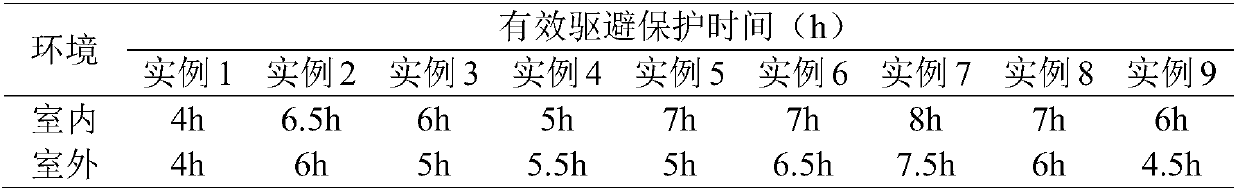
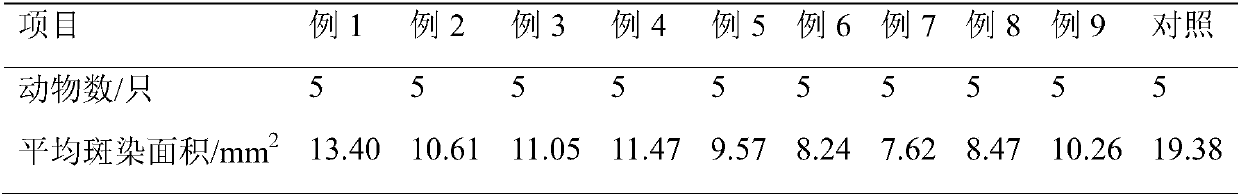
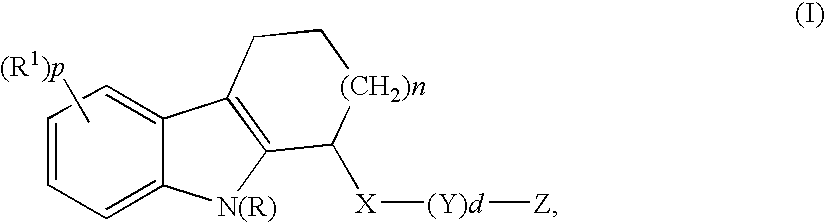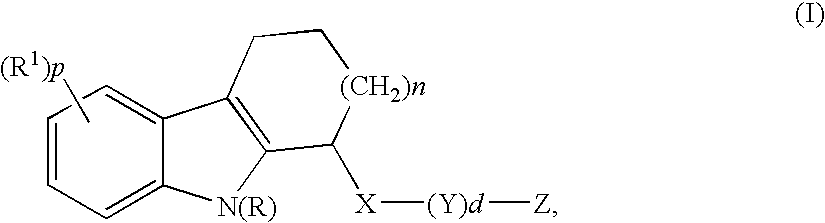Patents
Literature
125 results about "Dengue fever" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A mosquito borne viral disease.
Mosquito-repelling bactericide
InactiveCN104585255AInhibition of abnormal fermentationPromote peristalsisBiocideAntisepticsDiseaseAdditive ingredient
The invention relates to a mosquito-repelling bactericide. The raw material formula comprises main ingredients, wherein the main ingredients comprise the following components: clove, folium artemisiae argyi, honeysuckle, elsholtzia, radix angelicae, purple perilla, calamus, mint and agastache rugosus. The mosquito-repelling bactericide disclosed by the invention has the beneficial effects that according to the common sense and principle that mosquitoes bite to suck blood and spread diseases, the bactericide has the main effect of repelling mosquitoes, and harmful pathogenic bacteria and bacteria in air can be killed by virtue of the flavor volatilized by the traditional Chinese medicines, so that the family members are prevented from being troubled by the mosquitoes and are even prevented from suffering from various diseases due to mosquito bites, and people are kept away from diseases such as malaria, filariasis, epidemic encephalitis B and dengue fever.
Owner:刘向上
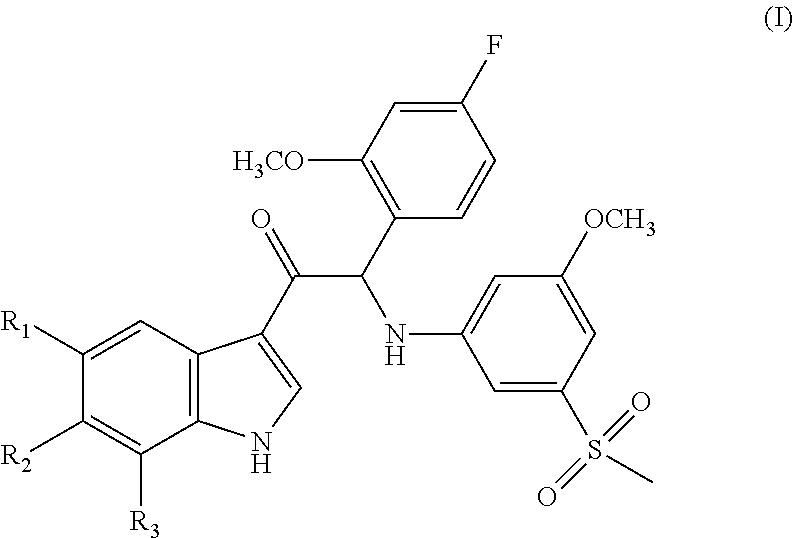
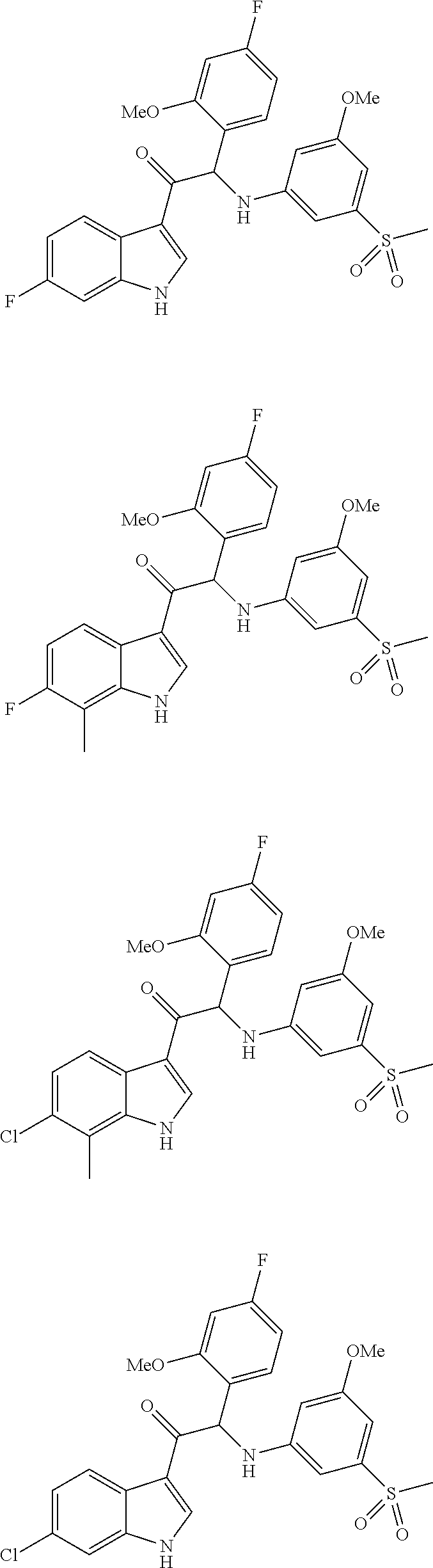
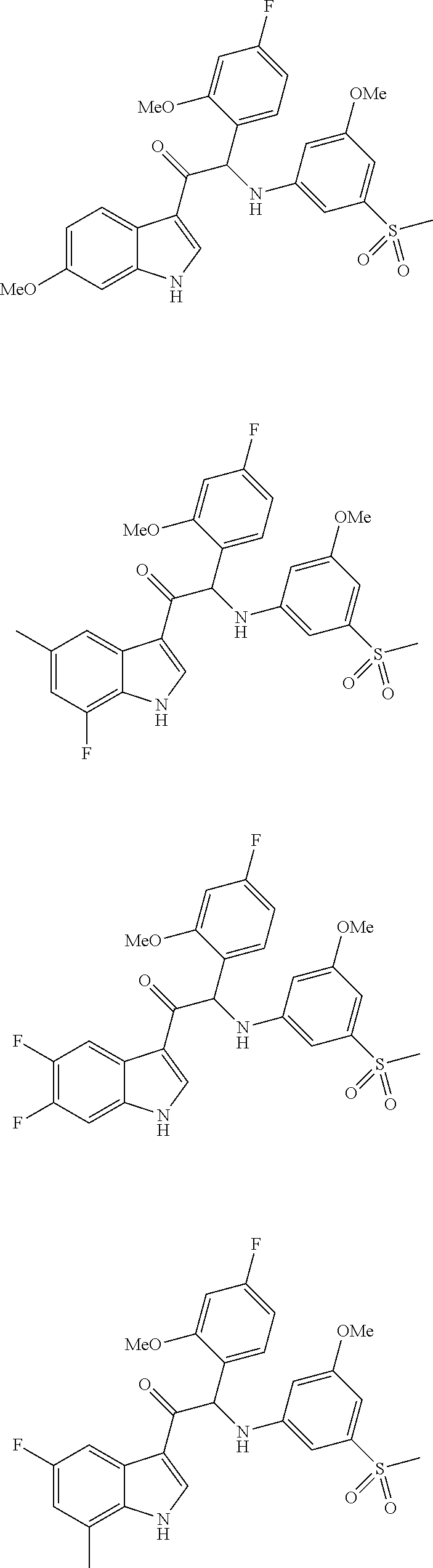
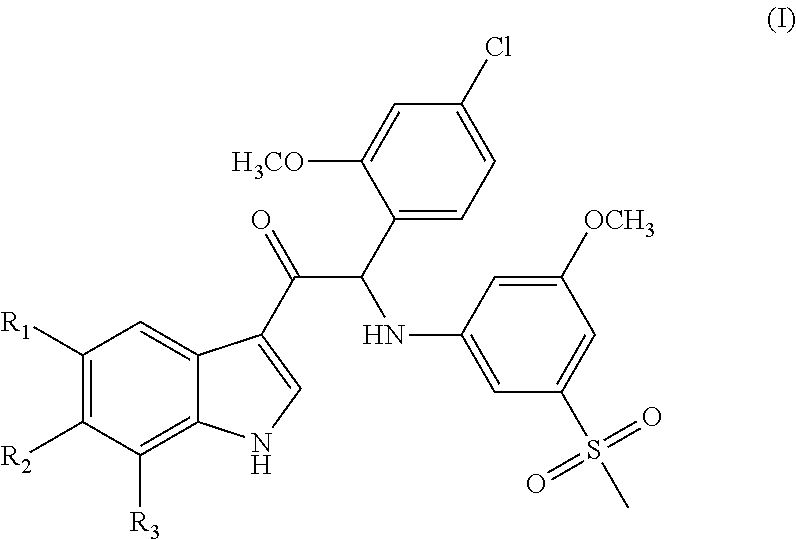
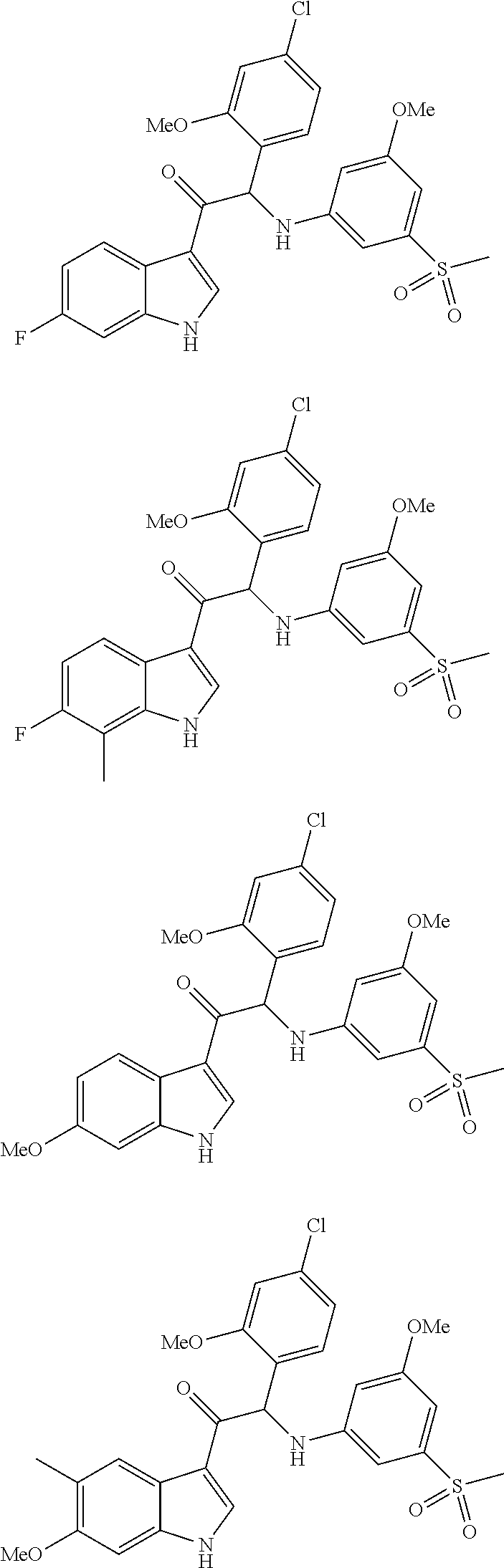
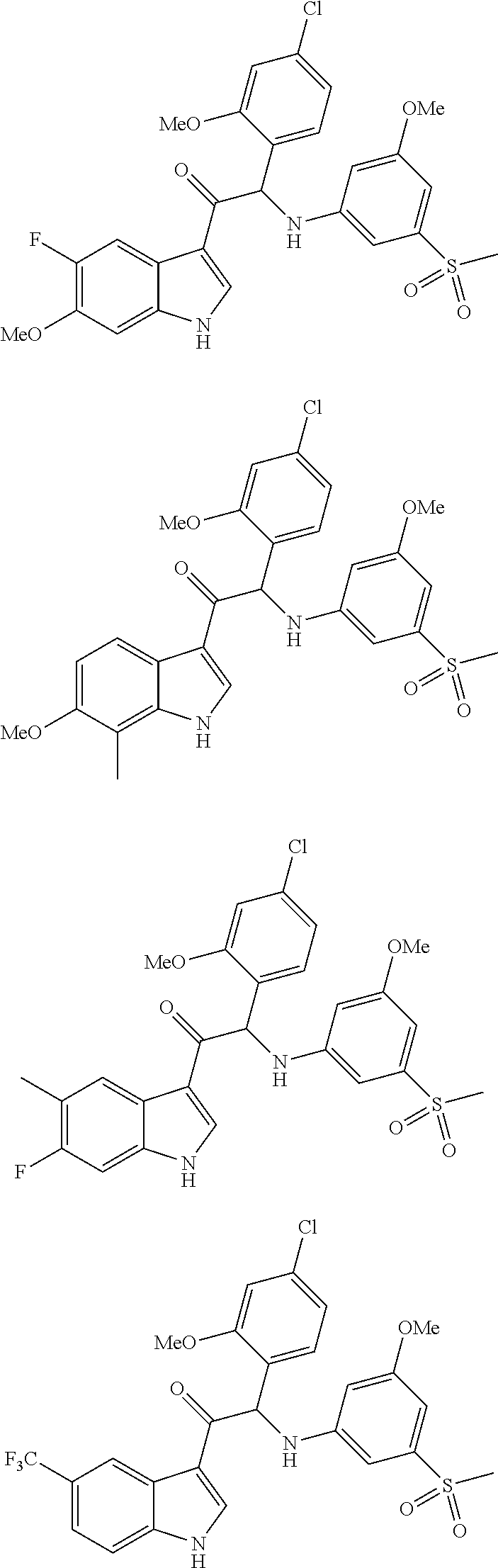
Mono- or di-substituted indole derivatives as dengue viral replication inhibitors
ActiveUS10029984B2Strong antiviral activityPromote efficient proliferationBiocideOrganic chemistry methodsMedicineViral infection
The present invention relates to mono- or di-substituted indole compounds, methods to prevent or treat dengue viral infections by using said compounds and also relates to said compounds for use as a medicine, more preferably for use as a medicine to treat or prevent dengue viral infections. The present invention furthermore relates to pharmaceutical compositions or combination preparations of the compounds, to the compositions or preparations for use as a medicine, more preferably for the prevention or treatment of dengue viral infections. The invention also relates to processes for preparation of the compounds.
Owner:JANSSEN PHARMA INC +1
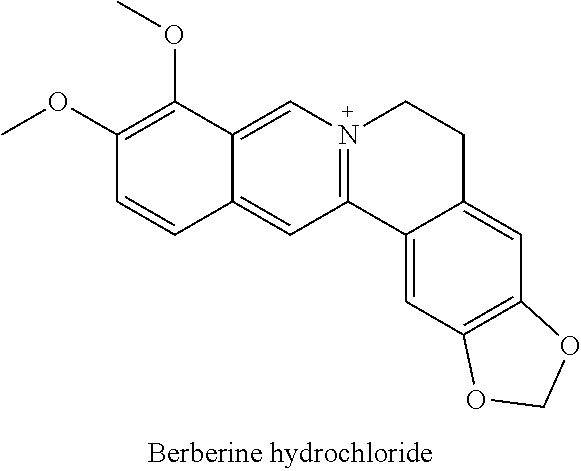
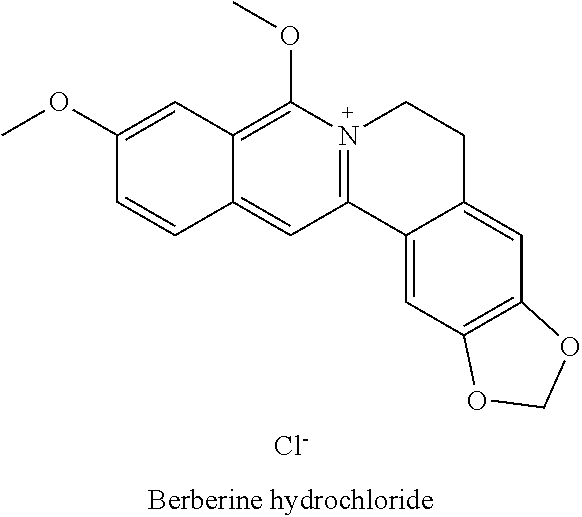
COMBINATIONS OF BERBERINE, ARTEMISININ, Loperamide AND THEIR DERIVATIVES TO TREAT MALARIA, DIARRHEA, TRAVELERS' DIARRHEA, DYSENTERY, DENGUE FEVER, PARASITES, CHOLERA AND VIRUSES
This invention relates to compositions and methods of combining berberine, artemisinin and loperamide or their derivatives in a therapeutic product for mammals suffering from malaria, diarrhea, travelers' diarrhea, dysentery, dengue fever, parasites, cholera and viruses by administration of a therapeutically effective amount of the composition.
Owner:SEUBERT KIRK MR +1
Mono- or Di-Substituted Indole Derivatives As Dengue Viral Replication Inhibitors
ActiveUS20180346419A1Ease of administrationImprove uniformityOrganic active ingredientsOrganic chemistryMedicineViral replication
The present invention relates to mono- or di-substituted indole compounds, methods to prevent or treat dengue viral infections by using said compounds and also relates to said compounds for use as a medicine, more preferably for use as a medicine to treat or prevent dengue viral infections. The present invention furthermore relates to pharmaceutical compositions or combination preparations of the compounds, to the compositions or preparations for use as a medicine, more preferably for the prevention or treatment of dengue viral infections. The invention also relates to processes for preparation of the compounds.
Owner:KATHOLIEKE UNIV LEUVEN +2
Antiviral activity from medicinal mushrooms
Compounds having unique antiviral properties are prepared from medicinal mushroom mycelium, extracts and derivatives. The compositions are derived from Fomitopsis, Piptoporus, Ganoderma and blends of medicinal mushroom species and are useful in preventing and treating viruses including Orthopox viruses, influenza, avian influenza, Venezuelan Equine Encephalitis, yellow fever, West Nile, Dengue, New World and Old World arenaviruses, hantavirus, Rift Valley fever, sandfly fever, hantavirus, SARS, Rhinovirus and other viruses.
Owner:TURTLE BEAR HLDG LLC
COMBINATIONS OF BERBERINE, ARTEMISININ, Loperamide AND THEIR DERIVATIVES TO TREAT MALARIA, DIARRHEA, TRAVELERS' DIARRHEA, DYSENTERY, DENGUE FEVER, PARASITES, CHOLERA AND VIRUSES
This invention relates to compositions and methods of combining berberine, artemisinin and loperamide or their derivatives in a therapeutic product for mammals suffering from malaria, diarrhea, travelers' diarrhea, dysentery, dengue fever, parasites, cholera and viruses by administration of a therapeutically effective amount of the composition.
Owner:SEUBERT KIRK MR +1
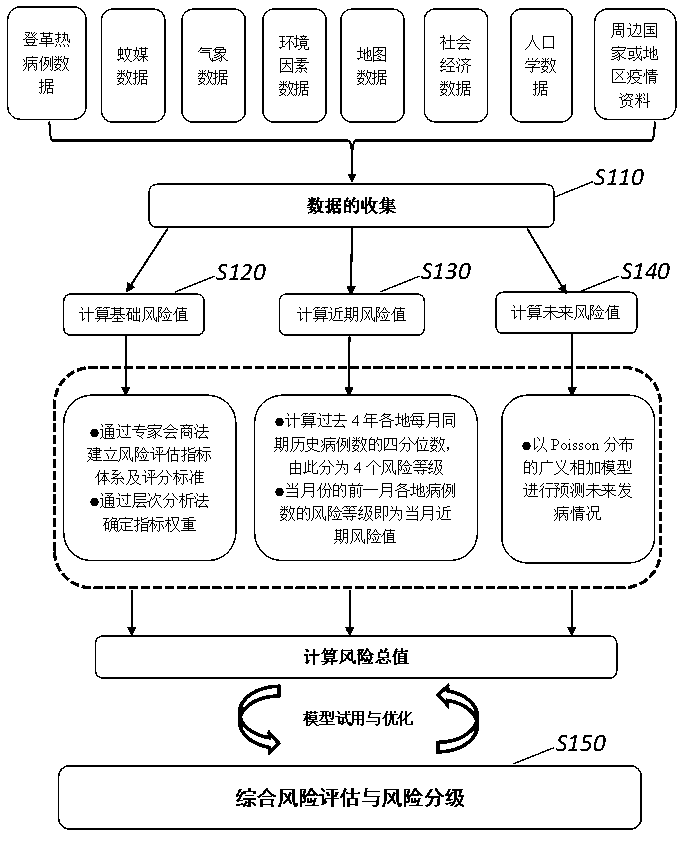
Comprehensive evaluation method for dengue risk
ActiveCN110459329AAccurate assessmentImprove accuracyHealth-index calculationEpidemiological alert systemsTotal riskWeight coefficient
The invention provides a comprehensive evaluation method for a dengue risk. The method comprises the steps that dengue case data, mosquito-borne data, meteorological data, map data, environment factordata, social economy data, demography data and surrounding country or area epidemic situation data are collected; a basic risk value, a nearby risk value and a future prediction risk value are calculated, and weight coefficients of the three risk values are calculated; the comprehensive evaluation method is established, and a total dengue risk value is calculated; with reference to meteorologicaldisaster level early warning, the total risk value is evaluated for the dengue risk, and grading is conducted; based on a generalized addition model, a smooth function can be used for conducting non-linear processing on variables; the variables such as meteorological factors, dengue case numbers and mosquito-borne factors are comprehensively considered and comprise more information related to attack; in combination with the three risk values, a dengue risk evaluation model is established, the dengue spreading risk is identified at multiple angles, the influence of multiple factors on dengue spreading is comprehensively considered, and the accuracy of dengue risk evaluation is effectively improved.
Owner:广东省公共卫生研究院
Method of Immunization Against the 4 Serotypes of Dengue Fever
The invention relates to a method for inducing protection against the 4 serotypes of dengue fever in a patient, comprising:(a) the administration of a monovalent vaccine comprising a vaccinal virus of a first serotype of dengue fever, and(b) the administration of a tetravalent vaccine comprising vaccinal viruses of the four serotypes of dengue fever,in which administration (b) is made between at least 30 days and not more than 12 months following the first administration (a).
Owner:SANOFI PASTEUR SA
Heavy chain single-domain antibody of type-II dengue fever virus NS1 protein as well as preparation method and application of heavy chain single-domain antibody
ActiveCN103396481AImprove detection accuracyImmunoglobulins against virusesVector-based foreign material introductionEscherichia coli3-deoxyribose
The invention belongs to the technical field of biology and relates to a heavy chain single-domain antibody of a type-II dengue fever virus NS1 protein as well as a preparation method and application of the heavy chain single-domain antibody. The preparation method comprises the following steps of: biologically screening the recombination expressed dengue fever virus NS1 protein for many times by using an alpacas non-immunity heavy chain single-domain antibody library; picking by using an ELISA (Enzyme Linked Immunosorbent Assay) to obtain a specific bonded phage monoclone, and determining a DNA (Deoxyribose Nucleic Acid) sequence; and then, converting the DNA sequence into escherichia coli to express to obtain the heavy chain single-domain antibody. The heavy chain single-domain antibody obtained through screening can be efficiently expressed in the escherichia coli, can be used for preparing a colloid gold detection kit for detecting type-II dengue fever virus infection, and is higher in detection accuracy.
Owner:SOUTH CHINA UNIV OF TECH
Chinese material medicine preparation for treating dengue fever and preparation method thereof
InactiveCN104940793AAdvanced dosage formEasy to takeAntiviralsUnknown materialsToxic materialGLYCYRRHIZA EXTRACT
The invention belongs to the technical field of medicines and particularly relates to a Chinese material medicine preparation for treating dengue fever and a preparation method thereof. The Chinese material medicine preparation is prepared by bezoar, honeysuckle, coptis chinensis, folium isatidis, lalang grass rhizome, semen coicis, fructus amomi, the roots of Chinese thorowax, radix scrophulariae and liquorice. The Chinese material medicine preparation is reasonable in formula and easy to prepare, has remarkable treating effects of clearing away heat and toxic materials, invigorating spleen to eliminate dampness and eliminating phlegm and fever and is an effective medicine especially for eliminating dampness, fever and repression and treating dengue.
Owner:QINGDAO YUNTIAN BIOTECH
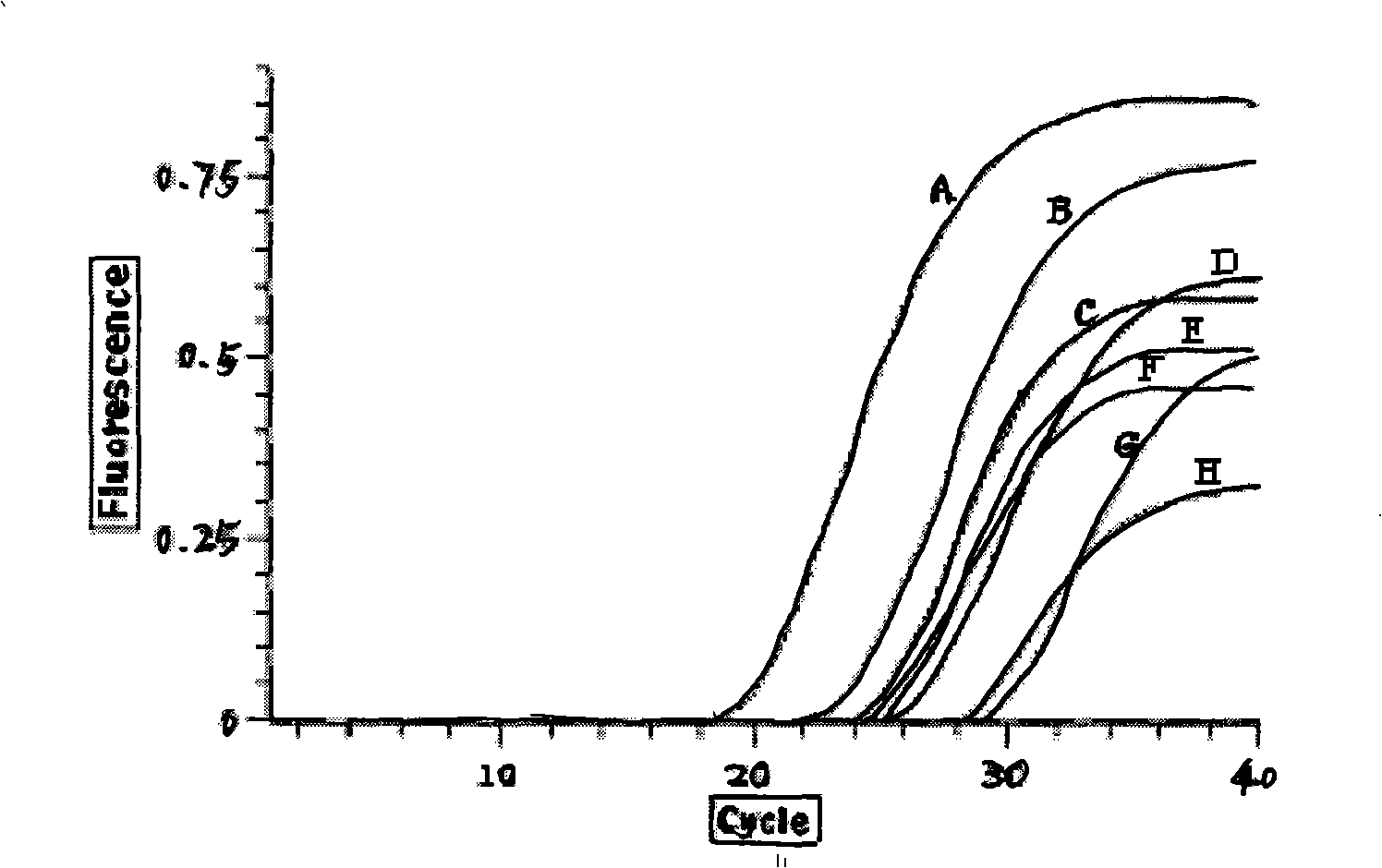
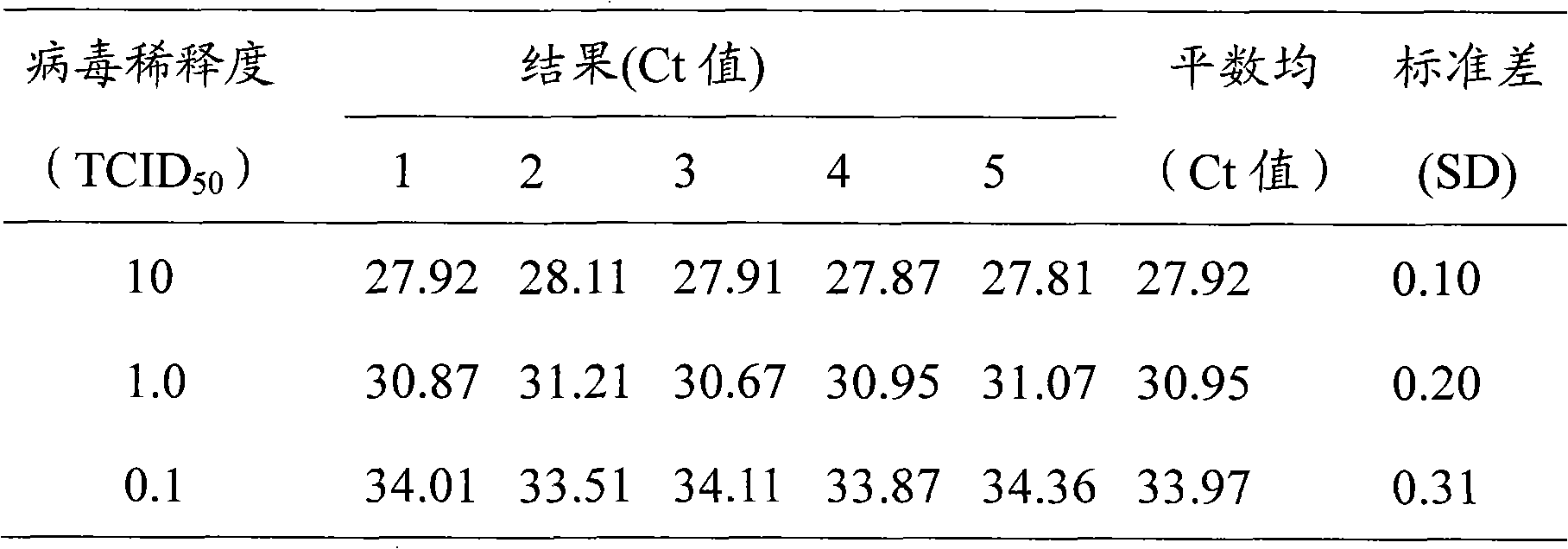
Fluorescence quantitative polymerase chain reaction (PCR) new method for detecting multiple viruses of yellow fever, dengue fever and epidemicencephalitis B and multiple virus detection PCR system
ActiveCN102191338AMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceYellow fever
The invention discloses a fluorescence quantitative polymerase chain reaction (PCR) new method for detecting multiple viruses of yellow fever, dengue fever and epidemicencephalitis B and multiple virus detection PCR system consisting of primers, probes, a Premix Ex Taq reaction solution and a sterilized Tris buffer. With good singularity and high sensitivity, three pairs of primers and probes are very suitable for simultaneously detecting viruses of yellow fever, dengue fever and epidemicencephalitis B. And there is no cross reaction between the primers and probes and several other entomophily hemorrhagic fever viruses, such as Marburg virus and Rift Valley fever virus.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Flavivirus vaccine delivery system
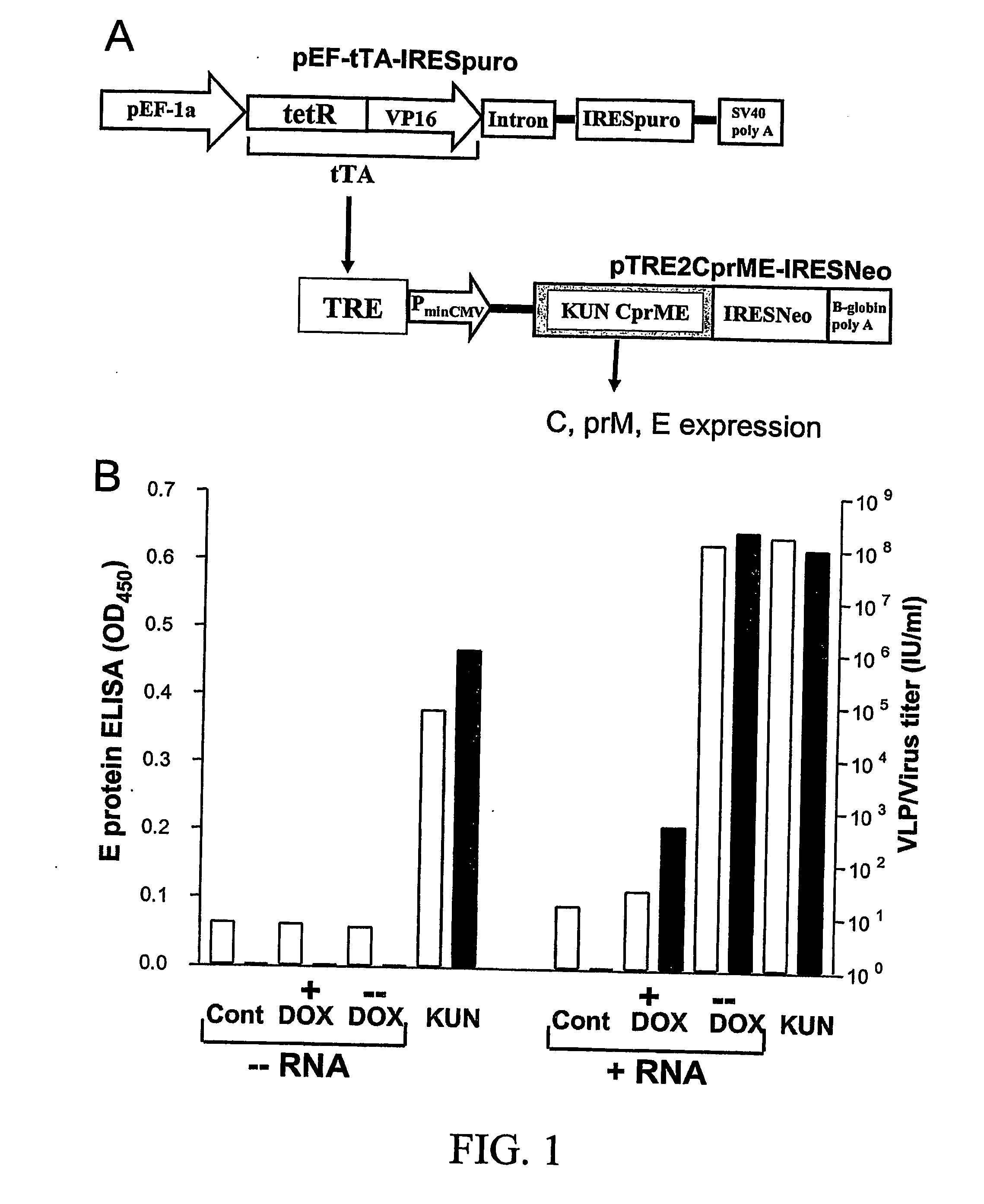
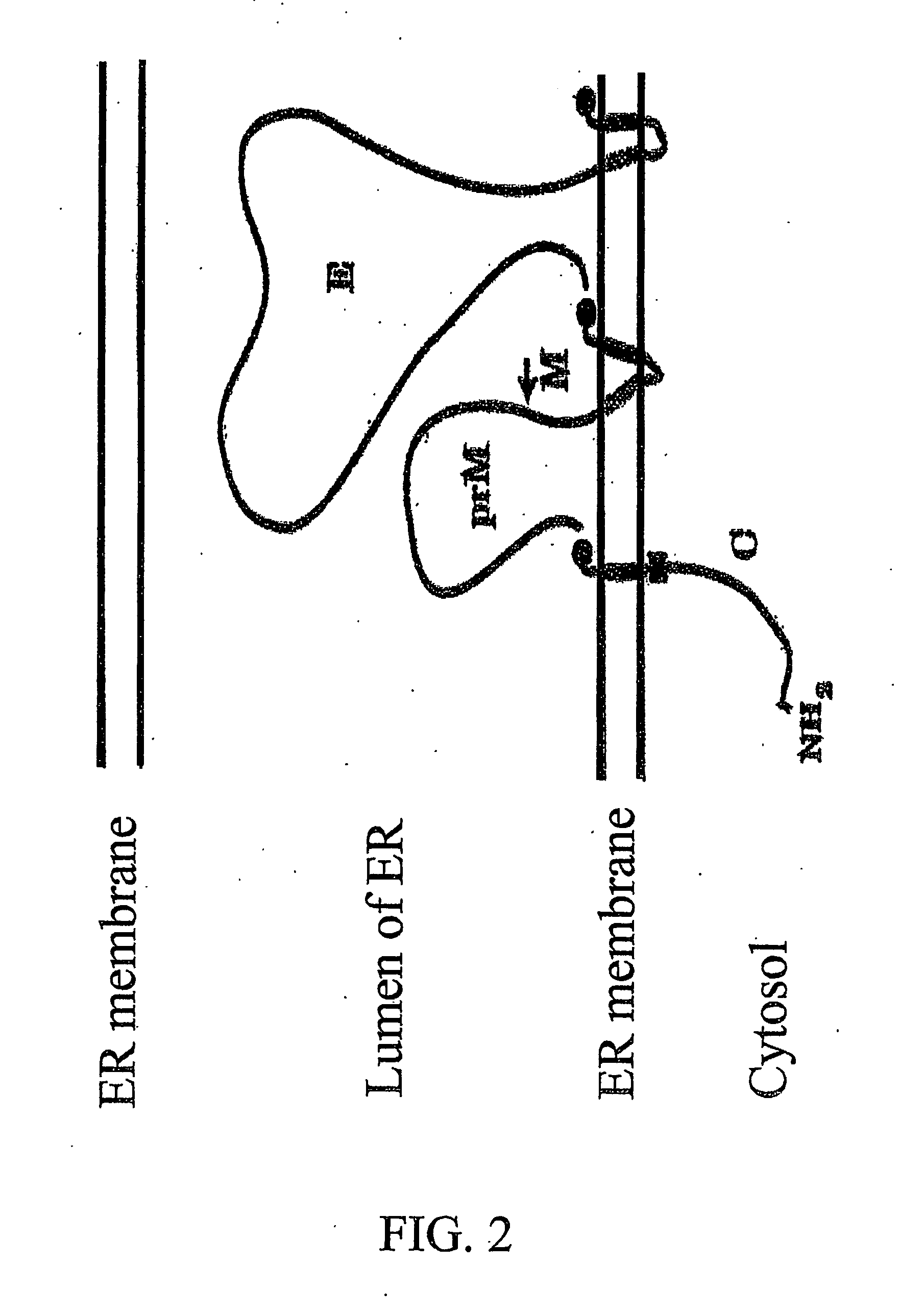
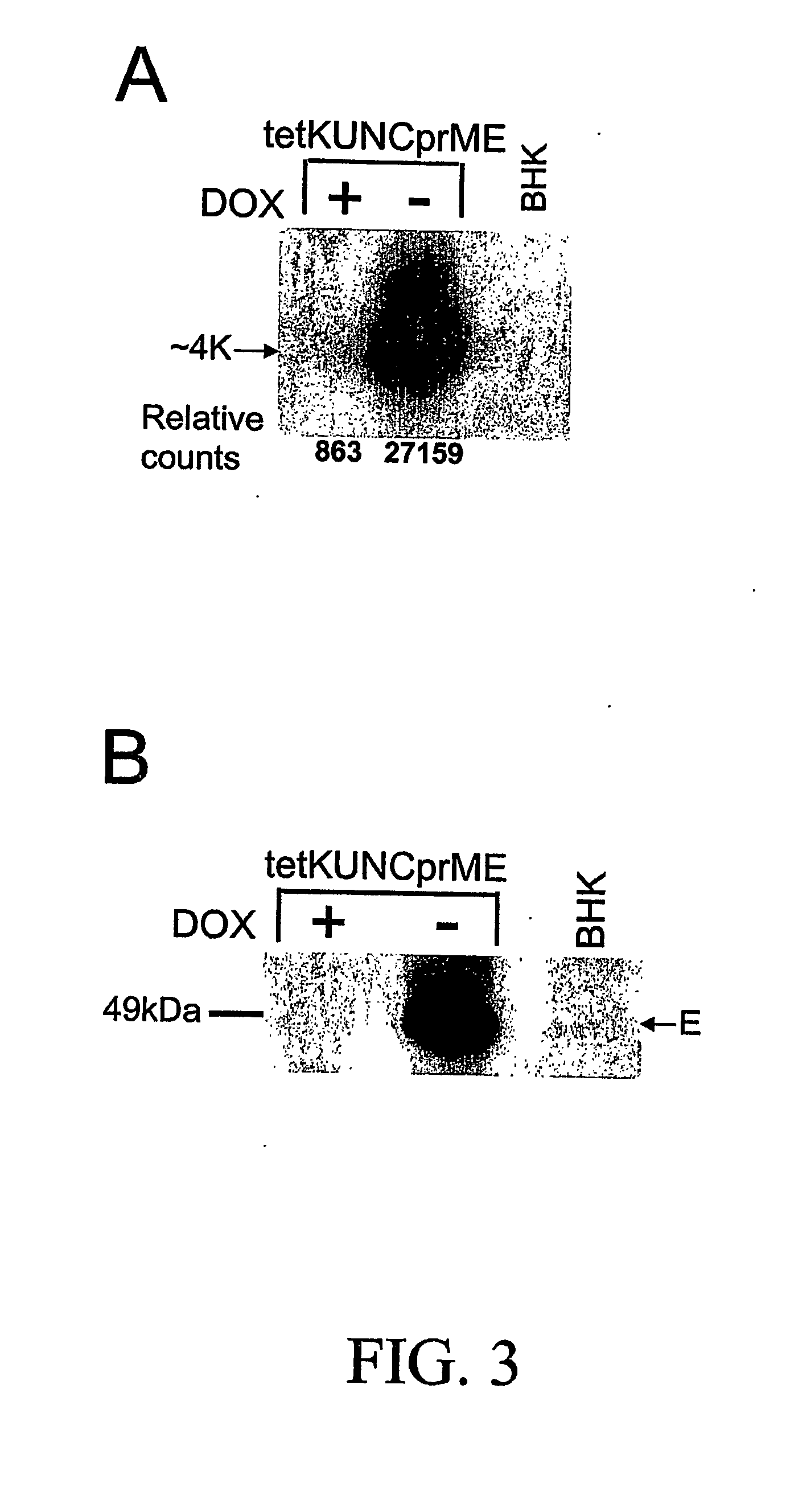
A tetracycline regulatable flaviviral packaging system is provided that facilitates expression of flaviviral structural proteins necessary for flaviviral RNA replicon packaging and virus like particle production in animal cells. This regulatable packaging system is compatible with Kunjin, Dengue and West Nile virus and other flaviviral replicon-based expression systems and produces unexpectedly high titres of virus-like particles. A particular application of this packaging system is the production of virus-like particles that package RNA comprising a flaviviral replicon and encoding a heterologous protein or peptide for expression in animal cells. Even more particularly, the packaging system is capable of delivering immunogens that induce a protective CD8 T cell-mediated immune response.
Owner:REPLIKUN BIOTECH
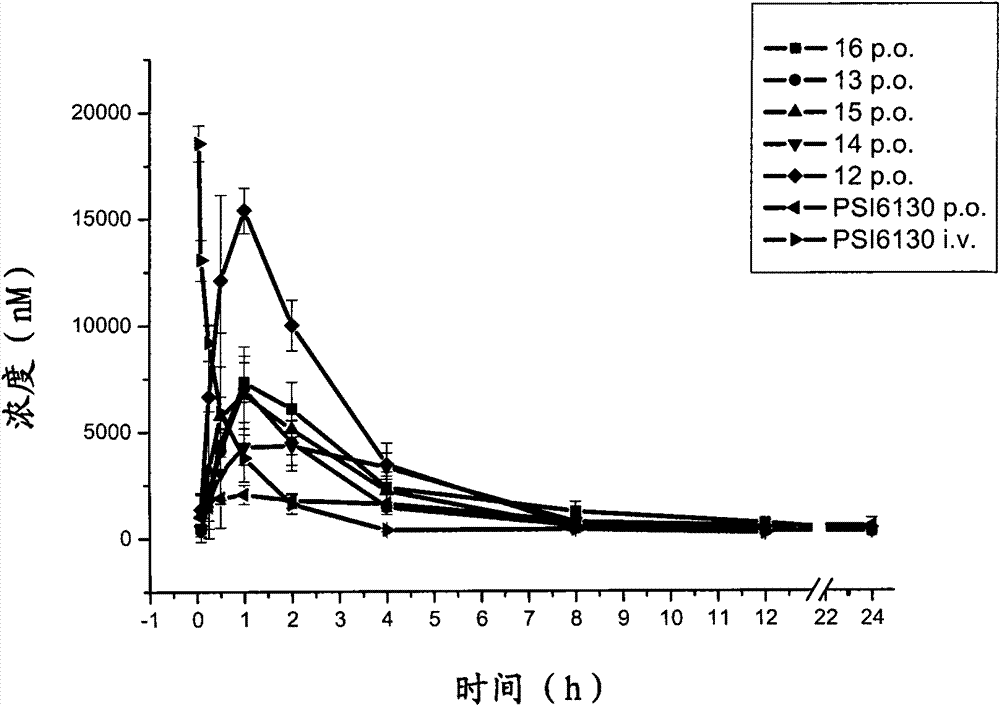
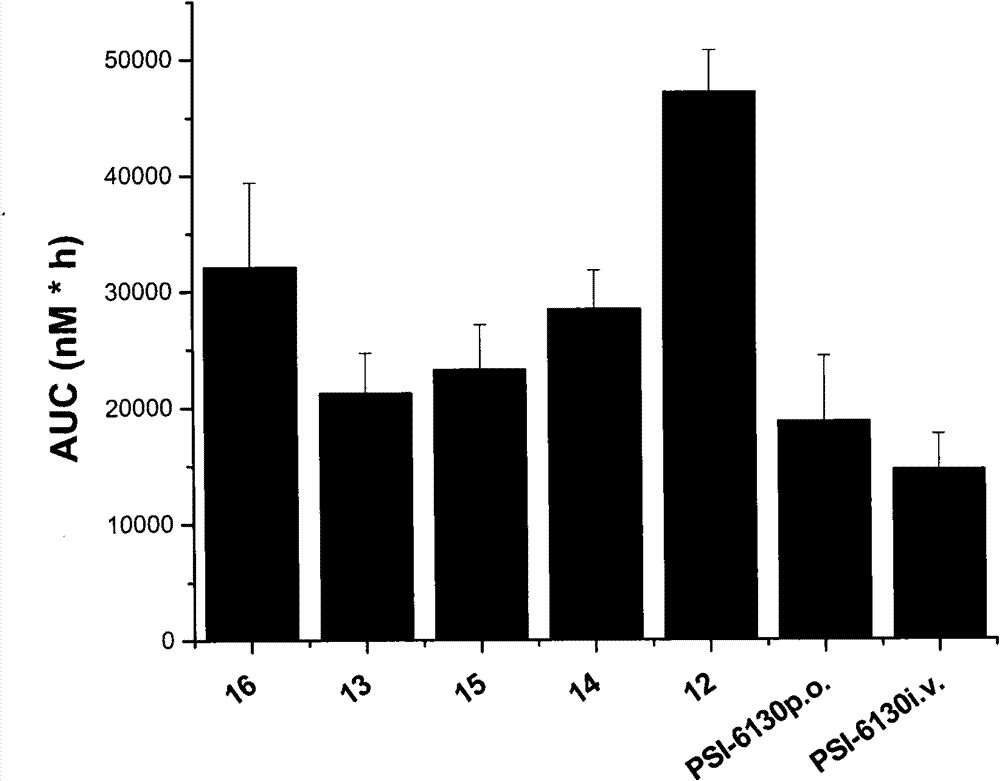
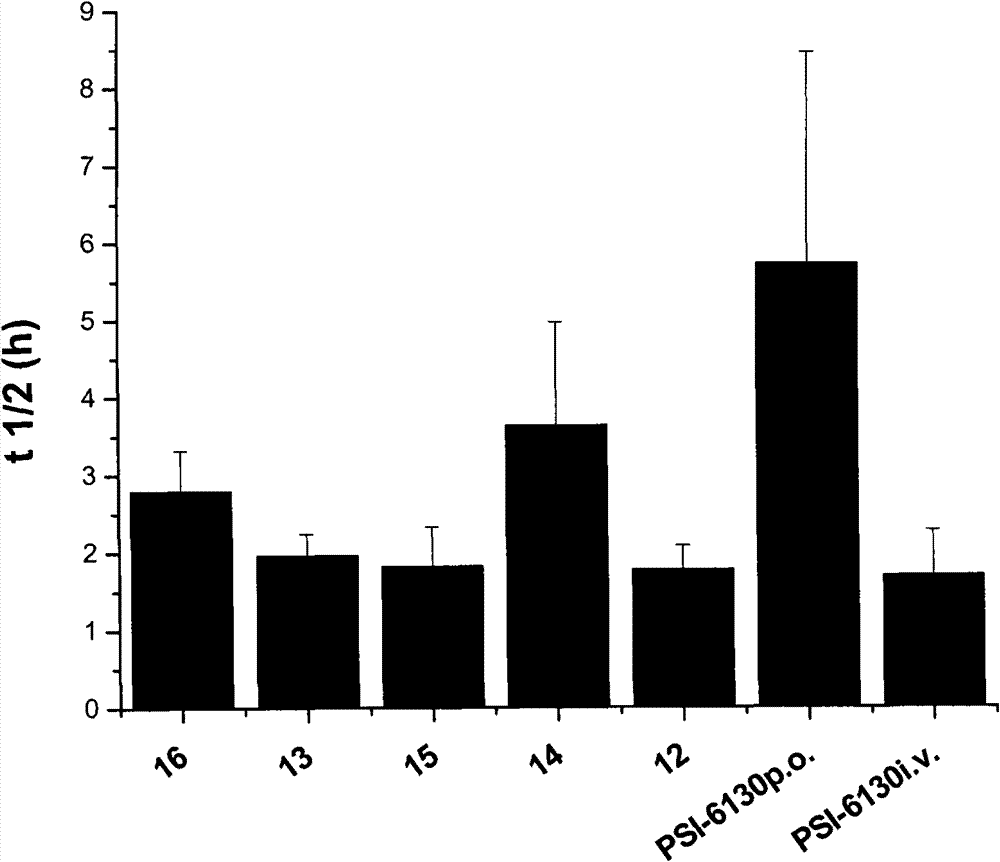
Preparation and application of beta-D-(2'R)-2'-deoxy-2'-fluoro-2'-C-methylcytidine derivatives
The invention relates to compounds as represented by the general formula (A) or their hydrates, solvates or medicinal salts formed with acids, a preparation method thereof, compositions containing the same and application of the same in treating flavivirus infections (such as hepatitis c, West Nile disease and Dengue fever), especially in treating hepatitis c virus (HCV) infected disease. X, Y and Z in the general formula (A) are defined by the specification.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Method of immunization against the 4 serotypes of dengue fever
The invention relates to a method for inducing protection against the 4 serotypes of dengue fever in a patient, comprising:(a) the administration of a monovalent vaccine comprising a vaccinal virus of a first serotype of dengue fever, and(b) the administration of a tetravalent vaccine comprising vaccinal viruses of the four serotypes of dengue fever,in which administration (b) is made between at least 30 days and not more than 12 months following the first administration (a).
Owner:SANOFI PASTEUR SA
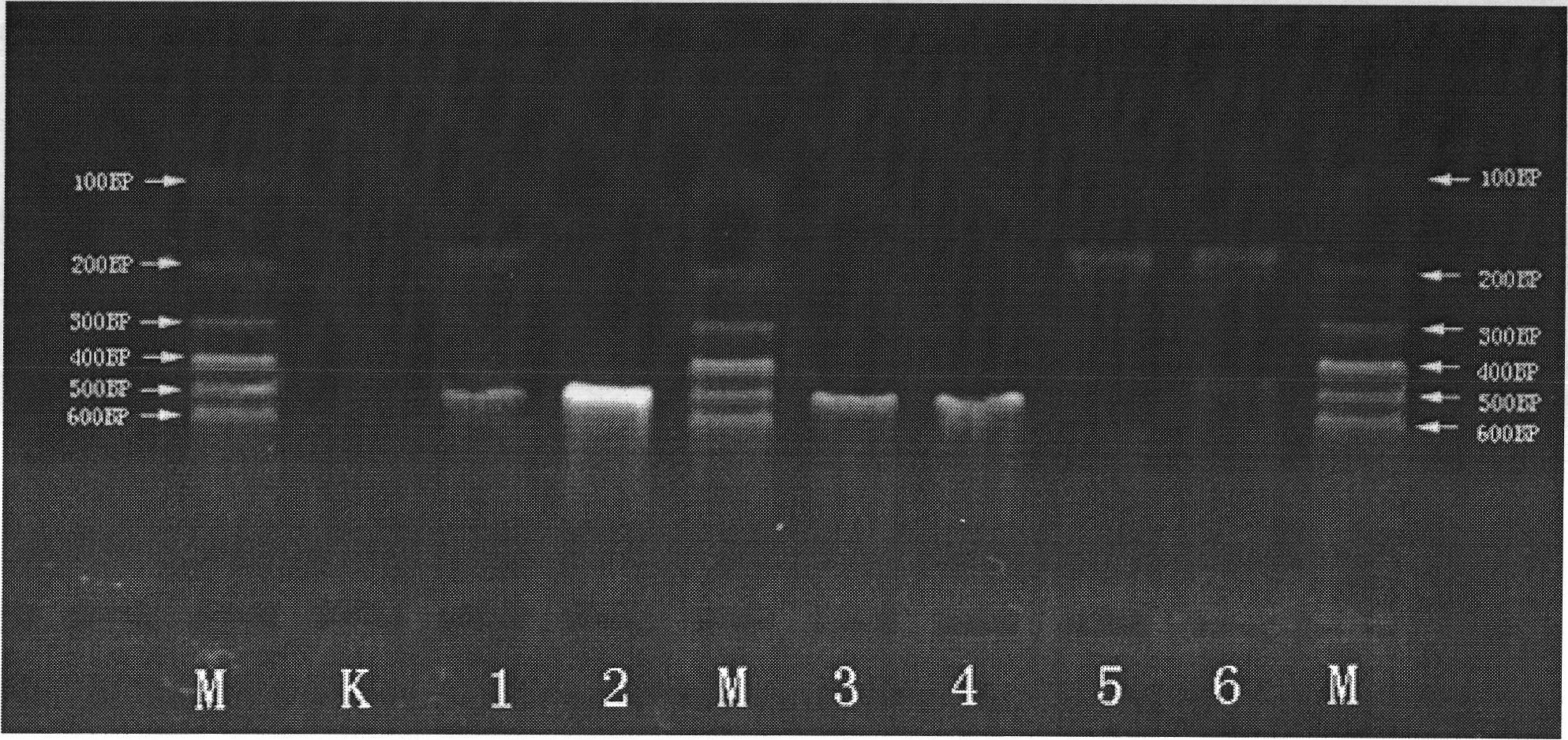
Multiple polymerase chain reaction (PCR) kit and method for detecting mosquito-borne pathogens
InactiveCN101979665AReliable informationEffective early warning dataMicrobiological testing/measurementAgainst vector-borne diseasesTissue fluidMultiplex pcrs
The invention provides a multiple polymerase chain reaction (PCR) kit for detecting mosquito-borne pathogens. The kit comprises six pairs of specific primers. The invention also provides a method for detecting the mosquito-borne pathogens. Electrophoresis is performed on a PCR-amplified product. Whether pathogens, such as encephalitis B virus, dengue fever virus, yellow fever virus, plasmodium falciparum, plasmodium vivax, plasmodium knowlesi, plasmodium ovale, plasmodium malariae, wuchereria malayi, wuchereria bancrofti and the like, exists or not is detected and identified according to the length of a PCR-amplified fragment. By the method, various reported mosquito-borne pathogens, and the yellow fever virus and west nile virus which come from other countries can be detected quickly, accurately and sensitively at the same time, and can be applied to the detection of various samples, such as mosquitoes, blood of patients, tissue fluid and the like. The invention provides a low-cost and high-efficient method for early monitoring and finding mosquito-borne disease prevalence for prevention and control work of mosquito-borne diseases in China.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
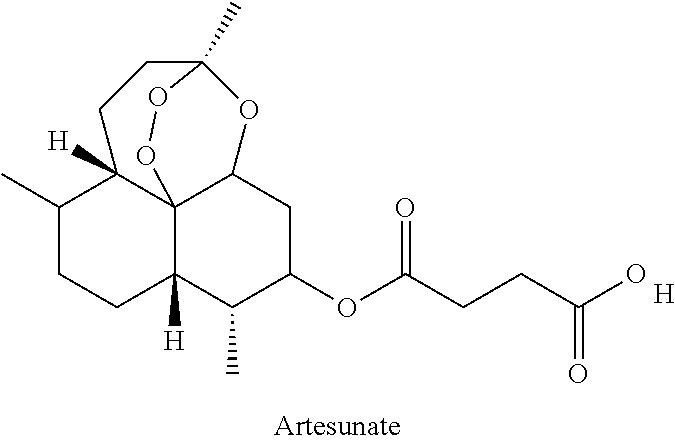
Artemisinin-Based Combination Therapy For Treating Viral Mediated Disease
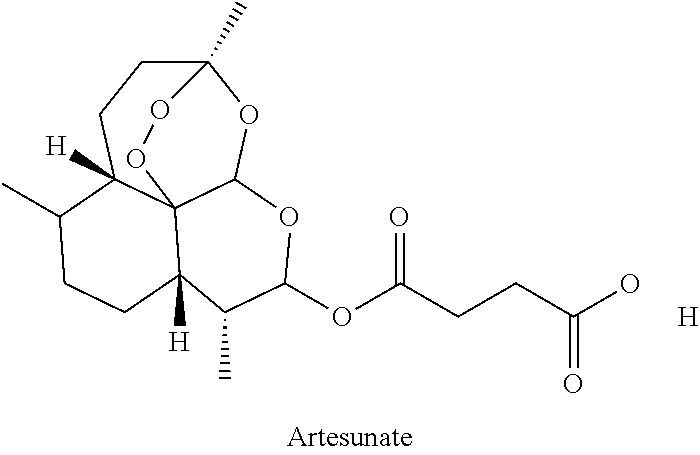
The present invention describes a method of treating individuals suffering from microbial infections, including a viral infection such as Dengue Fever, using an improved Artemisinin Combination Therapy (ACT), known as Tri-ACT. The improved ACT therapy includes administering a combination of three drugs. In one embodiment of the present invention, the method includes administering to an individual a first composition comprising a therapeutically effective amount of an artemether spray sublingually. The individual is then administered a second composition, a therapeutically effective amount of artesunate. A third composition, an effective amount of berberine, or its pharmaceutically acceptable derivatives or salts is then administered to the individual.
Owner:KRYPTONITE GRP
Tetravalent Dengue Vaccines
InactiveUS20100158938A1Immune responseLong durationSsRNA viruses positive-senseViral antigen ingredientsDengue vaccineDengue fever
Owner:GUIRAKHOO FARSHAD
Methods of treatment and diagnosis using modulators of virus-induced cellular gene sequences
InactiveUS20070087982A1Reduce expressionReducing and preventing expression of mRNABiocideGenetic material ingredientsDiseaseImmunodeficiency virus
Applicants have used microarrays, gene expression profiling, and gene silencing methods to identify and provide a plurality of ‘validated’ virus-induced cellular gene sequences (e.g., HMG20B, HRH1, NP and c-YES (src family kinases)) and pathways useful as therapeutic targets for modulation of viral-mediated cellular effects. Particular embodiments provide therapeutic compositions, and methods for modulation of viral infection, replication, maturation, progression, or other virally-related conditions or diseases, comprising inhibition of virally-induced gene sequences and gene products. Additional embodiments provide screening assays for compounds useful to modulate viral infection, replication, maturation or progression, or viral-related conditions or diseases. Further embodiments provide diagnostic and / or prognostic assays for viral infection, replication, maturation or progression. Preferably, the viruses all selected from the group consisting of retroviruses (e.g., human immunodeficiency virus (HIV), and viruses of the family Flaviviridae that includes the flaviviruses (e.g., West Nile virus (WNV), Japanese encephalitis virus (JEV), yellow fever virus (YFV) and Dengue fever virus (DEN)), and hepatitis C virus (HCV).
Owner:OREGON HEALTH & SCI UNIV
Fluorescent quantitative RT-PCR detection kit and detection method for enterovirus
InactiveCN101407846ANo cross reactionQuick checkMicrobiological testing/measurementFluorescence/phosphorescenceCerebrospinal fluidHand-foot-and-mouth disease
The invention provides a fluorescence quantitative RT-PCR detecting kit for an enterovirus and a detecting method thereof; and the sequences of an upstream primer and a downstream primer and a specific probe of the fluorescence quantitative RT-PCR detecting kit are as follows: the upstream primer EV(YG)F is 5'-GGCTGCGYTGGCGGCC-3', the downstream primer EV(YG)R is 5'-CCAAAGTAGT CGGTTCCGC-3' and the specific probe EV(YG)PB is 5'-CTCCGGCCCCTGAATGCGG-3'. The method has high specificity on detecting the enterovirus and does not have cross reactions with other enteroviruses such as hepatitis A, hives, rubella, parotitis, encephalitis, dengue fever, adenovirus, and the like. The detection sensitivity of the method achieves 0.1TCID50; the method can directly detect the nucleic acid of the enterovirus from the samples of ncurolymph, herpes liquid, dejecta and the like of suspected patients; only about 3h is needed from extracting the nucleic acid of the enterovirus to finishing the detection; and the detecting kit and the detecting method are suitable for the lab early diagnosis of sudden epidemic caused by the infection of the enteroviruses such as the hand-foot-and-mouth disease and the like.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Tetravalent Dengue Vaccines
InactiveUS20130095136A1Immune responseLong durationSsRNA viruses positive-senseViral antigen ingredientsMedicineDengue vaccine
Owner:SANOFI PASTEUR BIOLOGICS CO
Artemisinin with Berberine Compositions and Methods of Making
ActiveUS20130102625A1Effective treatmentBiocideSolid materialProgressive multifocal leukoencephalopathyYellow fever
An all-natural herbal composition and methods of preparing the same are provided. The novel Artemisinin Combination Therapy (ACT) consists of artemisinin and its derivatives and berberine, the two active substances mixed with various selected excipient compounds to form a single pill, tablet or capsule for treatment and prevention of malaria, dengue fever, yellow fever, dysentery, Lyme disease, babesiosis, progressive multifocal leukoencephalopathy, Helicobacter Pylori, and colitis, in adults and children. A tablet or pill for children is formulated to be chewable.
Owner:U S PHYTOTHERAPY
Competitive enzyme linked immunosorbent assay (C-ELISA) for the detection of a flavivirus specific antibody
InactiveCN101449165ASsRNA viruses positive-senseBiological material analysisSpecific immunitySecondary Infections
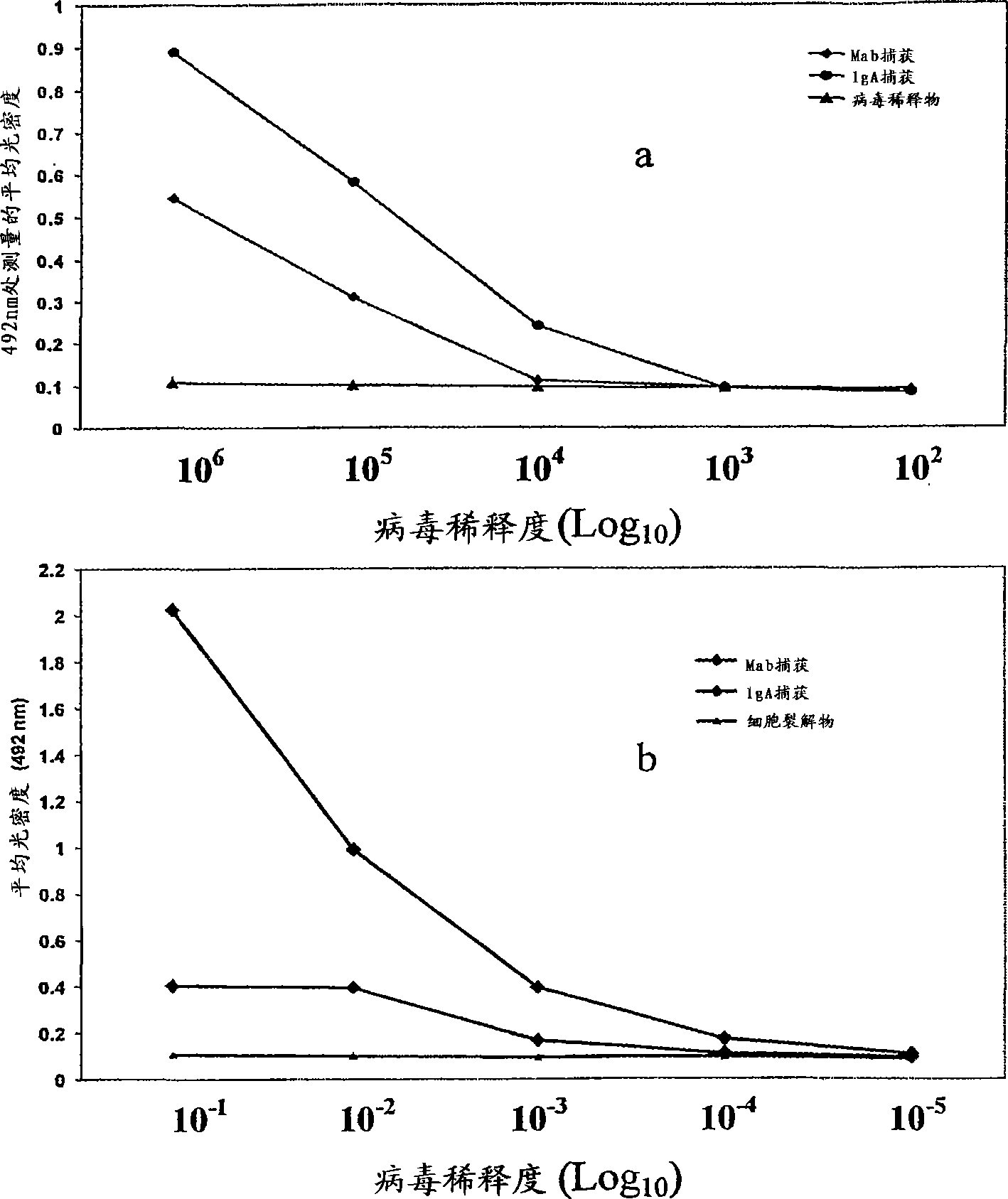
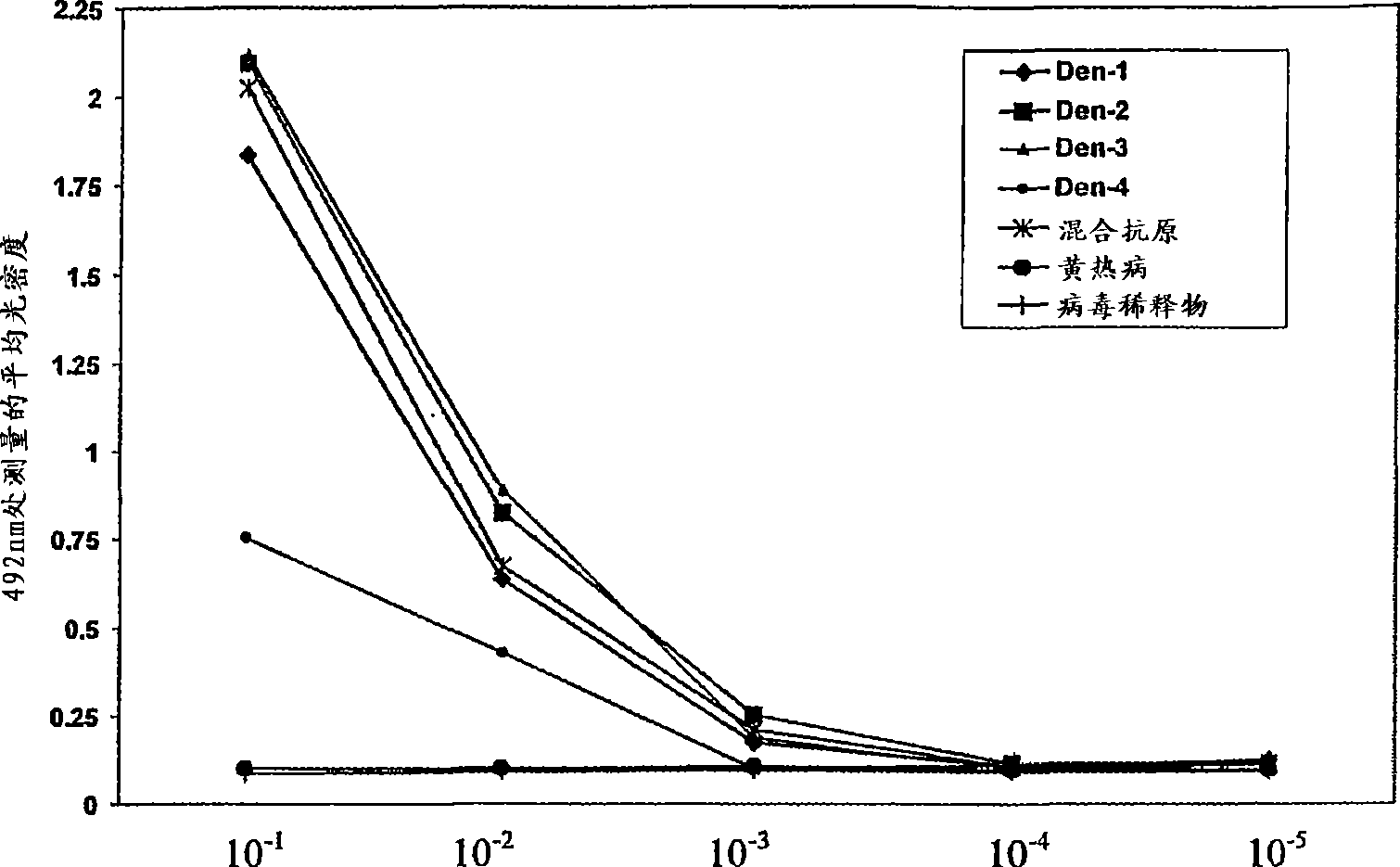
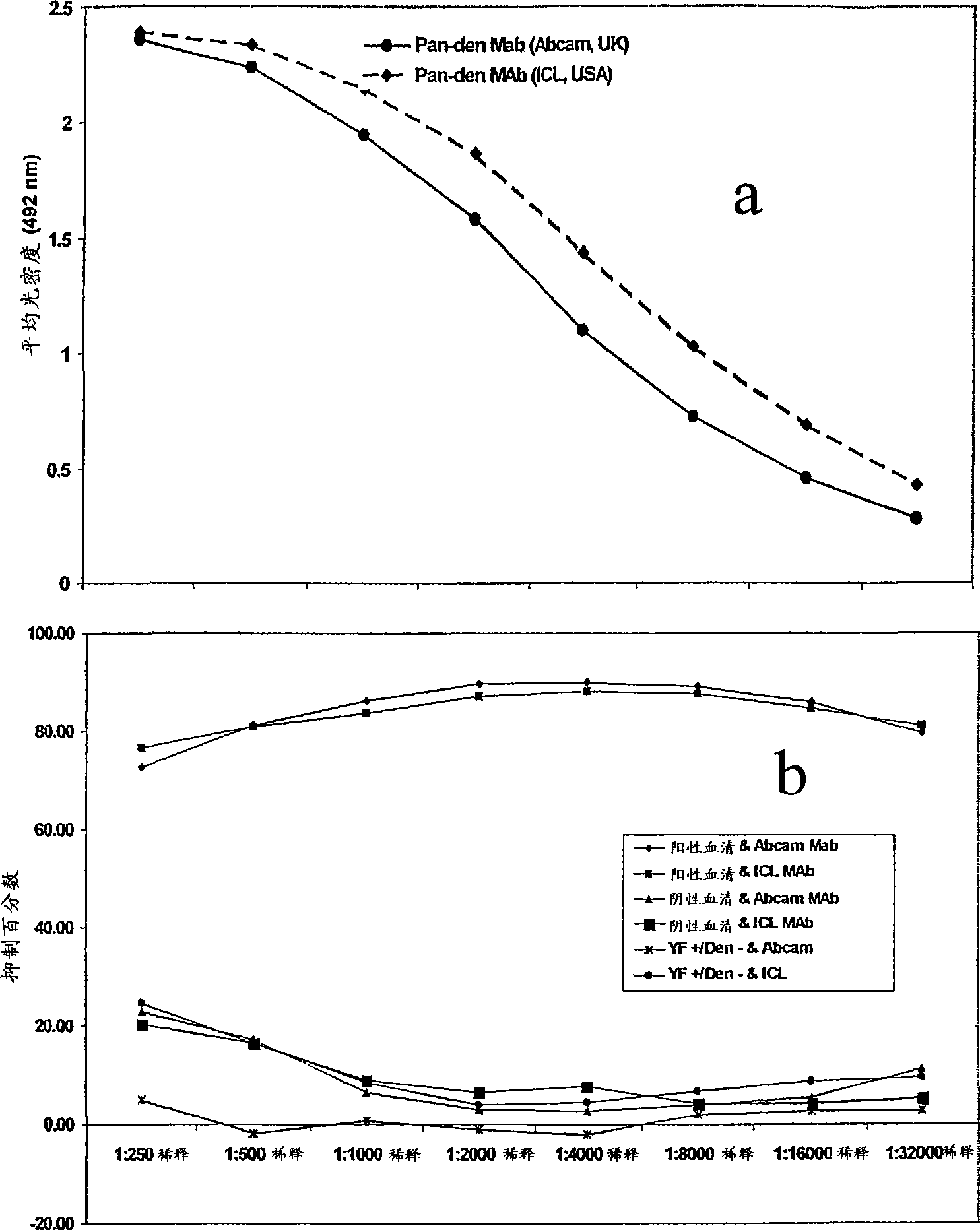
A competitive enzyme-linked immunosorbent assay (C-ELISA), using flavivirus member specific immunological agents was developed to detect antibody specific to members of the flaviviruses indicative of exposure to flavivirus. The test is based on a competition for epitope binding on the envelope protein of the flavivirus antigen captured using anti-flavivirus IgA in the presence of flavivirus positive serum. This test has comparable sensitivity specificity and speed to the virus neutralization assay (VNT). C-ELISA is a versatile technique, which could have various applications. Slight modifications of this protocol could lead to a C-ELISA-based detection method of secondary infection or one that could be used for serotype specific sero-epidemiological studies and / or vaccine evaluation. The protocol developed for C-ELISA was demonstrated using dengue lysate antigen and dengue specific monoclonal antibody. This can be used against other flaviviruses and the results for Japanese encephalitis illustrates this.
Owner:NATIONAL ENVIRONMENT AGENCY
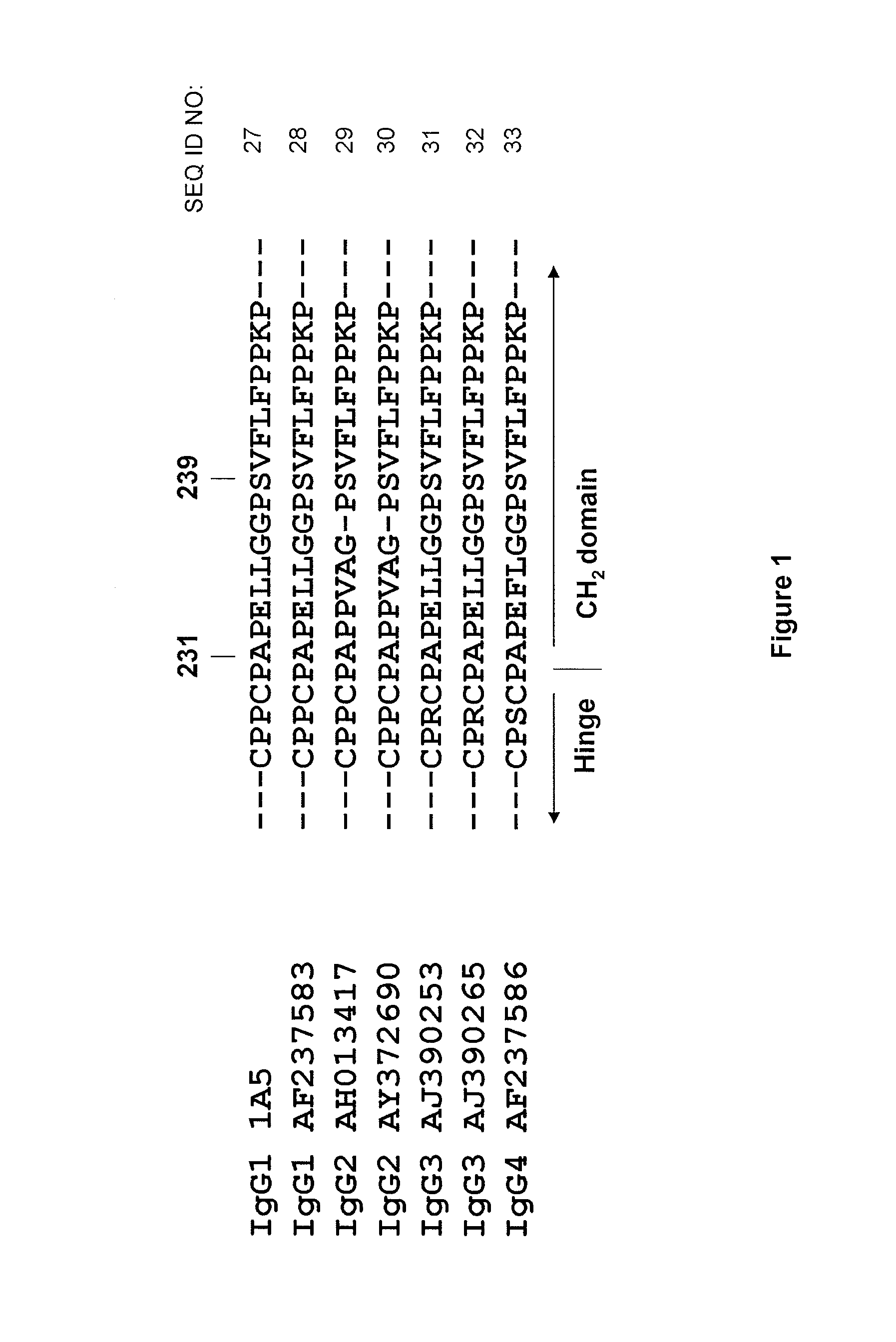
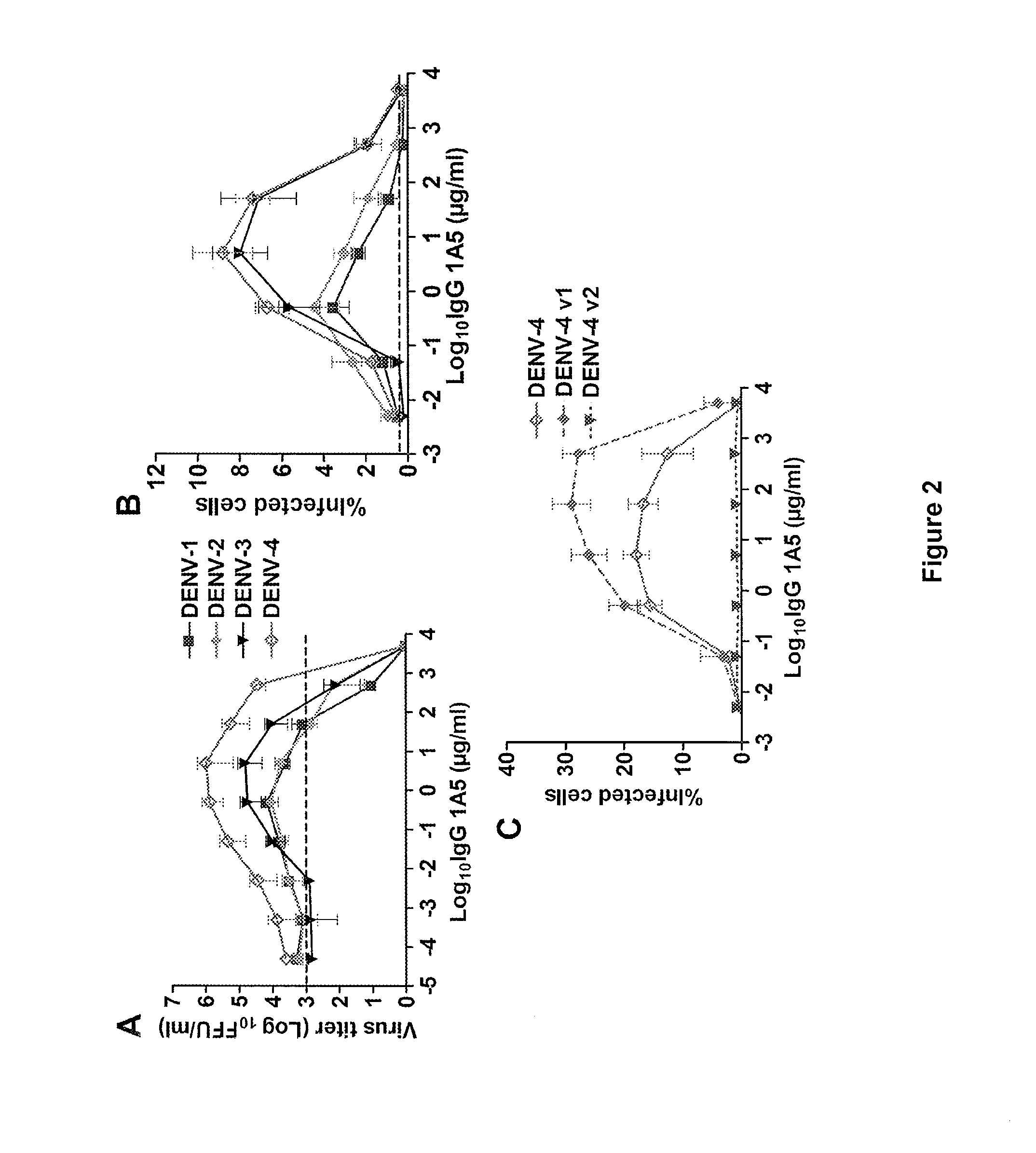
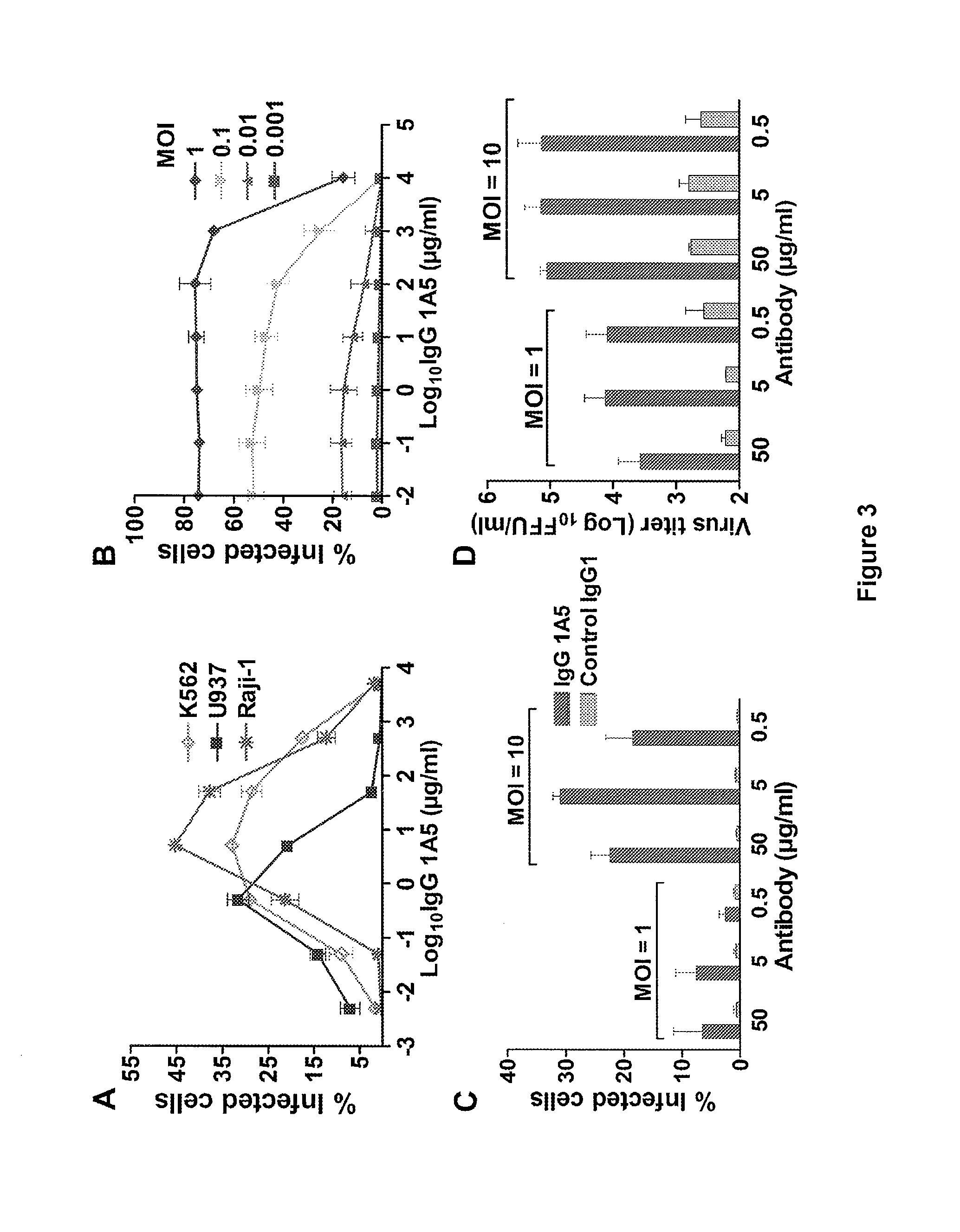
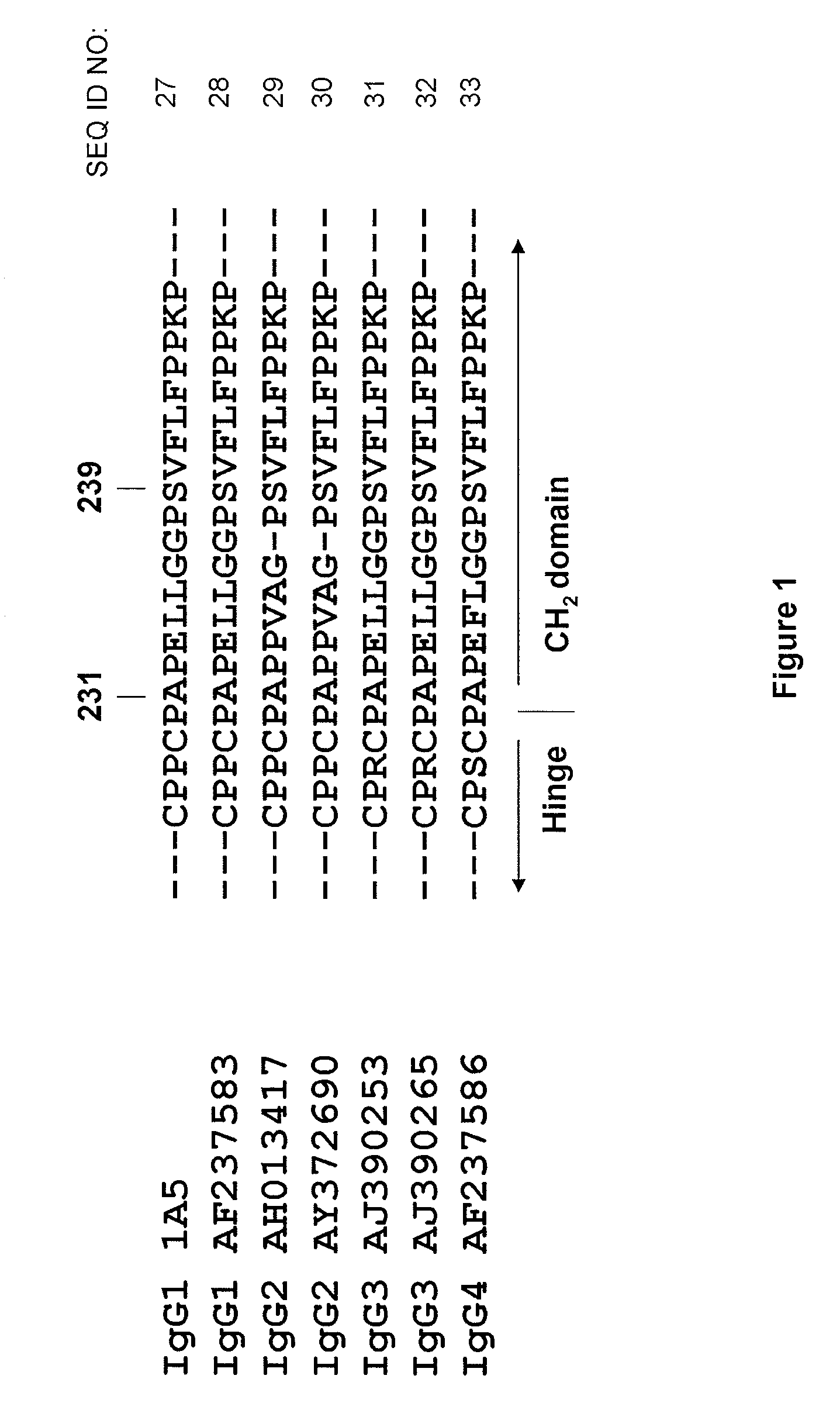
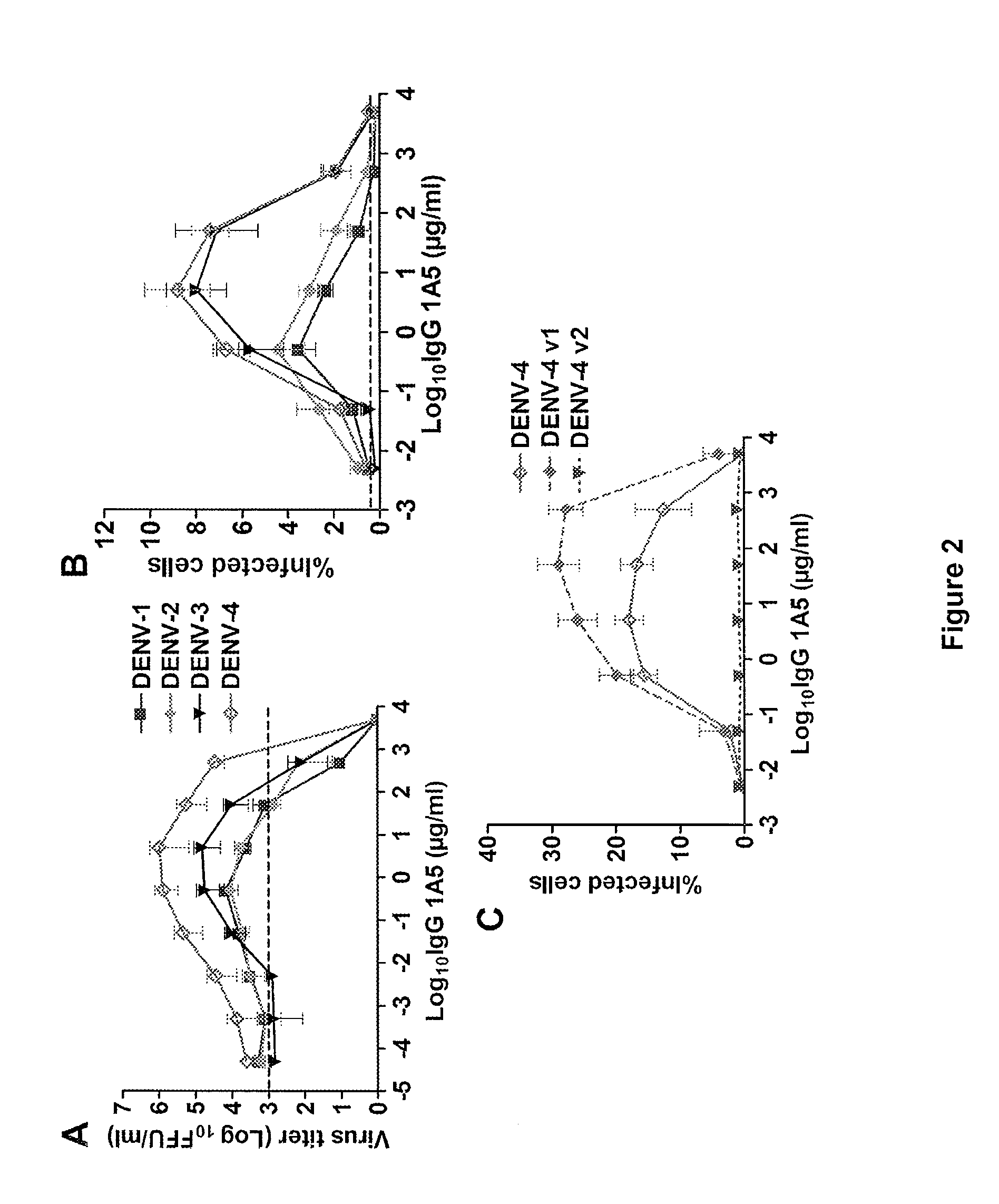
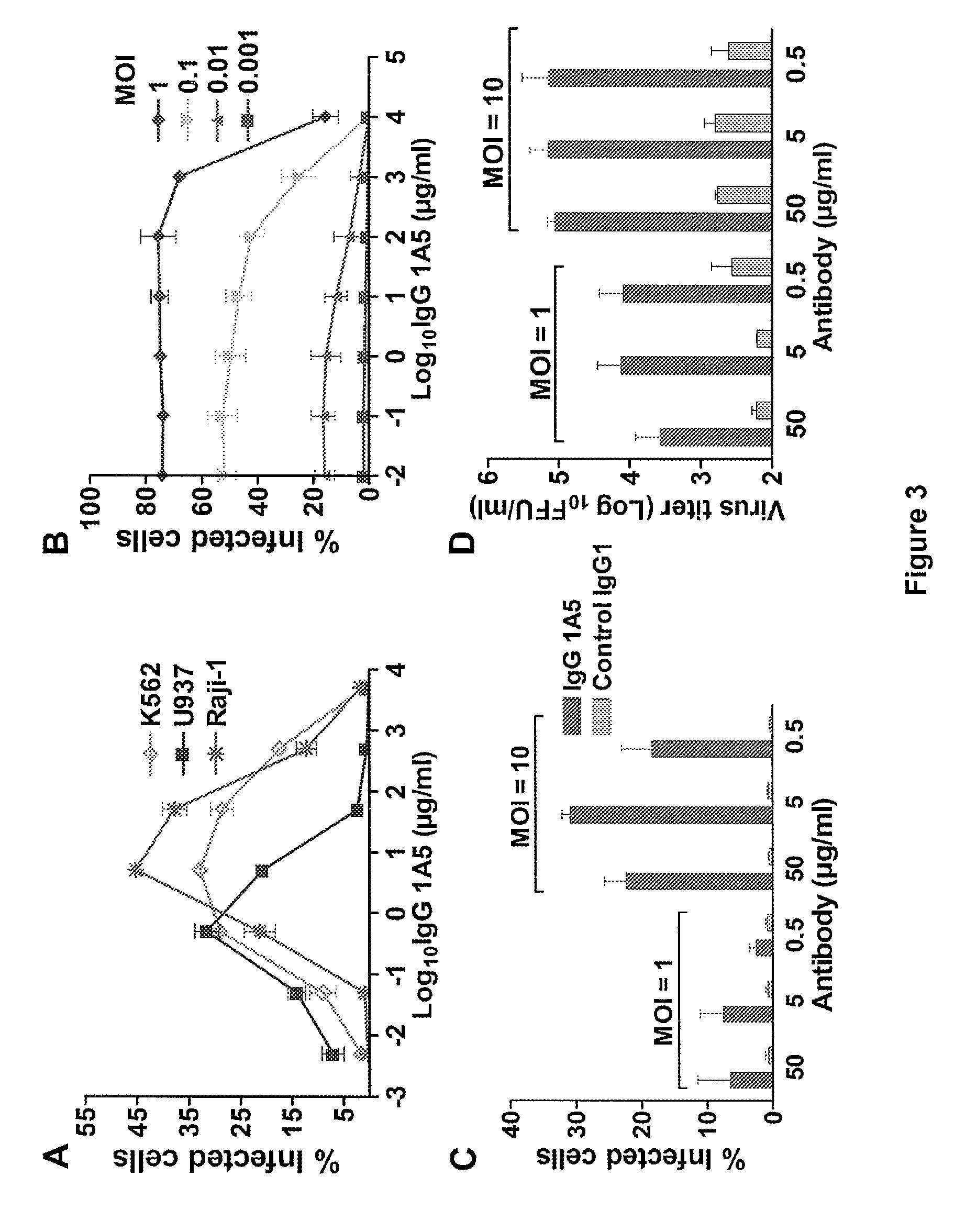
Monoclonal antibodies against dengue and other viruses with deletion in fc region
Owner:THE U S AS REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES
Mosquito repellent
The invention discloses mosquito repellent. The mosquito repellent is made of raw materials including, by weight, 5-15 parts of flos caryophylli, 10-15 parts of folium artemisiae argyi, 8-20 parts of flos lonicerae, 5-7 parts of herba moslae, 10-12 parts of herba menthae, 6-9 parts of agastache rugosa and 10-30 parts of realgar. The mosquito repellent has the advantages that the mosquito repellent is prepared from purely natural Chinese herbal medicines and chemical products, mosquitoes mainly can be expelled according to mosquito biting and blood sucking and disease spreading common sense and principles, harmful germs, bacteria and the like in air can be killed by odor volatilized from the traditional Chinese medicines, accordingly, family members can be guaranteed against being disturbed by the mosquitoes and can be prevented from suffering from diversified diseases due to mosquito biting, effects of keeping the family members away from diseases such as malaria, filariasis, epidemic encephalitis and dengue fever can be realized, methods for manufacturing the mosquito repellent are simple, raw materials for the mosquito repellent are easily available, and the like.
Owner:GUANGXI UNIV FOR NATITIES
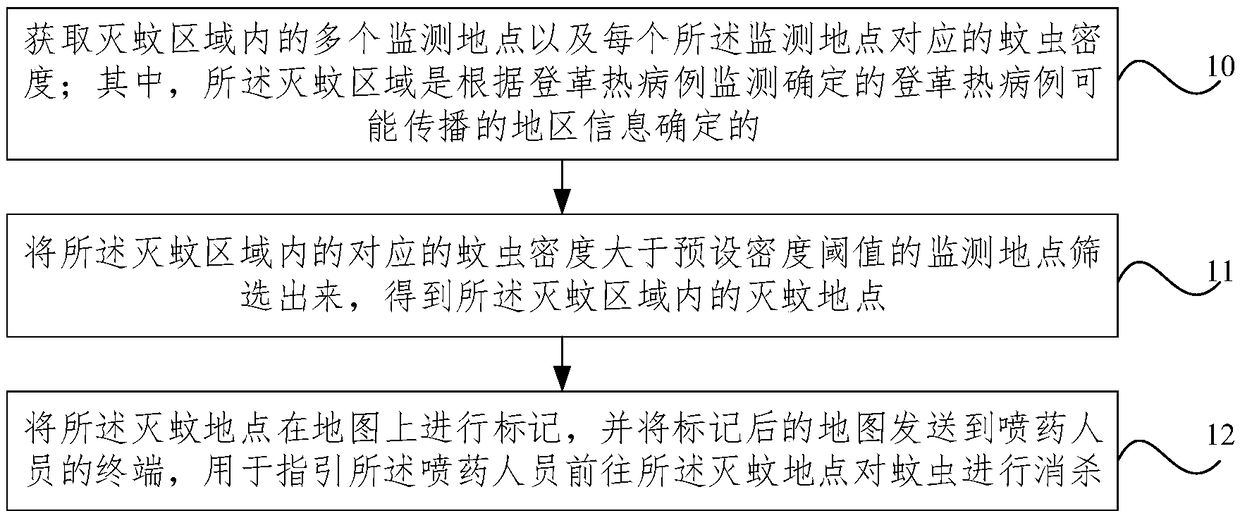
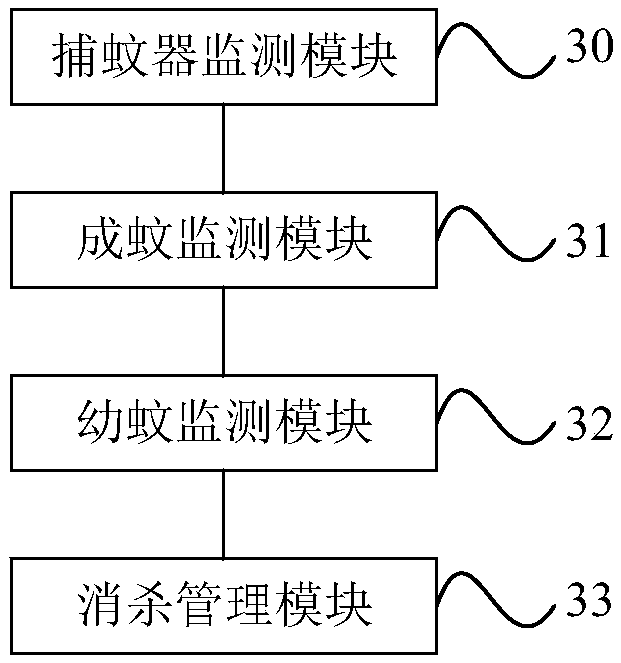
Dengue fever prevention and control method and system
ActiveCN108711455AReduce usagePrecision SprayingEpidemiological alert systemsICT adaptationWork qualityQuantitative assessment
The invention provides a Dengue fever prevention and control method and system. The method comprises steps of acquiring multiple monitoring places in a mosquito eradication region and the mosquito density corresponding to each monitoring place, and screening out the monitoring place where the corresponding mosquito density in the mosquito eradication region is larger than the preset density threshold value so as to obtain a key mosquito eradication place in the mosquito eradication region; marking the mosquito eradication place on a map, and sending the marked map to a terminal of a drug spraying worker so as to guide the drug spraying worker to go to the mosquito eradication place to eradicate mosquitoes. According to the invention, drugs are mainly sprayed to a monitoring place with highmosquito density, so the mosquito eradication work is allowed to be quite targeted, the drug spraying efficiency and drug eradication effects are improved, quantitative estimation of the mosquito density monitoring and mosquito eradication work is achieved and working quality is improved.
Owner:ICDC CHINA CDC
Vaccine compositions
The present invention relates to vaccine compositions that are useful in a method of protecting a human subject against dengue disease.
Owner:SANOFI PASTEUR SA
Monoclonal antibodies against dengue and other viruses with deletion in Fc region
The present invention relates to a variant of a parent polypeptide comprising an Fc region, which variant binds an Fc gamma receptor (FcγR) with lower affinity than the parent polypeptide and comprises a deletion of at least one amino acid in about position 100 to about position 150 in the Fc region and related nucleic acids, vectors, host cells and methods of producing the variant and methods for preventing or treating a disorder in a mammal.
Owner:UNITED STATES OF AMERICA
Sweet wormwood ointment with anti-mosquito and anti-malaria effects
InactiveCN107802755AAvoid bitesAvoid spreadingHydroxy compound active ingredientsAerosol deliveryNepetaEugenol
The invention relates to a sweet wormwood ointment with anti-mosquito and anti-malaria effects. The ointment is mainly prepared from the following raw materials: nepeta oil, menthol, dementholized peppermint oil, sweet wormwood oil, blumea oil, lemongrass oil, eugenol, artesunate, alpha-bisabolol and an ointment excipient, prevent mosquito bite well, and further prevent rapid spread of diseases which mainly take mosquitos as media such as malaria, dengue fever, filariasis or epidemic encephalitis B. The sweet wormwood ointment further has effect of rapidly relieving itching and removing swelling on itching and swelling which are caused by mosquito bite.
Owner:江西龙卿堂科技有限公司
Hcv inhibitors
The present invention relates to compounds that are useful in the treatment of viruses belonging to Flaviviridae, including flaviviruses, pestiviruses, and hepaciviruses. The invention includes compounds useful for the treatment or prophylaxis of dengue fever, yellow fever, West Nile virus, and HCV.
Owner:SMITHKLINE BECKMAN CORP
Methods and Biomarkers for the Detection of Dengue Hemorrhagic Fever
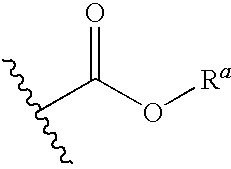
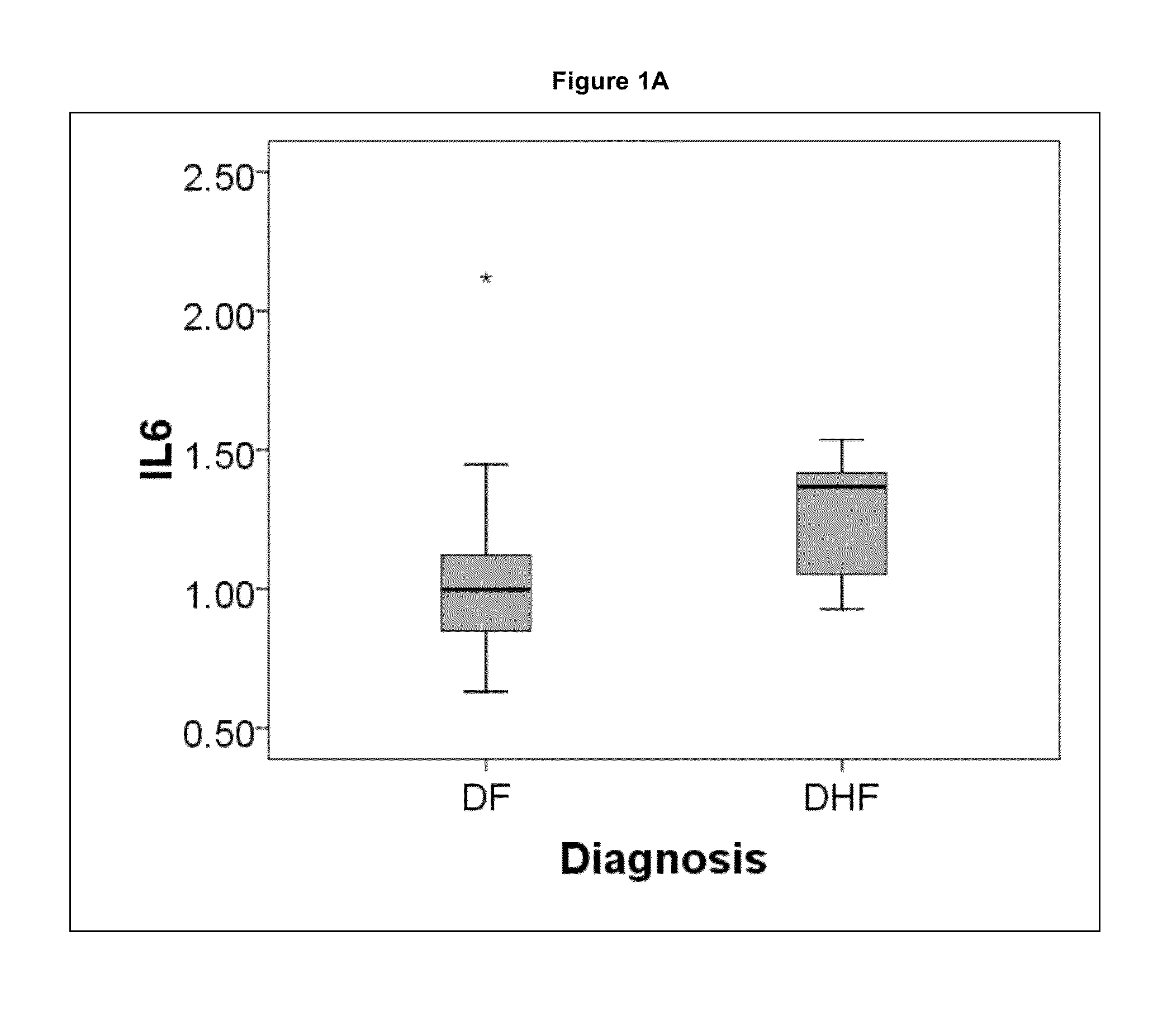
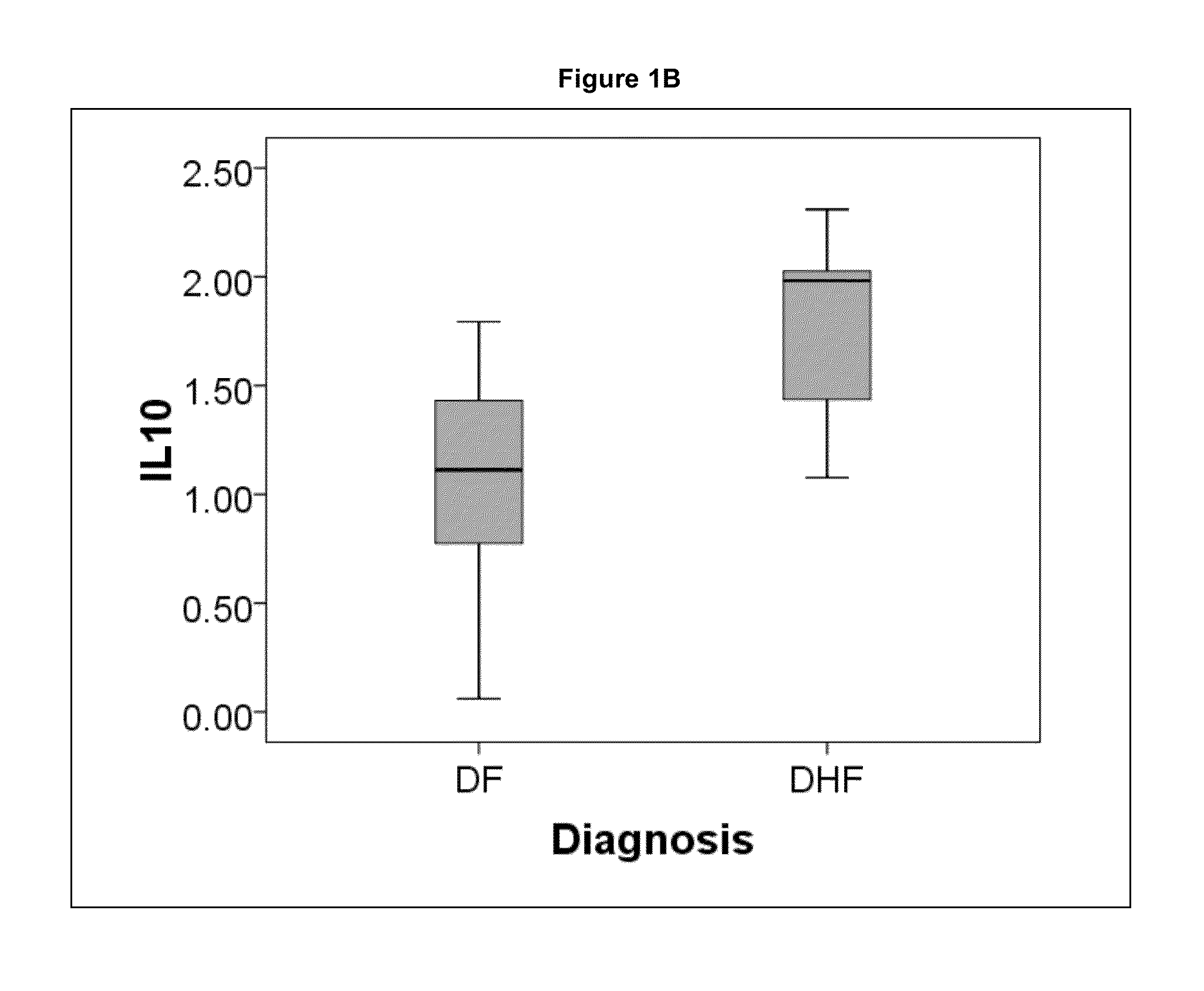
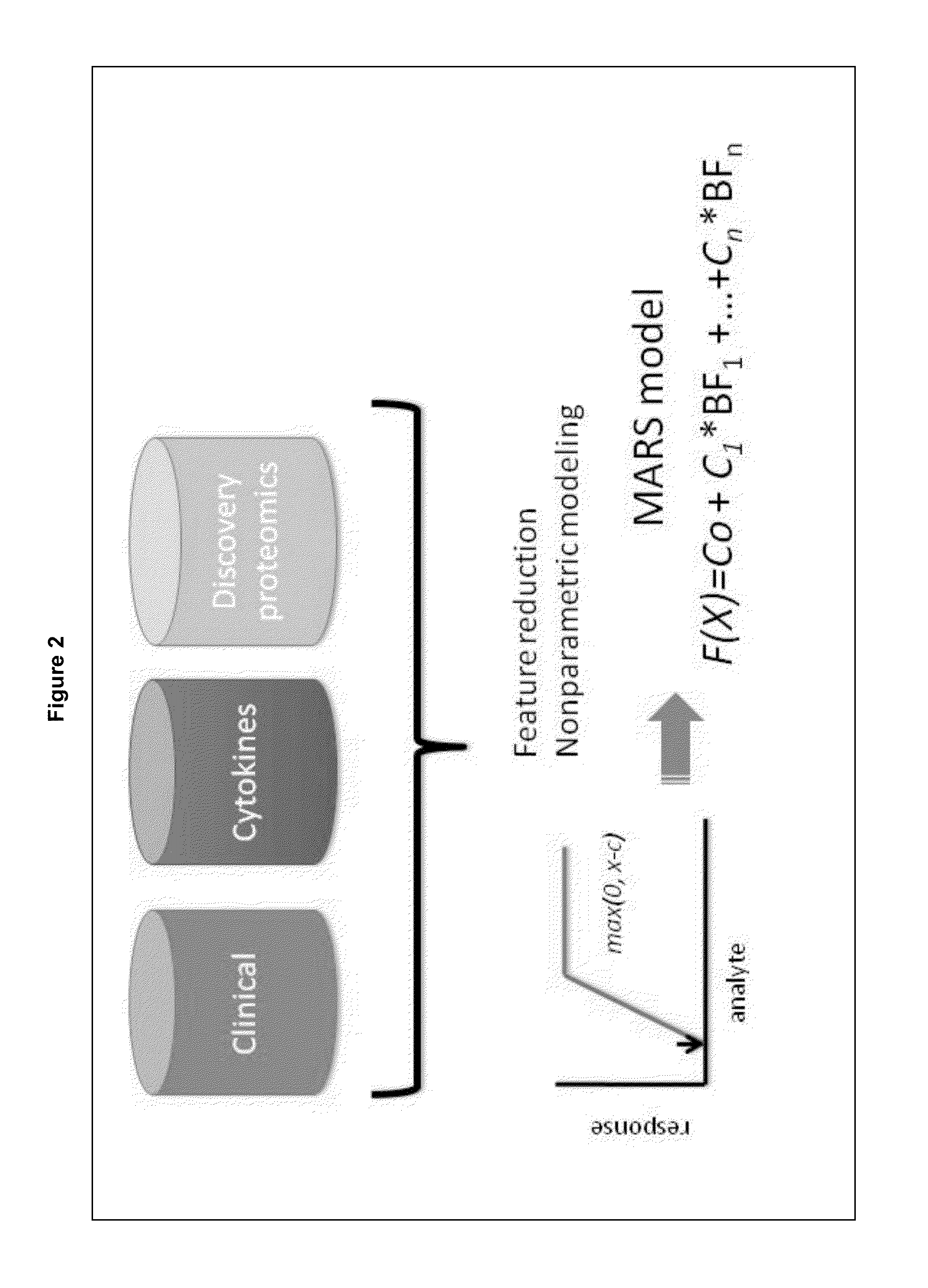
The present invention provides methods for detecting, analyzing, and identifying biomolecules used to identifying patient with dengue-like symptom who are at risk of DHF. The inventive method comprises detecting in a sample from a subject dengue infected patient one or more biomarkers selected from the group consisting of IL-10, fibrinogen, C4A, immunoglobulin, tropomyosin, and three isoforms of albumin, and which are used in a predictive MARS model to detect patients with risk of developing DHF.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com