Pharmaceutical compositions and method for treating rheumathoid arthritis
a technology of rheumatoid arthritis and compositions, applied in the field of pharmaceutical compositions and methods for treating rheumatoid arthritis, can solve the problem of immunogenicity of chronic diseases treated with xenogenic neutralizing antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Results of Experiments Conducted With the Chemokines MIP-1.alpha., MCP-1, MIP-1.beta., and RANTES
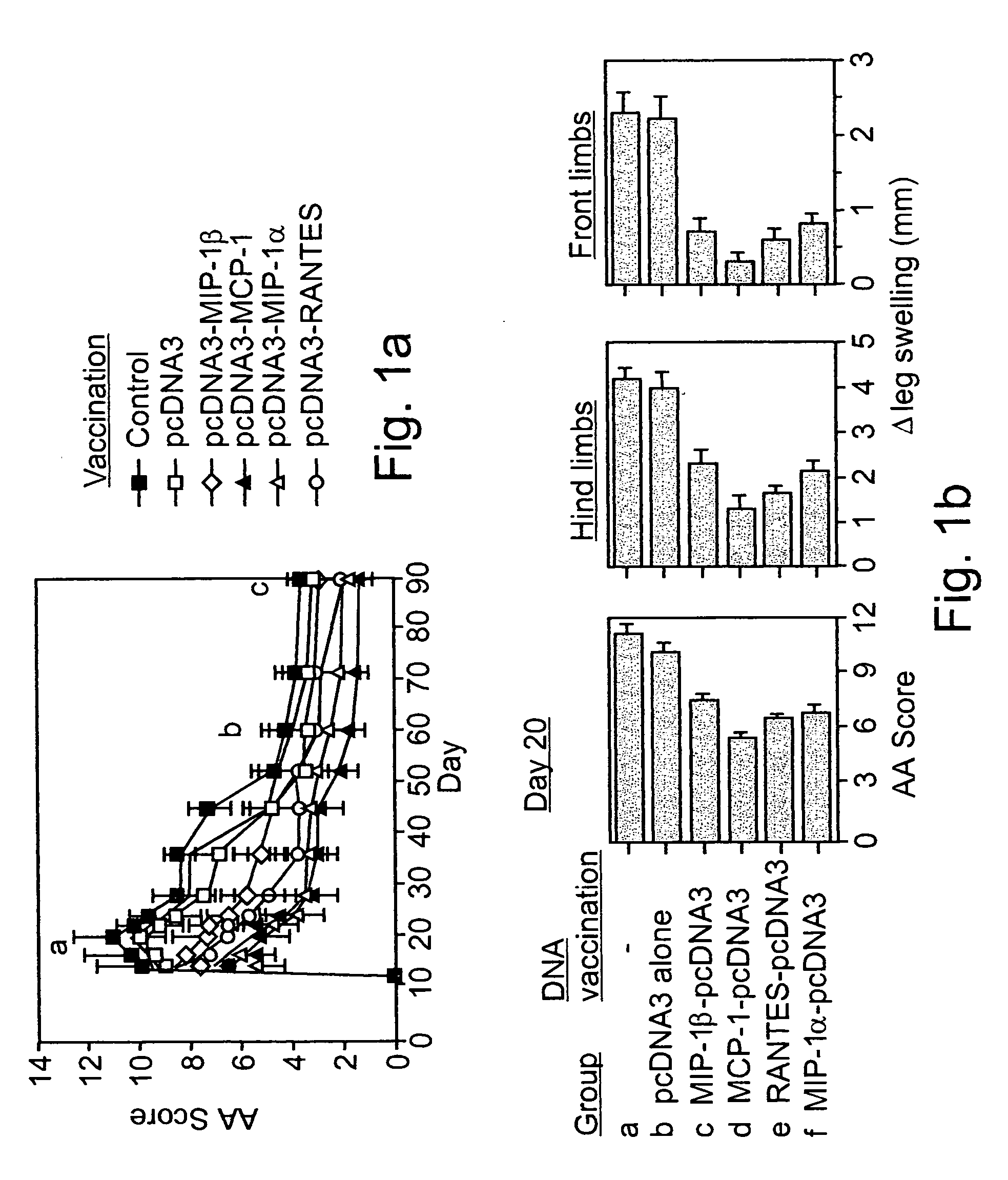
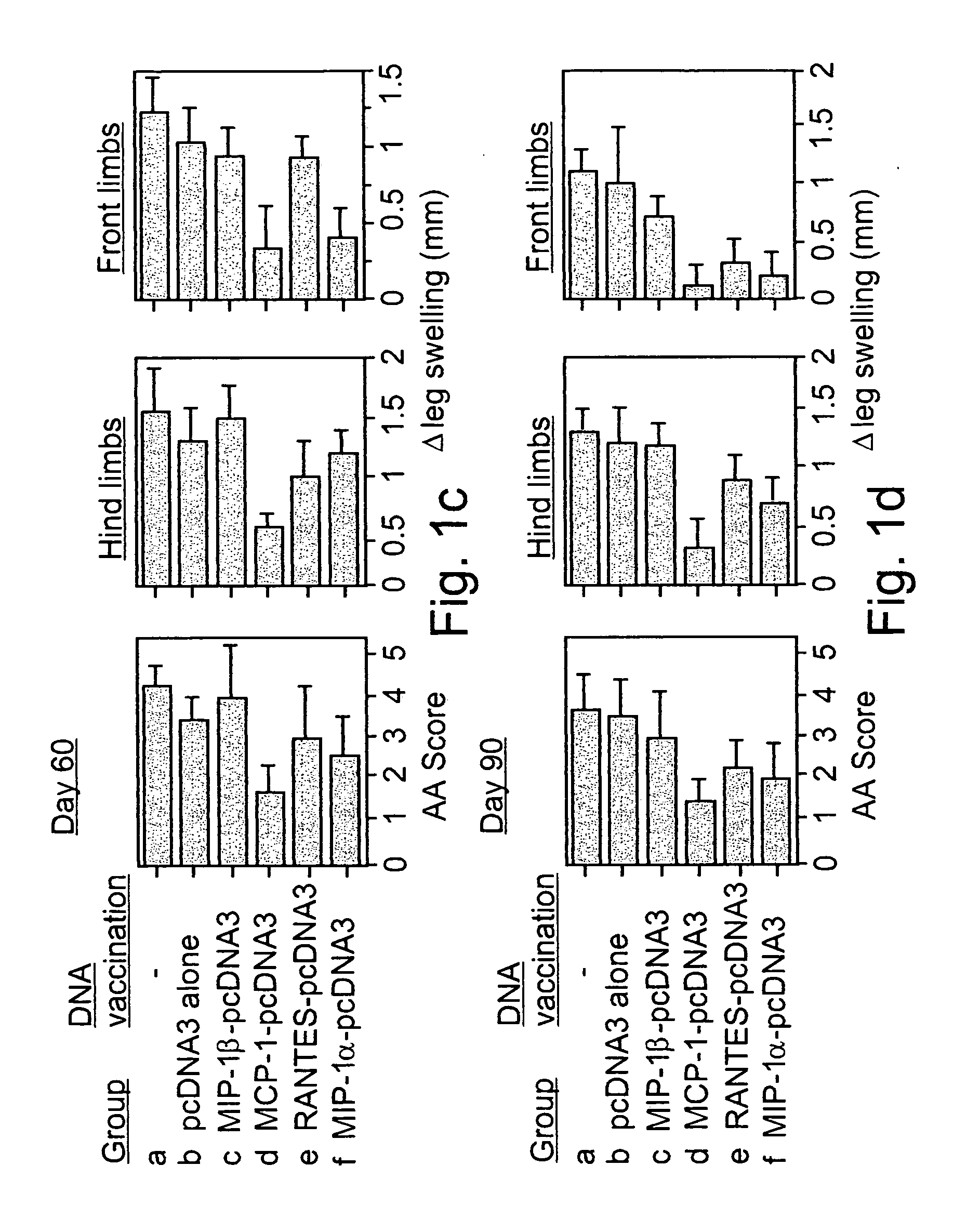
[0119] Prevention of AA Using Naked DNA Vaccines:
[0120] Cloned PCR products of the MIP-1.alpha., MCP-1, MIP-1.beta., RANTES C-C chemokines (Youssef et al., 1998) were ligated into a pcDNA3 mammalian expression vector and used as constructs for naked DNA vaccination (FIG. 1a). Lewis rats were exposed to four weekly administrations of various naked DNA vaccines. Control rats were either injected with the pcDNA3 vector alone, or with PBS. Three weeks after the last immunization all rats were immunized with CFA to induce AA. Under working conditions established in the present study, AA manifests a long lasting form of disease that includes an acute phase, peaking around day 20, and a chronic phase that persists for more than 100 days (FIG. 1a). All control (PBS immunized) and pcDNA3 vaccinated rats (10 per group) developed a severe form of disease with a maximal clinical score (day 20) of 11...
example 2
Results of Experiments Conducted With TNF-.alpha.
[0135] Prevention of AA using TNF-.alpha. Naked DNA Vaccine:
[0136] The cloned PCR product of TNF-.alpha. was ligated into a pcDNA3 mammalian expression vector and used as constructs for naked DNA vaccination. Lewis rats were exposed to four weekly administrations of this construct. Control rats were injected with either the .beta.-actin construct, the pcDNA3 vector alone, or with PBS. Three weeks following the last immunization all rats were immunized with CFA to induce AA. Under working conditions established by the present study, AA manifests a long lasting form of disease that includes an acute phase, peaking around day 20, and a chronic phase that persists for more than 100 days (FIGS. 10a-d). All of the control rats which were treated with either PBS, pcDNA3 alone or .beta.-actin pcDNA3 (12 per group) developed a severe form of disease with a maximal clinical score (day 20) of 13.5.+-.1.8, 13.+-.1.52 and 13.+-.1.52 respectively (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com