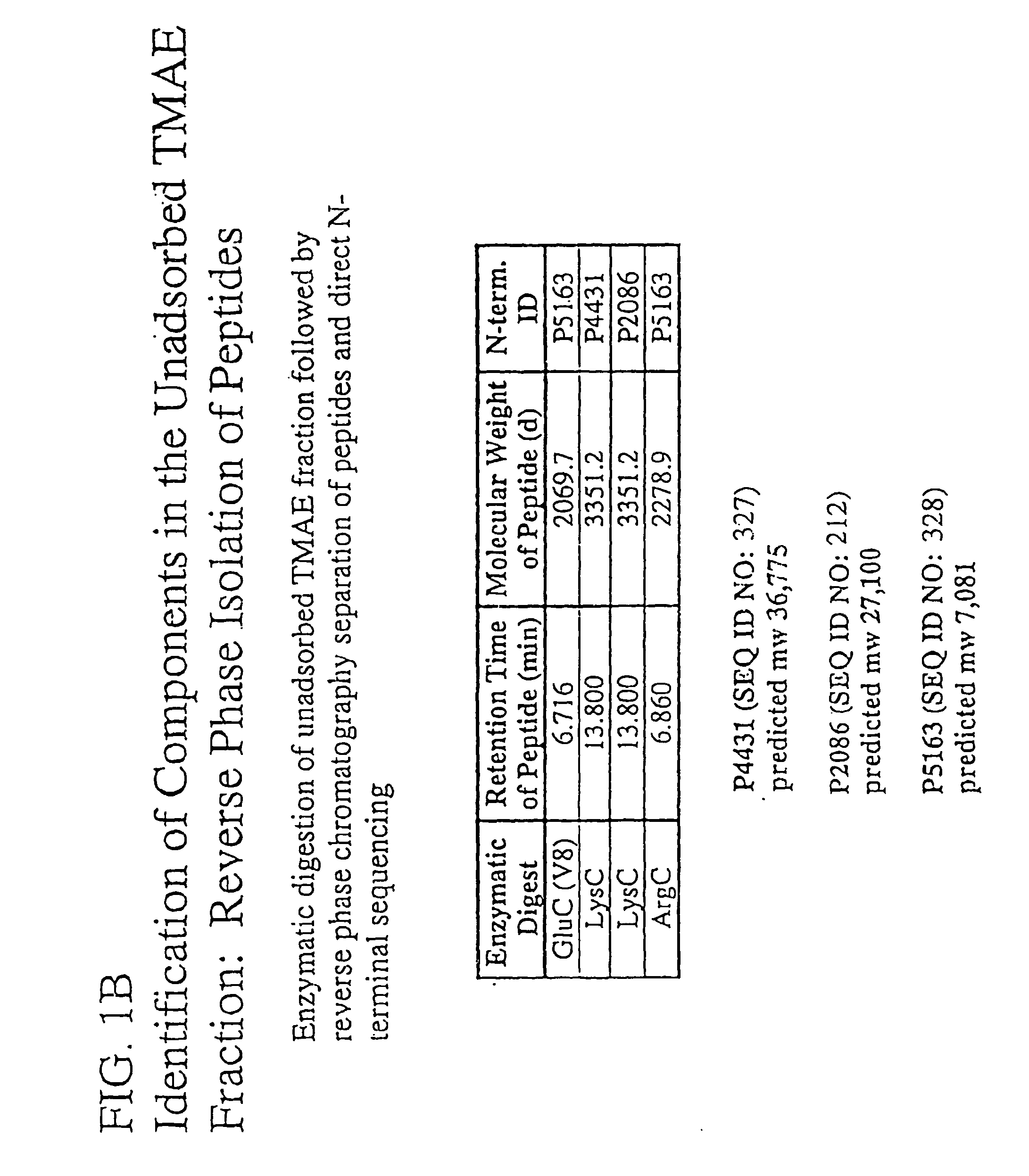
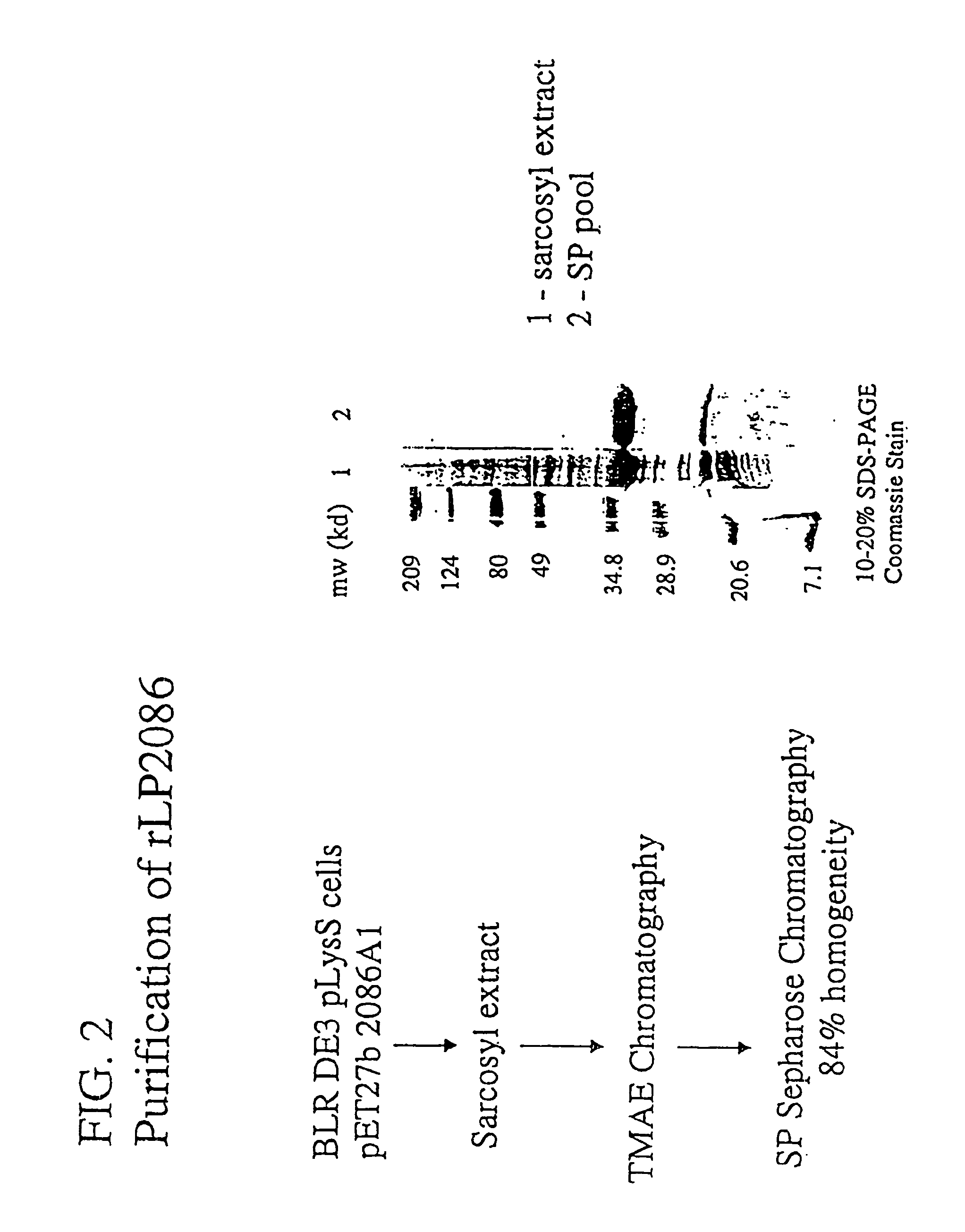
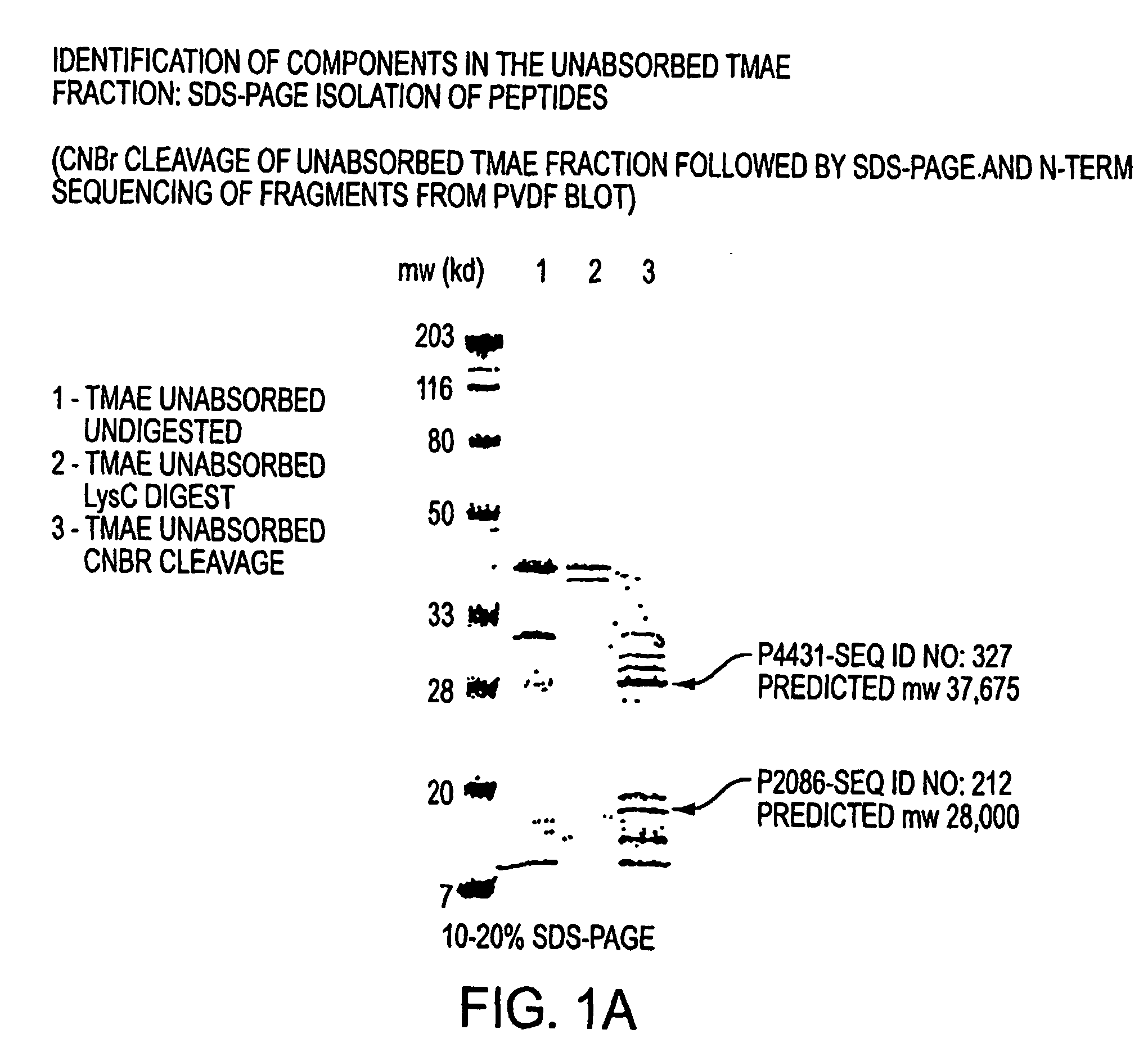
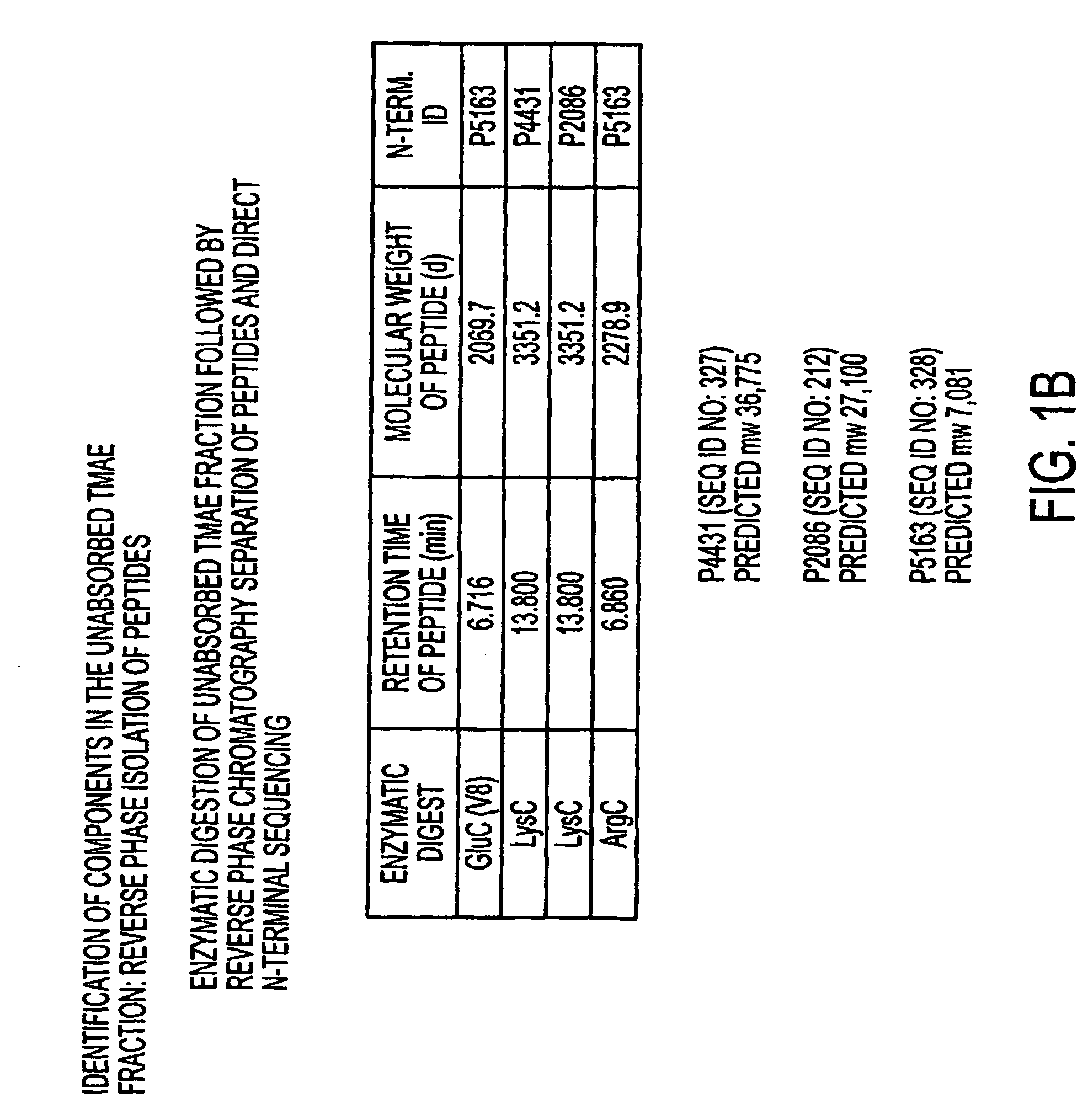
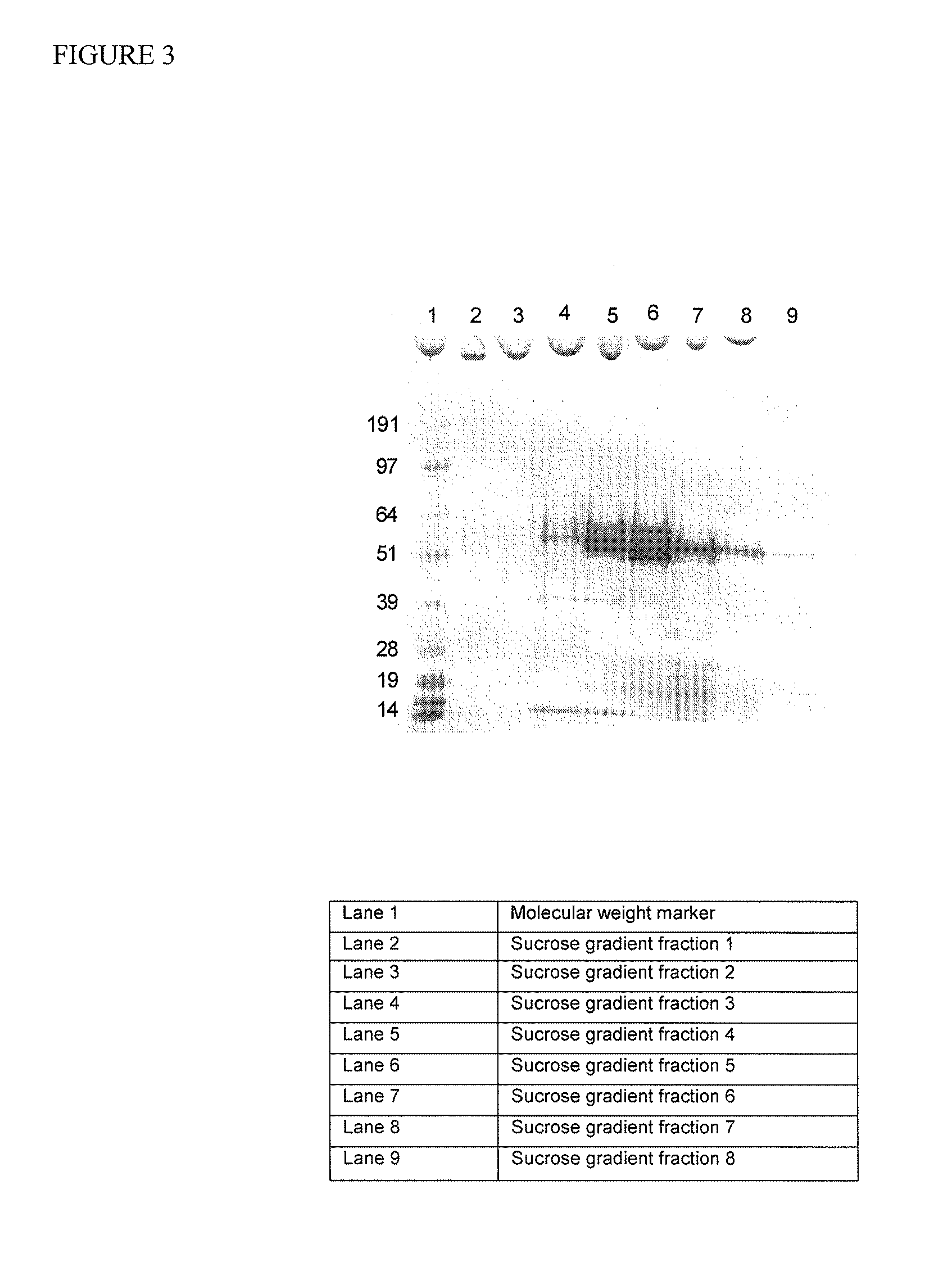
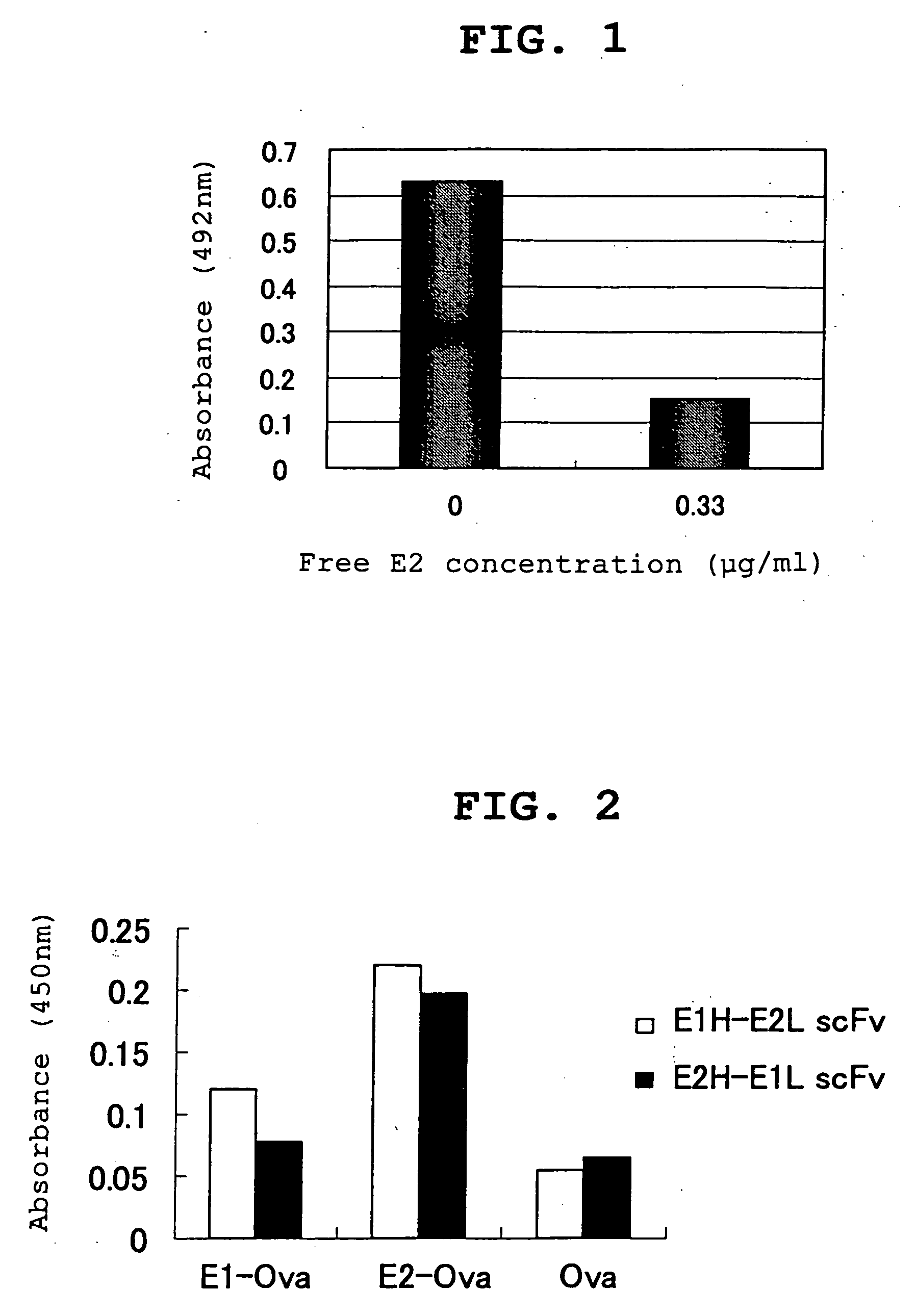
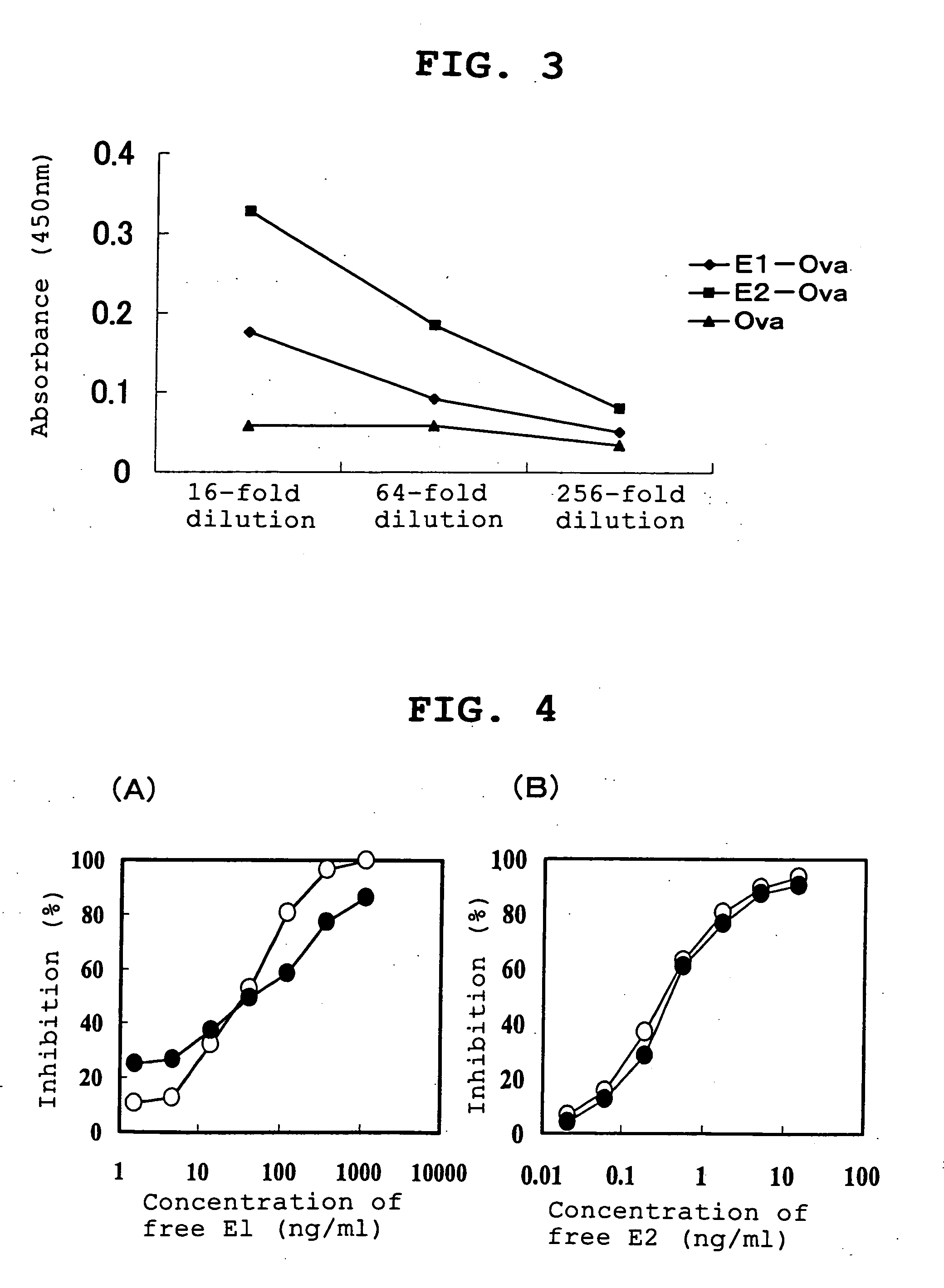
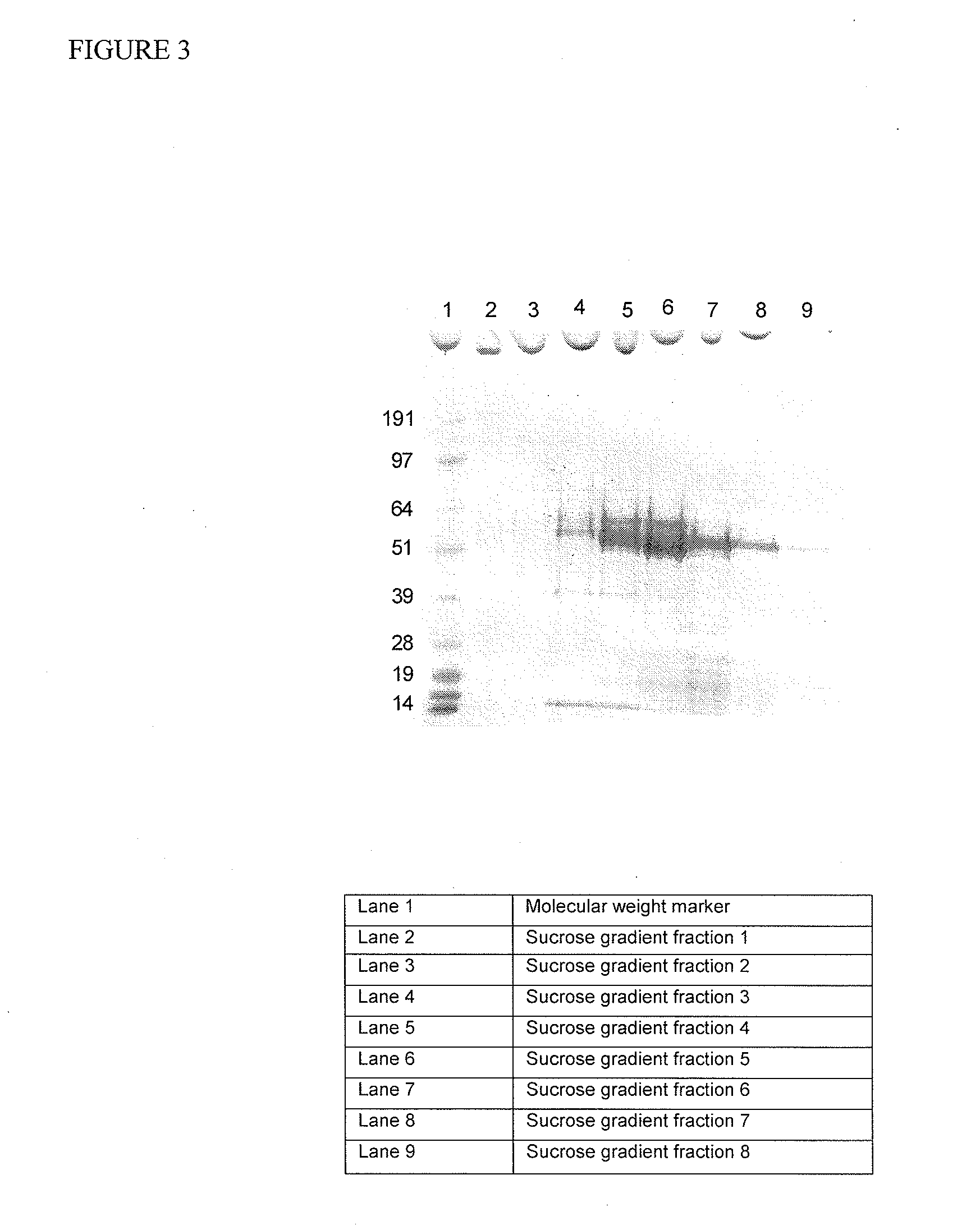
Patents
Literature
325 results about "Cross-reactivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cross-reactivity, in a general sense, is the reactivity of an observed agent which initiates reactions outside the main reaction expected. In immunology, the cross-reactivity has a more narrow meaning of the reaction between an antibody and an antigen that differs from the immunogen. It is sometimes also referred to as crossimmunity or cross-protective immunity, although cross-reactivity does not necessarily infer cross-protection. A few examples of cross-reactivity have been confirmed in humans, one of which involves influenza virus-specific CD8+ T cell and hepatitis C virus antigens.
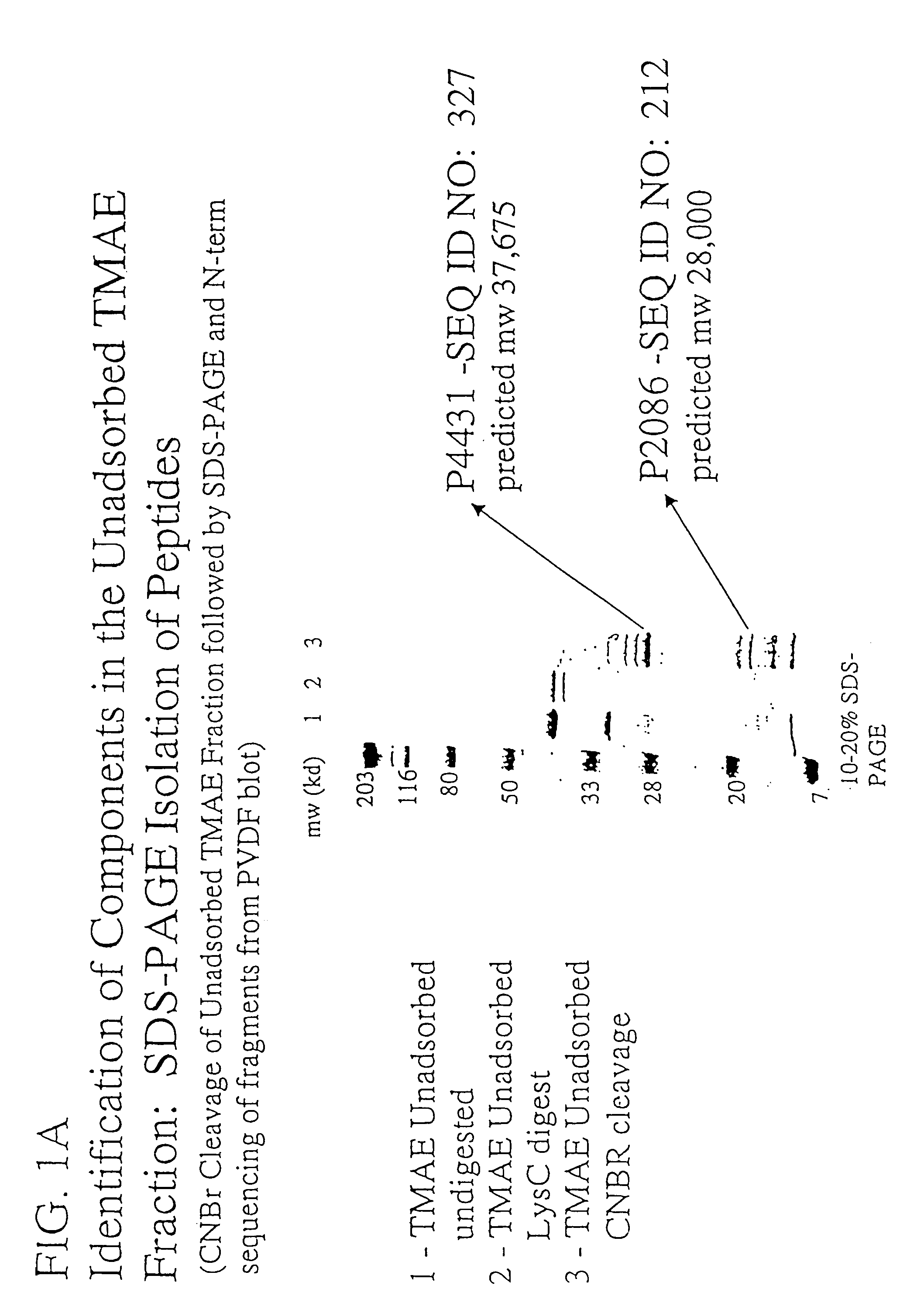
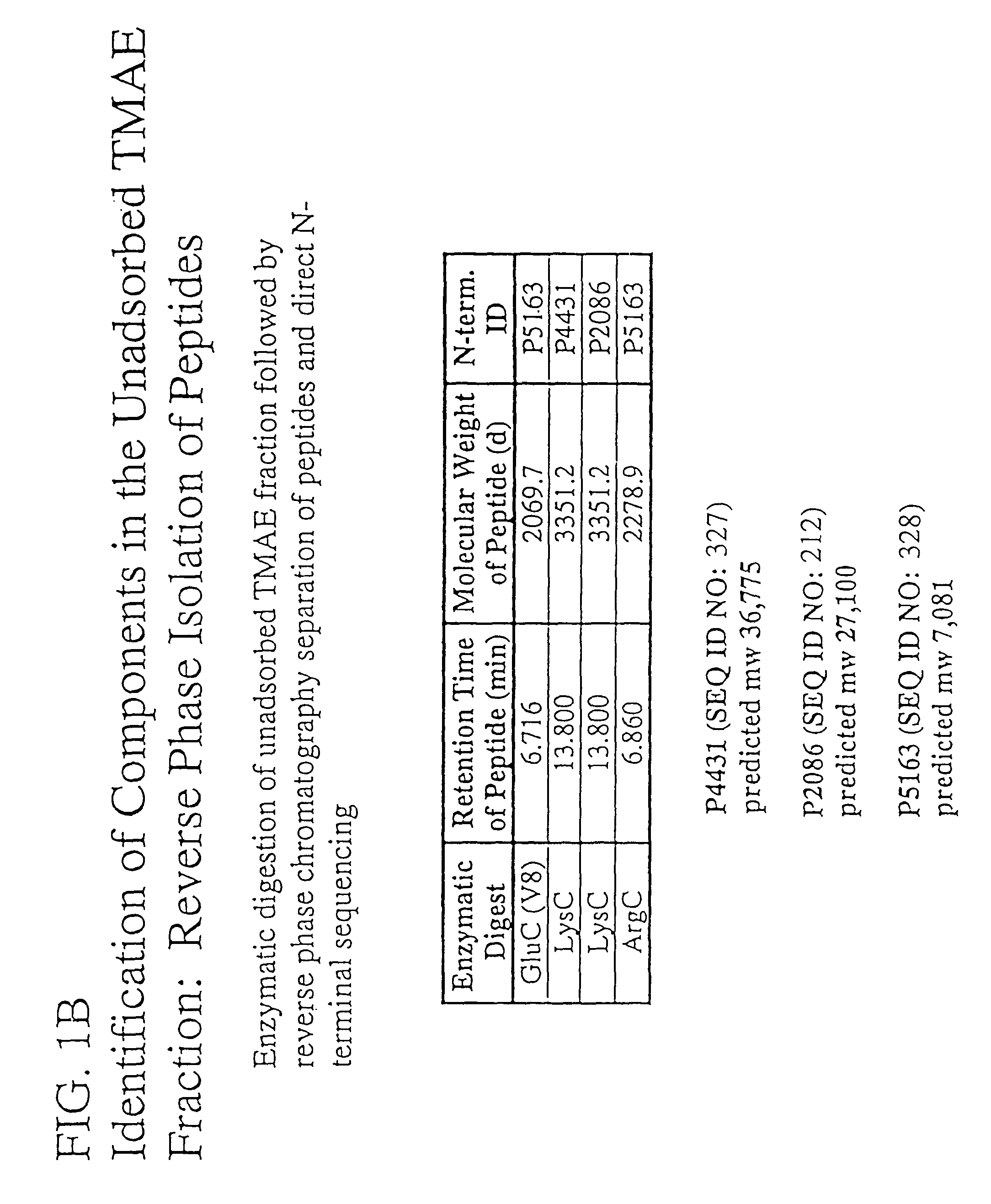
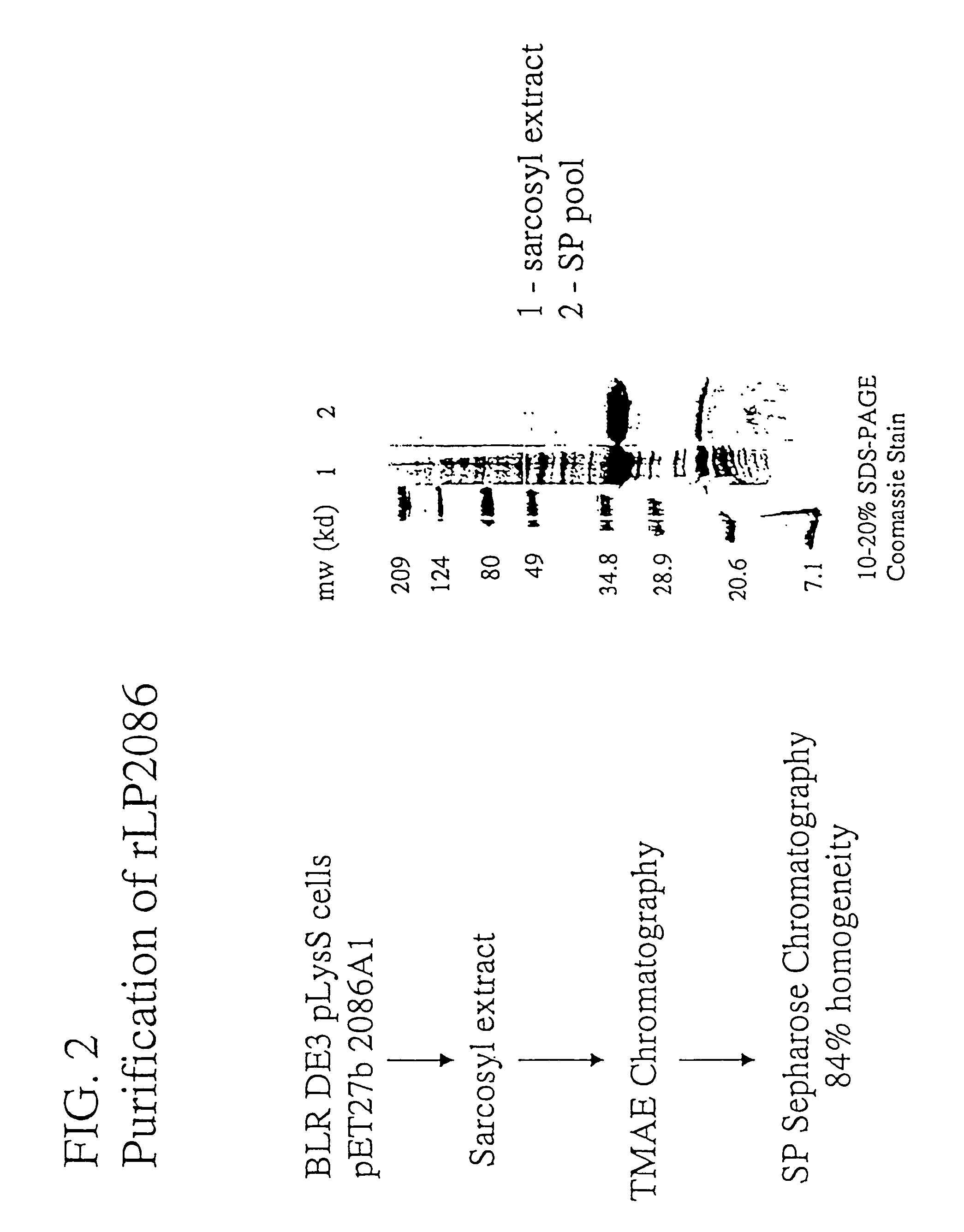
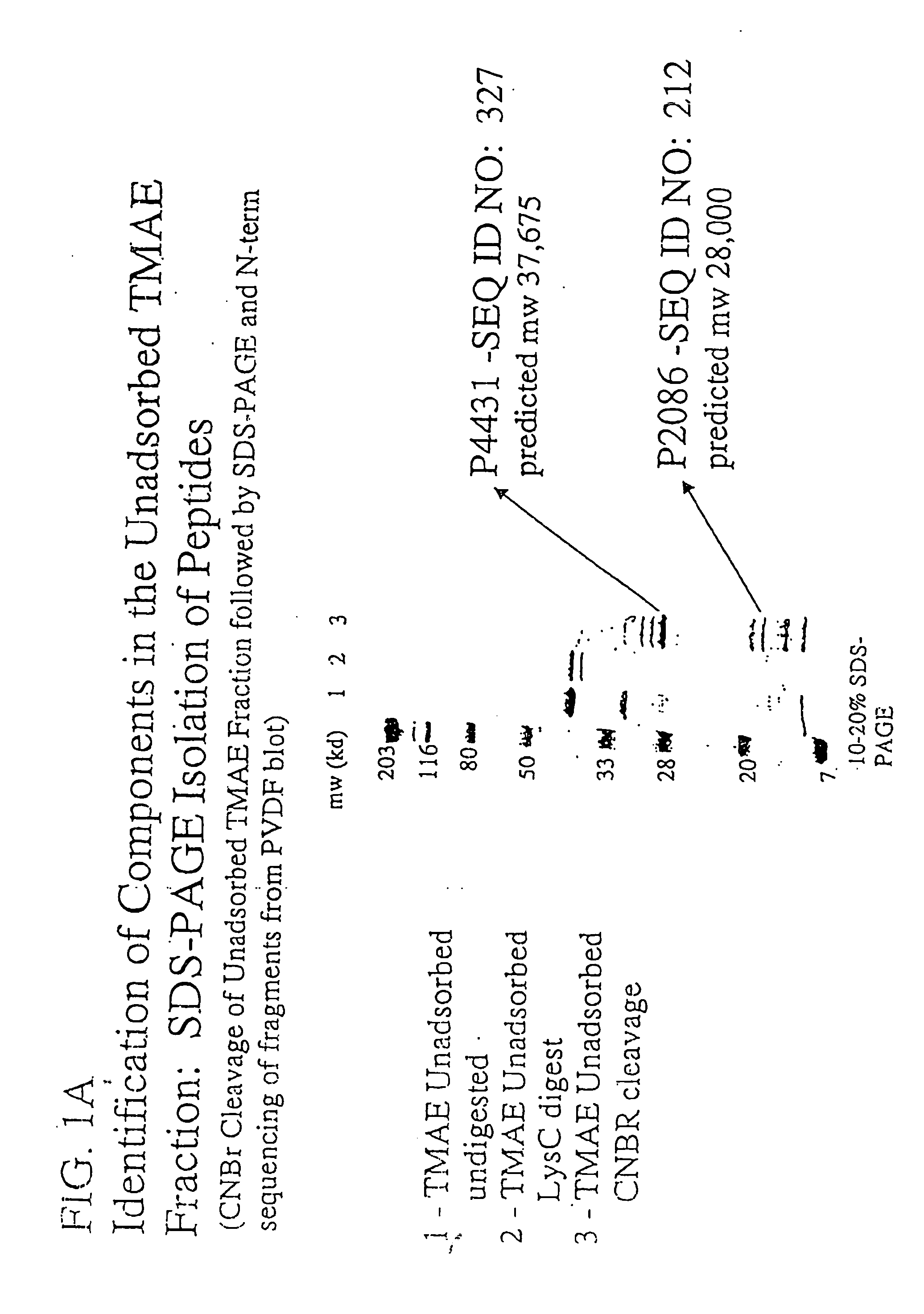
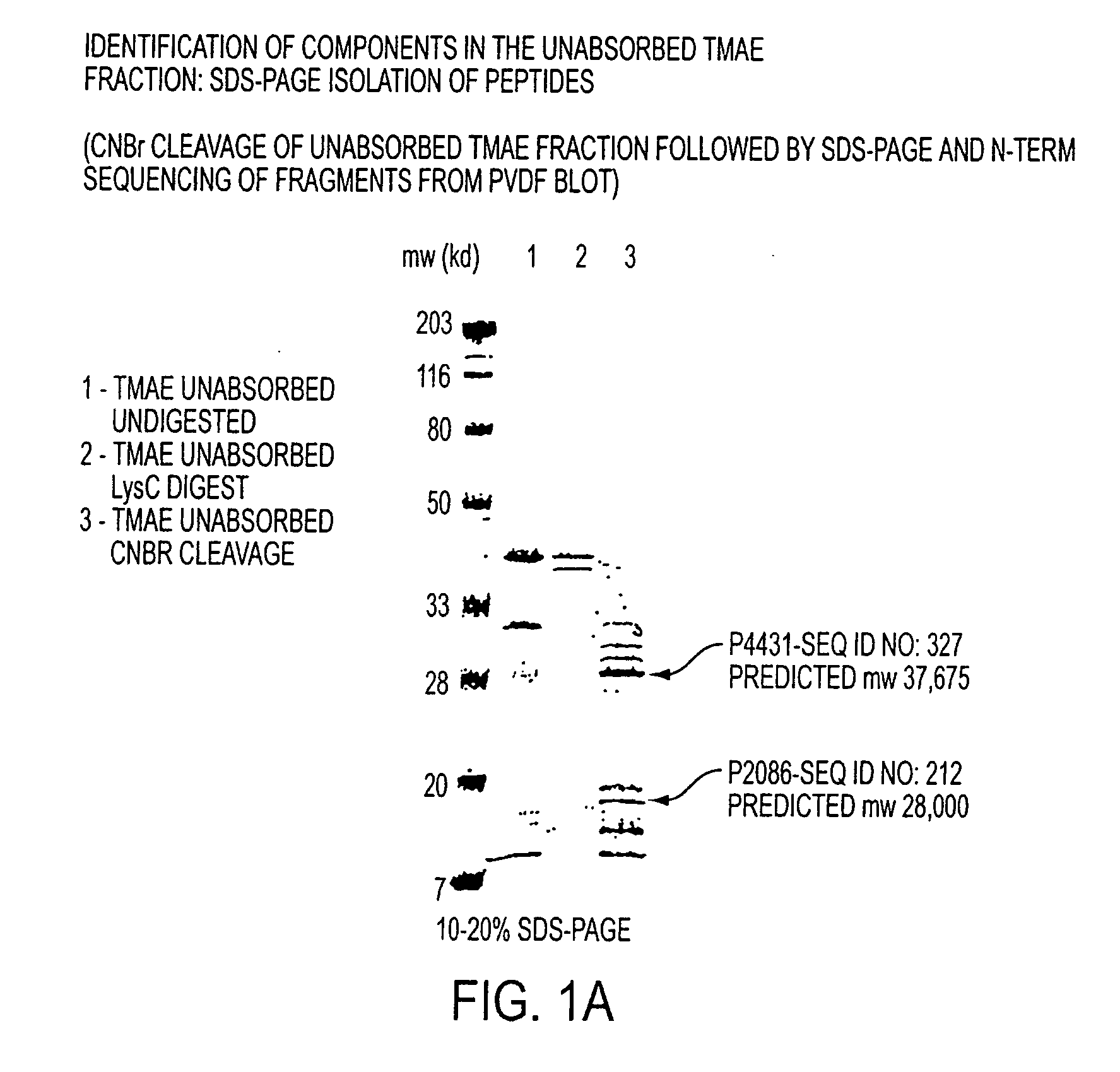
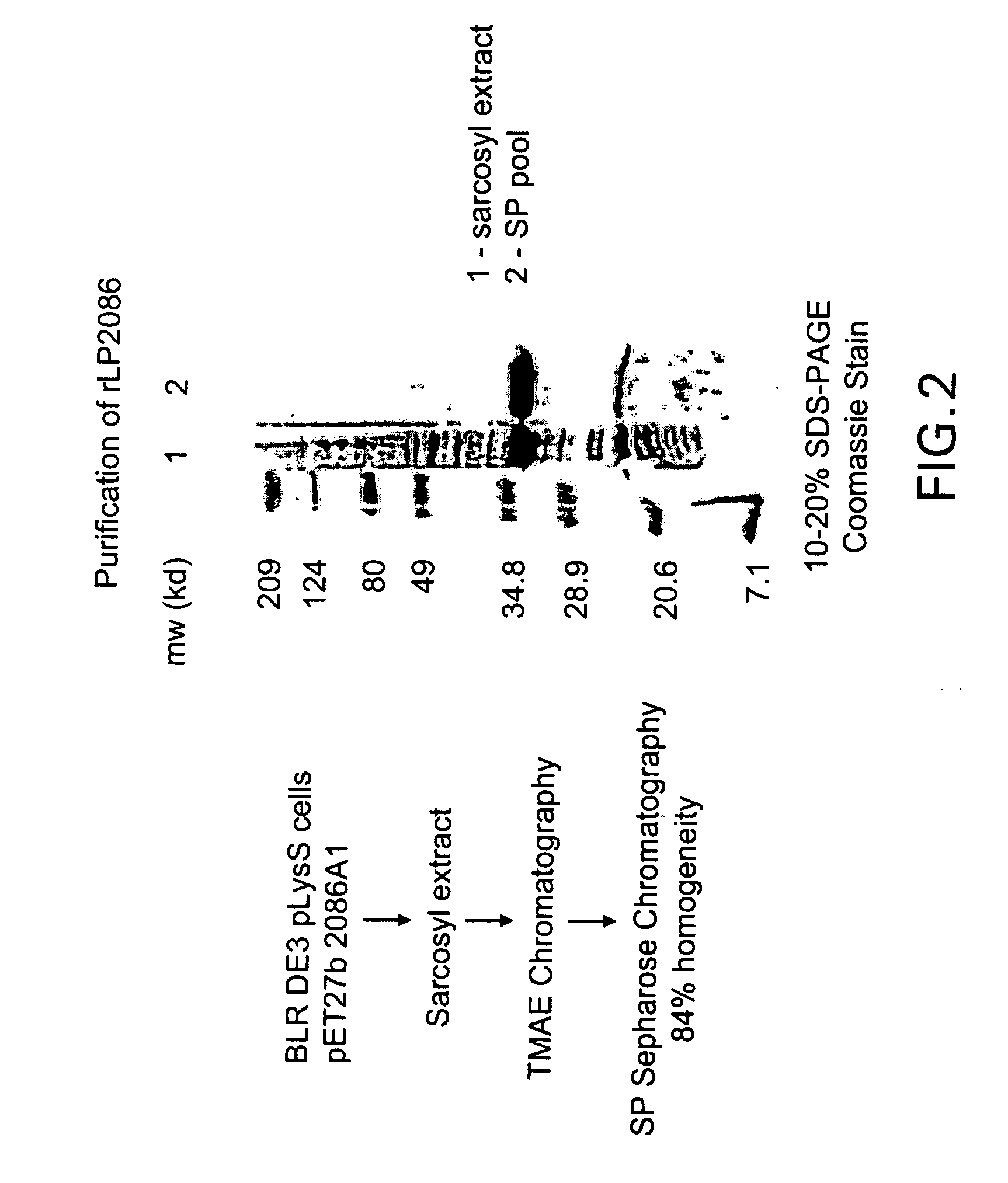
Immunogenic compositions for the prevention and treatment of meningococcal disease
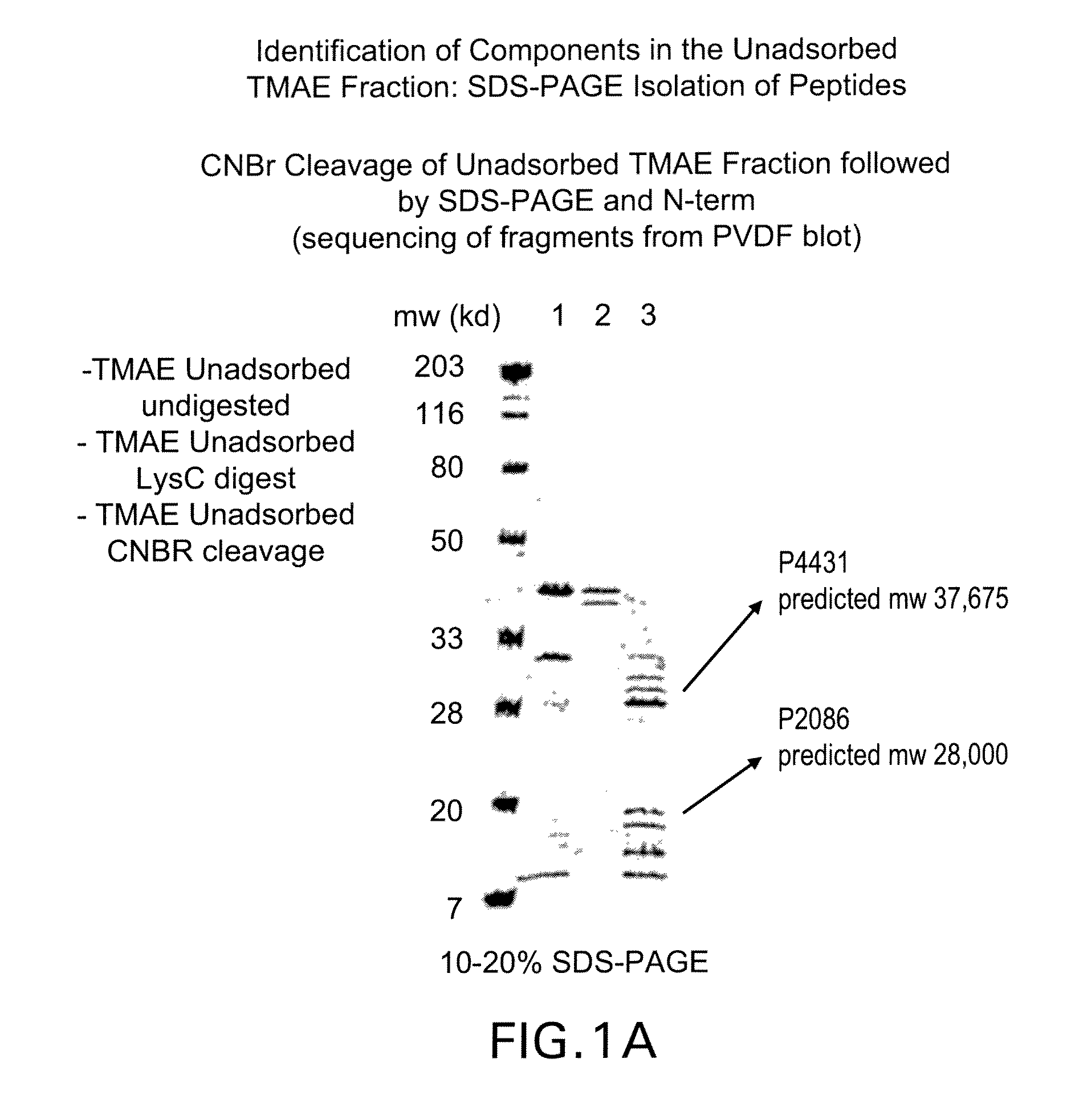
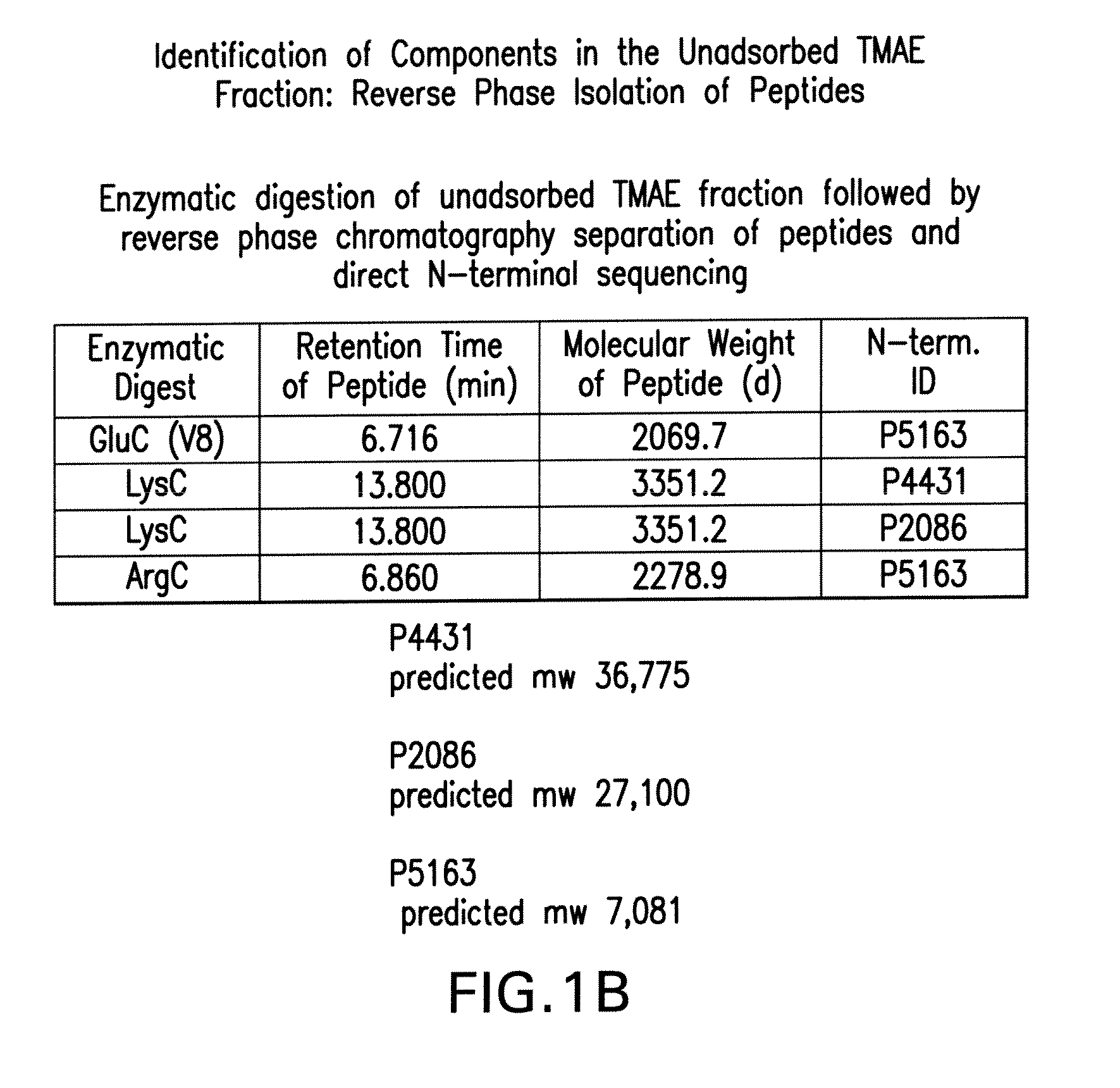
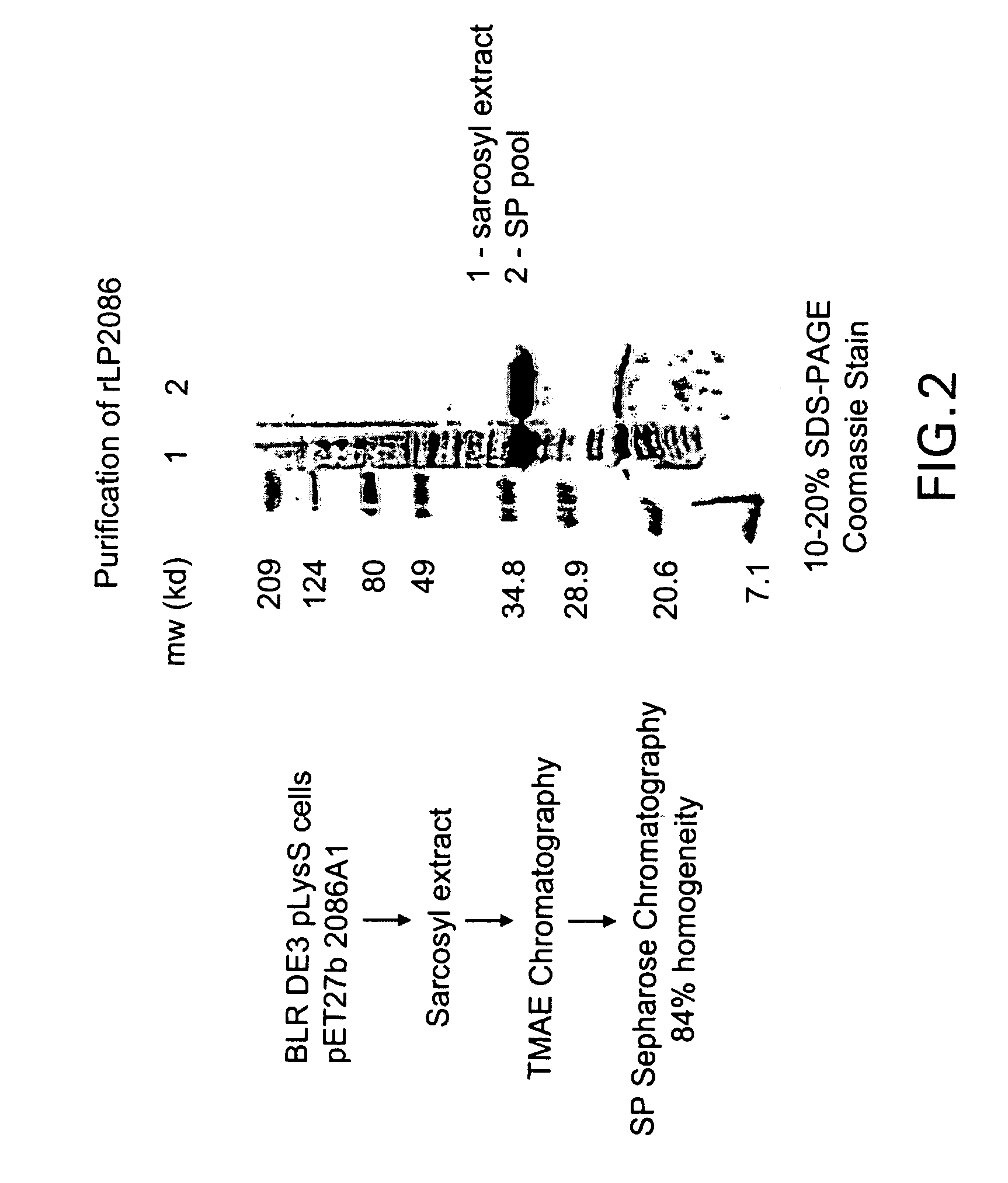
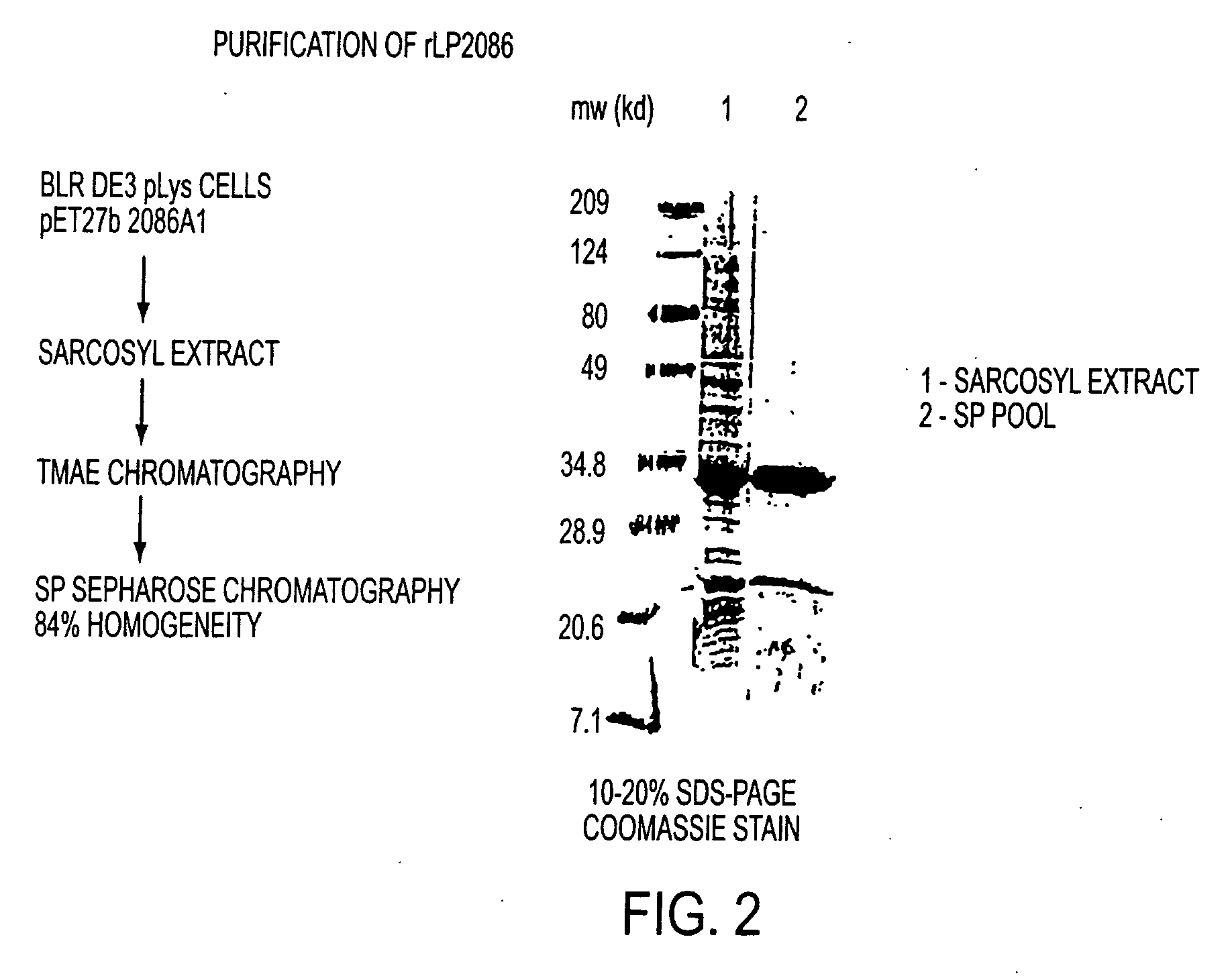
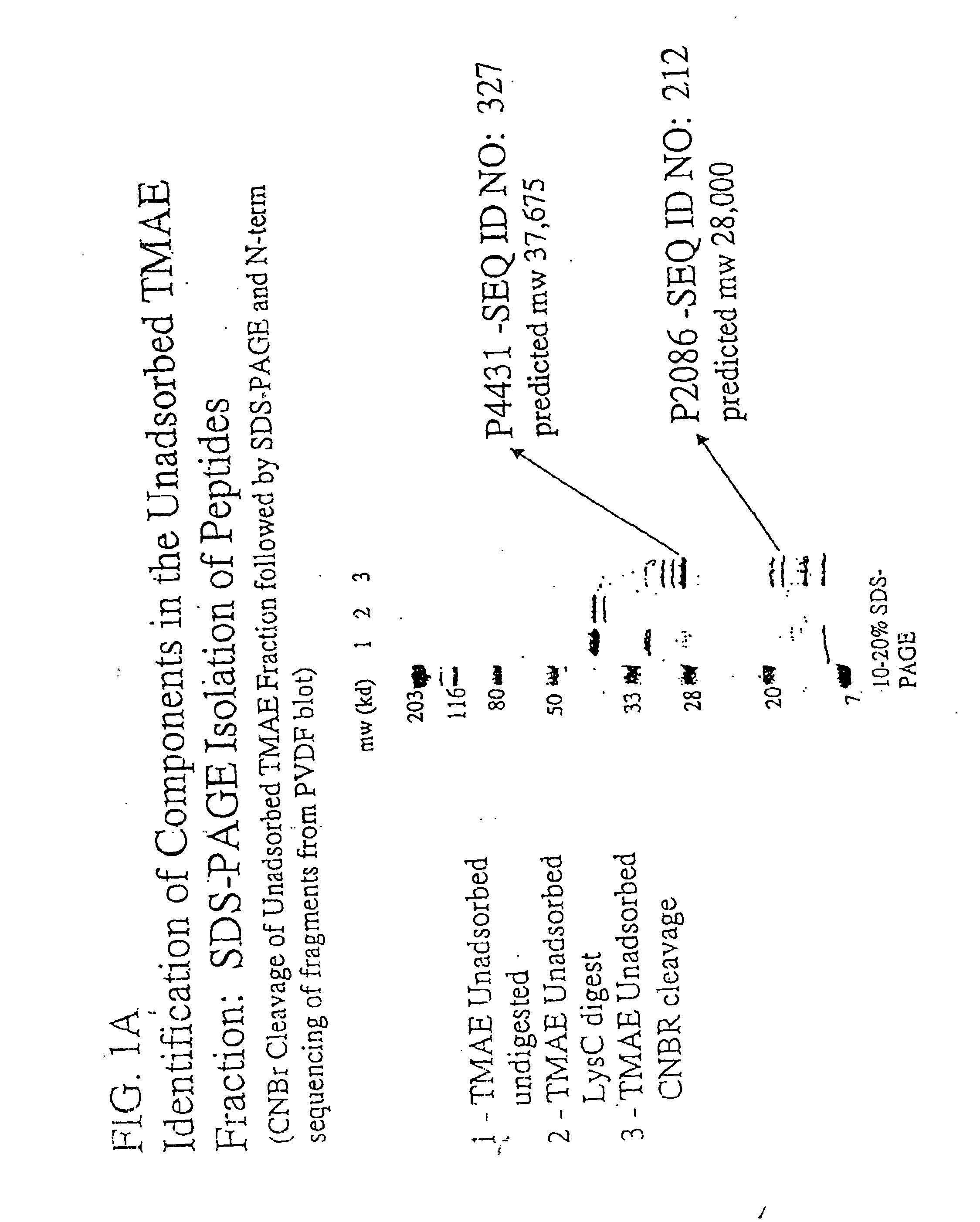
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH HOLDINGS LLC
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH HOLDINGS LLC
Aflatoxin nano antibody gene pool, construction method and application of aflatoxin nano antibody gene pool as well as aflatoxin B1 nano antibody 2014AFB-G15
ActiveCN103866401AEasy to buildResistant to organic reagentsImmunoglobulins against fungi/algae/lichensBiological testingGene poolAntibody variable region
The invention relates to an aflatoxin nano antibody gene pool, a construction method and application of the aflatoxin nano antibody gene pool as well as an aflatoxin B1 nano antibody 2014AFB-G15. The aflatoxin nano antibody gene pool is prepared by extracting RNA (ribonucleic acid) in alpaca blood after immunization of an aflatoxin B1 antigen, performing specific amplification on a variable region gene of an alpaca heavy chain antibody by adopting an RT-PCR (reverse transcription-polymerase chain reaction) method to obtain an aflatoxin nano antibody VHH gene, and then performing transformation after connecting with a pCANTAB5E (his) vector. The aflatoxin B1 nano antibody 2014AFB-G15 obtained by screening, disclosed by the invention, has the characteristics of organic reagent resistance, high temperature resistance and the like, and is good in stability; the IC50 (half maximal inhibitory concentration) of the aflatoxin B1 nano antibody 2014AFB-G15 to the aflatoxin B1 is 0.66ng / mL, and the cross reactivity of the aflatoxin B1 nano antibody 2014AFB-G15 to the aflatoxins B2, G1,G2 and M1 is 22.6%, 0.95%, 32.1% and 26% respectively.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Composition of a first non-labeled monoclonal antibody binding to a tumor antigen and a non-cross reactive second monoclonal antibody labeled with a nir fluorescence label
ActiveUS20110027190A1Maximizes tissue penetrationMinimize absorptionUltrasonic/sonic/infrasonic diagnosticsSurgeryCo administrationMonoclonal antibody
This invention relates to a composition of a non-labeled monoclonal antibody binding to a tumor antigen and a second monoclonal antibody labeled with a NIR fluorescence label, binding to the same tumor antigen, wherein the first and second antibody exhibit no cross reactivity. The composition can be used for the treatment of patients suffering of solid tumors which are associated with an overexpression of such a tumor antigen. The invention further relates to a the co-administration of said first and second antibody as wells as to a method of acquiring a NIR fluorescence images of such tumors or the patients suffering from such tumors during the treatment of said patient with such composition.
Owner:F HOFFMANN LA ROCHE & CO AG
Immunogenic compositions derived from poxviruses and methods of using same
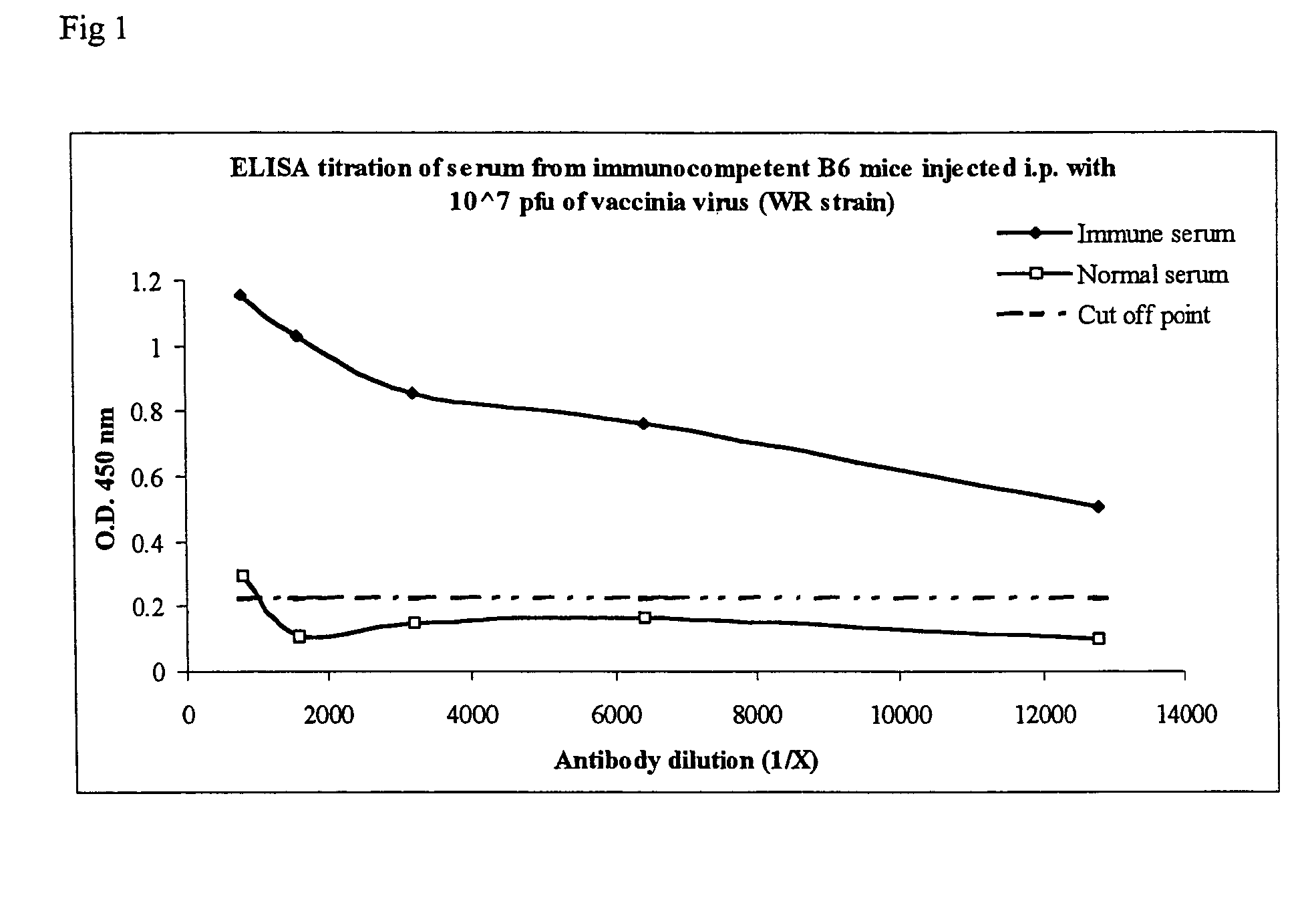
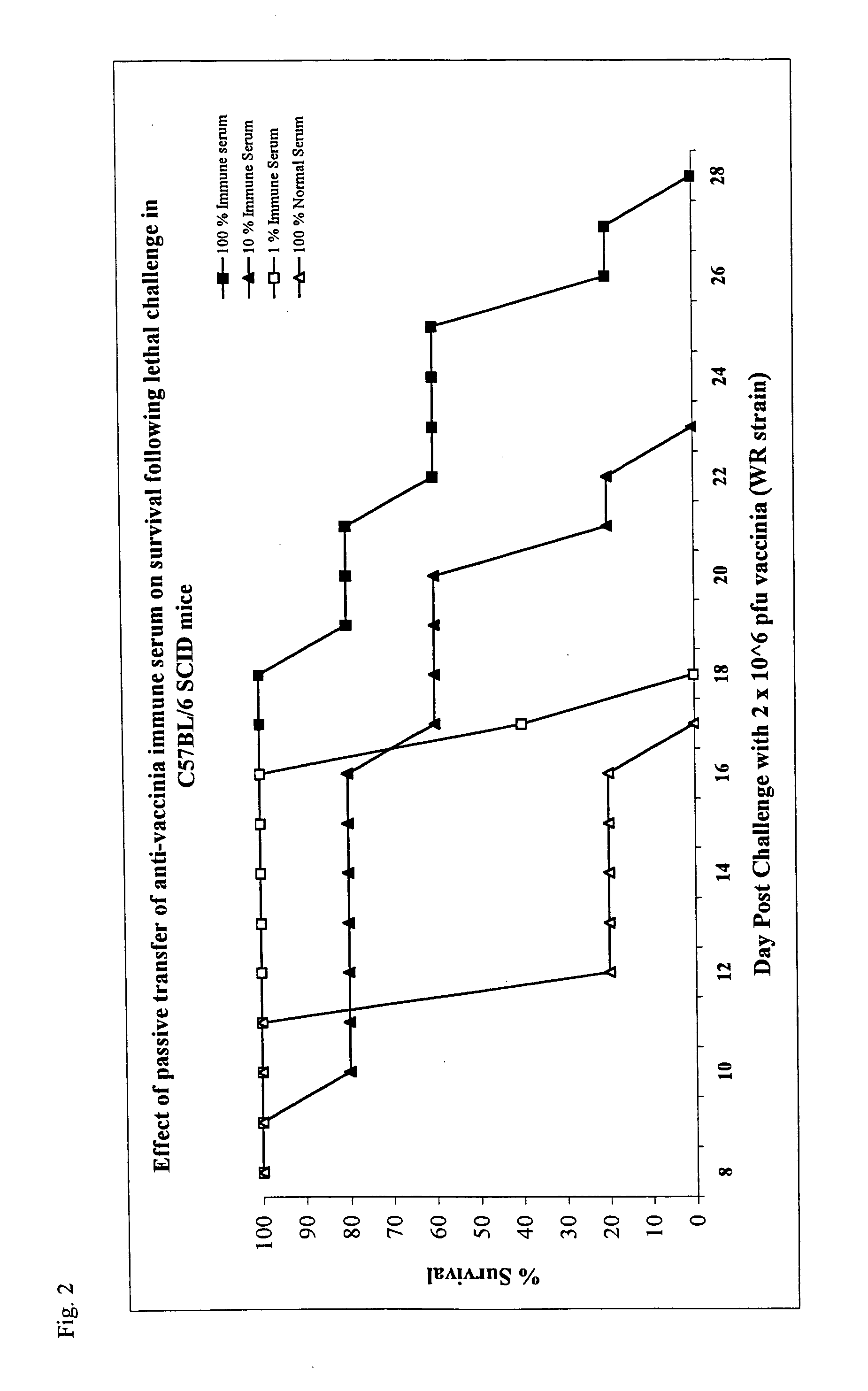
Immunogenic compostions composed of poxvirus immunogens and related methods are disclosed. Specifically, immunogenic compostions useful in eliciting immune responses in animals are disclosed. In one embodiment the immunogenic compostions include viral antigens derived from vaccinia and / or variola that elicit cross-reactive immune responses. The immunogens can be made synthetically, by using recombinant DNA technology or derived from purified virus. Moreover, methods of using the immunogenic compostions are also disclosed.
Owner:MANNKIND CORP
Antagonists to IL-17A, IL-17F, and IL-23P19 and methods of use
Owner:ZYMOGENETICS INC
Immunogenic compositions for the prevention and treatment of meningococcal disease
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH LLC
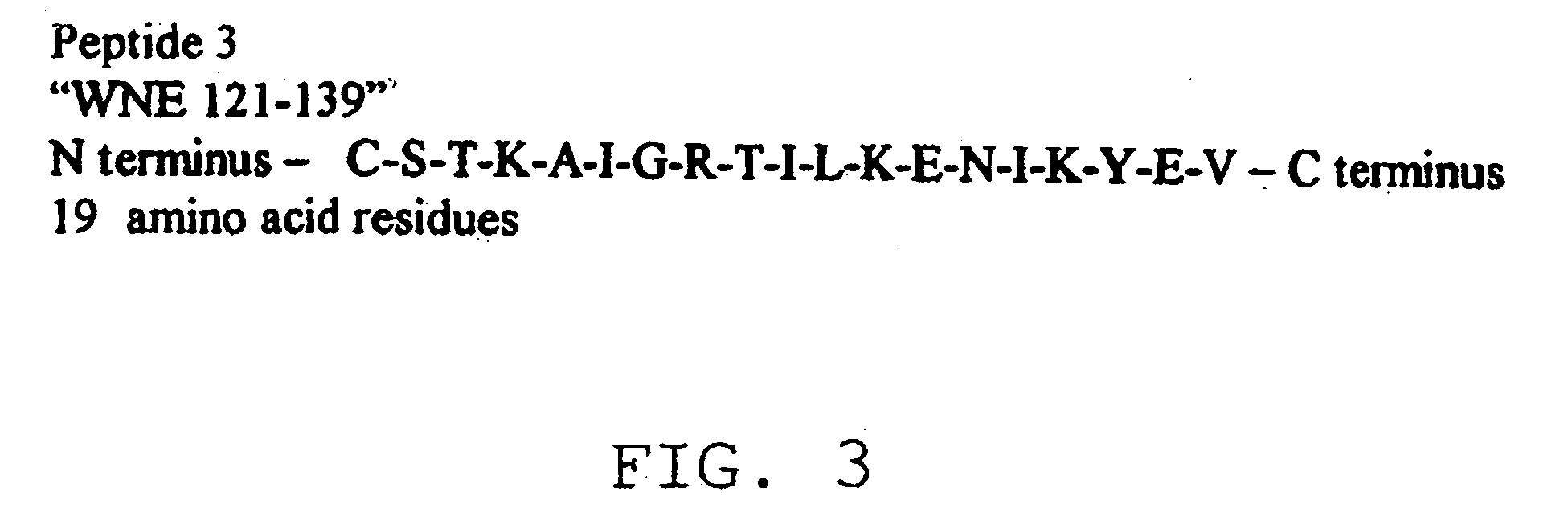
Diagnostic test for West Nile virus
InactiveUS20040197769A1More sensitiveEasy to useViral antigen ingredientsMicrobiological testing/measurementSt Louis encephalitis virusSerum ige
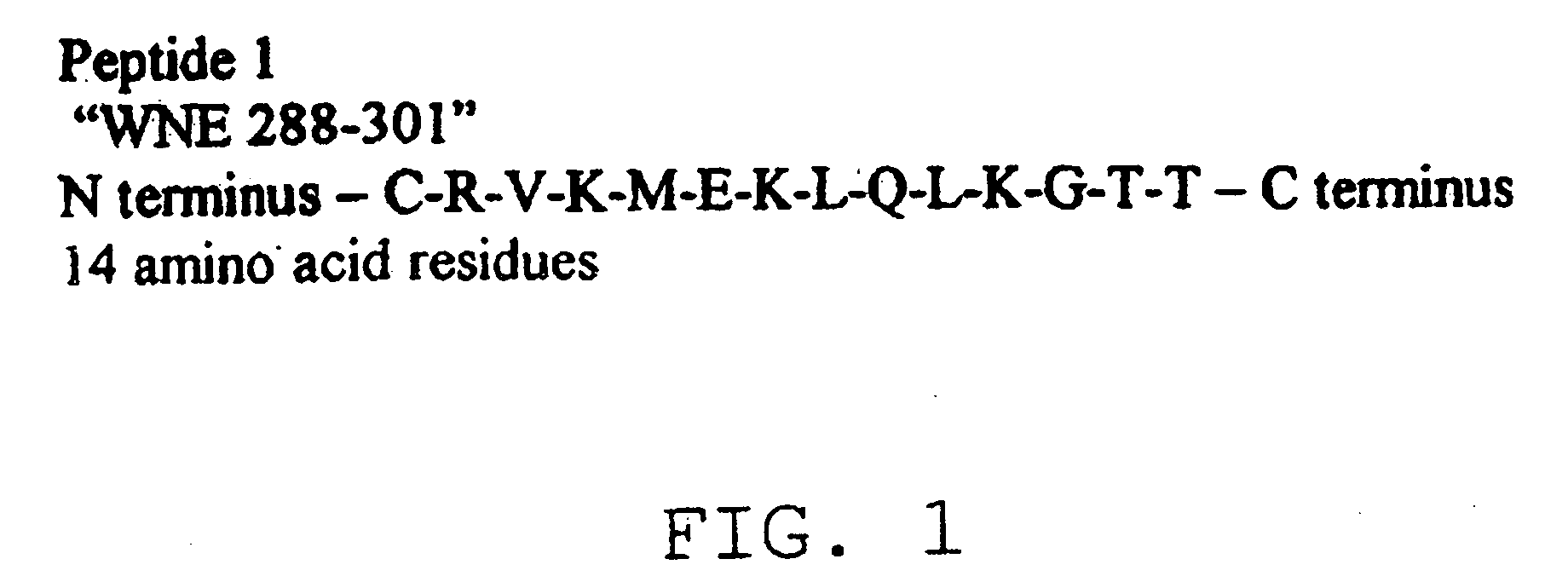
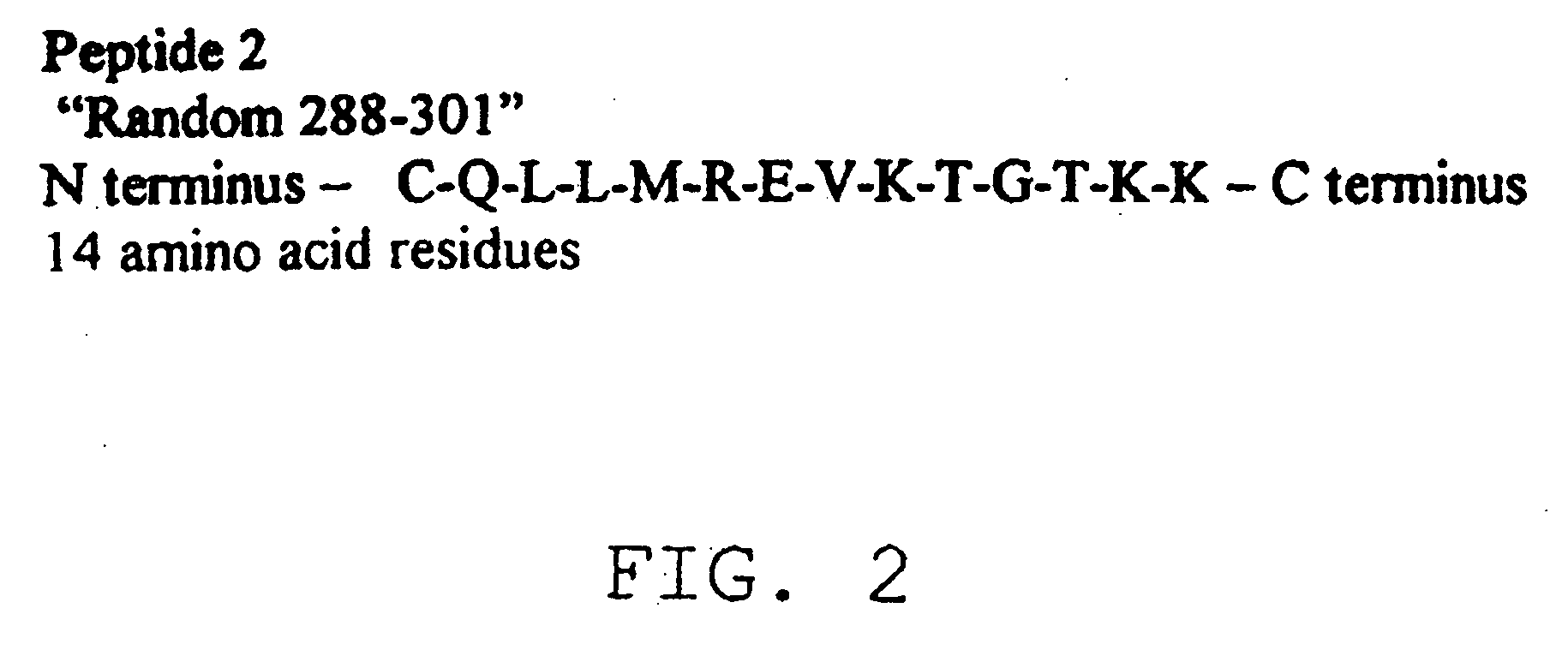
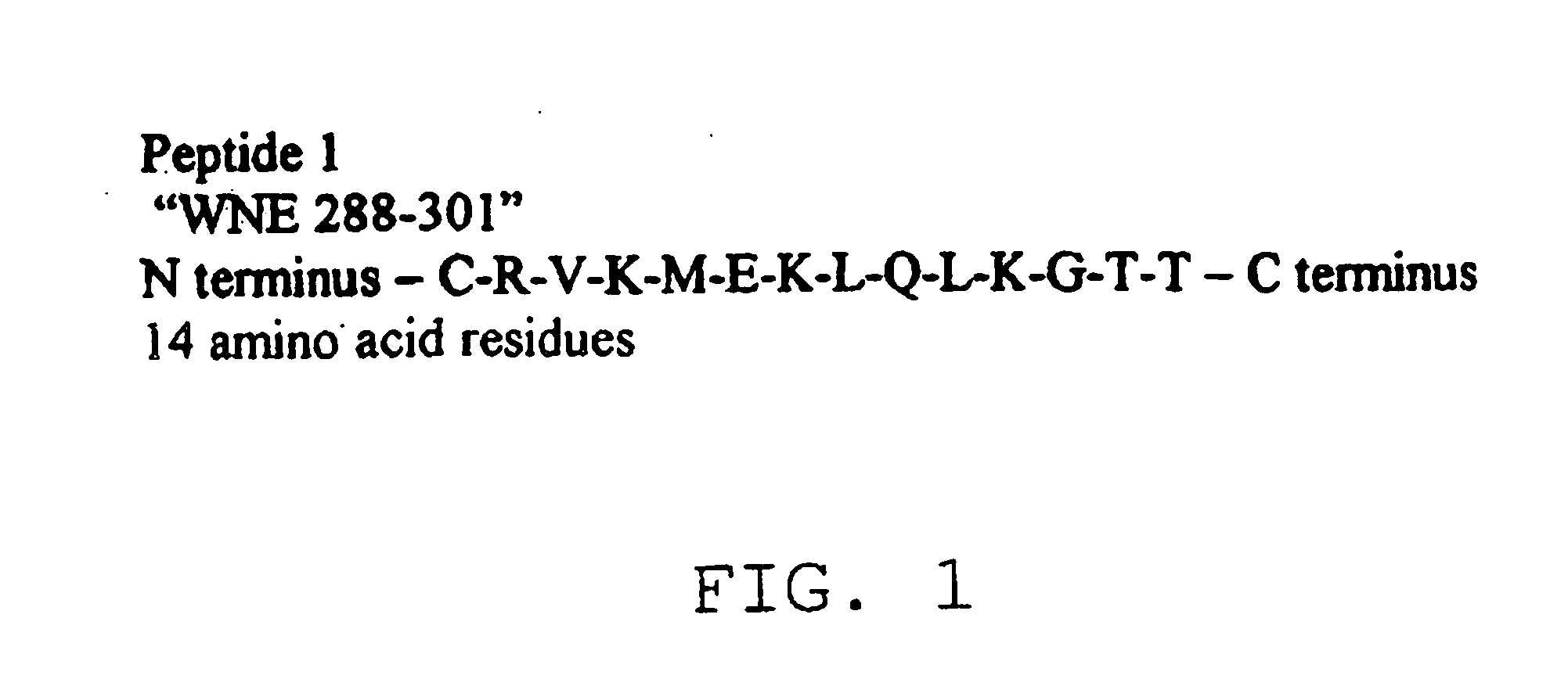
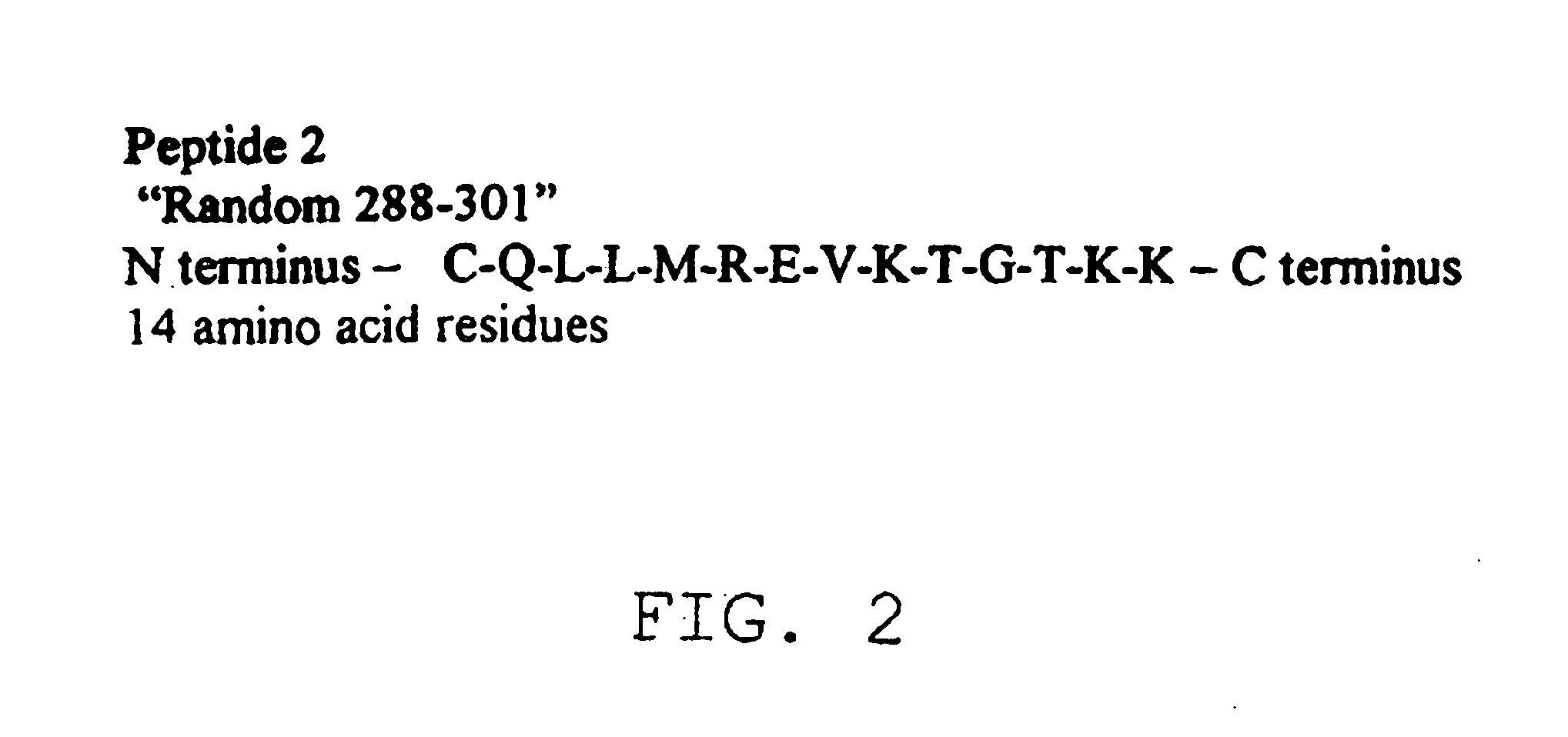
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result.
Owner:HEALTH RES INC
Controlled modulation of amino acid side chain length of peptide antigens
ActiveUS20050169934A1Extend and shortenReduce decreaseBacterial antigen ingredientsPeptide/protein ingredientsPeptide antigenEpitope
The invention provides a method for the creation of peptide antigens comprising epitopes with at least a first modification comprising a shortened or lengthened amino acid side chain. By extension or shortening of the side chain with CH3 / CH2 groups, for example, made by computer assisted modeling of the tumor antigen (peptide) bound in the MHC-I-groove, immunogenicity can be improved with minimal modification of adjacent tertiary structure, thereby avoiding cross-reactivity. Provided by the invention are methods of creating such antigens, as well as methods for therapeutic or prophylactic treatment of various conditions comprising administration of the antigens.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Antagonists to il-17a, il-17f, and il-23p19 and methods of use
The present invention relates to blocking, inhibiting, reducing, antagonizing or neutralizing the activity of IL-17A, IL-17F, and IL-23. Antagonists include antibodies and antibody fragments that bind IL-23 and that bind IL-17A or IL-17F, such as antibodies that are cross-reactive for IL-17A and Il-17F. Antagonists that include an antibody or antibody fragment that binds IL-23 and an antibody or antibody fragment that binds IL-17A or IL-17F on one molecule are also disclosed. Antibodies and antibody fragments that bind IL-23 and IL-17F but that do not bind IL-17A are also disclosed. IL-17 and IL-23 are cytokines that are involved in inflammatory processes and human disease.
Owner:ZYMOGENETICS INC
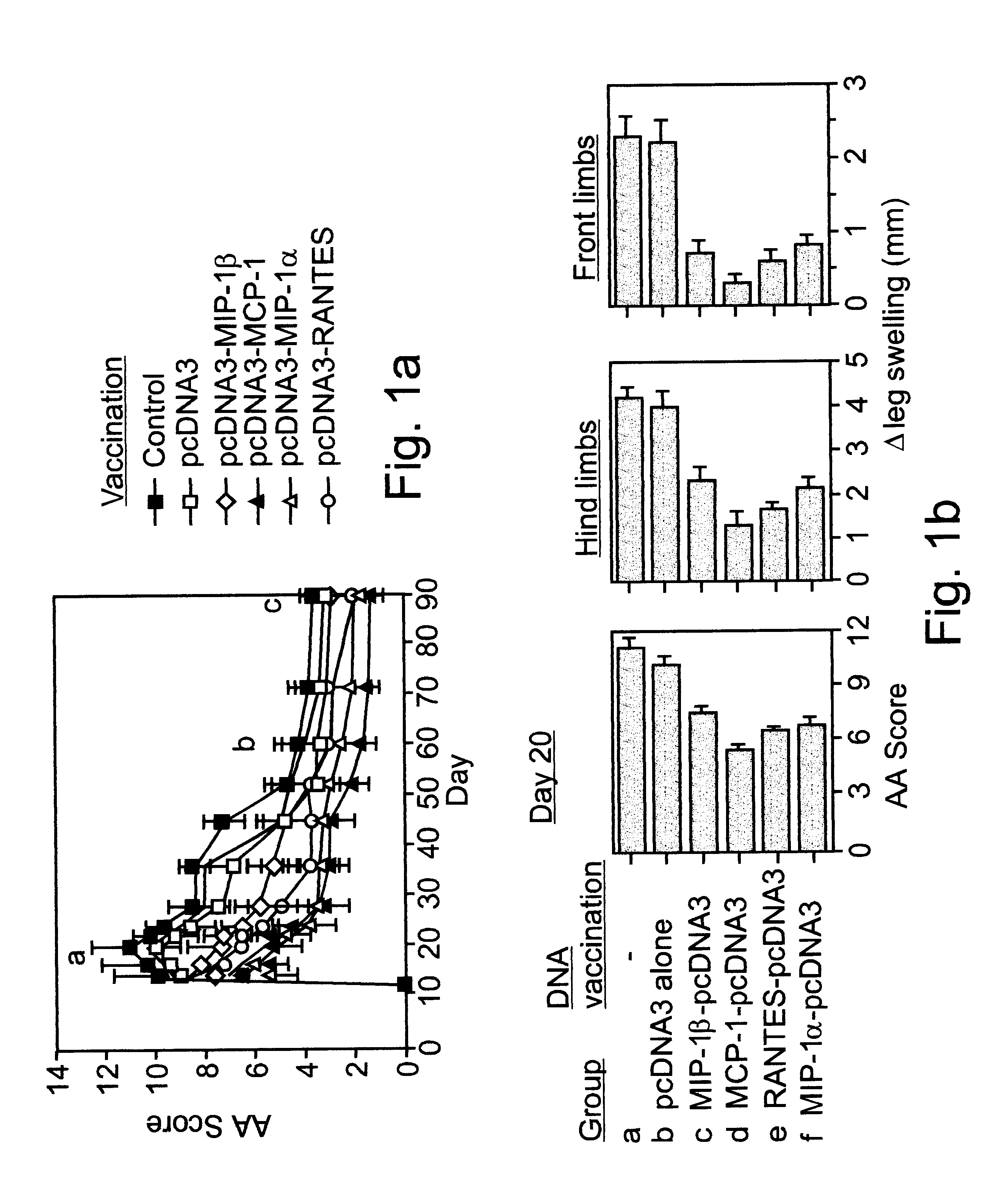
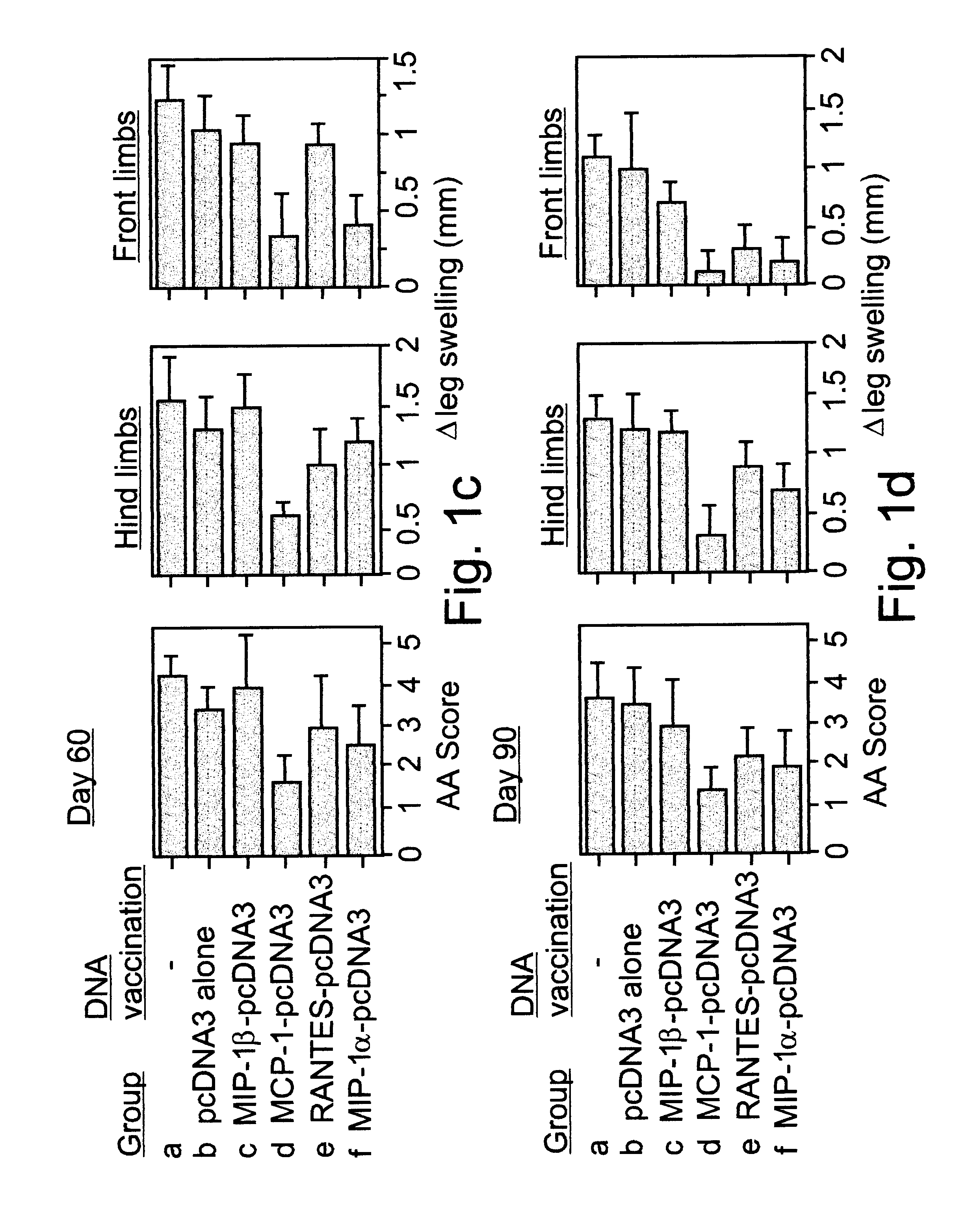
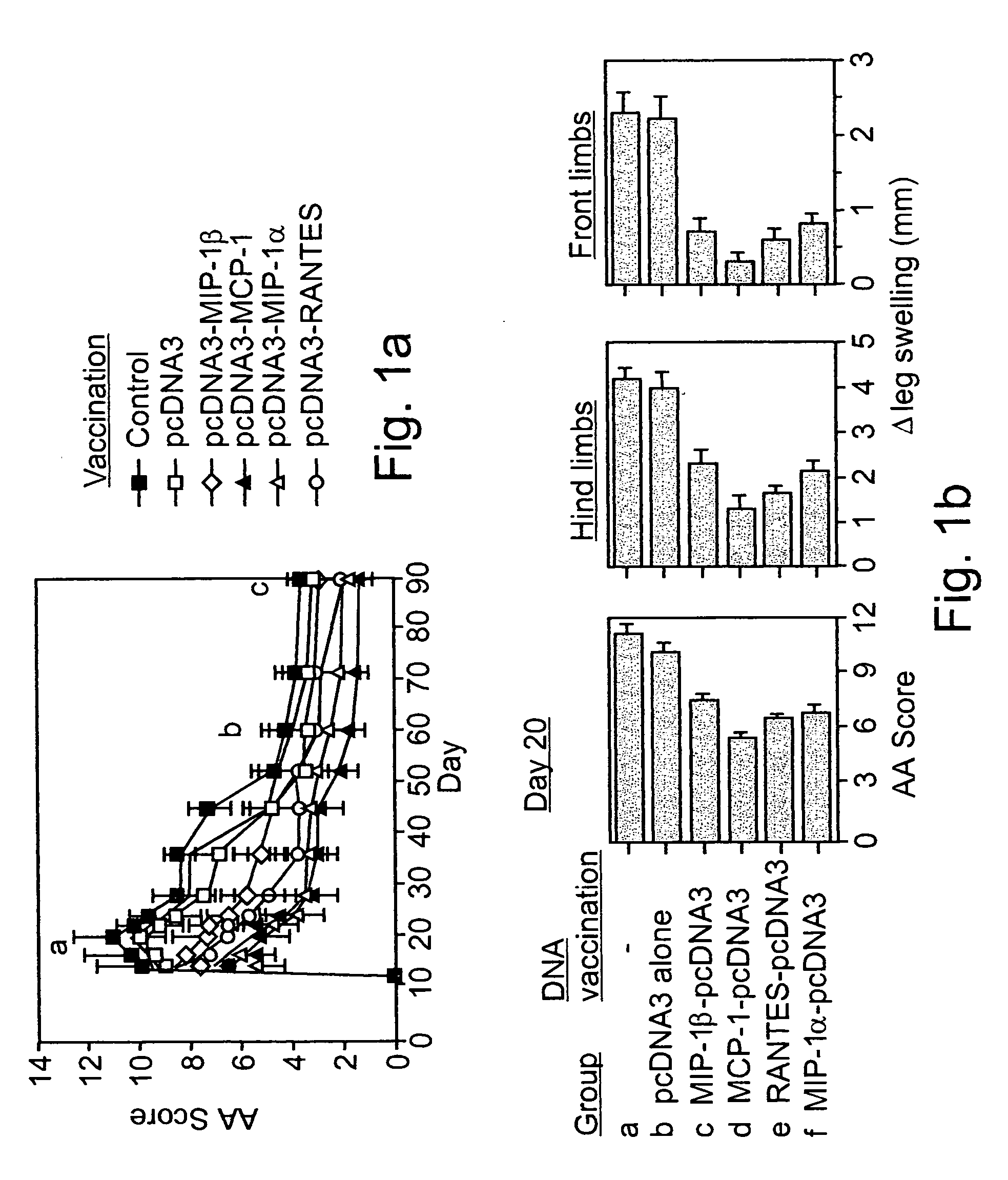
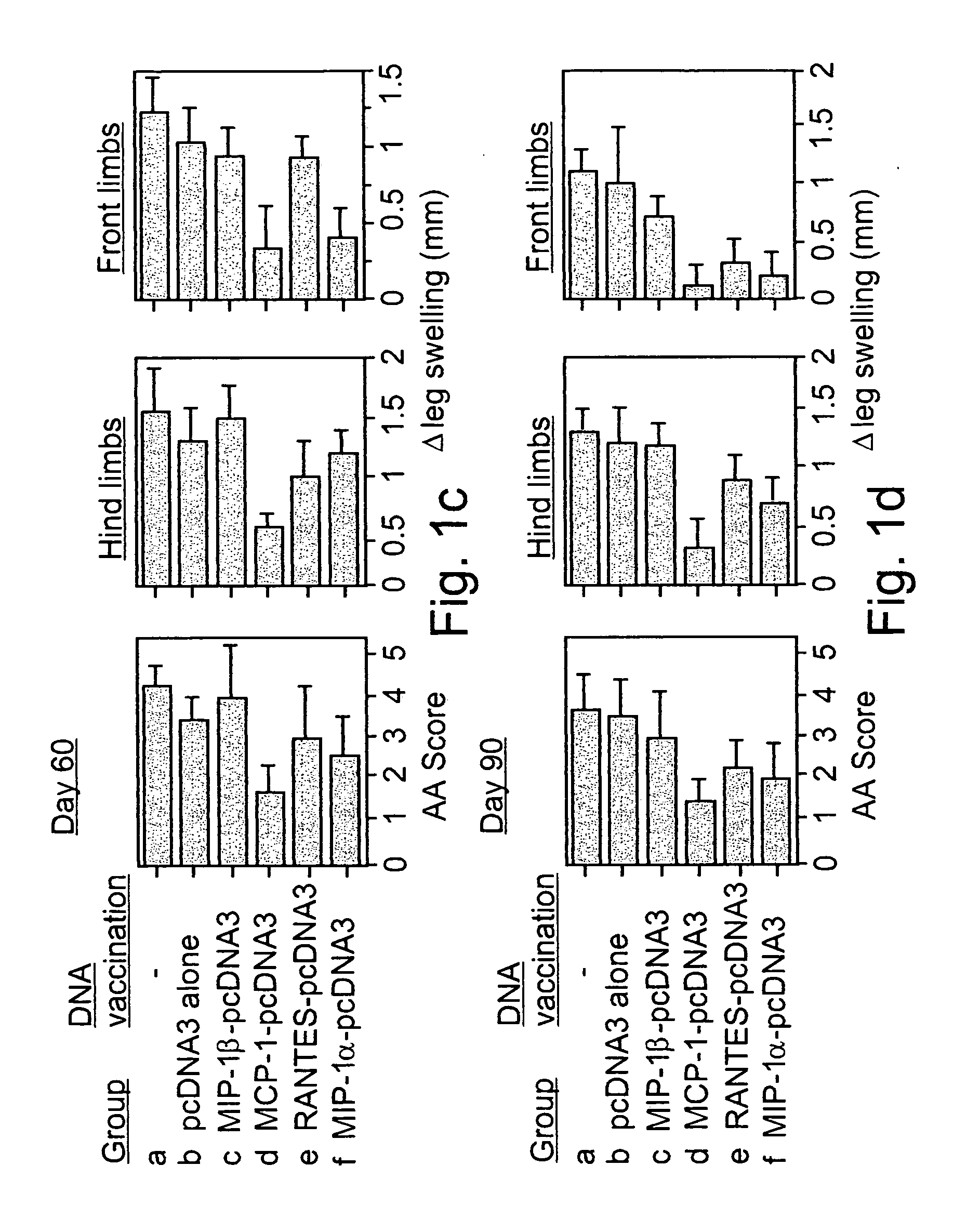
Polynucleotides encoding MIP-1alpha, MCP-1, MIP-1beta, Rantes and TNF-alpha, and methods for treating rheumatoid arthritis
A method of treating rheumatoid arthritis of an individual is disclosed. The method comprises the step of expressing within the individual at least an immunologically recognizable portion of a cytokine from an exogenous polynucleotide encoding the at least a portion of the cytokine, wherein a level of expression of the at least a portion of the cytokine is sufficient to induce the formation of anti-cytokine immunoglobulins which serve for neutralizing or ameliorating the activity of a respective and / or cross reactive endogenous cytokine, to thereby treat rheumatoid arthritis.
Owner:RAPPAPORT FAMILY INSTITUTE FOR RESEACH IN THE MEDICAL SCIENCES
Diagnostic test for west nile virus
ActiveUS20060115896A1More sensitiveEasy to useAnimal cellsMicrobiological testing/measurementDiagnostic testFlavivirus
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result. The invention also provides monoclonal antibodies against WNV NS5 and DENV NS5 antigen and their use in detecting WNV and DENV infections in a biological sample.
Owner:HEALTH RES INC
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
InactiveUS20070082007A1Avoid infectionInhibition of colonizationAntibacterial agentsNervous disorderDiseaseSalmonella serotype typhi
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:ZLOTNICK GARY W +5
Immunogenic peptide composition comprising measles virus Fprotein Thelper cell epitope (MUFThl-16) and N-terminus of β-amyloid peptide
InactiveUS6906169B2Increase the gapHigh cross reactivityNervous disorderPeptide/protein ingredientsFibrilDisease patient
The present invention relates to a composition comprsing a peptide immunogen useful for the prevention and treatment of Alzheimer's Disease. More particularly, the peptide immunogen comprises a main functional / regulatory site, an N-terminal fragment of Amyloid β (Aβ) peptide linked to a helper T cell epitope (Th) having multiple class II MHC binding motifs. The peptide immunogen elicit a site-directed immune response against the main functional / regulatory site of the Aβ peptide and generate antibodies, which are highly cross-reactive to the soluble Aβ1-42 peptide and the amyloid plaques formed in the brain of Alzheimer's Disease patients. The antibodies elicited being cross reactive to the soluble Aβ1-42 peptide, promote fibril disaggregation and inhibit fibrillar aggregation leading to immunoneutralization of the “soluble Aβ-derived toxins”; and being cross-reactive to the amyloid plaques, accelerate the clearance of these plaques from the brain. Thus, the composition of the invention comprising the peptide immunogen is useful for the prevention and treatment of Alzheimer's Disease.
Owner:UNITED NEUROSCIENCE LIMITED
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
InactiveUS20110076299A1Antibacterial agentsOrganic active ingredientsDiseaseSalmonella serotype typhi
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH HOLDINGS LLC
Novel immunogenic compositions for the prevention and treatment of meningococcal disease
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from nesserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH LLC
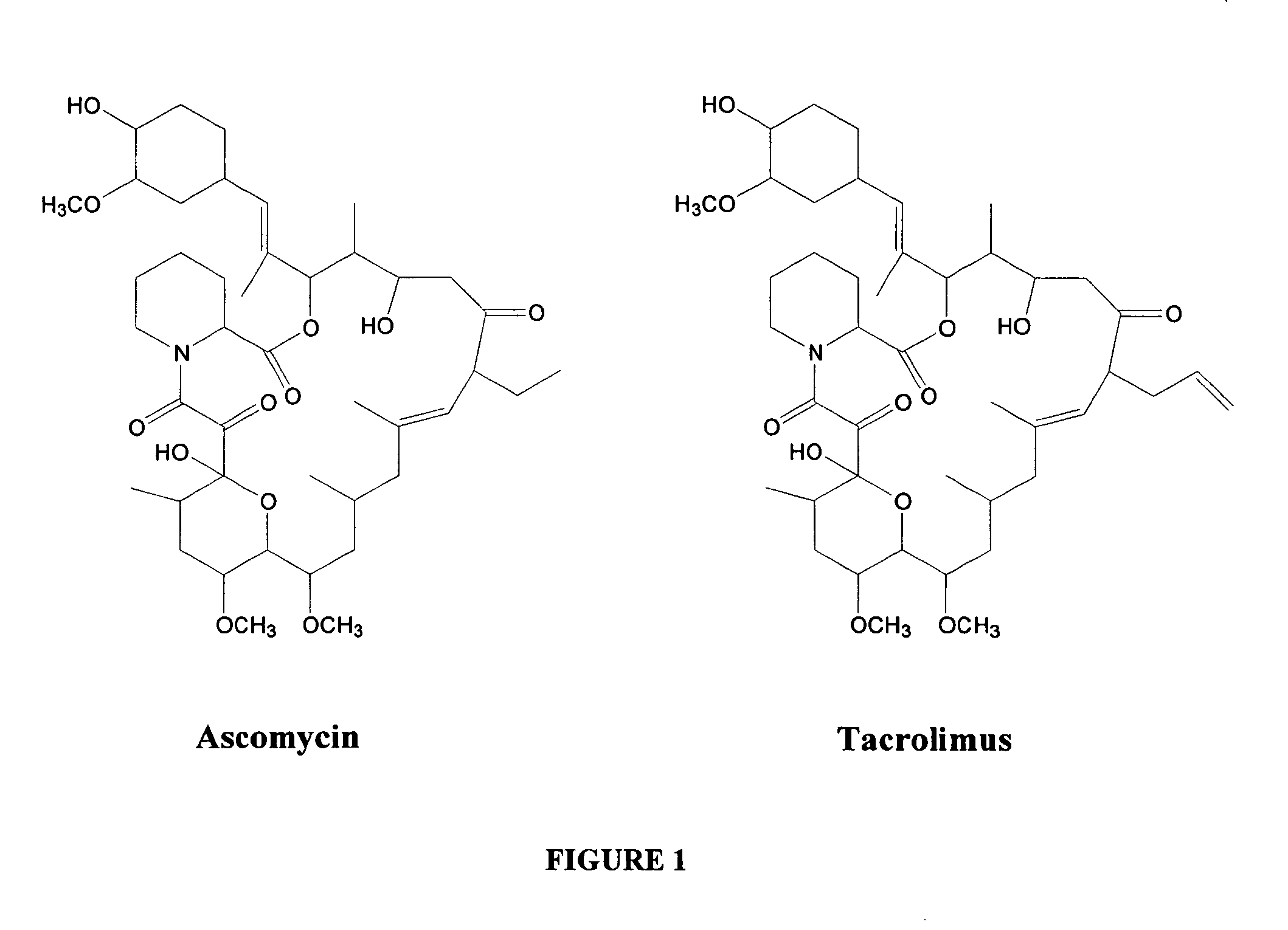
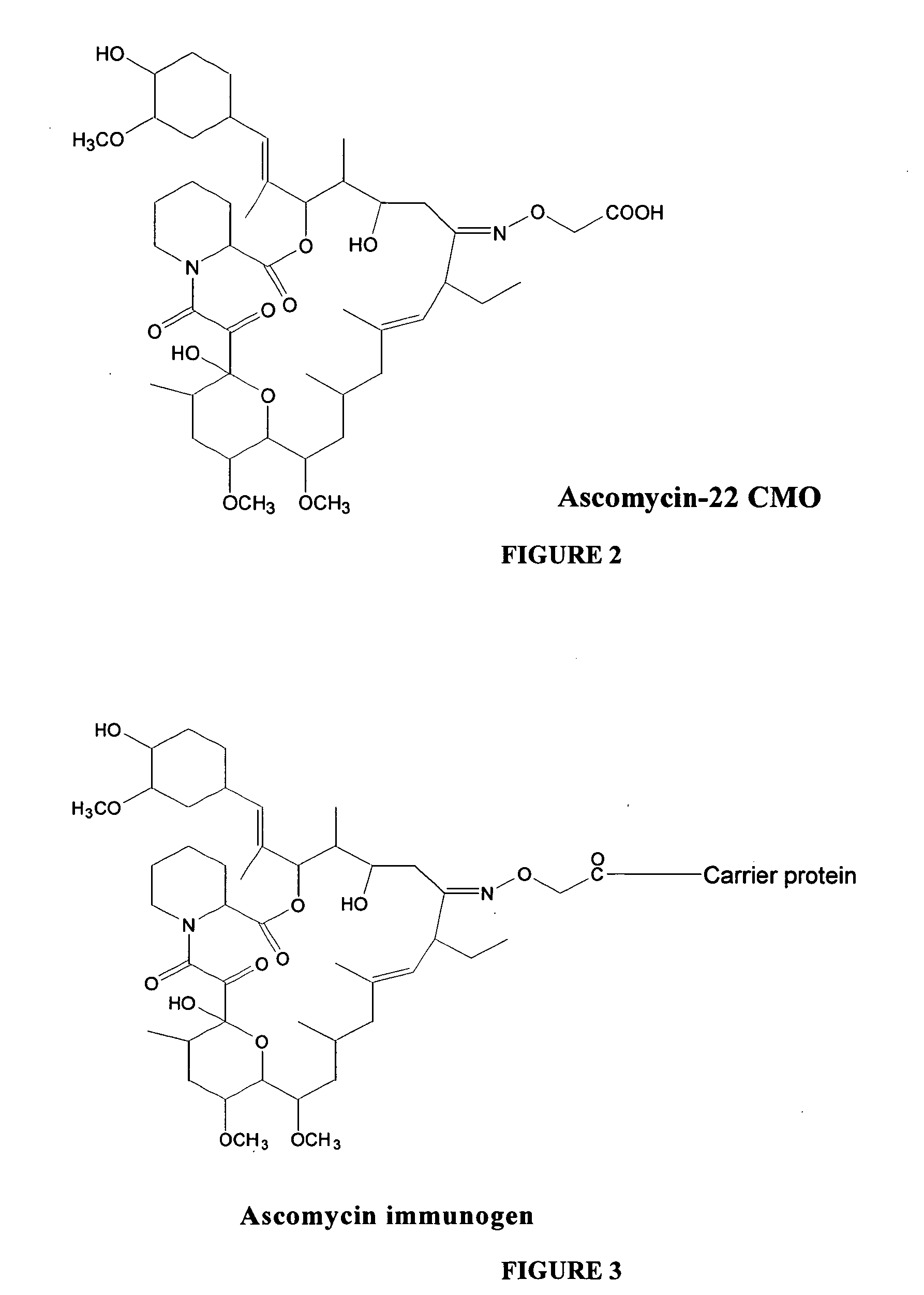
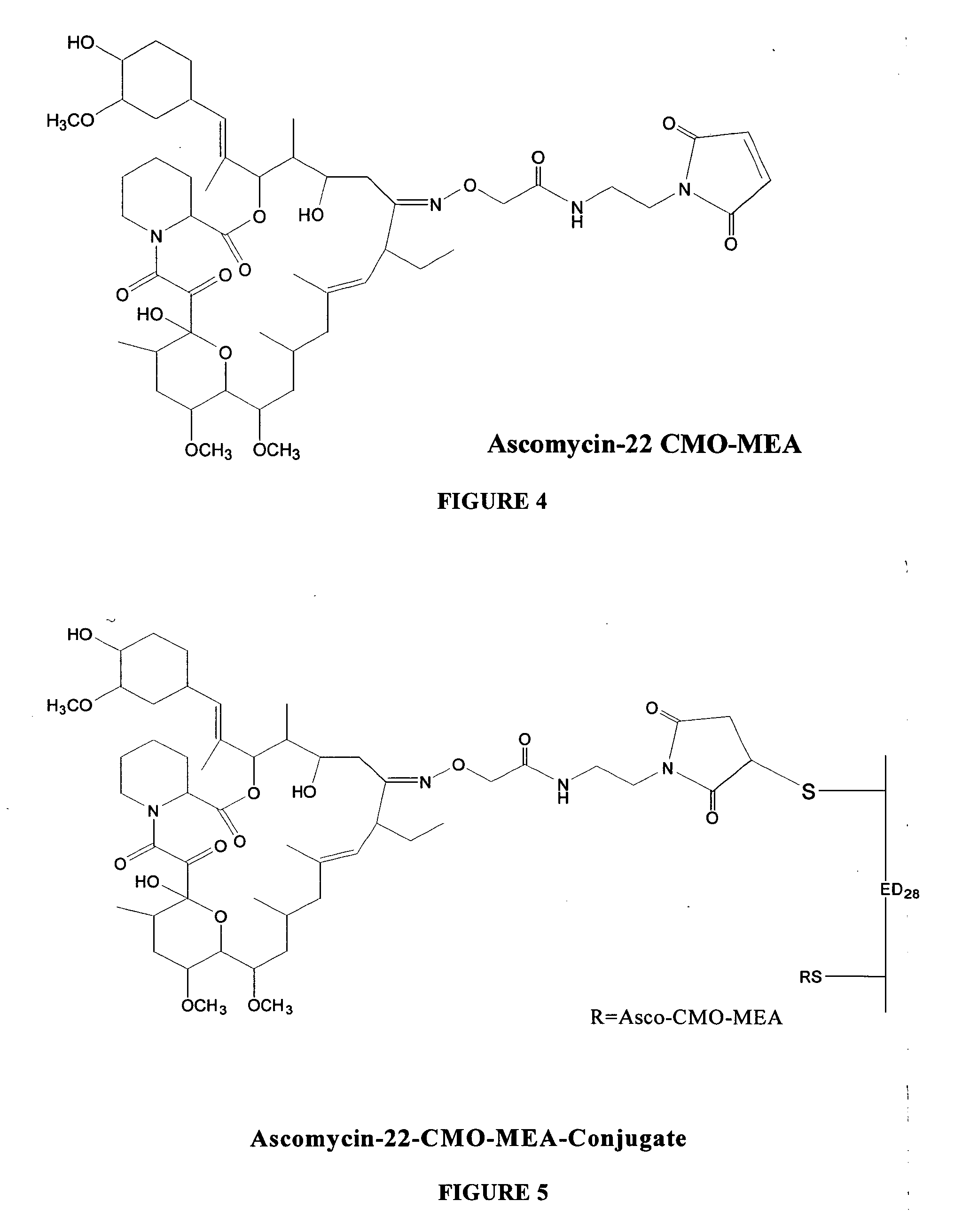
Hapten, immunogens and derivatives of ascomycin useful for preparation of antibodies and immunoassays
The invention teaches derivatives of ascomycin and methods of preparing immunogens and other conjugates useful in immunoassays for quantitatively measuring concentrations of tacrolimus in patient specimens. Antibodies produced from the disclosed immunogens capable of binding to tacrolimus with cross-reactivity of no more than 5% with each of 15-O-demethyl tacrolimus, 31-O-demethyl tacrolimus, and 13,31-O-didemethyl tacrolimus, less than 40% with 13-O-demethyl tacrolimus, and less than 1% with cyclosporin, rapamycin, mycophenolic acid, prednisone, hydrocortisol, and prednisolone are described. Further, immunoassays for measuring the concentration of tacrolimus using such antibodies are taught.
Owner:MICROGENICS CORP
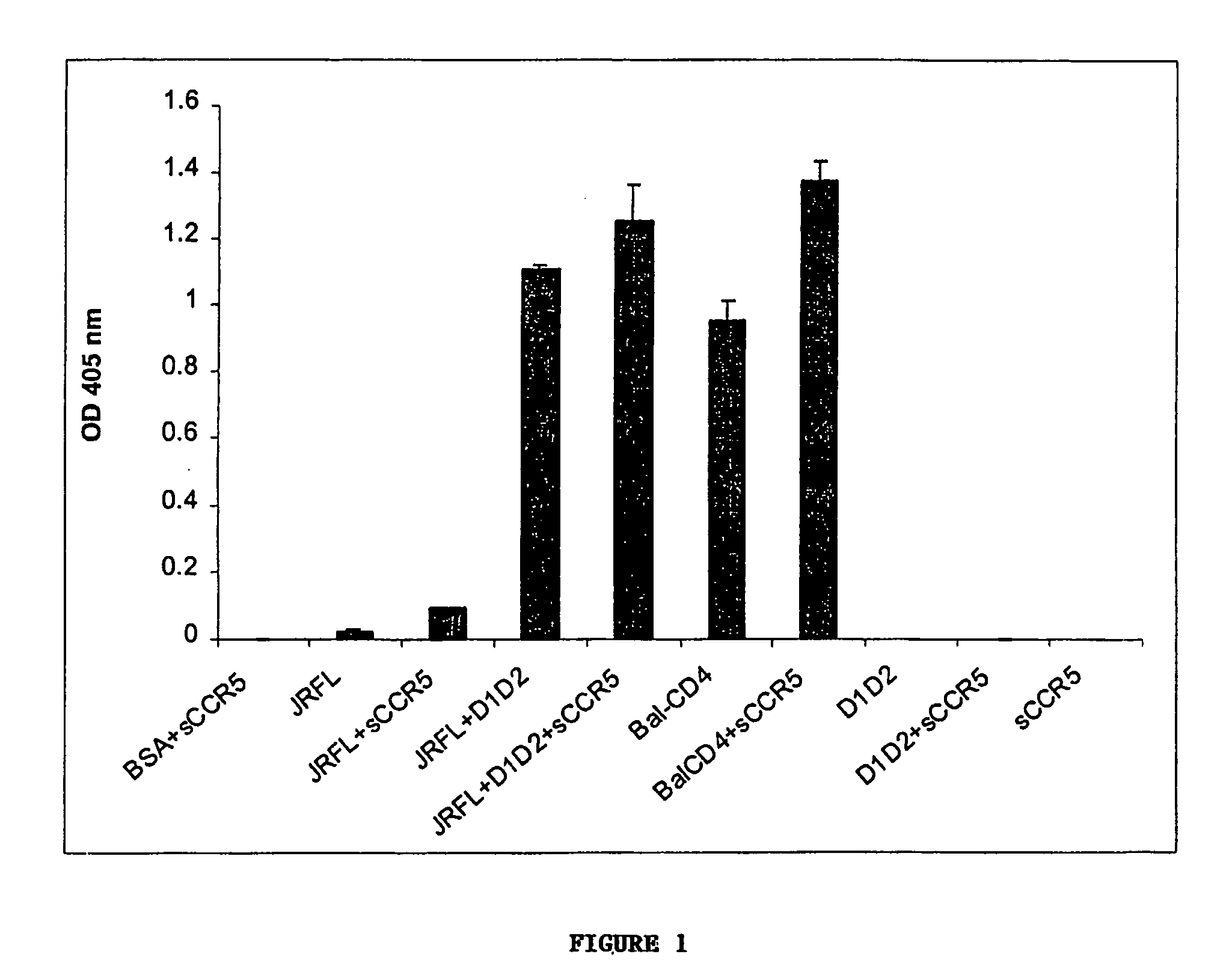
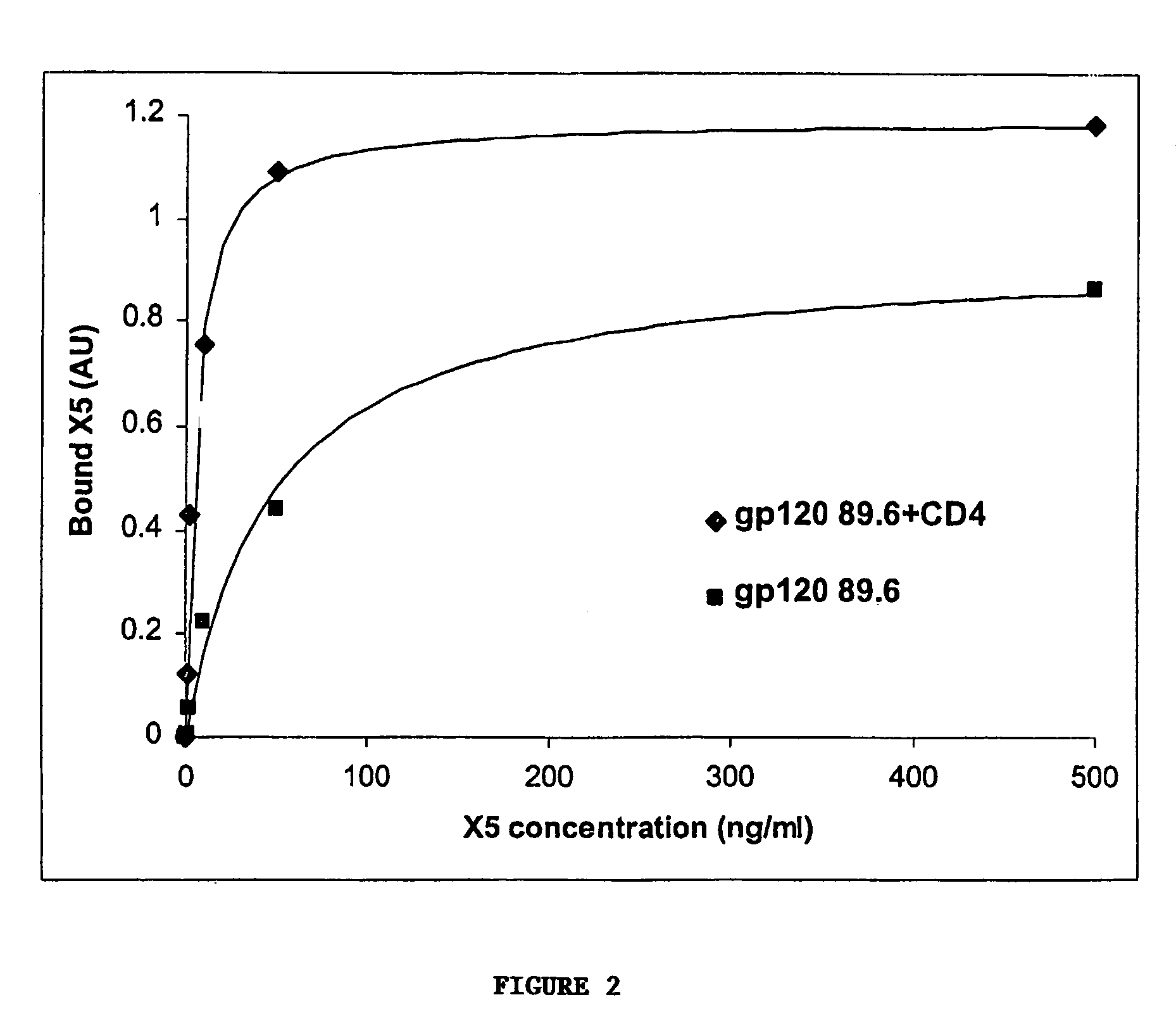
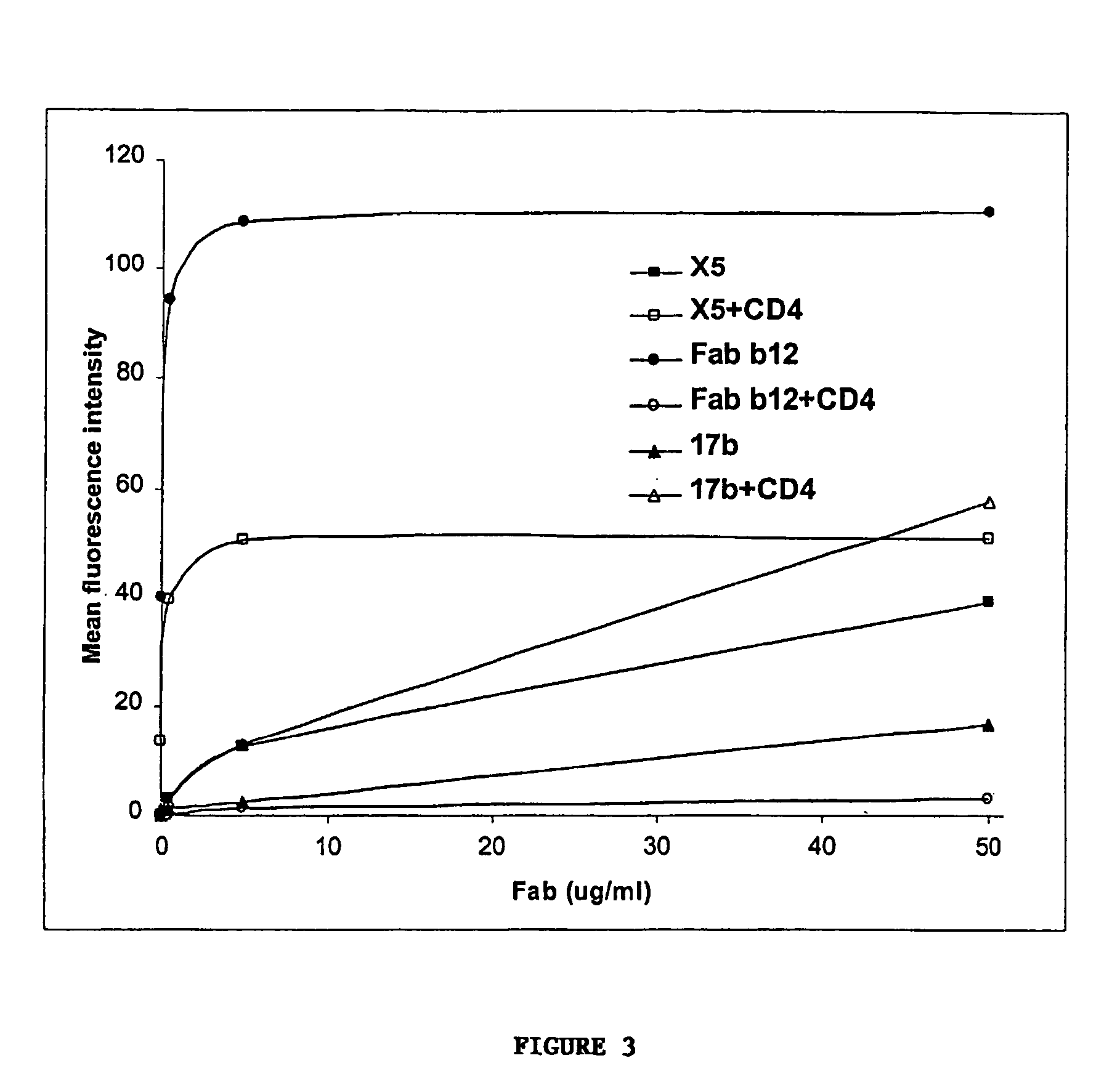
Broadly cross-reactive neutralizing antibodies against human immunodeficiency virus selected by Env-CD4-co-receptor complexes
InactiveUS7223844B2Increase exposurePeptide/protein ingredientsAntibody mimetics/scaffoldsAntibody fragmentsMammal
The present invention features antibodies and antibody fragments that specifically bind a CD4-inducible HIV gp120 epitope that is enhanced by binding a co-receptor for HIV, such as CCR5 or CXCR4, and pharmaceutical compositions comprising the antibodies or antibody fragments. The invention also features nucleic acids encoding the antibodies or antibody fragments, pharmaceutical compositions comprising the nucleic acids encoding the antibodies or antibody fragments, vectors comprising the nucleic acids, and cells comprising the vectors. The invention further features methods of identifying antibodies or antibody fragments with broadly neutralizing activity against HIV. The invention also features methods of inhibiting HIV entry into cells and methods of inhibiting replication of HIV in mammals, using the antibodies and nucleic acids of the invention.
Owner:THE SCRIPPS RES INST +1
Novel Immunogenic Compositions for the Prevention and Treatment of Meningococcal Disease
InactiveUS20070253964A1Antibacterial agentsPeptide/protein ingredientsNucleic acid sequencingImmunogenicity
The present invention relates to Neisseria ORF2086 proteins, crossreactive immunogenic proteins which can be isolated from neisserial strains or prepared recombinantly, including immunogenic portions thereof, biological equivalents thereof, antibodies that immunospecifically bind to the foregoing and nucleic acid sequences encoding each of the foregoing, as well as the use of same in immunogenic compositions that are effective against infection by Neisseria meningitidis serogroup B.
Owner:WYETH LLC
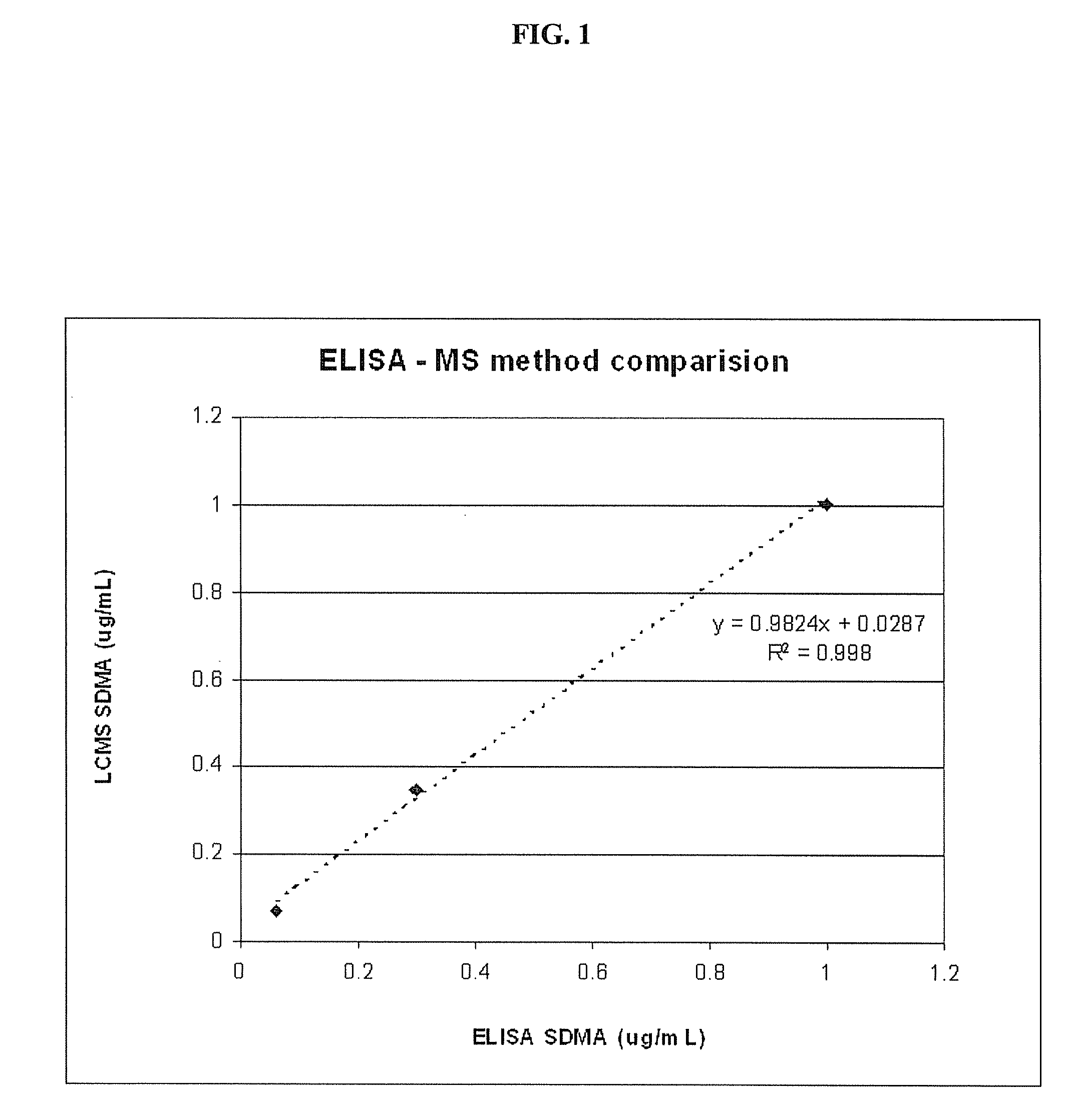
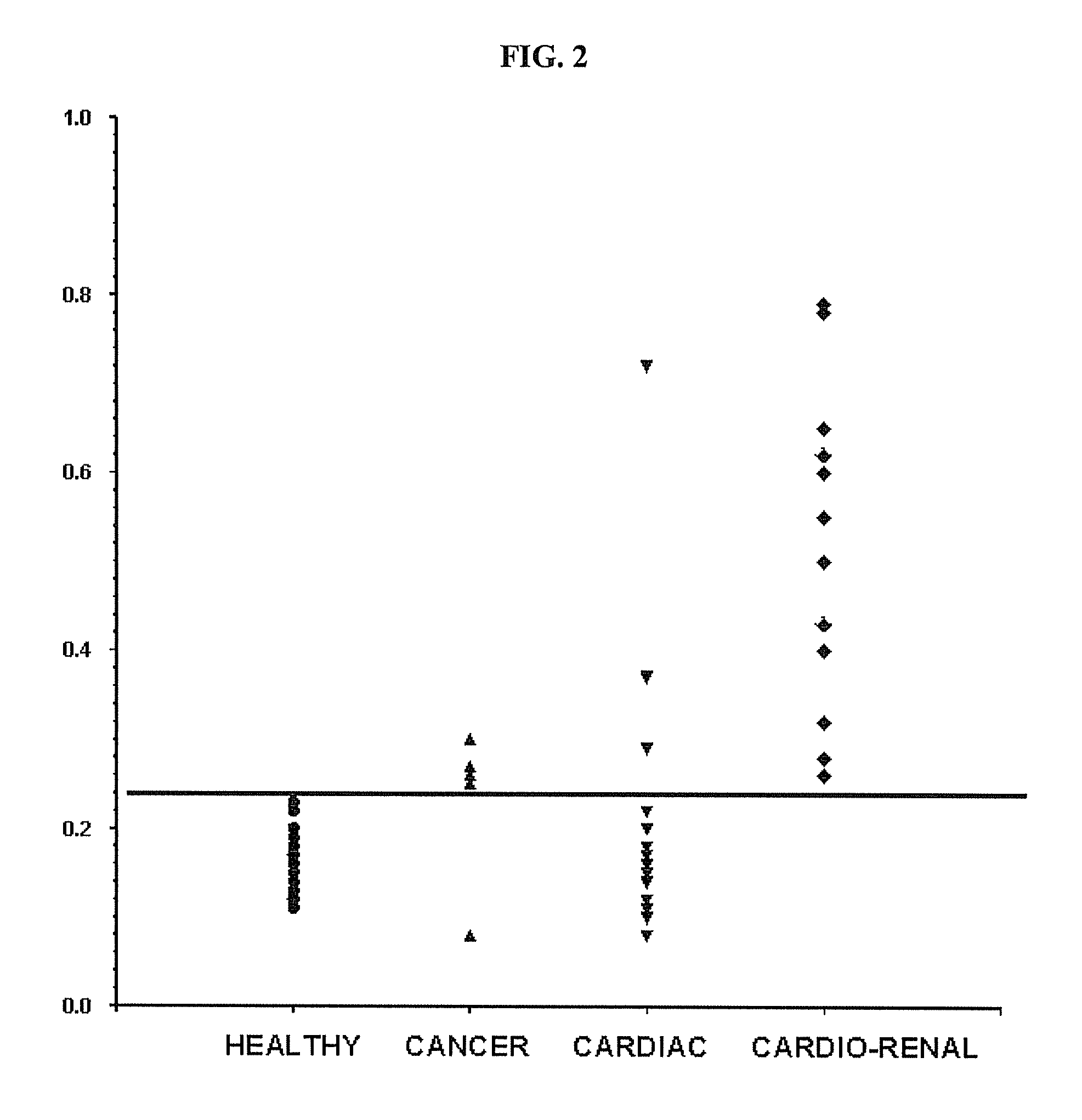
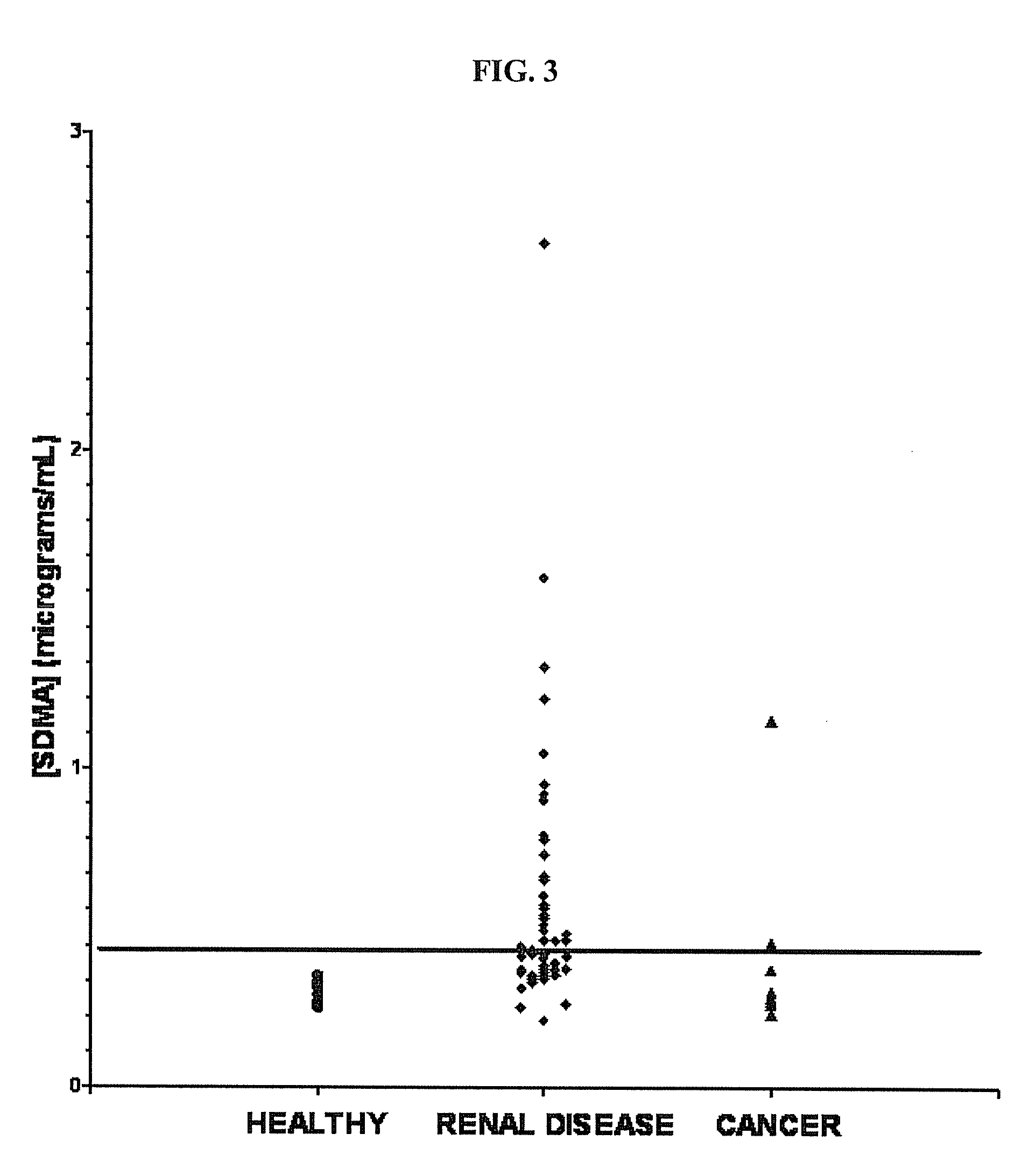
Methods for Detecting Symmetrical Dimethylarginine
ActiveUS20100035274A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseHydroxy compound
Method of detecting Symmetrical dimethyl arginine (SDMA) in biological samples. SDMA analogs for generating anti-SDMA antibodies having little or no cross-reactivity with asymmetrical dimethyl arginine, arginine, and monomethylarginine. The analogs have a protected or free thiol (—SH) group or hydroxyl (—OH) group that allow them to be linked to a suitable conjugation target which can be, for example, a protein containing molecule of a label. The anti-SDMA antibodies can be used in diagnostic immunoassay for the diagnosis of SDMA associated disorders and / or diseases.
Owner:IDEXX LABORATORIES
Method and apparatus for enhanced bacteriophage-based diagnostic assays by selective inhibition of potential cross-reactive organisms
InactiveUS20080241819A1Growth inhibitionInhibition of attachmentMicrobiological testing/measurementMicroorganismBacteriophage
A sample to be tested for the presence of a target microorganism is exposed to bacteriophage and conditions are provided to inhibit phage attachment to or replication in a potentially cross-reactive, non-target microorganism. The sample is incubated and assayed to detect the presence or absence of a bacteriophage marker to determine the presence or absence of the target microorganism. The inhibiting may comprise the addition of an inhibiting substance or the use of an inhibiting process. It may include inhibiting the growth of potentially cross-reactive bacteria while allowing growth of the target bacteria, selectively removing or blocking potential cross-reactive bacteria using selective binding agents or selectively destroying potentially cross-reactive bacteria.
Owner:MICROPHAGE
Virus-like particles comprising composite capsid amino acid sequences for enhanced cross reactivity
ActiveUS8841120B2Strong immune responseImprove protectionVirusesMicroorganismsEpitopeVirus-like particle
The present invention provides polypeptides having a composite amino acid sequence derived from a consensus sequence representing the capsid proteins of two or more circulating strains of a non-enveloped virus. In particular, the invention provides virus-like particles comprising at least one composite polypeptide. Such virus-like particles have antigenic epitopes of two or more circulating strains of a non-enveloped virus and produce an increase in antisera cross-reactivity to one or more circulating strains of the non-enveloped virus. Methods of making composite virus-like particles and vaccine formulations comprising composite virus-like particles are also disclosed.
Owner:TAKEDA VACCINES INC
Proteins capable of binding to female sex hormones and process for producing the same
InactiveUS20060110771A1High sensitivityLow cross-reactivityFungiBacteriaBiotechnologySexual hormones
The present invention provides a protein capable of binding to a female sex hormone, which protein is supplemented with useful properties for measuring, quantifying and concentrating female sex hormones, such as high sensitivity, low cross-reactivity, unlikelihood of being influenced by interfering substances, and unlikelihood of being influenced by solvents. Specifically, the present invention provides a recombinant protein prepared by obtaining various genes for various antibodies against female sex hormones, and modifying, by gene recombination technology, various properties of the original antibody, such as affinity, avidity, cross-reactivity, resistance for interfering substance on antigen-antibody reaction, resistance for interfering substance on enzymatic color developing reaction, resistance for solvent and the like.
Owner:JAPAN ENVIROCHEM
Virus-like particles comprising composite capsid amino acid sequences for enhanced cross reactivity
ActiveUS20110195113A1Enhance immune responseHigh IgG levelSsRNA viruses positive-senseMicroorganismsEpitopeVirus-like particle
The present invention provides polypeptides having a composite amino acid sequence derived from a consensus sequence representing the capsid proteins of two or more circulating strains of a non-enveloped virus. In particular, the invention provides virus-like particles comprising at least one composite polypeptide. Such virus-like particles have antigenic epitopes of two or more circulating strains of a non-enveloped virus and produce an increase in antisera cross-reactivity to one or more circulating strains of the non-enveloped virus. Methods of making composite virus-like particles and vaccine formulations comprising composite virus-like particles are also disclosed.
Owner:TAKEDA VACCINES INC
Meningococcal fhbp polypeptides
ActiveUS20110020390A1Stable maintenanceSugar derivativesBacteriaAntiendomysial antibodiesMeningococcal carriage
fHBP is a protein in Neisseria meningitidis. Three families of fHBP are known. To increase the ability of a fHBP protein to elicit antibodies that are cross-reactive between the families, fHBP is selected or engineered to have a sequence which can elicit broad-spectrum bactericidal anti-meningococcal antibodies after administration to a host animal.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Pharmaceutical compositions and method for treating rheumathoid arthritis
A method of treating rheumatoid arthritis of an individual is disclosed. The method comprises the step of expressing within the individual at least an immunologically recognizable portion of a cytokine from an exogenous polynucleotide encoding the at least a portion of the cytokine, wherein a level of expression of the at least a portion of the cytokine is sufficient to induce the formation of anti-cytokine immunoglobulins which serve for neutralizing or ameliorating the activity of a respective and / or cross reactive endogenous cytokine, to thereby treat rheumatoid arthritis.
Owner:RAPPAPORT FAMILY INSTITUTE FOR RESEACH IN THE MEDICAL SCIENCES
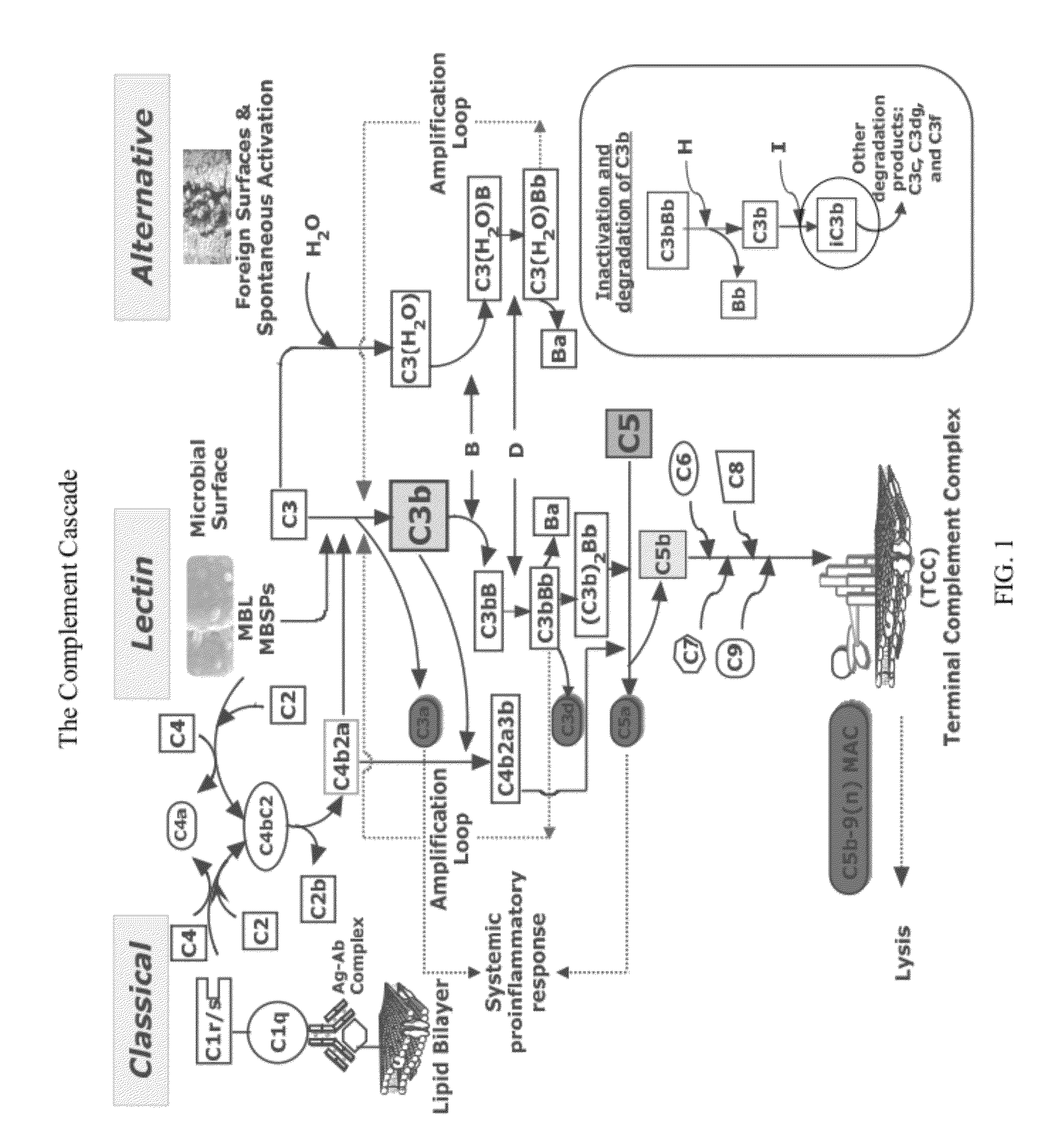
Detecting complement activation
ActiveUS20120315266A1Minimizing spontaneous complement activationReduce materialSenses disorderNervous disorderActivation methodCross reactive antibodies
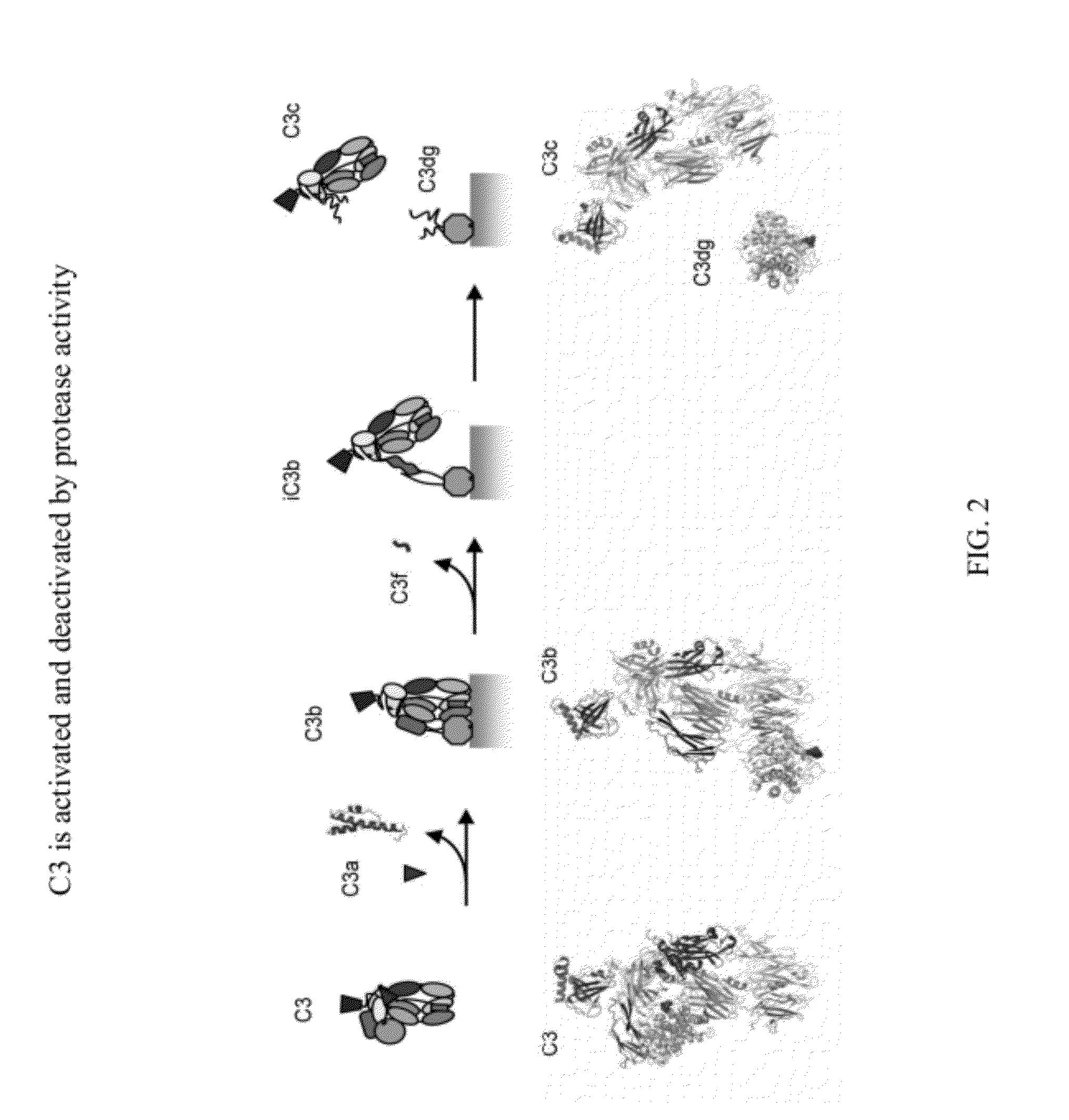
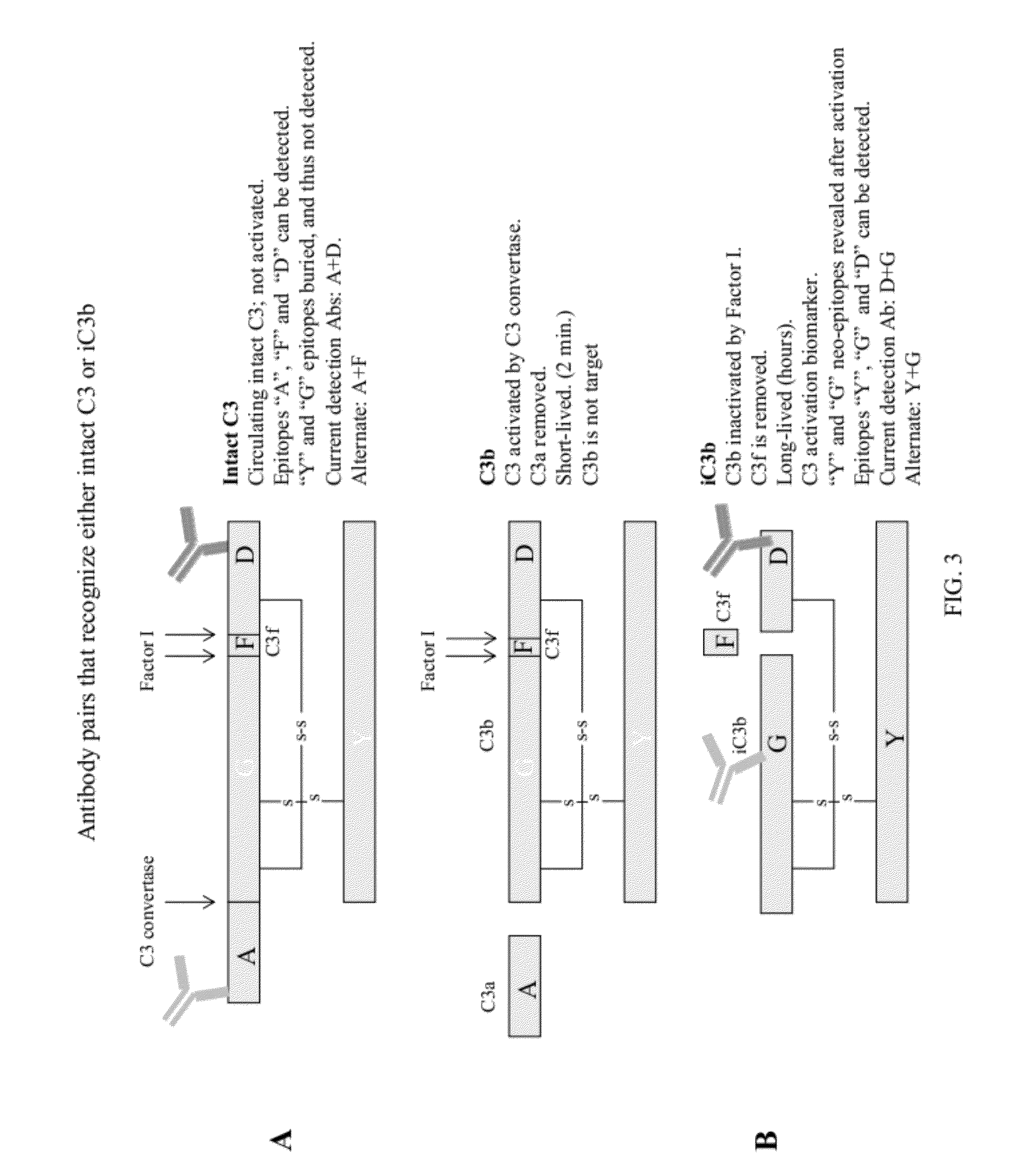
Methods of detecting complement activation including steps of detecting in a sample from a subject a level of iC3b wherein the detecting involves specific interaction between the iC3b and a non-cross-reactive antibody thereto, comparing the detected level with a reference level, which reference level is within a range of about 10 ng / ml to about 5,000 ng / ml, wherein determination that the detected level is above the reference level indicates that the subject is suffering from or susceptible to undesirable and / or pathologic complement activation, and administering treatment to treat undesired complement activation if the detected level is above the reference level. Other methods of detecting complement activation with or without measuring iC3b are also provided.
Owner:KYPHA
Anti-EpCAM antibodies
InactiveCN102549017AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHeavy chainCam
Disclosed are antibodies that bind to Epithelial Cell Adhesion Molecule (Ep CAM) and display certain advantages over known antibodies which bind to Ep CAM, for example, the antibodies of the invention show good affinity, good cross-reactivity profiles and excellent ADCC and CDCC activity. Antibodies comprising specific heavy and light chain CDRs are disclosed. The invention thus relates to these antibodies and all uses thereof, in particular in the treatment of cancer. The present invention thus provides new antibody-based compositions, methods and combined protocols for treating cancer. Advantageous immunoconjugate compositions and methods using the new anti-Ep CAM antibodies are also provided.
Owner:奥菲技术科学研究院
Hybridoma cell strain 2D3, monoclonal antibody to zearalenone secreted by same and application of monoclonal antibody
The invention provides a hybridoma cell strain 2D3, a monoclonal antibody to zearalenone secreted by the hybridoma cell strain 2D3 and application of the monoclonal antibody. The hybridoma cell strain 2D3 is preserved in China Center for Type Culture Collection with an accession number of CCTCC No. C201328 and can be used for preparation of a high-titer monoclonal antibody to zearalenone. According to detection results of enzyme linked immunosorbent assay (ELISA), the titer of the monoclonal antibody to zearalenone prepared through purification of mouse ascites can reach 1.5 * 10<5>. The monoclonal antibody to zearalenone has high sensitivity, half maximal inhibitory concentration IC50 of 20 pg / mL to zearalenone and cross reactivity of 4.9%, 3.3% and 3.2% with beta-zearalanel, alpha-zearalanel and beta-zeranol, respectively. The monoclonal antibody to zearalenone can be used for determination of the content of zearalenone.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
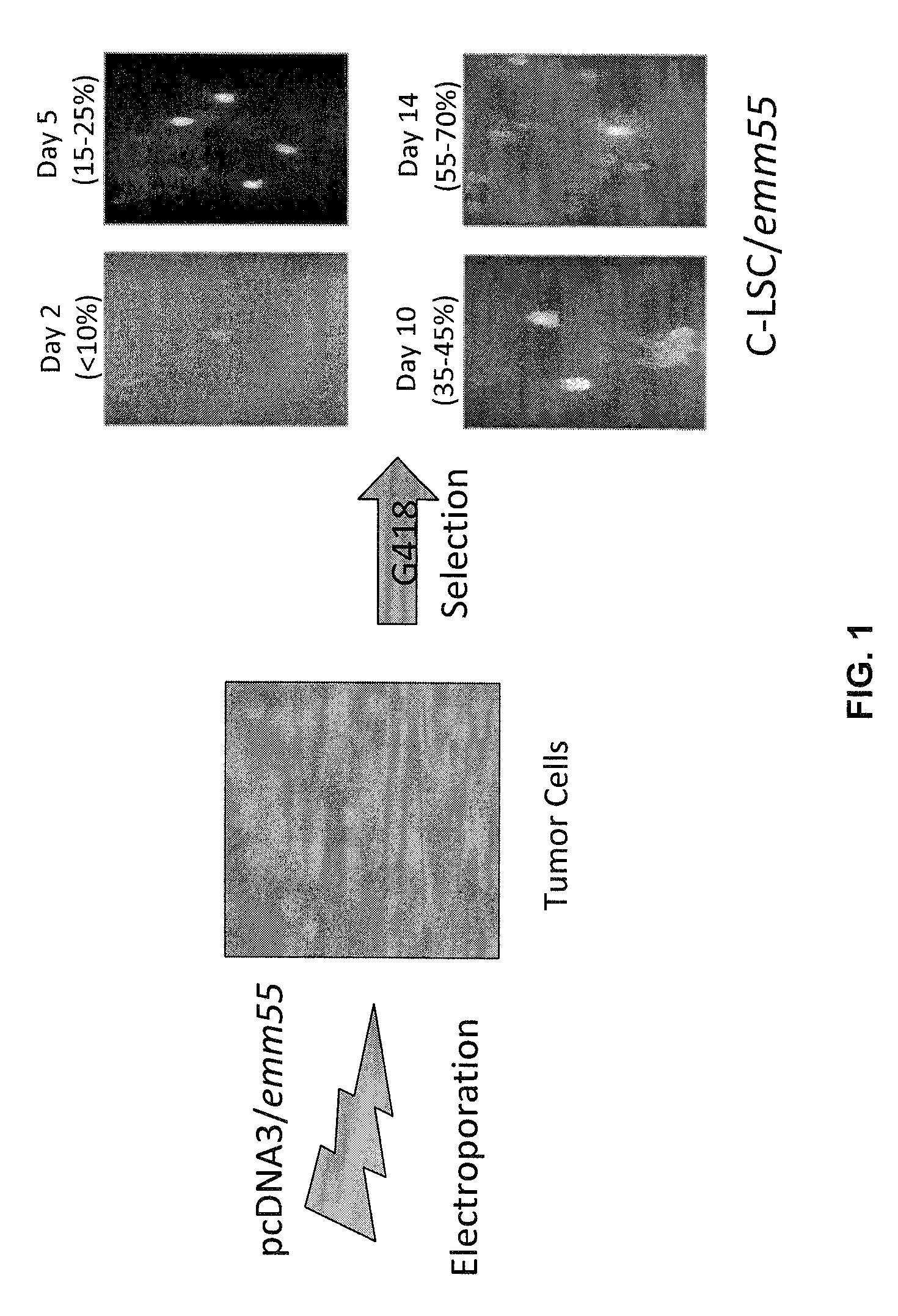
Tumor cell vaccines
InactiveUS7795020B2Maximize potentialExtend your lifeBiocidePeptide/protein ingredientsCancer cellCanis lupus familiaris
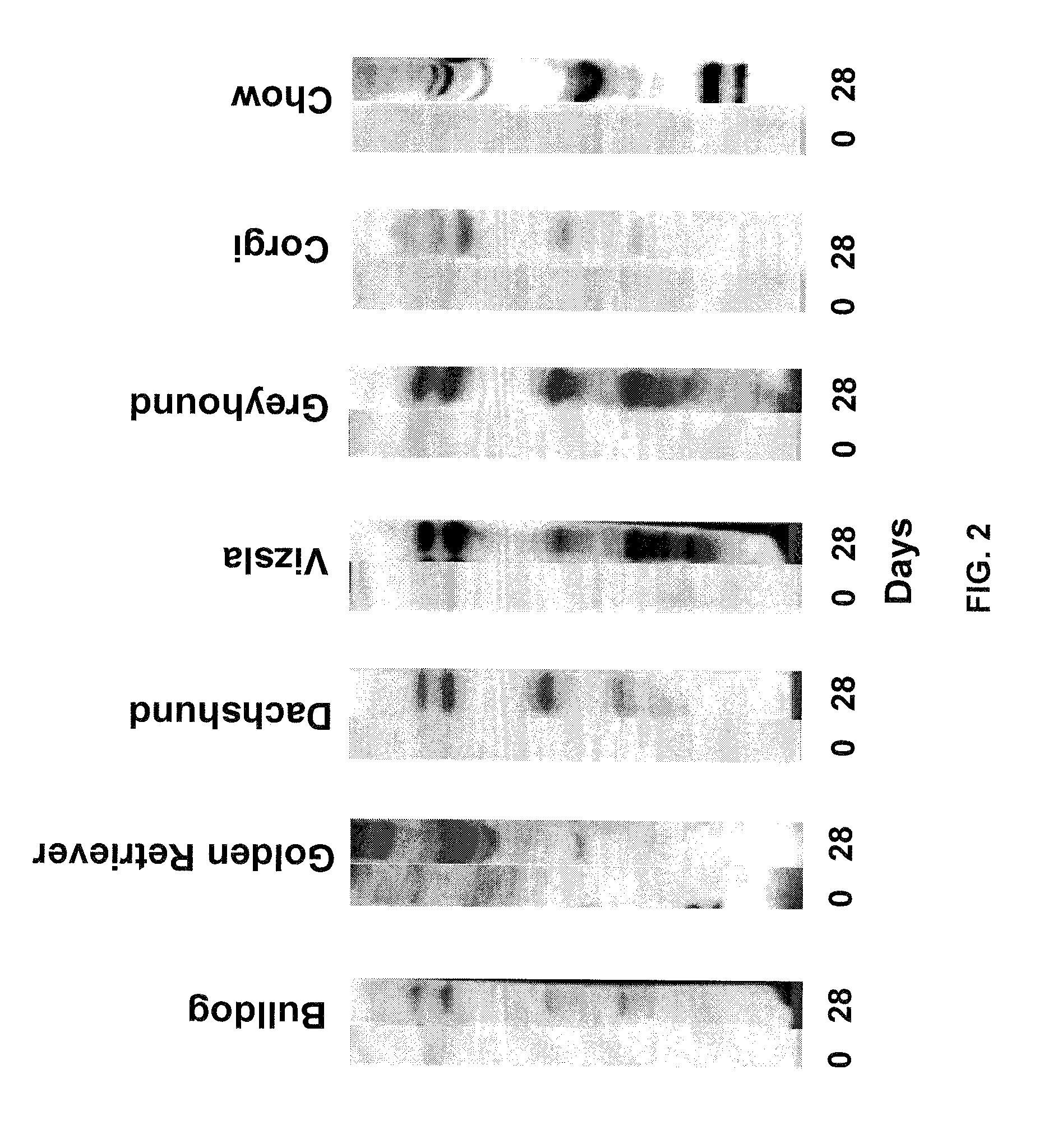
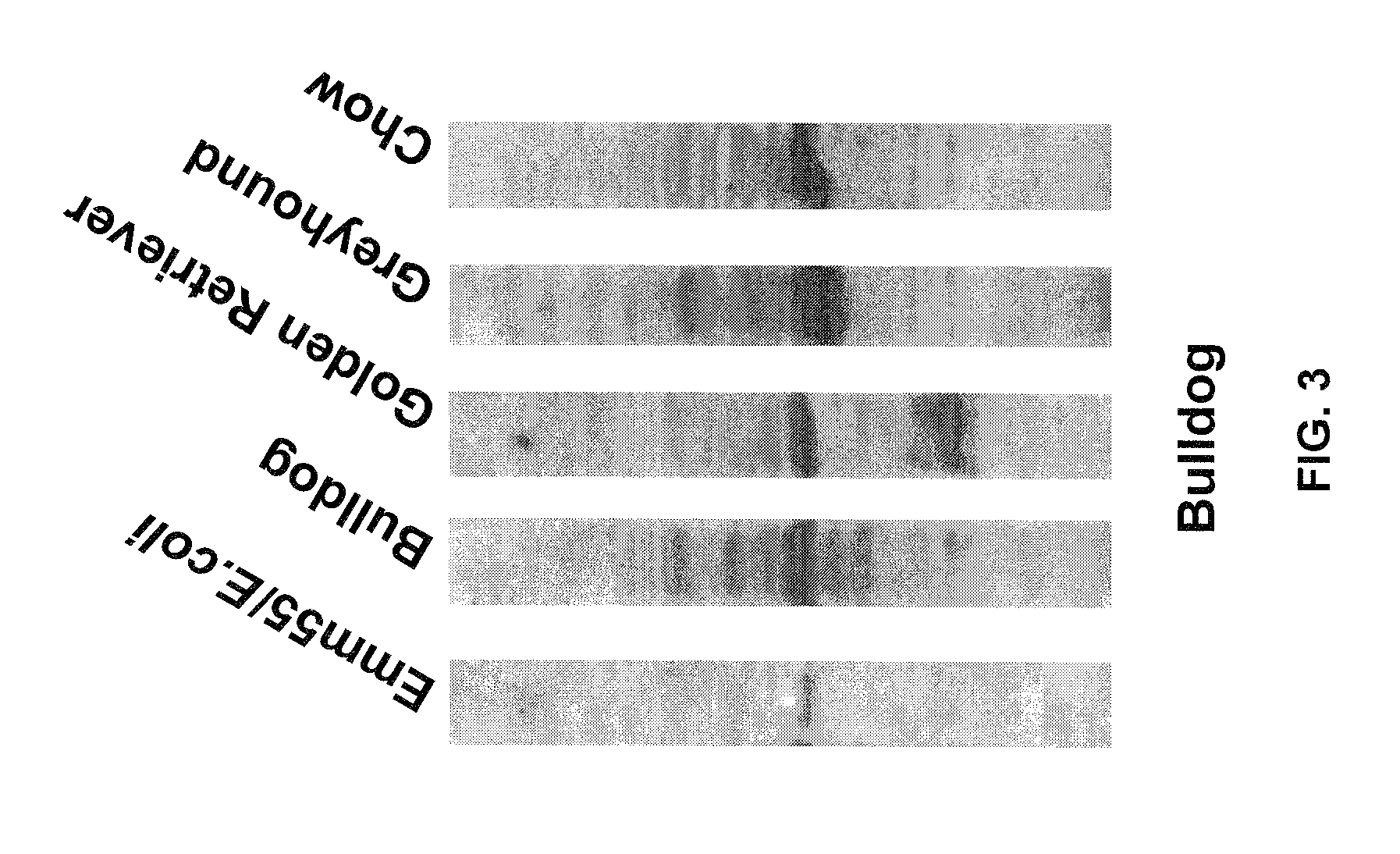
An effective cancer cell vaccine for canines has been developed. The vaccine is prepared from autologous lymphoma cells transfected with emm55. Once an animal is vaccinated, the expressed Emm55 antigen stimulates an immunogenic response to the tumor cells resulting in significantly increased survival, strong autologous and cross reactive humoral and cell mediated responses in several breeds of dogs diagnosed with later stage lymphomas.
Owner:MORPHOGENESIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com