Patents
Literature
193 results about "Encephalitis Viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
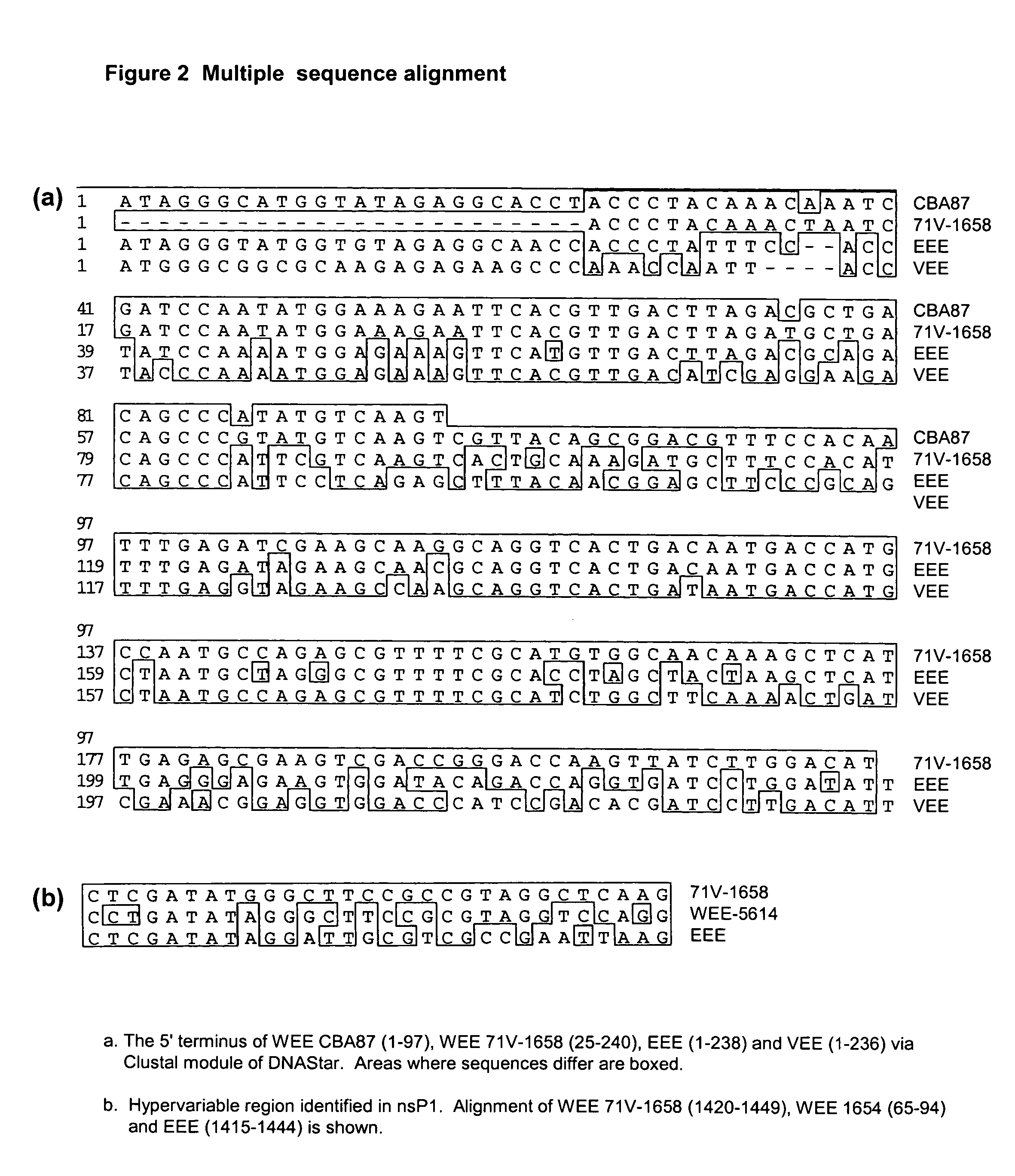
A collection of single-stranded RNA viruses scattered across the Bunyaviridae, Flaviviridae, and Togaviridae families whose common property is the ability to induce encephalitic conditions in infected hosts.
Diagnostic test for West Nile virus
InactiveUS20040197769A1More sensitiveEasy to useViral antigen ingredientsMicrobiological testing/measurementSt Louis encephalitis virusSerum ige
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result.
Owner:HEALTH RES INC
Diagnostic test for west nile virus
ActiveUS20060115896A1More sensitiveEasy to useAnimal cellsMicrobiological testing/measurementDiagnostic testFlavivirus
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result. The invention also provides monoclonal antibodies against WNV NS5 and DENV NS5 antigen and their use in detecting WNV and DENV infections in a biological sample.
Owner:HEALTH RES INC
Thienopyridine Derivatives for the Treatment and Prevention of Dengue Virus Infections
Methods and pharmaceutical compositions for treating viral infections, by administering certain thienopyridine derivative compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
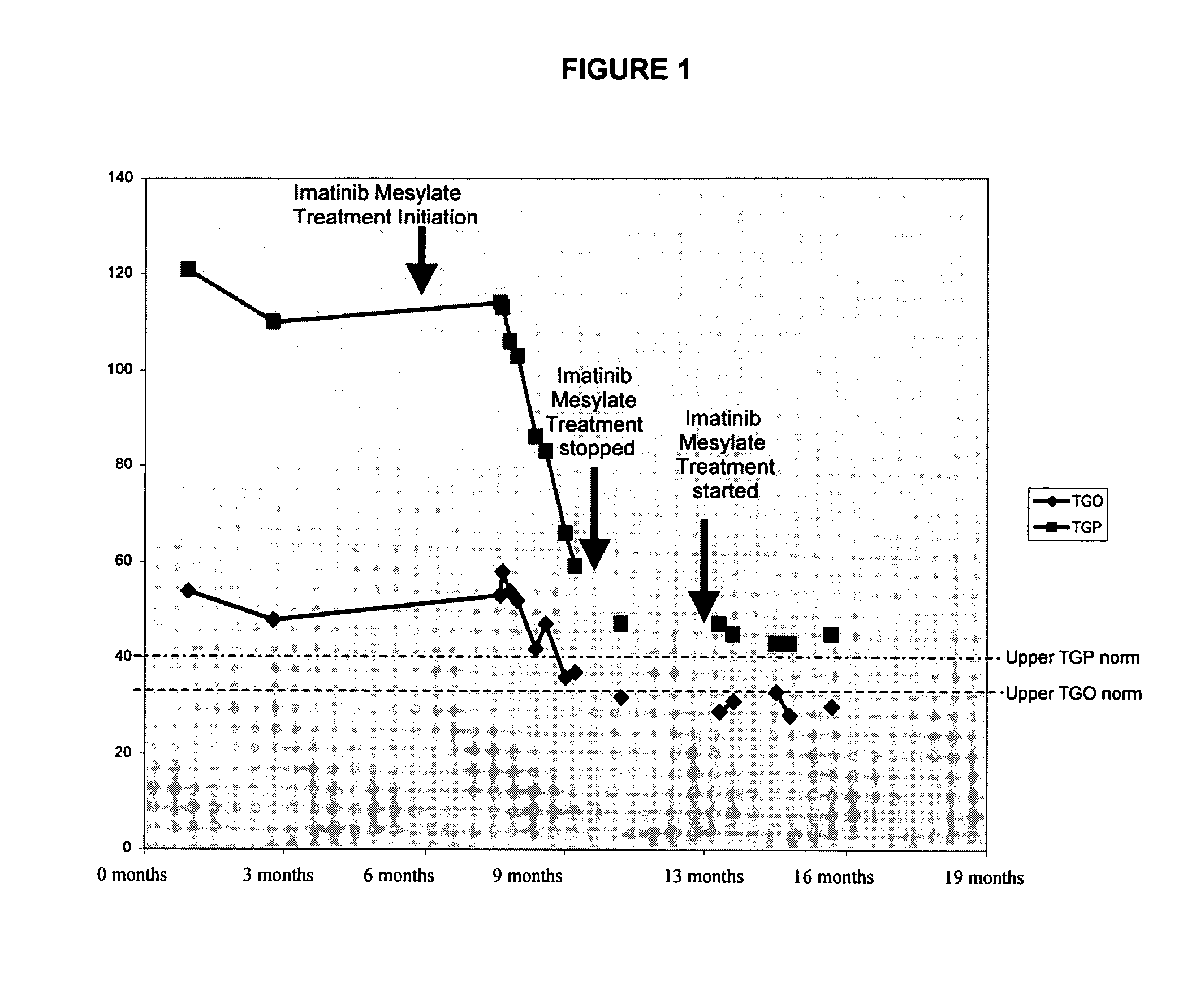
Use of imatinib to treat liver disorders and viral infections
The present invention relates to the use of imatinib for treating viral liver diseases and in particular for viral hepatitis. The invention provides the use of imatinib for inhibiting replication, transmission or both of hepatitis viruses. The invention further relates to the use of imatinib for inhibiting replication, transmission or both of other viruses including herpes virus, poxvirus, influenza virus, para influenza virus, respiratory syncytial virus, rhinovirus, yellow fever virus, west nile virus, and encephalitis virus.
Owner:BIONICHE LIFE SCI
Kit for rapid joint detection of epidemic JEV, DEV and WNV and detection method thereof
InactiveCN101629215AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesDeoxyribonucleoside triphosphateDensovirus
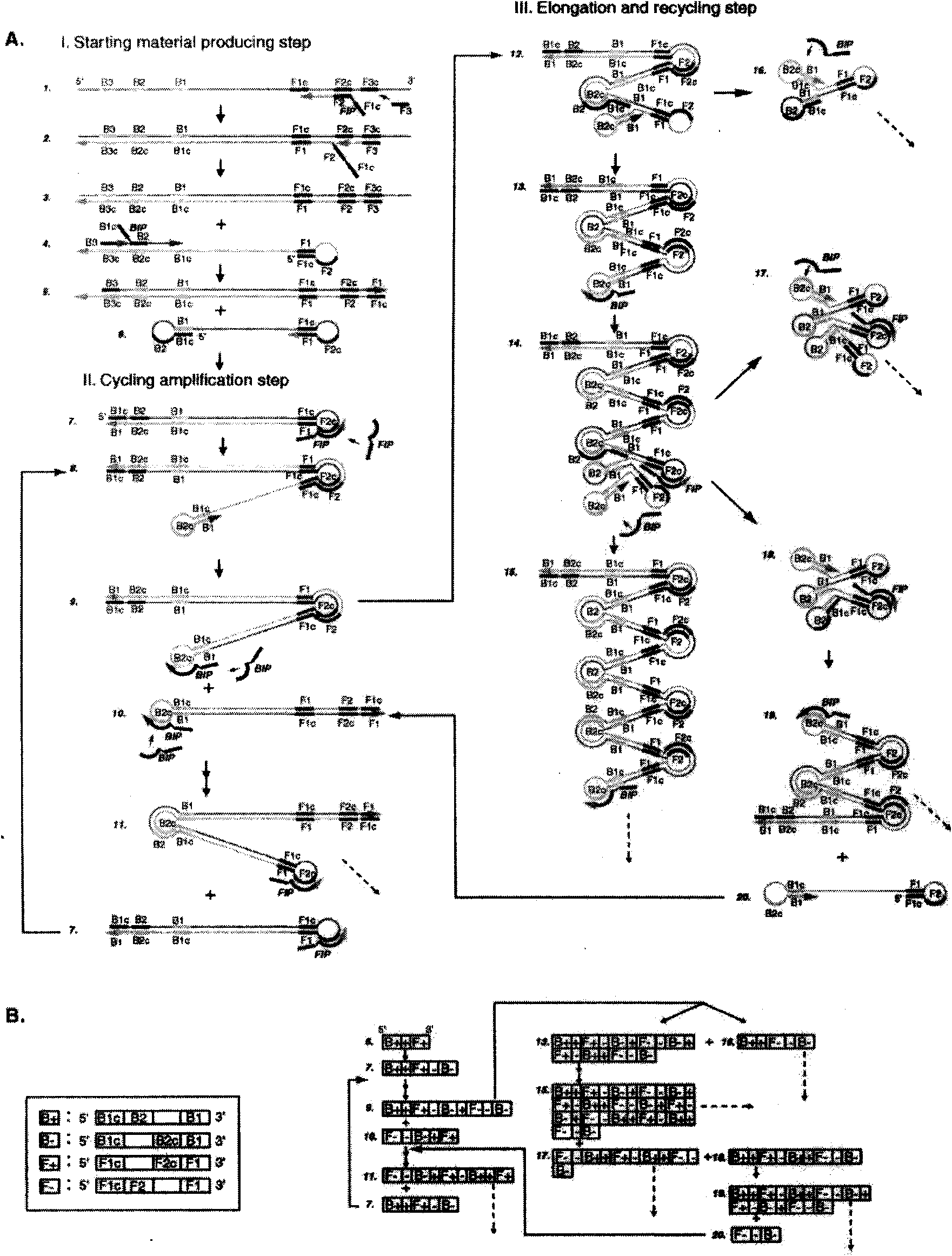
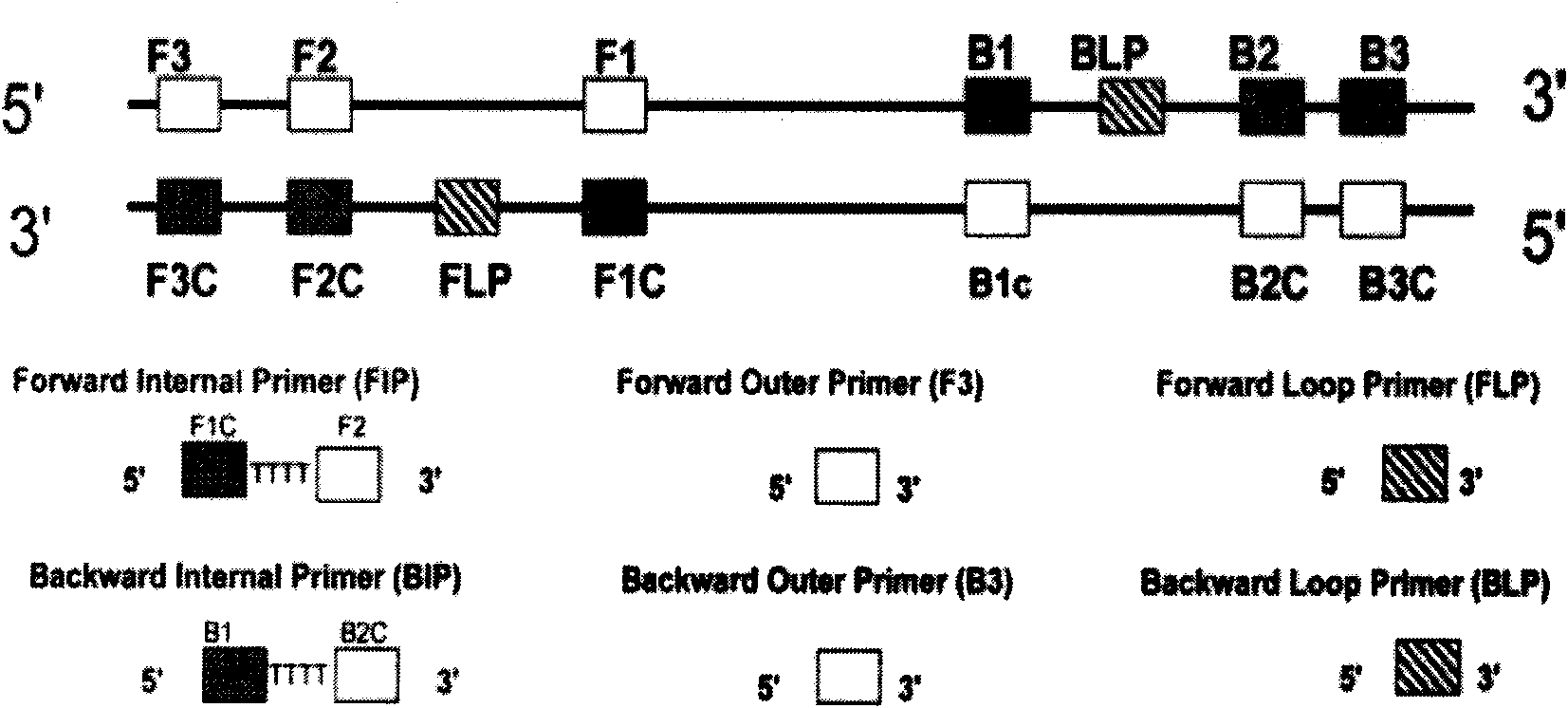
The invention relates to a kit for the rapid joint detection of epidemic JEV (Japanese Encephalitis Virus), DEV (Dengue Virus) and WNV (West Nile Virus). The kit consists of a main reaction solution and specific primers, wherein, the main reaction solution comprises a reaction buffer, AMV (Avian Myeloblastosis Virus) reverse transcriptase, a nuclease inhibitor, Bst (Bacillus stearothermophilus) DNA polymerase, dNTP (deoxyribonucleoside triphosphate), magnesium sulfate, betaine and DEPC (diethylpyrocarbonate) and conditioning water; and the specific primers comprise a specific amplification primer of JEV (PJEV), a specific amplification primer of DEV (PDV) and a specific amplification primer of WNV (PWNV). The invention is capable of rapidly detecting three viruses of flaviviridae by employing the LAMP (Loop-mediated Isothermal Amplification) technology and designing high-degree specific primers, thereby achieving the purpose of highly-efficient specific amplification. The invention further provides a detection method in which an ultraviolet-visible spectrophotometer is utilized for detecting the absorbance value of the reaction system and for indirectly reflecting the DNA amplification of target genes. Therefore, the invention is applicable to rapid detection at primary and field levels and is of greater application value.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
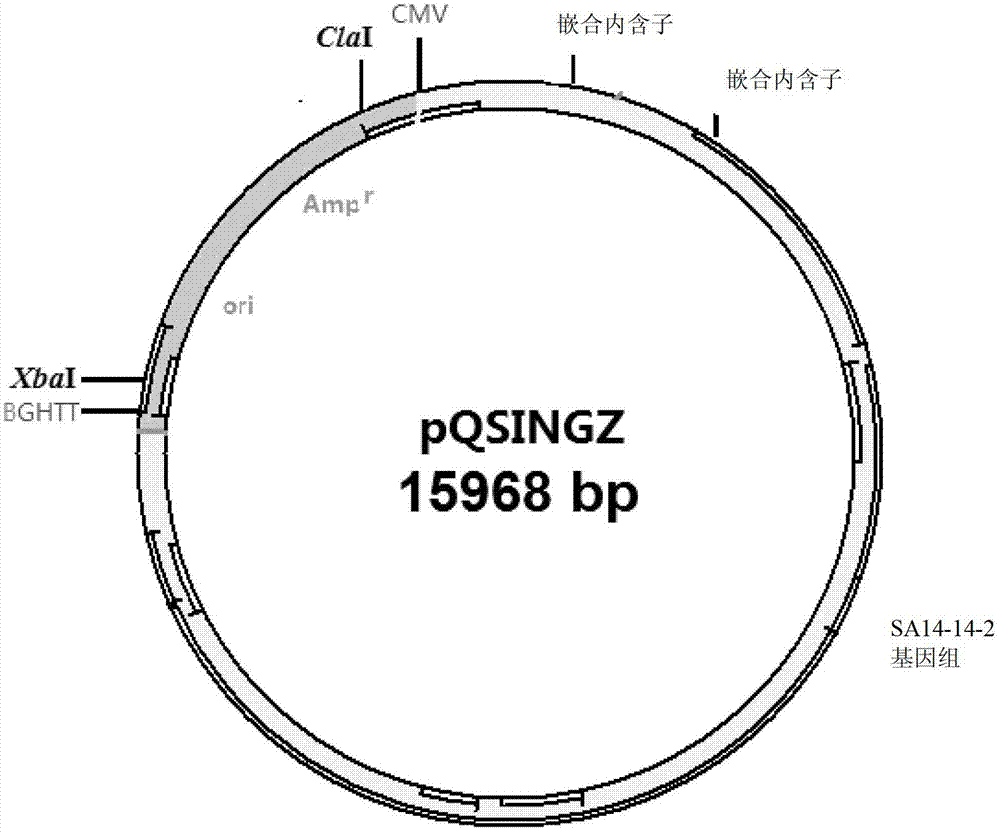
DNA (Deoxyribose Nucleic Acid)-based infectious clone of a Japanese encephalitis virus SA14-14-2 strain, as well as construction method and application thereof
InactiveCN103088049AInfectiousViral antigen ingredientsMicroorganism based processesPolyadenylationJapanese encephalitis viruses
The invention relates to a DNA (Deoxyribose Nucleic Acid)-based infectious clone of a Japanese encephalitis virus SA14-14-2 strain and a construction method of the infectious clone. The infectious clone is constructed by adopting a pBR322 plasmid as a framework vector and then inserting the full-length cDNA of the Japanese encephalitis virus SA14-14-2 vaccine strain; the 5' end of the full-length cDNA of the SA214-14-2 vaccine strain is connected with a CMV (Cucumber Mosaic Virus) promoter, a BGH (Bovine Growth Hormone) polyadenylation sequence is added at the 3' end, and gomphosis intron sequences for stabilizing are respectively added on the 356 locus and the 2217 locus of the genome cDNA. The invention also provides application of infectious clone serving as a novel viral vector, and thus a solid foundation is provided for developing a plurality of novel vaccines for preventing and treating tumours and viral diseases.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE +1
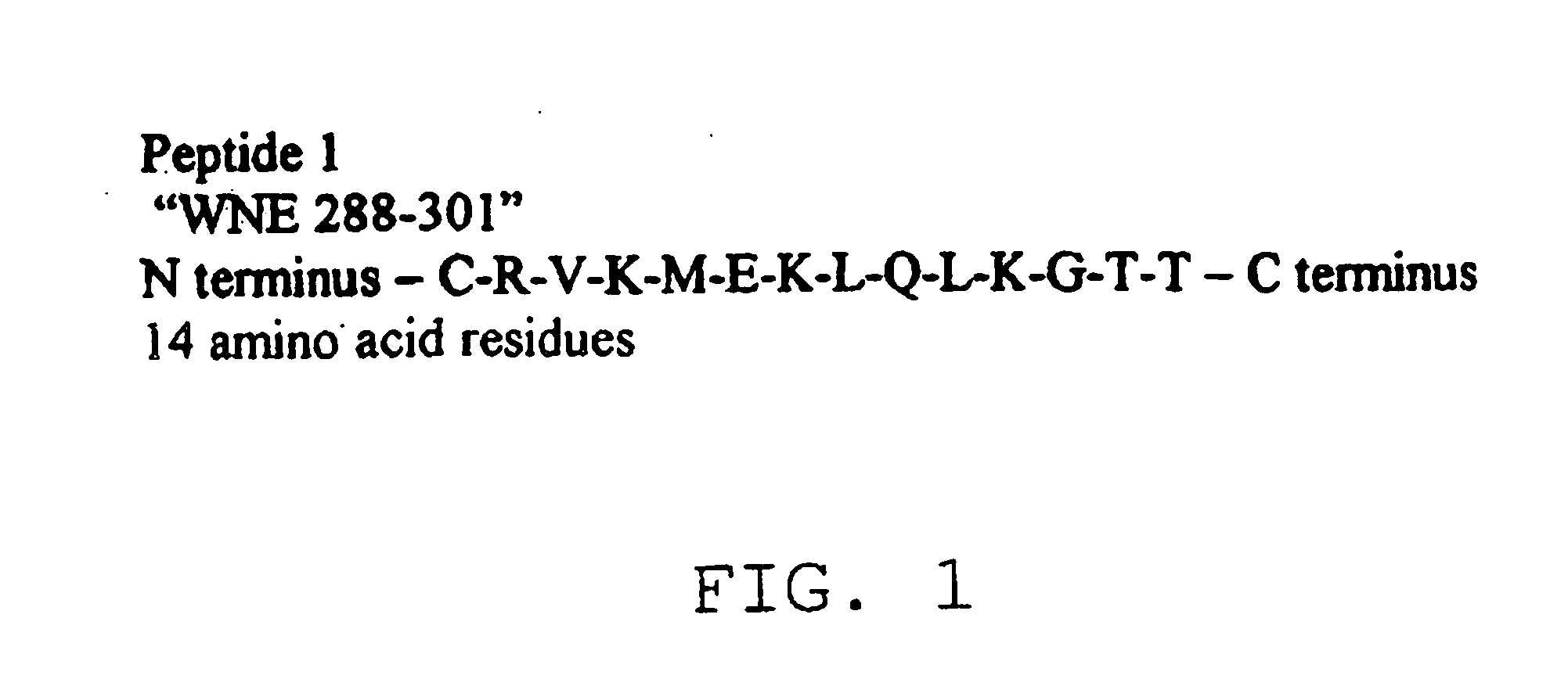
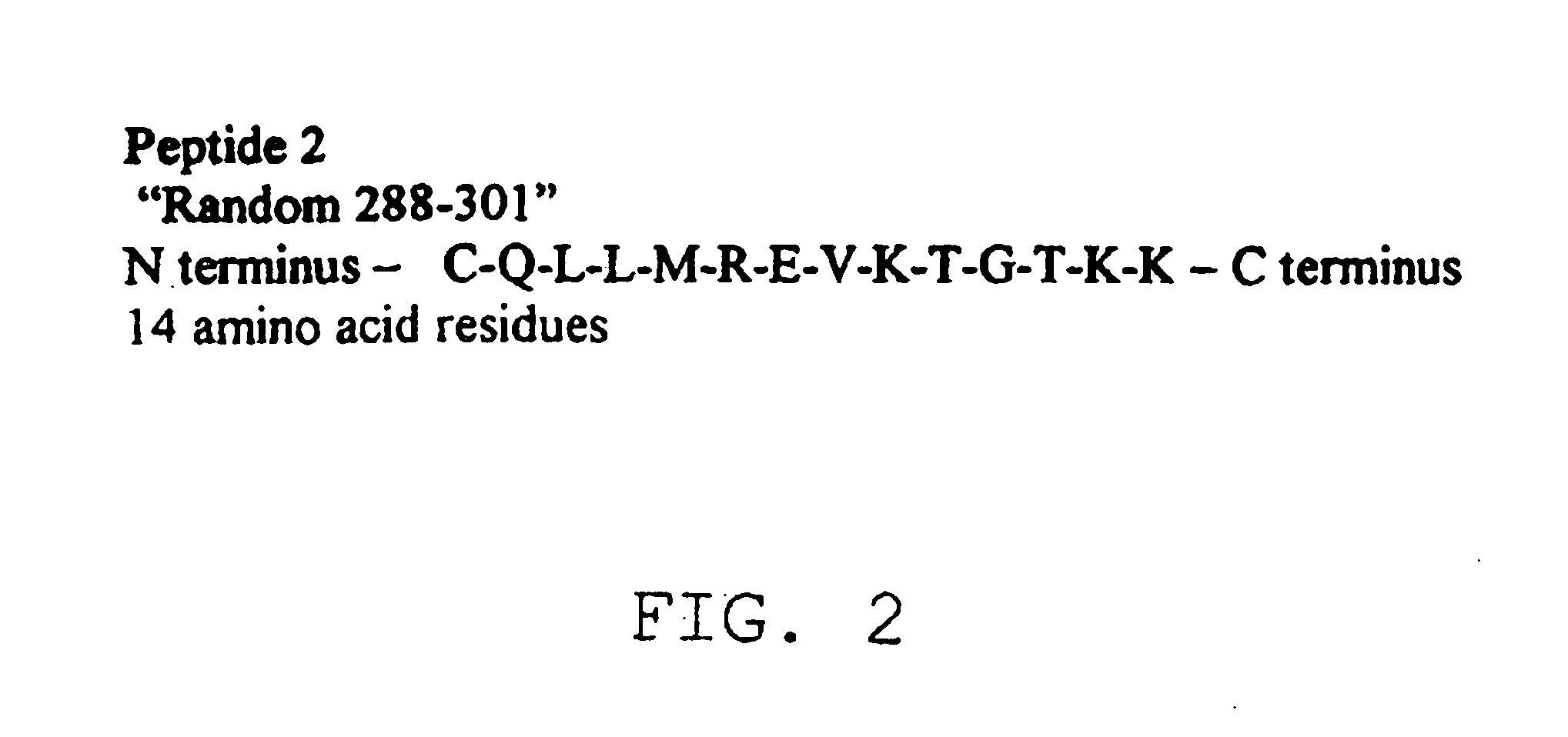
A group of antigen epitope polypeptide and uses thereof
InactiveCN101492495AViral antigen ingredientsMicroorganism based processesCarrier proteinB-Cell Epitopes
The invention discloses a set of epitope polypeptides, particularly relates to a neutralizing B cell epitope sequence of encephalitis B virus E protein, and further discloses application of controlling and diagnosing encephalitis B virus by these epitopes. The amino acid sequences of the epitope polypeptides in the invention are respectively any amino acid sequence of SEQ ID NO: 64, SEQ ID NO: 79, SEQ ID NO: 95, SEQ ID NO: 108, SEQ ID NO: 124 and SEQ ID NO: 139. After the epitope polypeptides in the invention are coupled or fused with carrier proteins to be expressed as immunogenic or vaccine immuno animal organism, neutralizing antibodies aiming at JEV can be generated, and the JEV can be neutralized in vivo or in vitro so as to prevent virus from infecting animal organism. The epitope polypeptides in the invention or the junctional complex thereof can be used as reagents for detecting encephalitis B virus antibodies or encephalitis B virus polypeptide antibodies.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Nucleic acid vaccines for prevention of flavivirus infection
InactiveUS7417136B1Easy to administerEasy to prepareOrganic active ingredientsVirusesActive agentEncephalitis Viruses
The invention encompasses nucleic acid molecules containing transcription units which encode the flavivirus M and E protein antigens. The flaviviruses include Japanese encephalitis virus, dengue, yellow fever virus and St. Louis encephalitis virus. The nucleic acids function to provide the M and E protein antigens when the nucleic acid resides in an appropriate host cell, especially when the host cell is the cell of a subject. The invention also encompasses a vaccine whose active agent is the nucleic acid. The invention further encompasses the cultured host cells when they contain within them nucleic acid molecules containing the transcription units. The invention in addition encompasses a method of immunizing a subject against flavivirus infection by administering to the subject an effective amount of a vaccine containing a nucleic acid molecule containing the transcription unit of the invention.
Owner:HEALTH & HUMAN SERVICES DEPT OF UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC THE
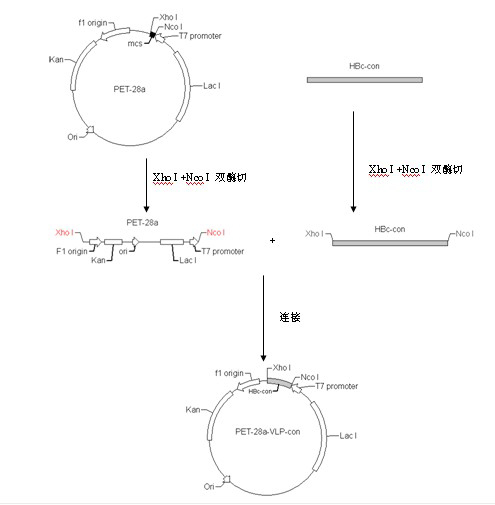
Japanese encephalitis particle vaccine and preparation method and application thereof
InactiveCN102127554AImprove the level ofStrong immune memoryViral antigen ingredientsVirus peptidesHepatitis B virus core AntigenEscherichia coli
The invention belongs to the field of biotechnology, and relates to a vaccine embedded with virus-like particles expressing multi-epitope antigen for Japanese encephalitis, and a preparation method and application thereof. The antigen of the vaccine is virus-like particles formed through spontaneous assembly of hepatitis B virus core antigen embedded with neutralizing antigen epitope expressing Japanese encephalitis virus and cytotoxic lymphocyte (CTL) antigen epitope, and is prepared through soluble expression of escherichia coli and purification. The Japanese encephalitis virus-like particle antigen is properly diluted with physiological saline, or is compatible with immunologic adjuvants to be prepared into the Japanese encephalitis particle vaccine. Animal experiments show that: the vaccine is safe and high-efficiency; mice inoculate with the vaccine generate high-level neutralizing antigen for the Japanese encephalitis virus to protect the mice against the attack of strong Japanese encephalitis virus by 100 percent.
Owner:NANJING AGRICULTURAL UNIVERSITY
Attenuated recombinant alphaviruses incapable of replicating in mosquitoes and uses thereof
ActiveUS20110052634A1SsRNA viruses positive-senseMutant preparationEastern equine encephalitis virusEncephalitis Viruses
The present invention discloses an attenuated recombinant alphavirus that is incapable of replicating in mosquito cells and of transmission by mosquito vectors. These attenuated alphavirus may include but is not limited to Western Equine Encephalitis virus, Eastern equine encephalitis virus, Venezuelan equine encephalitis virus or Chikungunya virus. The present invention also discloses the method of generating such alphaviruses and their use as immunogenic compositions.
ELISA (Enzyme-Linked Immuno Sorbent Assay) kit for detecting Japanese encephalitis virus antigens in swine, human and mosquitoes and application
InactiveCN102464716AEfficient detectionShort period of bloodMicroorganism based processesImmunoglobulins against virusesAnimal virusProtein.monoclonal
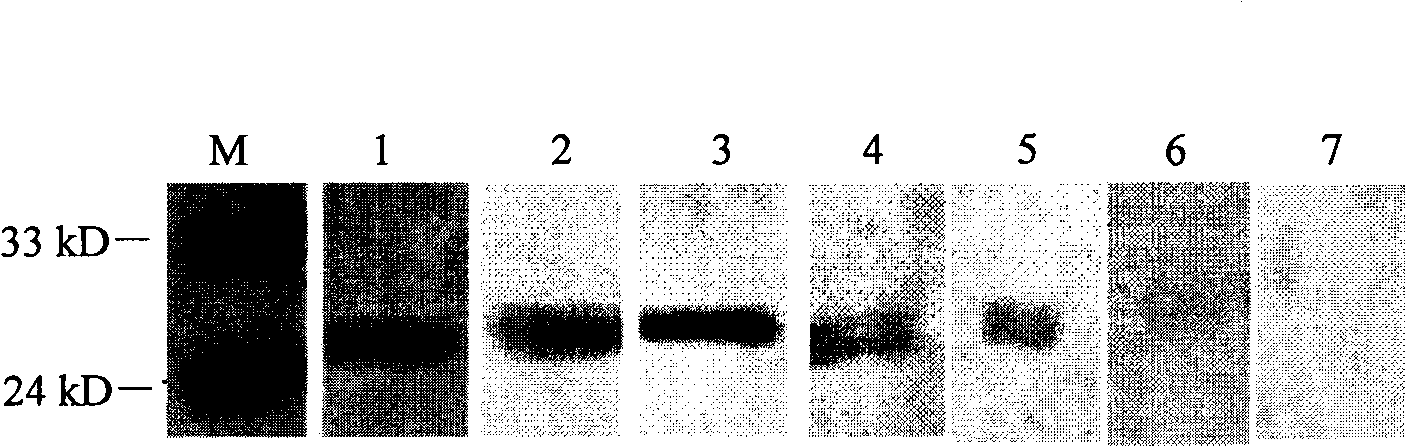
The invention belongs to the technical field of animal virology and immunology, in particular to a double-antibody sandwich ELISA (Enzyme-Linked Immuno Sorbent Assay) kit for detecting Japanese encephalitis virus antigens in swine, human and mosquitoes. Core reagents of the kit comprise a Japanese encephalitis virus E protein monoclonal antibody serving as a primary antibody and a rabbit polyclonal antibody serving as a secondary antibody. The invention discloses a preparation and purification method of the Japanese encephalitis virus E protein monoclonal antibody and the rabbit polyclonal antibody. The invention also discloses a detection method of the double-antibody sandwich ELISA. A hybridoma cell strain secreting the monoclonal antibody is preserved in China Center for Type Culture Collection with CCTCC NO. C2010114.
Owner:HUAZHONG AGRI UNIV
Novel DNA-based vaccine against the encephalitis alphaviruses
InactiveUS20050118251A1BiocideSsRNA viruses positive-senseCell freeEastern equine encephalitis virus
This invention relates to the development of a mammalian expression vector, under which expression of the structural genes of western equine encephalitis virus have been placed under the control of an eucaryotic promoter. When the recombinant vector is administered to mammalian cell culture or using a cell-free transcription / translation system, in vitro, authentic structural proteins of western equine encephalitis virus are produced as verified by reactivity with monoclonal antibodies developed to western equine encephalitis virus. When the recombinant DNA molecule is administered in vivo, a protective immune response is induced, thereby enhancing protection of the individual against subsequent infection by western equine encephalitis virus. In a similar manner, DNA vaccines to related alphaviruses (Venezuelan and eastern equine encephalitis viruses) could also be developed.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
Gene VIb subtype Rubulavirus Newcastle disease virus attenuated strain VIbI4 and construction method thereof
ActiveCN102776156AImprove reproductive performanceSuitable for mass productionMicroorganism based processesViruses/bacteriophagesNewcastle disease virus NDVEncephalitis Viruses
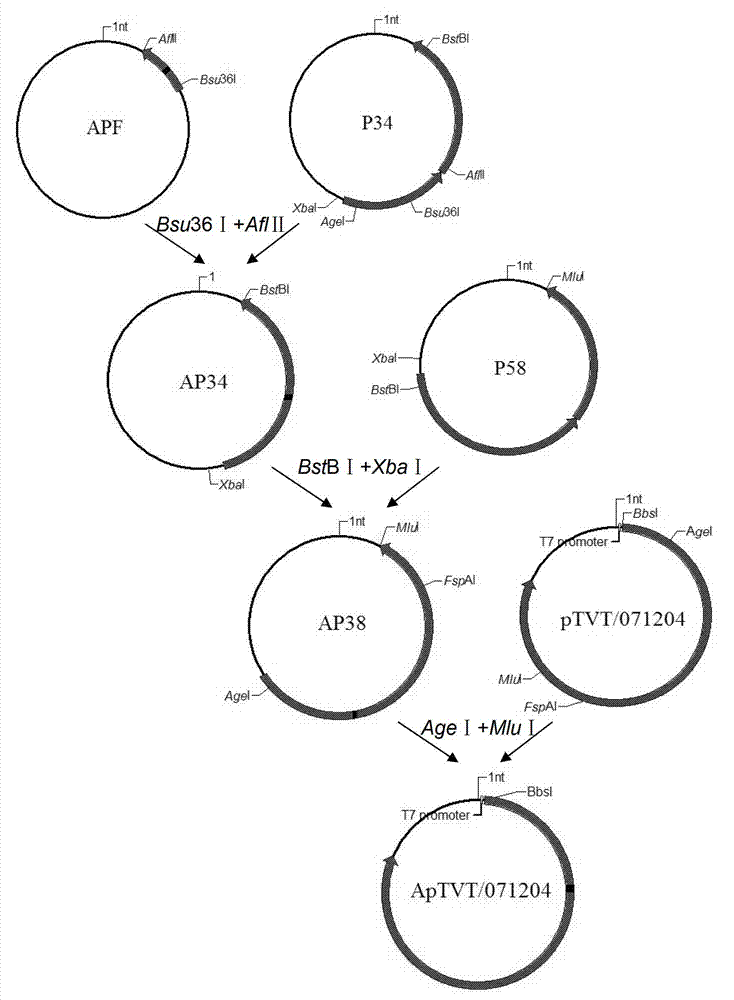
The invention relates to gene VIb subtype Avian pneumo-encephalitis virus attenuated strain VIbI4 and a construction method thereof. The preserving number of the gene VIb subtype scriber set Avian pneumo-encephalitis virus attenuated strain VIbI4 is CGMCCNo: 6149. The gene VIb subtype Rubulavirus Newcastle disease virus attenuated strain VIbI4 and the construction method thereof relate to a technology for applying reverse genetics; and the method comprises the following steps: carrying out mutation on an F gene by using a transcription carrier Ptvt / 071204 containing Avian pneumo-encephalitis virus JS / 07 / 04 / Pi full gene genome from pigeons so as to obtain to a transcription carrier ApTVT / 071204, and then substituting corresponding parts on the ApTVT / 071204 by the NP, P and L genetic fragments of Avian pneumo-encephalitis virus rNDV / I4 so as to obtain recombinant plasmids ApTVT / VIbI4, wherein the plasmids successfully save a recombinant virus VIbI4 after transfecting a BSR-T7 / 5 cell together with auxiliary plasmids. The virus is relatively high in propagating titre, is suitable for large-scale production of vaccines and can be used for manufacturing vaccines.
Owner:YANGZHOU UNIV
Multiple polymerase chain reaction (PCR) kit and method for detecting mosquito-borne pathogens
InactiveCN101979665AReliable informationEffective early warning dataMicrobiological testing/measurementAgainst vector-borne diseasesTissue fluidMultiplex pcrs
The invention provides a multiple polymerase chain reaction (PCR) kit for detecting mosquito-borne pathogens. The kit comprises six pairs of specific primers. The invention also provides a method for detecting the mosquito-borne pathogens. Electrophoresis is performed on a PCR-amplified product. Whether pathogens, such as encephalitis B virus, dengue fever virus, yellow fever virus, plasmodium falciparum, plasmodium vivax, plasmodium knowlesi, plasmodium ovale, plasmodium malariae, wuchereria malayi, wuchereria bancrofti and the like, exists or not is detected and identified according to the length of a PCR-amplified fragment. By the method, various reported mosquito-borne pathogens, and the yellow fever virus and west nile virus which come from other countries can be detected quickly, accurately and sensitively at the same time, and can be applied to the detection of various samples, such as mosquitoes, blood of patients, tissue fluid and the like. The invention provides a low-cost and high-efficient method for early monitoring and finding mosquito-borne disease prevalence for prevention and control work of mosquito-borne diseases in China.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
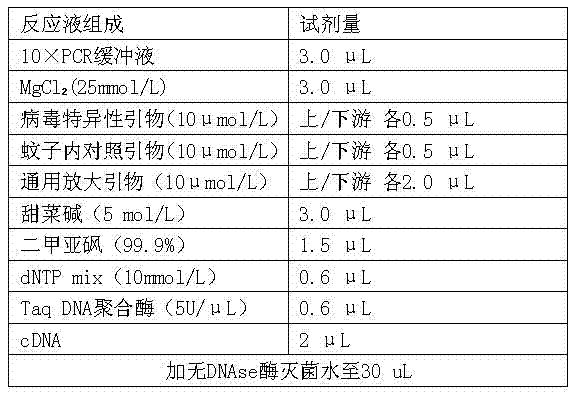
Oligonucleotide primers for detecting arbo encephalitis viruses and detection method thereof
InactiveCN102399905AEasy to detectStrong specificityMicrobiological testing/measurementMicroorganism based processesReverse transcriptaseOligonucleotide primers
The invention relates to oligonucleotide primers for detecting arbo encephalitis viruses, which comprise 12 pairs of virus specific primers, 1 pair of universal amplification primers and 1 pair of contrast primers inside a mosquito. In the detection step, firstly, an object to be detected is required to carry out an RT (Reverse Transcriptase) amplification reaction and a PCR (Polymerase Chain Reaction) amplification reaction, and a signal to be detected is amplified. The amplification product is detected by using an electrophoresis system, and the rapid detection and the synchronous identification of 12 kinds of viruses are realized by analyzing the segment length of the amplification product. The primers can be used for rapidly realizing detection and identification about whether the unknown sample carries the 12 kinds of arbo encephalitis viruses. The detection has high sensitivity and strong specificity. The primers provided by the invention can be applied to the efficient detection and virus identification of mosquitoes and other blood sucking insects under different environments such as departure and entry ports, baffle fields, livestock houses, rainforests, swamps and the like.
Owner:CHONGQING UNIV +1
ELISA-Array method for detection of encephalitis viruses, and special kit thereof
InactiveCN102426237AChemiluminescene/bioluminescenceAgainst vector-borne diseasesEncephalitis VirusesCapture antibody
The present invention discloses an ELISA-Array method for detection of encephalitis viruses, and a special kit thereof. The kit of the present invention comprises six capture antibodies, wherein the solution of each capture antibody is a mixing solution prepared by mixing the capture antibody and a spotting solution, the concentration of each capture antibody in the corresponding capture antibody solution is 0.05 mg / ml. Experimental results of the present invention show that: the six specific encephalitis virus monoclonal antibodies are prepared by the method of the present invention; with adopting the ELISA-Array technology platform, and optimizing the experimental conditions, the ELISA-Array technology capable of concurrent detection of the six encephalitis viruses is established, and can be adopted for detection of the six encephalitis-related viruses; compared to the common ELISA, the ELISA-Array method of the present invention has the following advantages that: the specificity of the ELISA-Array method is the same as the specificity of the common ELISA, the sensitivity is high, the highest sensitivity is more than 10 times the sensitivity of the common ELISA, and the ELISA-Array method has a high clinical application prospect.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Epidemic encephalitis B/forest encephalitis hybrid virus and application of virus
InactiveCN103352029AImprove securityStable attenuation characteristicsViral antigen ingredientsMicrobiological testing/measurementLethal doseEncephalitis Viruses
The invention discloses an epidemic encephalitis B / forest encephalitis hybrid virus and an application of the virus. The epidemic encephalitis B / forest encephalitis hybrid virus takes an epidemic encephalitis virus attenuated strain as a gene skeleton, and is a recombinant virus obtained by replacing a deoxyribonucleotide fragment of an encoded epidemic encephalitis virus prM-E delta 3 protein in epidemic encephalitis virus genome RNA (Ribonucleic Acid) with a deoxyribonucleotide fragment of an encoded forest encephalitis virus prM-E delta 3 protein in forest encephalitis virus genome RNA. The epidemic encephalitis B / forest encephalitis hybrid virus has the advantages that the virus is high in safety, and strong in stability, and can be induced to generate a good immune response to a forest encephalitis virus envelope protein, and protect an animal from being attacked by the exogenous forest encephalitis virus of lethal dose. The epidemic encephalitis B / forest encephalitis hybrid virus has a good application prospect in preventing and / or treating forest encephalitis virus infection as a vaccine.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
EIII-indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) antibody detection kit for detecting swine Japanese encephalitis virus and application thereof
ActiveCN103616509AImproving immunogenicityEvade attackBiological material analysisElisa kitJapanese encephalitis virus Antibody
The invention discloses an EIII-indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) antibody detection kit for detecting a swine Japanese encephalitis virus and an application thereof. The kit disclosed by the invention consists of an elisa plate which takes a purified JEV-EIII protein as an envelope antigen, a cleaning liquid, a serum diluent, rabbit anti-pig elisa secondary antibody, a primer developing liquid, a stop buffer, a JEV positive serum and a JEV negative serum. The envelope antigen used by the invention is easy to be obtained stably and massively, the purifying method is simple and easy to realize, and concentration of recombinant proteins is easy to test and control, thereby facilitating industrialized production on a large scale. The kit disclosed by the invention is used for detecting the swine Japanese encephalitis virus antibody, and the detection coincidence rate with the ELISA kit in the prior art is 90%. The kit disclosed by the invention is convenient to operate, high in sensitivity, good in specificity, low in using cost, good in repeatability and suitable for popularization and application, and provides a reliable means for clinical rapid detection of the JEV antibody.
Owner:广州易安生物技术有限公司
Japanese encephalitis virus like particles as well as preparation method and application thereof
InactiveCN102329784AReserve spaceReserved epitopeViral antigen ingredientsInactivation/attenuationJapanese B Encephalitis VirusReverse transcriptase
The invention discloses Japanese encephalitis virus like particles as well as a preparation method and an application thereof. The Japanese encephalitis virus like particles are prepared by assembling a PrM / E gene of a Japanese Encephalitis virus (JEV) and a JEVRNA replicor with a nucleotide sequence as shown in SEQ ID NO: 1. The preparation method of the Japanese encephalitis virus like particles comprises the steps of: connecting the JEV PrM / E gene amplified by an RT-PCR (Reverse Transcriptase Polymerase Chain Reaction) to a plasmid pTRE-Tight of a Tet-on advance expression system to form a recombination vector, transfecting a Hela Tet-on advanced cell by using an Xfect method, after screening and identifying through HygB, obtaining a Hela cell line for controllably and stably expressing the PrM / E gene through a limiting dilution process, inducing for expressing a PrM / E protein, and packaging to obtain the conventional virus like particles; and transfecting the Hela cell line by using a JEV replicor RNA vector and packaging to obtain the virus like particles with a capacity of transient infection. The Japanese encephalitis virus like particles can be used for preparing a vaccine or used as a detection agent, has better immunogenicity and reactivity, and is safe and reliable.
Owner:SOUTH CHINA AGRI UNIV
Japanese encephalitis virus JEV replicon vector and application thereof
InactiveCN101712965AFree from virus attacksViral antigen ingredientsGenetic material ingredientsForeign proteinTerra firma
The invention provides a replicon vector taking Japanese encephalitis virus genome as a framework, as well as a cell line for packaging the same and a packaging system. The JEV replicon vector has the capability of efficiently expressing foreign protein. Mice are immunized with the replicon vector, and the titer of an anti-JEV antibody reaches 1:1280 after three immunizations so as to protect 75 percent of suckling mice from virus attack. The cell line provided can produce 1.6*105 U / ml of pseudovirus particles after packaging JEV replicon, and the titer of the anti-JEV antibody reaches 1:2560 after two immunizations so as to protect 73 percent of suckling mice from virus attack. The invention establishes a technical platform for a JEV replicon vector system for the first time, and explores the feasibility of researching replicon vaccines and pseudovirus vaccines through the JEV replicon vector system, thereby laying a solid foundation for developing and researching a plurality of novel vaccines used to prevent and treat tumors and viral diseases in future.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Multi-RT-PCR method for monitoring pollution of six viruses in pig farm environment through one system and application
InactiveCN105002297AMicrobiological testing/measurementMicroorganism based processesBiotechnologyPig farms
The invention provides a multi-RT-PCR method for monitoring pollution of six main viruses in a pig farm environment through one system and application. According to the method, primer design is conducted for CSFV, JEV, PRRSV, PCV-2, PRV and PPV, a multi-RT-PCR reaction system for the six viruses is established, air samples are collected through liquid collision, the viruses are subjected to separation concentration through ultrafiltration centrifugation, and the method for detecting the pollution of the main viruses in the pig farm environment is established. The method can be used for monitoring the pollution of the six viruses in air, fodder, drinking water, appliances, excrement and pig groups in the pig house and pig farm environment, and technical support is provided for establishing a main virus pollution early-warning mechanism and a main virus disease preventing, controlling and purifying system in the pig farm environment.
Owner:HENAN INST OF SCI & TECH
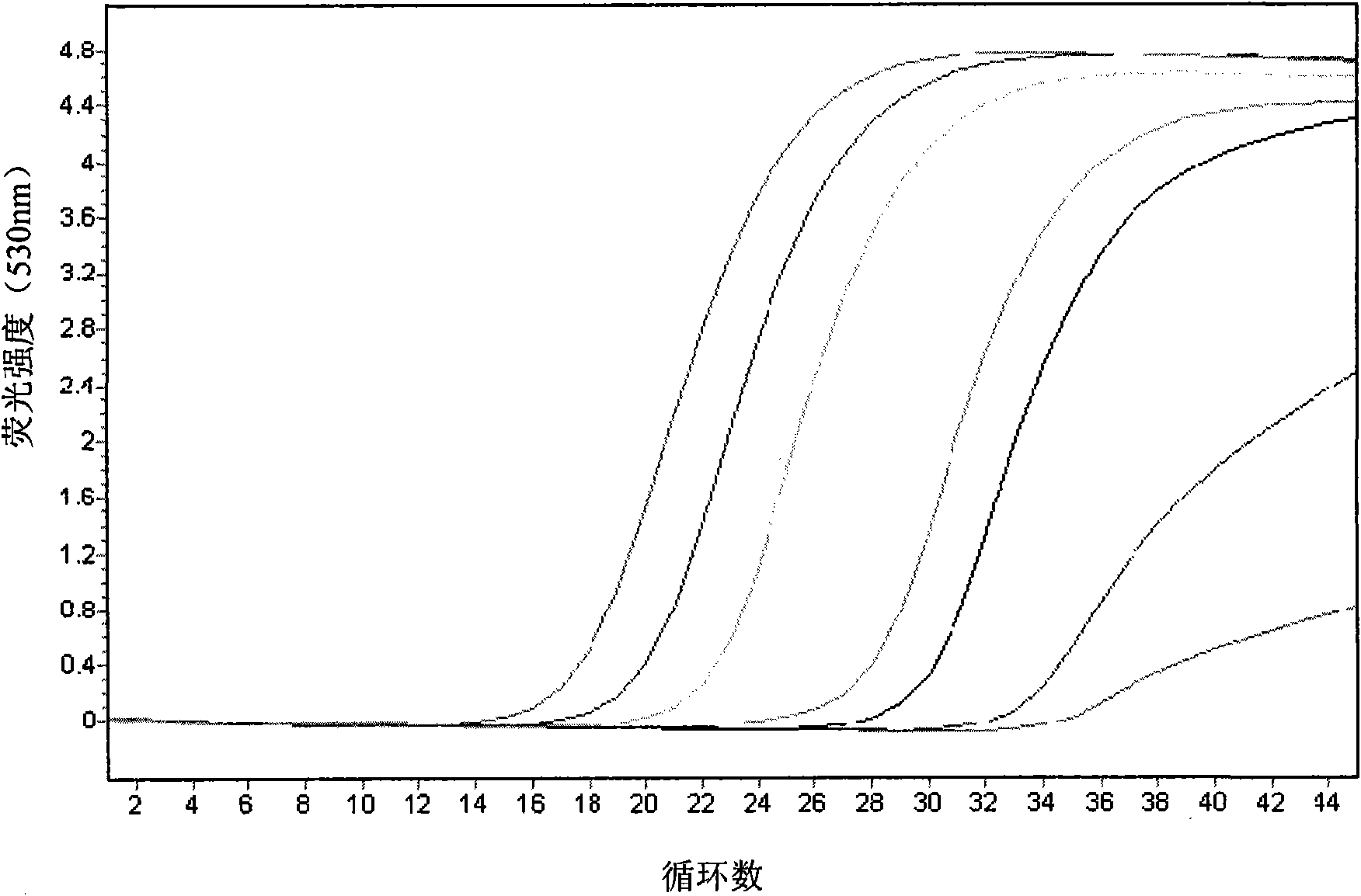
Kit for detecting eastern equine encephalitis virus and west equine encephalitis virus by real-time fluorescence quantitative RT-PCR
InactiveCN101967524AMicrobiological testing/measurementFluorescence/phosphorescenceForward primerFluorescence
The invention discloses a kit for detecting eastern equine encephalitis virus and west equine encephalitis virus by real-time fluorescence quantitative RT-PCR. The kit provided by the invention comprises a primer pair as shown in the following (1) and (2): (1) EEEV forward primer: TGTGCGTACCTCCTCATCGTT, EEEV reverse primer: GACTGGCGTGAATCTCTGCT; (2) WEEV forward primer: AGGGATACCCCCGAAGGTT, WEEV reverse primer: GTGAATAGCACACGGGTGGTT. The kit can be applied to detecting EEEV and WEEV and has good sensitivity and specificity; in addition, the lowest EEEV virus titer that the kit can detect is 0.2TCID50 / ml and the WEEV virus titer that the kit can detect is 1TCID50 / ml.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Compositions and methods for detecting certain flaviviruses, including members of the japanese encephalitis virus serogroup
ActiveCN1795275AMicrobiological testing/measurementAgainst vector-borne diseasesSalmonella serotype typhiEncephalitis Viruses
The present invention provides rapid and accurate methods, primers, probes and kits for detecting certain flaviviruses in samples. Detectable flaviviruses include Japanese encephalitis virus serogroup members, dengue virus, St. Louis encephalitis virus, Montana myotis leukoencephalitis virus, Modoc virus, and yellow fever virus. The primers and probes of the present invention can hybridize to the region within the 3' untranslated region of the genome of the virus to be detected.
Owner:F HOFFMANN LA ROCHE & CO AG
JEV (Japanese type B encephalitis virus) nucleic acid assay kit and application thereof
ActiveCN105349696AReduce sensitivityLow specificityMicrobiological testing/measurementFreeze-dryingMagnesium salt
The invention discloses a JEV (Japanese type B encephalitis virus) nucleic acid assay kit and application thereof. The kit comprises RPA (recombinase polymerase amplification) freeze-dried reagents, PRA reaction primers, an RPA reaction probe, buffer solution, magnesium salt solution and double-distilled water. The RPA reaction probe is used for real-time quantitative detection of RPA nucleic acid amplification products. Low contamination is achieved due to the fact that users do not need to open caps during the whole detection. The whole reaction is performed in a constant temperature of 38 DEG C, and users can determine and read results within 5-20 minutes by observing fluorescent curves. The JEV nucleic acid assay kit has the advantages of high sensitivity and specificity, operational convenience, quick reaction, portability, low contamination and the like.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
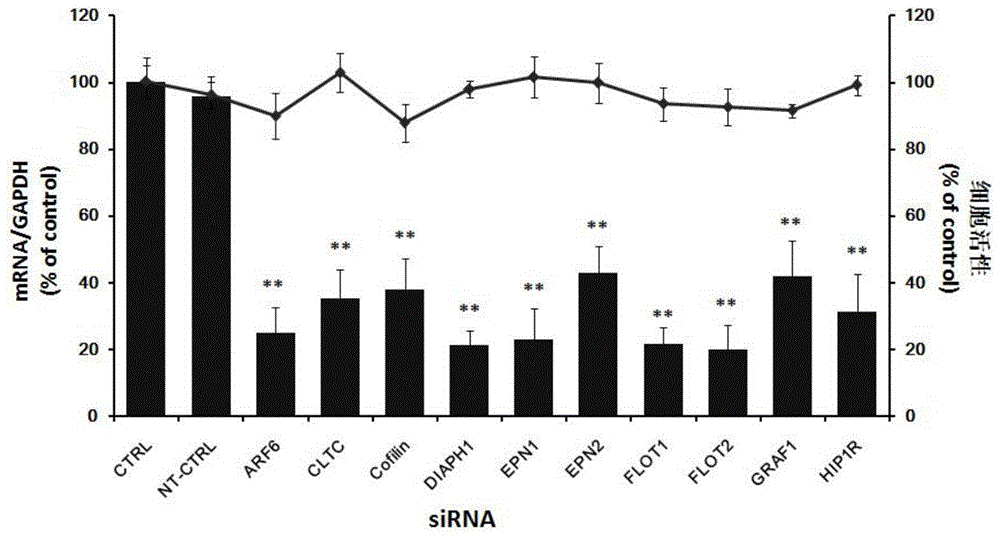
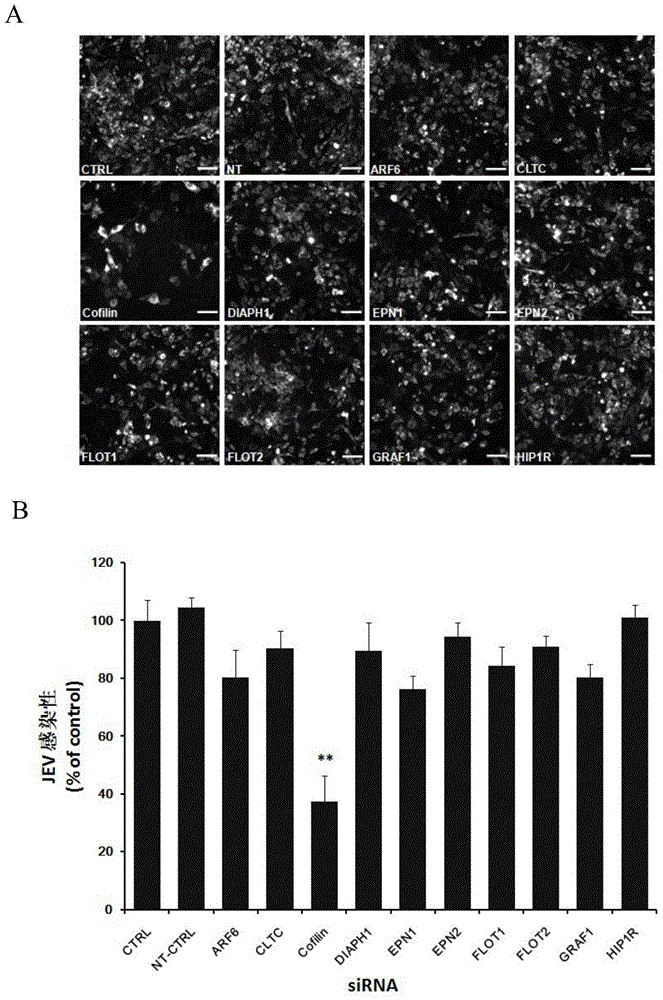
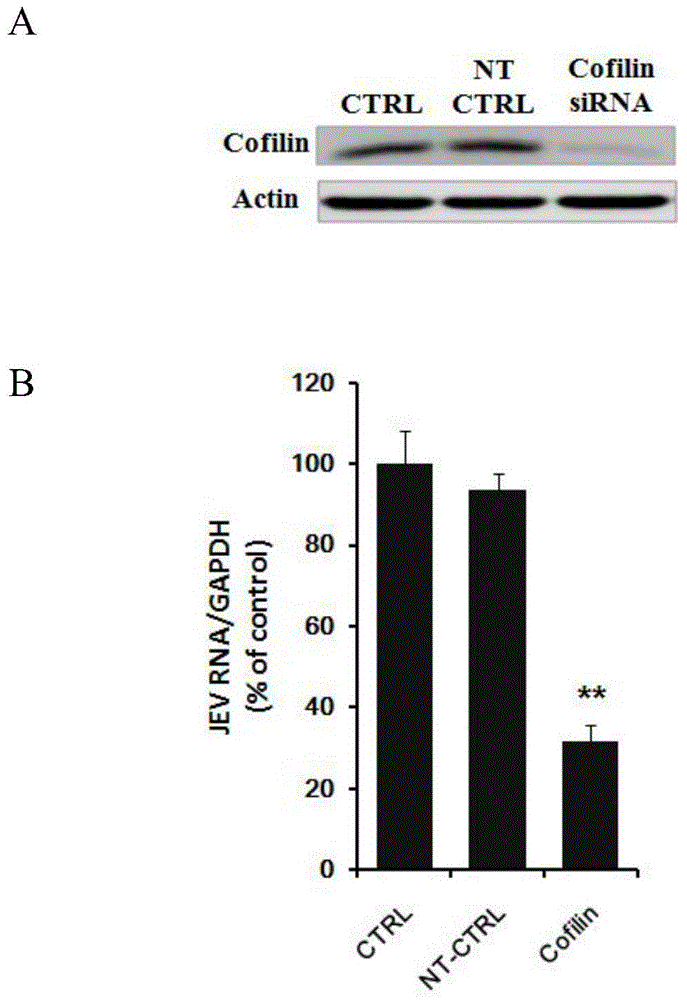
Application of Cofilin in preventing and treating Japanese encephalitis virus infection
The invention relates to the technical field of biomedicine, in particular to a novel target resistant to Japanese encephalitis virus (JEV) infection and application. Human neuroblastoma cells (SK-N-SH) are taken as target cells, RNA technology is adopted to reduce expression of host protein of the target cells to find host factors capable of effectively inhibiting JEV-infected human neuroblastoma cells (SK-N-SH) so as to protect functions of a nerve system and prevent virus from infecting a central nerve system to cause inflammation. The invention provides application of Cofilin in preventing and treating JEV infection, and experiments find that Cofilin plays an important role in JEV-infected SK-N-SH, and JEV infection can be inhibited remarkably by reducing expression of Cofilin. The invention further provides application of Cofilin in preparing drug for preventing or treating JEV infection.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Novel technology for detecting viruses of arbovirus encephalitis
ActiveCN102199672AReduce usageHigh sensitivityMicrobiological testing/measurementEncephalitis VirusesVirus
The invention relates to a method capable of detecting common viruses of arbovirus encephalitis. The invention also relates to an amplification primer and an extension primer which are used for detecting the common viruses of arbovirus encephalitis, and a kit containing the primers.
Owner:BGI GENOMICS CO LTD +1
Epidemic encephalitis B and enterovirus 71 (EV71) combined vaccine
InactiveCN102160892ANo abnormal reactionViral antigen ingredientsAntiviralsHand-foot-and-mouth diseaseEncephalitis Viruses
The invention relates to a combined vaccine prepared from epidemic encephalitis B viral vaccines and enterovirus 71 (EV71) viral vaccines and a preparation method thereof. An epidemic encephalitis B viral stock solution prepared from the epidemic encephalitis B virus through culture, harvest, inactivation, ultrafilter concentration and purification and an EV71 viral stock solution prepared from the EV71 virus through culture, harvest, inactivation, ultrafilter concentration and purification are prepared according to a certain proportion to form the combined vaccine. The combined vaccine can be used for simultaneously preventing and treating the hand-foot-and-mouth disease suffered by infants and children caused by the epidemic encephalitis B and the EV71 virus.
Method and kit for detecting multiple encephalitis related viruses
ActiveCN102796827AImprove throughputAddresses issues with reduced sensitivityMicrobiological testing/measurementDNA/RNA fragmentationEastern equine encephalitis virusMicrosphere
The invention provides a method and a kit for detecting multiple encephalitis related viruses. In particular, the invention discloses a method for simultaneously detecting multiple encephalitis related viruses. The method comprises the following steps of: performing polymerase chain reaction (PCR) by using a specific primer set aiming at the encephalitis related viruses in a polymerase reaction system to obtain an amplification product; and detecting with specific probes or probe microspheres. The invention also provides the corresponding kit. The method and the kit can be used for sensitively and simply detecting and identifying multiple encephalitis related viruses comprising eastern equine encephalitis viruses, western equine encephalitis viruses, Venezuelan equine encephalitis viruses, forest encephalitis viruses and Japanese encephalitis viruses.
Owner:SHANGHAI TELLGEN LIFE SCI CO LTD
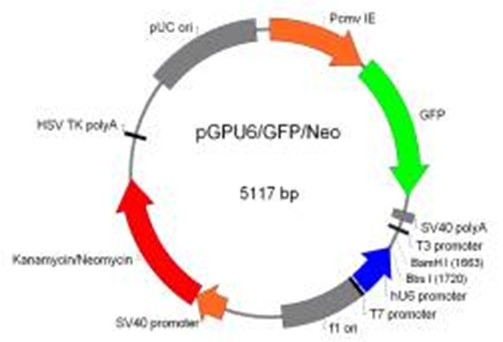
shRNA capable of inhibiting replication and infection of Japanese encephalitis virus and application thereof
InactiveCN102002499AImprove protectionInhibition of replicationGenetic material ingredientsAntiviralsConserved sequenceEncephalitis Viruses
The invention belongs to the technical field of genetic engineering and discloses shRNA capable of inhibiting replication and infection of Japanese encephalitis virus and application thereof. The shRNA takes a highly-conserved sequence in a Japanese encephalitis virus genome as a target sequence which is SEQIDNO.9, SEQIDNO.10, SEQIDNO.11 or SEQIDNO.12. The shRNA eukaryotic expression plasmid can be obtained by connecting a template of the shRNA with a linearized vector pGPU6 / GFP / Neo and respectively aims at Japanese encephalitis virus E and NS3 or NS4b gene mRNA sequence. The shRNA and the shRNA eukaryotic expression plasmid provided by the invention can be applied to a medicament for treating and / or preventing JEV (Japanese Encephalitis Virus) infection and provides a novel selectable medicament for treating and preventing the JEV infection.
Owner:NANJING AGRICULTURAL UNIVERSITY
RT-PCR primer and probe combination for simultaneous detection of 8 arbo encephalitis viruses and kit
InactiveCN106167833AAmplification efficiency is comparableGuaranteed high sensitivityMicrobiological testing/measurementDNA/RNA fragmentationEncephalitis VirusesArbovirus Infections
The invention specifically discloses an RT-PCR primer and probe combination for simultaneous detection of 8 arbo encephalitis viruses and a kit. The primer and probe have sequences shown as SEQ ID NO:1-24. The invention is based on the primer and probe combination, combines the sensitive and reliable advantages of real-time quantitative PCR technology and the advantages of simultaneous detection of multi-gene expression amount by microarray technology, and develops an RT-PCR array kit for simultaneous detection of 8 arbo encephalitis viruses. The kit can rapidly detect 8 arbo encephalitis viruses simultaneously by one RT-PCR reaction so as to realize integration and high throughput in RT-PCR detection of multiple pathogens. The kit provided by the invention can be applied to large-scale pathogen screening, provides technical support for diagnose of infection of pathogenic encephalitis arbo virus infection and determination of infection sources, and has important practical significance to clinical prevention and control of pathogenic encephalitis.
Owner:SOUTHERN MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com