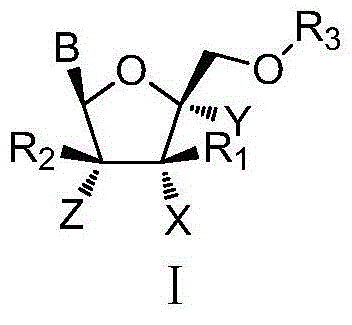
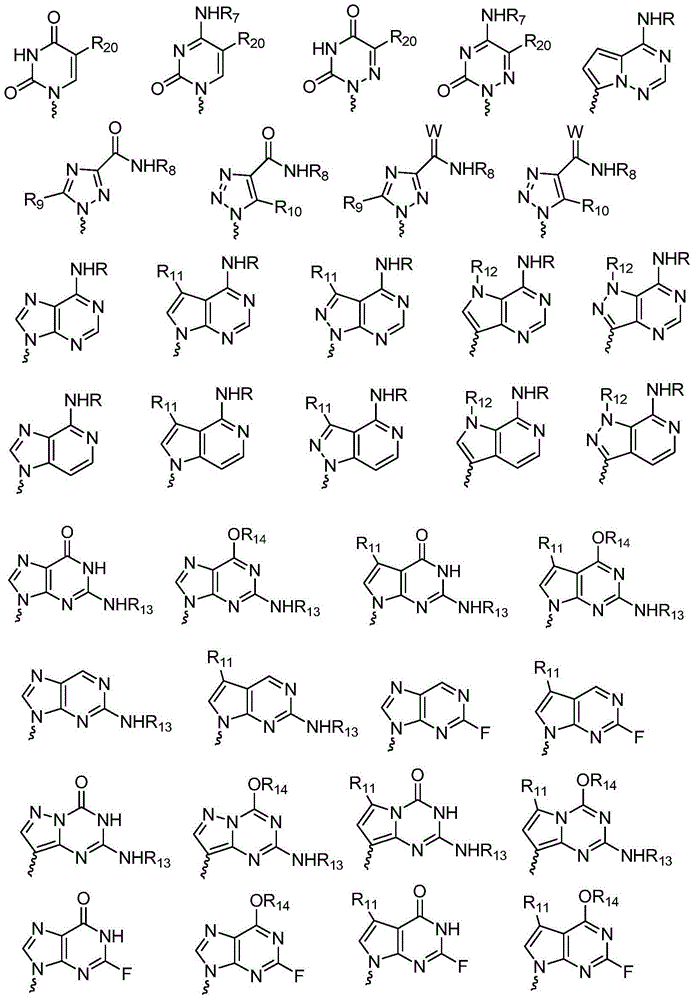
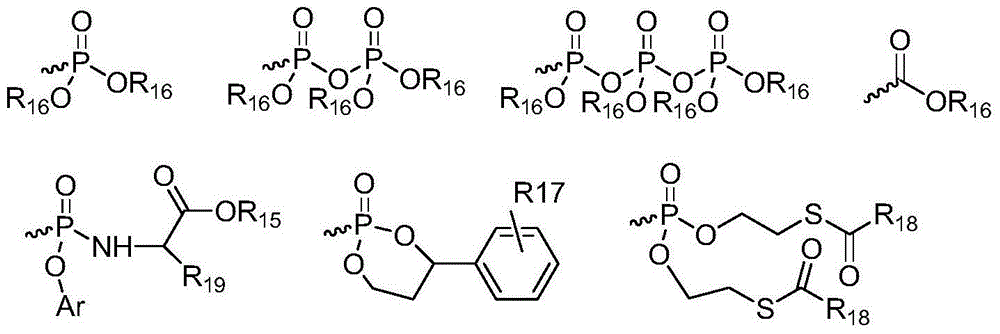
L-nucleoside compounds and application thereof
A technology of nucleosides and compounds, applied in the field of medicinal chemistry, can solve problems such as difficulty in controlling the epidemic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Preparation of L-3-deoxynucleoside compounds.
[0073] Prepare according to the following reaction scheme:
[0074]
[0075] (1) Preparation of 1,2-O-isopropylidene-α-L-xylofuranose (compound 1)
[0076] Take a 500mL three-necked flask, add L-xylose (20g, 133mmol) and acetone (300mL), cool down to 0°C, add 20mL of concentrated sulfuric acid dropwise under stirring, stir at 0°C for 6h, the reaction solution is clear, cool down to -10°C , add 1M (i.e. 1mol / L) NaOH solution to adjust the pH to neutral, evaporate acetone, add saturated brine, EA (ethyl acetate) for extraction, dry, and spin dry to obtain a colorless oily liquid, which is a monoacetonide protected Mixture of xylose (compound 1) and diacetonylidene-protected xylose. The mixture was dissolved in 100 mL of methanol, 50 mL of water and 40 g of 732-type cation exchange resin were added, stirred at room temperature for one day, filtered, and the filtrate was spin-dried to obtain 24.77 g of a light yellow oily...
Embodiment 2
[0101] Preparation of L-2-methyl-2-fluoronucleoside compounds.
[0102] Prepare according to the following reaction scheme:
[0103]
[0104] (1) (2E)-3-[(4R)-2,2-Dimethyl-1,3-dioxolan-4-yl]-2-methyl-2-propenoic acid ethyl ester (compound 10) preparation of
[0105] Ph 3 PC(Me)CO 2 Et (152g, 419.85mmol, 2.2 equivalents) was dissolved in 500mL redistilled DCM, cooled to -40°C, and compound 9 ((S)-(-)-2,2-dimethyl- 1,3-dioxolane-4-carbaldehyde) in DCM (50 g, 190.84 mmol, 100 mL), raised to room temperature, and stirred for 17 h. The reaction solution was spin-dried, added EA, stirred for 1 h, and filtered. The filtrate was spin-dried again, added tert-butyl methyl ether, stirred overnight, filtered, and spin-dried to obtain 81.86 g of an oily liquid. A small amount was passed through the column (PE:EA=10:1) to determine the structure and content, and the calculated yield was 90.07%.
[0106] Characterization data of compound 10: 1 HNMR (400MHz, Chloroform-d): δ6.69(d, ...
Embodiment 3
[0136] Preparation of 3-beta-methyluracil compounds.
[0137] Prepare according to the following reaction scheme:
[0138]
[0139] (1) Preparation of compound 23
[0140] Suspend L-ribose (10 g) in 100 mL of anhydrous methanol, add 0.8 mL of acetyl chloride, heat to reflux for 2 h, and spin dry to obtain crude compound 21.
[0141] The crude compound 21 was dissolved in 100 mL of dry pyridine, a catalytic amount of DMAP was added, the temperature was lowered to 0°C, and benzoyl chloride (24 mL, 206.7 mmol, 3.1 equivalents) was slowly added dropwise. After the dropwise addition, it was raised to room temperature and reacted overnight. Spin the reaction solution to dryness, add DCM, saturated NaHCO 3 solution, stirred for 10 min and then separated, the aqueous phase was extracted with DCM, the combined organic phase was washed with 1N dilute hydrochloric acid, saturated NaHCO 3 solution, washed with saturated brine, dried, and spin-dried to obtain the crude compound 22. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com