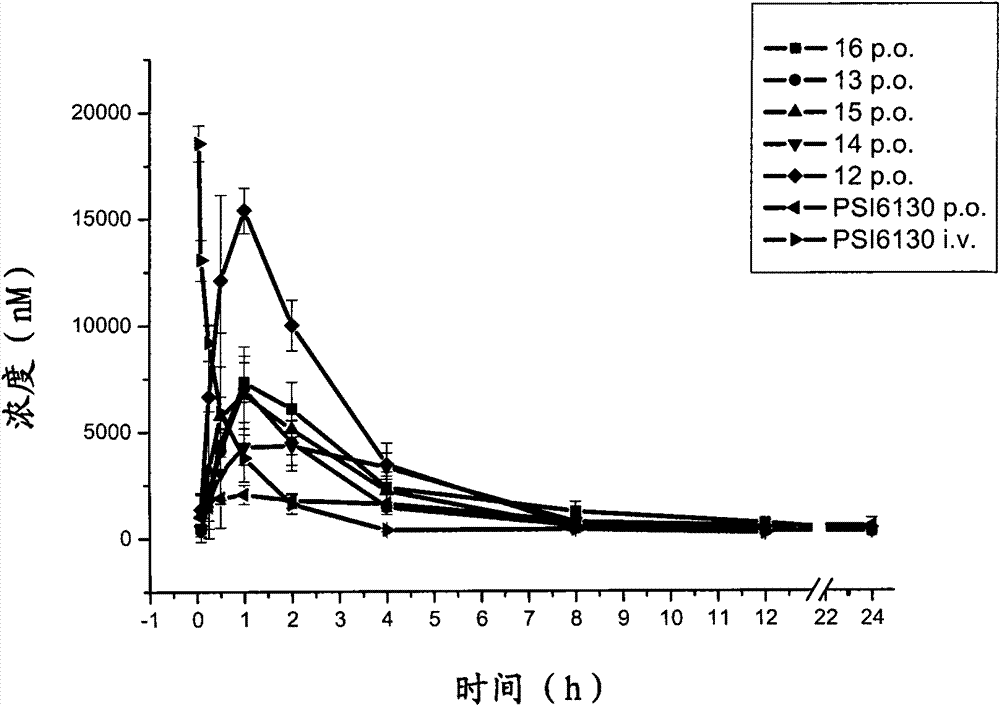
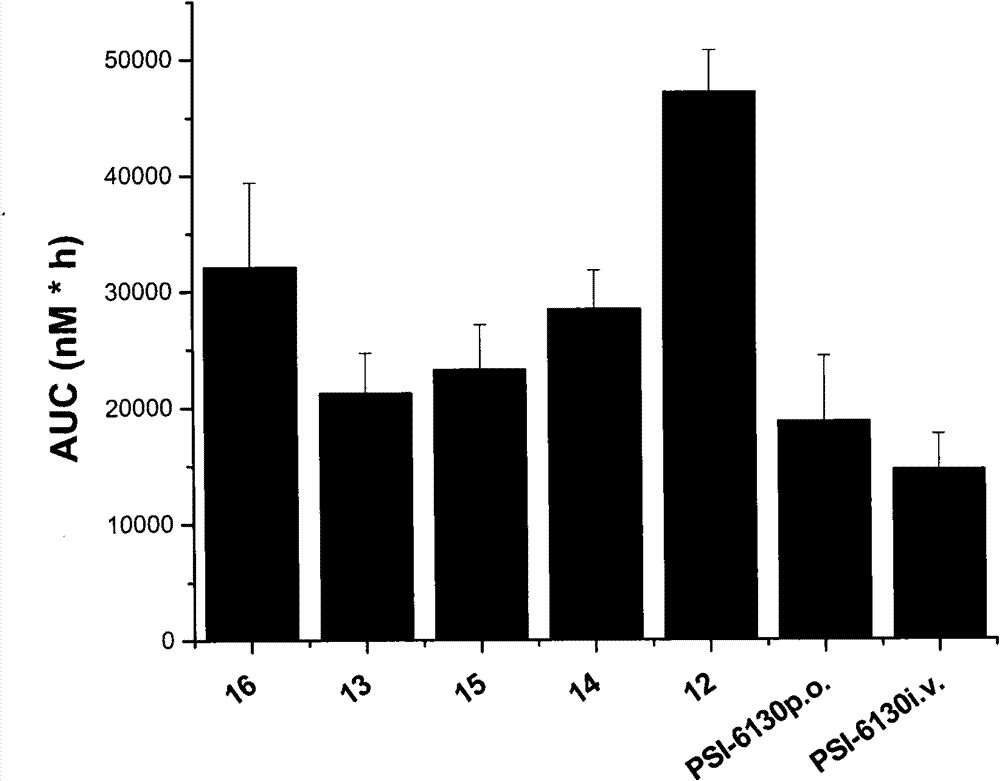
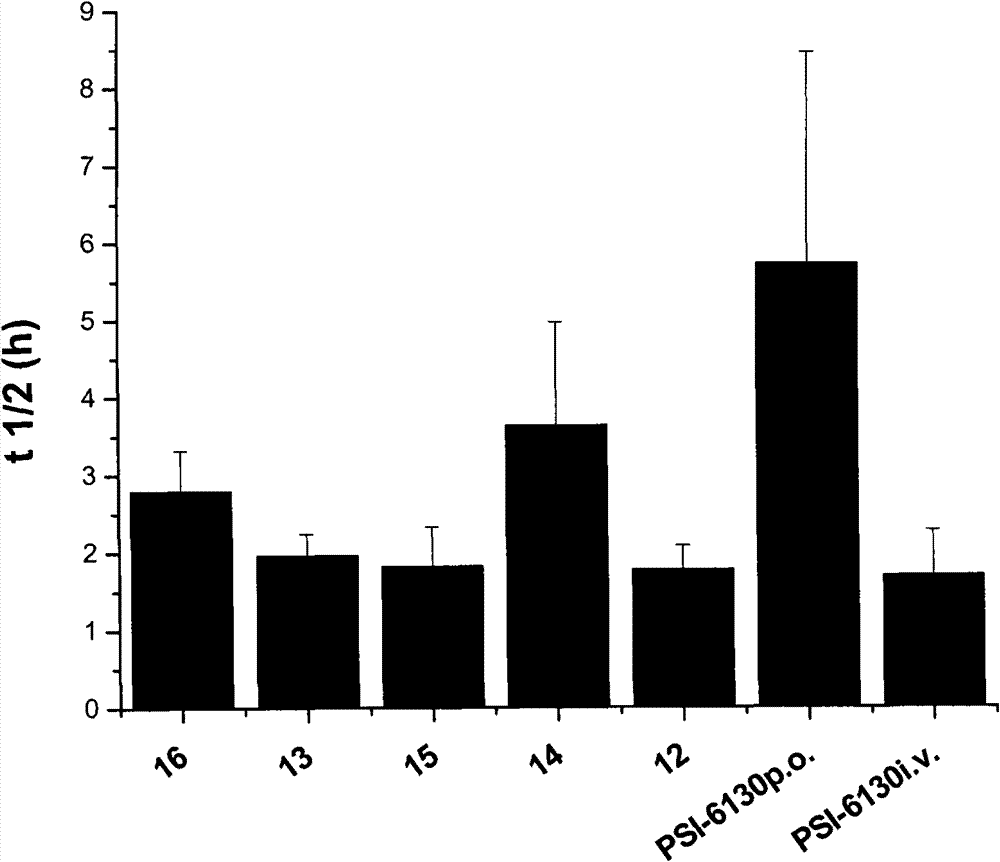
Preparation and application of beta-D-(2'R)-2'-deoxy-2'-fluoro-2'-C-methylcytidine derivatives
A R2-C, R3-C technology, applied in the field of hepatitis C virus infection, can solve complex multidisciplinary tasks, a large number of experiments and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0107] The following specific examples are preferred embodiments of the present invention, which should not be construed as constituting any limitation to the present invention.
[0108] The melting point of the compound was determined by RY-1 melting point apparatus, and the thermometer was not corrected. Mass spectra were determined by a Micromass ZabSpec high-resolution mass spectrometer (resolution 1000). 1 H-NMR is measured by JNM-ECA-400 superconducting NMR instrument, working frequency 1 H-NMR 400MHz.
[0109] Abbreviations or symbols used in this application have the following meanings:
[0110] DAST: Diethylaminosulfur trifluoride
[0111] TIPDSCl 2 : 1,3-dichloro-1,1,3,3-tetraisopropyl disiloxane
[0112] TBAF: Tetrabutylammonium fluoride
[0113] HOBt: 1-Hydroxybenzotriazole
[0114] DCC: Dicyclohexylcarbodiimide
[0115]DMAP: 4-Dimethylaminopyridine
[0116] TFAA: Trifluoroacetic anhydride
[0117] Bz: benzoyl
preparation example
[0119] Preparation of compound 9
[0120] Preparation of β-D-4-N-benzoylcytidine (2)
[0121] Dissolve 2.5g of cytidine (1) (10mmol), 2.5g of benzoic anhydride (11mmol) in 50ml of anhydrous pyridine to form a suspension, and stir at room temperature for 48h. The solvent was distilled off under reduced pressure. A small amount of ether was added to the residue and filtered. The filter cake was washed with ethyl acetate, water and acetone. The obtained white solid was recrystallized from ethanol-water (3:1) to obtain 2.7 g of a white solid. Yield 77%. mp: 232-233°C. 1 H-NMR (400MHz, DMSO) δ (ppm): 3.69 (2H, m, 5'-CH2), 3.90 (3H, m, 2', 3', 4'-CH), 5.07 (1H, d, J =5.6Hz, OH), 5.20 (1H, t, J=4.8, 5′-OH), 5.53 (1H, d, J=4.4Hz, OH), 5.81 (1H, d, J=2.8, 1′- CH), 7.35(1H, d, J=7.2Hz), 7.52(2H, t, J=7.6Hz), 7.63(1H, t, J=7.2Hz), 8.01(2H, d, J=7.2Hz) , 8.51 (1H, d, J=7.6Hz), 11.26 (1H, s, NH).
[0122] Preparation of β-D-4-N-benzoyl-3',5'-O-(tetraisopropyldisiloxane-1,3-diether...
Embodiment 14
[0138] Example 14-N-valyl-2'-methyl-2'-fluorocytidine hydrochloride (compound 12)
[0139]
[0140] Under nitrogen protection, 380mg (1.75mmol) N-tert-butoxycarbonyl valine, 473mg (3.5mmol) 1-hydroxybenzotriazole (HOBt) and 90mg (0.35mmol) 2′-methanol were added to a 10mL three-necked flask. base-2'-flucytidine. Add 5 mL of a mixed solution of anhydrous tetrahydrofuran and anhydrous N,N-dimethylformamide. At an internal temperature of 0° C., 360 mg (1.75 mmol) of dicyclohexylcarbodiimide (DCC) was added dropwise, and reacted for 1 hour, then reacted overnight at room temperature. The solvent was distilled off under reduced pressure, and 100 mL of ethyl acetate was added for dissolution, followed by washing with 50 mL of water. The organic layer was washed with 50 mL each of saturated sodium bicarbonate and saturated sodium chloride solutions. The organic layer was dried over anhydrous sodium sulfate overnight. Filtration, the mother liquor was evaporated to remove the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com