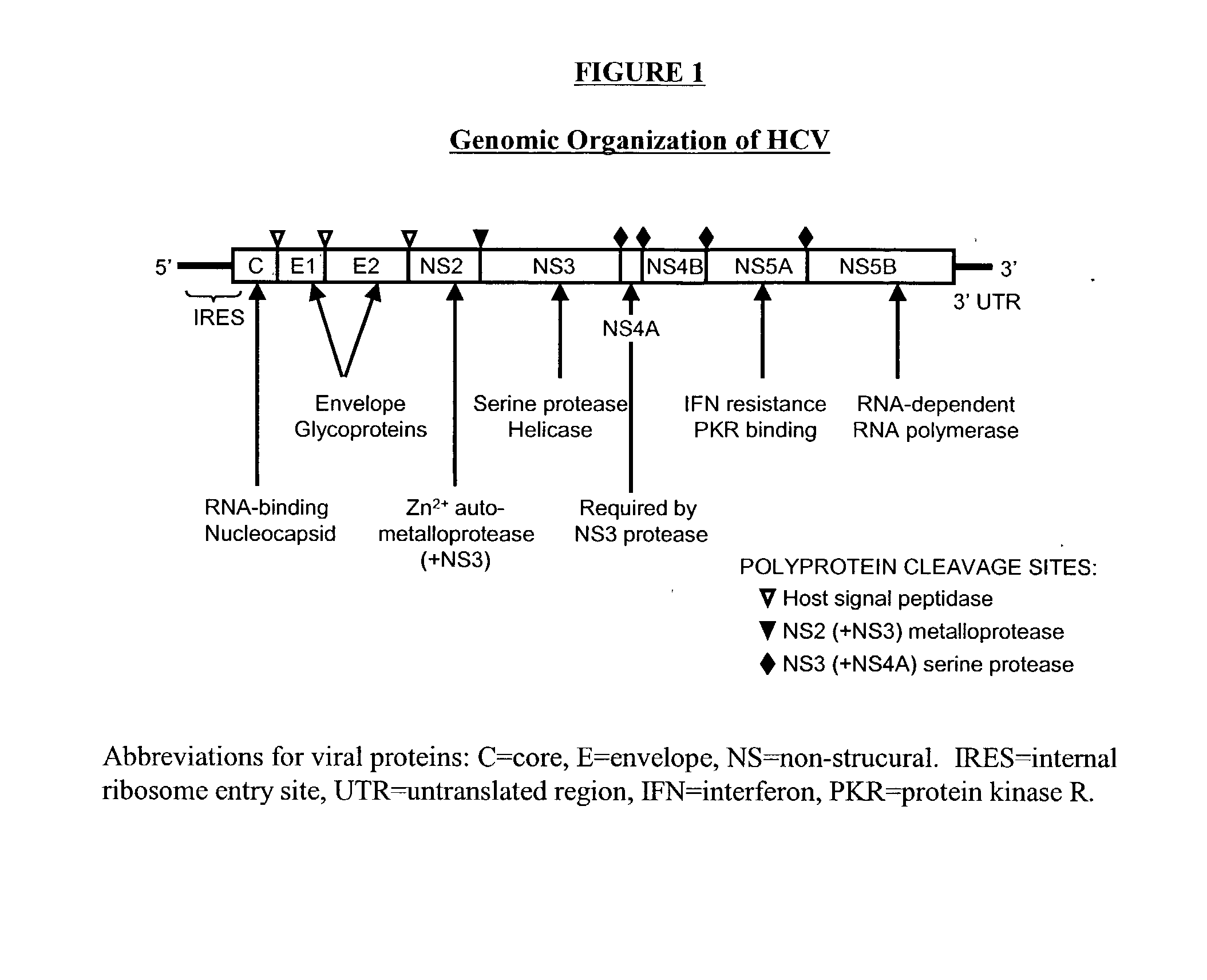
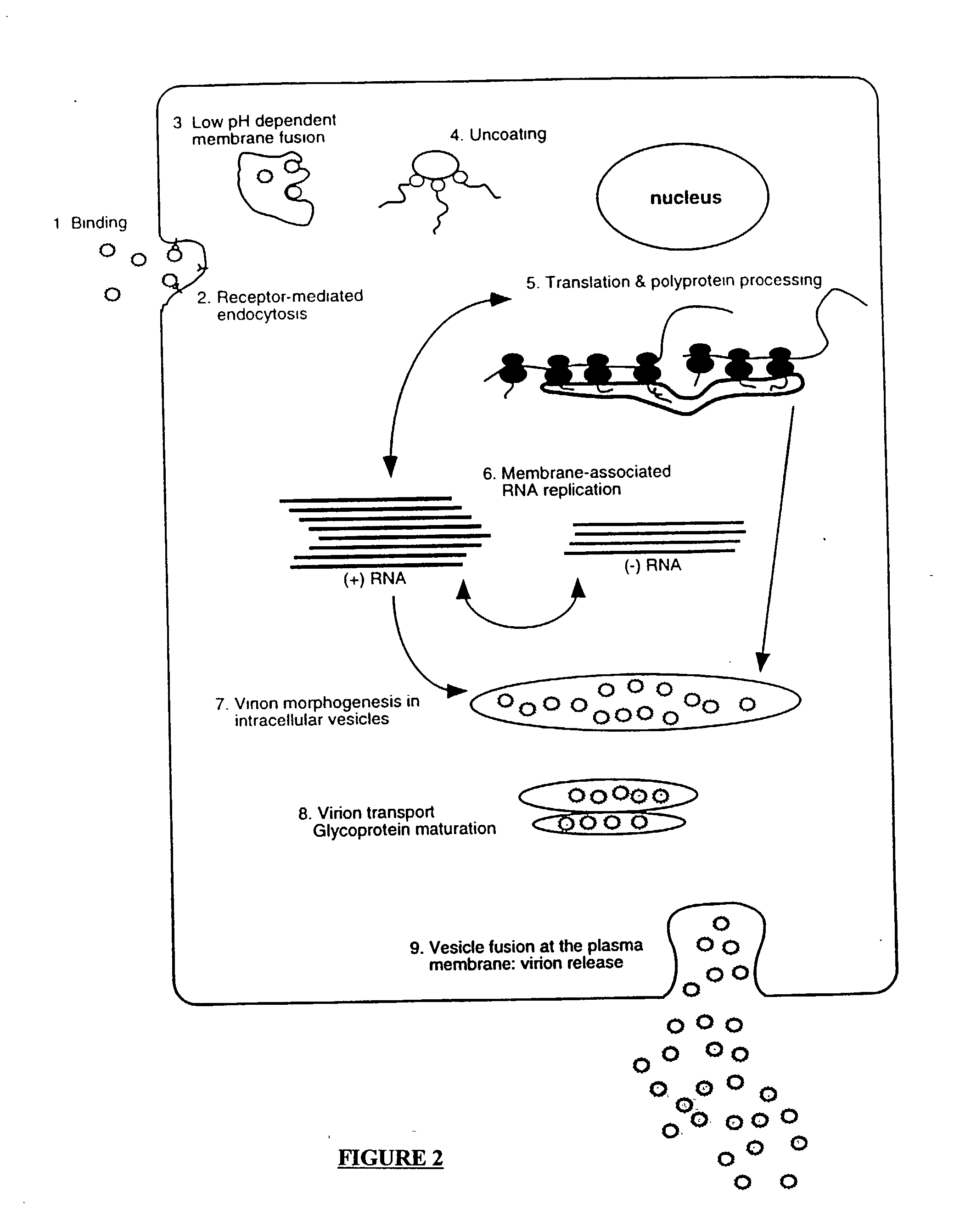
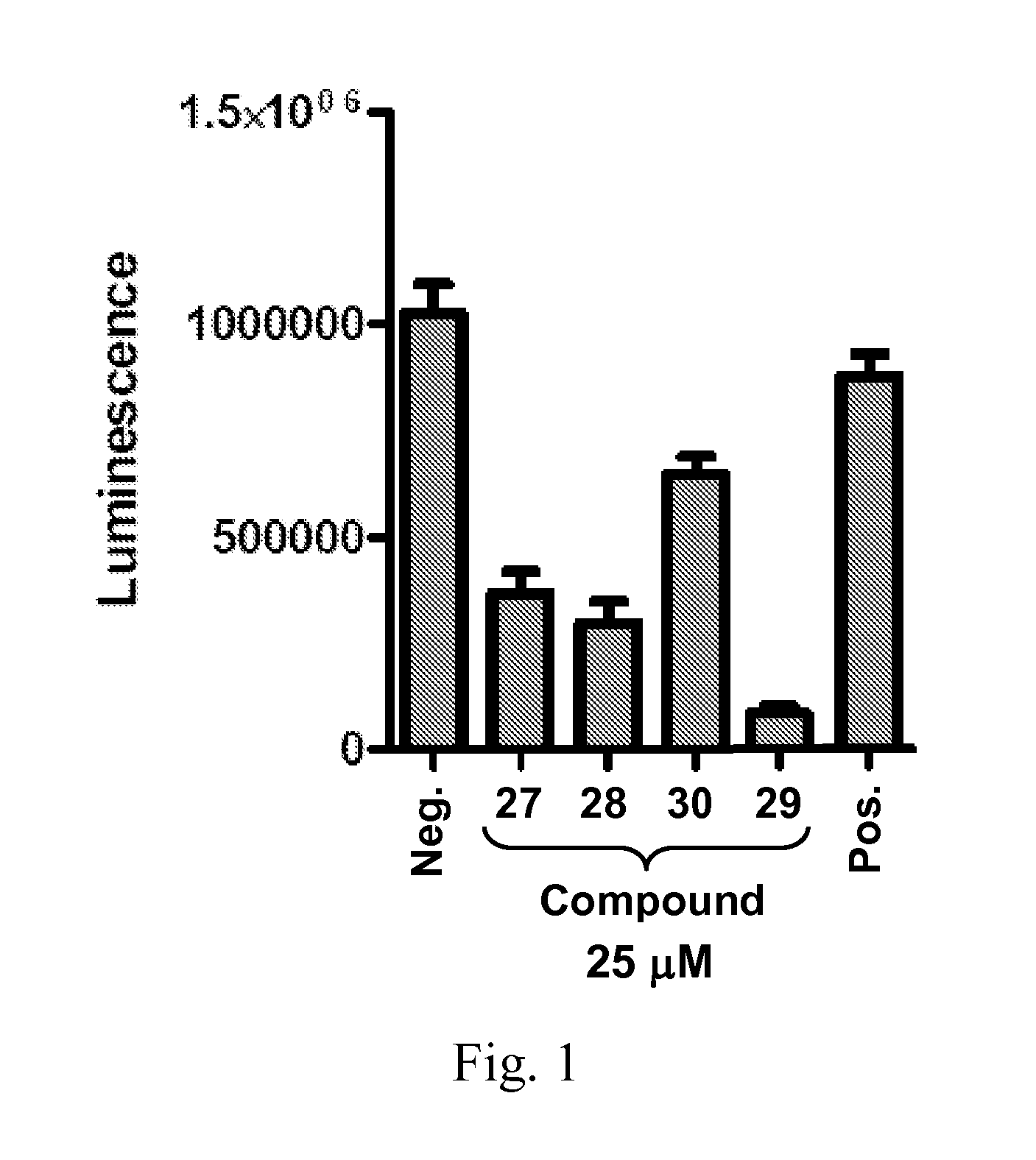
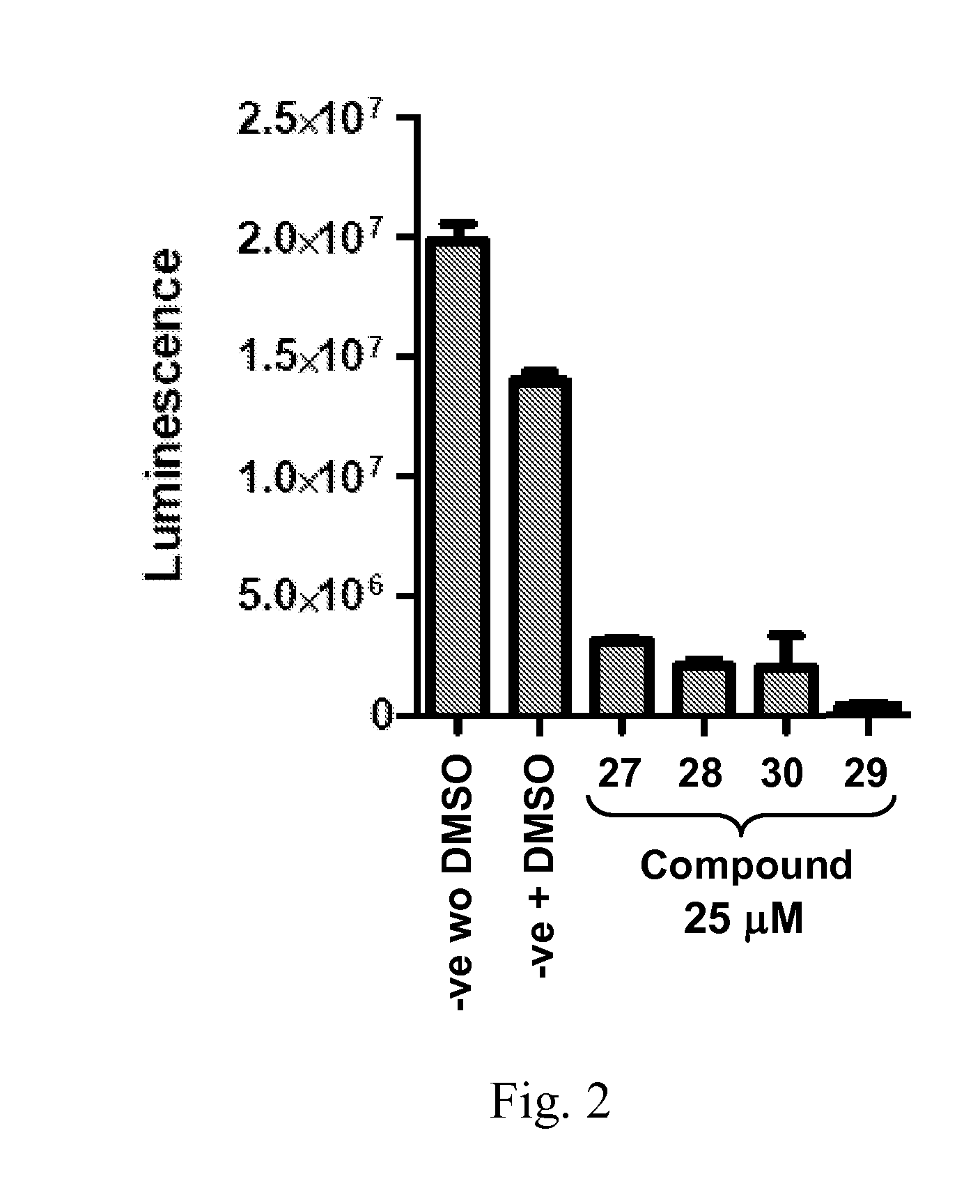
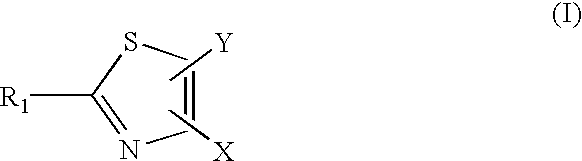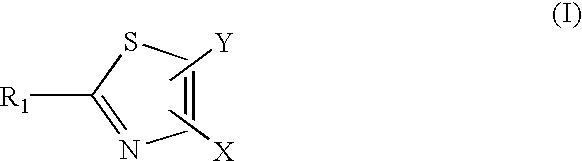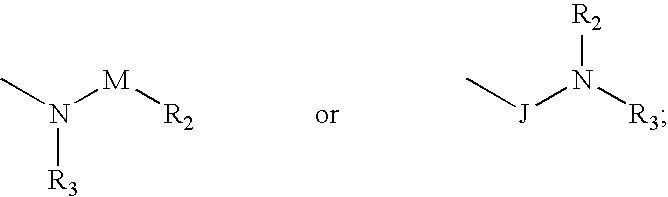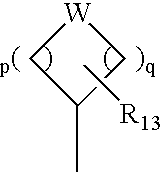Patents
Literature
134 results about "Flavivirus Infections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
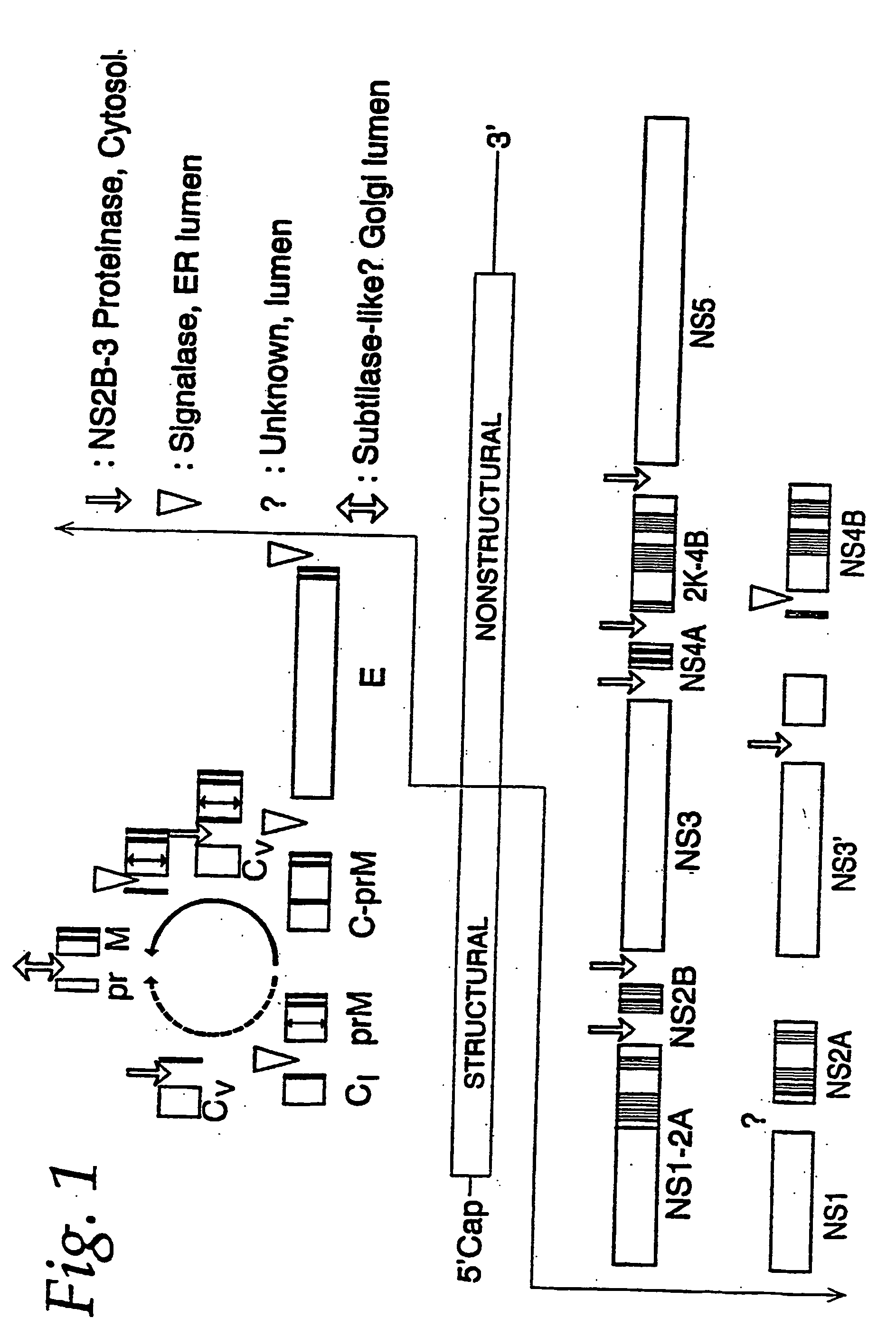
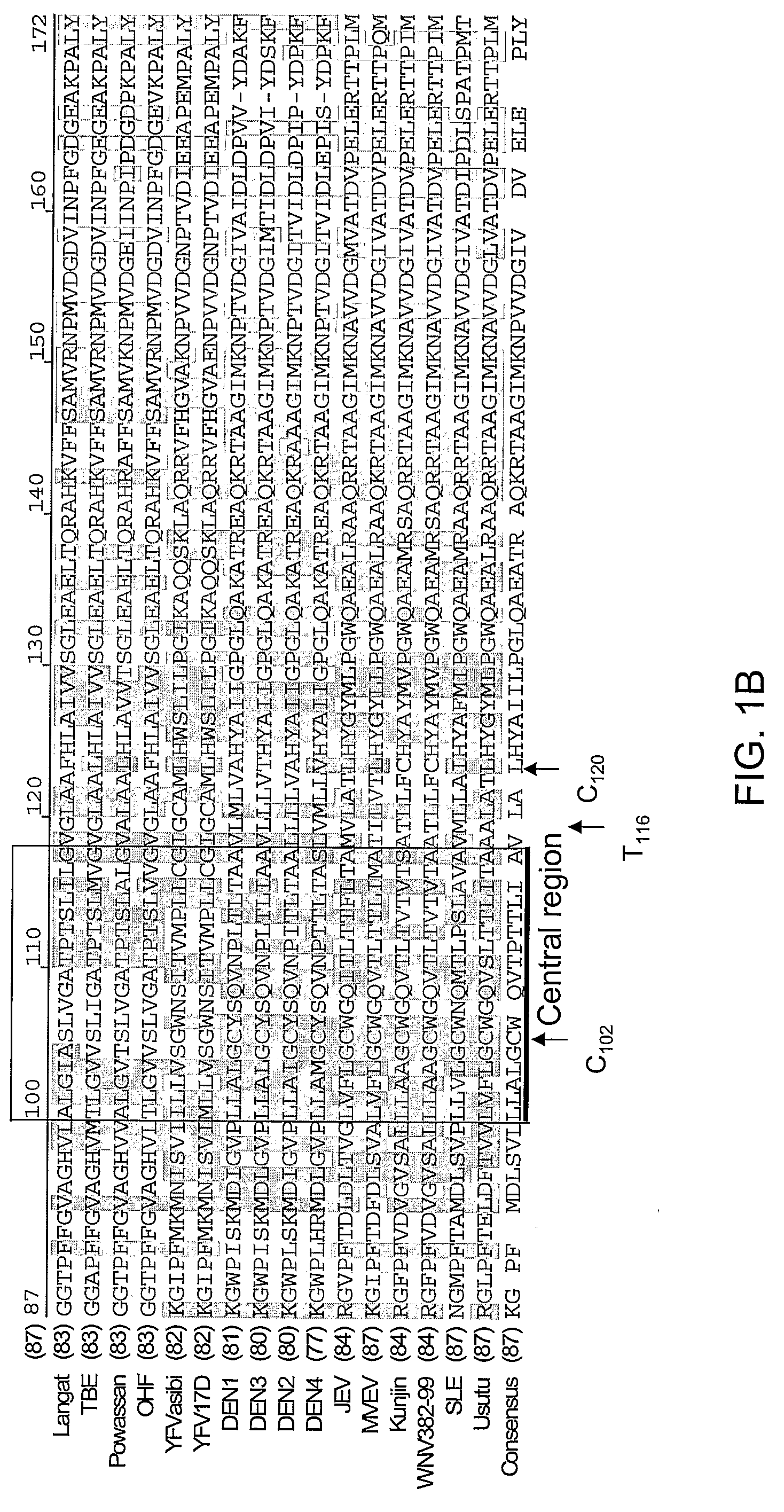
Infections with viruses of the genus FLAVIVIRUS, family FLAVIVIRIDAE.
Macrocyclic compounds as inhibitors of viral replication
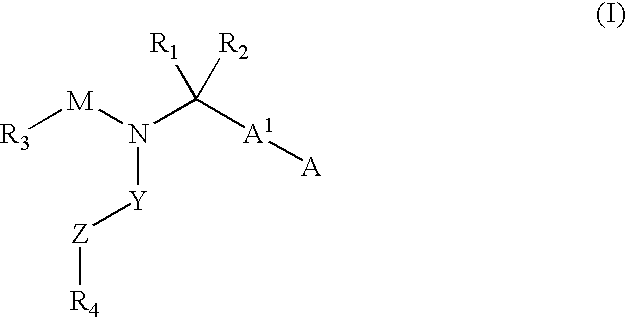
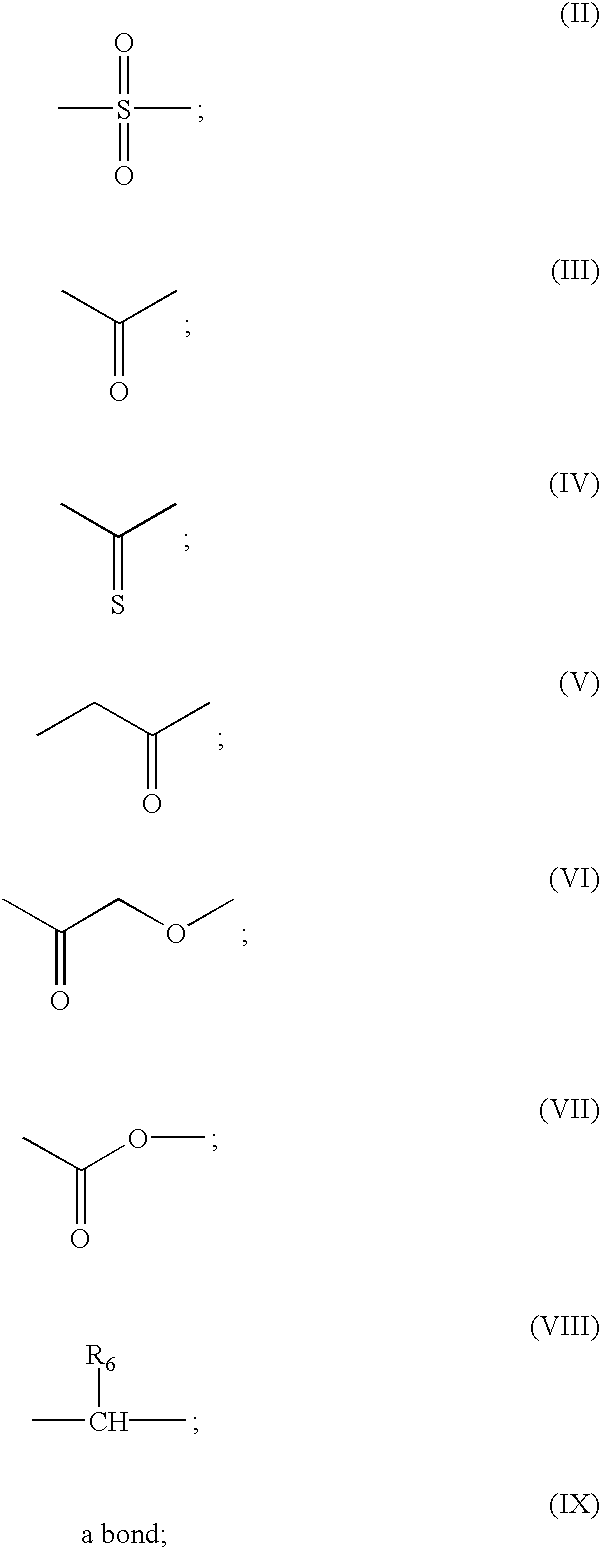
The embodiments provide compounds of the general formulas I-XIX, as well as compositions, including pharmaceutical compositions, comprising a subject compound. The embodiments further provide treatment methods, including methods of treating flaviviral infection, including hepatitis C virus infection and methods of treating liver fibrosis, the methods generally involving administering to an individual in need thereof an effective amount of a subject compound or composition.
Owner:F HOFFMANN LA ROCHE & CO AG
Macrocyclic compounds as inhibitors of viral replication
The embodiments provide compounds of the general formulas I-XIX, as well as compositions, including pharmaceutical compositions, comprising a subject compound. The embodiments further provide treatment methods, including methods of treating flaviviral infection, including hepatitis C virus infection and methods of treating liver fibrosis, the methods generally involving administering to an individual in need thereof an effective amount of a subject compound or composition.
Owner:F HOFFMANN LA ROCHE & CO AG
Compounds and methods for the treatment or prevention of Flavivirus infections
InactiveUS6881741B2Useful in therapyInhibit and reduce activityGroup 4/14 element organic compoundsBiocideMedicineViral infection
The present invention provides novel compounds represented by formula I: or pharmaceutically acceptable salts thereof useful for treating flaviviridae viral infection.
Owner:VERTEX PHARMA CANADA
Screening for west nile virus antiviral therapy
InactiveUS20050058987A1Improve efficiencySsRNA viruses positive-senseVectorsHigh-Throughput Screening MethodsImmunogenicity
The instant invention provides stable and novel lineage I WNV reverse genetics systems, and methods for making the reverse genetics systems, specifically, a fully-infectious lineage I WNV cDNA or replicon system engineered with one or more nucleotide sequences each encoding a reporter gene to be used in high throughput cell-based screening assays for the identification of novel antiflaviviral chemotherapeutics and / or vaccines effective to treat and / or immunize against infections by WNV and other emerging flaviviruses, such as, for example, JEV, SLEV, AV, KV, JV, CV, YV, TBEV, DENV-1, DENV-2, DENV-3, DENV-4, YFV and MVEV. The present invention further provides methods of high throughput screening of antiflaviviral compounds or improved derivatives thereof using novel lineage I WNV reverse genetics systems and / or cell lines stably containing the reverse genetics systems. Also, the invention provides novel pharmaceutical compositions comprising an attenuated lineage I WNV that is less virulent but similarly immunogenic as the parent WNV and is capable of providing a protective immune response in a host.
Owner:HEALTH RES INC
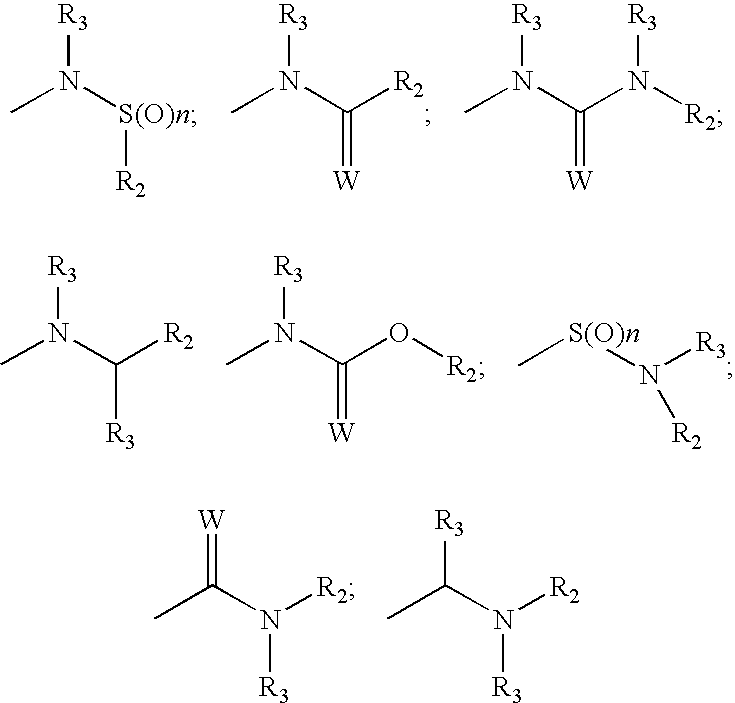
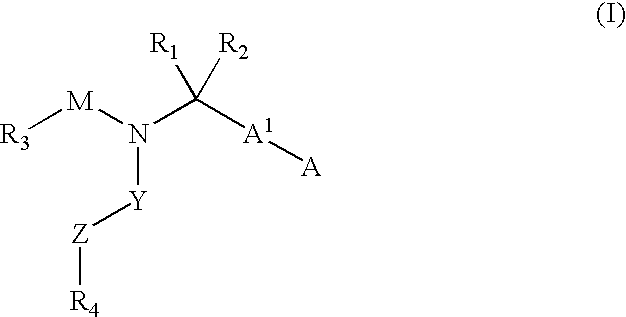
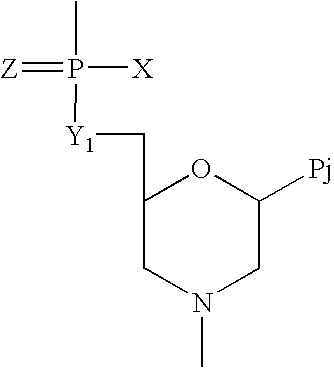
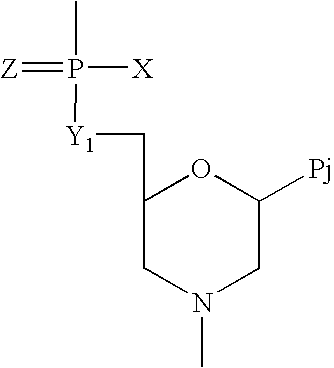
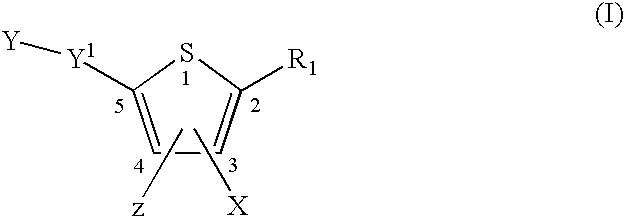
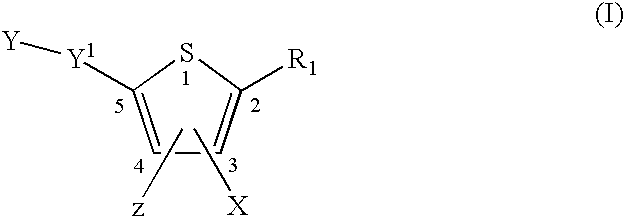
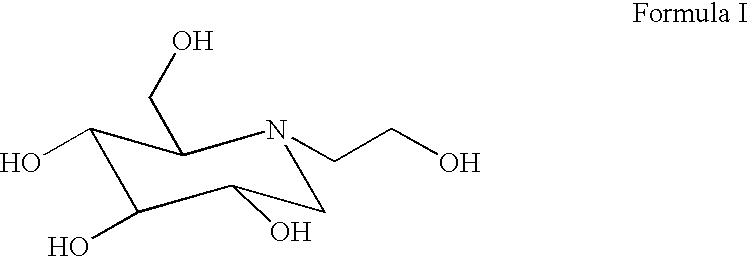
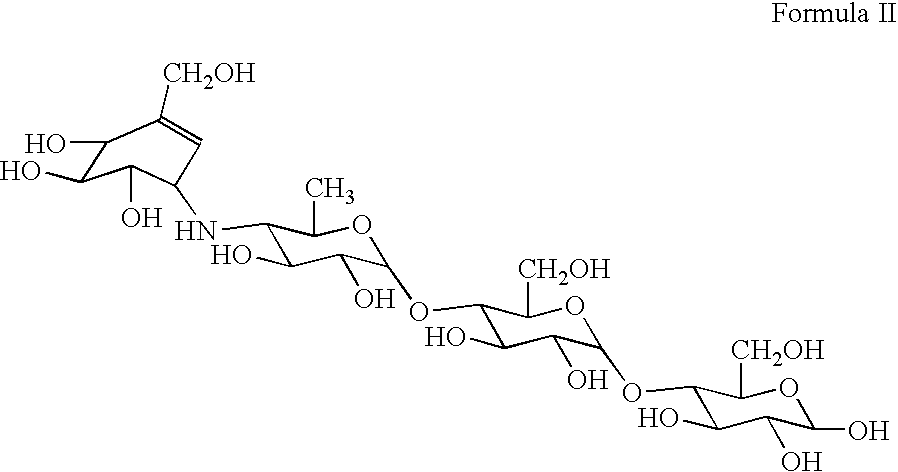
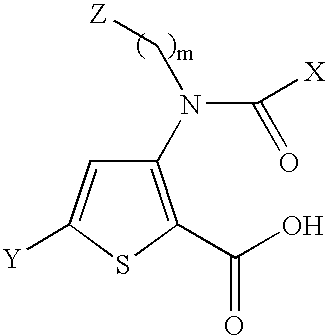
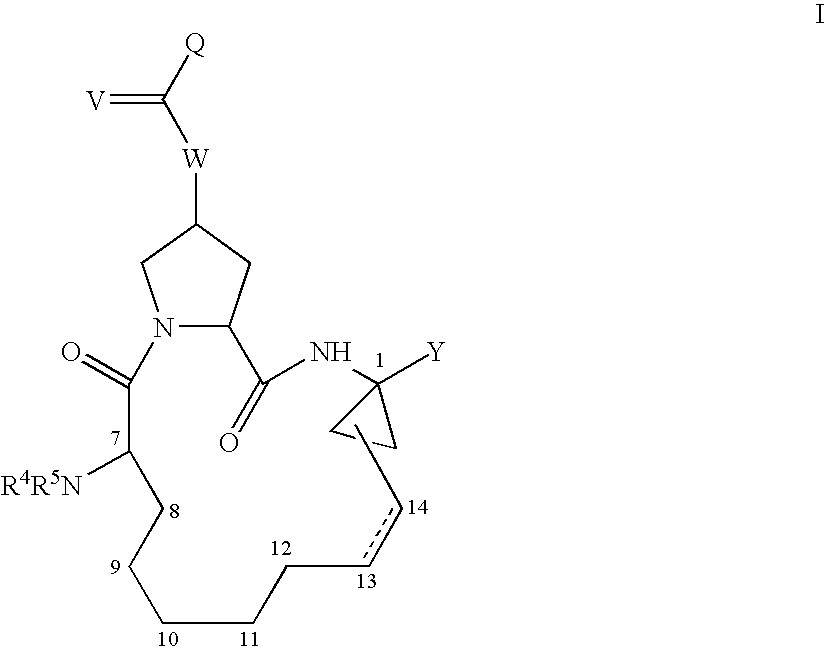
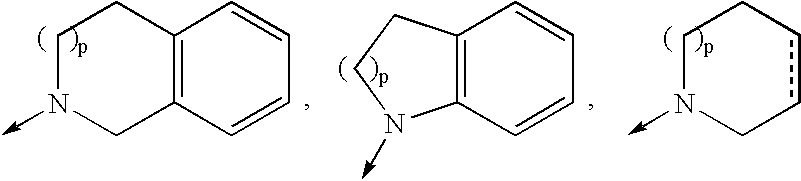
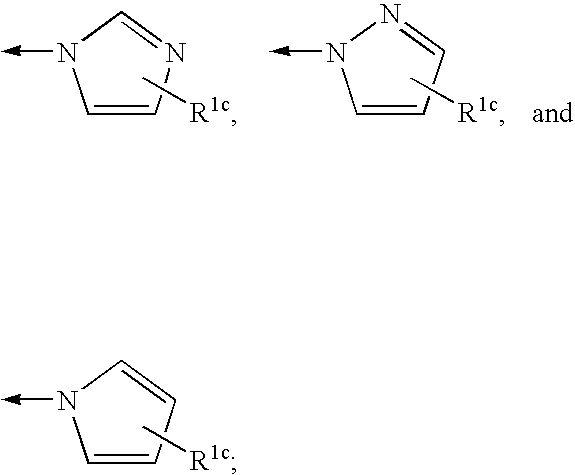
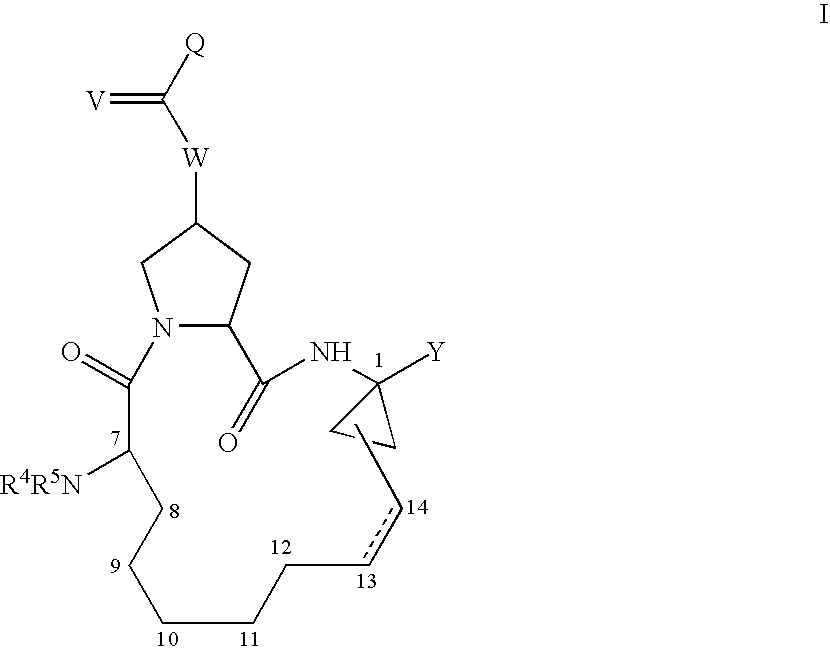
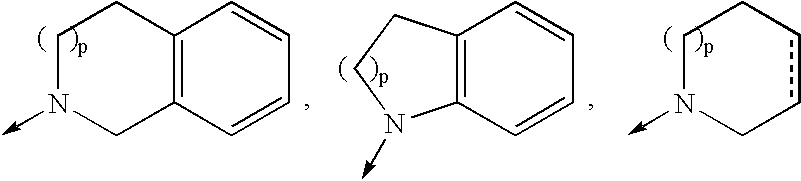
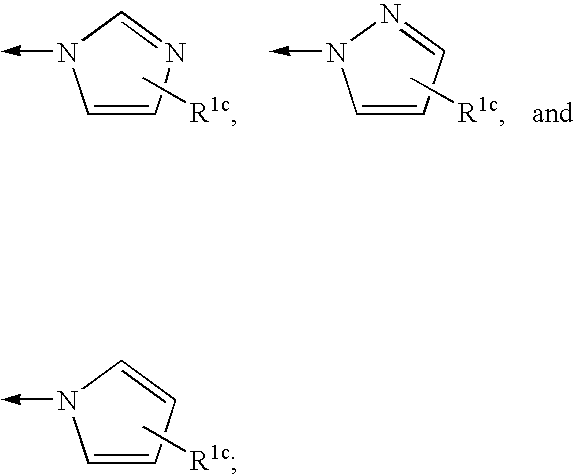
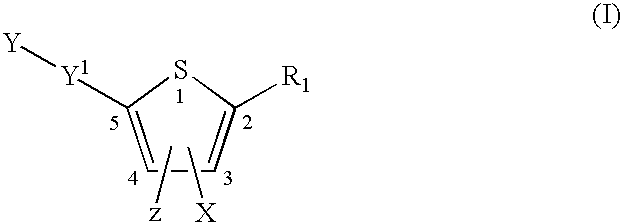
Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections
InactiveUS20040082574A1Potent and selective activityPotent activityBiocideSugar derivativesPestivirusMedicine
The disclosed invention is a bicyclo[4.2.1]nonane and its pharmaceutically acceptable salt or prodrug, and its composition and method of use to treat Flaviviridae (Hepacivirus, Flavivirus, and Pestivirus) infections in a host, including animals, and especially humans.
Owner:PHARMASSET
Compounds and methods for the treatment or prevention of flavivirus infections
The present invention provides novel compounds represented by formula I: or pharmaceutically acceptable salts thereof useful for treating flaviviridae viral infection.
Owner:VIROCHEM PHARMA INC (CA)
Compounds and methods for the treatment or prevention of Flavivirus infections
The present invention provides novel compounds represented by formula I: or pharmaceutically acceptable salts thereof useful for treating Flaviviridae viral infection.
Owner:VIROCHEM PHARMA INC (CA)
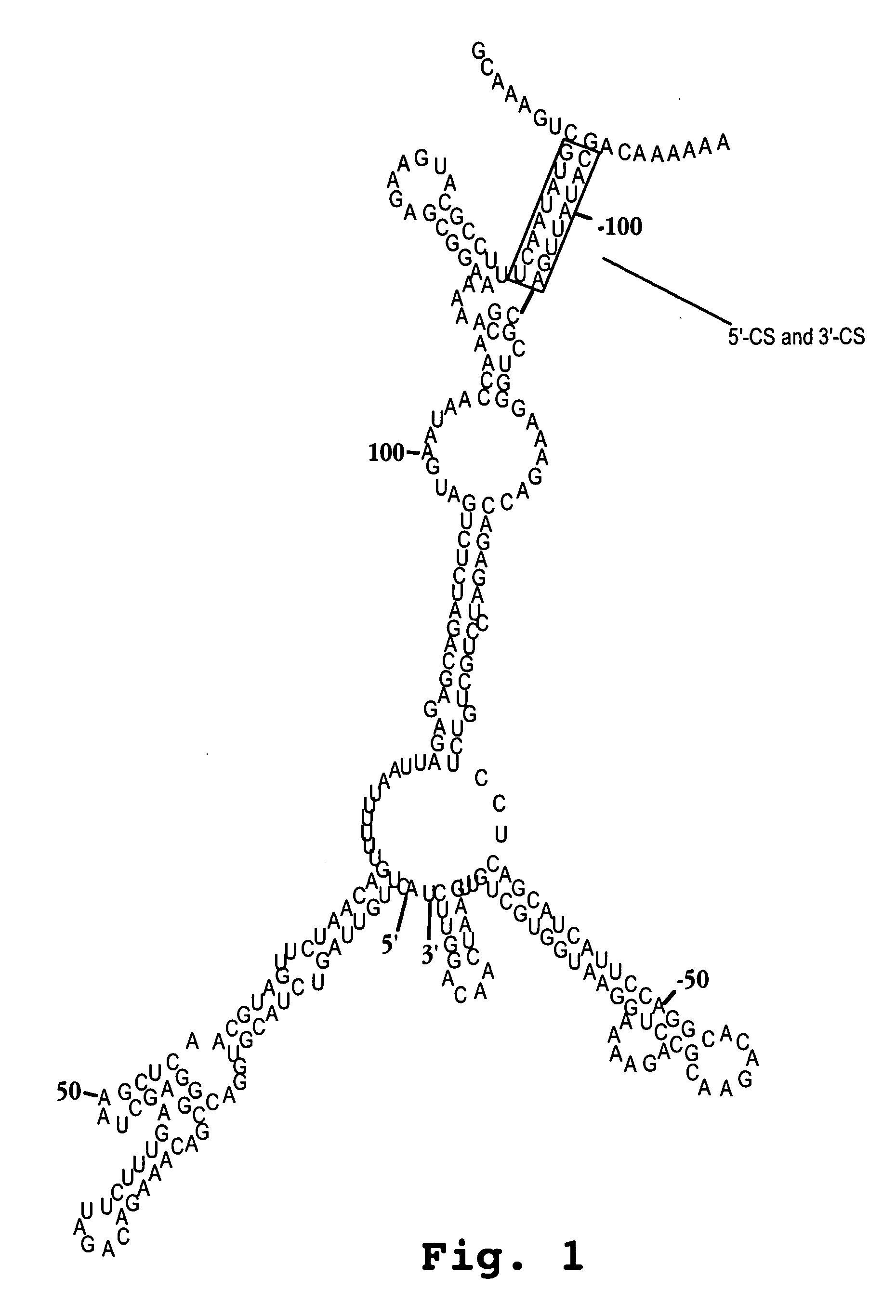
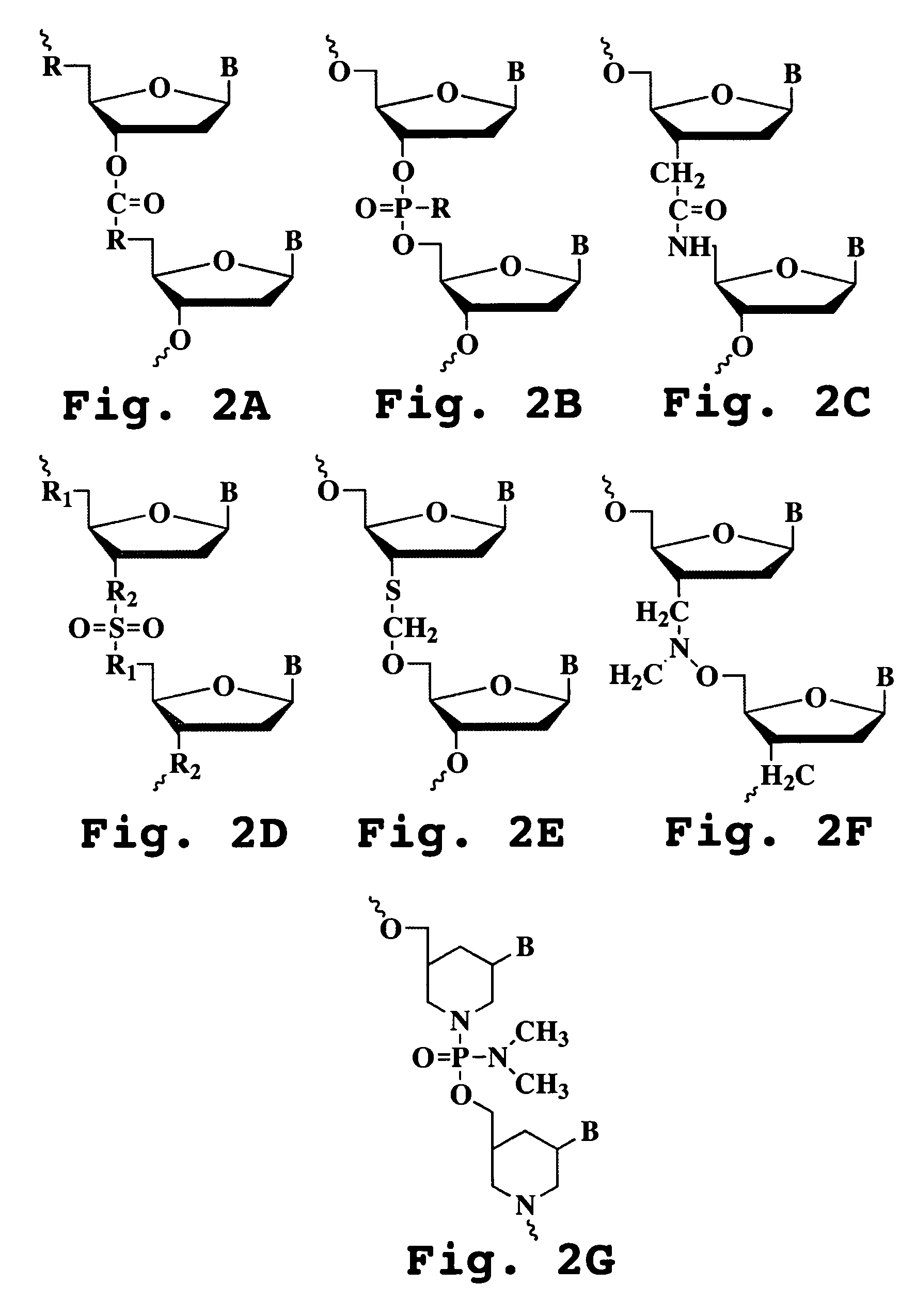
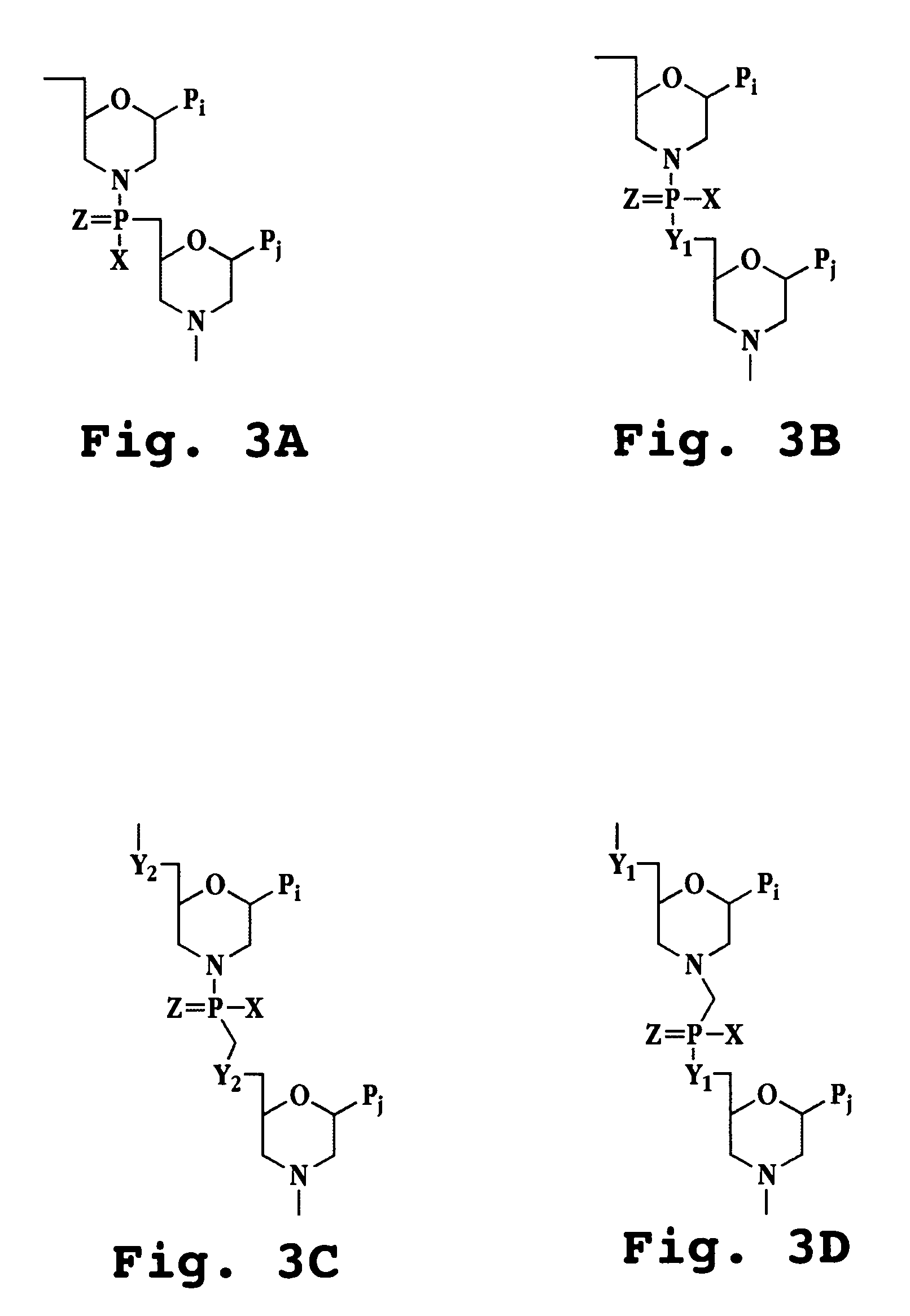
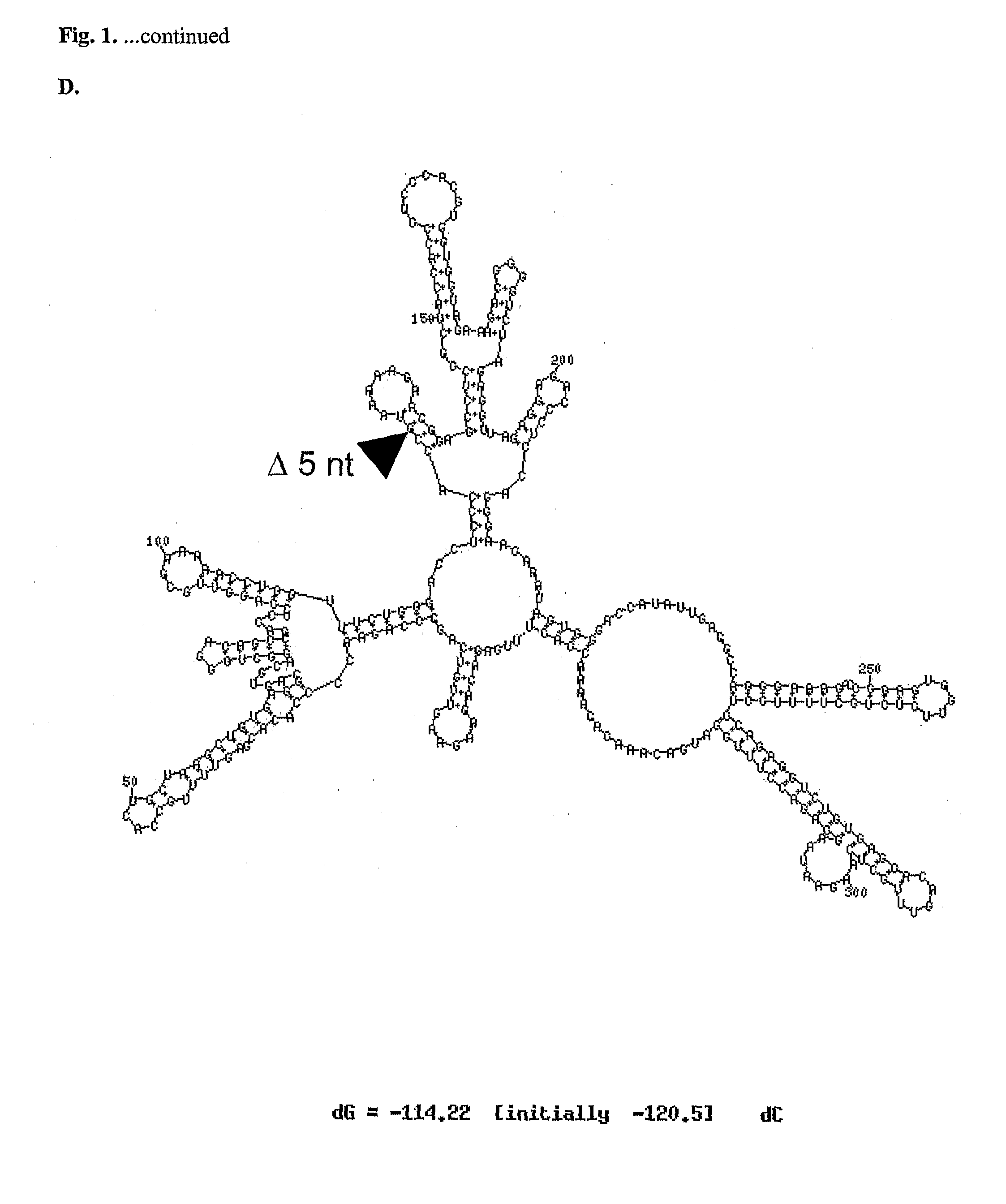
Oligonucleotide analog and method for treating flavivirus infections
InactiveUS20050096291A1Inhibition of replicationSugar derivativesMicrobiological testing/measurementHeterologousHeteroduplex
A method of inhibiting replication of a flavivirus in animal cells, and an oligonucleotide compound for use in the method are disclosed. The oligonucleotide analog (i) has a nuclease-resistant backbone, (ii) is capable of uptake by the cells, (iii) contains between 8-40 nucleotide bases, and (iv) has a sequence of at least 8 bases complementary to a region of the virus' positive strand RNA genome that includes at least a portion of SEQ ID NOS:1-4. Exposure of cells infected with a flavivirus to the analog is effective to form within the cells, a heteroduplex structure composed of the virus ssRNA and the oligonucleotide, characterized by a Tm of dissociation of at least 45 ° C., and having disrupted base pairing between the virus' 5′ and 3′ cyclization sequences.
Owner:SAREPTA THERAPEUTICS INC
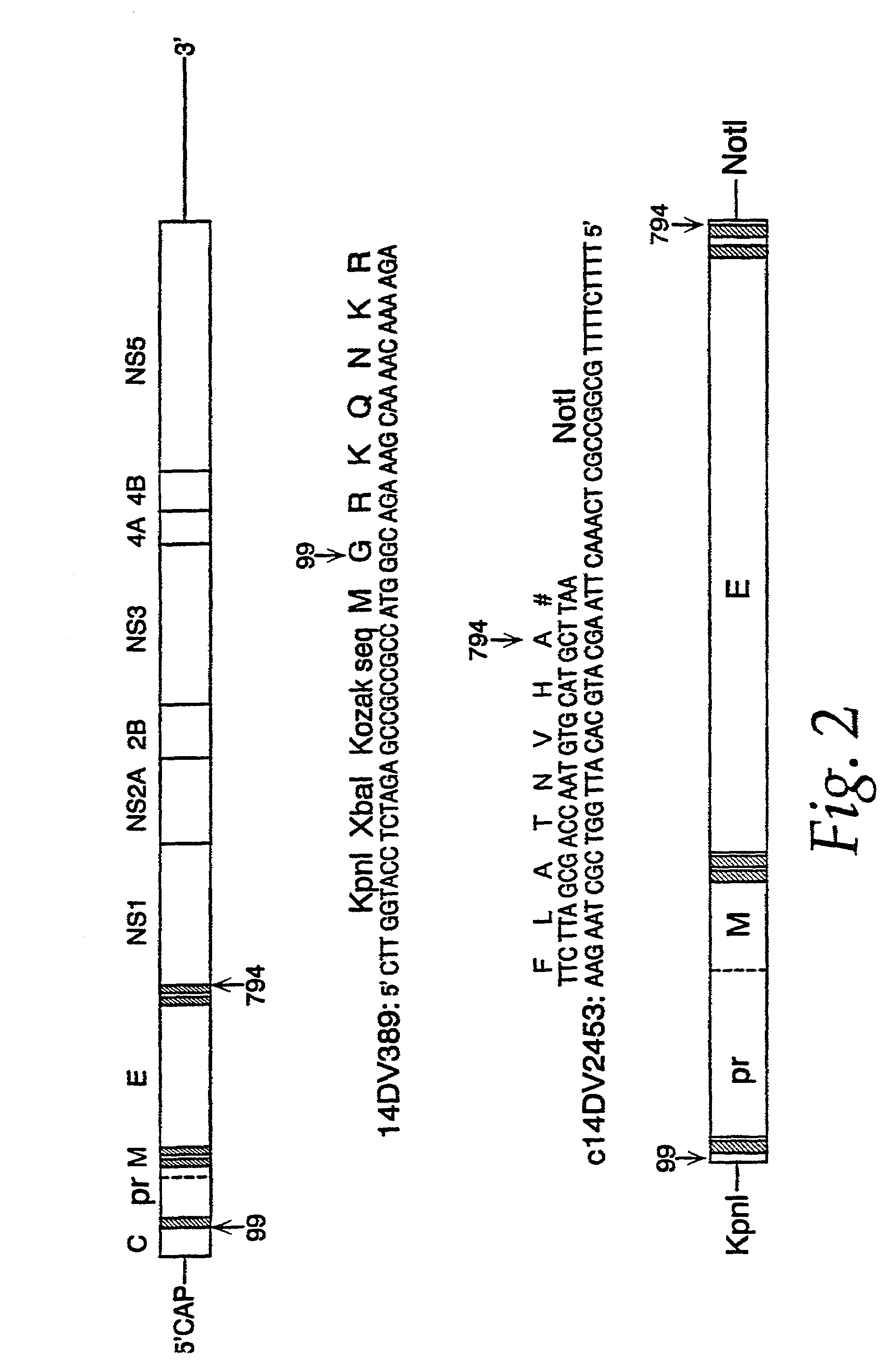
Nucleic acid vaccines for prevention of flavivirus infection
InactiveUS7227011B2Improve translationLarge structureOrganic active ingredientsFungiImmunogenicityVirology
The present invention encompasses isolated nucleic acids containing transcriptional units which encode a signal sequence of one flavivirus and an immunogenic flavivirus antigen of a second flavivirus. The invention further encompasses a nucleic acid and protein vaccine and the use of the vaccine to immunize a subject against flavivirus infection. The invention also provides antigens encoded by nucleic acids of the invention, antibodies elicited in response to the antigens and use of the antigens and / or antibodies in detecting flavivirus or diagnosing flavivirus infection.
Owner:HEALTH & HUMAN SERVICES THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC +1
Compounds and methods for the treatment or prevention of Flavivirus infections
InactiveUS20060142347A1Inhibit and reduce activityBiocideGroup 4/14 element organic compoundsMedicineViral infection
The present invention provides novel compounds represented by formula I: or pharmaceutically acceptable salts thereof useful for treating flaviviridae viral infection.
Owner:VERTEX PHARMA CANADA
Oligonucleotide analog and method for treating flavivirus infections
Owner:SAREPTA THERAPEUTICS INC
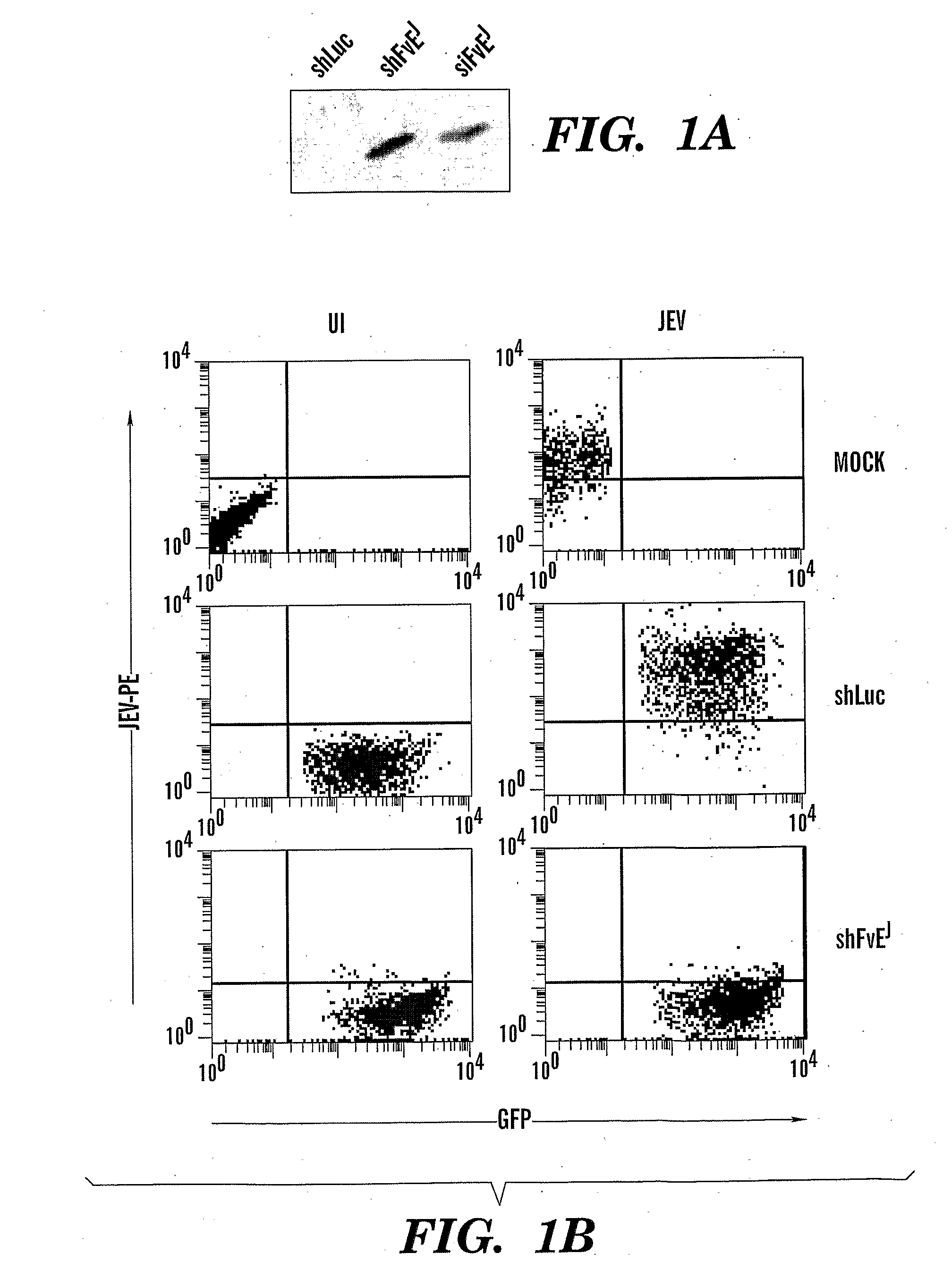
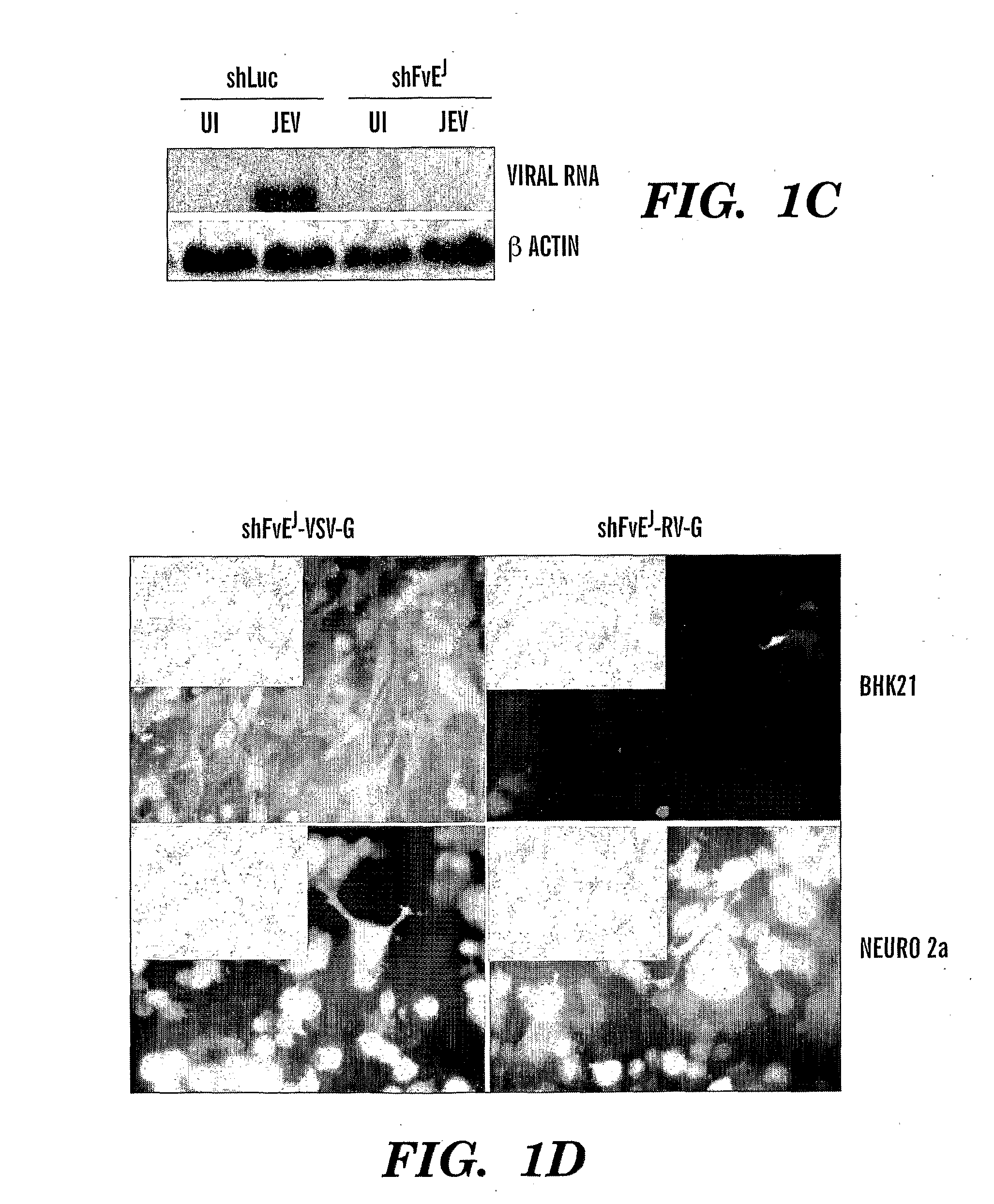
Method to Treat Flavivirus Infection with siRNA
InactiveUS20090047338A1Avoid infectionInhibit expressionOrganic active ingredientsSsRNA viruses positive-senseAntisense RNADisease
The present invention is directed to methods of treating flavivirus mediated diseases using siRNAs. The invention is based upon our findings in a mouse model that siRNAs directed against sequences conserved among multiple flaviviruses prevents and treats flavivirus infections. Accordingly, the present invention provides an isolated siRNA comprising a sense RNA and an antisense RNA strand or a single strand. The sense and the antisense RNA strands, or the single RNA strand, form an RNA duplex, and wherein the RNA strand comprises a nucleotide sequence identical to a target sequence of about 15 to about 30 contiguous nucleotides in flavivirus mRNA or mutant or variant thereof.
Owner:IMMUNE DISEASE INST INC
Nucleic acid vaccines for prevention of flavivirus infection
InactiveUS20050163804A1Easy to prepareEasy to manageOrganic active ingredientsFungiImmunogenicityViral infection
The present invention encompasses isolated nucleic acids containing transcriptional units which encode a signal sequence of one flavivirus and an immunogenic flavivirus antigen of a second flavivirus or of a chimeric immunogenic flavivirus antigen comprising sequence from more than one flavivirus. The invention further encompasses a nucleic acid and protein vaccine and the use of the vaccine to immunize a subject against flavivirus infection. The invention also provides antigens encoded by nucleic acids of the invention, antibodies elicited in response to the antigens and use of the antigens and / or antibodies in detecting flavivirus or diagnosing flavivirus infection.
Owner:THE GOVERNMENT OF THE US SEC THE DEPT OF HEALTH & HUMAN SERVICE CENTS FOR DISEASE CONTROL & PREVENTION
Flavivirus host range mutations and uses thereof
ActiveUS20110236421A1Easy to understandSsRNA viruses positive-senseSugar derivativesVaccinationNucleotide sequencing
Methods and compositions concerning mutant flaviviruses with host range mutations. In some embodiments the invention concerns nucleotide sequences that encode mutant flavivirus proteins. Viruses comprising these sequences that display reduced replication in mammalian cells are provided. In further aspects of the invention, flavivirus vaccine compositions are provided. In another embodiment the invention provides methods for vaccination against flavivirus infection.
Owner:ARBOVAX +1
Detection of mutations in a gene associated with resistance to viral infection, OAS2 and OAS3
Compositions and methods are provided for detecting a mutation in a human oligoadenylate synthetase gene, particularly OAS2 or OAS3, wherein the mutation confers resistance to flavivirus infection, including infection by hepatitis C virus, and the mutation relates to other disease states including prostate cancer and diabetes, and uses of the encoded proteins and antibodies thereto.
Owner:IB SECURITYHOLDERS
Method to block the infection by flaviviruses, molecules and uses
ActiveUS20110212105A1Modulate the DV infectionReducing and increasing interactionSsRNA viruses positive-sensePeptide/protein ingredientsMolecular biologyCarrier protein
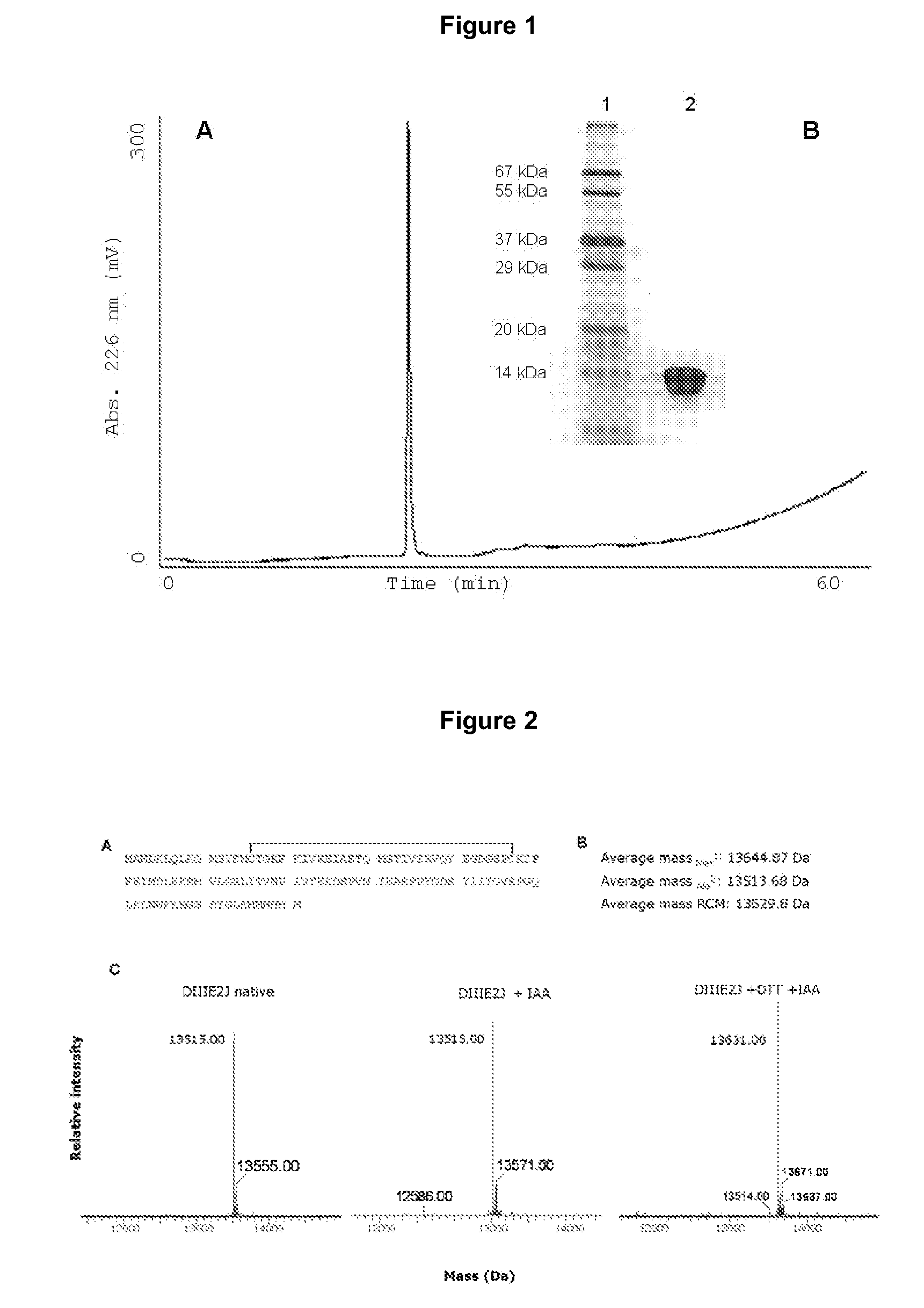
The present invention is related to a method for blocking the infection of cells by dengue virus, based on interfering the direct interaction of the viral envelope protein with a cellular receptor or its indirect interaction with said cellular receptor through a carrier protein, as well as related uses; wherein said cellular receptor is the alpha-2 macroglobulin receptor, also known as the low density receptor-related protein or as CD91, and said carrier protein is human alpha-2 macroglobulin.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Nucleic acid vaccines for prevention of flavivirus infection
InactiveUS7417136B1Easy to administerEasy to prepareOrganic active ingredientsVirusesActive agentEncephalitis Viruses
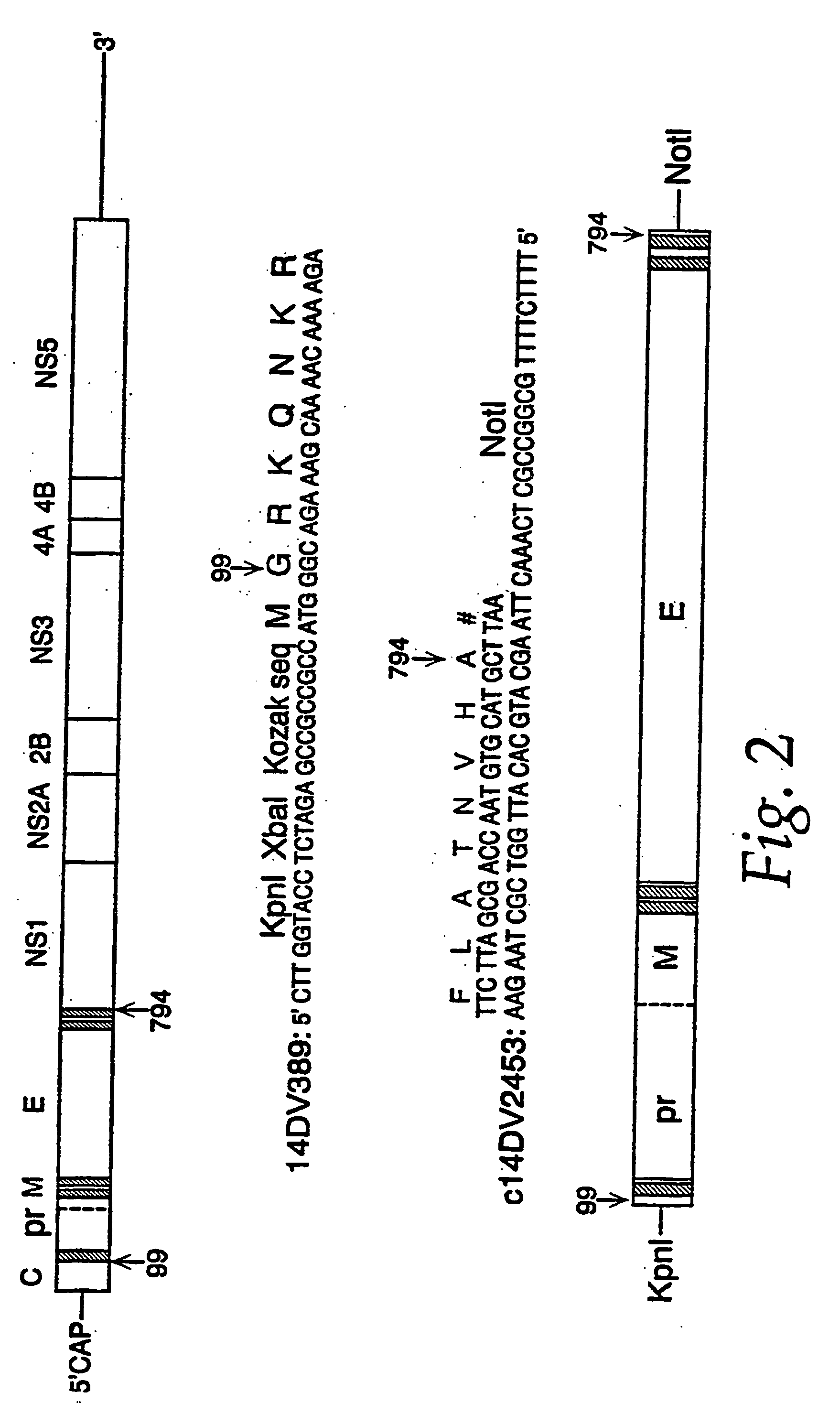
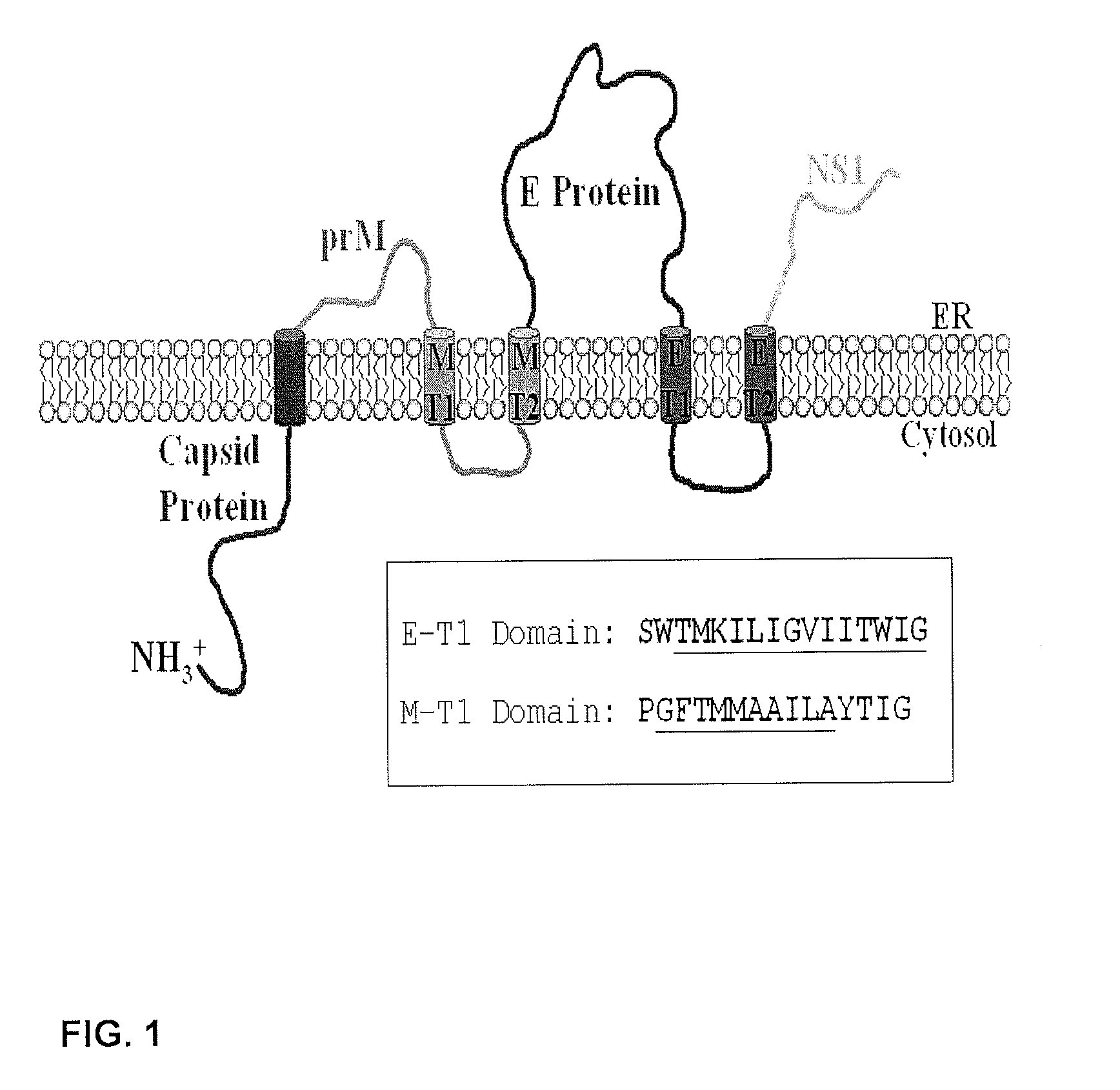
The invention encompasses nucleic acid molecules containing transcription units which encode the flavivirus M and E protein antigens. The flaviviruses include Japanese encephalitis virus, dengue, yellow fever virus and St. Louis encephalitis virus. The nucleic acids function to provide the M and E protein antigens when the nucleic acid resides in an appropriate host cell, especially when the host cell is the cell of a subject. The invention also encompasses a vaccine whose active agent is the nucleic acid. The invention further encompasses the cultured host cells when they contain within them nucleic acid molecules containing the transcription units. The invention in addition encompasses a method of immunizing a subject against flavivirus infection by administering to the subject an effective amount of a vaccine containing a nucleic acid molecule containing the transcription unit of the invention.
Owner:HEALTH & HUMAN SERVICES DEPT OF UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC THE
Flavivirus vaccine
PendingUS20210069315A1Eliminate and prevent growthStrong attackSsRNA viruses positive-senseViral antigen ingredientsDiseaseImmunogenicity
The present invention is directed to an artificial nucleic acid and to a polypeptide suitable for use in the treatment or prophylaxis of an infection with a flavivirus, in particular an infection with yellow fever virus or with dengue virus, or of a disorder related to such an infection. The present invention is also directed to a composition, preferably an immunogenic composition, comprising the artificial nucleic acid or the inventive polypeptide. In particular, the present invention concerns an immunogenic composition against a flavivirus, such as yellow fever virus or dengue virus. Further, the invention concerns a kit, particularly a kit of parts, comprising the artificial nucleic acid, polypeptide or (immunogenic) composition. The invention is further directed to a method of treating or preventing a disorder or a disease, first and second medical uses of the artificial nucleic acid, polypeptide, composition, in particular the first and second medical uses of the immunogenic composition according to the invention.
Owner:CUREVAC SE +1
Preparation and application of beta-D-(2'R)-2'-deoxy-2'-fluoro-2'-C-methylcytidine derivatives
The invention relates to compounds as represented by the general formula (A) or their hydrates, solvates or medicinal salts formed with acids, a preparation method thereof, compositions containing the same and application of the same in treating flavivirus infections (such as hepatitis c, West Nile disease and Dengue fever), especially in treating hepatitis c virus (HCV) infected disease. X, Y and Z in the general formula (A) are defined by the specification.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
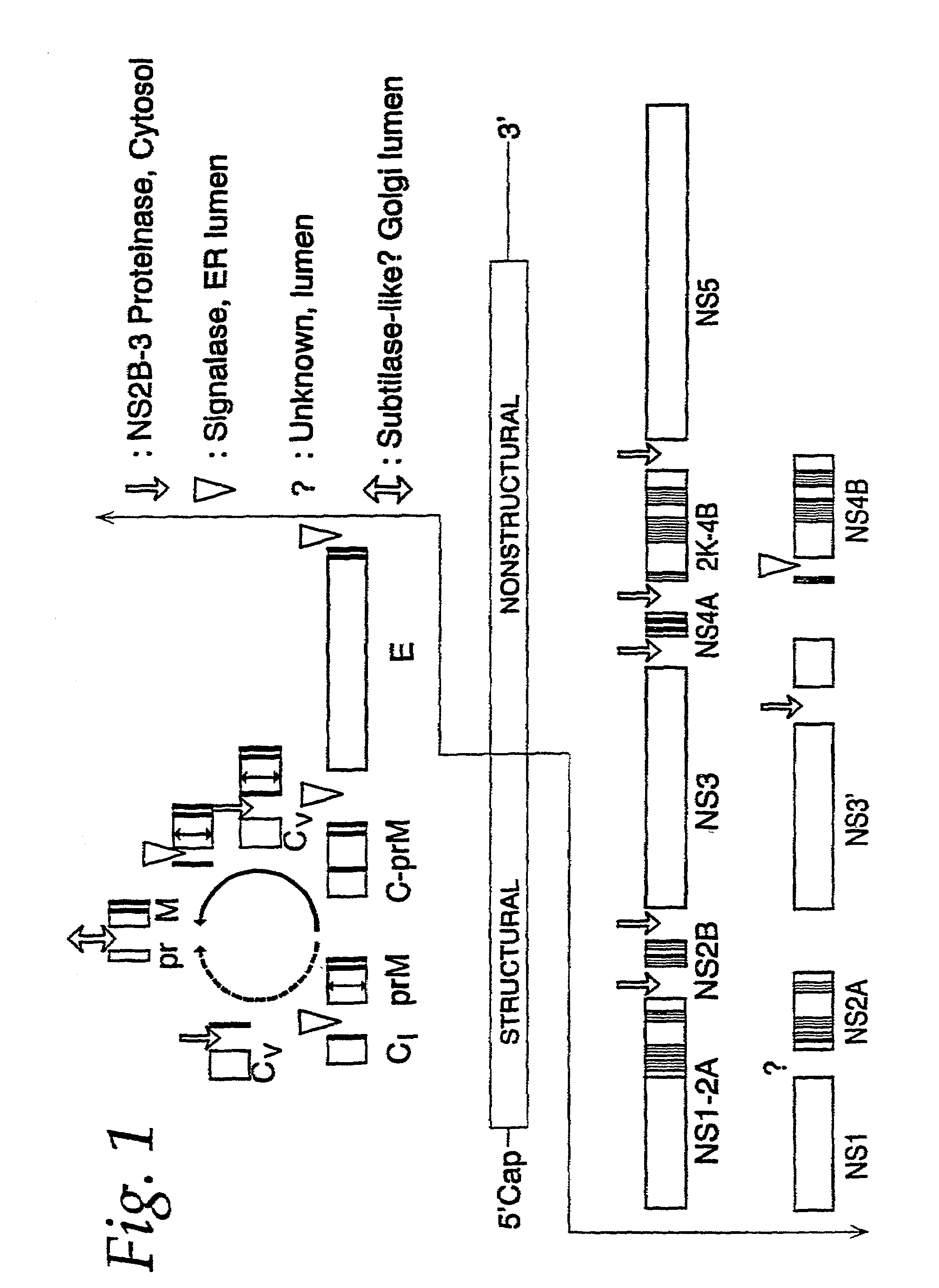
Methods of inhibiting viral replication comprising the signal peptidase complex
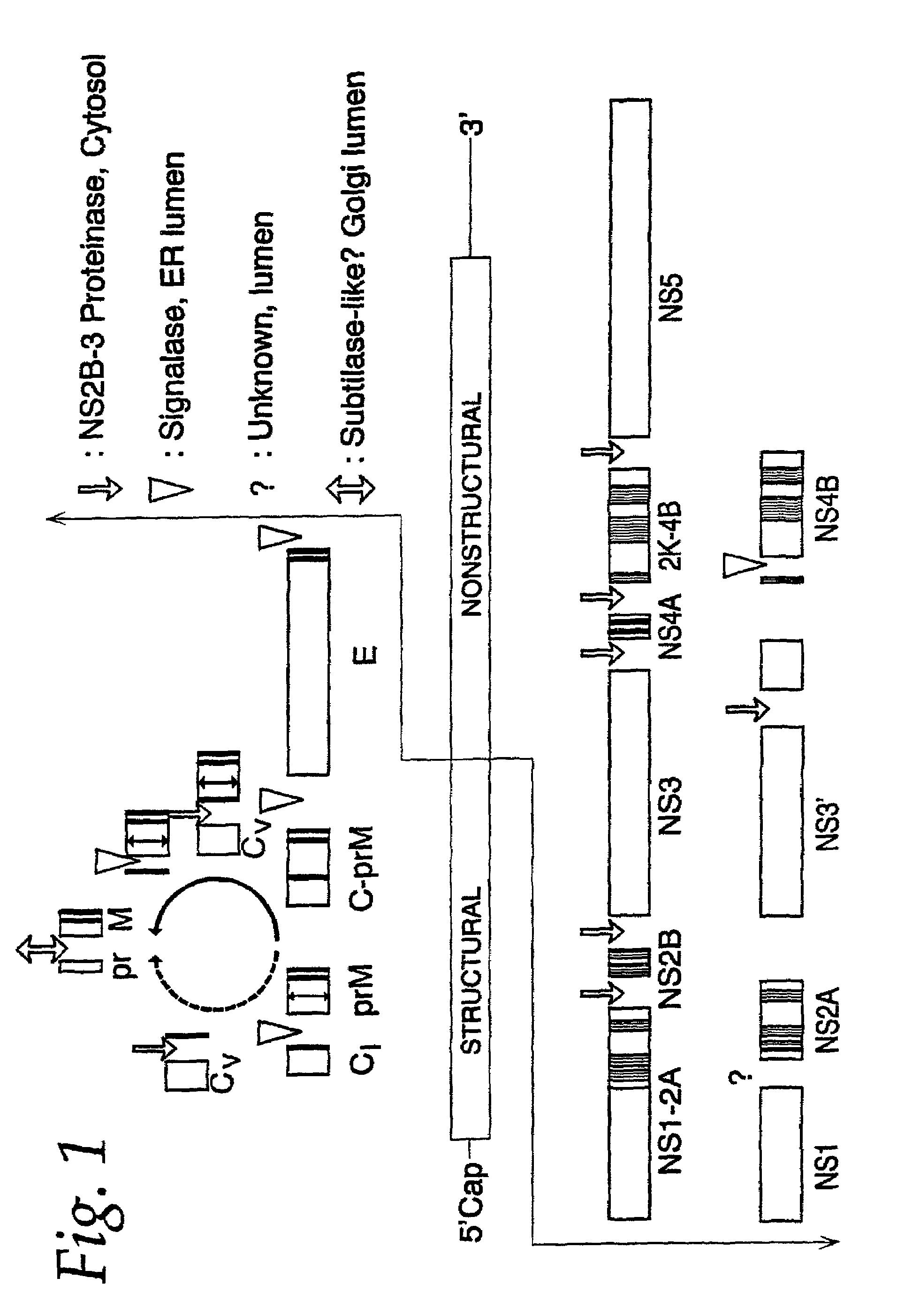
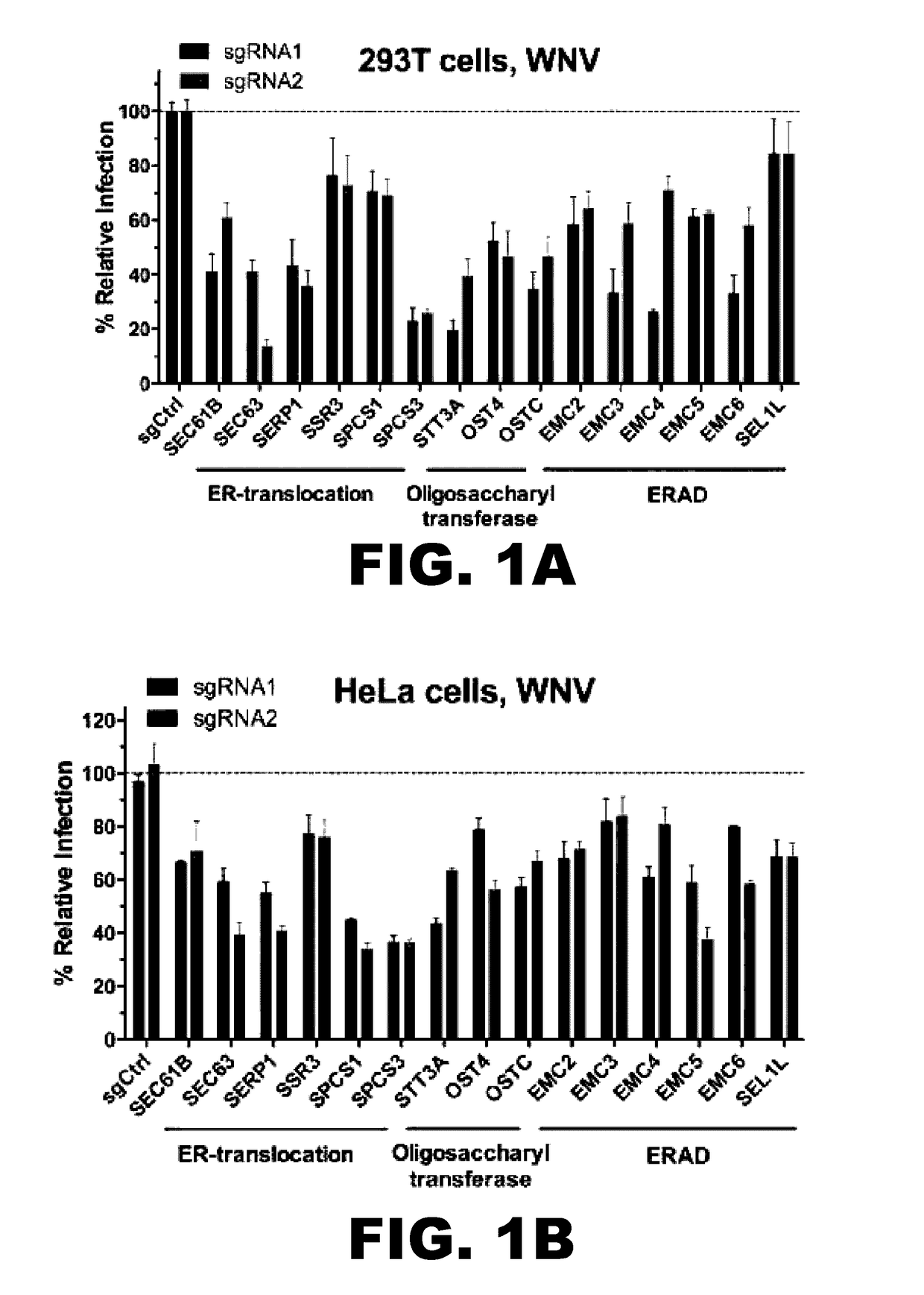
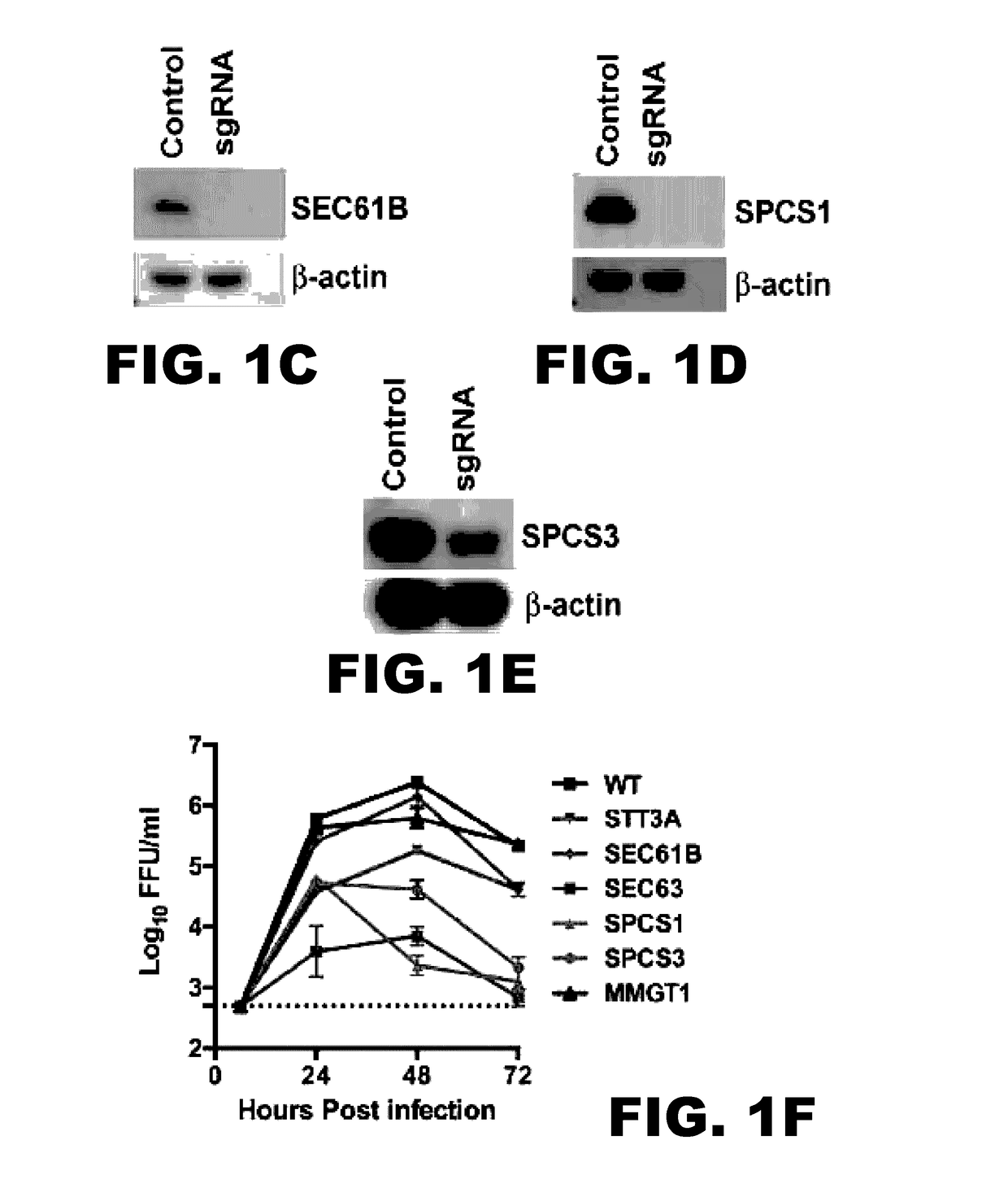
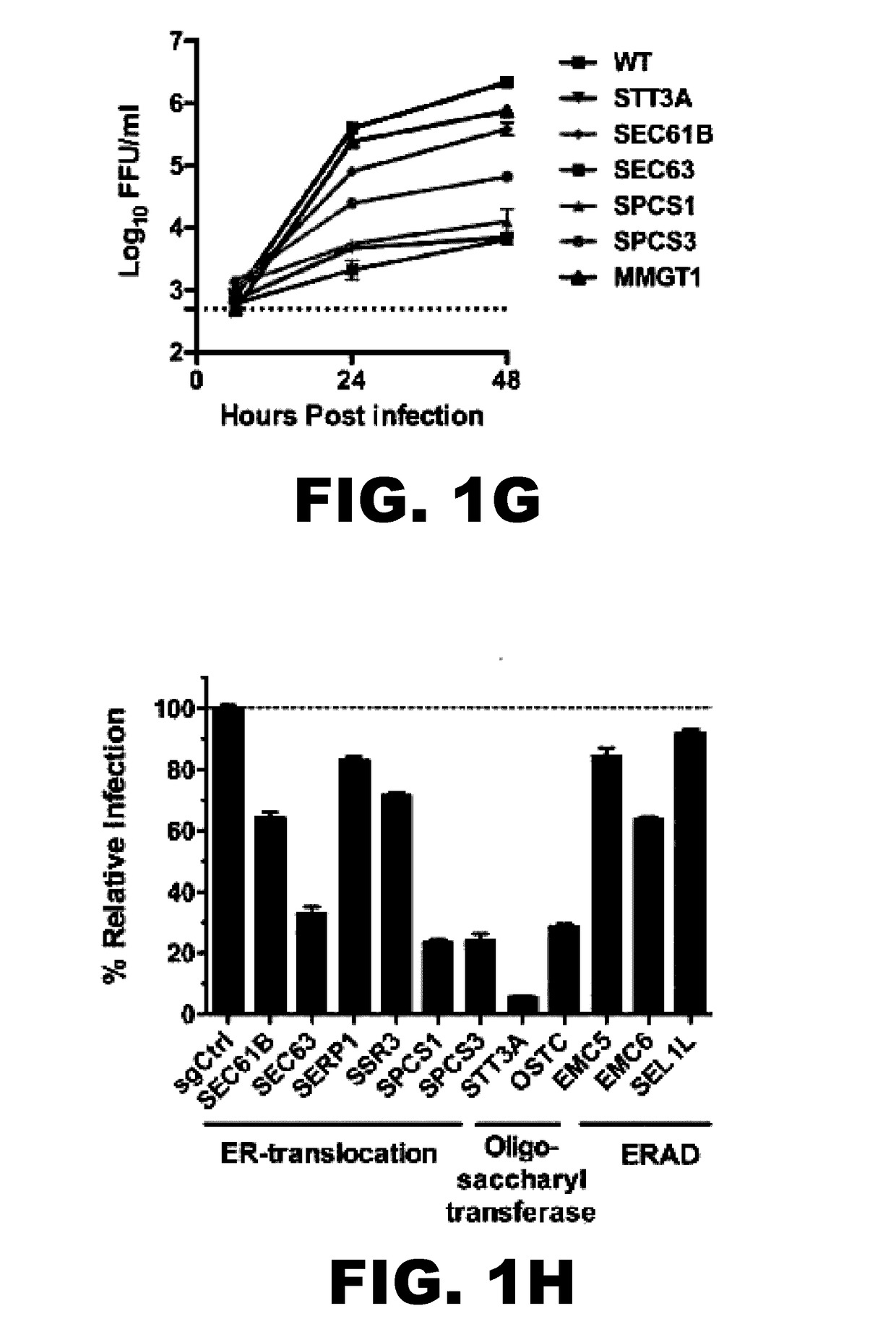
InactiveUS20170101642A1Resume normal productionScreening processDNA/RNA fragmentationViral replicationViral infection
The present invention is directed to compositions targeting the signal peptidase complex and methods of use in treating and preventing flavivirus infection.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes and methods for their use
Disclosed herein is a method for identifying flavivirus cross-reactive epitopes. Also provided are flavivirus E-glycoprotein cross-reactive epitopes and flavivirus E-glycoprotein cross-reactive epitopes having reduced or ablated cross-reactivity (and polypeptides comprising such epitopes), as well as methods of using these molecules to elicit an immune response against a flavivirus and to detect a flaviviral infection.
Owner:UNITED STATES OF AMERICA
Recombinant Flavivirus Vaccines
InactiveUS20080175862A1Promote safe productionSufficient attenuationSsRNA viruses positive-senseSugar derivativesViral VaccineViral infection
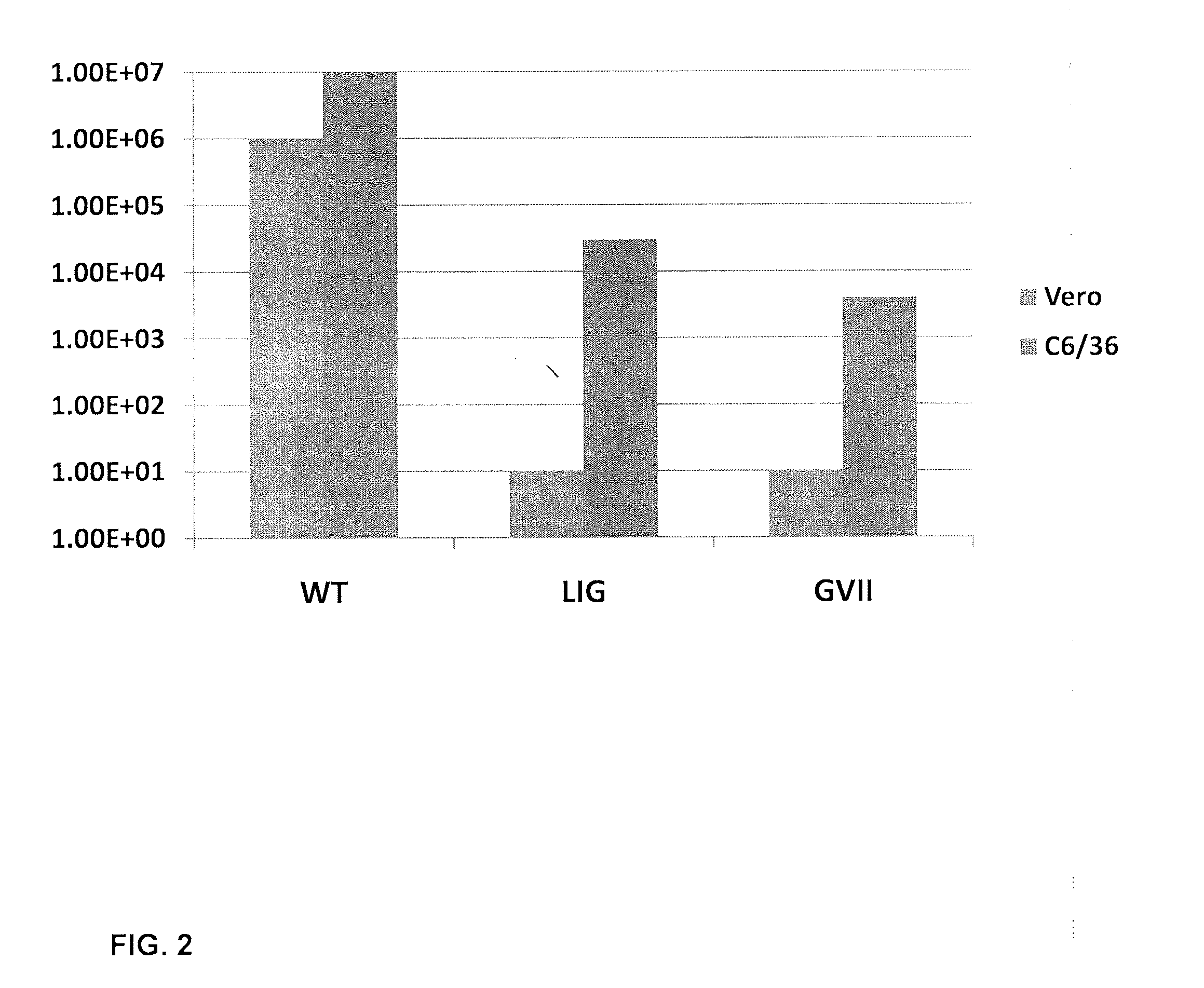
The invention provides recombinant flavivirus vaccines that can be used in the prevention and treatment of flavivirus infection. The vaccines of the invention contain recombinant flaviviruses including attenuating mutations.
Owner:SANOFI PASTEUR BIOLOGICS CO
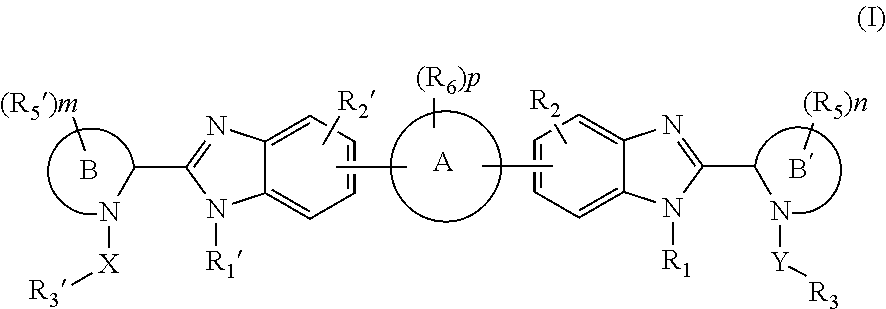
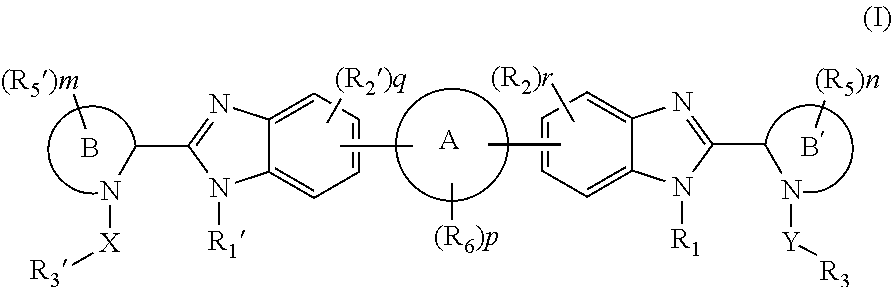
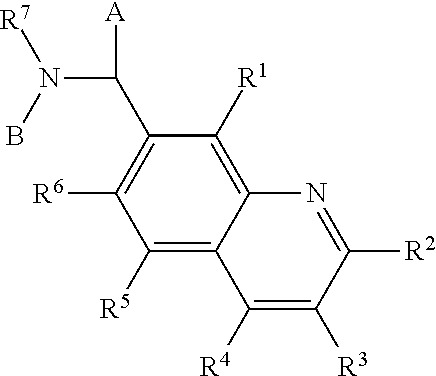
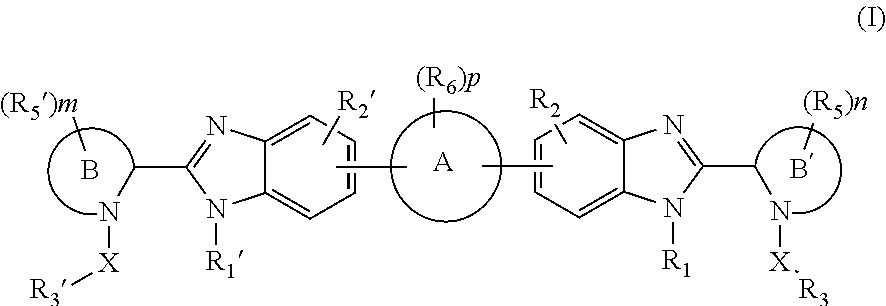
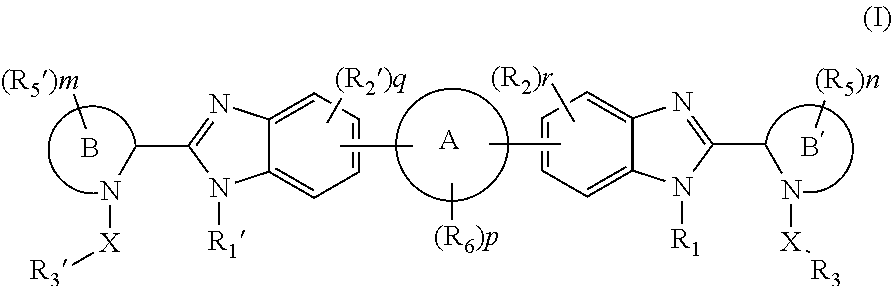
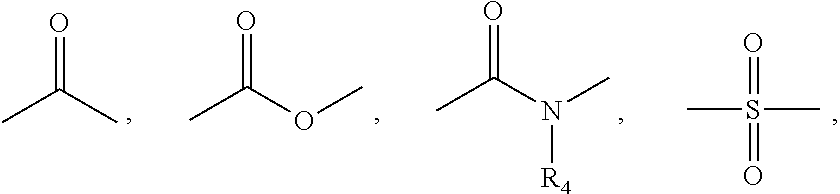
Benzimidazole analogues for the treatment or prevention of flavivirus infections
Owner:VERTEX PHARMA INC
Novel attenuated virus strains and uses thereof
ActiveUS20090117149A1Reducing glycosylationReduce probabilityOrganic active ingredientsSsRNA viruses positive-senseVaccinationVirulent characteristics
Methods and compositions concerning mutant flaviviruses with reduced virulence. In some embodiments the invention concerns nucleotide sequences that encode mutant flaviviral proteins. Viruses comprising mutant NS1 and NS4B genes display reduced virulence are provided. In further aspects of the invention, flavivirus vaccine compositions such as West Nile virus vaccines are provided. In another embodiment the invention provides methods for vaccination against flavivirus infection.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Compositions and methods for determining susceptibility of hepatitis C virus to anti-viral drugs
InactiveUS20030028011A1Change activityAltered susceptibilityOrganic active ingredientsSsRNA viruses positive-senseAntiviral drugDrug compound
The present invention provides methods for determining the susceptibility of a pathogenic flavivirus to anti-viral compounds. This invention also provides methods for determining anti-viral drug susceptibility in a patient infected with a flavivirus. This invention also provides a method for evaluating the biological effectiveness of a candidate anti-viral drug compound. The methods are useful for identifying effective drug regimens for the treatment of flaviviral infections, and identifying and assessing the biological effectiveness of potential therapeutic compounds. Compositions including resistance test vectors and host cells transformed with the resistance test vectors are provided.
Owner:VIROLOGIC INCORPORATED
Flavivirus inhibitors and methods for their use
Methods of treating, preventing, and / or ameliorating a Flavivirus infection in a subject are disclosed. The methods comprise administering to the subject a therapeutically effective amount of a Flavivirus inhibitor, e.g., a Flavivirus serine protease inhibitor. These methods are useful in treating, preventing, and / or ameliorating Flavivurs infections such as, for example, West Nile Virus, Dengue Virus, and Japanese Encephalitis Virus.
Owner:GEORGETOWN UNIV
Benzimidazole analogues for the treatment or prevention of flavivirus infections
Compounds represented by formula I:or pharmaceutically acceptable salts and solvates thereof, wherein A, B, B′, X, Y, R1, R1′, R2, R2′, R3, R3′, R5, R5′, R6, m, n, or p are as defined herein, are useful for treating flaviviridae viral infections.
Owner:VERTEX PHARMA INC
Use of alpha-glucosidase inhibitors to treat alphavirus infections
The present invention provides methods for treating a flavivirus infection, including hepatitis C virus (HCV) infection, in an individual suffering from a flavivirus infection. In some embodiments, the methods involve administering to an individual in need thereof an effective amount of an agent that inhibits enzymatic activity of a membrane-bound α-glucosidase inhibitor. In other embodiments, the methods involve administering to an individual in need thereof effective amounts of an α-glucosidase inhibitor and at least one additional therapeutic agent.
Owner:INTERMUNE INC
Amino acid sites in Flavivirus E proteins useful for development of diagnostics and vaccines
InactiveUS7943148B1Efficient productionEasy to purifySsRNA viruses positive-senseViral antigen ingredientsComputational analysisAmino acid
Highly immunoreactive viral peptides are disclosed which are derived from the E protein of major groups of the Flavivirus genus by computational analyses. These peptides are used in reliable diagnostic methods for the detection and diagnosis of Flavivirus, detecting the presence of antibodies against Flavivirus, and to form vaccine composition(s) for the prevention of Flavivirus infections in humans.
Owner:UNITED STATES OF AMERICA
Compounds and methods for the treatment or prevention of Flavivirus infections
InactiveUS20080269481A1Organic chemistryHeterocyclic compound active ingredientsMedicineFlaviviridae
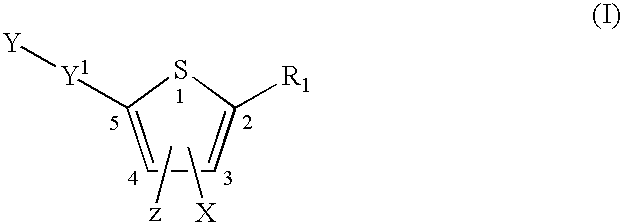
Compounds represented by formula:wherein X, Y and Z are as defined herein, pharmaceutically acceptable salts thereof, and related compounds, are suitable for use in treating or preventing a Flaviviridae viral infection in a host.
Owner:VERTEX PHARMA CANADA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
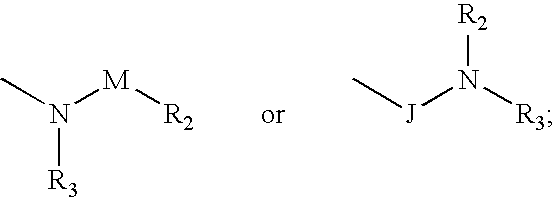

![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka.patsnap.com/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00001.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka.patsnap.com/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00002.png)
![Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections Compounds with the bicyclo[4.2.1]nonane system for the treatment of flavivridae infections](https://images-eureka.patsnap.com/patent_img/48441fda-b6e5-4967-b334-797833136516/US20040082574A1-20040429-C00003.png)