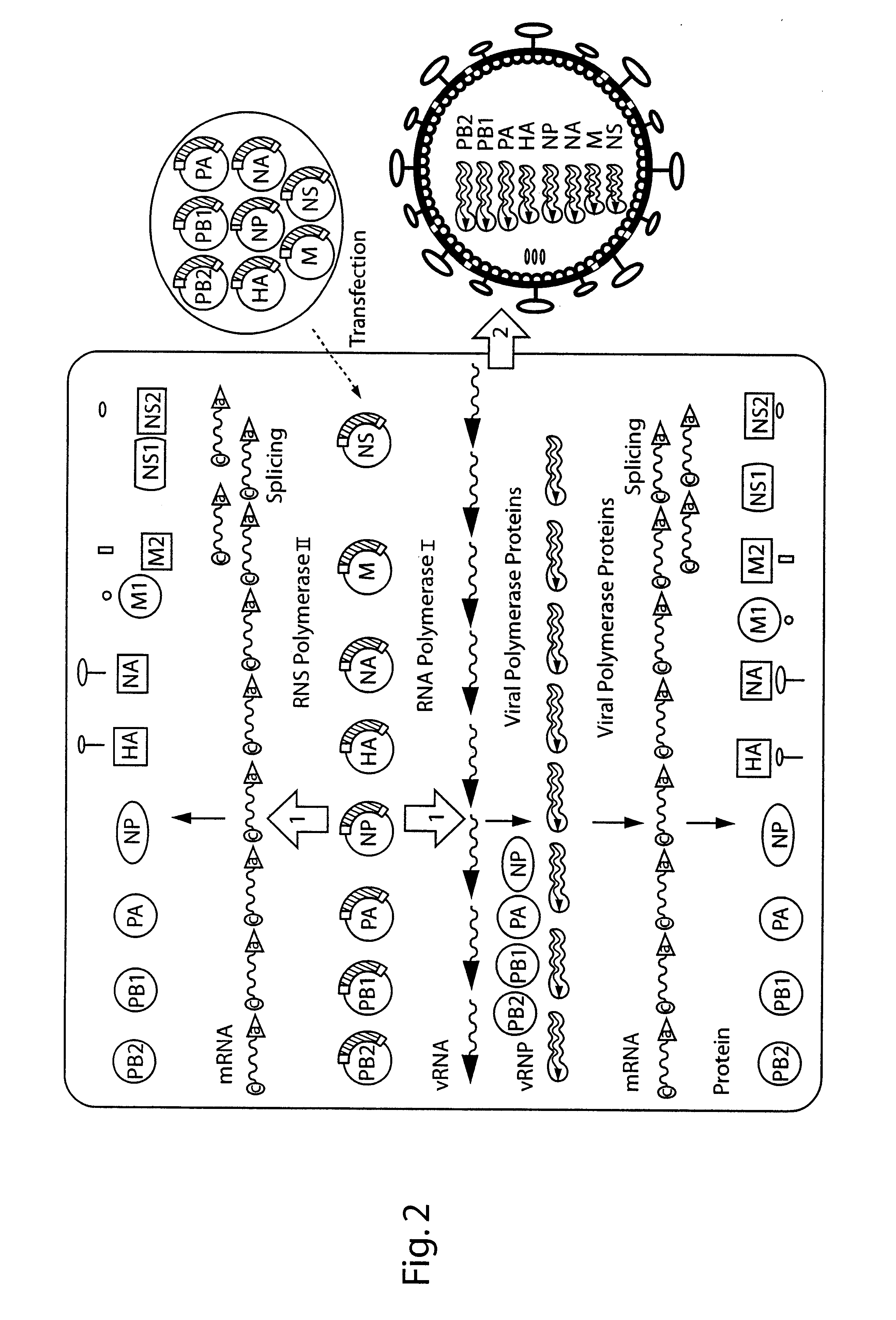
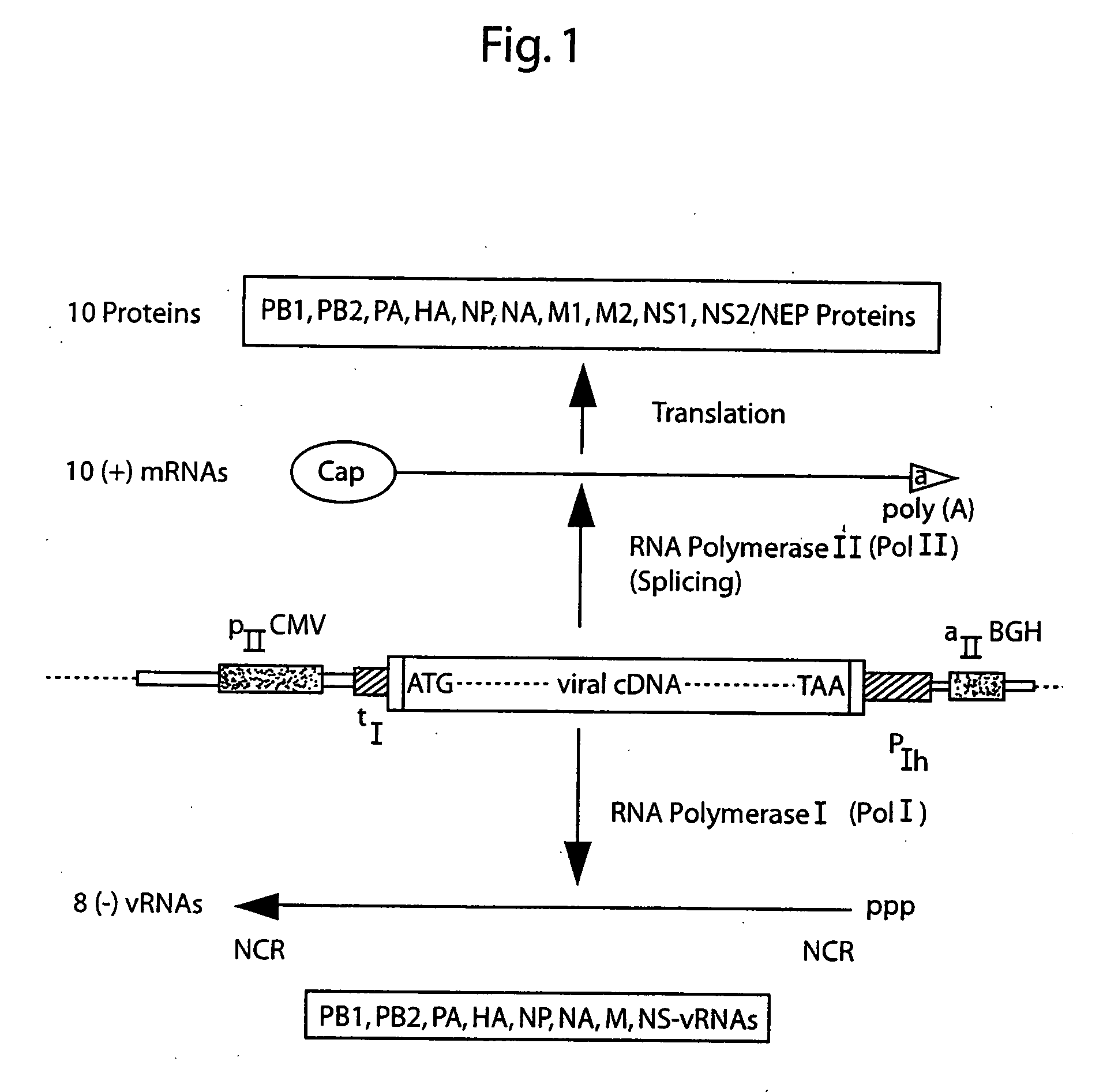
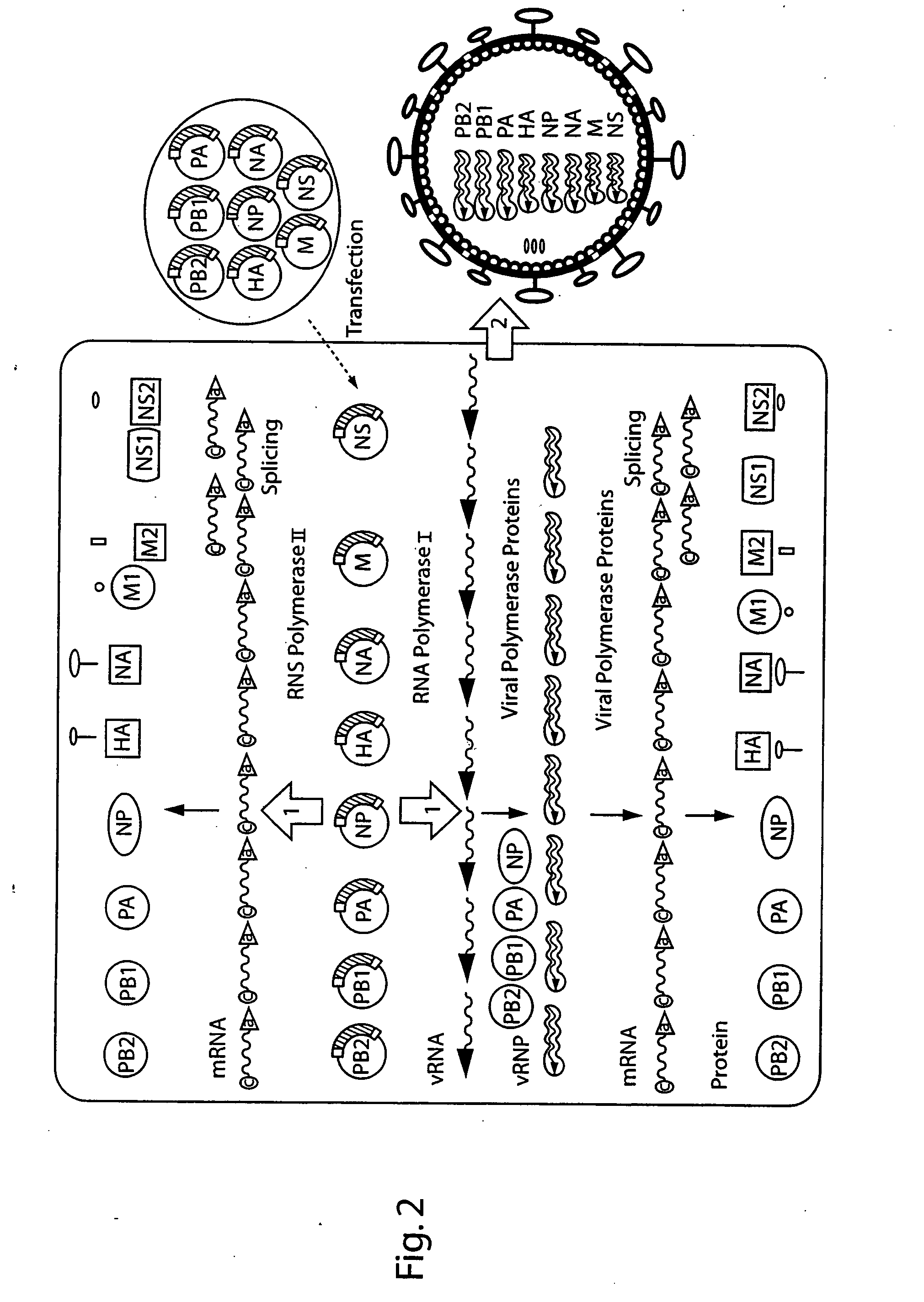
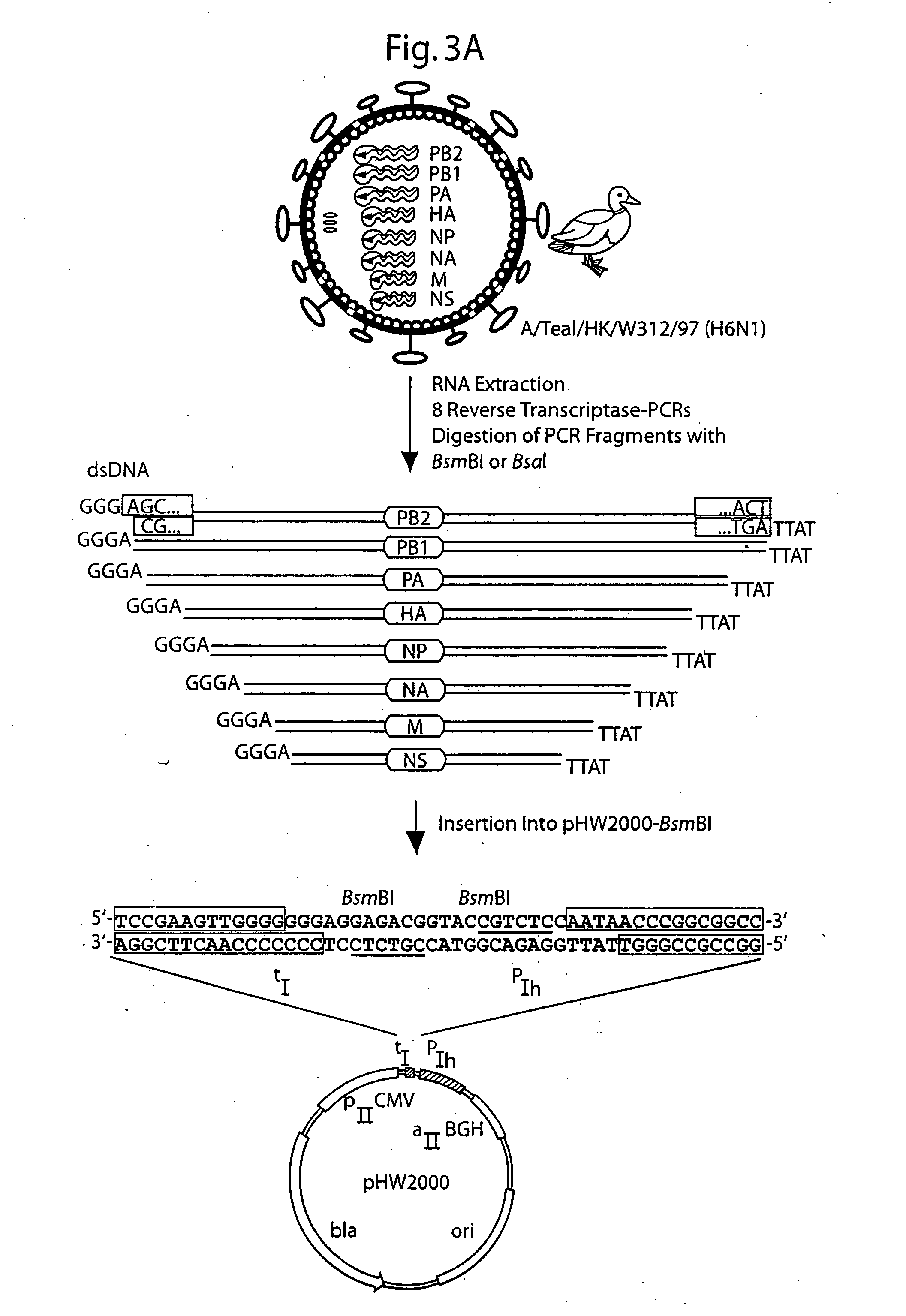
DNA transfection system for the generation of infectious influenza virus
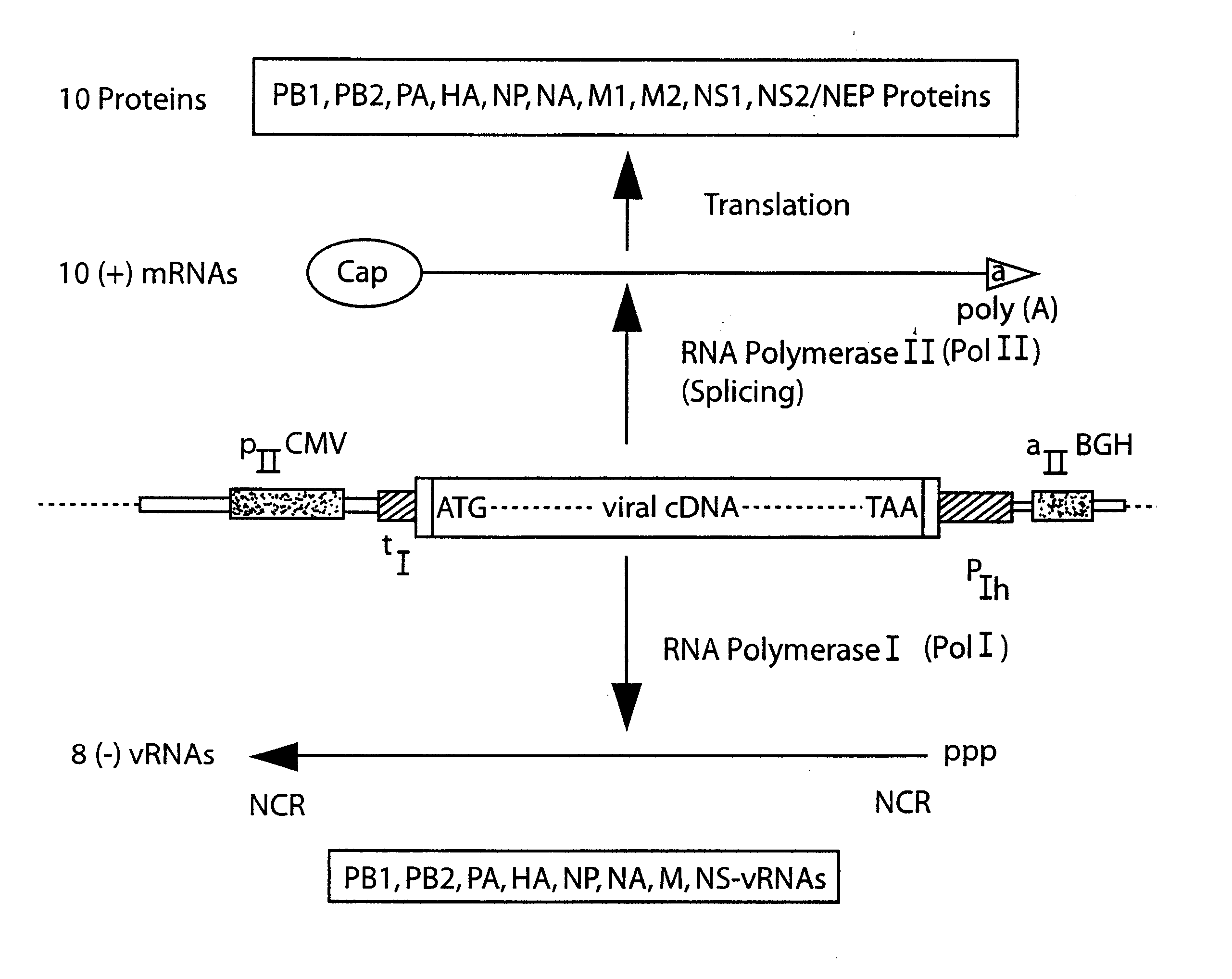
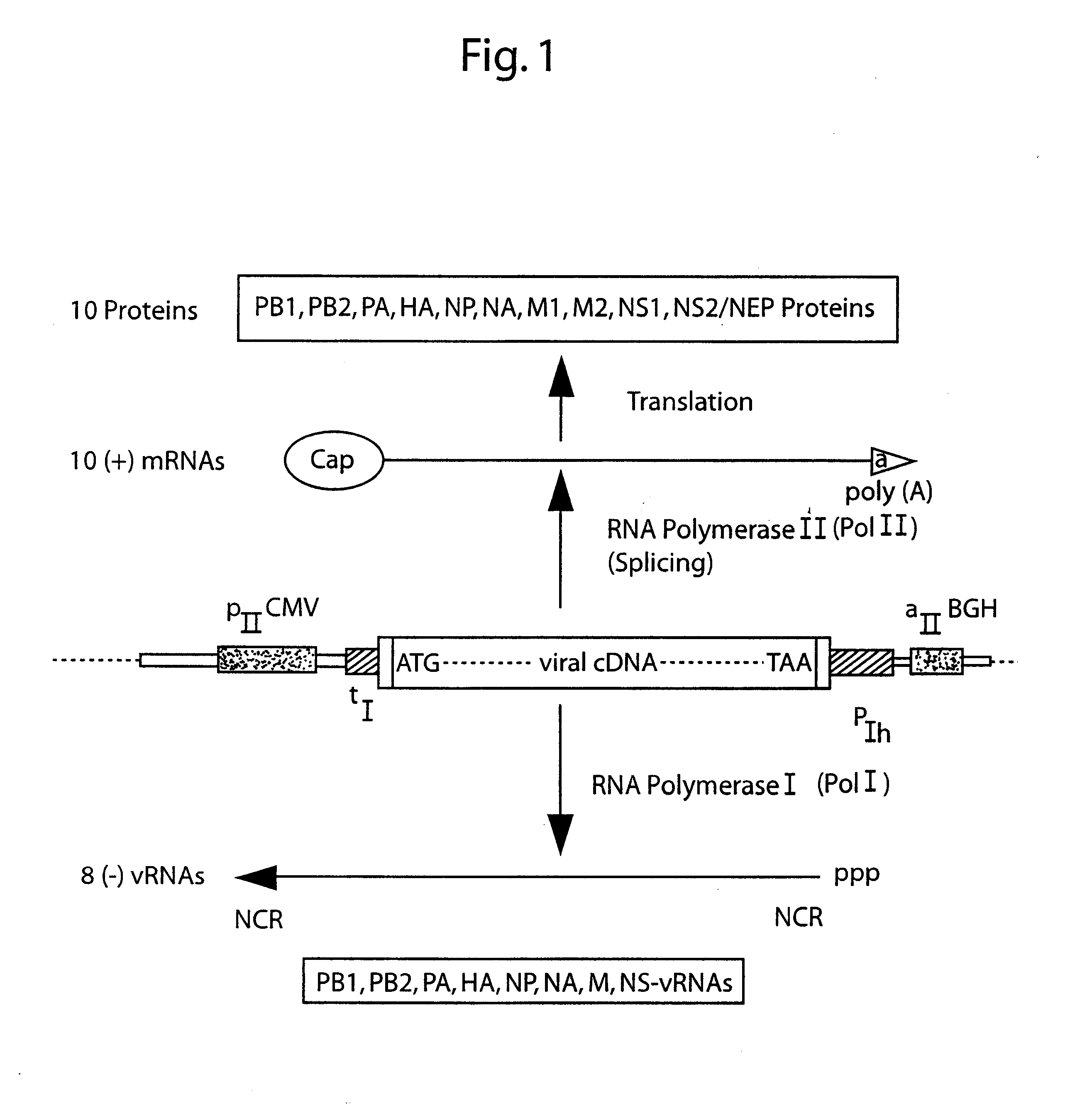
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuramimidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
DNA transfection system for the generation of infectious influenza virus
InactiveUS20050186563A1Improve effectivenessElicit protective immunitySsRNA viruses negative-senseFungiDual promoterSingle-Stranded RNA
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuraminidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Compositions and methods for inhibiting viral replication
The present invention relates to a double-stranded ribonucleic acid (dsRNA) having a nucleotide sequence which is less that 30 nucleotides in length and which is substantially identical to at least a part of a 3′-untranslated region (3′-UTR) of a (+) strand RNA virus, such as HCV, as well as pharmaceutical compositions comprising the dsRNA, together with a pharmaceutically acceptable carrier. The pharmaceutical compositions are useful for treating infections and diseases caused by the replication or activity of the (+) strand RNA virus, as well as methods for inhibiting viral replication.
Owner:ALNYLAM PHARMA INC
Primer, probe and kit for detecting novel coronavirus
ActiveCN111197112AStrong conservativeEasy to detectMicrobiological testing/measurementAgainst vector-borne diseasesMolecular biologyRNA virus
The invention provides a primer, a probe and a kit for detecting a novel coronavirus. The specific primer and the probe are designed according to an ORF1ab gene, an E gene, an N gene, an S gene and anM gene of the novel coronavirus 2019-nCoV and are used for identifying and detecting the novel coronavirus. The specific primer / probe and the kit for detecting the 2019-nCoV are high in sensitivity,precision and accuracy, good in specificity and stability, small in template amount required by detection and objective and accurate in detection result. The five-target design can well overcome falsenegative caused by variation in the RNA virus passage process, the positive detection rate can be effectively improved, and missing detection is avoided as much as possible. High clinical applicationvalues are realized.
Owner:GUANGZHOU LBP MEDICINE SCI & TECH
Method of preventing virus: cell fusion by inhibiting the function of the fusion initiation region in rna viruses having class i membrane fusogenic envelope proteins
ActiveUS20060280754A1Prevent and inhibit infectionSsRNA viruses negative-senseSsRNA viruses positive-senseCell membraneDisease cause
The present invention relates to a method of preventing or inhibiting viral infection of a cell and / or fusion between the envelope of a virus and the membranes of a cell targeted by the virus (thereby preventing delivery of the viral genome into the cell cytoplasm, a step required for. viral infection). The present invention particularly relates to the families of RNA viruses, including the arenaviruses, coronaviruses, filoviruses, orthomyxoviruses, paramyxoviruses, and retroviruses, having Class I membrane fusion proteins as the fusion proteins that mediate this fusion process. The present invention provides for a method of identifying a conserved motif or domain called the fusion initiation region (FIR) in these viruses. The present invention further provides for methods of preventing infection by such viruses, by interfering with their FIR. The present invention further provides for methods of treatment and prophylaxis of diseases induced by such viruses.
Owner:TULANE EDUCATIONAL FUND THE ADMINISTRATORS OF THE
Induction of viral mutation by incorporation of miscoding ribonucleoside analogs into viral RNA
InactiveUS20050187180A1High mutation rateReduced viabilityOrganic active ingredientsSsRNA viruses positive-senseRibonucleosideViral replication
Owner:UNIV OF WASHINGTON
Protein expression system
ActiveUS20100287670A1Improve expression levelBoost protein levelsVirus peptidesVaccinesStart codonForeign protein
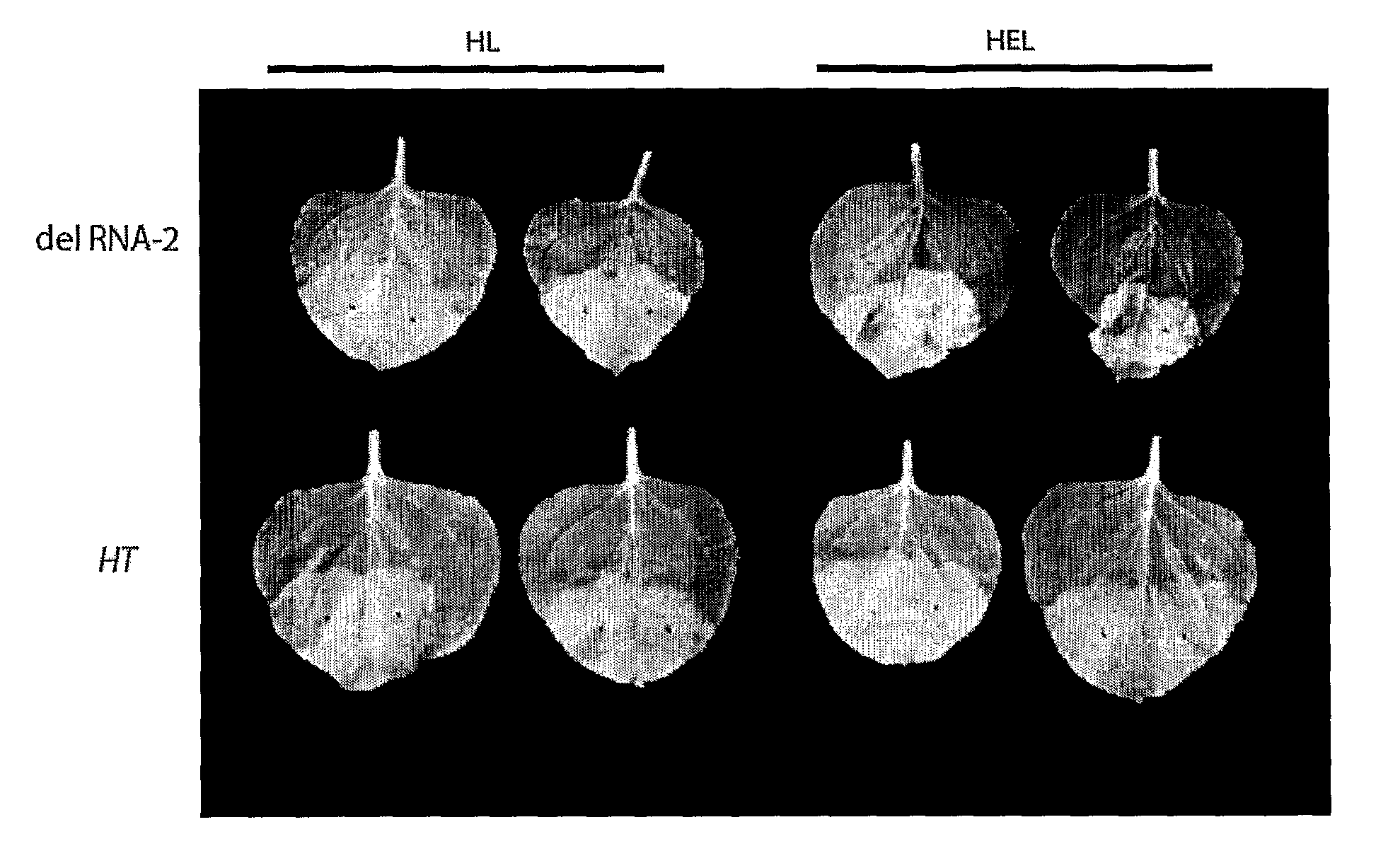
The inventions is based on an expression enhancer sequence derived from the RNA-2 genome segment of a bipartite RNA virus, in which a target initiation site in the RNA-2 genome segment has been mutated. Deletion of appropriate start codons upstream of the main RNA2 translation initiation can greatly increase in foreign protein accumulation without the need for viral replication. Also provided are methods, vectors and systems, including the ‘hyper-translatable’ Cowpea Mosaic Virus (‘CPMV-HT’) based protein expression system.
Owner:PLANT BIOSCI LTD
Compositions and methods for detecting severe acute respiratory syndrome coronavirus
InactiveUS20050095582A1Lower Level RequirementsReduce rateSsRNA viruses positive-senseMicrobiological testing/measurementSARS coronavirusSevere acute respiratory syndrome coronavirus
The invention provides compositions and methods for detecting the presence of SARS-coronavirus, for screening anti-SARS coronavirus agents and vaccines, and for reducing infection with plus-strand RNA viruses such as SARS-coronavirus.
Owner:DIAGNOSTIC HYBRIDS +1
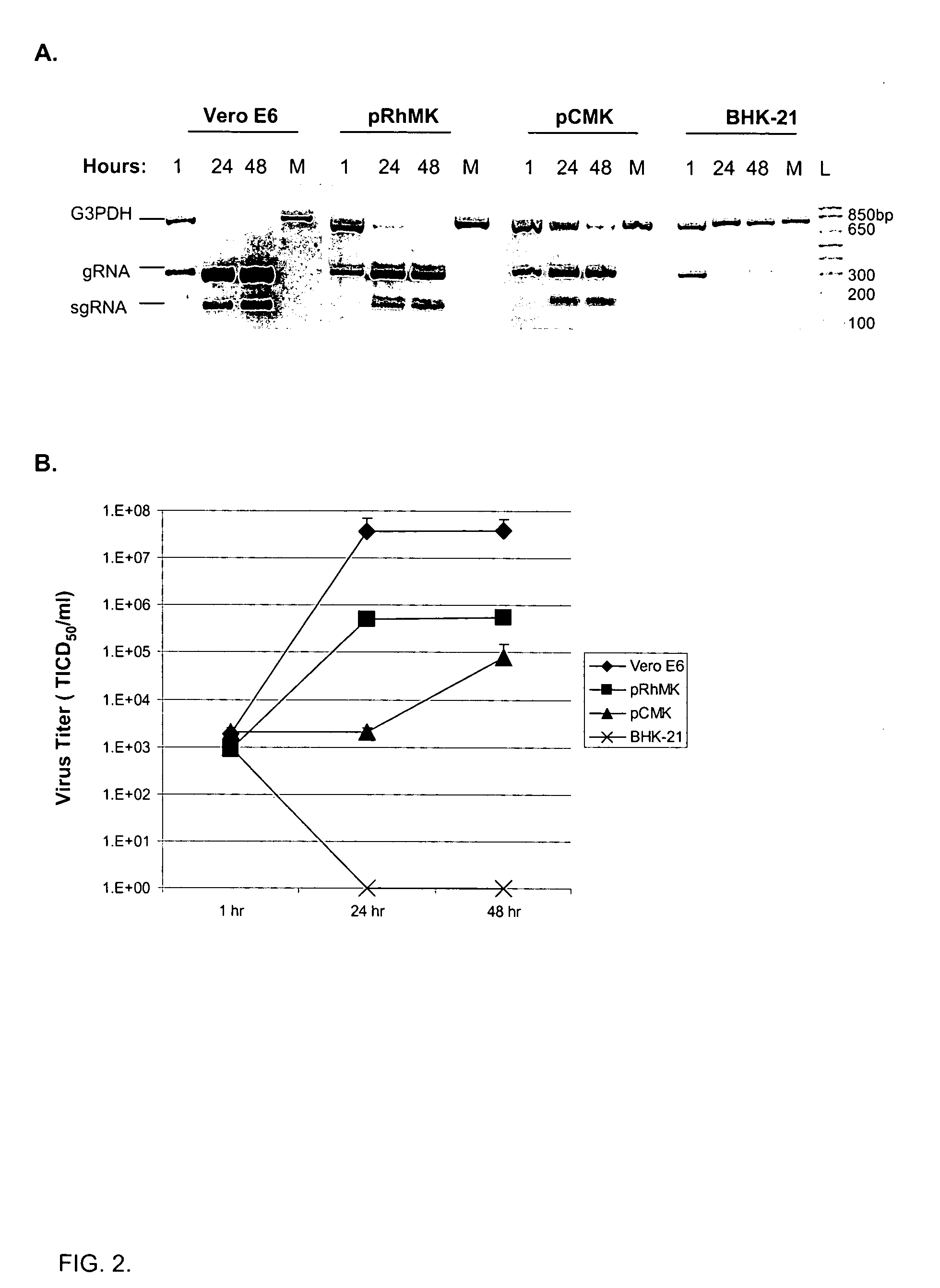
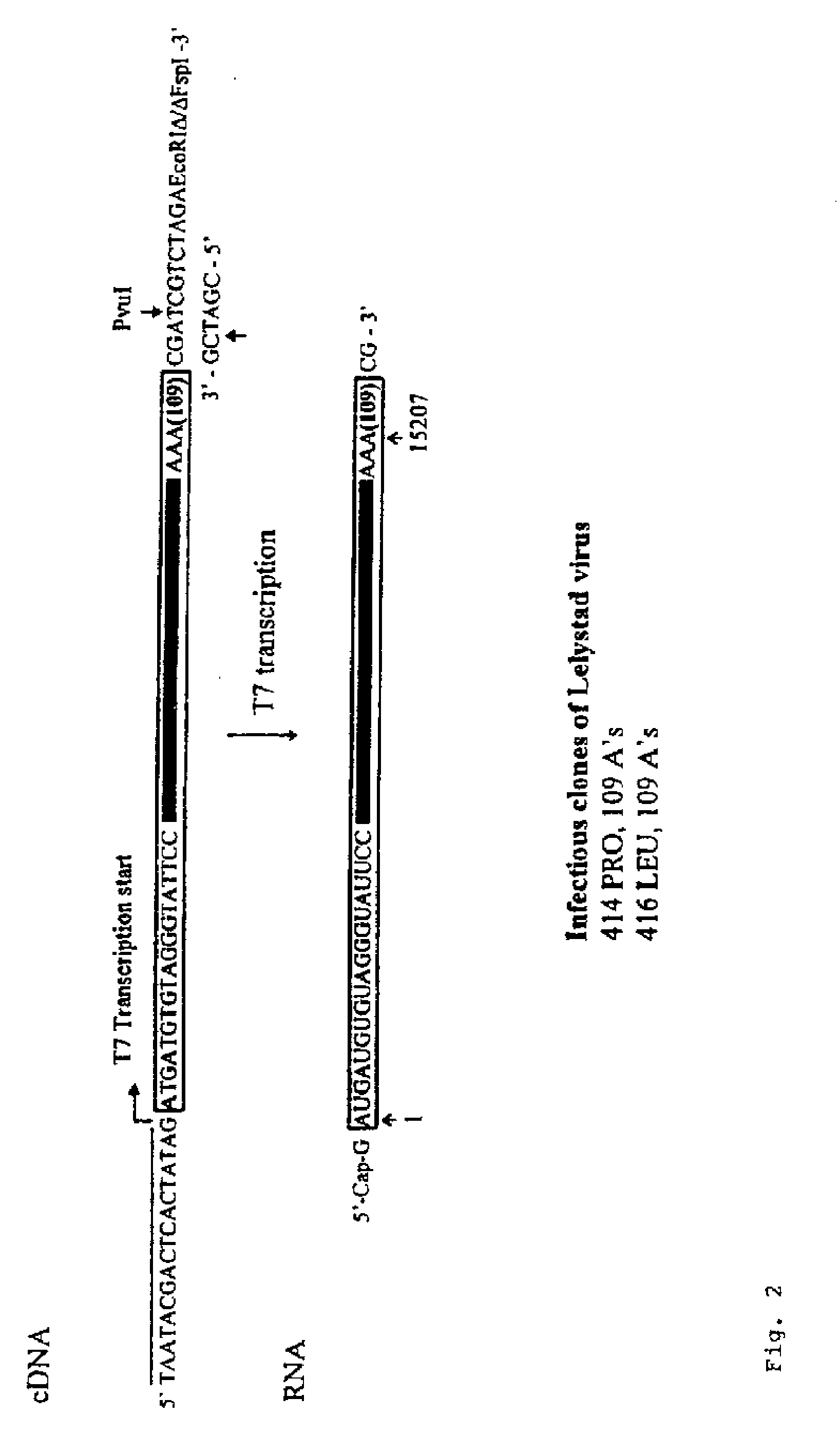
Infectious clones of RNA viruses and vaccines and diagnostic assays derived thereof
InactiveUS20060205033A1SsRNA viruses positive-senseViral antigen ingredientsRNA Virus InfectionsWild type
An infectious clone based on the genome of a wild-type RNA virus is produced by the process of providing a host cell not susceptible to infection by the wild-type RNA virus, providing a recombinant nucleic acid based on the genome of the wild-type RNA virus, transfecting the host cell with the recombinant nucleic acid and selecting for infectious clones. The recombinant nucleic acid comprises at least one full-length DNA copy or in vitro-transcribed RNA copy or a derivative of either. The infectious clones can be used in single or dual purpose vaccines and in viral vector vaccines.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
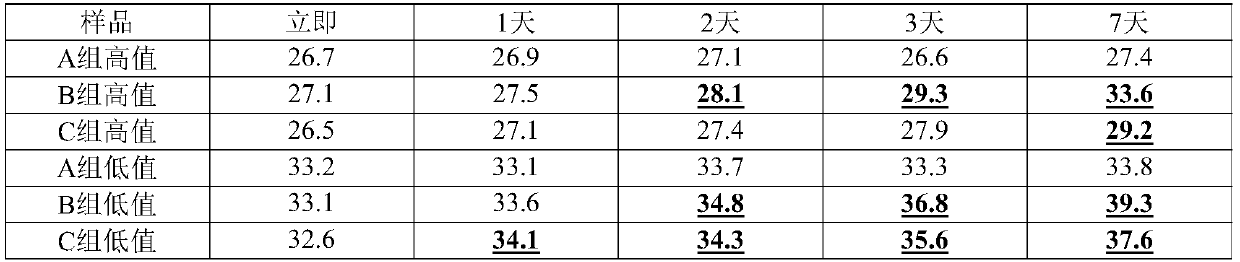
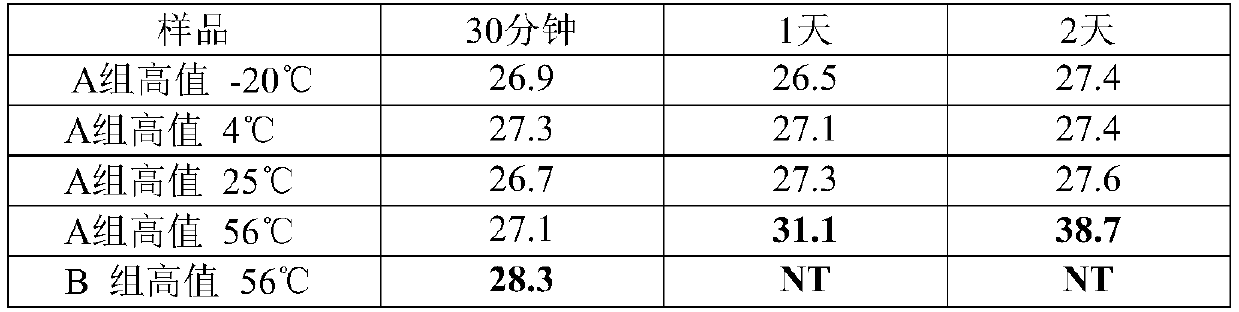
RNA virus inactivation preservation solution and application thereof
ActiveCN111363729AAchieve immediate lysis and inactivationHigh sensitivityMicrobiological testing/measurementInactivation/attenuationLithium chlorideActive agent
The present invention discloses an RNA virus inactivation preservation solution. The inactivation preservation solution comprises ammonium salt, 1-ethyl-3-methylimidazolium bisulfate, lithium chloride, ethylenediaminetetraacetic acid, an RNase inhibitor, a surfactant, a reducing agent, a buffer agent and water. The present invention also provides an RNA virus sample inactivation and preservation method and an RNA virus detection method, and the method is conducted by using the inactivation preservation solution. The preservation solution has low cost, inhibits RNase activity, preserves RNA integrity, realizes preservation and transportation of samples at room temperature, can also quickly inactivate pathogens at room temperature, reduces infection risks of medical personnel, and reduces false negatives in detection. At the same time, the inactivation preservation solution can realize a lysis function, does not contain chaotropic reagents and alcohols, is compatible with mainstream nucleic acid extraction kits on the market, greatly saves time of an entire process, and is particularly suitable for 2019 new coronavirus (COVID-19) inactivation and lysis steps after swab sampling.
Owner:苏州白垩纪生物科技有限公司
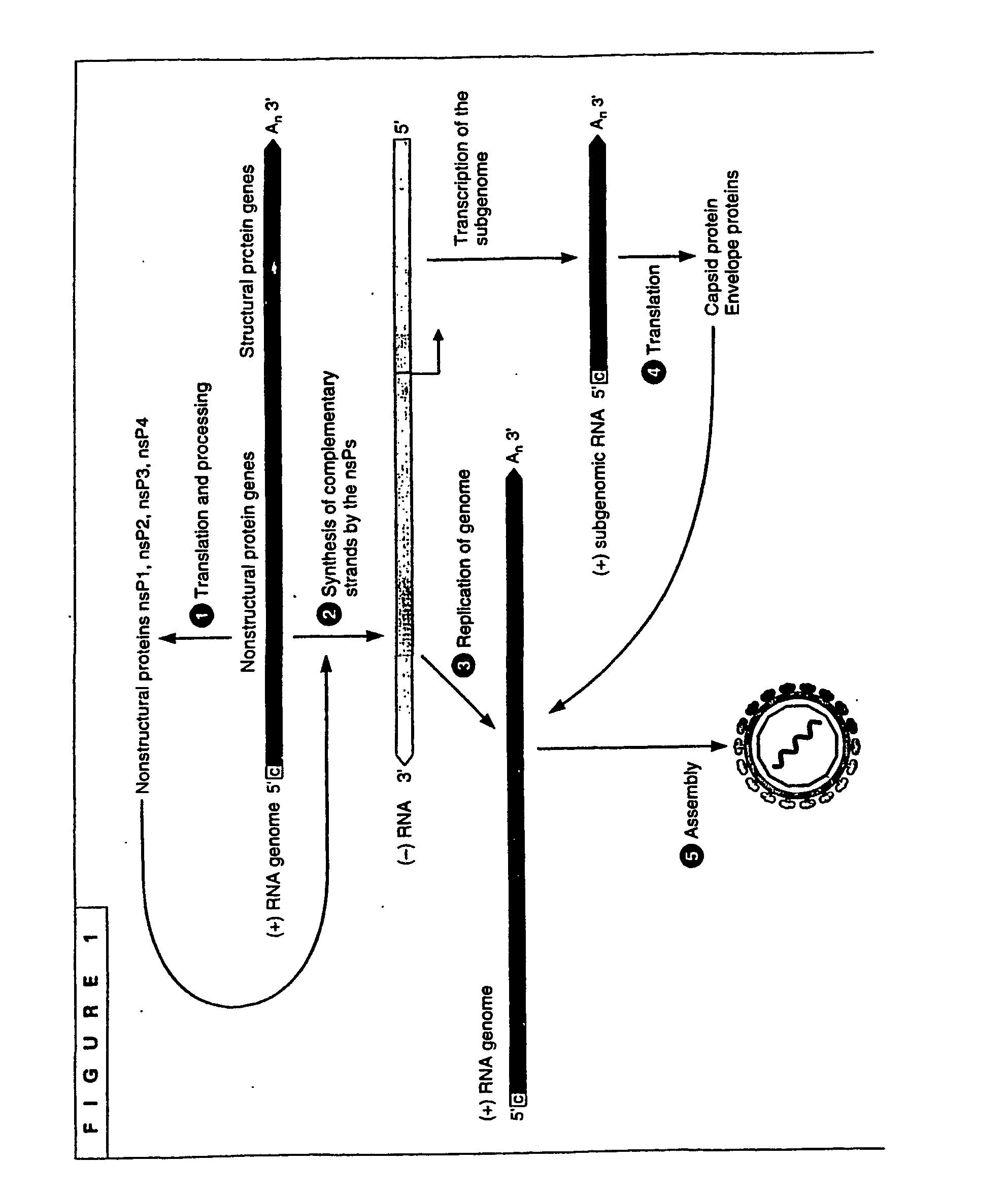
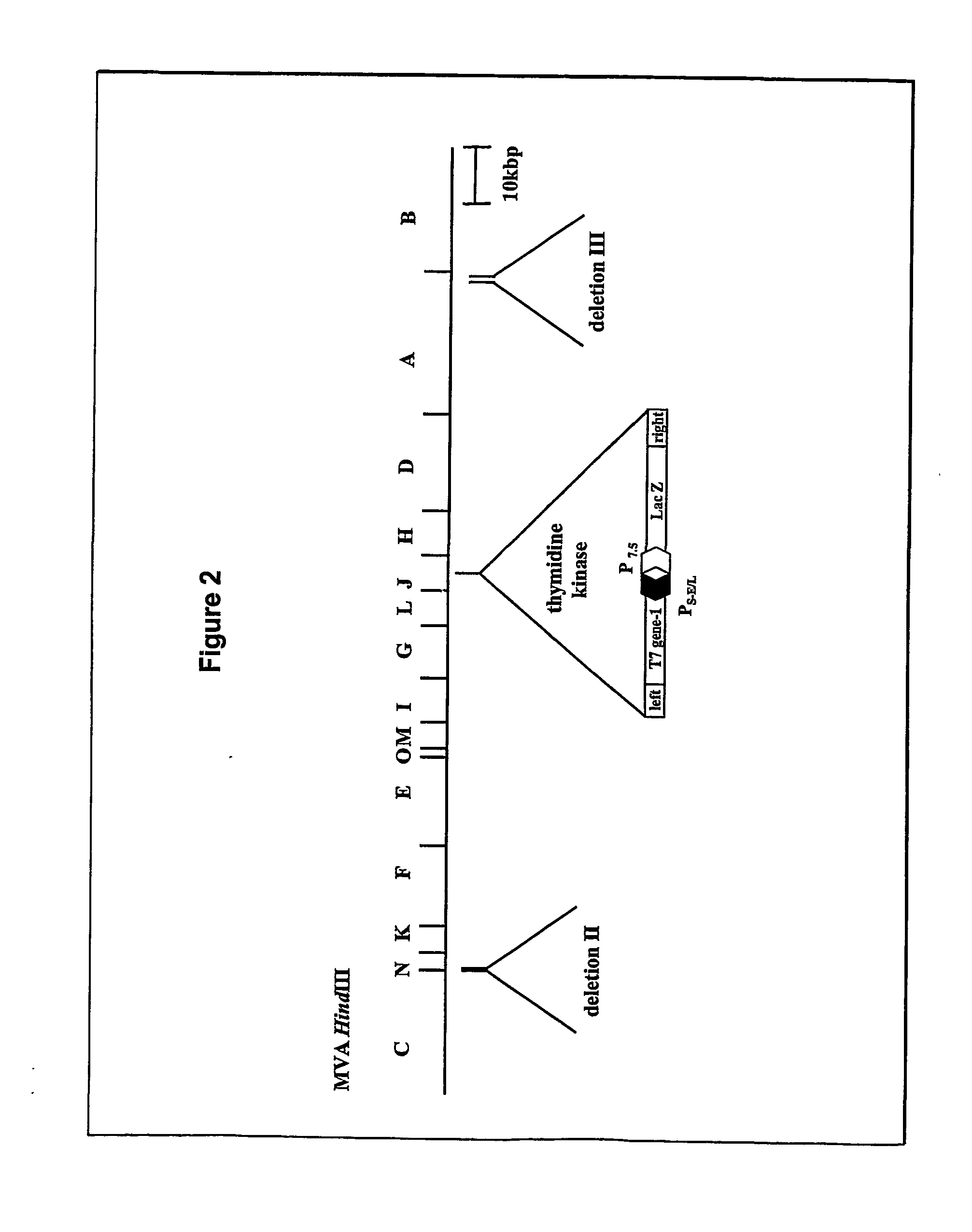
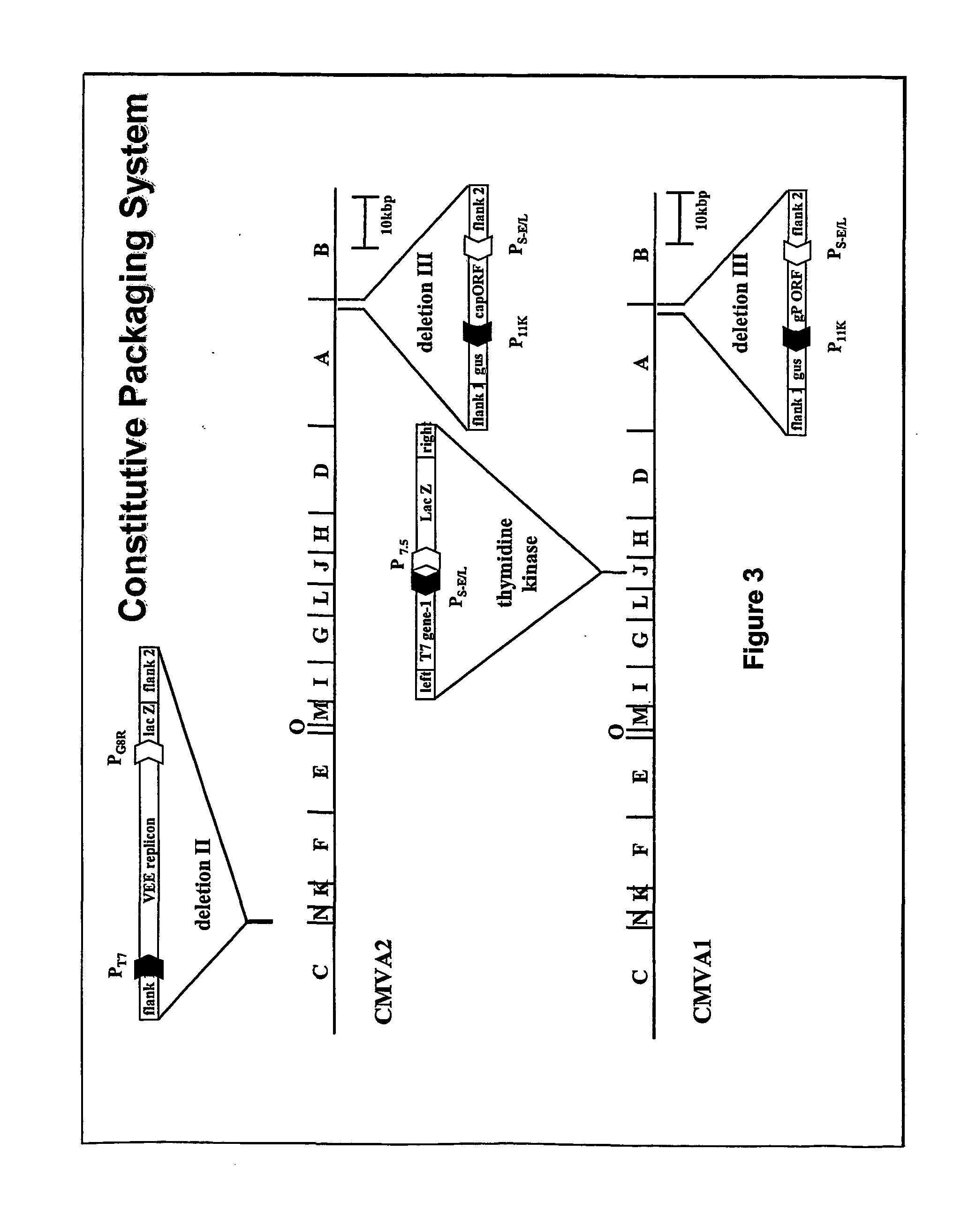
Packaging of positive-strand rna virus replicon particles
InactiveUS20040029279A1Great titerProbability of generatingFungiSsRNA viruses positive-sensePharmaceutical formulationViral vector
The invention generally relates to recombinant polynucleotides, positive-strand RNA virus (psRNAV) recombinant expression vectors, and packaging systems. The packaging systems are based on the expression of helper functions by coinfecting re-combinant poxvirus vectors comprising recombinant polynucleotides. Methods for obtaining psRNAV replicon particles using these packaging systems are disclosed. Immunogenic compositions and pharmaceutical formulations are provided that comprise replicon particles of the invention. Methods for generating an immune response or producing a pharmaceutical effect are also provided.
Owner:WYETH HOLDINGS CORP
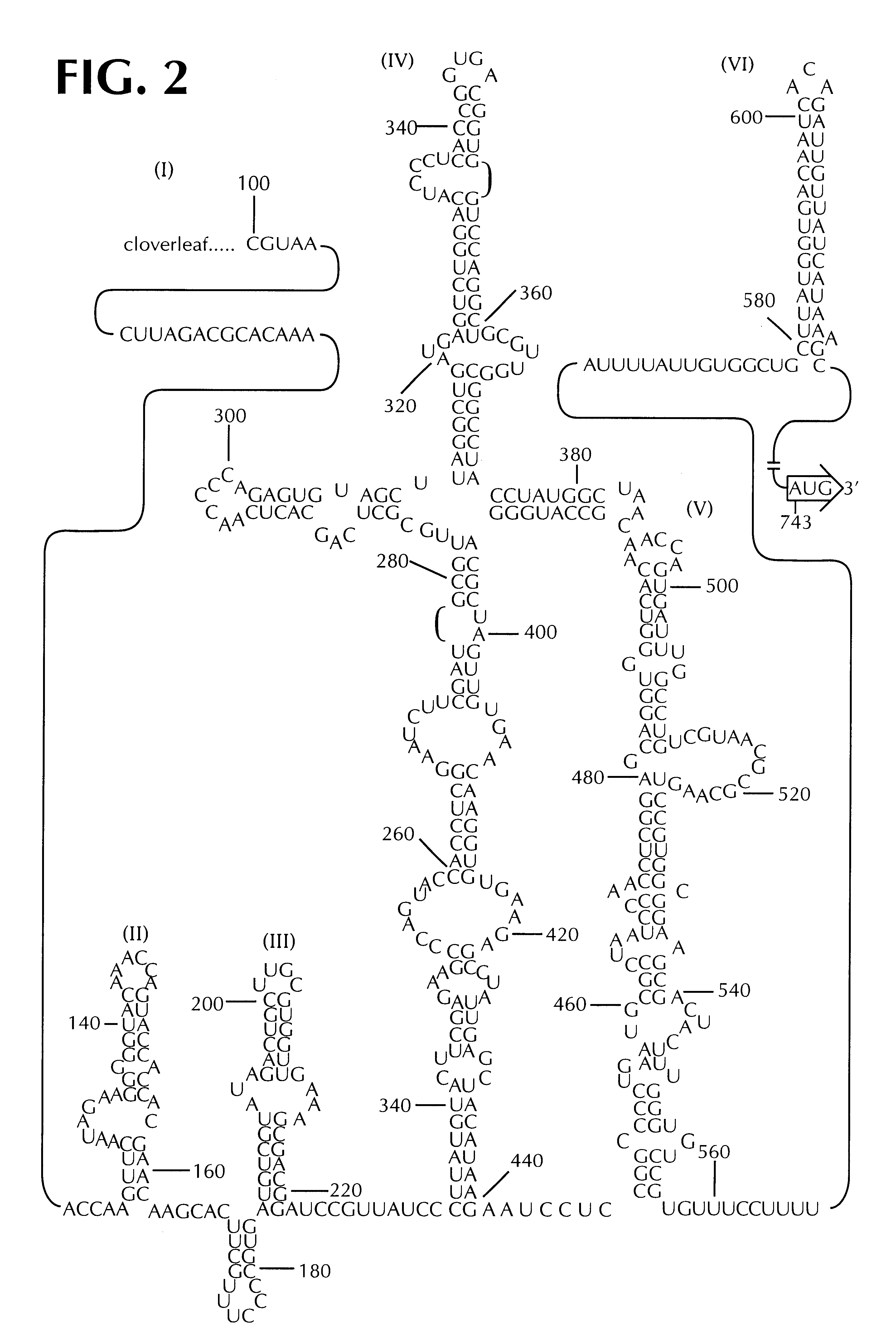
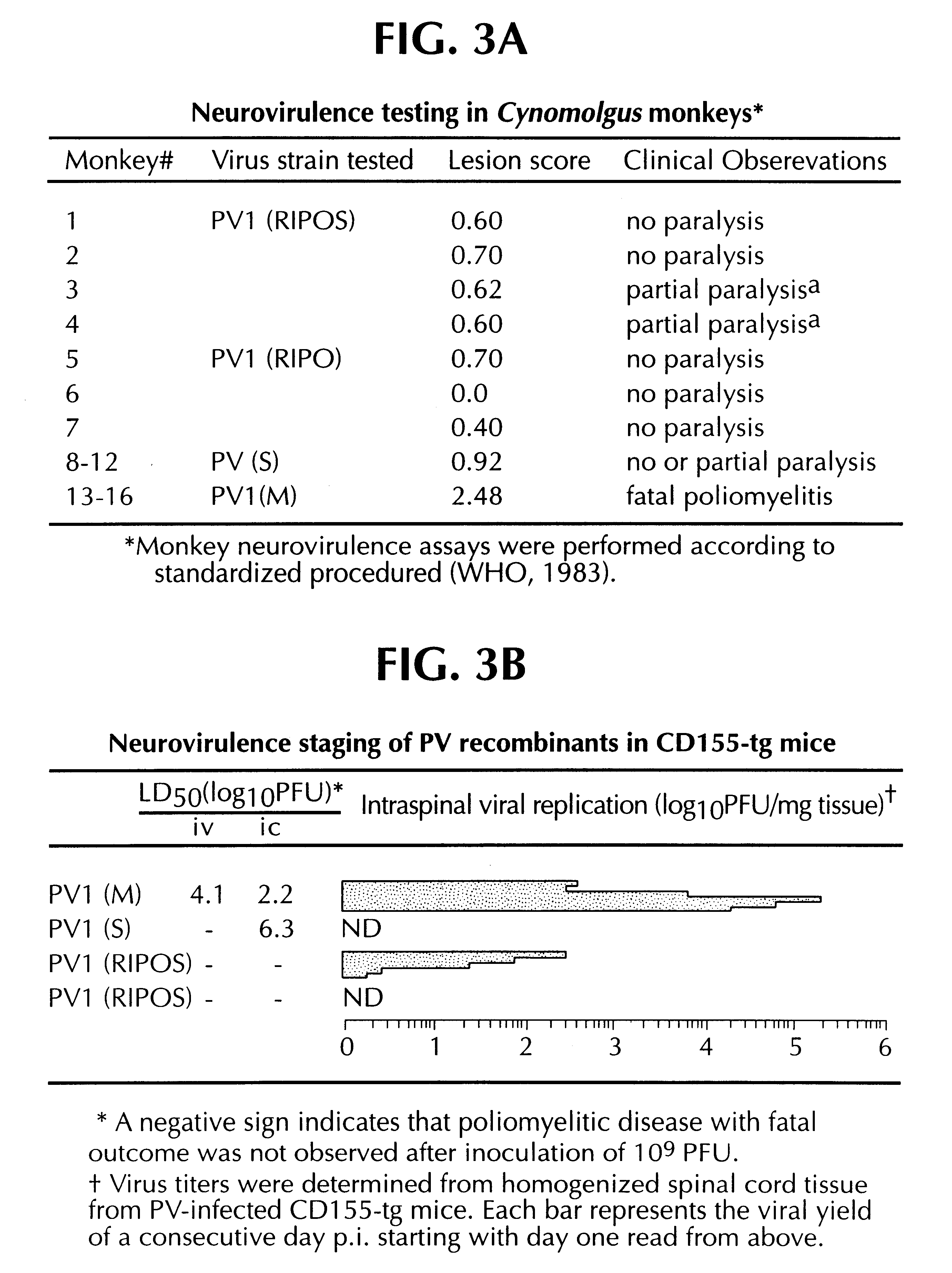
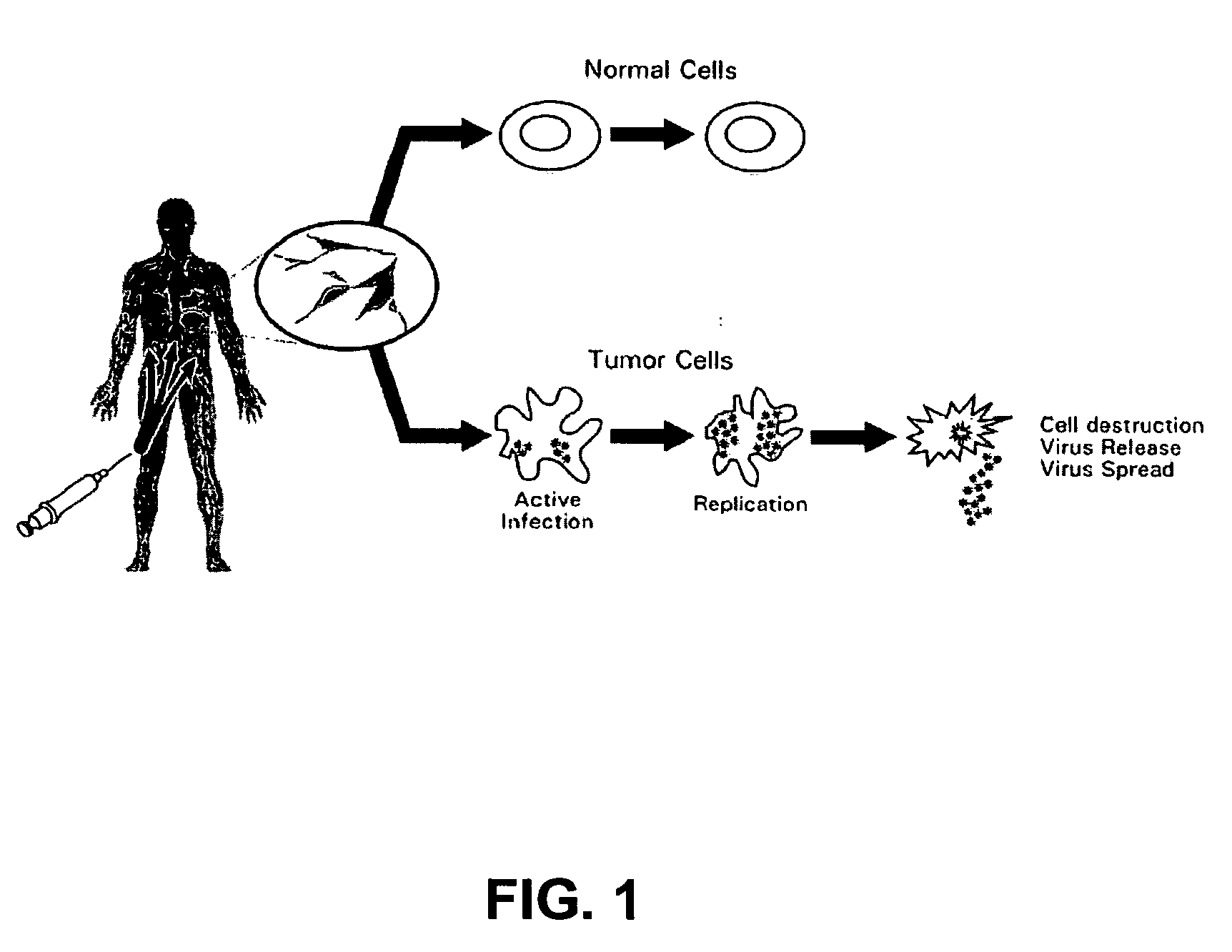
Recombinant poliovirus for the treatment of cancer
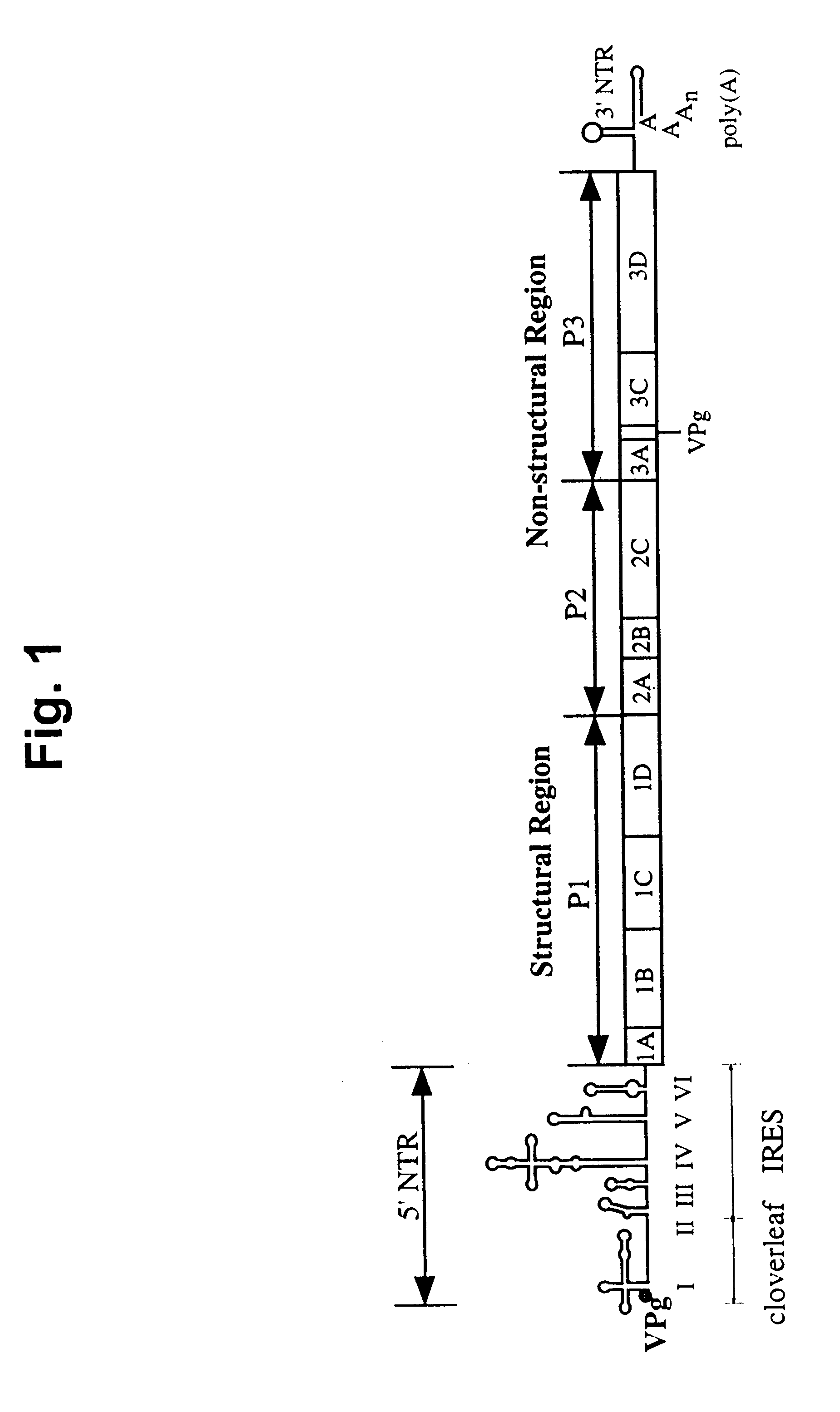
The present invention is directed to non-pathogenic, oncolytic, recombinant polioviruses for the treatment of various forms of malignant tumors. The recombinant polioviruses of the invention are those in which the internal ribosomal entry site (IRES) of the wild type poliovirus was exchanged with the IRES of other picornaviruses, and optionally P1, P3 or the 3'NTR thereof was exchanged with that of poliovirus Sabin type. More particularly, the present invention is directed to the administration of the non-pathogenic, oncolytic, recombinant poliovirus to the tumor directly, intrathecally or intravenously to cause tumor necrosis. The method of the present invention is particularly useful for the treatment of malignant tumors in various organs, such as: breast, colon, bronchial passage, epithelial lining of the gastrointestinal, upper respiratory and genito-urinary tracts, liver, prostate and the brain. Astounding remissions in experimental animals have been demonstrated for the treatment of malignant glioblastoma multiforme, an almost universally fatal neoplasm of the central nervous system.
Owner:NEW YORK UNIV OF RES FOUND OF THE
Seneca valley virus based compositions and methods for treating disease
ActiveUS20060159659A1High therapeutic indexSafe and effective and new lineBiocideSsRNA viruses positive-senseAbnormal tissue growthProtein detection
The present invention relates to a novel RNA picornavirus that is called Seneca Valley virus (“SVV”). The invention provides isolated SVV nucleic acids and proteins encoded by these nucleic acids. Further, the invention provides antibodies that are raised against the SVV proteins. Because SVV has the ability to selectively kill some types of tumors, the invention provides methods of using SVV and SVV polypeptides to treat cancer. Because SVV specifically targets certain tumors, the invention provides methods of using SVV nucleic acids and proteins to detect cancer. Additionally, due to the information provided by the tumor-specific mechanisms of SVV, the invention provides methods of making new oncolytic virus derivatives and of altering viruses to have tumor-specific tropisms.
Owner:PERCEIVER PHARMA +1
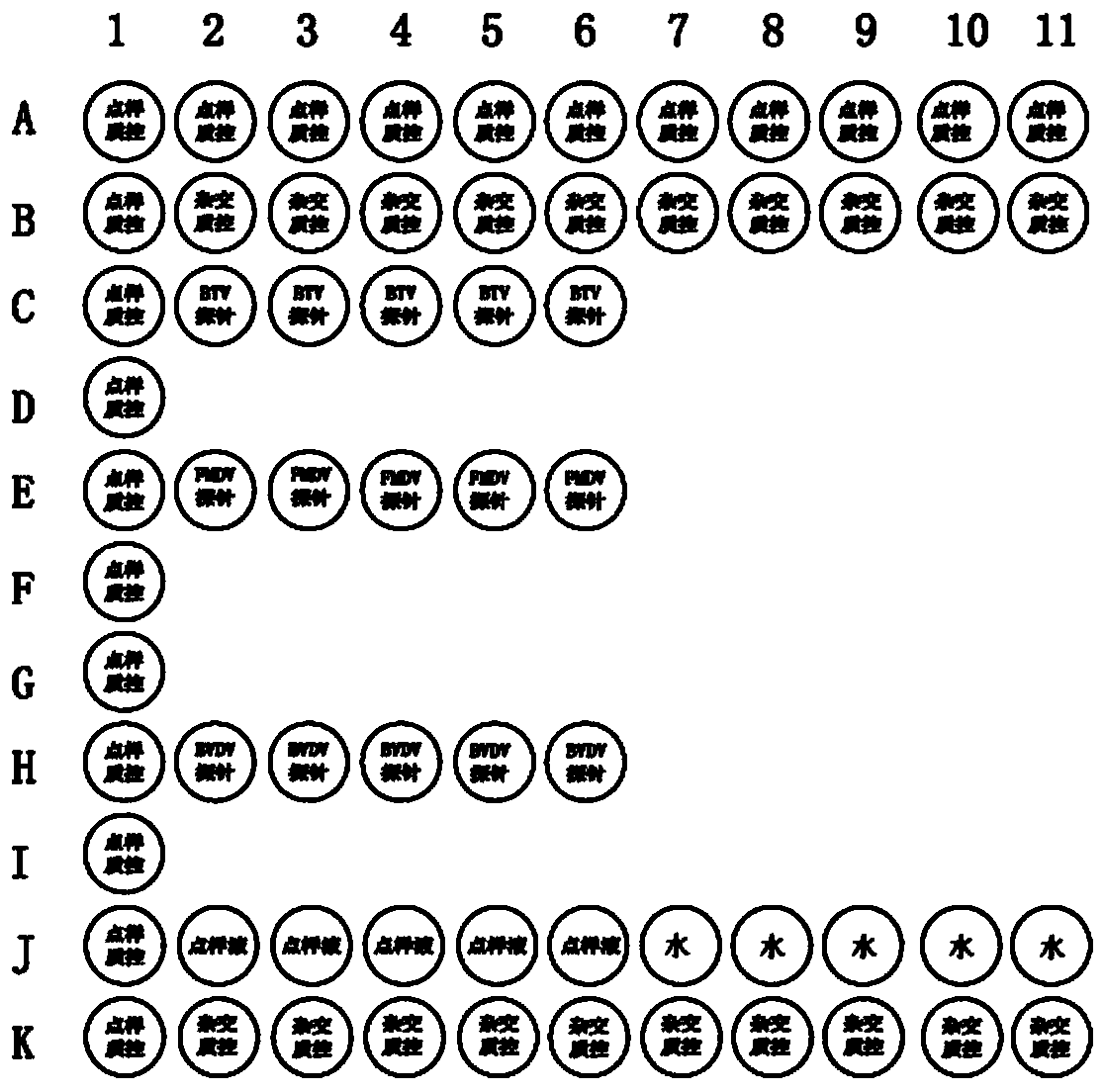
Multiplex PCR (polymerase chain reaction) primer, probe and gene chip for detecting bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus
InactiveCN103695566AImprove throughputShorten diagnostic timeMicrobiological testing/measurementDNA/RNA fragmentationForward primerMultiplex
The invention relates to a multiplex PCR (polymerase chain reaction) primer, a probe and a gene chip for detecting the bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus. The multiplex PCR primer and probe have the nucleotide sequences shown by SEQ ID No.1 to SEQ ID and No.9. The gene chip comprises a solid-phase carrier, a sample application quality control probe, a positive hybrid quality control probe and a multiplex PCR primer for detecting the bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus and the corresponding probe. In the invention, the forward primers of three viruses are marked with fluorescence, a gene chip detection technology carrying three viruses in animal fur is established based on multiplex RT-PCR (reverse transcription-polymerase chain reaction), and the RNA virus in the fur can be sensitively and specifically detected with high flux; the three viruses are screened at the same time in detection once, and the situation that a specific method is required for each virus before is changed, thereby saving the diagnosis time, meeting the needs for quick detection of mass imported / exported fur samples of the exit-entry inspection and quarantine departments and the fur import and export enterprises, and realizing relatively high application values.
Owner:徐超
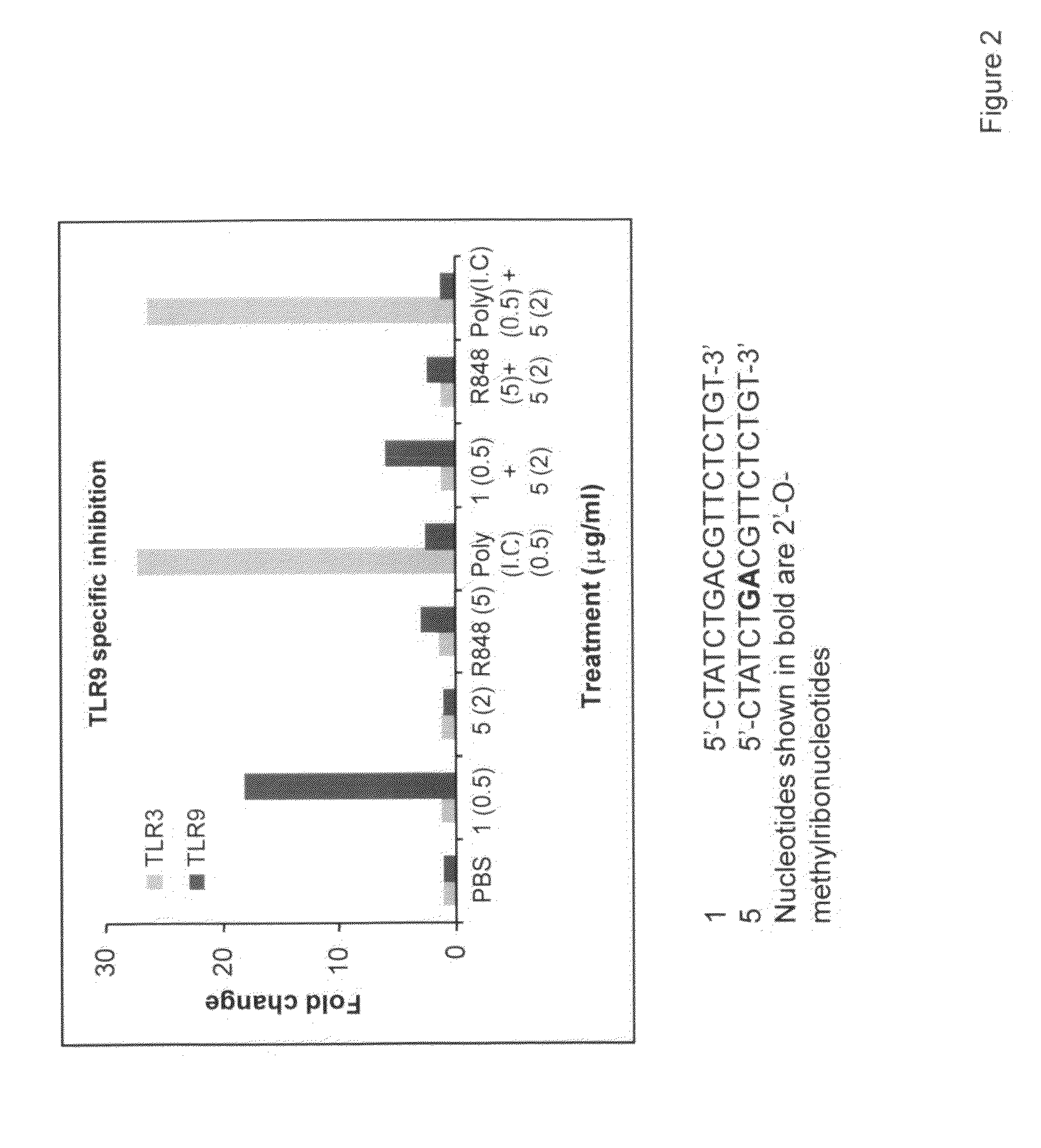
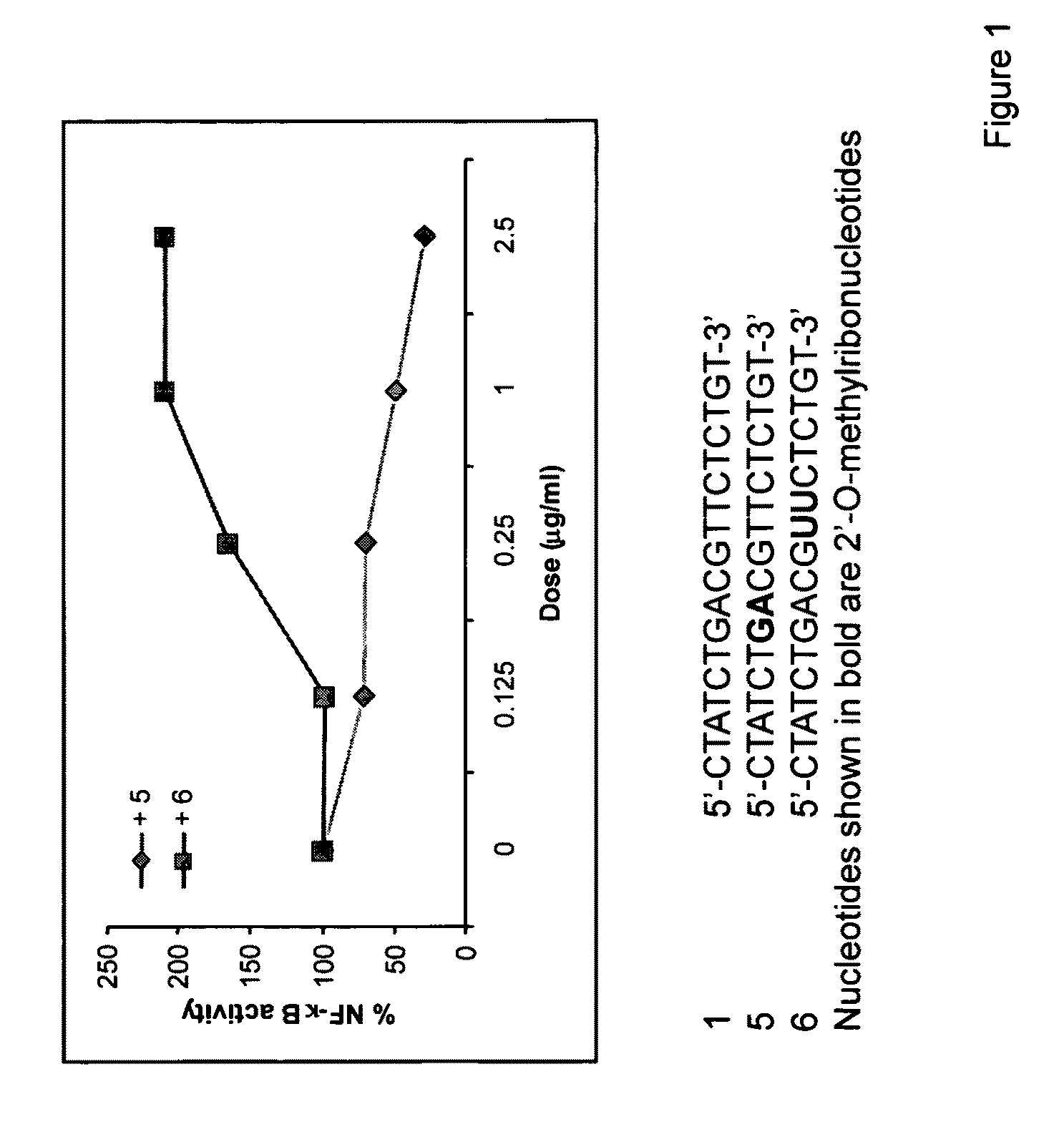
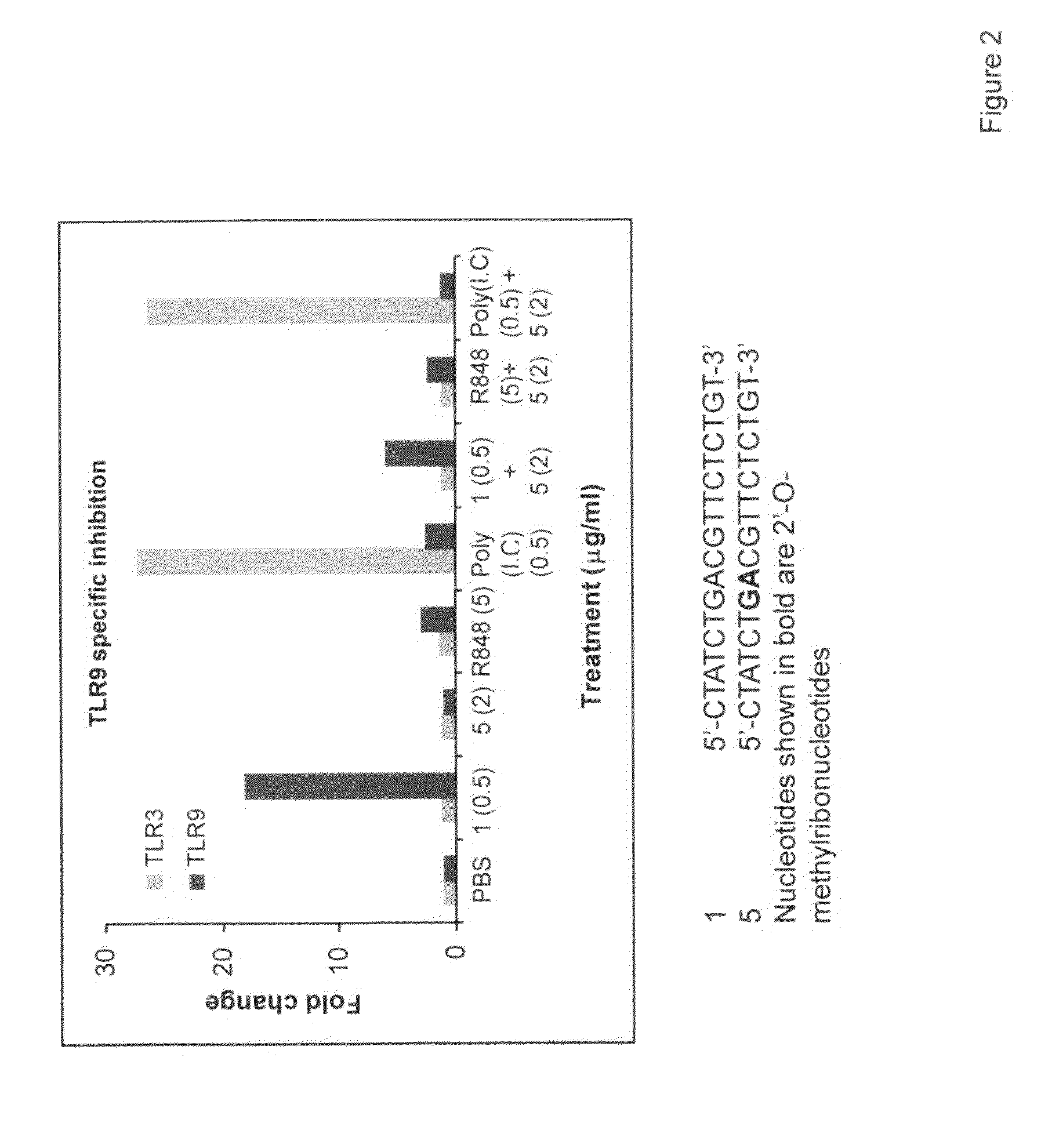
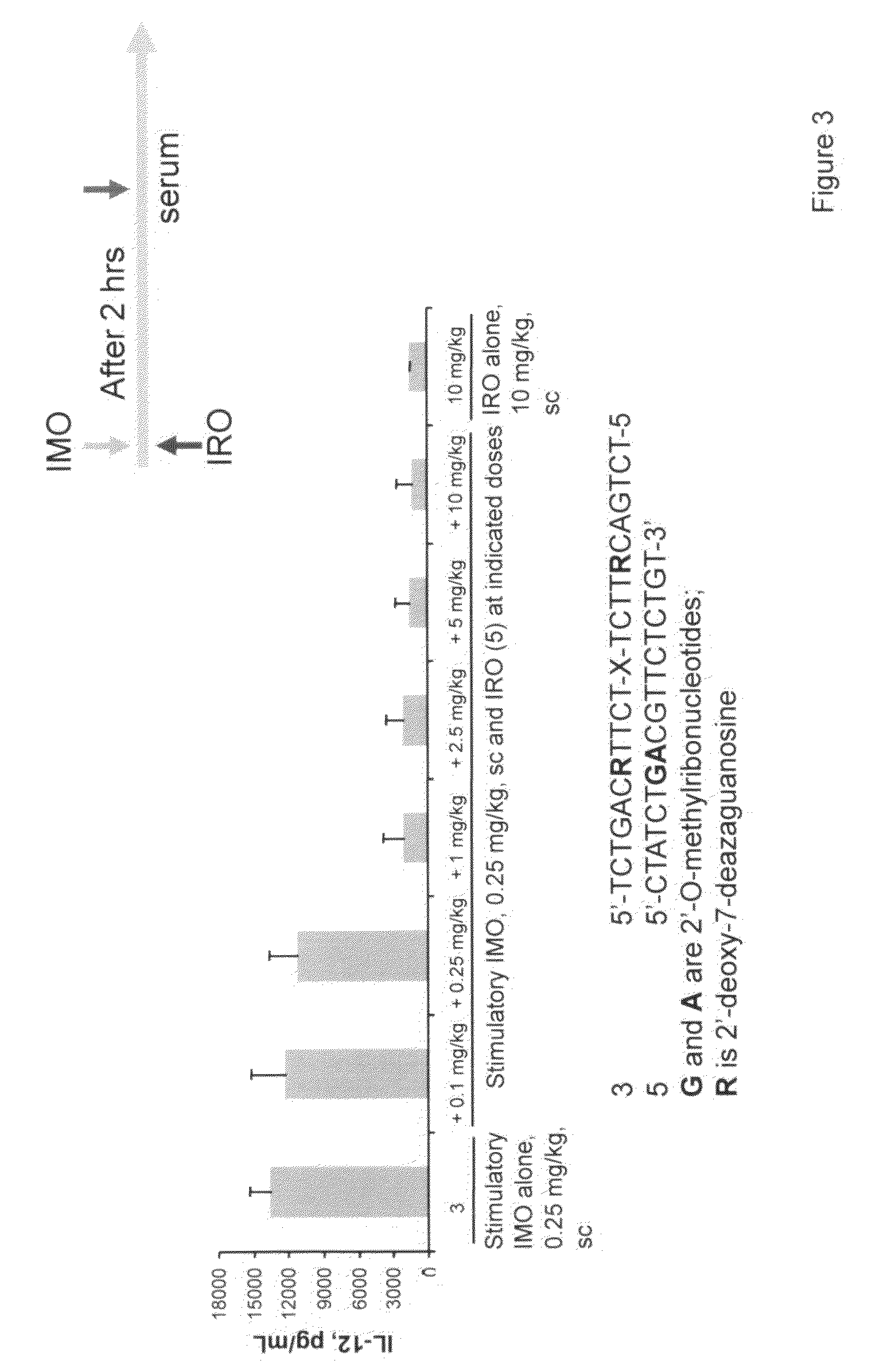
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
ActiveUS20090098063A1Suppressing TLR activationInhibition of thrombin activityOrganic active ingredientsCosmetic preparationsDiseaseRIG-I-like receptor
Owner:IDERA PHARMA INC
L-nucleoside compounds and application thereof
InactiveCN105646629AInhibitory activityOrganic active ingredientsSugar derivativesEbola virusRNA Virus Infections
The invention discloses L-nucleoside compounds having the structure characteristic represented by the formula (I) or pharmaceutically acceptable salts thereof, and belongs to the technical field of pharmaceutical chemistry. The compounds can inhibit the activity of RNA viral polymerase, so the compounds can be used as potential drugs for prevention and treatment of infection of RNA viruses such as HCV, influenza virus, HRV (rhinovirus), RSV, Ebola virus, dengue virus, intestinal virus and the like.
Owner:GUANGZHOU HENOVCOM BIOSCI CO LTD
Infectious DNA as a vaccine against west nile and other flavivirues
InactiveUS20050276816A1Easy to prepareStable and safe vaccineSsRNA viruses positive-senseViral antigen ingredientsDna encodingInfectious agent
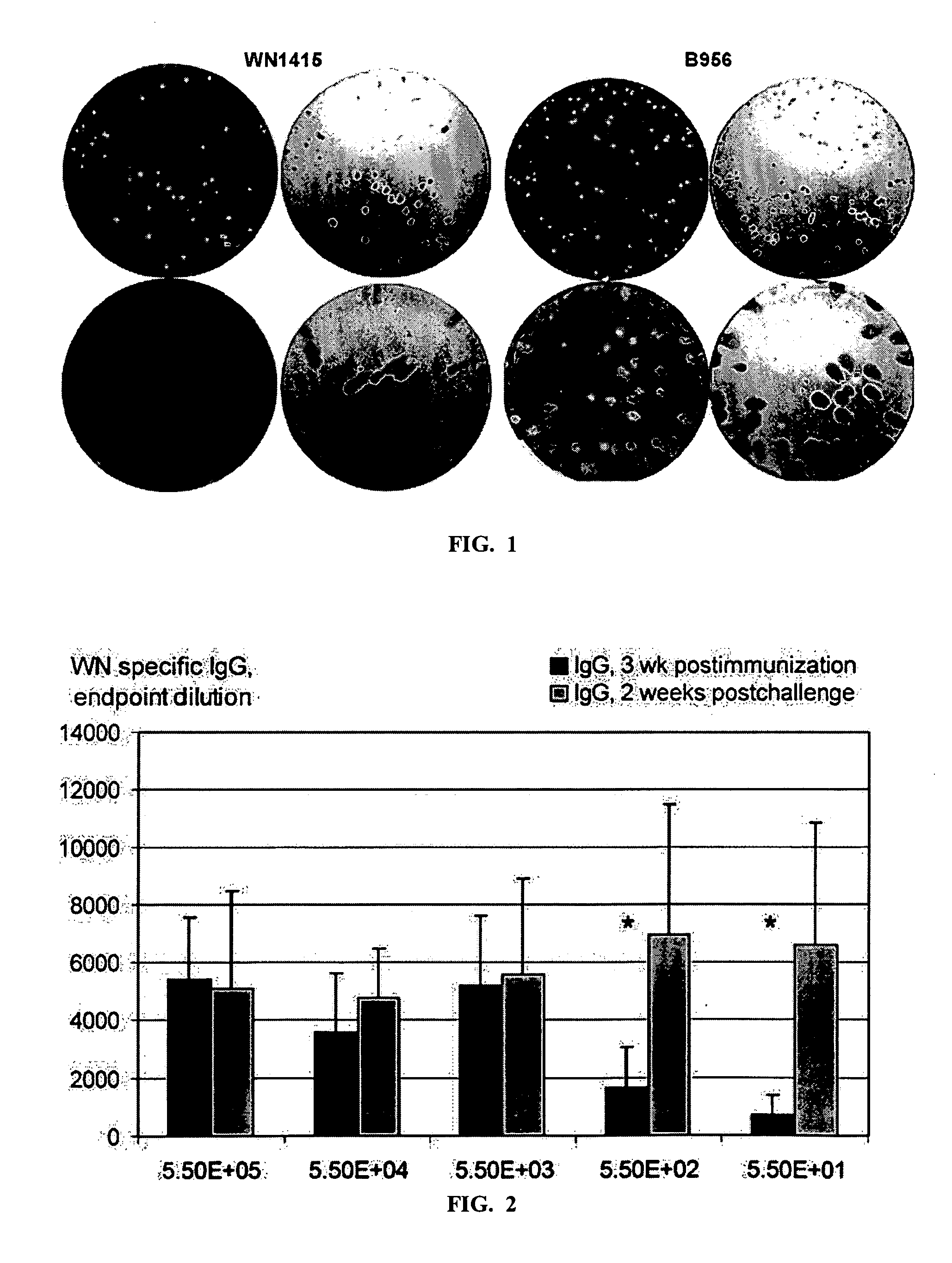
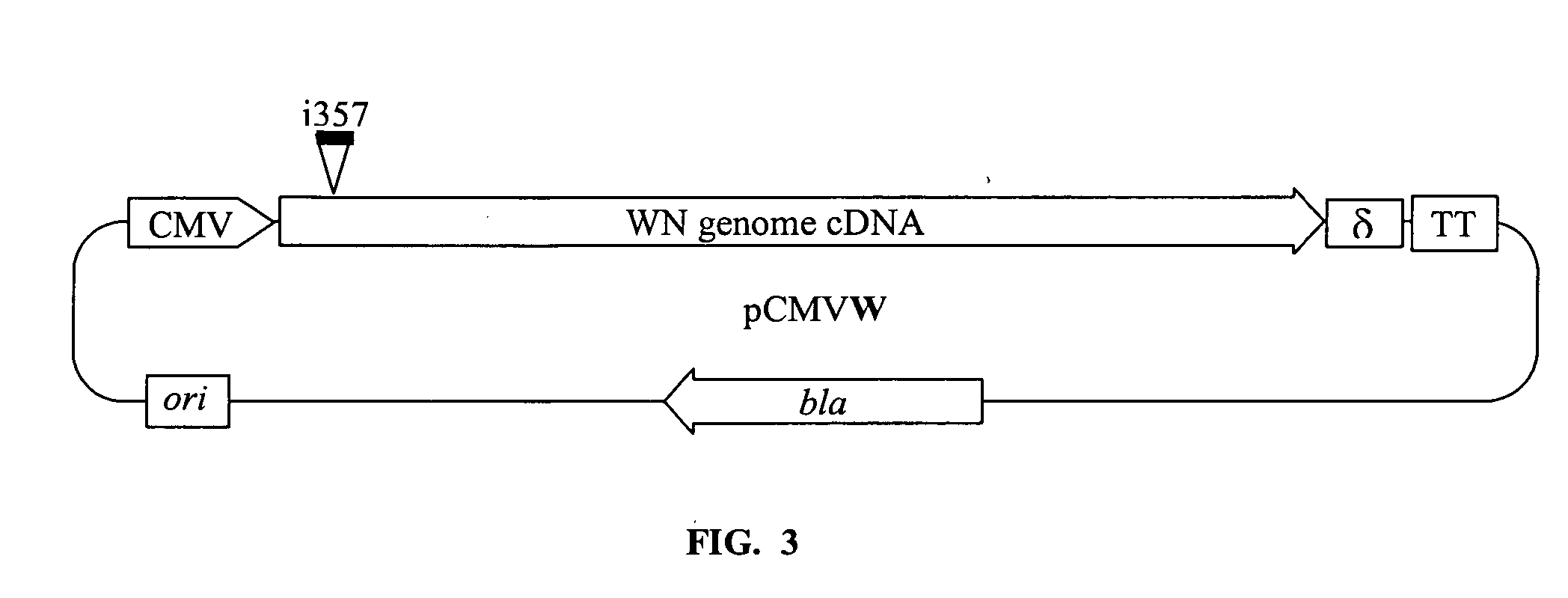
A vaccine for West Nile virus that protects a subject against West Nile infection comprising an a pharmaceutically acceptable carrier and a therapeutically effective does of an infectious agent selected from the group consisting of: a live attenuated infectious (+) RNA virus designated as WN1415, a vector comprising infectious DNA encoding an infectious (+) RNA molecule encoding the West Nile virus, and the West Nile (+) RNA virus designated as WN956D117B3 (GenBank #M12294).
Owner:UNIVERSITY OF KANSAS
Method for co-extracting DNA/RNA (Deoxyribonucleic Acid/Ribonucleic Acid) virus nucleic acid
InactiveCN105925568AImprove throughputHigh-throughput extractionMicrobiological testing/measurementDNA preparationMagnetic beadBiology
The invention provides a method for co-extracting DNA / RNA (Deoxyribonucleic Acid / Ribonucleic Acid) virus nucleic acid. The method comprises the following steps: cracking a sample, which is diluted with saline, with a guanidine isothiocyanate cracking solution containing an RNA precipitating aid agent; releasing and dissociating DNA or RNA of different nucleic acid viruses in the sample into a cracking solution; adding magnetic beads to form a magnetic bead-nucleic acid compound; washing to obtain a nucleic acid eluting solution. The novel method for co-extruding the DNA and the RNA from a respiratory tract sample is established based on the nano magnetic beads; in a whole process, toxic reagents including phenol, chloroform, beta-mercaptoethanol and the like are not used, and time and labor are saved. The method is used for commonly extracting the DNA and the RNA from the respiratory tract sample and is also applicable to nucleic acid co-extraction of other samples with different types, such as urine and blood; impurities including protein and the like are efficiently removed and the degradation of the nucleic acid is reduced; the aim of detecting DNA viruses and RNA viruses in the same tube at the same time is realized. The method is simple, convenient, rapid and safe, and is particularly suitable for fluorescent quantitative PCR (Polymerase Chain Reaction) detection and the like.
Owner:HANGZHOU FIRST PEOPLES HOSPITAL
Compositions and methods for detecting severe acute respiratory syndrome coronavirus
InactiveUS7129042B2Improve productivityHigh sensitivitySsRNA viruses positive-senseMicrobiological testing/measurementSARS coronavirusSevere acute respiratory syndrome coronavirus
The invention provides compositions and methods for detecting the presence of SARS-coronavirus, for screening anti-SARS coronavirus agents and vaccines, and for reducing infection with plus-strand RNA viruses such as SARS-coronavirus.
Owner:DIAGNOSTIC HYBRIDS +1
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
Owner:IDERA PHARMA INC
Novel viral replication inhibitors
ActiveUS20160297810A1Promote efficient proliferationOrganic chemistryAntiviralsRNA Virus InfectionsMedicine
The present invention relates to a series of novel compounds, methods to prevent or treat viral infections in animals by using the novel compounds and to said novel compounds for use as a medicine, more preferably for use as a medicine to treat or prevent viral infections, particularly infections with RNA viruses, more particularly infections with viruses belonging to the family of the Flaviviridae, and yet more particularly infections with the Dengue virus. The present invention furthermore relates to pharmaceutical compositions or combination preparations of the novel compounds, to the compositions or preparations for use as a medicine, more preferably for the prevention or treatment of viral infections. The invention also relates to processes for preparation of the compounds.
Owner:KATHOLIEKE UNIV LEUVEN
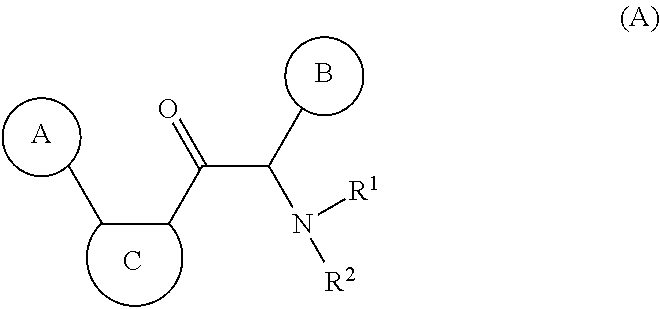
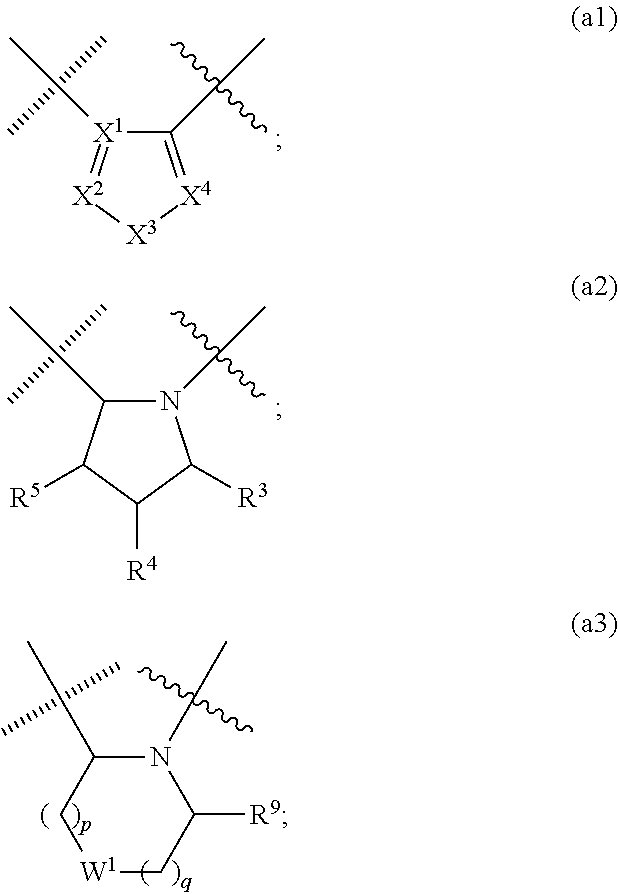
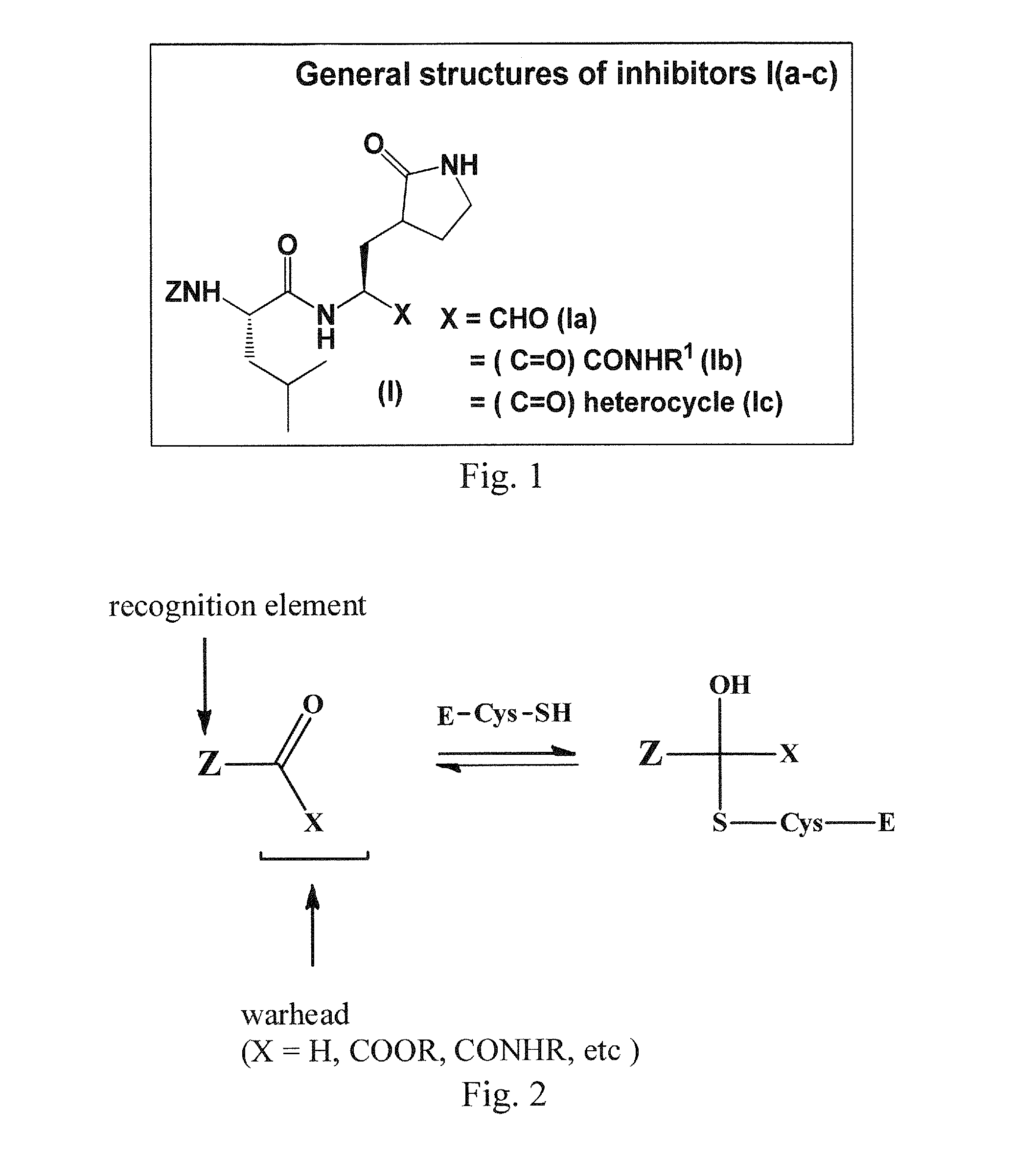
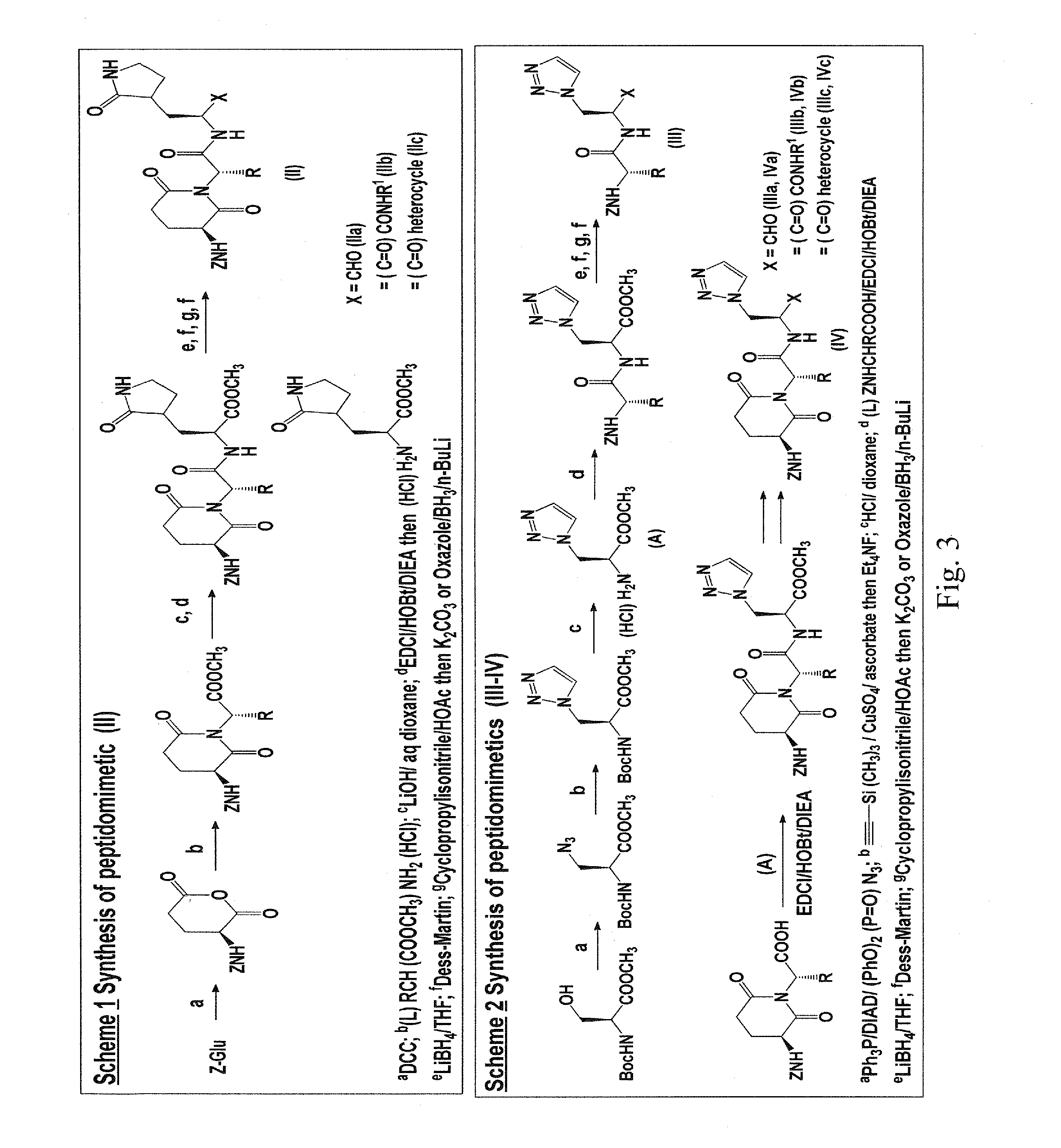
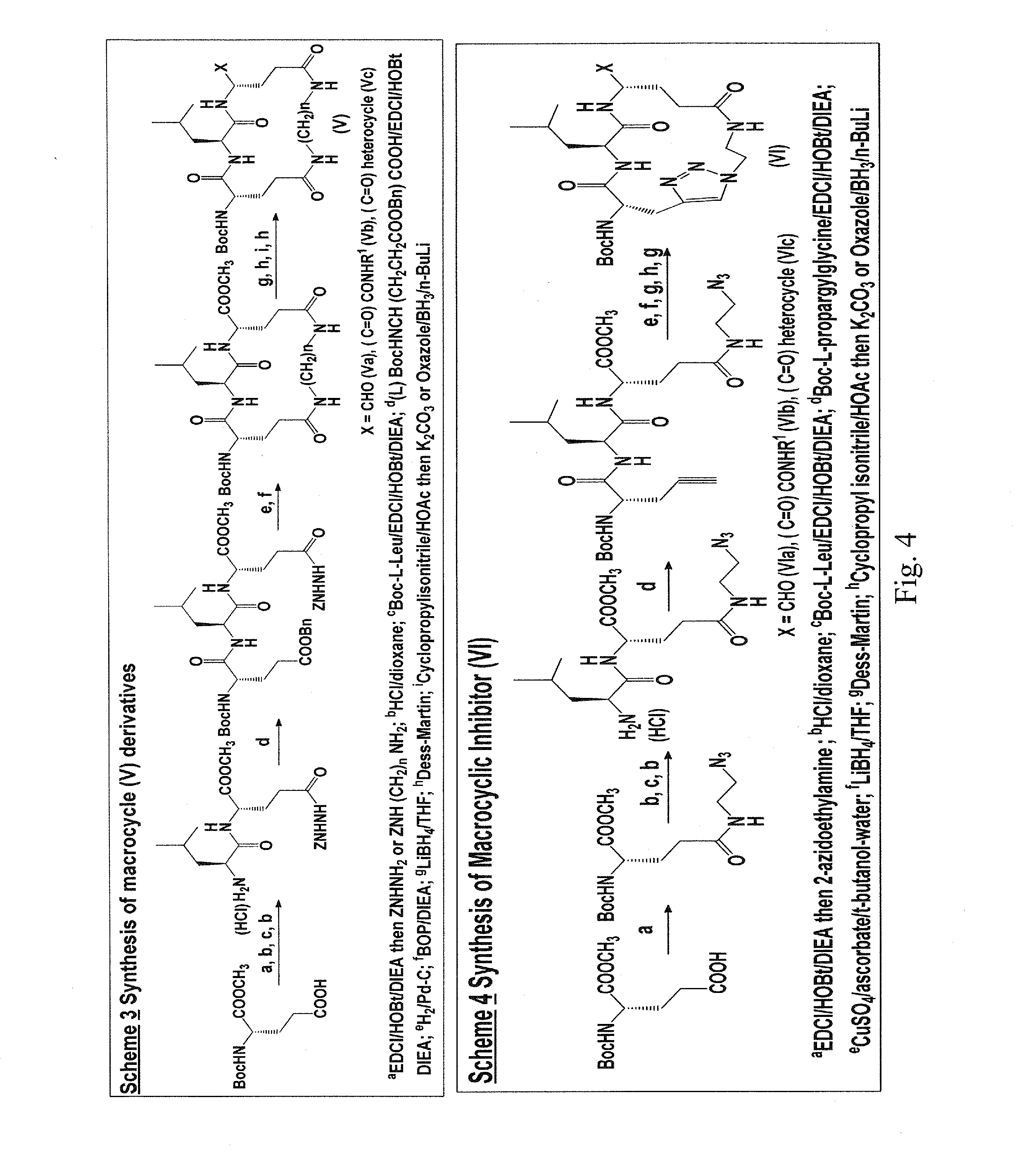
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:THE WICHITA STATE UNIV +1
Artificial cpg single-stranded oligodeoxynucleotide and antiviral use thereof
The present invention provides a series of artificial CpG-containing single-stranded oligodeoxynucleotides (ODNs), each of which is consisted of single-stranded oligodeoxynucleotide DNA molecule containing one or more CpG(s), wherein said ODNs can stimulate human peripheral blood mononuclear cell (PBMC) to produce antiviral substances. These ODNs can protect the cells against the attack from virus, wherein said virus is preferably selected from the group consisted of influenza virus and single-stranded positive strand RNA virus such as SARS virus, hepatitis C virus, dengue virus and Japanese encephalitis virus. Moreover, the antiviral use of artificial CpG ODNs and its use for treating and preventing viral infection are also provided.
Owner:CHANGCHUN HUAPU BIOTECHNOLOGY CO LTD
Method of simultaneously extracting animal DNA (Deoxyribonucleic Acid) virus and RNA (Ribonucleic Acid) virus nucleic acid in blood serum and double swabs
The invention discloses a method of simultaneously extracting animal DNA (Deoxyribonucleic Acid) virus and RNA (Ribonucleic Acid) virus nucleic acid in blood serum and double swabs. The method comprises the following steps of: carrying out lysis on a to-be-extracted substance by using guanidinium isothiocyanate lysate; adsorbing RNA by a silica gel membrane; removing impure protein by washing liquor I; removing impurities by washing liquor II; carrying out DEPC (Diethylpyrocarbonate) water-washing to remove nucleic acid, wherein the guanidinium isothiocyanate lysate comprises 3M-7M guanidinium isothiocyanate, 0.6%-1.0% TriTon-100, 30mM-50mM Tris-Cl, 5mM-15mM DTT (DL-Dithiothreitol), 60 mu g / mL-90 mu g / mL protease K, 10mM-30mM EDTA (Ethylene Diamine Tetraacetic Acid), and PH of the guanidinium isothiocyanate lysate is 4.3-4.6; the washing liquor I comprises 5M-6M guanidine hydrochloride, 53%-59% absolute ethyl alcohol, 70-90 mu g / mL protease K, and PH of the washing liquor I is 6.4-6.6; the washing liquor II comprises 70%-80% alcohol. The method disclosed by the invention has the advantages of being simple in extracting process, short in period, low in cost, and capable of simultaneously extracting RNA virus and DNA virus nucleic acid in an animal blood serum sample and a double-swab sample.
Owner:安徽华卫集团禽业有限公司
Coliphage MS2 standard sample and preparing method thereof
InactiveCN104774974AImprove uniformityImprove stabilityMicrobiological testing/measurementViruses/bacteriophagesEscherichia coliFood borne
The invention discloses a coliphage MS2 standard sample. The concentration of the coliphage MS2 standard sample is 1.57*1011 pfu / mL, and the standard sample can be used as a quality control substance for the detection process when food-borne RNA viruses are detected. According to a preparing method of the coliphage MS2 standard sample, evenness and stability are tested, and the guarantee period is 12 months.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
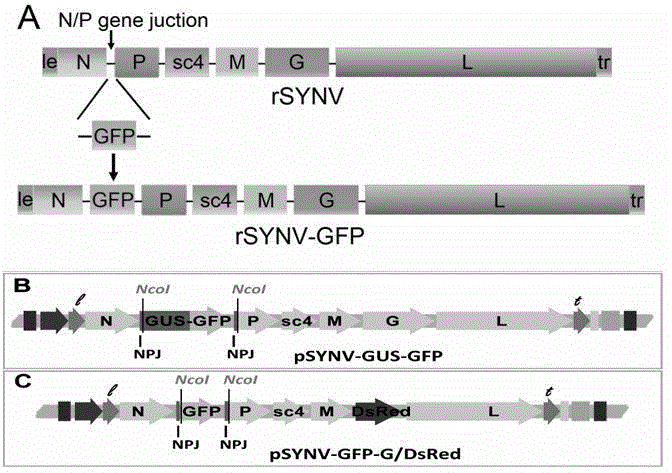
Recombinant plant rhabdovirus vector and construction method thereof
ActiveCN104962580AEasy to operateGood repeatabilityFermentationVector-based foreign material introductionHeterologousPlant rhabdovirus
The invention discloses a recombinant plant rhabdovirus vector and a construction method thereof. The recombinant plant rhabdovirus vector comprises a modified plant rhabdovirus genome, a transcription unit which is induced newly and a heteroduplex nucleotide sequence, wherein the transcription unit is inserted into the plant rhabdovirus genome to express the heteroduplex nucleotide sequence, the heteroduplex nucleotide sequence is replaced into a glycoprotein transcription unit of a recombinant plant rhabdovirus; the recombinant plant rhabdovirus has copying and infecting capacity, and one antigenic polypeptide or other protein is coded by the heteroduplex nucleotide sequence. The virus expression vector is the first one which can express a negative-sense RNA virus vector of foreign protein on plants, and the recombinant plant rhabdovirus vector has the advantages that the expression quantity is high, the expression stability is good, a relative long foreign gene segment can be inserted, and inserted foreign gene segment is not prone to loosing; the recombinant plant rhabdovirus vector can be used for expressing of multiple kinds of foreign protein and can also be used for preparing animal vaccines, and wide application values and application prospects are achieved.
Owner:ZHEJIANG UNIV
Two-Component Rna Virus-Derived Plant Expression System
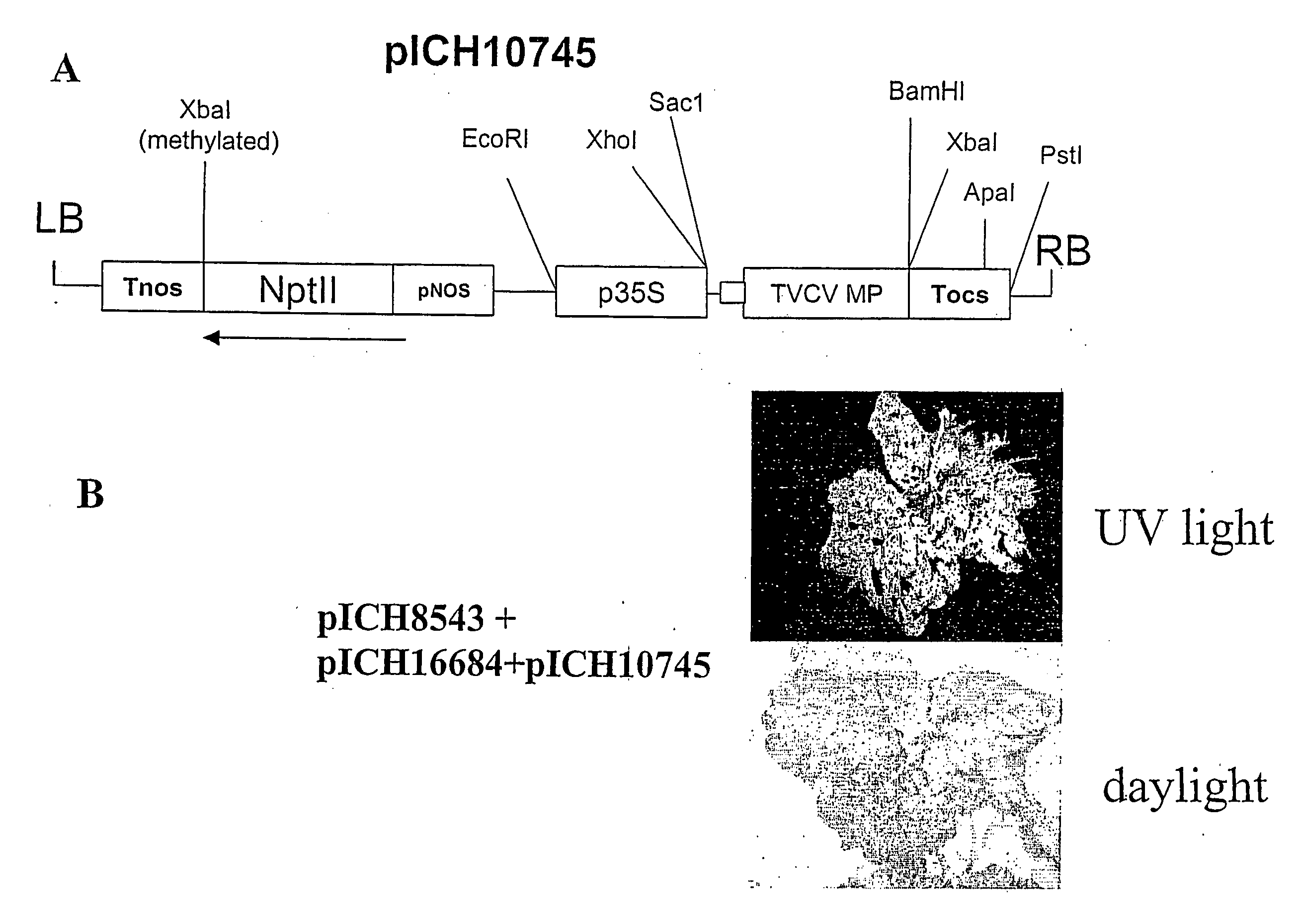
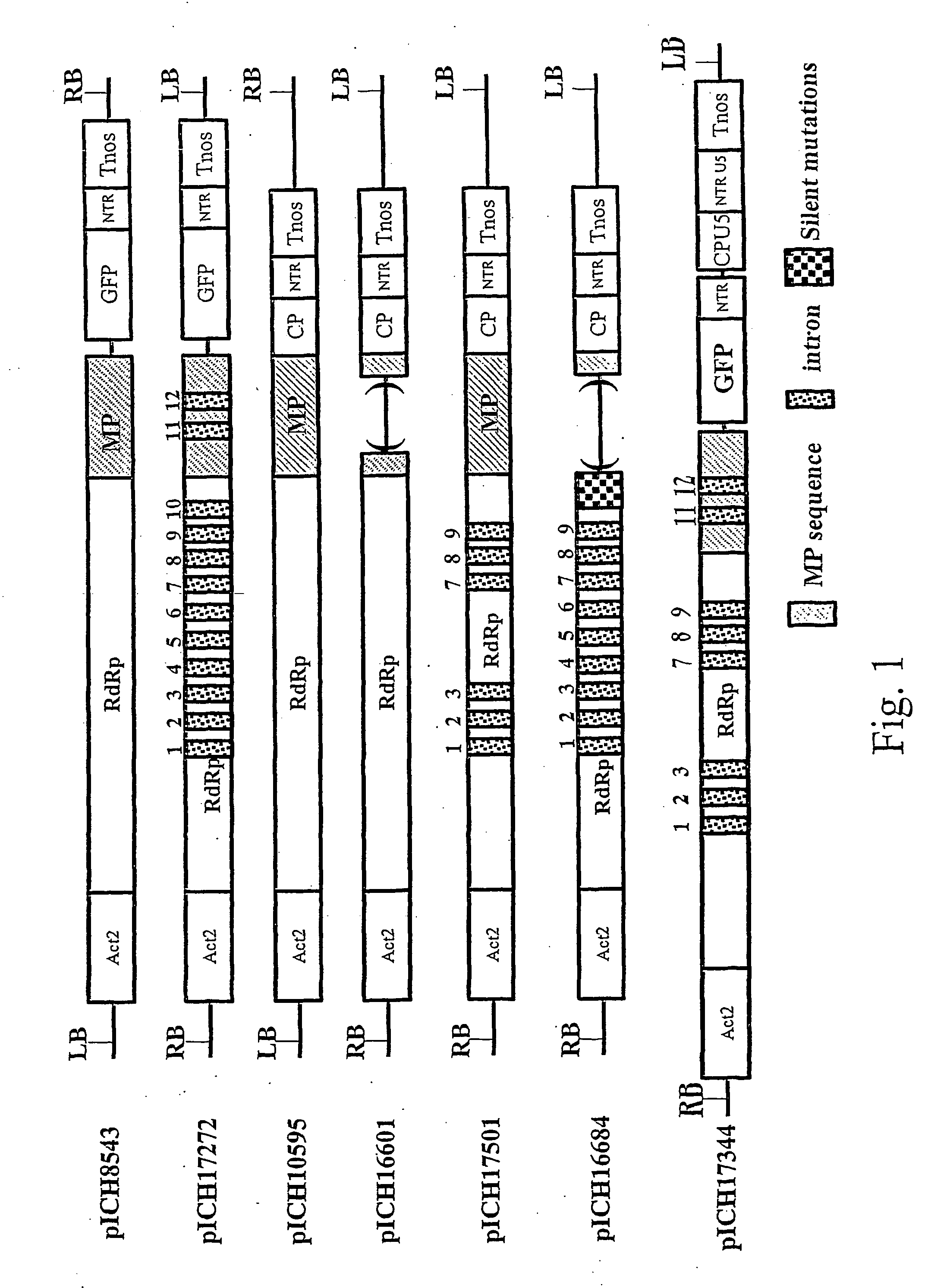
ActiveUS20070300330A1Improve efficiencyProvide efficiencySugar derivativesOther foreign material introduction processesSingle-Stranded RNAWhole body
A process for replicating or for replicating and expressing a sequence of interest in a plant, comprising: (i) an RNA replicon or a precursor thereof, said RNA replicon being derived from a plus-sense single stranded RNA virus and comprising at least one sequence of interest; and (ii) a helper replicon, or a precursor thereof, wherein said helper replicon is (a) incapable of systemic movement in said plant both in the presence and in the absence of said RNA replicon (i) and (b) capable of expressing in a plant one or more proteins necessary for systemic movement of said RNA replicon (i), whereby said RNA replicon (i) is capable of replicating or replicating and expressing said sequence of interest in said plant, but unable to move systemically in said plant in the absence of said one or more proteins expressed by said helper replicon (ii).
Owner:ICON GENETICS
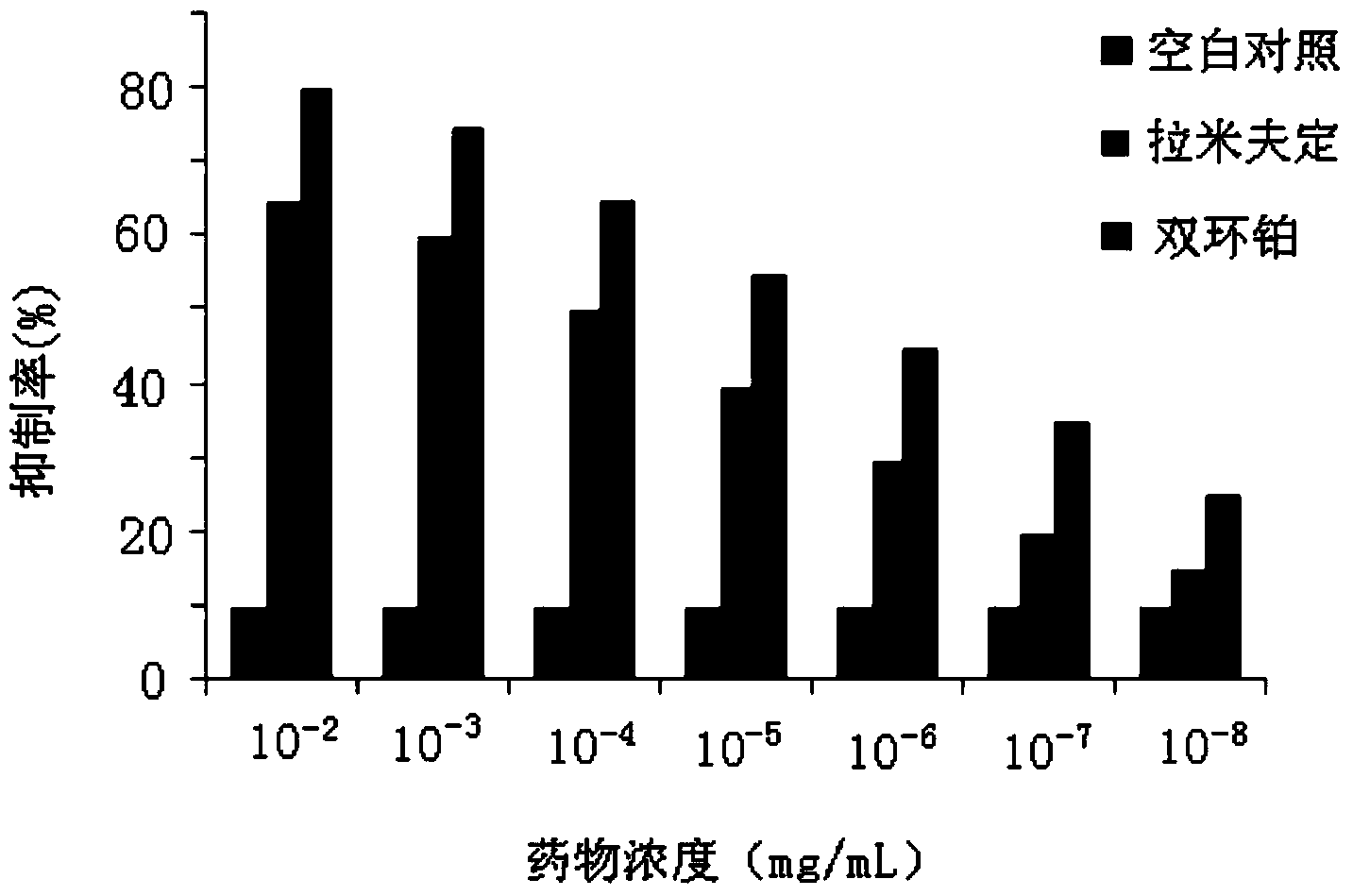
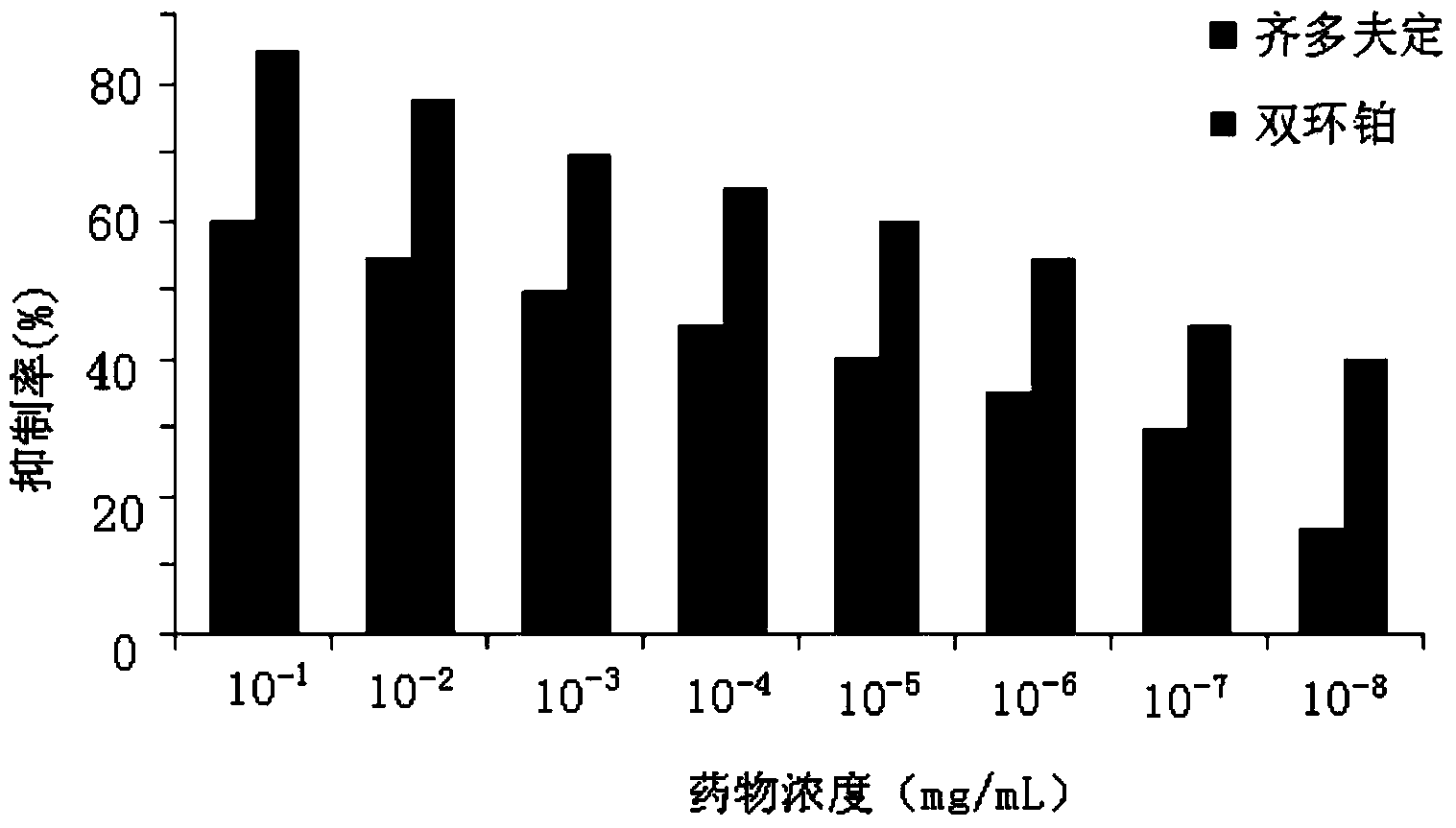
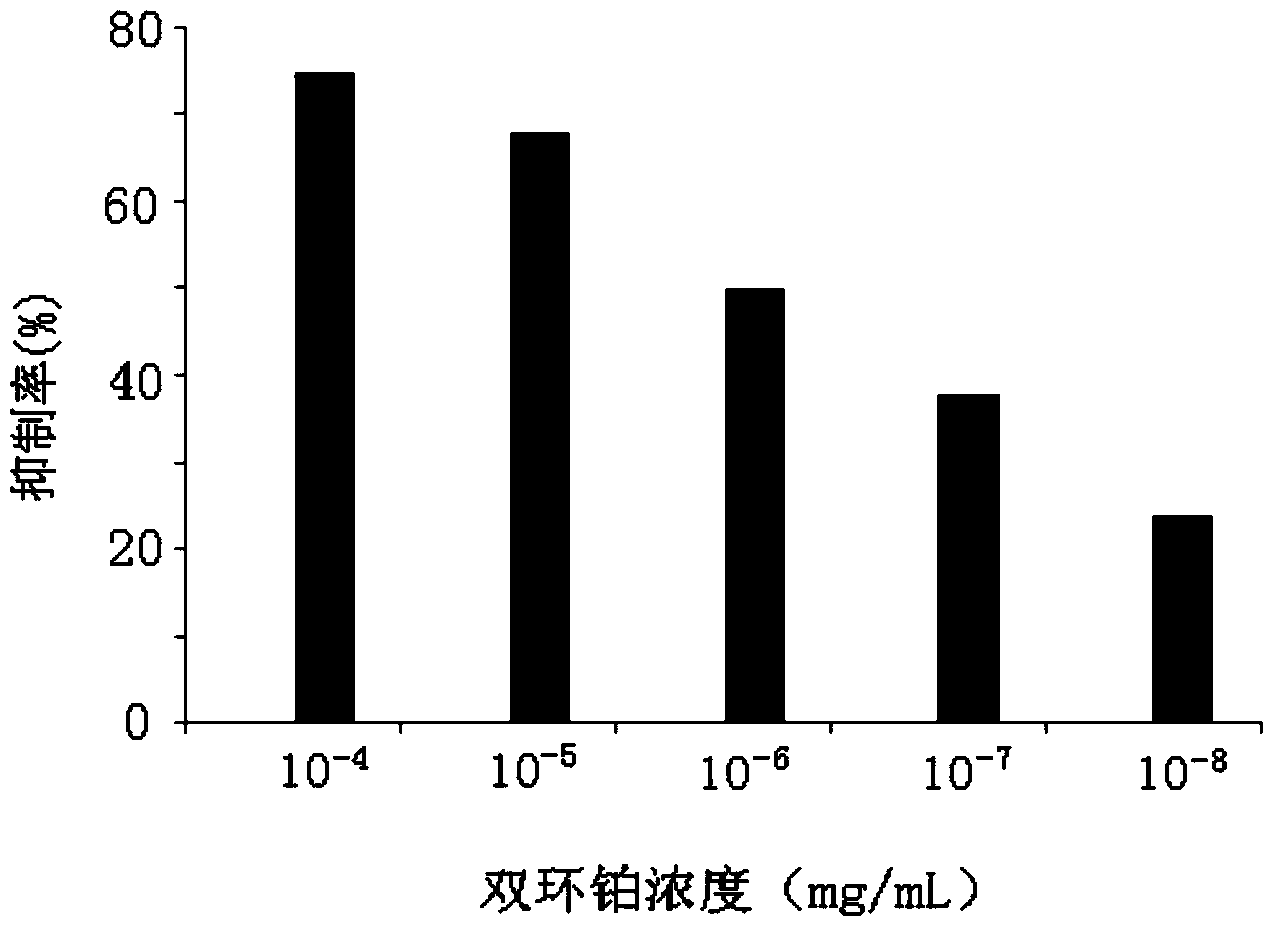
Application of dicycloplatin in preparation of antiviral drug and antibacterial drug
ActiveCN104127402AImprove antibacterial propertiesGood antiviral effectAntibacterial agentsAntiviralsBacteroidesAdjuvant
The invention provides application of dicycloplatin in preparation of an antiviral drug and / or antibacterial drug and application of the dicycloplatin in preparation of an antiviral adjuvant drug and / or antibacterial adjuvant drug. Viruses related in the invention are RNA viruses such as hepatitis B virus, hepatitis C virus, human immunodeficiency virus and influenza virus, and the antibacterial drug is resistant to mycobacterium tuberculosis and / or nontuberculosis mycobacteria, Research results prove that dicycloplatin has the effect of resisting multiple bacteria and viruses, especially has obvious inhibitory effect on drug-resistance bacteria, no obvious cytotoxicity and high medication safety.
Owner:北京默加农生物技术发展有限公司
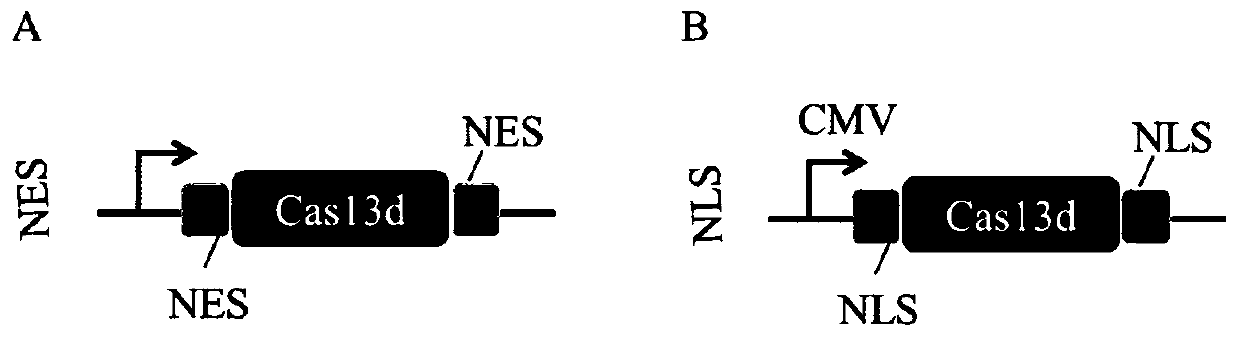
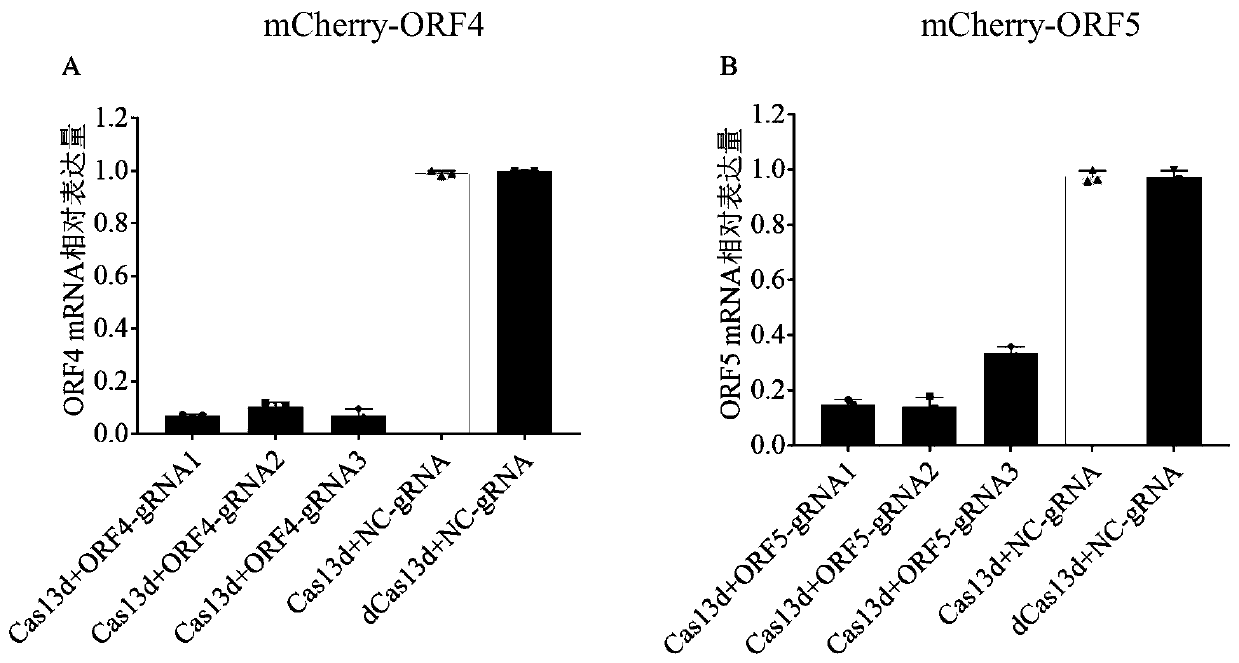
Screening method of sgRNA (small guide ribonucleic acid) efficient action target based on CRISPR-Cas13d (clustered red regularly interspaced short palindromic repeat) system and application
ActiveCN110656123AUniversalImprove efficiencyVector-based foreign material introductionDNA/RNA fragmentationVirusBioinformatics
The invention provides a screening method of a sgRNA (small guide ribonucleic acid) efficient action target based on a CRISPR-Cas13d (clustered red regularly interspaced short palindromic repeat) system and application, and particularly application in RNA virus knockdown. According to the screening method, two expression read frame sequences ORF4 and ORF5 of a PRRSV are respectively fused with a carbon end of a mCherry gene through a mCherry fluorescence report gene, a fluorescence report system for screening sgRNA efficient action targets of the CRISPR-Cas13d system is established, and by virtue of the property that mRNA is efficiently cut by CRISPR-Cas13d, rapid screening of sgRNAs with high efficiency is implemented. Additionally, PRRSV-GFP recombinant viruses are efficiently degraded by using the screened efficient targetedly combined sgRNA based on the CRISPR-Cas13d system. The RNA virus knockdown method provided by the invention has the advantages of being high in efficiency, high in precision and low in target missing rate.
Owner:CHINA AGRI UNIV
Materials and methods for detection of enterovirus and norovirus
The invention provides polynucleotides and methods for detecting and quantifying RNA viruses, such as enteroviruses and noroviruses. In one aspect, the invention provides amplification primers and labeled molecular beacons for amplification of viral nucleic acid sequences. In another aspect, the invention provides a synthetic RNA internal control. In another aspect, the invention provides a kit for detecting the presence of enterovirus and / or norovirus in a sample.
Owner:UNIV OF SOUTH FLORIDA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com