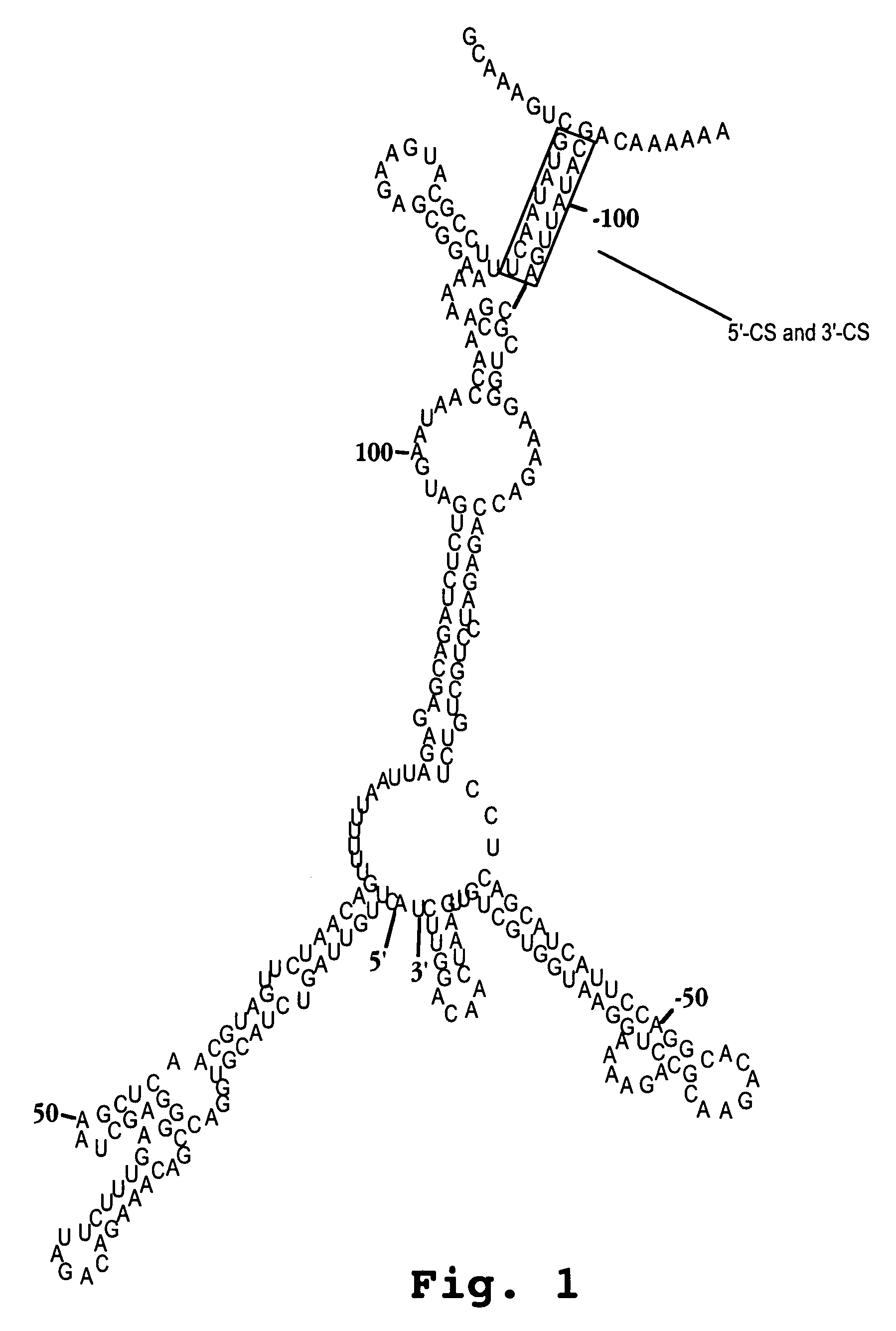
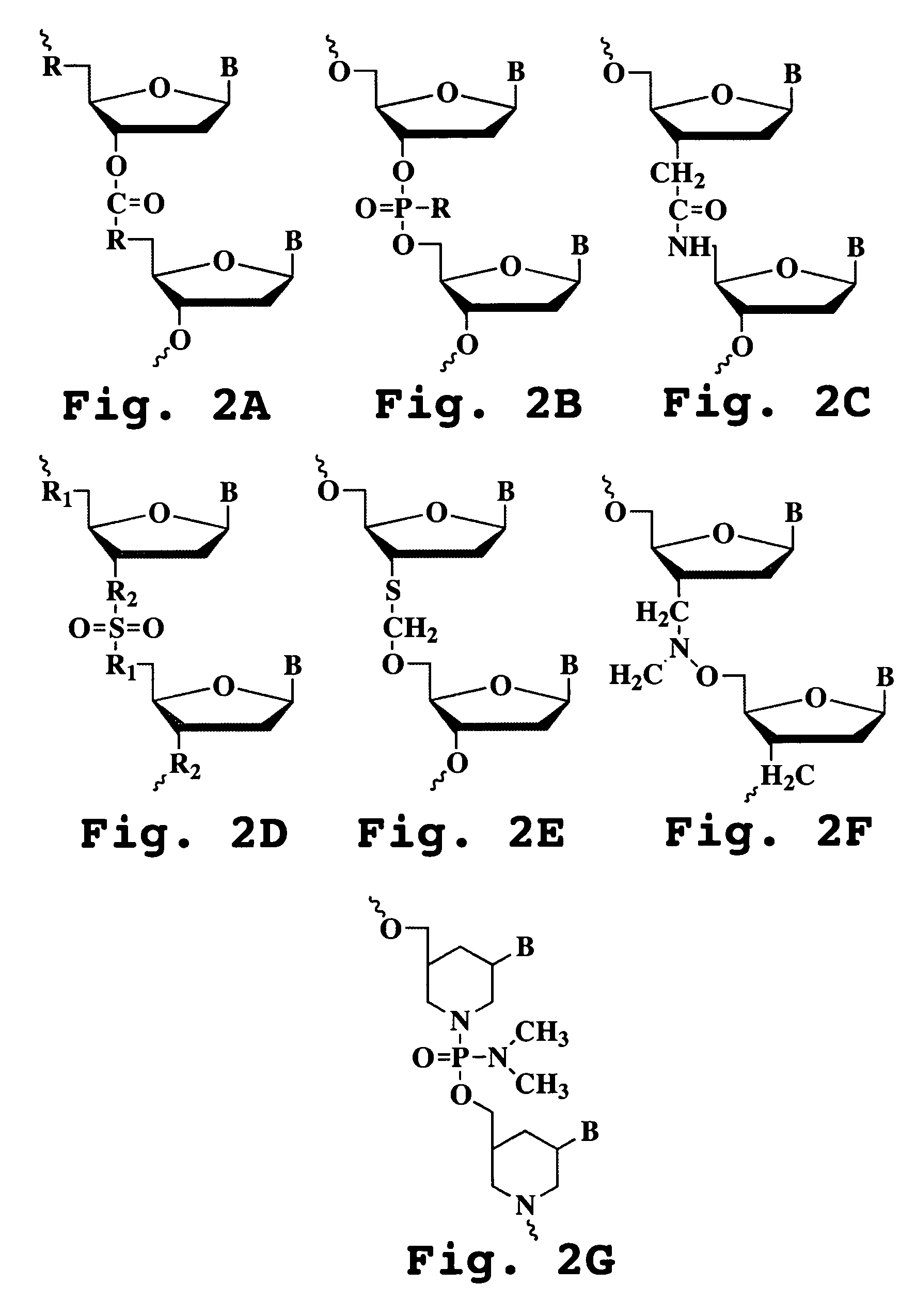
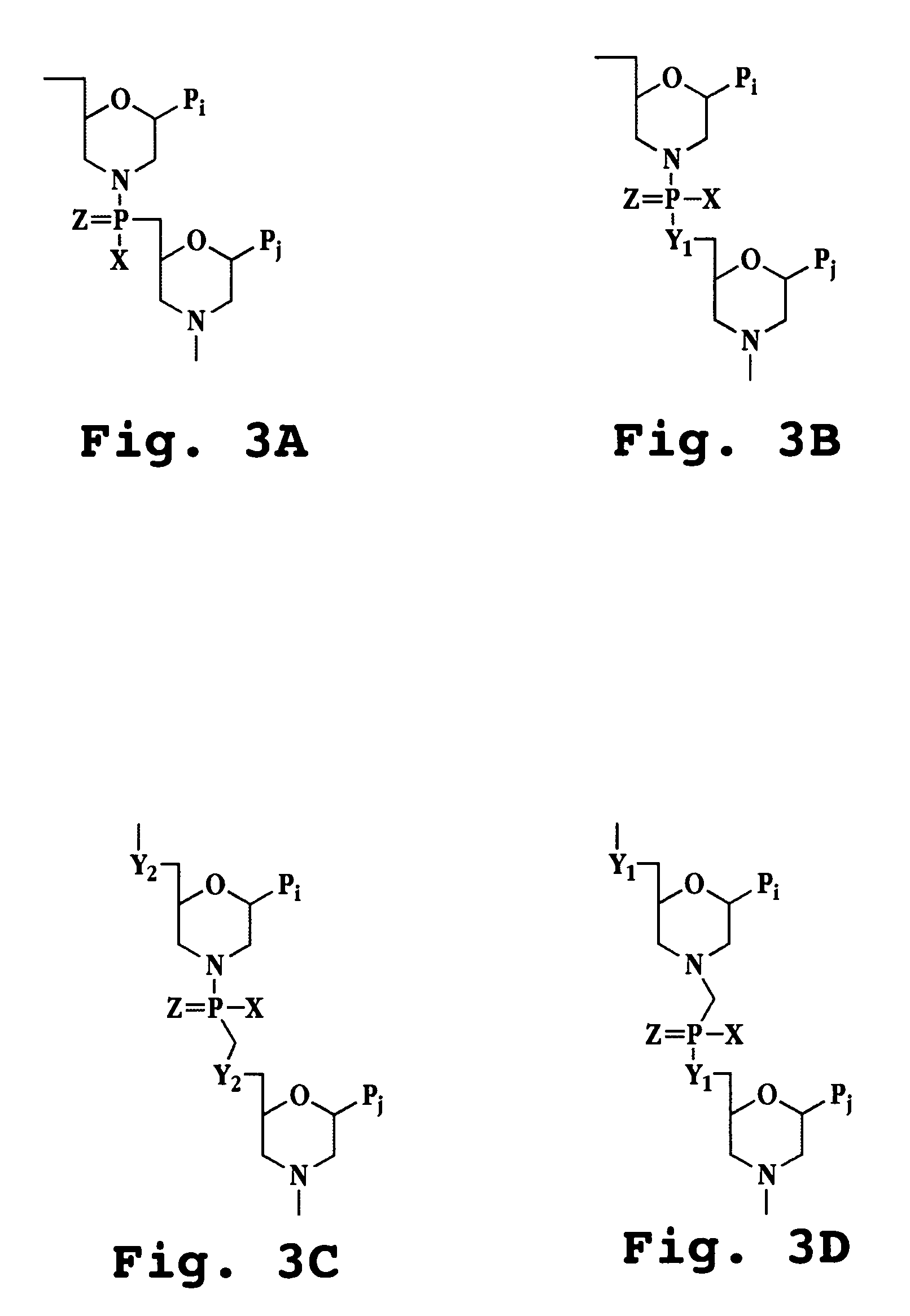
Oligonucleotide analog and method for treating flavivirus infections
a technology of oligonucleotide analogs and flaviviruses, applied in the field of oligonucleotide analogs, can solve the problems of weak viability and in vivo activity of ribavirin against yellow fever, and the inability to effectively treat most i>flaviviruses /i>such as dengue, and achieve the effect of inhibi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Antisense Inhibition of West Nile Virus In Vitro
[0155]Two PMO oligomers were evaluated for their activity against West Nile virus in cultured Vero cells. One 20 mer PMO oligomer targets the 3′-CS region of West Nile virus (WNV 3′ CS, SEQ ID NO. 26), the other 20 mer PMO compound was a “nonsense” sequence (5′-AGTCTCGACTTGCTACCTCA-3′) with no significant homology to any human, monkey or WNV genetic sequence (NC-1). Both PMO oligomers were conjugated at the 5′ end with a peptide (R9F2C-5′-PMO) to enhance cellular uptake in vitro. Two separate experiments, a “two point” and an “eight point dose response”, were performed by adding each PMO oligomer, along with virus inoculum, to cells suspended in standard mammalian tissue culture media supplemented with 2% fetal-calf serum. After 24 hrs. the cells were scored for cytopathic effect both visually under a microscope, and quantitatively with a microplate reader using the ‘neutral-red dye assay’ as described (Morrey, Smee et al. 2002). To th...
example 2
Antisense Inhibition of Tick Borne Encephalitis
[0158]This example describes a study that was devised to test the antiviral activity of antisense PMO compounds of the present invention against two flaviviruses; Tick Borne Encephalitis virus (TBE) and West Nile virus (WN). Two PMO oligomers were evaluated for antiviral activity; TBE 3′CS, SEQ ID NO:25 and; a scramble control sequence DS-scr (5′-AGTCTCGACTTGCTACCTCA-3′). Both PMO oligomers were conjugated at the 5′ end with an arginine-rich peptide (R9F2C-5′-PMO) to enhance cellular uptake as described (U.S. patent application Ser. No. 60 / 466,703 and Moulton, Nelson et al. 2004). The WN virus infection provided a negative control infection as there is no homology between WN and the TBE 3′CS targeting PMO. This control indicates the level of non-specific viral suppression of each of the PMOs. The PMO compounds were prepared to provide a 2 mM stock solution, which were then titrated against a standard dose of virus on tissue culture cell...
example 3
Inhibition of Dengue Virus Serotypes 1-4 with Antisense PMO
[0167]Dengue Fever / Dengue Hemorrhagic Fever (DF / DHF) has become a major global health problem over the past 20 years. Geographic distribution of the dengue virus (DEN), it's mosquito vectors and the disease burden it causes continue to increase. The World Health Organization estimates that there are 50-100 million new infections yearly. DF / DHF is now a leading cause of hospitalization and death among children in southern Asia, and it's incidence is sharply rising in the Americas. There is currently no vaccine or effective therapeutic. One requirement of a successful vaccine or therapeutic is that it be effective against all 4 human serotypes of DEN. The purpose of this study was to evaluate the efficacy and specificity of PMO that target the 3′ CS at inhibiting the replication of four serotypes of DEN in Vero cells in culture. The 5 PMO compounds were designed to target sequence elements in the positive-strand DEN2 RNA that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com