Patents
Literature
885 results about "Biological specimen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A biological specimen (also called a biospecimen) is a biological laboratory specimen held by a biorepository for research. Such a specimen would be taken by sampling so as to be representative of any other specimen taken from the source of the specimen. When biological specimens are stored, ideally they remain equivalent to freshly-collected specimens for the purposes of research.
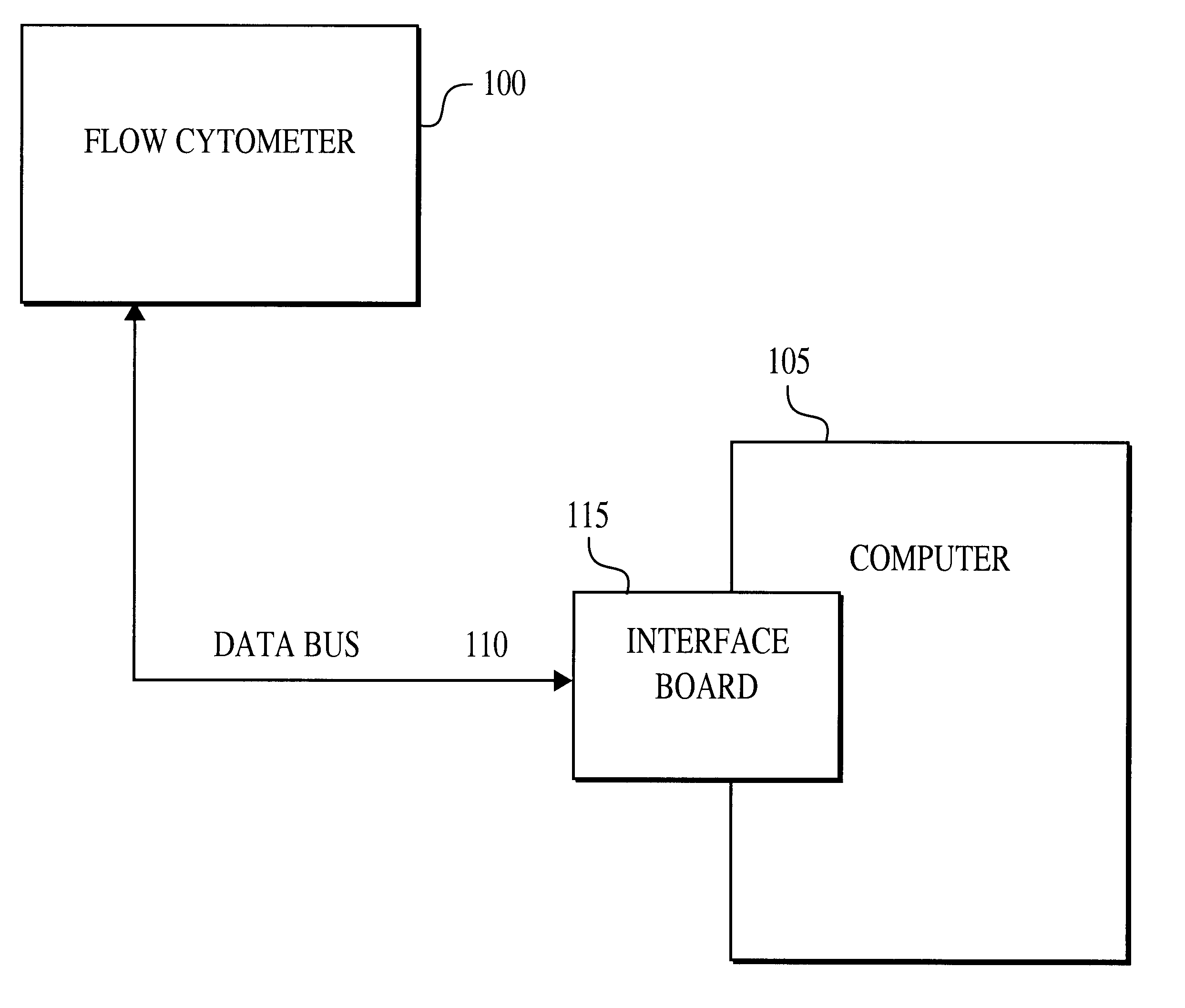
Multiplexed analysis of clinical specimens apparatus and method
InactiveUS6524793B1Simple methodSure easyMicrobiological testing/measurementEnzymologyReal time analysisDNA fragmentation
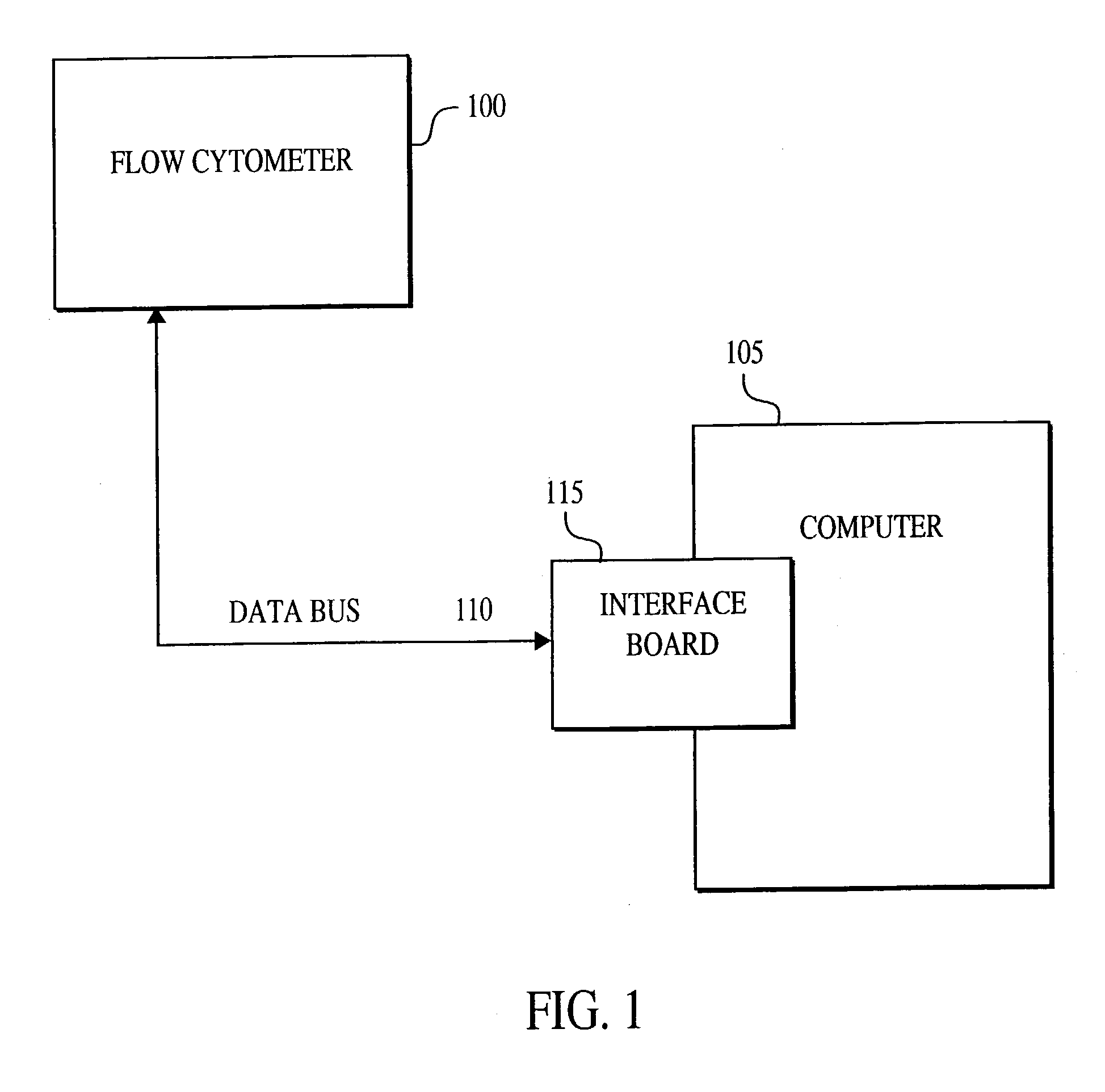
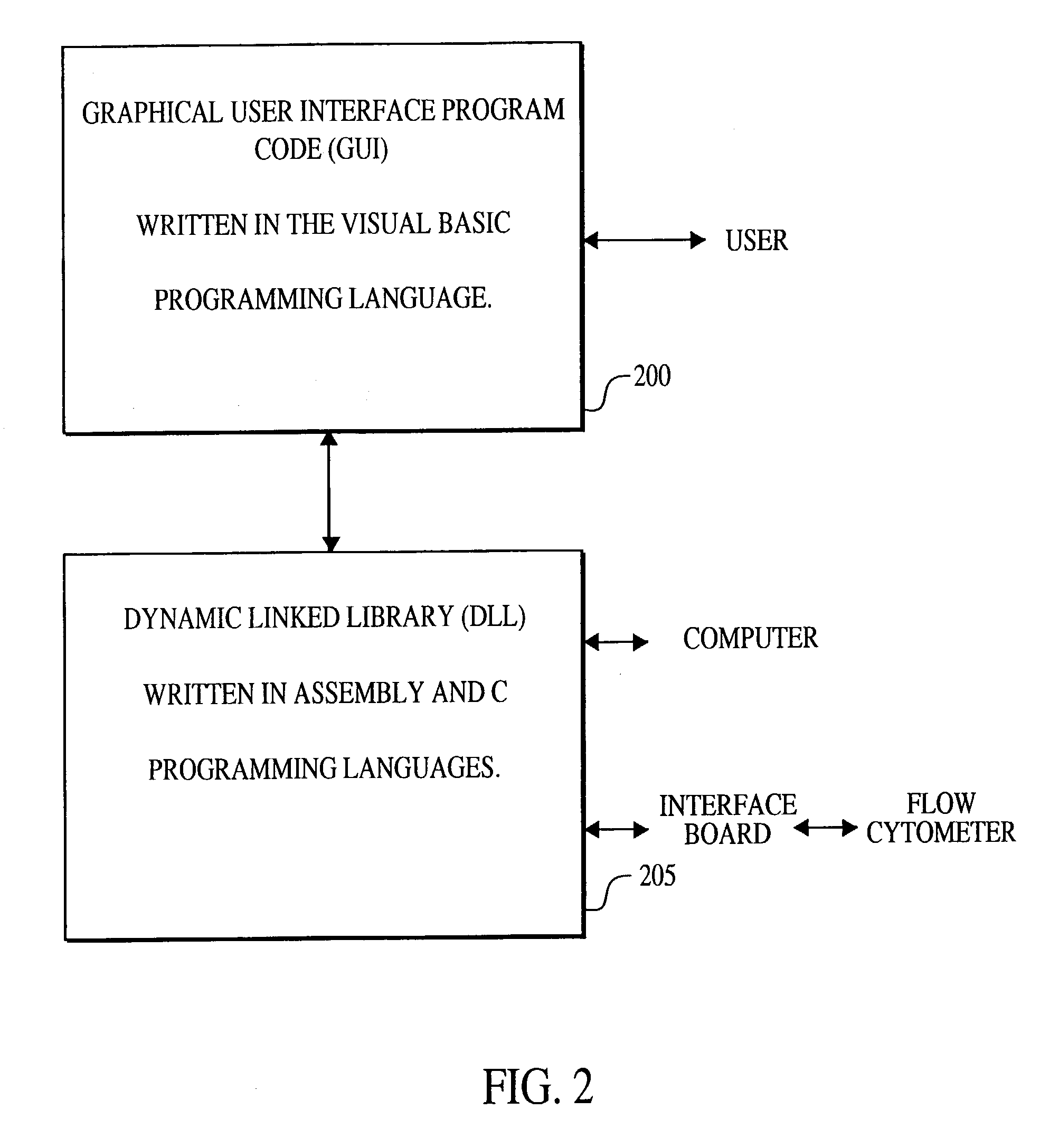
A method for the multiplexed diagnostic and genetic analysis of enzymes, DNA fragments, antibodies, and other biomolecules comprises the steps of constructing an appropriately labeled beadset, exposing the beadset to a clinical sample, and analyzing the combined sample / beadset by flow cytometry. Flow cytometric measurements are used to classify, in real-time, beads within an exposed beadset and textual explanations, based on the accumulated data obtained during real-time analysis, are generated for the user. The inventive technology enables the simultaneous, and automated, detection and interpretation of multiple biomolecules or DNA sequences in real-time while also reducing the cost of performing diagnostic and genetic assays.
Owner:LUMINEX
Multiplexed analysis of clinical specimens apparatus and method
InactiveUS6939720B2Sure easyMicrobiological testing/measurementEnzymologyReal time analysisDNA fragmentation
A method for the multiplexed diagnostic and genetic analysis of enzymes, DNA fragments, antibodies, and other biomolecules comprises the steps of constructing an appropriately labeled beadset, exposing the beadset to a clinical sample, and analyzing the combined sample / beadset by flow cytometry. Flow cytometric measurements are used to classify, in real-time, beads within an exposed beadset and textual explanations, based on the accumulated data obtained during real-time analysis, are generated for the user. The inventive technology enables the simultaneous, and automated, detection and interpretation of multiple biomolecules or DNA sequences in real-time while also reducing the cost of performing diagnostic and genetic assays.
Owner:LUMINEX
Sample Preparation System And Method for Processing Clinical Specimens
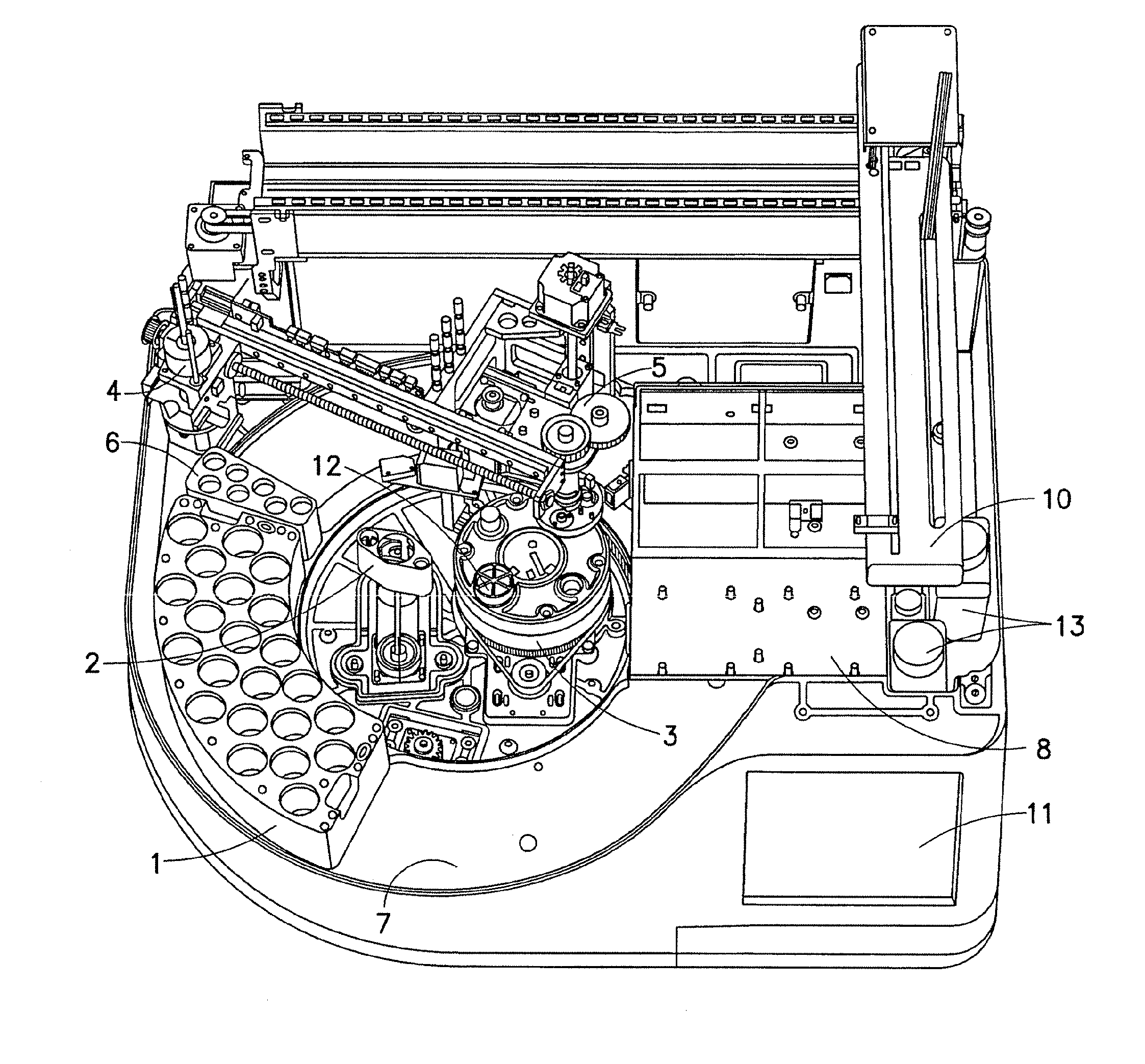
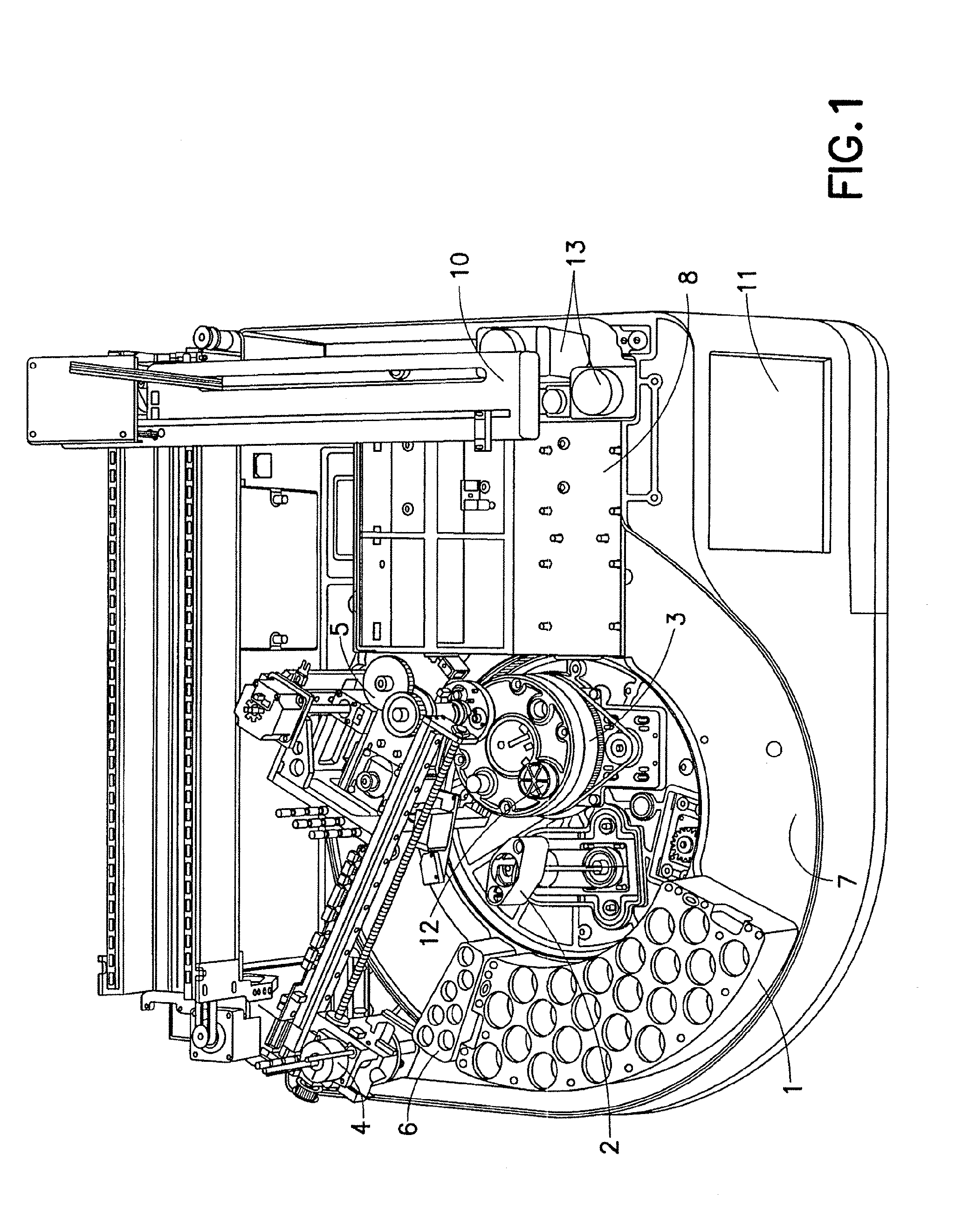
ActiveUS20080247914A1Reduce turnaround timeLow efficiencyAnalysis using chemical indicatorsMicrobiological testing/measurementRobotic systemsMolecular analysis
A system and method for automated handling of vials containing liquid medical specimens is disclosed. The robotic system processes the specimens for further downstream molecular analysis. The processing comprises automated vial cap removal, pipetting of the vial contents, transfer of the vial contents to a destination tray such as a multiwell plate, and recapping of the vial.
Owner:BECTON DICKINSON & CO
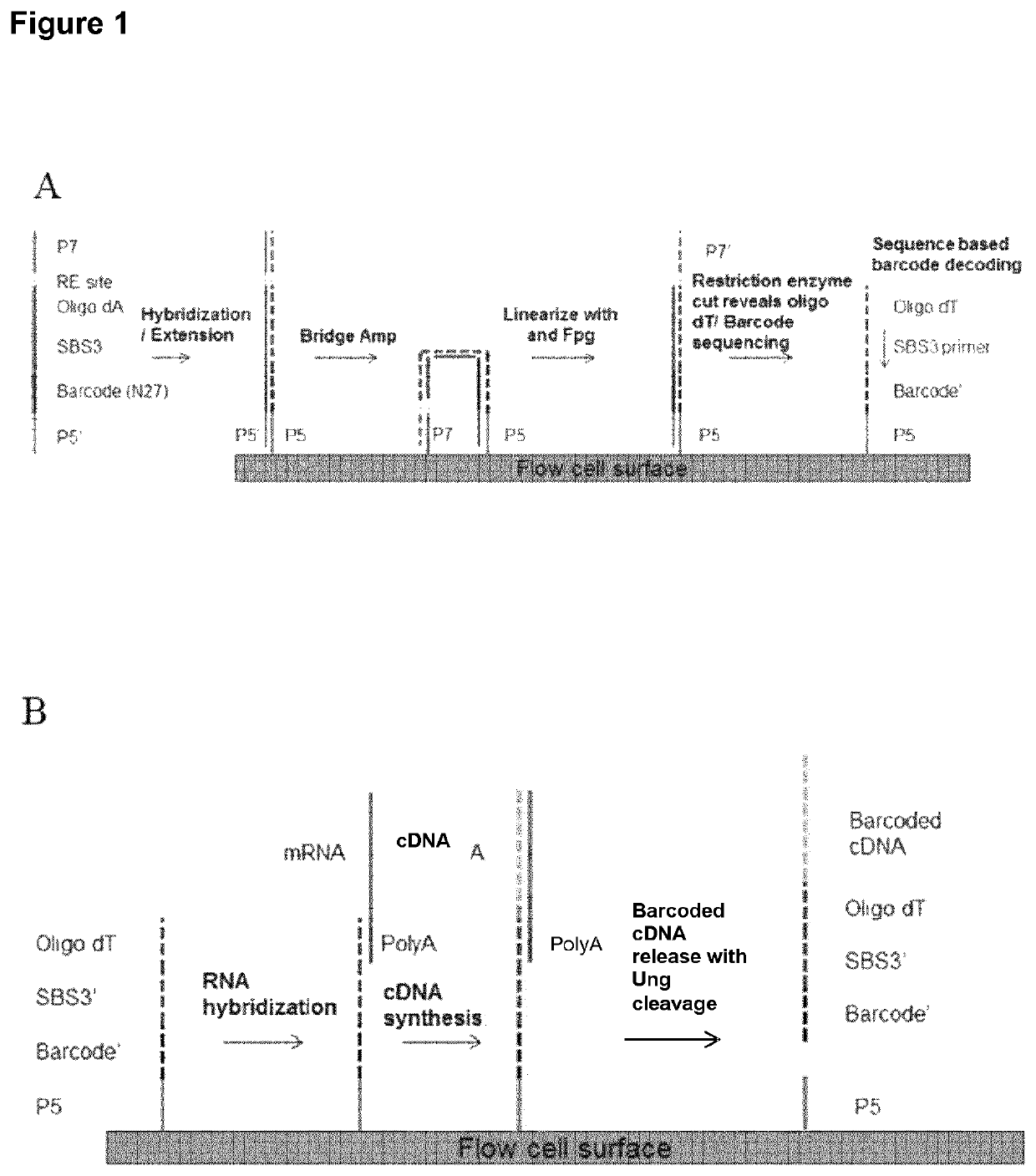
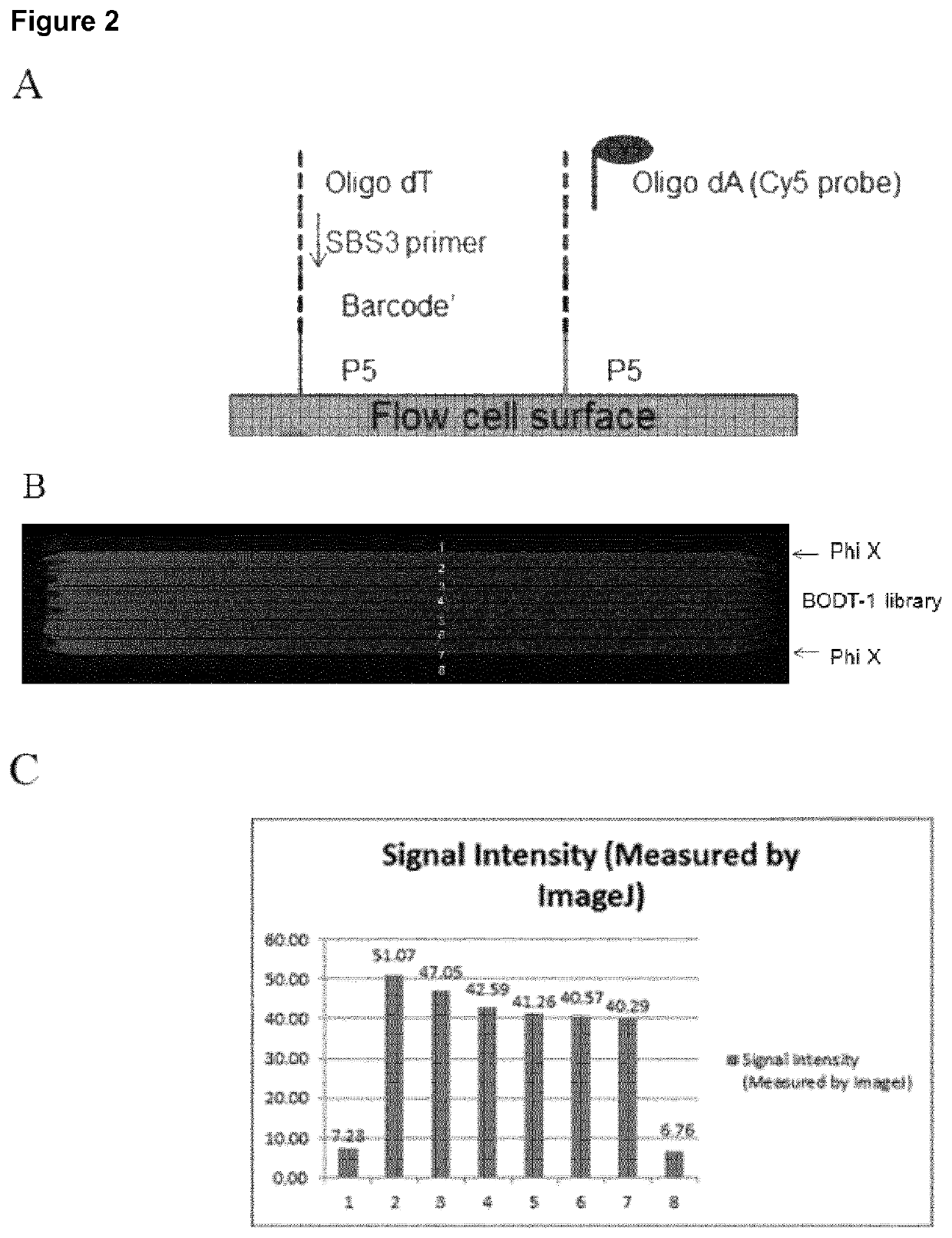
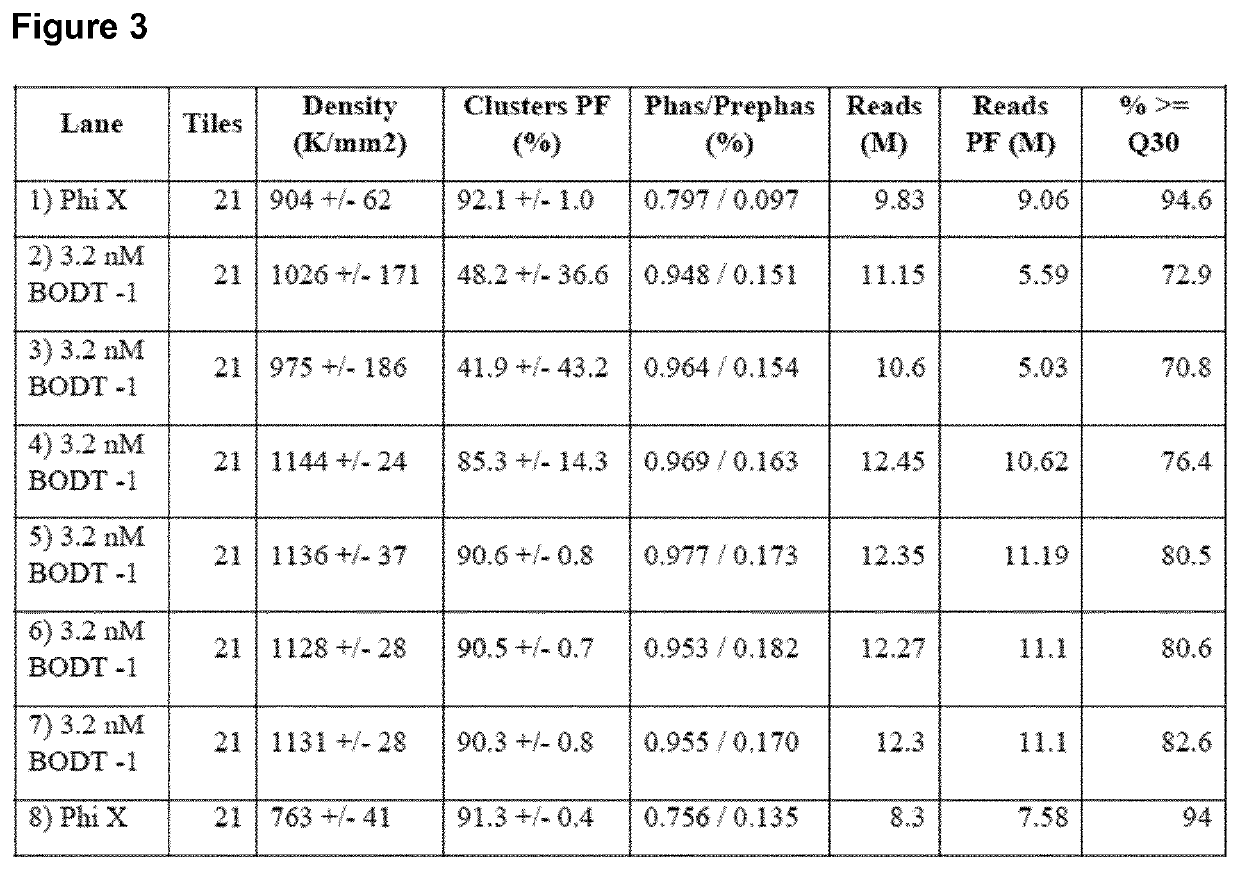
Spatially distinguished, multiplex nucleic acid analysis of biological specimens
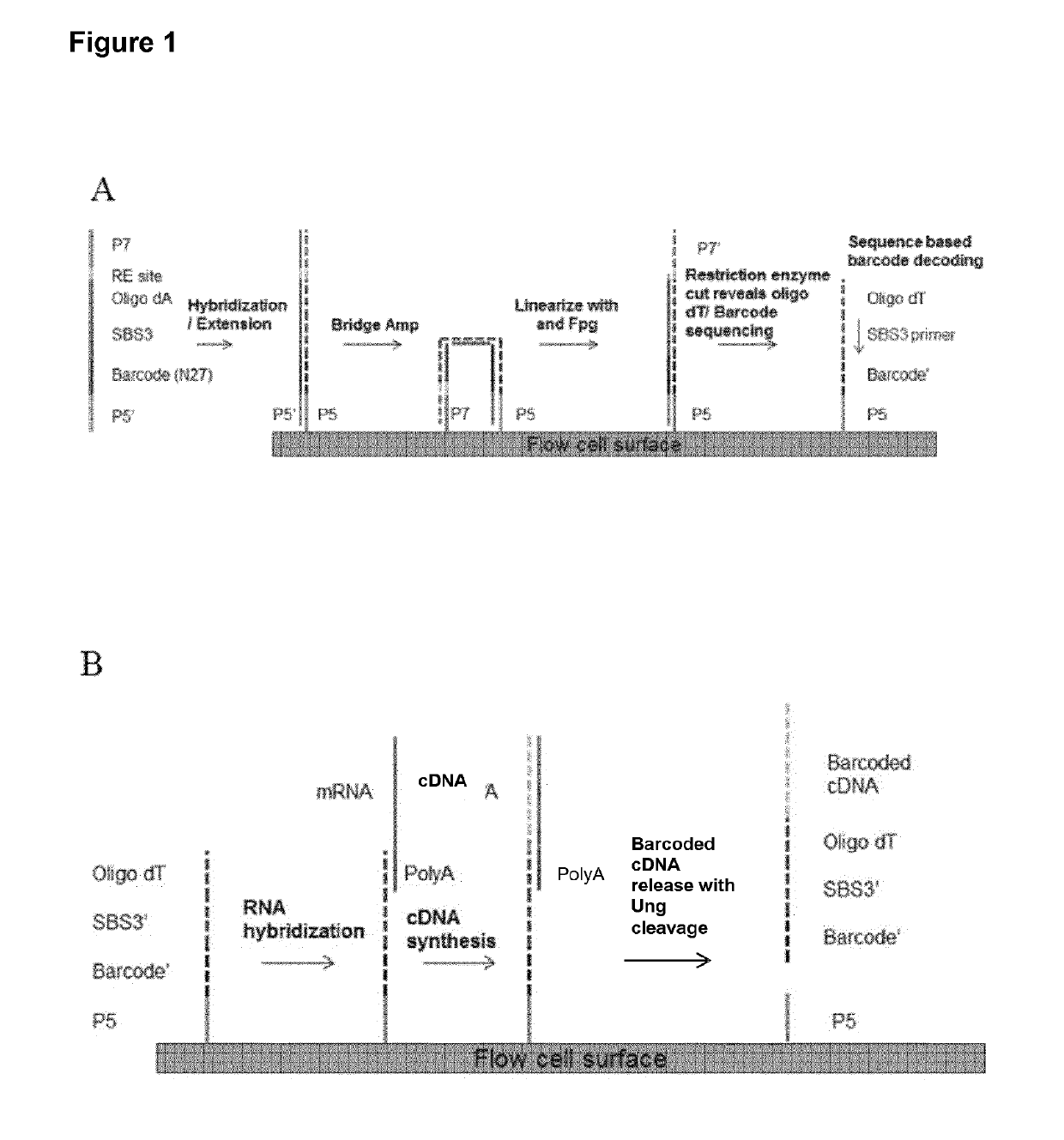
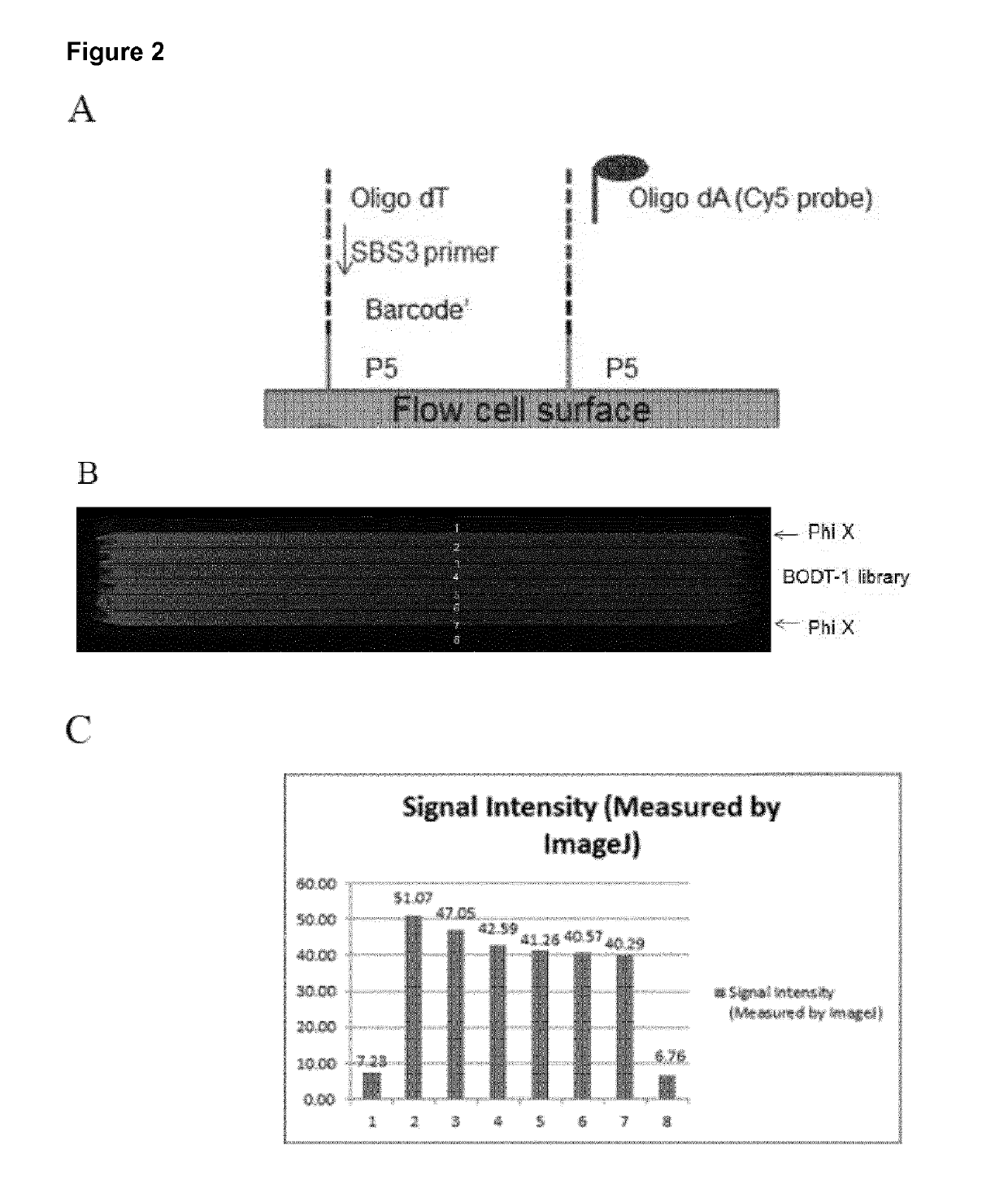
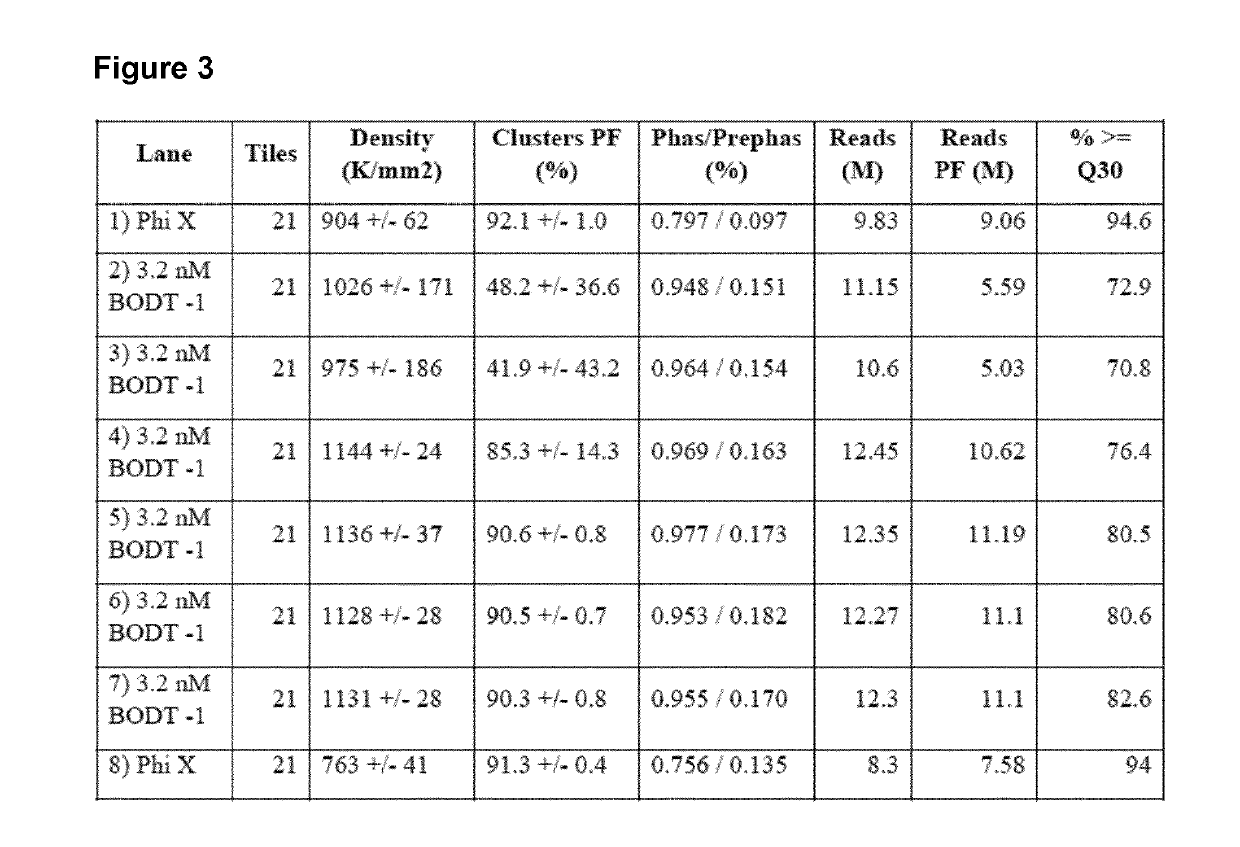
A method for spatially tagging nucleic acids of a biological specimen, including steps of (a) providing a solid support comprising different nucleic acid probes that are randomly located on the solid support, wherein the different nucleic acid probes each includes a barcode sequence that differs from the barcode sequence of other randomly located probes on the solid support; (b) performing a nucleic acid detection reaction on the solid support to locate the barcode sequences on the solid support; (c) contacting a biological specimen with the solid support that has the randomly located probes; (d) hybridizing the randomly located probes to target nucleic acids from portions of the biological specimen; and (e) modifying the randomly located probes that are hybridized to the target nucleic acids, thereby producing modified probes that include the barcode sequences and a target specific modification, thereby spatially tagging the nucleic acids of the biological specimen.
Owner:X GENOMICS SWEDEN AB +1
Analysis of circulating tumor cells, fragments, and debris
InactiveUS20050181463A1Avoid further damageInhibit further damageBioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceApoptosis
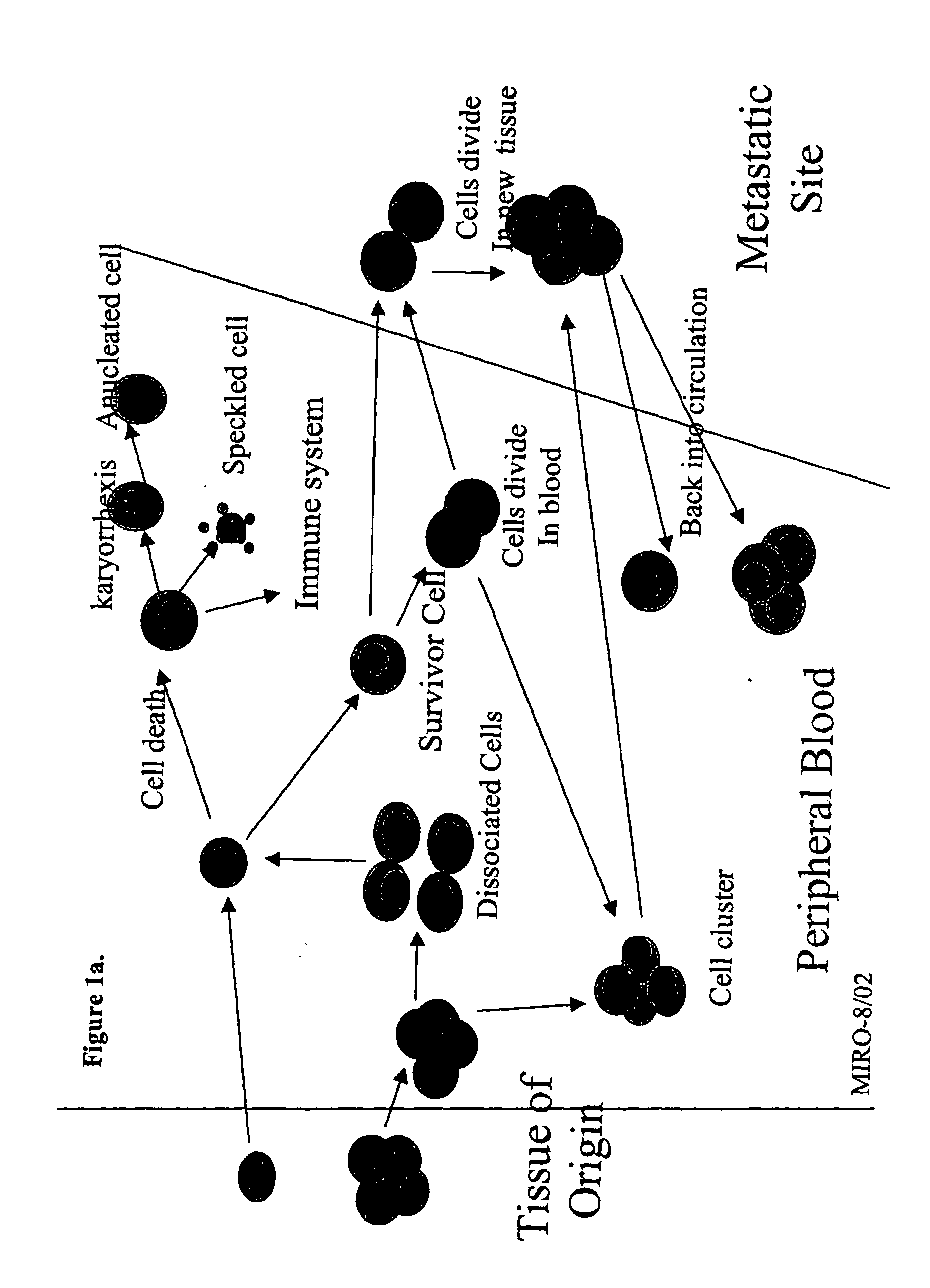
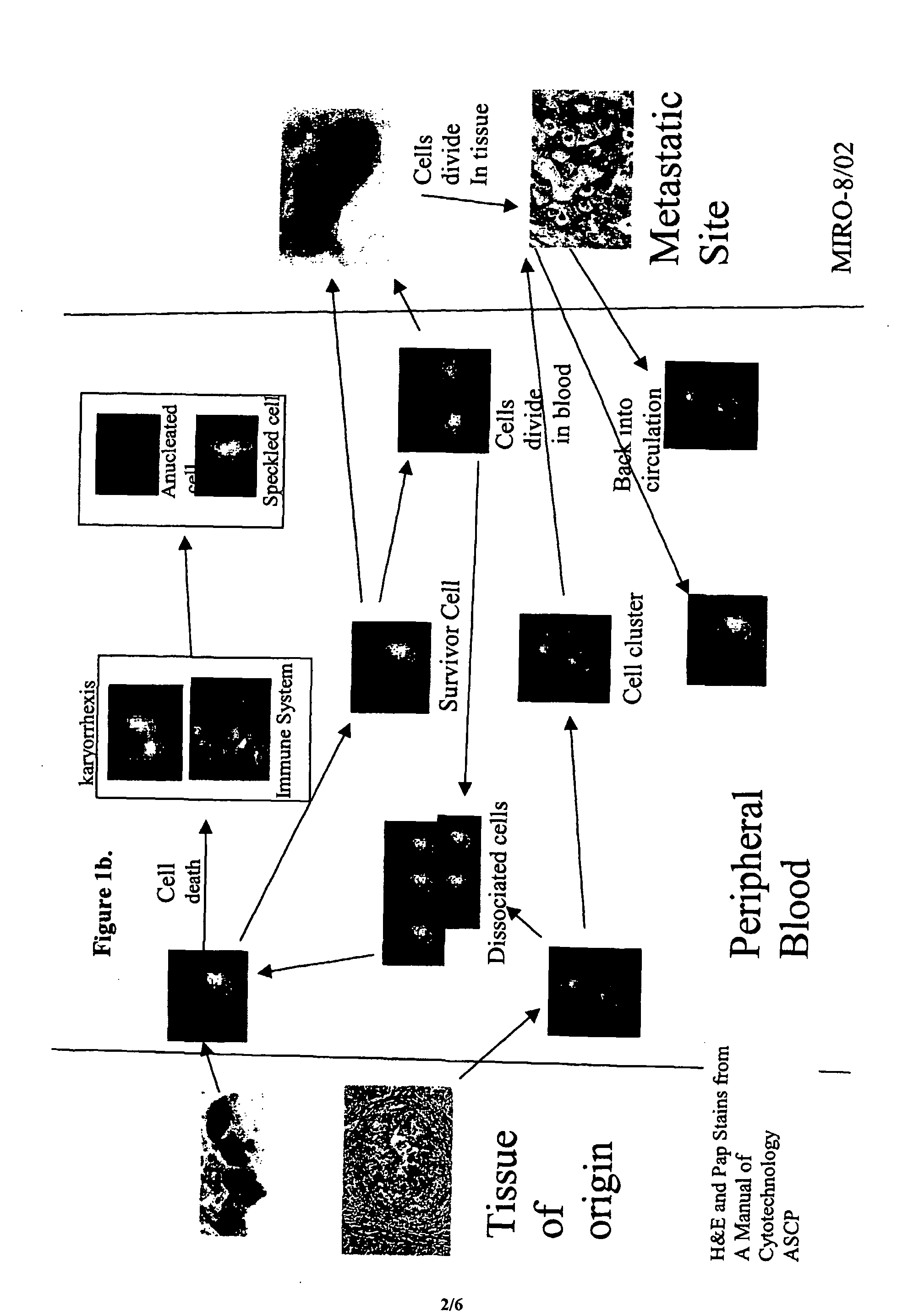
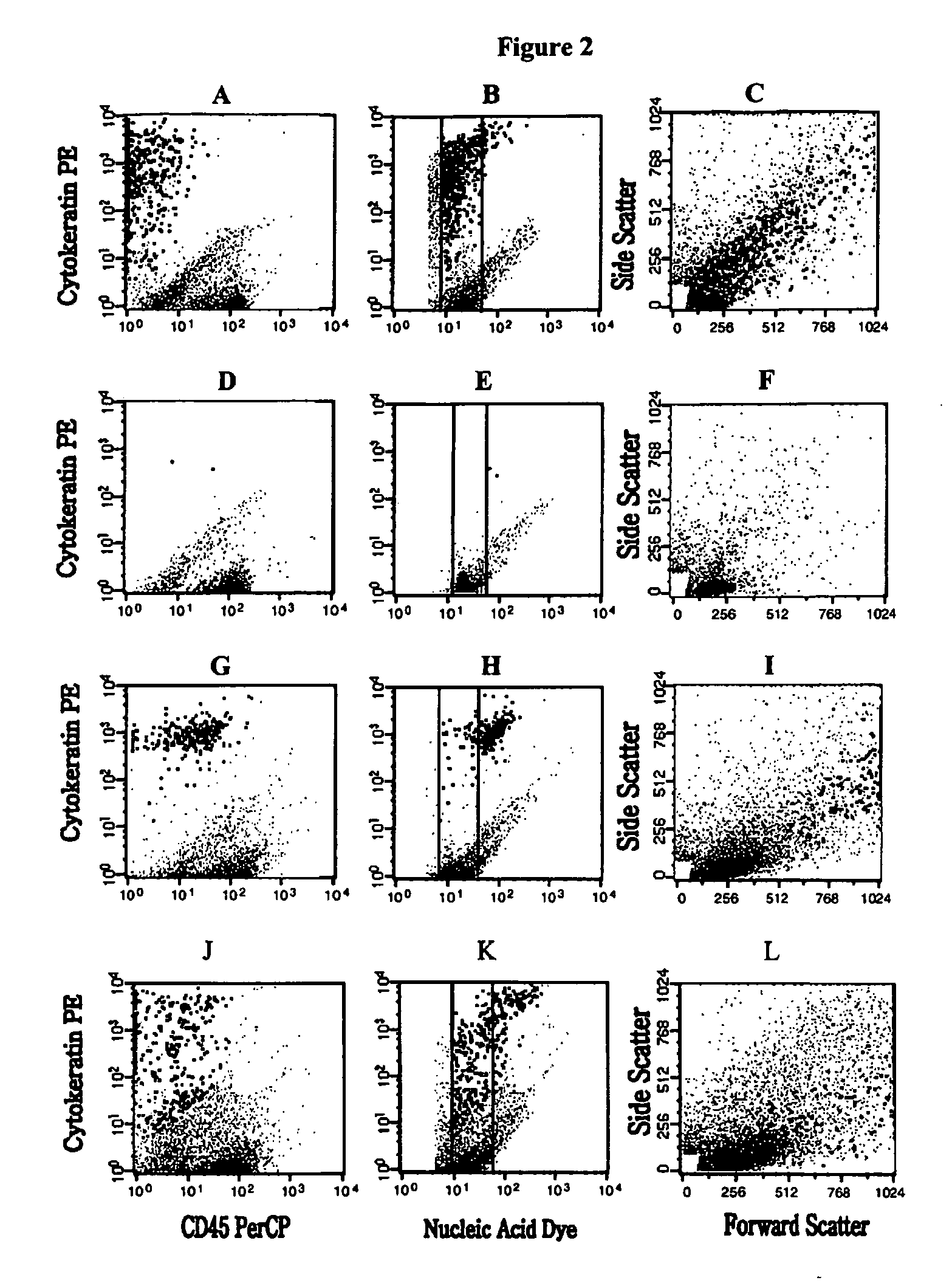
The methods and reagents described in this invention are used to analyze circulating tumor cells, clusters, fragments, and debris. Analysis is performed with a number of platforms, including flow cytometry and the CellSpotter® fluorescent microscopy imaging system. Analyzing damaged cells has shown to be important. However, there are two sources of damage: in vivo and in vitro. Damage in vivo occurs by apoptosis, necrosis, or immune response. Damage in vitro occurs during sample acquisition, handling, transport, processing, or analysis. It is therefore desirable to confine, reduce, eliminate, or at least qualify in vitro damage to prevent it from interfering in analysis. Described herein are methods to diagnose, monitor, and screen disease based on circulating rare cells, including malignancy as determined by CTC, clusters, fragments, and debris. Also provided are kits for assaying biological specimens using these methods.
Owner:MENARINI SILICON BIOSYSTEMS SPA
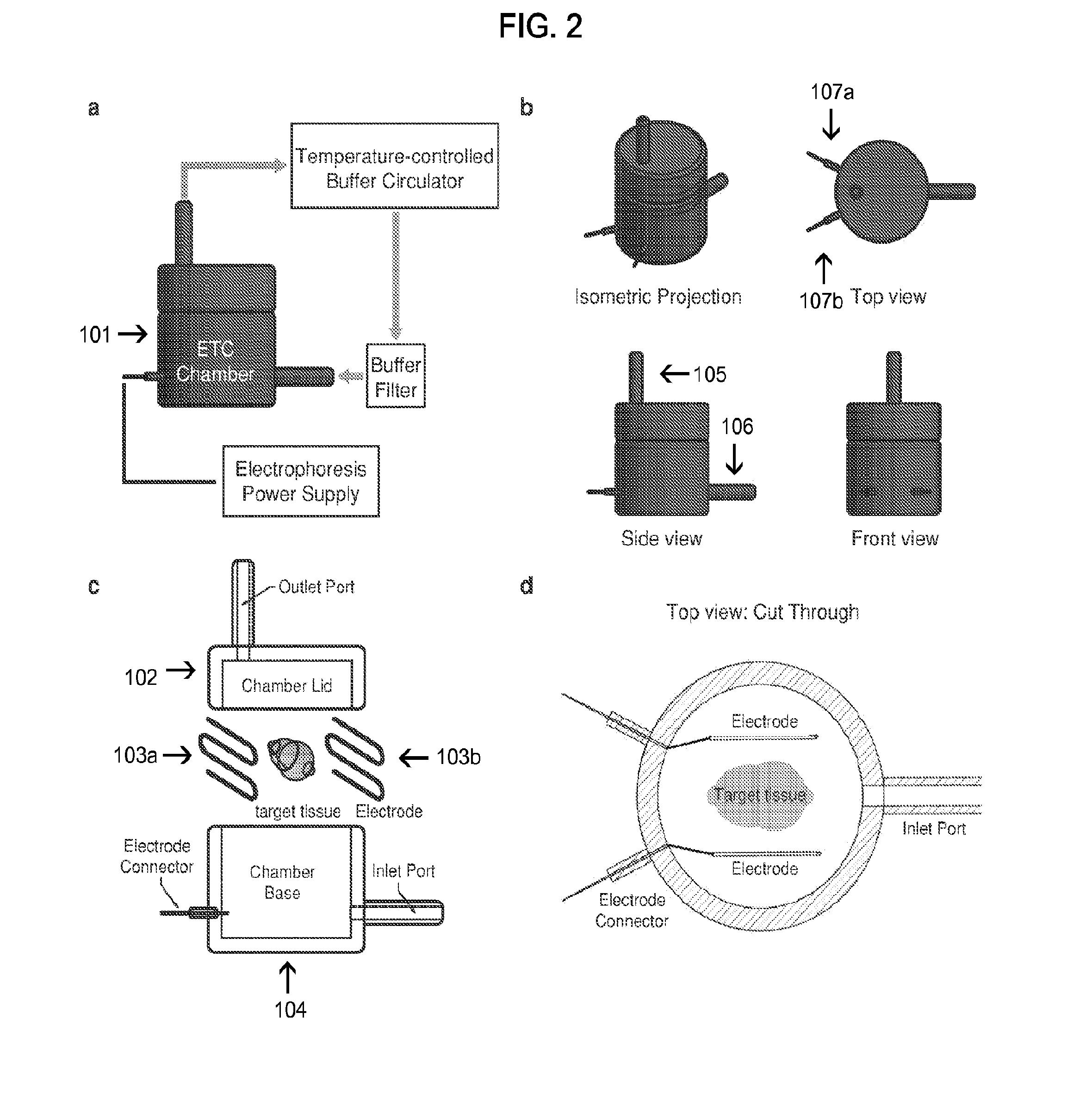
Biological specimen imaging method and biological specimen imaging apparatus
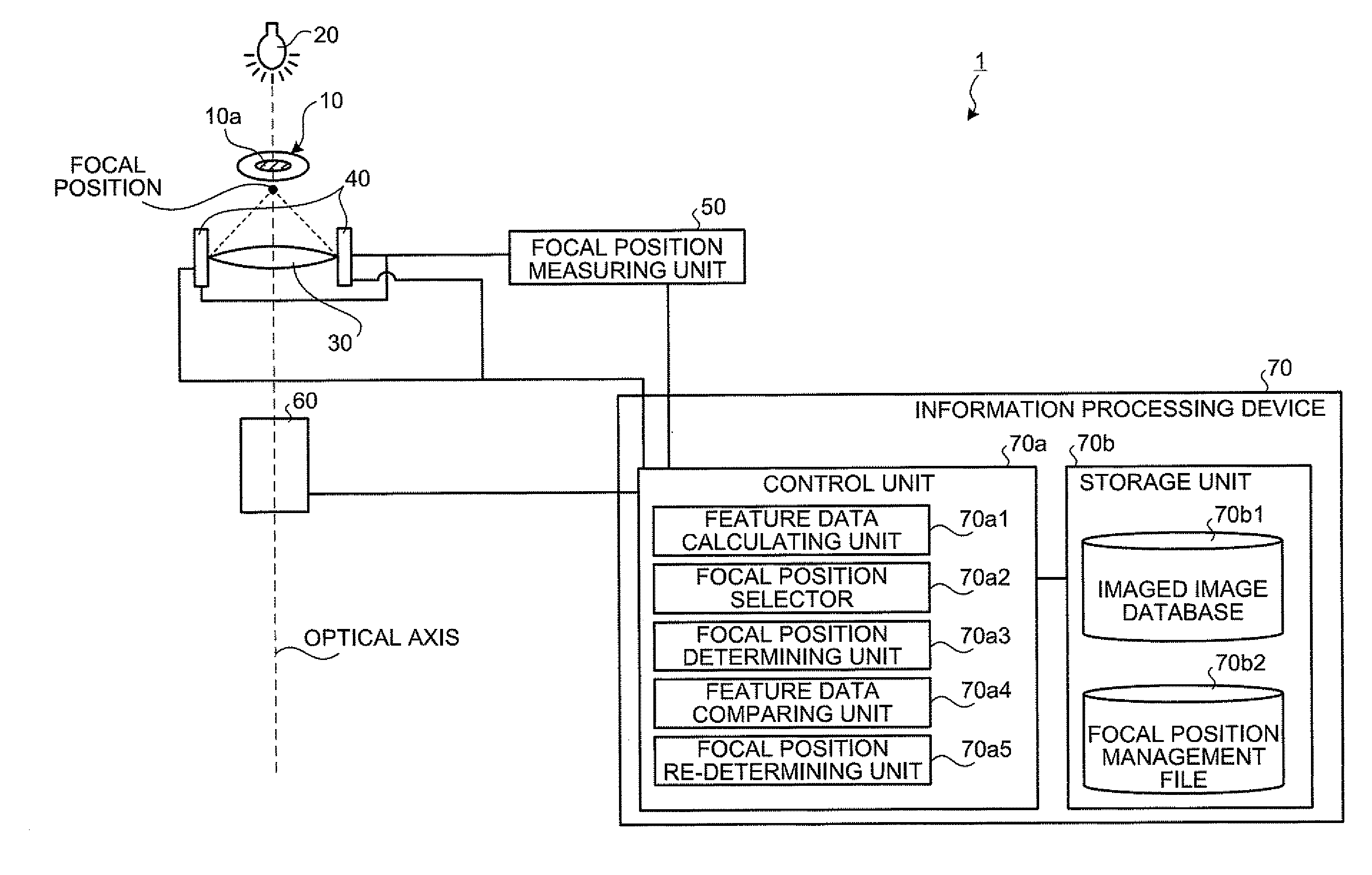
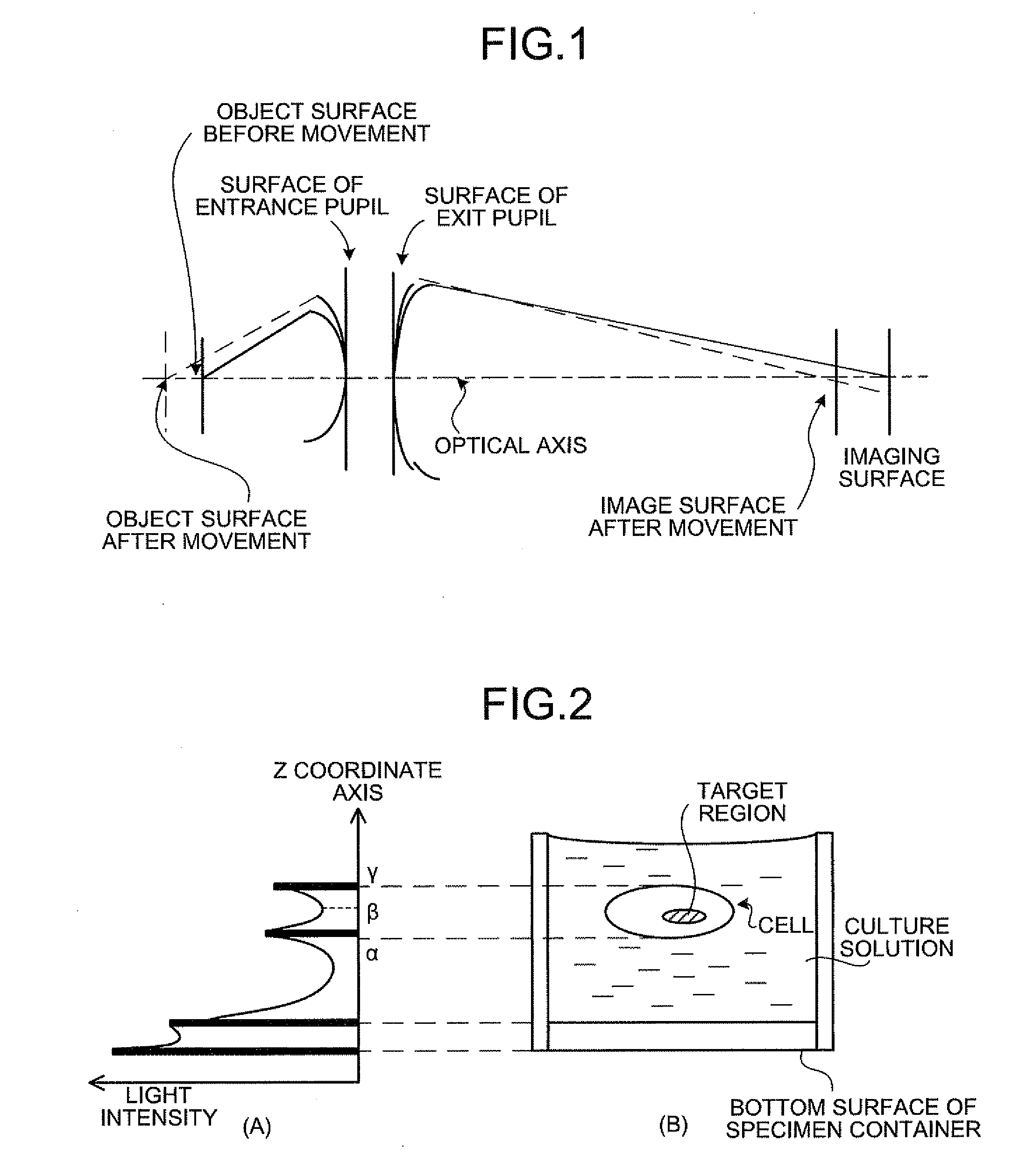
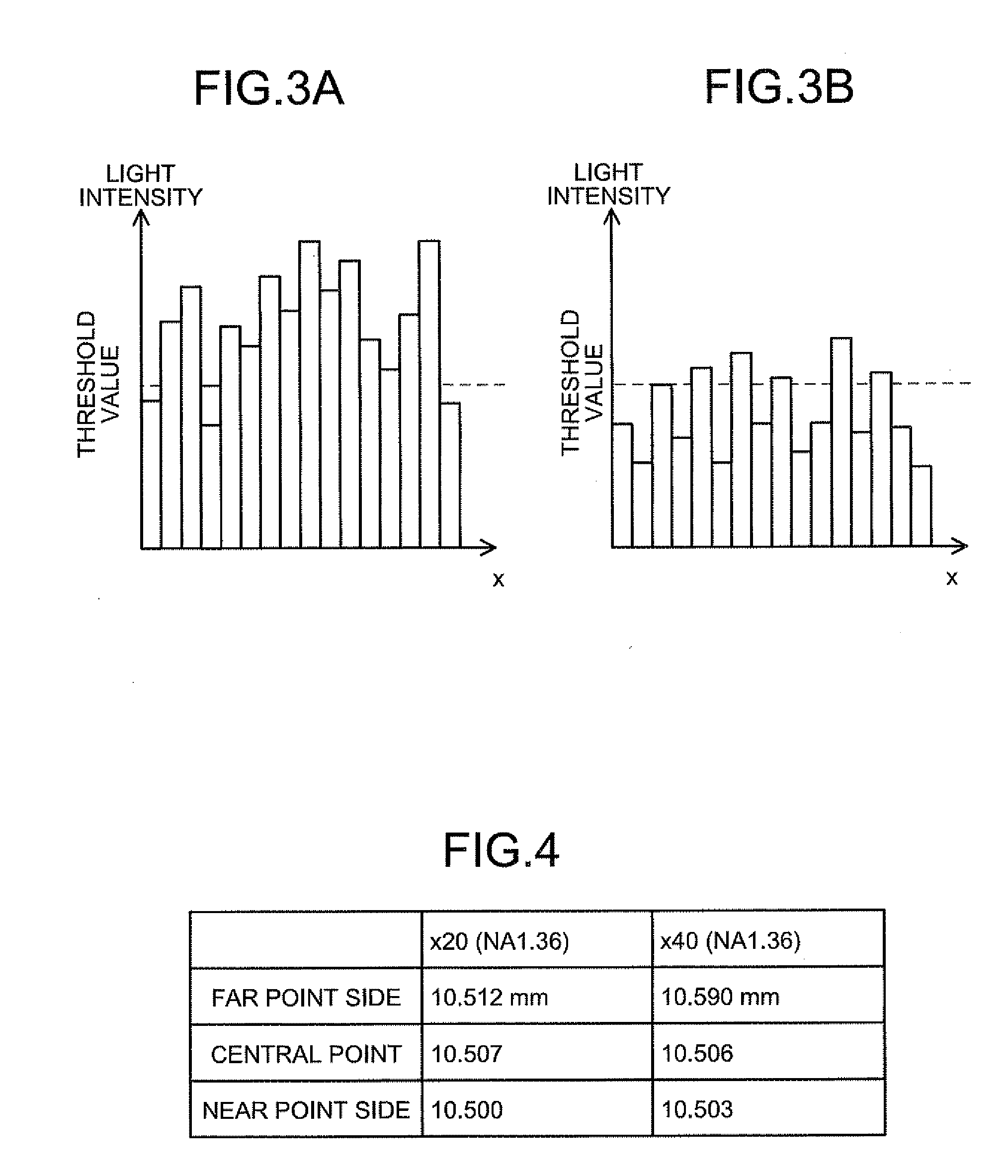
InactiveUS20090086314A1Improve spatial resolutionShort exposure timeMaterial analysis by optical meansMicroscopesFocal positionField of view
In a biological specimen imaging method, a biological specimen which is stored in a storing section of a substrate having plural storing sections and emitting a feeble light is imaged through an objective lens. The biological specimen imaging method includes moving any one of the substrate and the objective lens or both until the desired storing section falls within the field of view of the objective lens, measuring any one of a focal position at a near point and the focal position at a far point of the objective lens or both, determining the focal position of the objective lens focused on an observed target region in the biological specimen stored in the desired storing section based on the measured focal position, and adjusting the focal position of the objective lens to the determined focal position so as to image the biological specimen through the objective lens.
Owner:OLYMPUS CORP
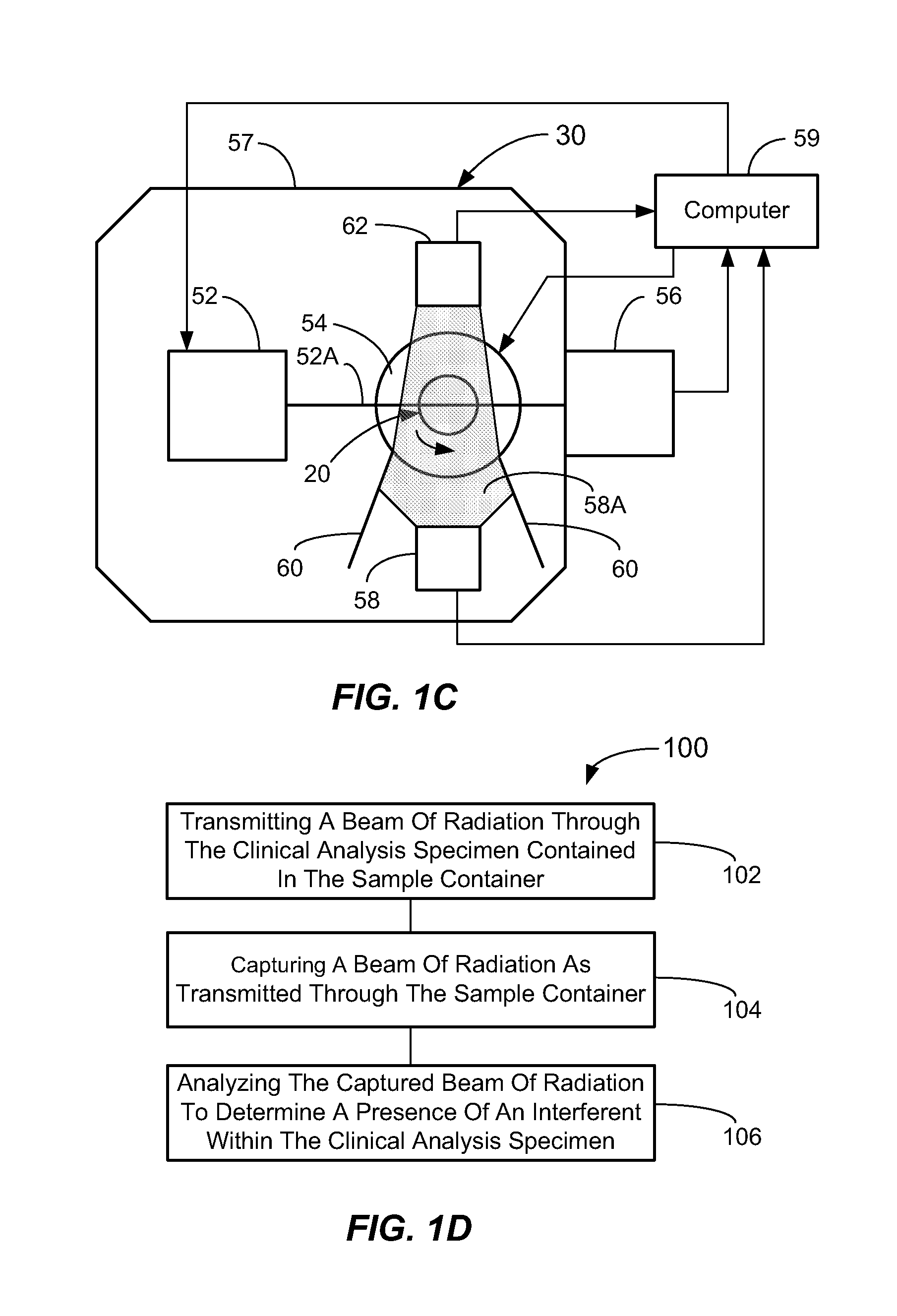
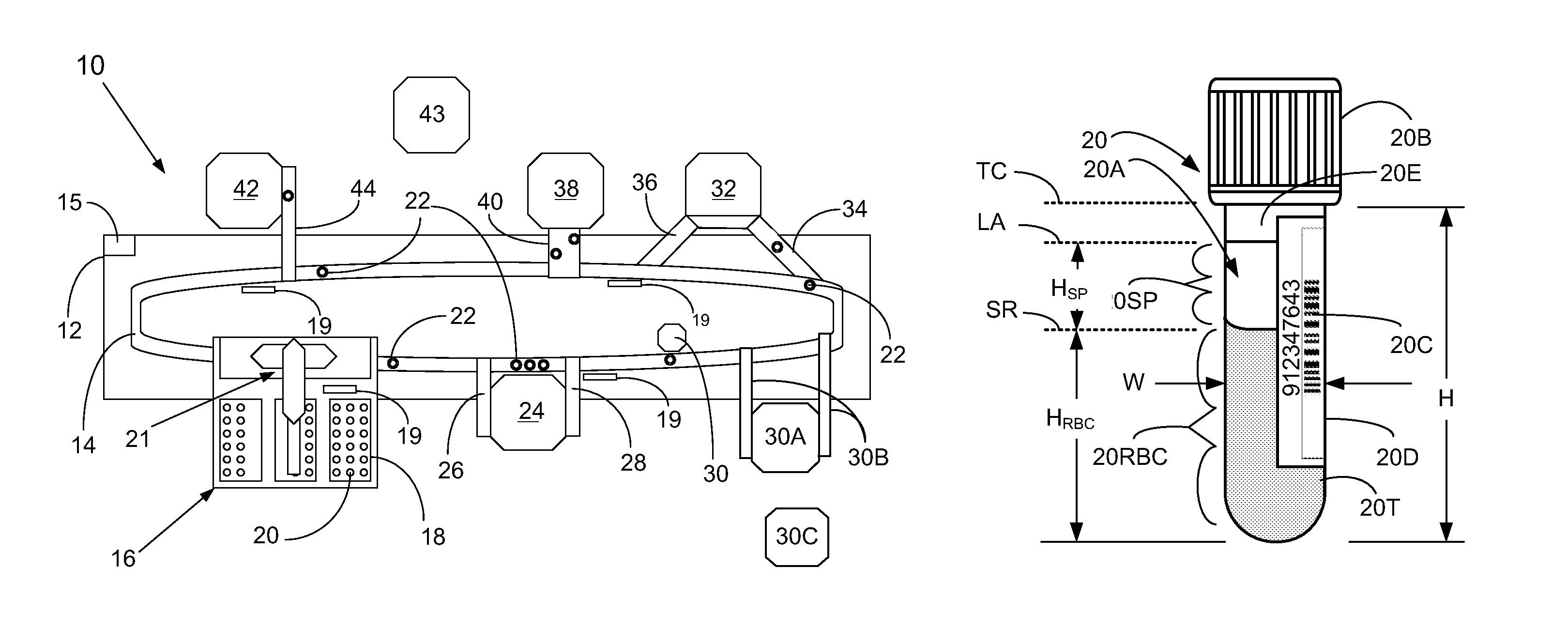
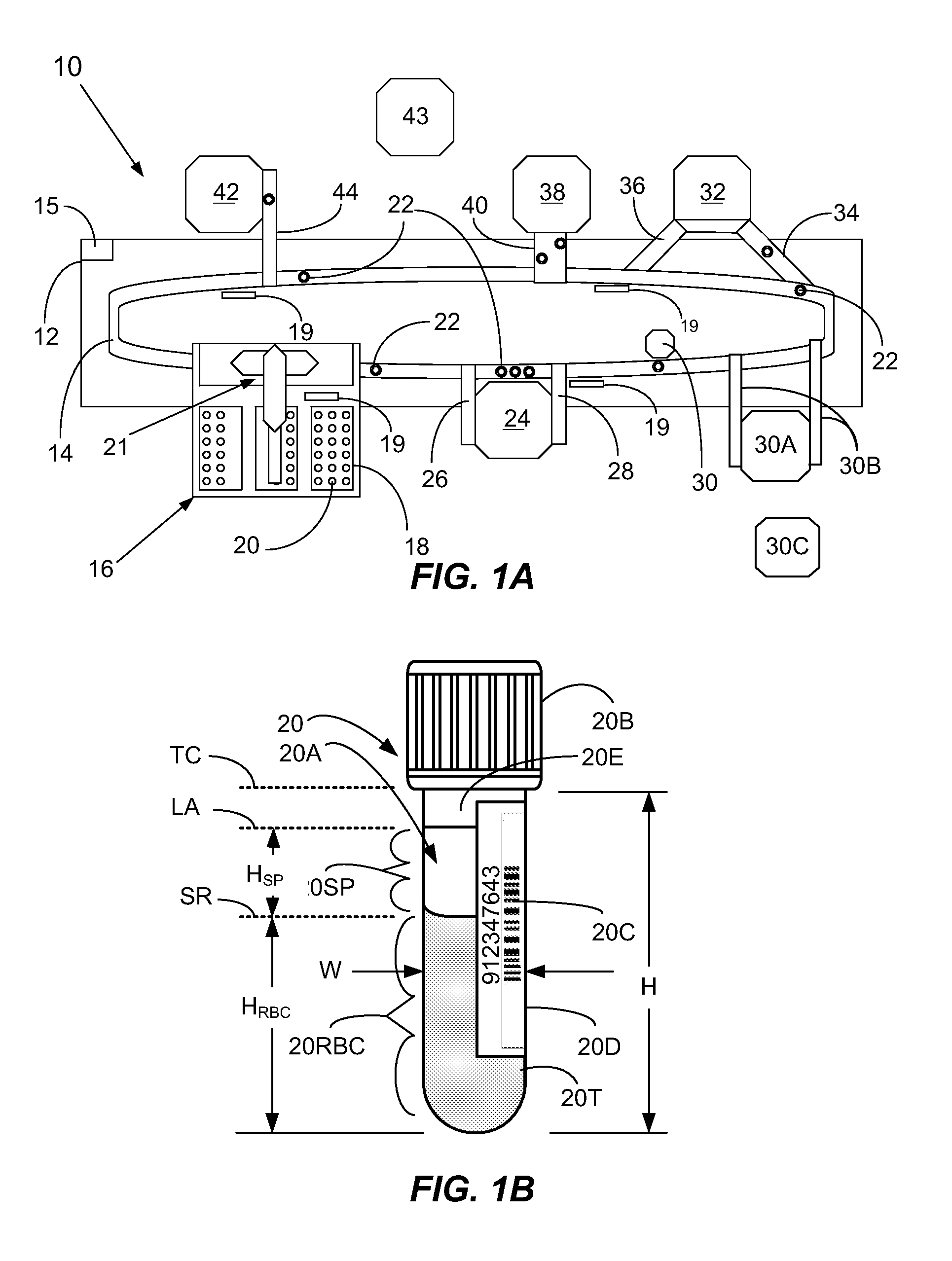
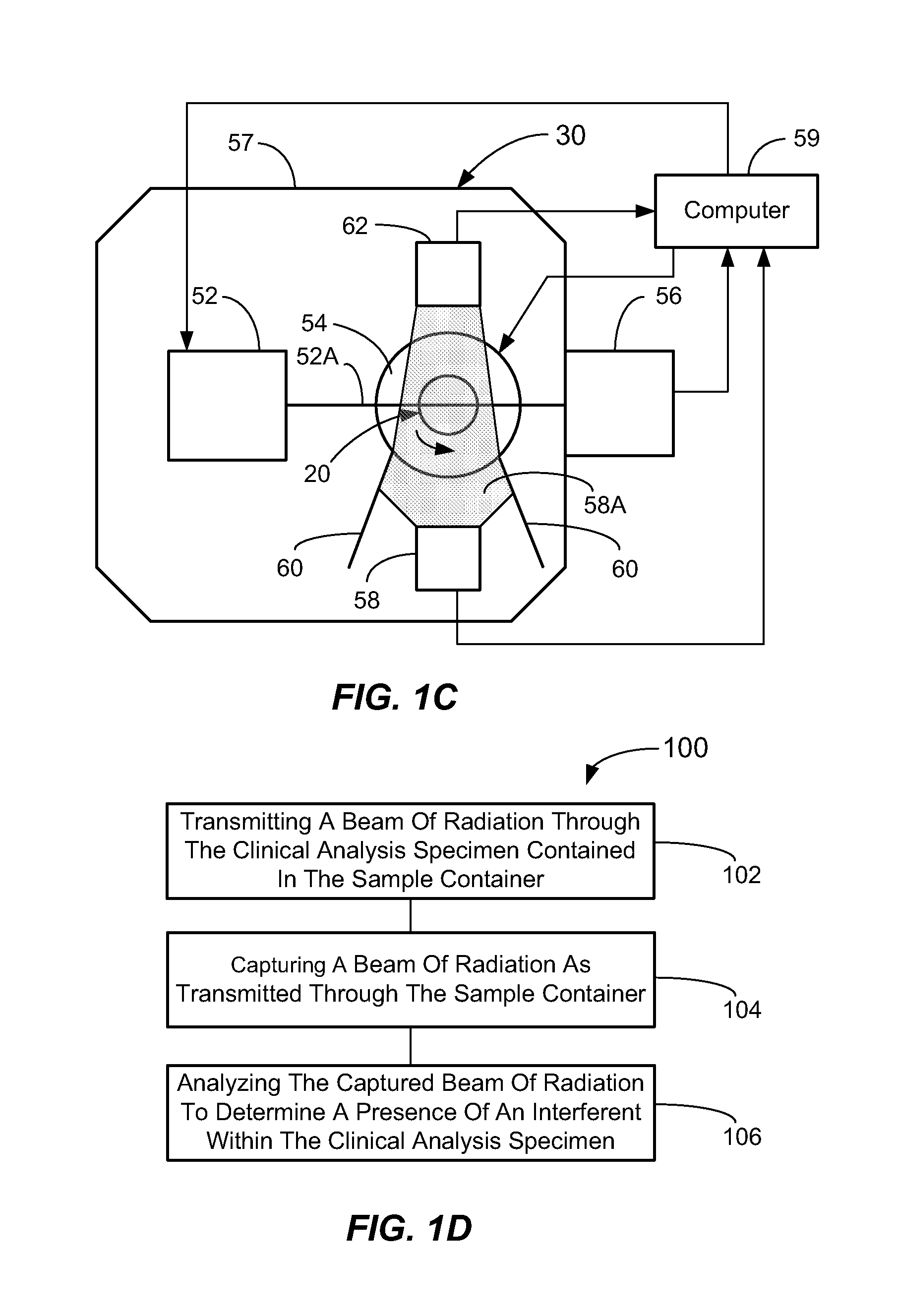
Methods And Apparatus For Ascertaining Interferents And Physical Dimensions In Liquid Samples And Containers To Be Analyzed By A Clinical Analyzer
A method of inspecting a clinical specimen for a presence of one or more interferents, such as those that might be found within clinical analytical blood specimens by subjecting the specimen to centrifugation to separate the specimen into a red blood cell portion and a blood serum or plasma portion is provided. Subsequent to the centrifuging procedure, the serum or plasma portion of the clinical analytical specimen may be tested for the presence of one or more interferents such as hemolysis, icterus, lipemia, or liquid nonuniformities therein. Additionally, physical dimensional characteristics of the sample container and / or specimen may be determined. Apparatus for carrying out the method are described, as are other aspects.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
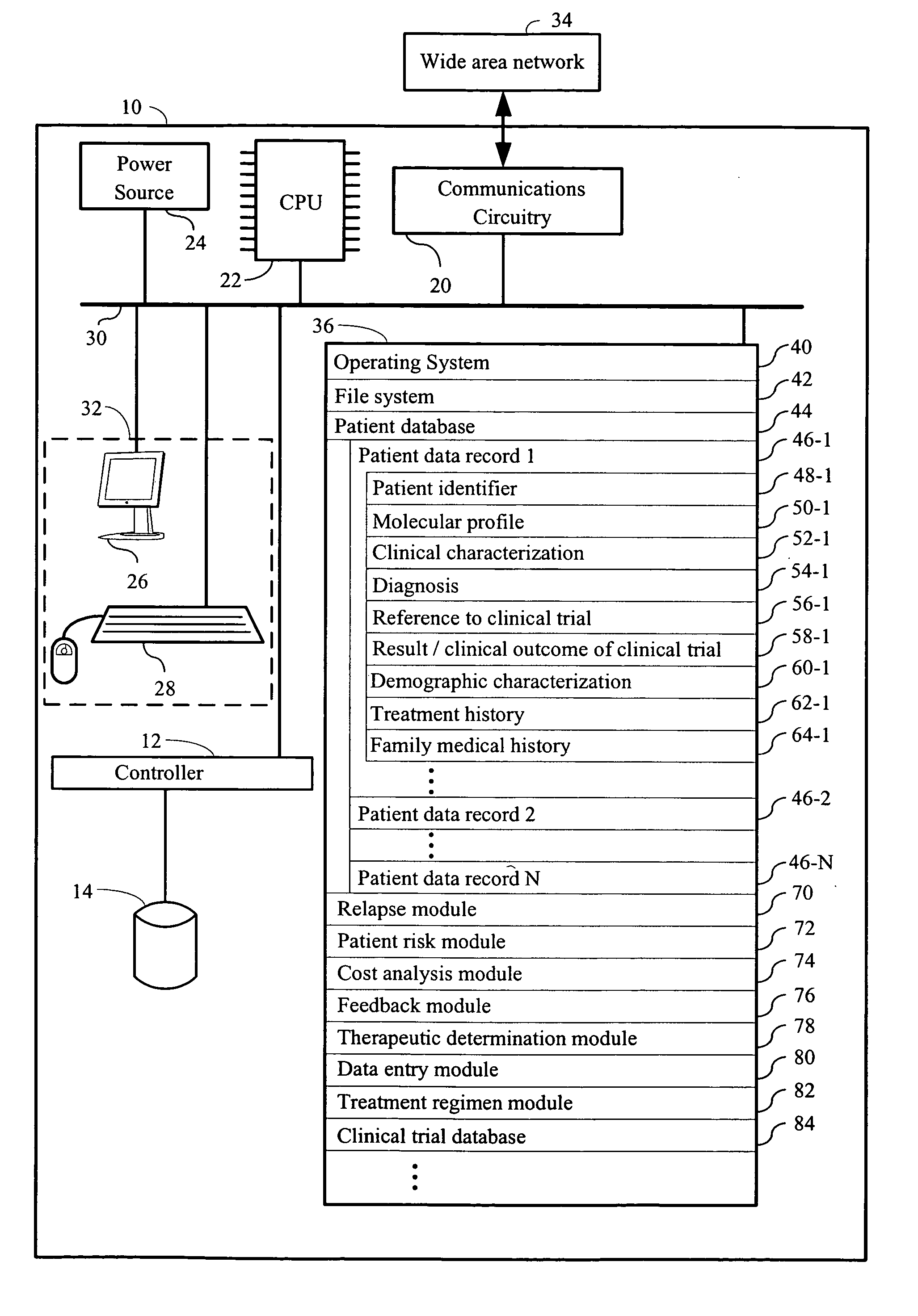
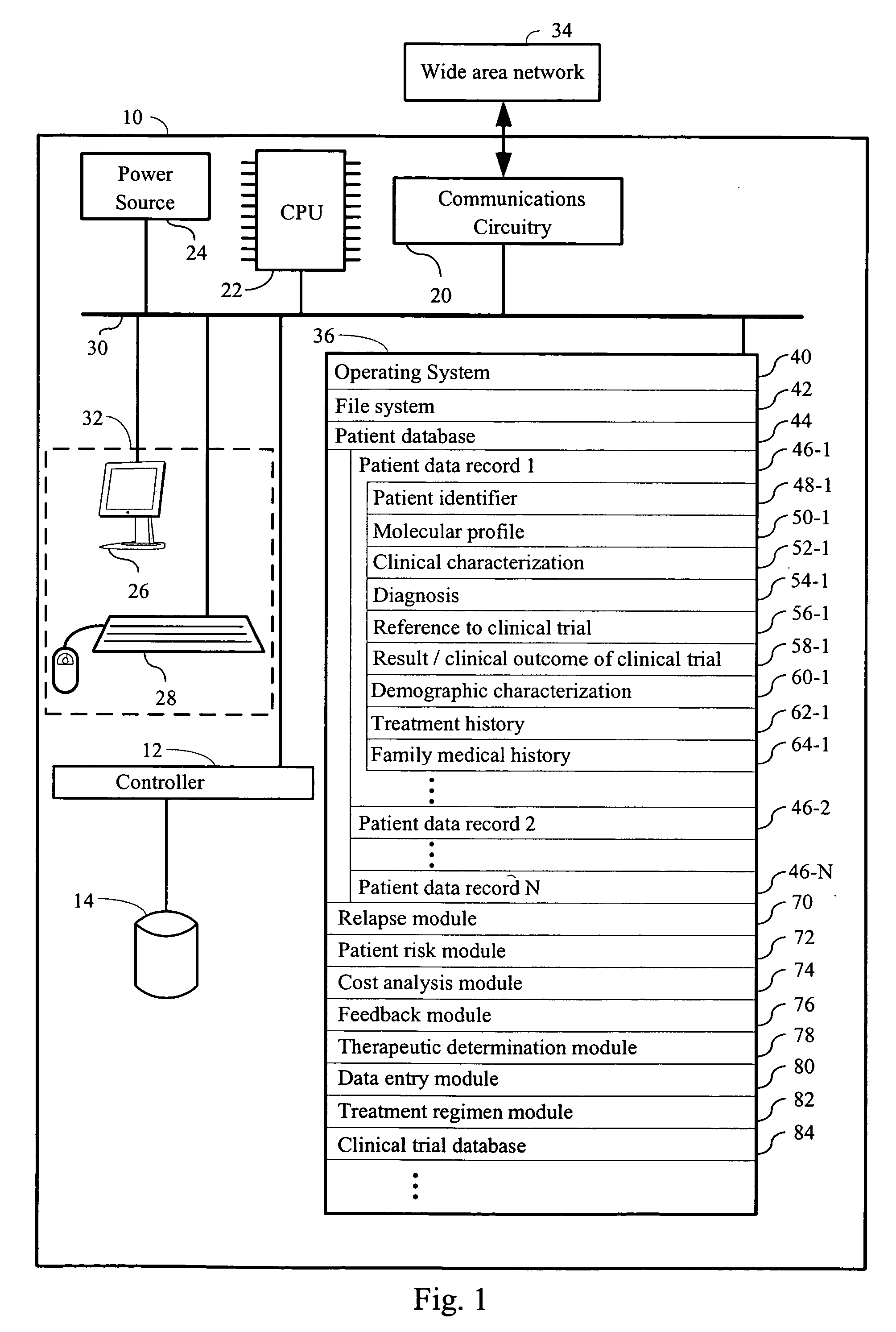
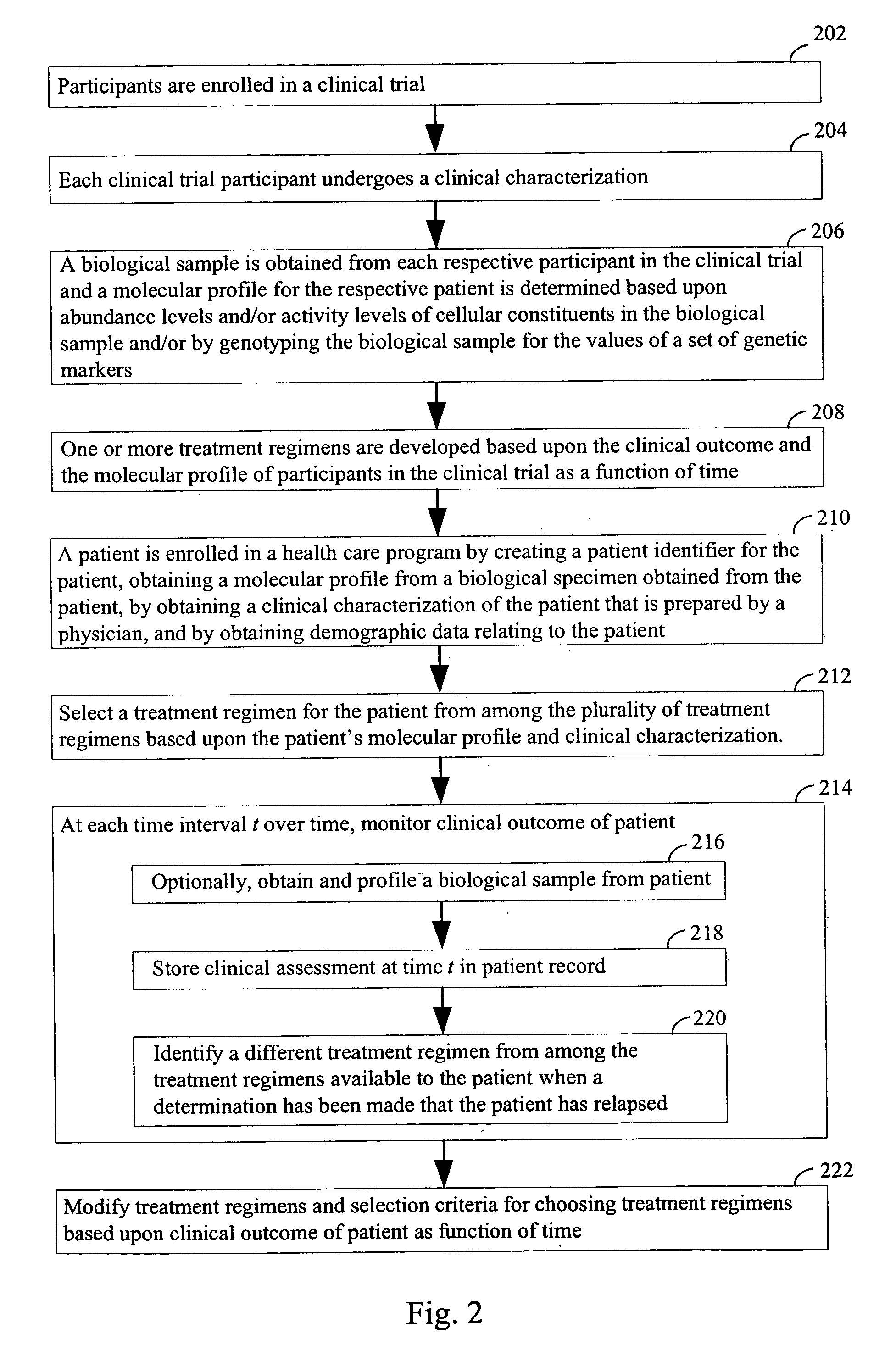
Computer systems and methods for providing health care
The invention provides a computer network comprising a first computer and one or more second computers that are in electronic communication with each other. The first computer is associated with a first health care facility and has instructions for retrieving, over a network, one or more data structures for a patient enrolled in a health care program. The one or more data structures for the patient collectively comprise (i) a patient identifier, (ii) a molecular profile from a biological specimen obtained from the patient at the first health care facility; and (iii) a clinical characterization of the patient that was made at the first health care facility. The first computer has instructions for retrieving, over the network connection, one or a plurality of treatment regimens that are deemed suitable for the patient based upon the molecular profile and the clinical characterization of the patient. The one or more second computers are at one or more locations other than the first health care facility and have one or more data structures for each patient in a plurality of patients enrolled in the health care program
Owner:H LEE MOFFITT CANCER CENT & RES INST INC
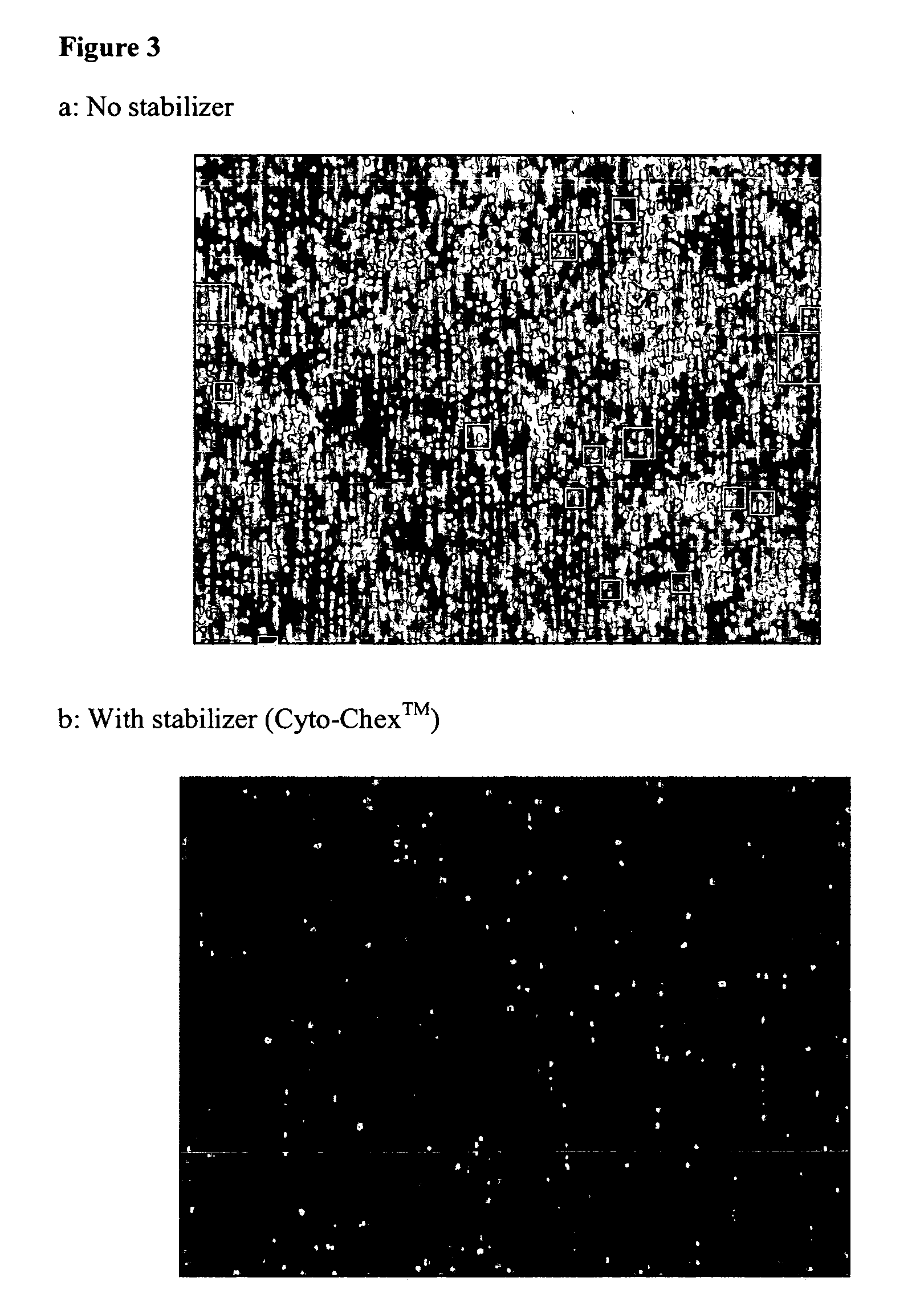
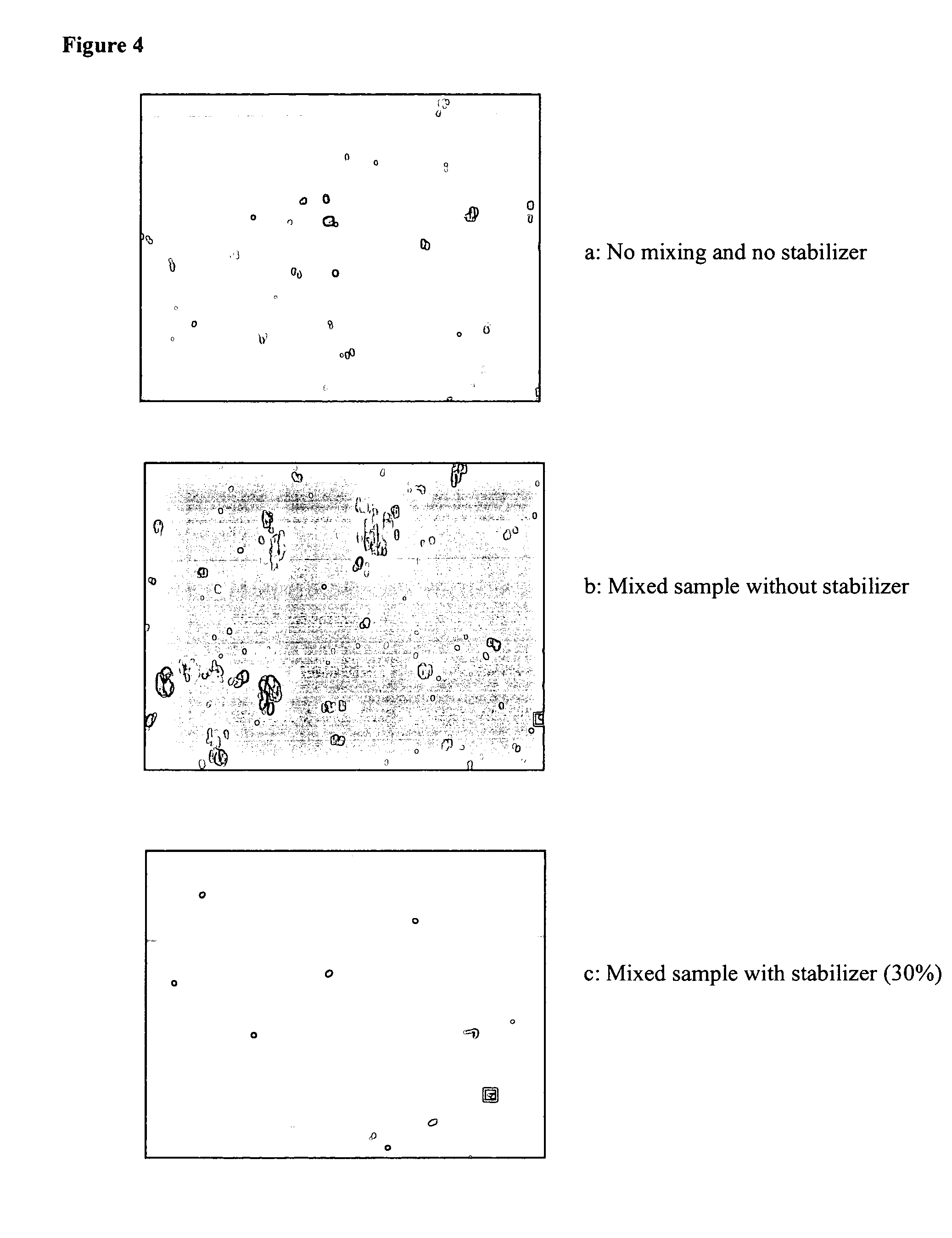
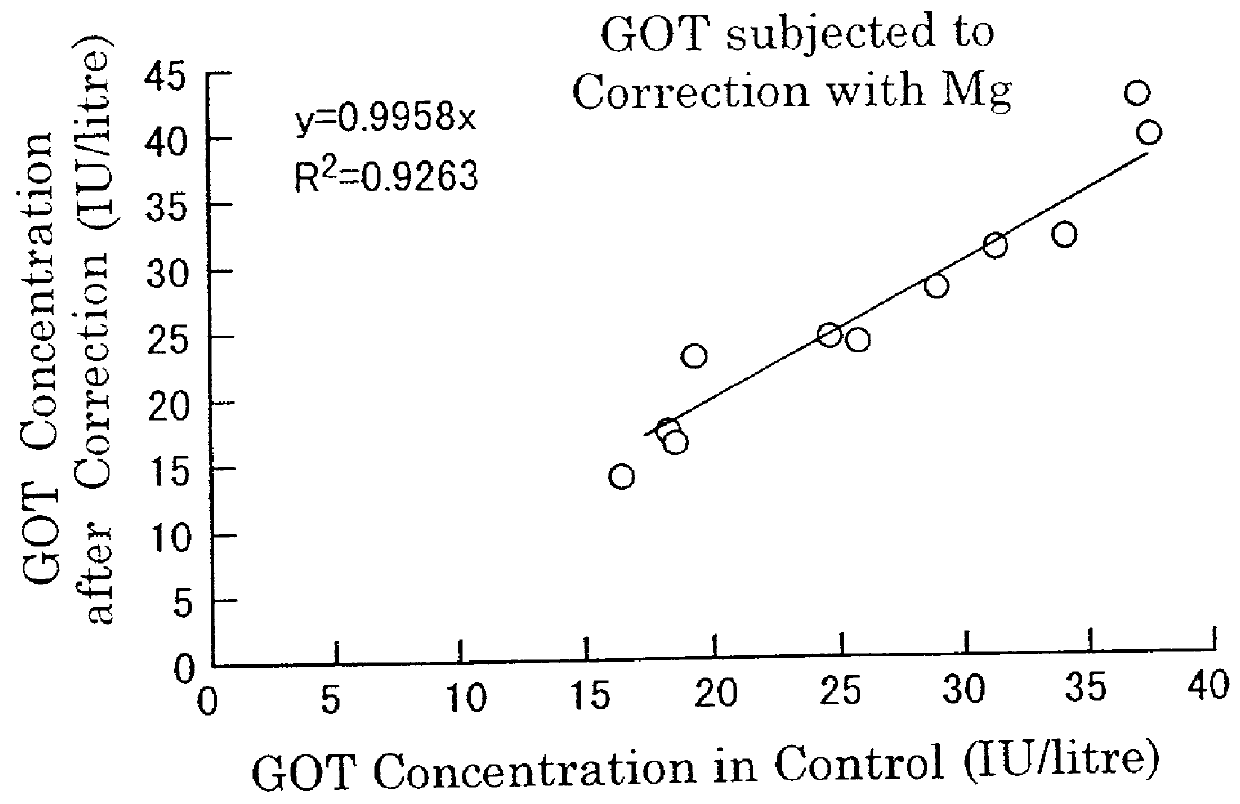
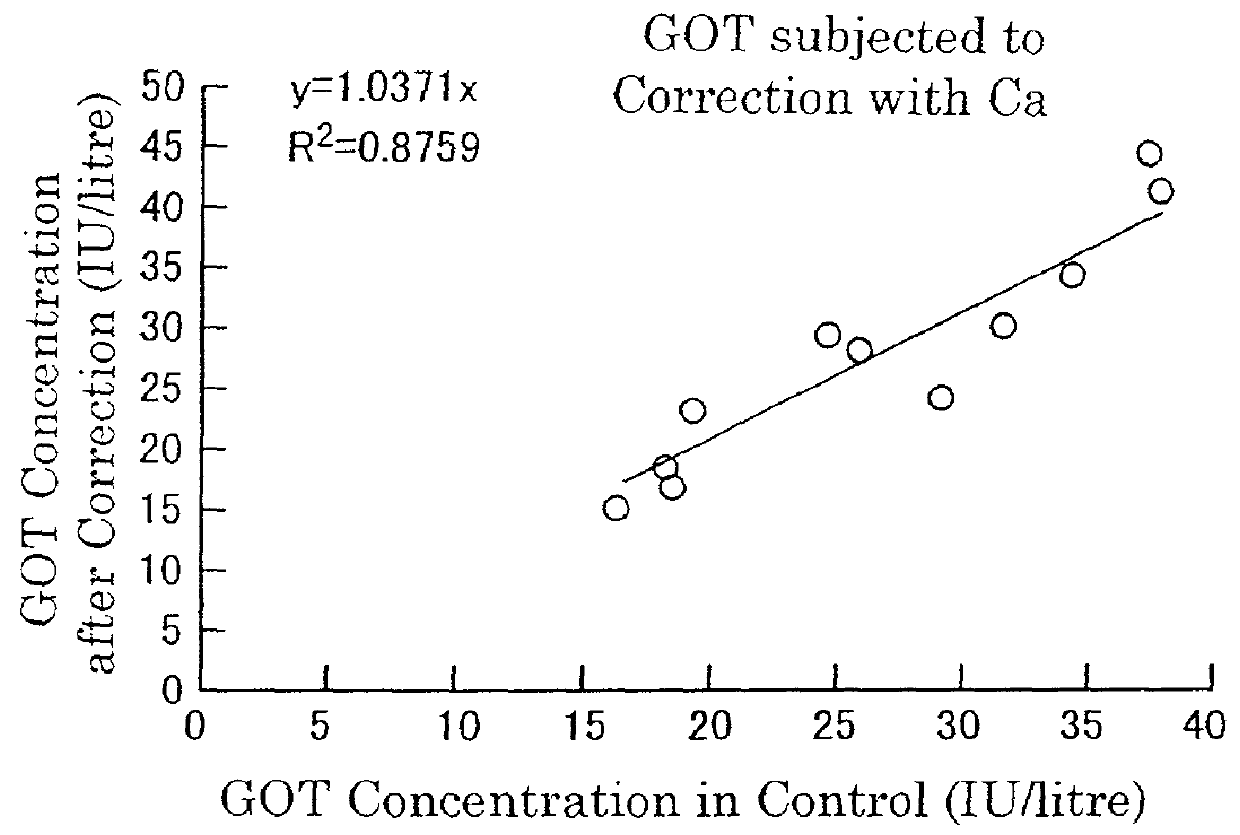
Stabilization of cells and biological specimens for analysis
InactiveUS20050181353A1Improve stabilityMaintain qualityOrganic active ingredientsBiocideAbnormal tissue growthIn vivo
Compositions and methods for stabilizing rare cells in blood specimens, preserving the quality of blood specimens, and also serving as cell fixatives are disclosed which minimize losses of target cells (for example, circulating tumor cells) and formation of debris and aggregates from target cells, non-target cells and plasma components, thereby allowing more accurate analysis and classification of circulating tumor cells (CTC) and, ultimately, of tumor burdens in cancer patients. Stabilization of specimens is particularly desirable in protocols requiring rare cell enrichment from blood specimens drawn from cancer patients. Exposure of such specimens to potentially stressful conditions encountered, for example, in normal processing, mixing, shaking, delays due to transporting the blood, has been observed to not only diminish the number of CTC but also to generate debris and aggregates in the blood specimens that were found to interfere with accurate enumeration of target cells, if present. Stabilizers are necessary to discriminate between in vivo CTC disintegration and in vitro sample degredation.
Owner:VERIDEX LCC
Process, device and reagent for cell separation
InactiveUS7316897B2Easy to implementConfirm diagnosisMicrobiological testing/measurementPreparing sample for investigationRare cellCell separation
A cell separation process for the isolation of pathogenic cells in a small concentration in a biological fluid specimen, including treating the biological specimen to modify in a differential manner selected rare cells and other cells and causing differential migration of cells that reacted during treatment versus cells that did not react during treatment.
Owner:LINSTITUTE NAT DE LA SANTE & DE LA RECH MEDICALE INSERM 45 OWNER +3
Spectral imaging of deep tissue
ActiveUS20050065440A1Easy to detectHigh detection sensitivityOptical radiation measurementNanoinformaticsDeep tissueSpectral analysis
Apparatus and methods are provided for the imaging of structures in deep tissue within biological specimens, using spectral imaging to provide highly sensitive detection. By acquiring data that provides a plurality of images of the sample with different spectral weightings, and subsequent spectral analysis, light emission from a target compound is separated from autofluorescence in the sample. With the autofluorescence reduced or eliminated, an improved measurement of the target compound is obtained.
Owner:CAMBRIDGE RES & INSTR
Spatially distinguished, multiplex nucleic acid analysis of biological specimens
ActiveUS20190203275A1Microbiological testing/measurementDNA preparationNucleic acid detectionNucleic Acid Probes
A method for spatially tagging nucleic acids of a biological specimen, including steps of (a) providing a solid support comprising different nucleic acid probes that are randomly located on the solid support, wherein the different nucleic acid probes each includes a barcode sequence that differs from the barcode sequence of other randomly located probes on the solid support; (b) performing a nucleic acid detection reaction on the solid support to locate the barcode sequences on the solid support; (c) contacting a biological specimen with the solid support that has the randomly located probes; (d) hybridizing the randomly located probes to target nucleic acids from portions of the biological specimen; and (e) modifying the randomly located probes that are hybridized to the target nucleic acids, thereby producing modified probes that include the barcode sequences and a target specific modification, thereby spatially tagging the nucleic acids of the biological specimen.
Owner:SPATIAL TRANSCRIPTOMICS +1
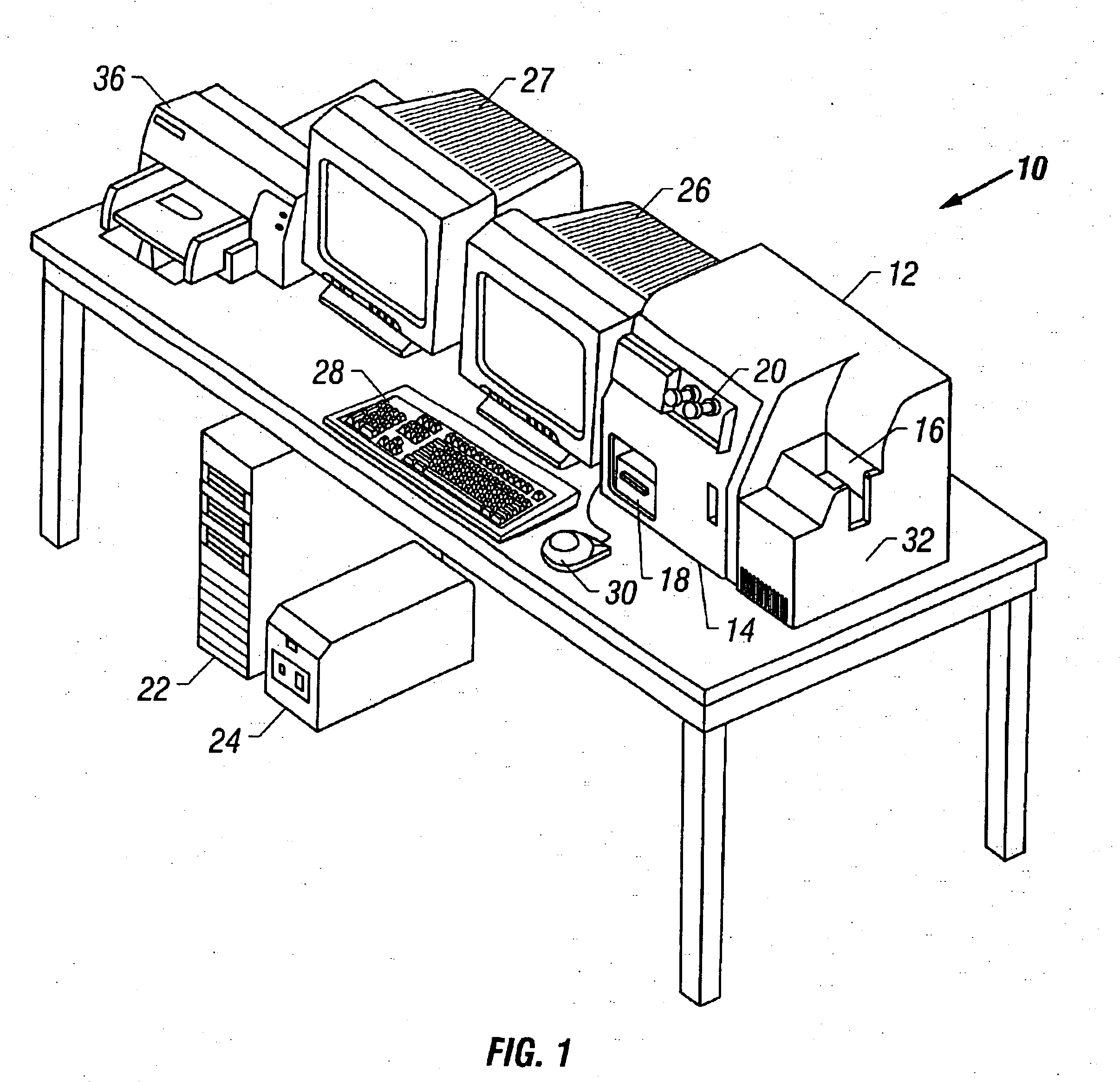
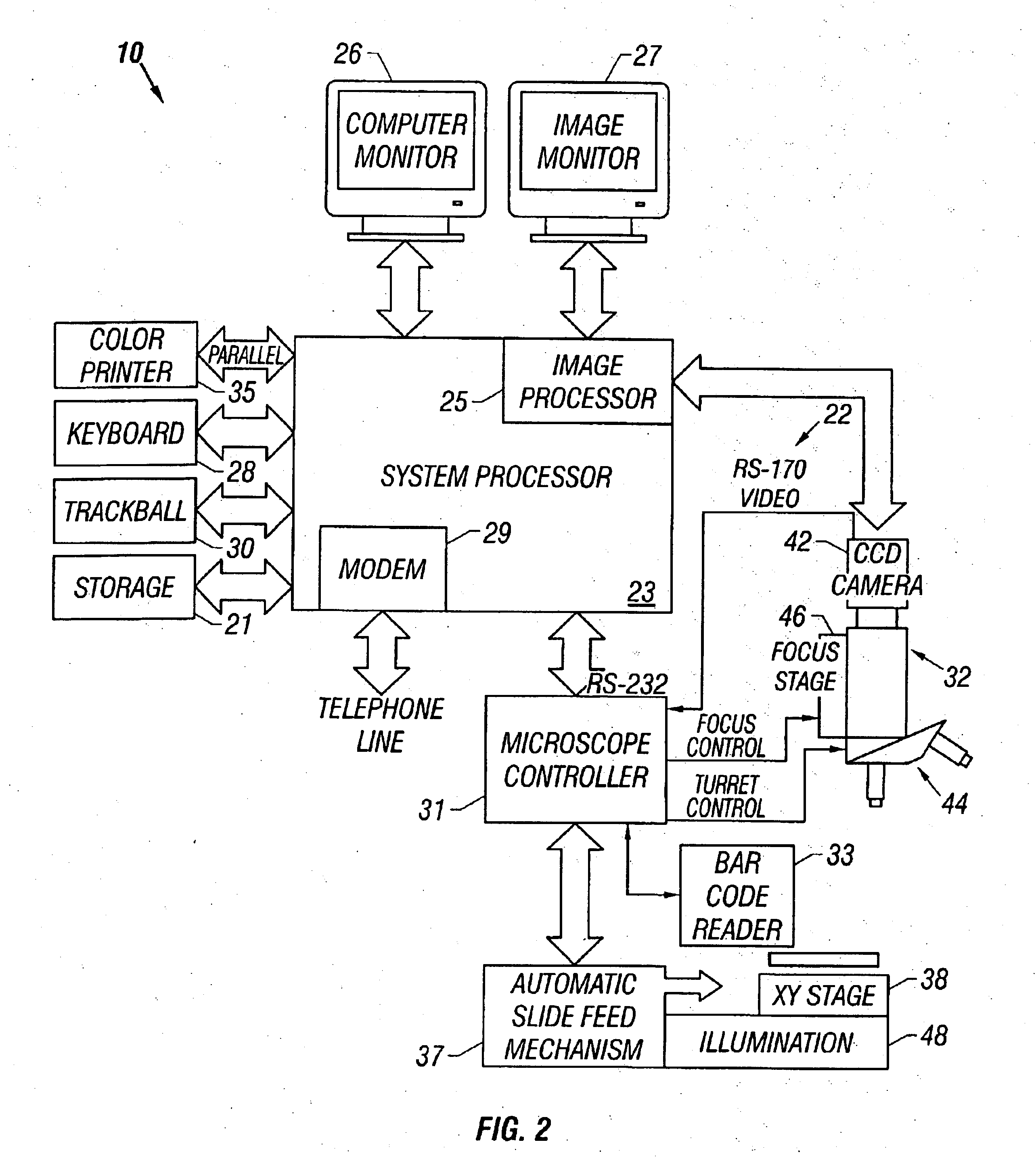
Method and apparatus for automated image analysis of biological specimens
InactiveUS6920239B2High degreeQuickly and accurately scanImage analysisPreparing sample for investigationColor transformationLow-pass filter
A method and apparatus for automated cell analysis of biological specimens automatically scans at a low magnification to acquire images which are analyzed to determine candidate cell objects of interest. The low magnification images are converted from a first color space to a second color space. The color space converted image is then low pass filtered and compared to a threshold to remove artifacts and background objects from the candidate object of interest pixels of the color converted image. The candidate object of interest pixels are morphologically processed to group candidate object of interest pixels together into groups which are compared to blob parameters to identify candidate objects of interest which correspond to cells or other structures relevant to medical diagnosis of the biological specimen. The location coordinates of the objects of interest are stored and additional images of the candidate cell objects are acquired at high magnification. The high magnification images are analyzed in the same manner as the low magnification images to confirm the candidate objects of interest which are objects of interest. A high magnification image of each confirmed object of interest is stored for later review and evaluation by a pathologist.
Owner:CARL ZEISS MICROSCOPY GMBH
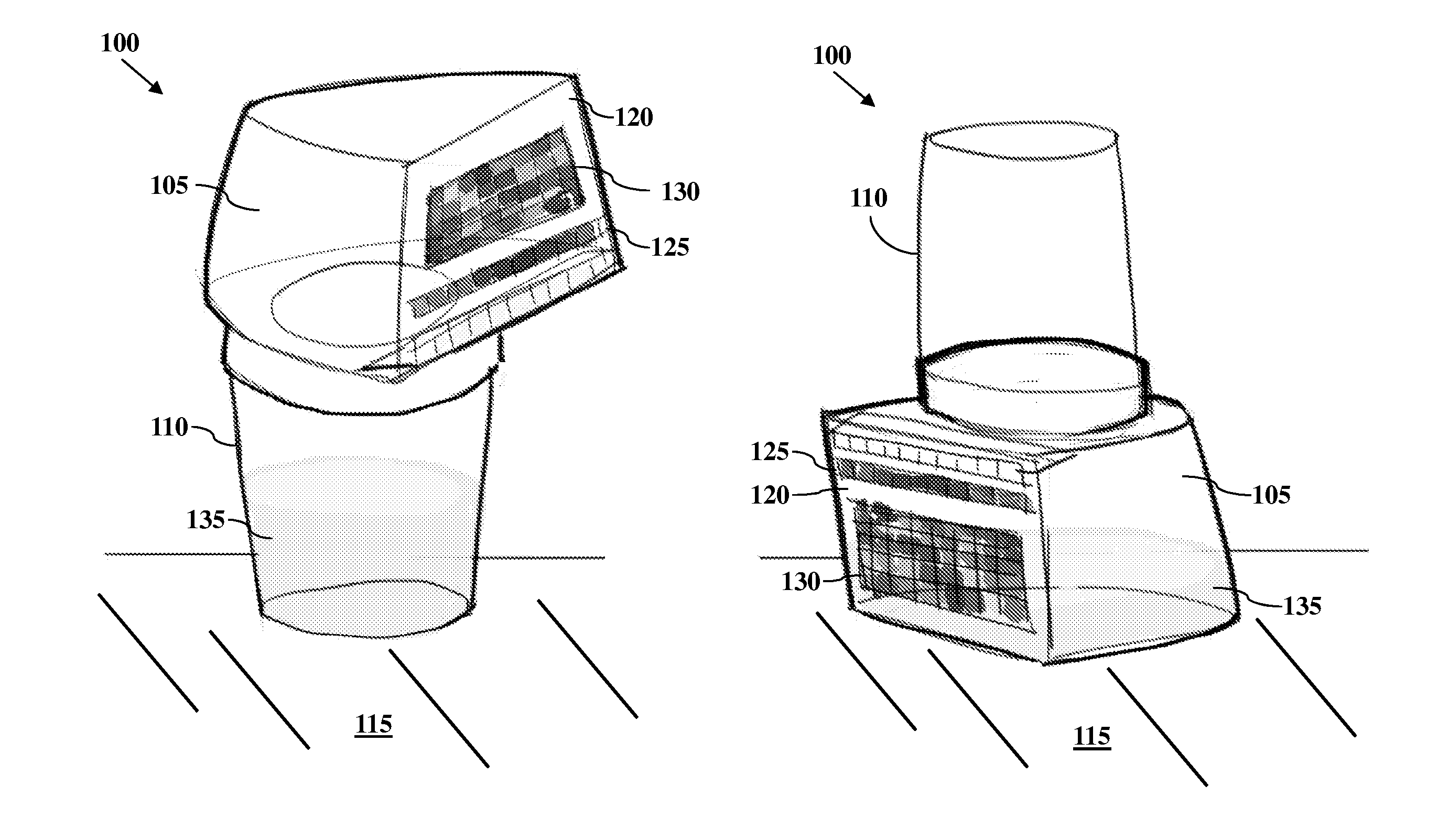
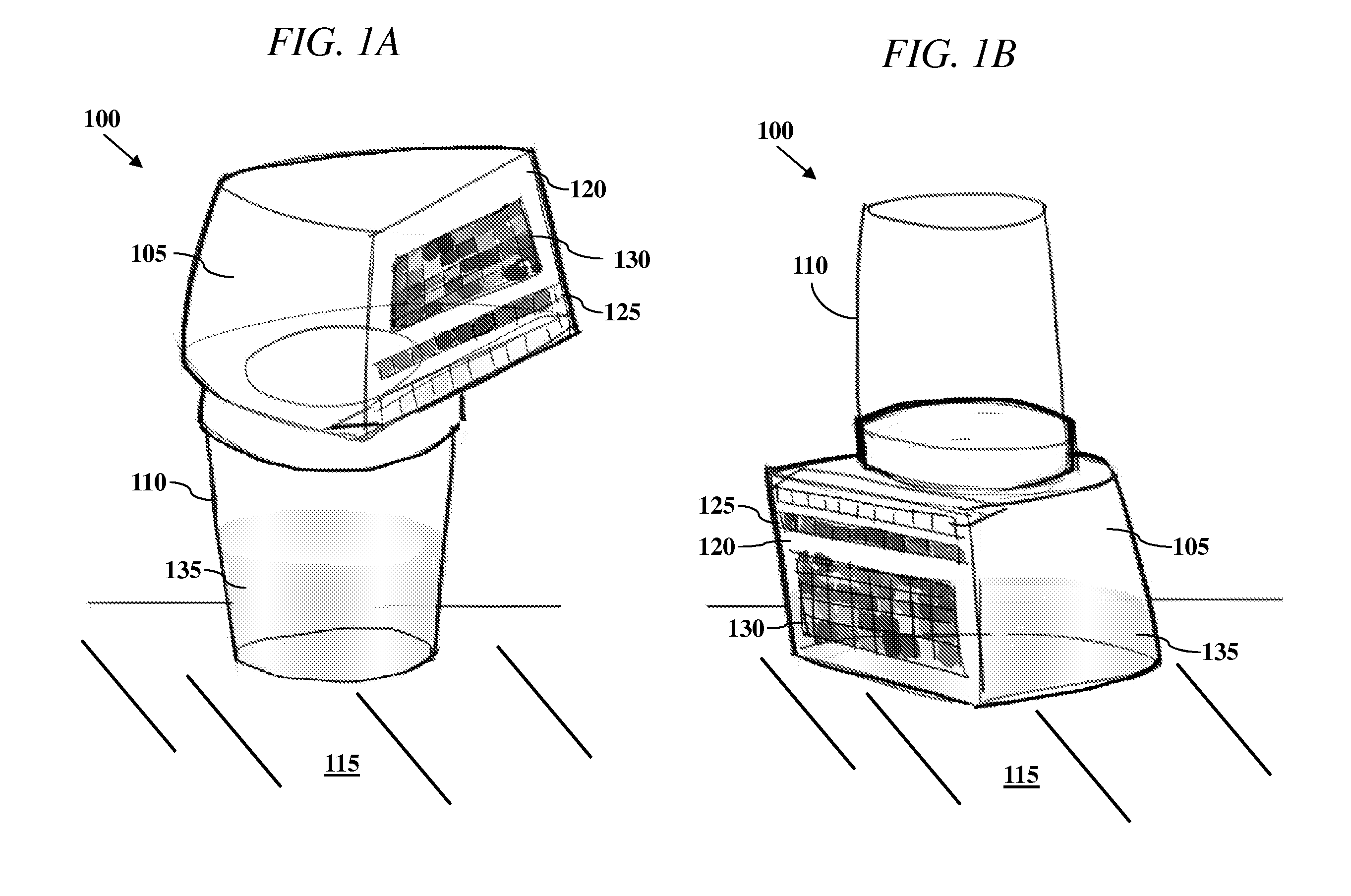
All-In-One Specimen Cup With Optically Readable Results
A biological material test strip and adjacently-located reference color chart are affixed to a lid portion of an all-in-one specimen cup to perform color-based reaction testing of collected biological specimens in an uncalibrated environment. After specimen collection, the lid portion is secured to a container portion of the specimen cup. The cup may then be rotated into an upside down position causing the specimen, under the force of gravity, to pass from the container portion and into a volume of the lid portion, such that the test strip is exposed to the specimen as it is received into the volume of the lid portion. An image of the exposed test strip and adjacently-located reference color chart may then be captured and processed to identify any color matches between the individual test pads on the test strip and the corresponding sequences of reference color blocks on the reference chart.
Owner:SCANWELL HEALTH INC
Diagnostic test device and method of using same
InactiveUS20050084842A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiagnostic testEngineering
A self contained diagnostic test device is provided for use in the collection and detection of a biological specimen or the like. The device comprises a tubular swab and reagent dispensing cap component for receiving specimens. The reagent dispensing cap component includes barrel, reagent chamber, and results window subcomponents, and delivers one or more selected reagents to a specimen testing chamber for contacting the collected specimen, upon the rotation of the reagent chamber component.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Biological specimen collection and transport system and methods of use
ActiveUS20090312285A1Prevent degradationStabilizes and preserves integrityBiocideBioreactor/fermenter combinationsCellular componentTransport system
Disclosed are compositions for isolating populations of nucleic acids from biological, forensic, and environmental samples. Also disclosed are methods for using these compositions as one-step formulations for killing pathogens, inactivating nucleases, and releasing polynucleotides from other cellular components within the sample, and stabilizing the nucleic acids prior to further processing or assay. The disclosed compositions safely facilitate rapid sample collection, and provide extended storage and transport of the samples at ambient or elevated temperature without contamination of the sample or degradation of the nucleic acids contained therein. This process particularly facilitates the collection of specimens from remote locations, and under conditions previously considered hostile for preserving the integrity of nucleic acids released from lysed biological samples without the need of refrigeration or freezing prior to molecular analysis.
Owner:LONGHORN VACCINES & DIAGNOSTICS LLC
Method and Apparatus for Multi-spectral Imaging and Analysis of Skin Lesions and Biological Tissues
ActiveUS20100042004A1Reduce in quantityWell representedDiagnostics using lightMedical automated diagnosisVirtual spaceElectromagnetic radiation
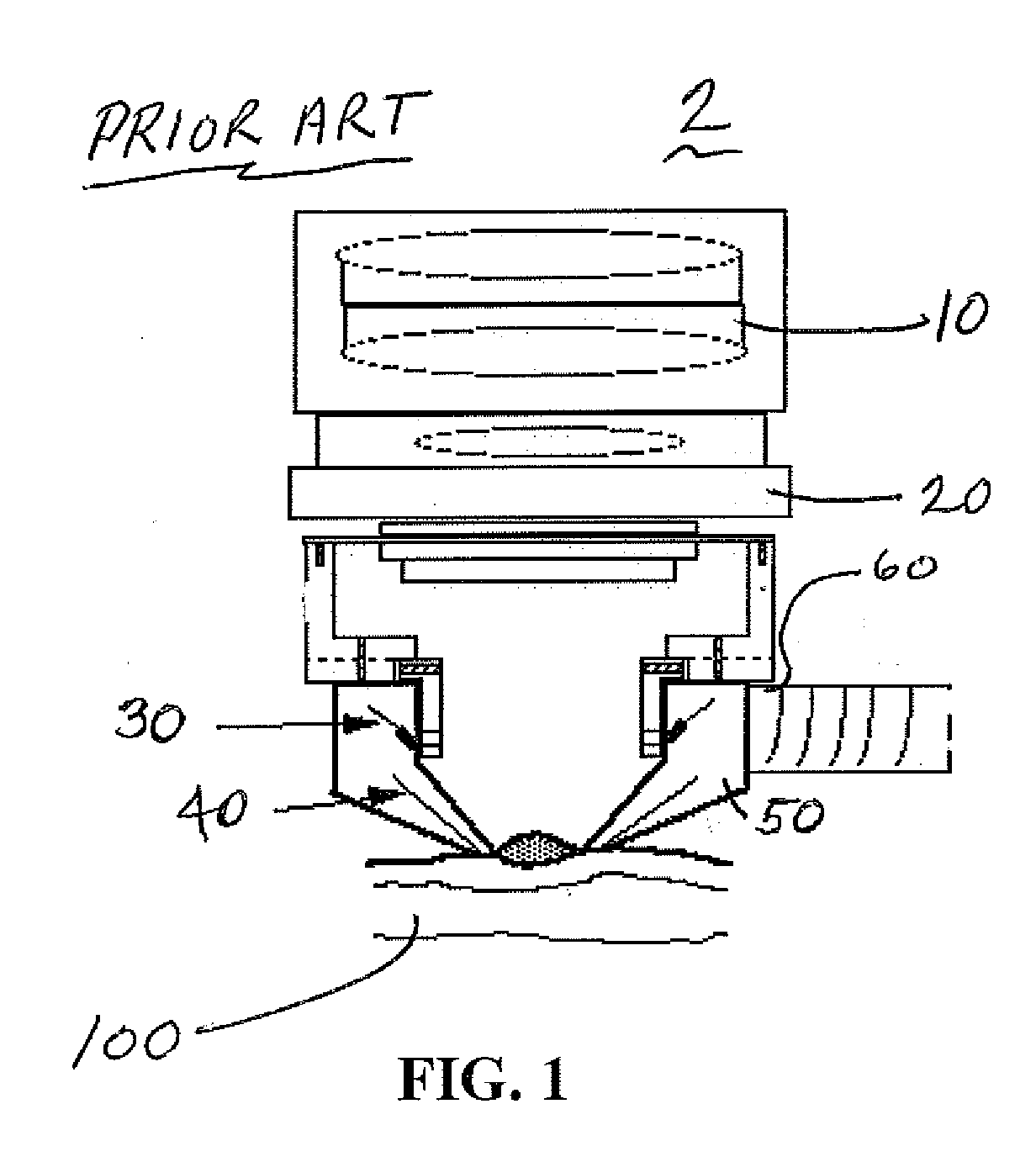
A multispectral nevoscope that uses specific wavelengths in the visible and infrared spectrum of electromagnetic radiation to transilluminate a skin-lesion or a biological tissue or specimen for imaging and maps multispectral 2-dimensional images into 3-dimensional virtual space for providing 3-D distributions of pre-defined parameters representing the characteristic properties (such as melanin, hemoglobin and deoxyhemoglobin, etc.) of a skin lesion. Methods are disclosed for analyzing and using the characteristic distributions of specific parameters for detection and management of skin-cancers, or characterization of a biological tissue or specimen.
Owner:NEW JERSEY INSTITUTE OF TECHNOLOGY
Simplified water-bag technique for magnetic susceptibility measurements on the human body and other specimens
InactiveUS7047059B2Less-expensive fabricationLess-expensive useMagnetic-field-controlled resistorsSolid-state devicesHuman bodyMagnetic susceptibility
A probe instrument using room-temperature sensor(s) that can measure variations in magnetic susceptibilities. The instrument has sufficient resolution to monitor paramagnetic materials in a human body, such as iron in a human liver, by noninvasively examining patients with iron-overload diseases. The instrument includes room temperature magnetic sensors, and detects the sample, that is, the tissue response to an alternating current field applied by an applied field coil. The applied field coil dimensions are chosen so that the applied field is optimized for maximum response from the liver while minimizing the effects due to the overlying abdominal tissue and at the same time not unduly increasing the sensitivity of the instrument to the lung. To overcome variations in the sensor output due to fluctuations in the applied field, change in the ambient temperature and mechanical relaxation of the instrument, the sensor-sample distance is modulated. The detector assembly is oscillated while the examined patient remains stationary. An improved water-bag technique is employed to eliminate background tissue response. The detector assembly forms part of a probe instrument for performing noninvasively the paramagnetic concentration of a patient.
Owner:QUANTUM MAGNETICS
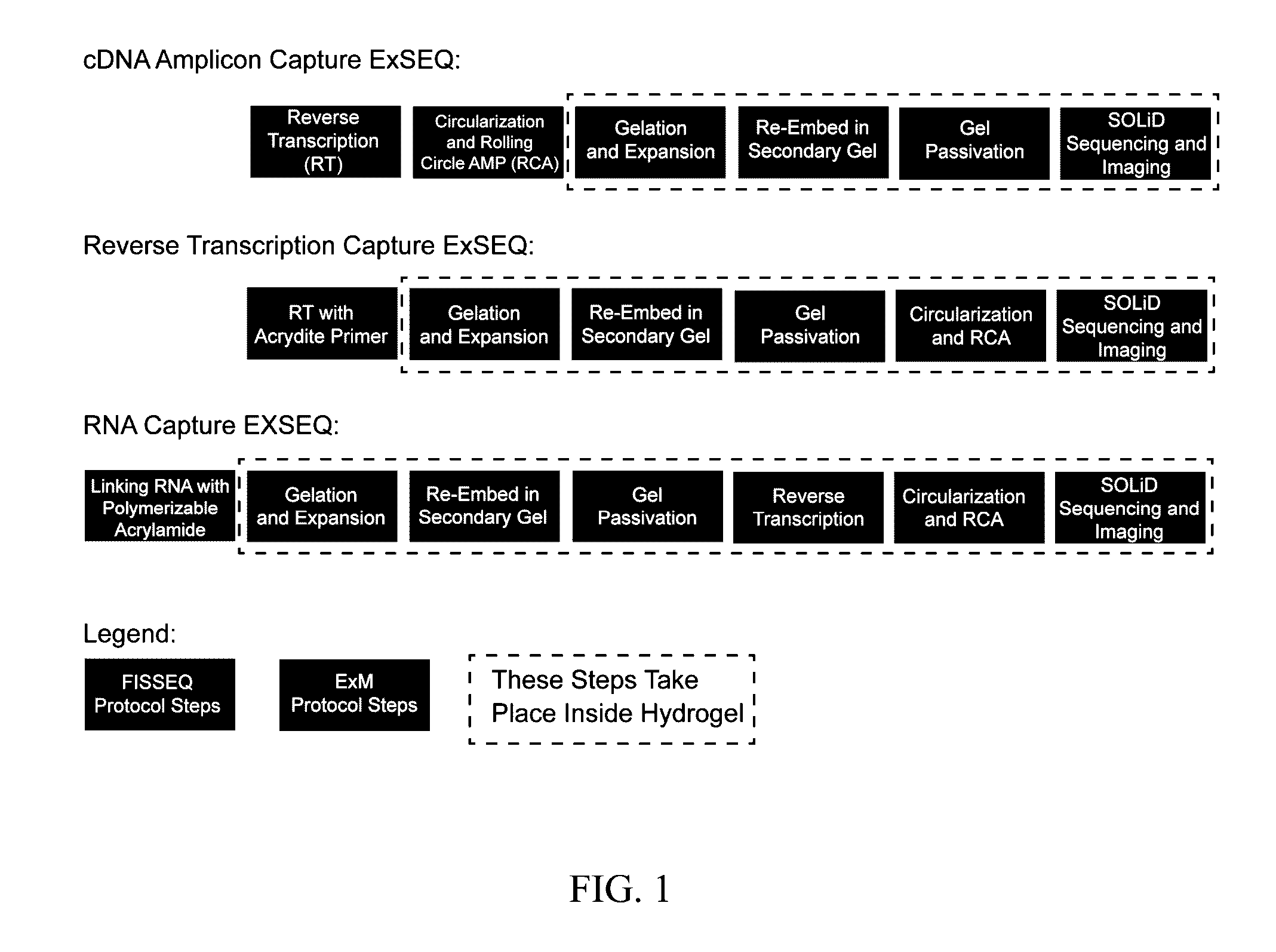
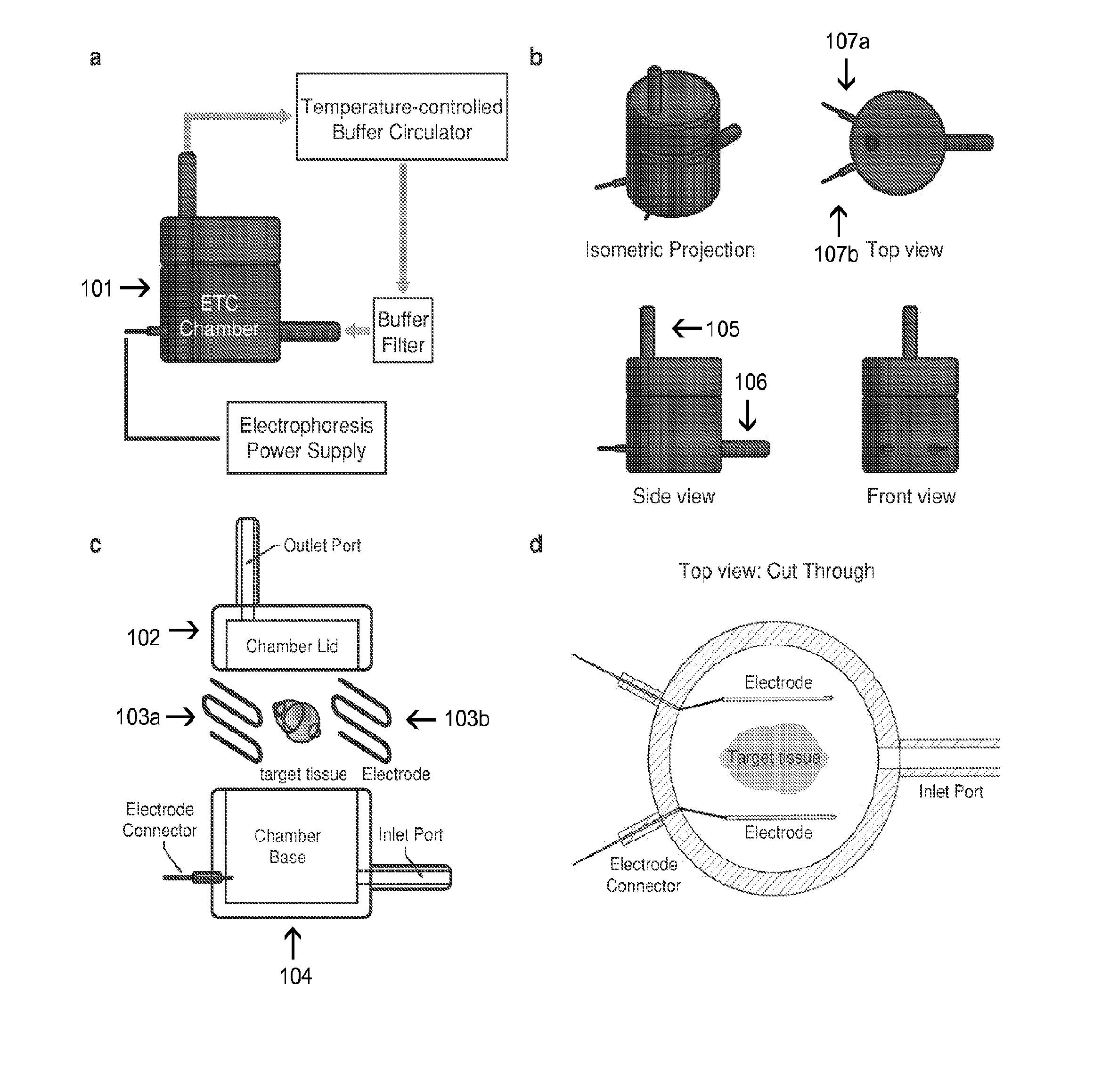
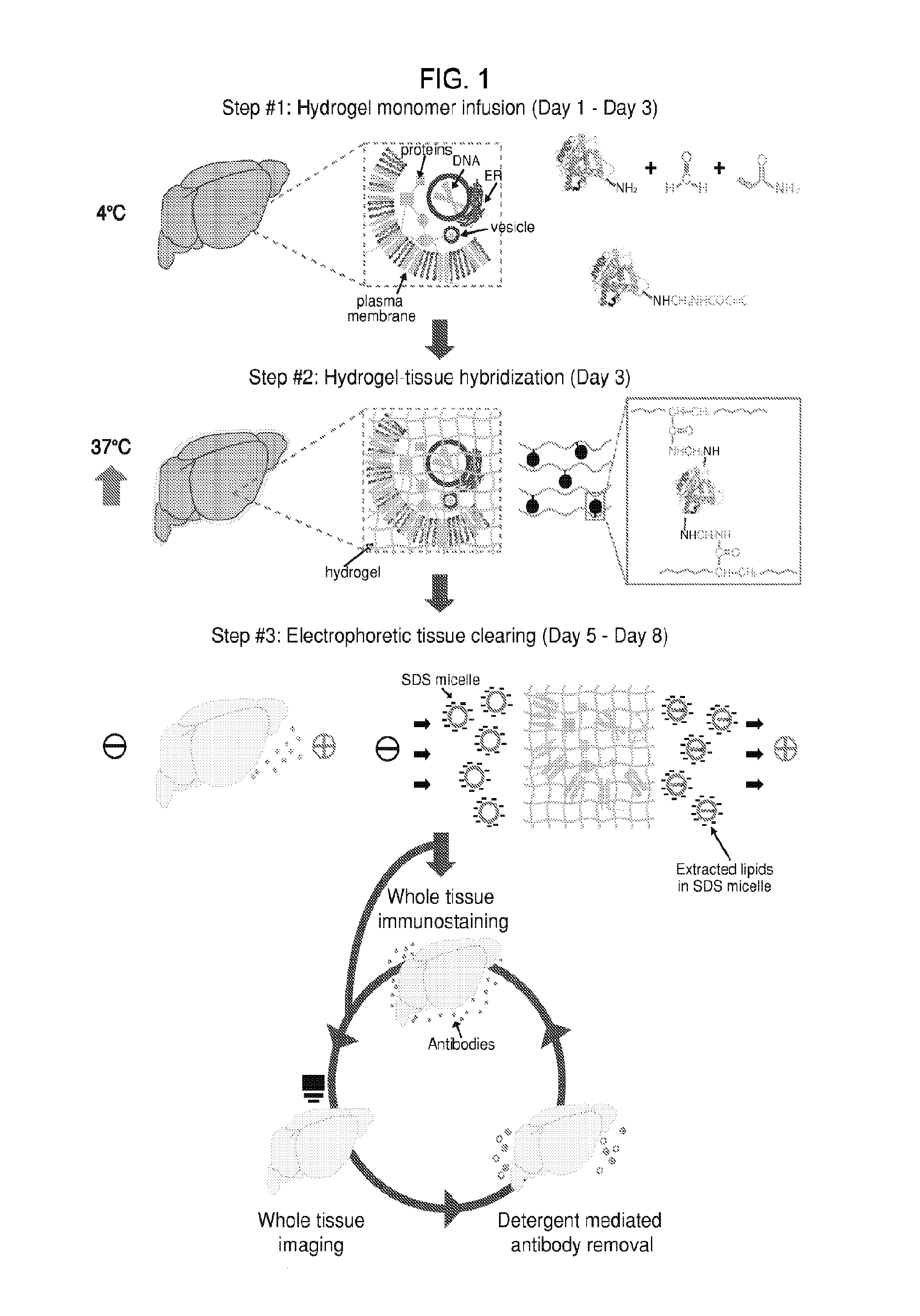
In situ nucleic acid sequencing of expanded biological samples
ActiveUS20160304952A1Microbiological testing/measurementLaboratory apparatusFluorescent in situ sequencingNucleic acid sequencing
The invention provides in situ nucleic acid sequencing to be conducted in biological specimens that have been physically expanded. The invention leverages the techniques for expansion microscopy (ExM) to provide new methods for in situ sequencing of nucleic acids as well as new methods for fluorescent in situ sequencing (FISSEQ) in a new process referred to herein as “expansion sequencing” (ExSEQ).
Owner:MASSACHUSETTS INST OF TECH +1
Methods and apparatus for ascertaining interferents and physical dimensions in liquid samples and containers to be analyzed by a clinical analyzer
A method of inspecting a clinical specimen for a presence of one or more interferents, such as those that might be found within clinical analytical blood specimens by subjecting the specimen to centrifugation to separate the specimen into a red blood cell portion and a blood serum or plasma portion is provided. Subsequent to the centrifuging procedure, the serum or plasma portion of the clinical analytical specimen may be tested for the presence of one or more interferents such as hemolysis, icterus, lipemia, or liquid nonuniformities therein. Additionally, physical dimensional characteristics of the sample container and / or specimen may be determined. Apparatus for carrying out the method are described, as are other aspects.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Sample preparation system and method for processing clinical specimens
ActiveUS7985375B2Reduce turnaround timeLow efficiencyAnalysis using chemical indicatorsMicrobiological testing/measurementRobotic systemsMolecular analysis
Owner:BECTON DICKINSON & CO
Method for preparing cell cultures from biological specimens for chemotherapeutic and other assays
InactiveUS6887680B2Promote growthMicrobiological testing/measurementMicroorganism separationParticulatesAnticarcinogen
An improved system for screening a multiple of candidate therapeutic or chemotherapeutic agents for efficacy as to a specific patient, in which a tissue sample from the patient is harvested, cultured and separately exposed to a plurality of treatments and / or therapeutic agents for the purpose of objectively identifying the best treatment or agent for the particular patient. Specific method innovations such as tissue sample preparation techniques render this method practically as well as theoretically useful. One particularly important tissue sample preparation technique is the initial preparation of cohesive multicellular particulates of the tissue sample, rather than enzymatically dissociated cell suspensions or preparations, for initial tissue culture monolayer preparation. With respect to the culturing of malignant cells, for example, it is believed (without any intention of being bound by the theory) that by maintaining the malignant cells within a multicellular particulate of the originating tissue, growth of the malignant cells themselves is facilitated versus the overgrowth of fibroblasts or other cells which tends to occur when suspended tumor cells are grown in culture. Practical monolayers of cells may thus be formed to enable meaningful screening of a plurality of treatments and / or agents. Growth of cells is monitored to ascertain the time to initiate the assay and to determine the growth rate of the cultured cells; sequence and timing of drug addition is also monitored and optimized. By subjecting uniform samples of cells to a wide variety of active agents (and concentrations thereof), the most promising agent and concentration for treatment of a particular patient can be determined. For assays concerning cancer treatment, a two-stage evaluation is contemplated in which both acute cytotoxic and longer term inhibitory effect of a given anti-cancer agent are investigated.
Owner:PRECISION THERAPEUTICS
Method for preparing cell cultures from biological specimens for chemotherapeutic and other assays
InactiveUS6900027B1Promote growthConvenient treatmentMicrobiological testing/measurementMicroorganism separationAnticarcinogenActive agent
An improved system for screening a multiple of candidate therapeutic or chemotherapeutic agents for efficacy as to a specific patient, in which a tissue sample from the patient is harvested, cultured and separately exposed to a plurality of treatments and / or therapeutic agents for the purpose of objectively identifying the best treatment or agent for the particular patient. The system includes the initial preparation of cohesive multicellular particulates of the tissue sample, rather than enzymatically dissociated cell suspensions or preparations, for initial tissue culture monolayer preparation. Practical monolayers of cells may thus be formed to enable meaningful screening of a plurality of treatments and / or agents. By subjecting uniform samples of cells to a wide variety of active agents (and concentrations thereof), the most promising agent and concentration for treatment of a particular patient can be determined. For assays concerning cancer treatment, a two-stage evaluation is contemplated in which both acute cytotoxic and longer term inhibitory effect of a given anti-cancer agent are investigated.
Owner:PRECISION THERAPEUTICS
Stabilization of cells and biological specimens for analysis
InactiveUS20060194192A1Improve stabilityMaintain qualityOrganic active ingredientsDead animal preservationAbnormal tissue growthIn vivo
Owner:JANSSEN DIAGNOSTICS LLC
Quantitative analysis
InactiveUS20010055784A1Shorten the timeSave energyMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementPhysicsBiological specimen
Owner:ARKRAY INC
A medical laboratory clinical biochemical detection automatic checking method and system
ActiveCN106126958AImprove pass rateImprove accuracyMedical automated diagnosisMedical equipmentMedical laboratoryProcess quality
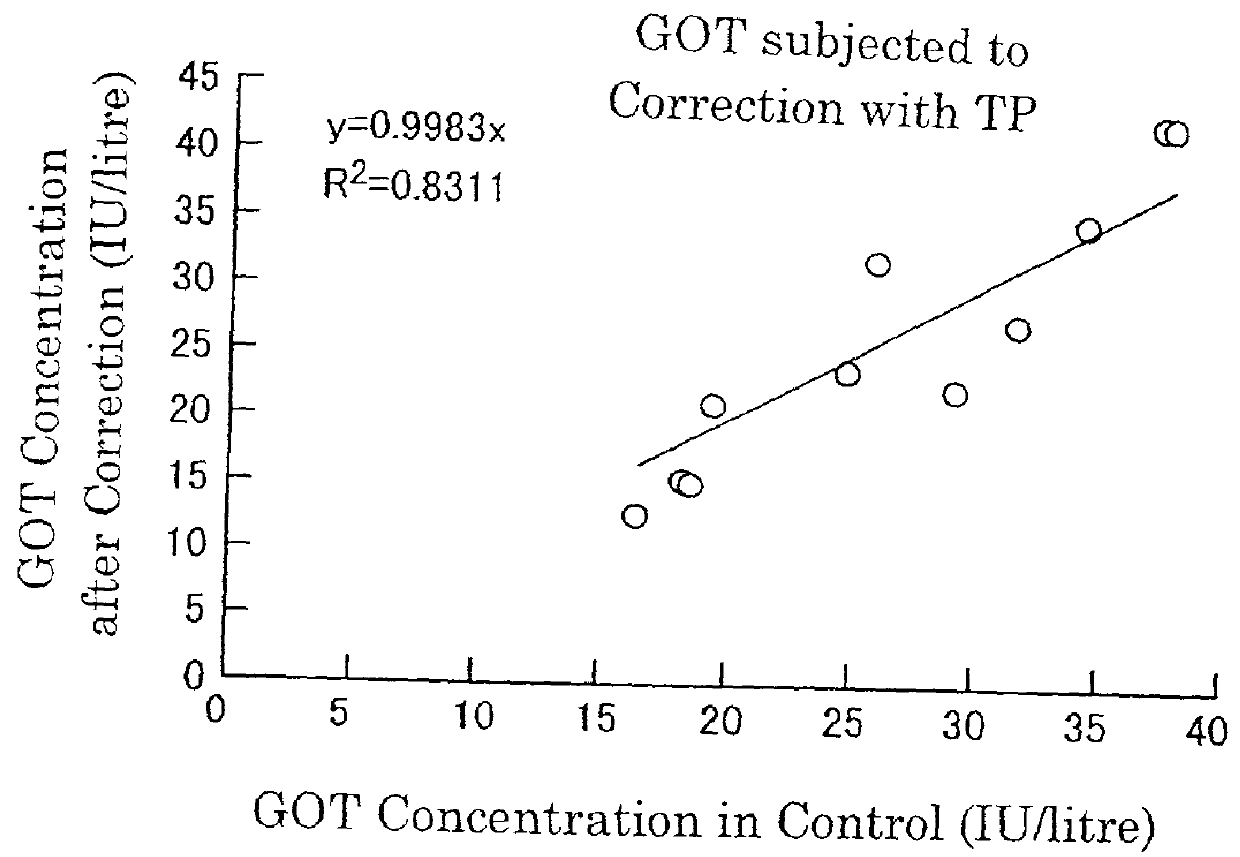
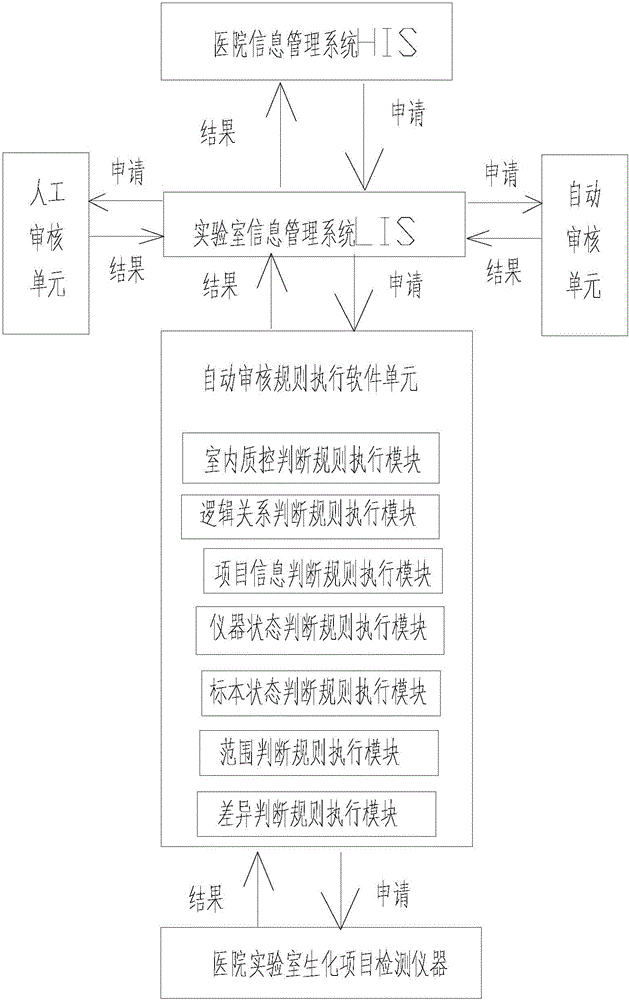
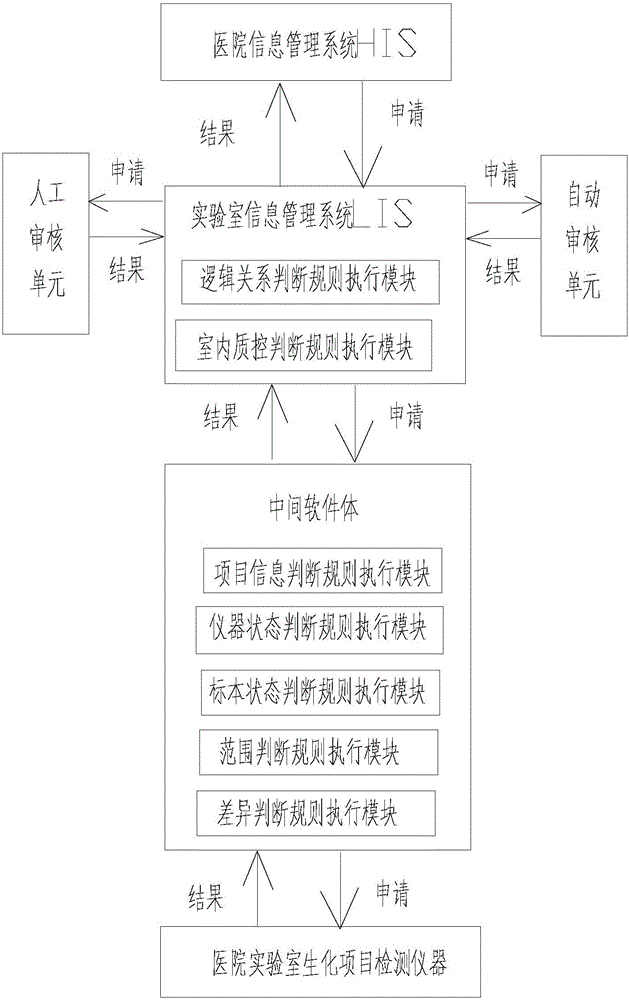
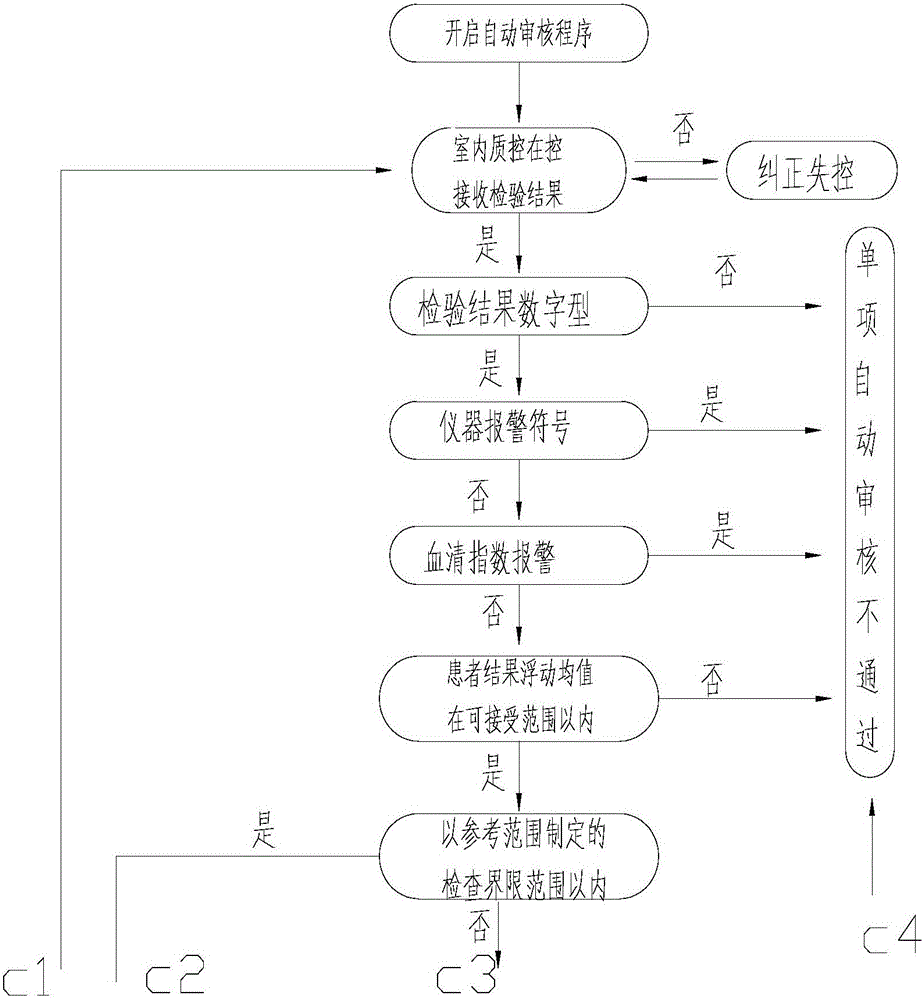
The invention provides a medical laboratory clinical biochemical detection automatic checking method and system. The method employs a computer software and hardware system and programs multiple checking rules to form a multiple checking rule execution module to automatically check the detection results of various biochemical immunity detection items. The detection results of the various biochemical immunity detection items cannot be sent to an automatic checking unit for automatic checking until they pass the multiple checking rules; if the detection results of the various biochemical immunity detection items cannot pass the above-mentioned checking rules, the detection results are transferred to a manual checking unit for checking; only after the detection results are checked by the manual checking unit or checked again after automatic treatment of dilution, reexamination, test item adding, unqualified sample return and the like by an assembly line can a detection report be issued; the multiple checking rules mainly include a clinical information judging rule, a sample state judging rule, an indoor quality control judging rule, an instrument state judging rule, a range judging rule, a difference judging rule, and a logical relationship judging rule. The checking rules are reasonable in design and cover the pre-analysis, analysis and post-analysis processes, thereby realizing whole-process quality control on a detection analysis process and guaranteeing the accuracy of detection results.
Owner:温冬梅
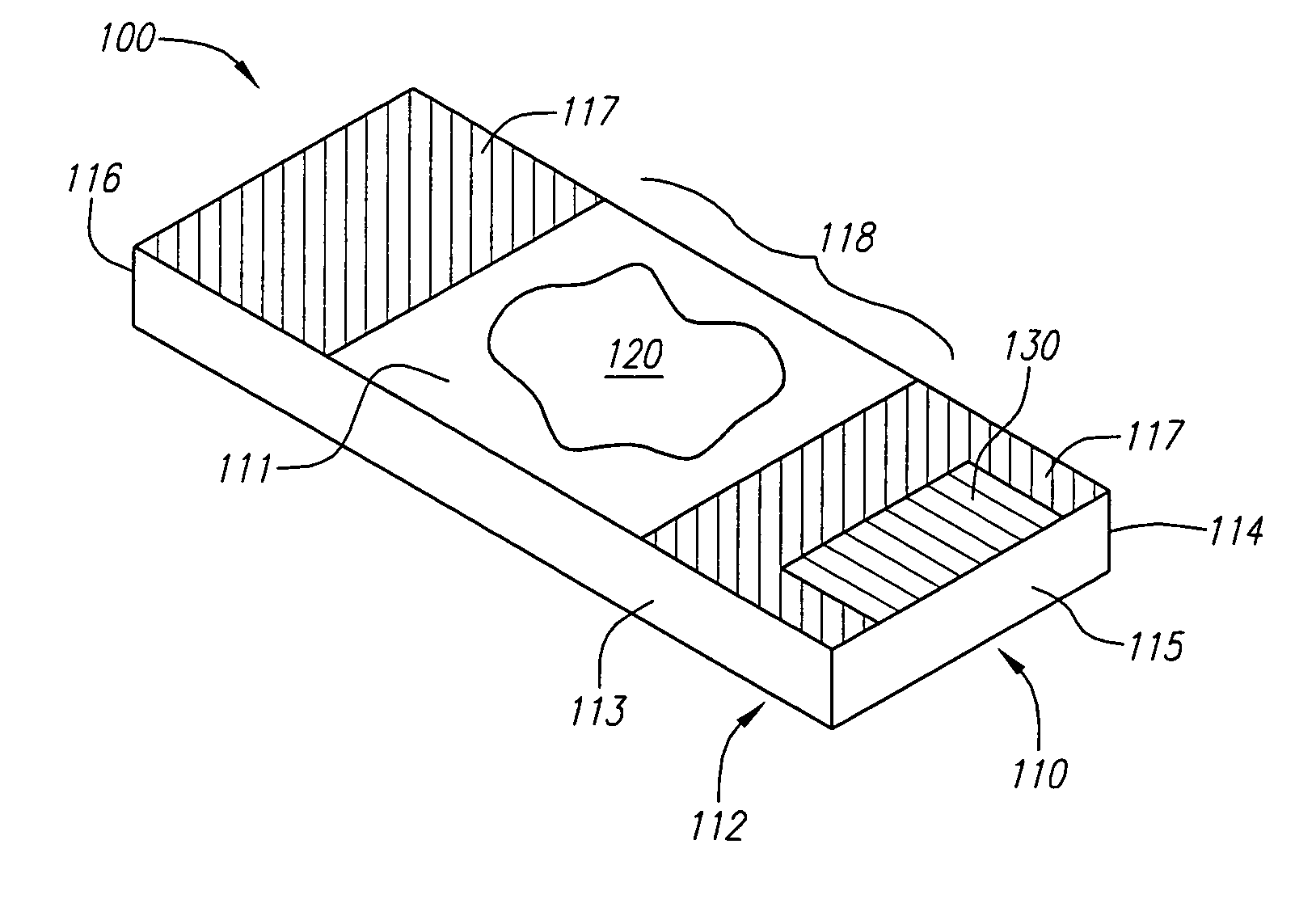
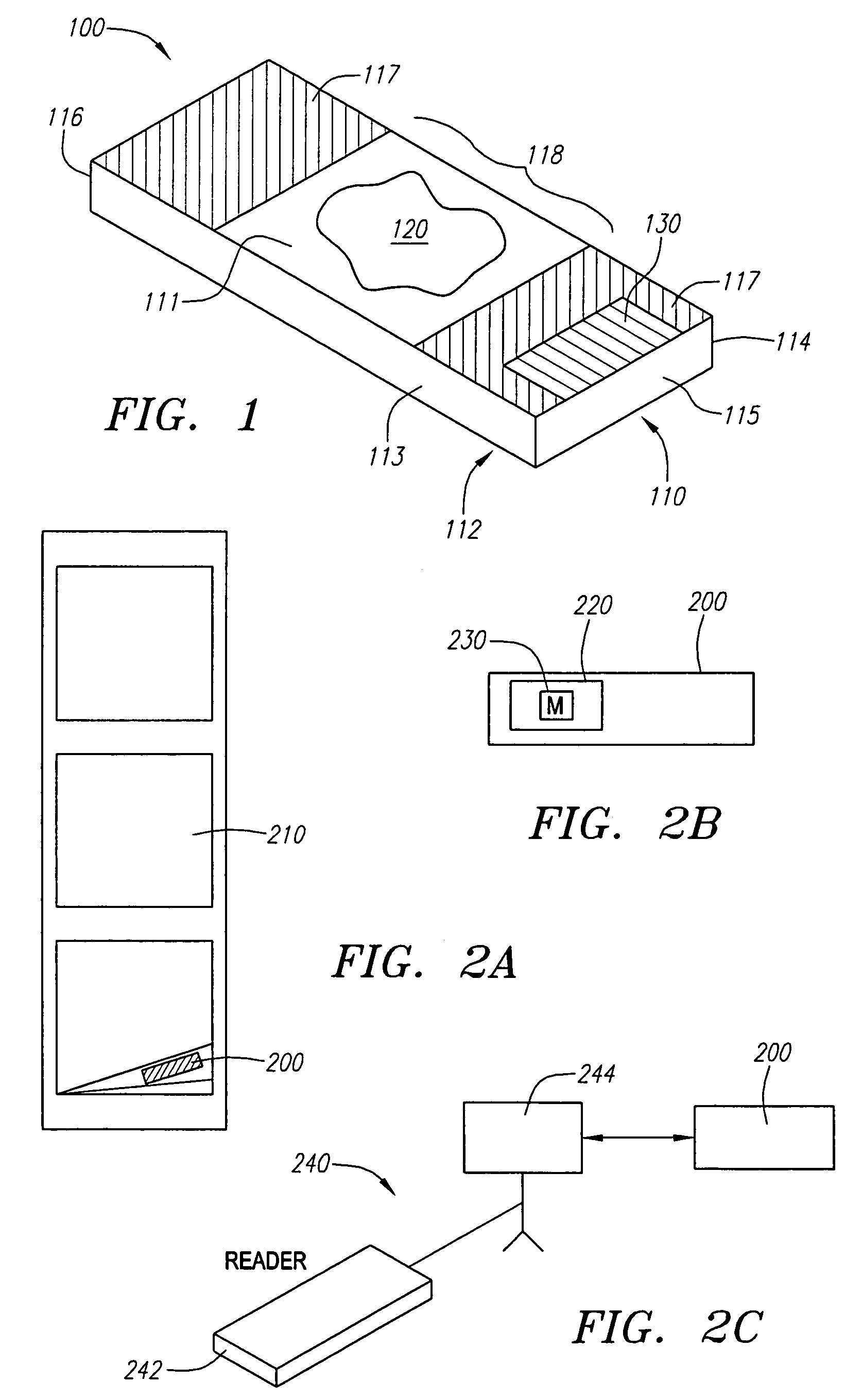
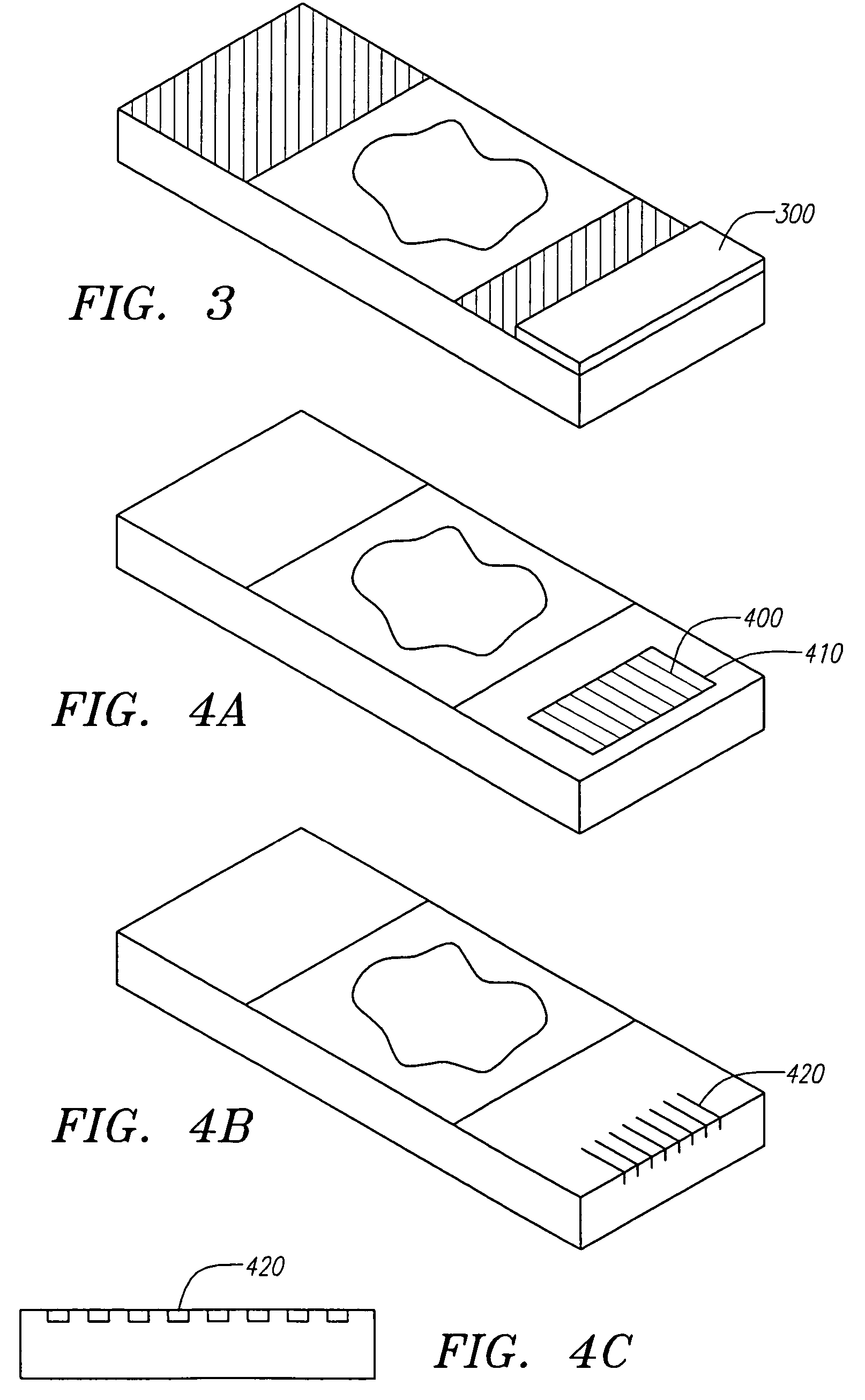
Locally storing biological specimen data to a slide
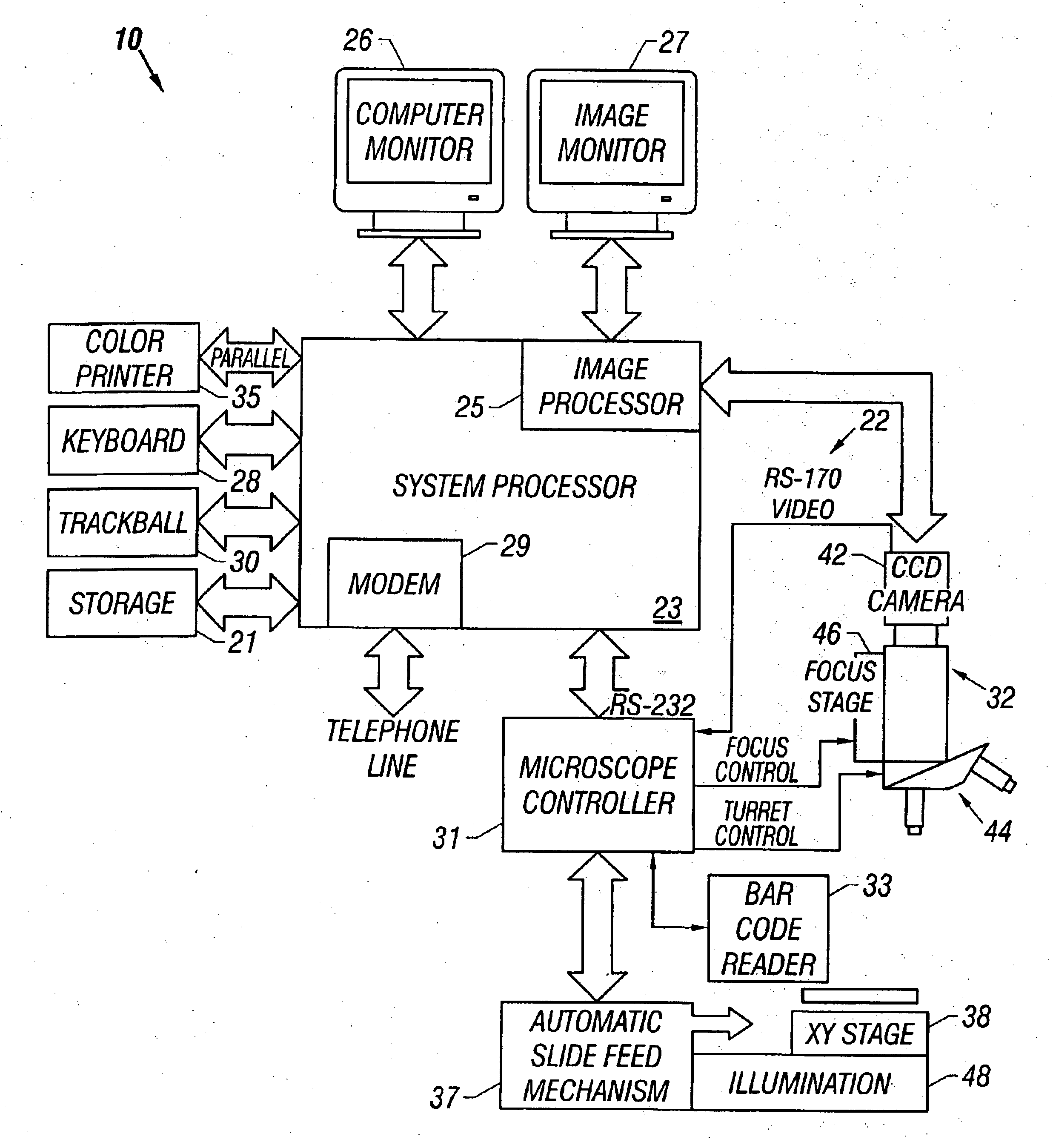
ActiveUS7083106B2Computer-assisted medical data acquisitionCharacter and pattern recognitionComputer scienceRadio-frequency identification
An apparatus, system and method for analyzing biological specimens, including storing data relating to the analysis in a read / write data storage device attached to specimen carrier. The data storage device can be a Radio Frequency Identification (RFID) tag, a magnetic device, or a an optical device.
Owner:CYTYC CORP
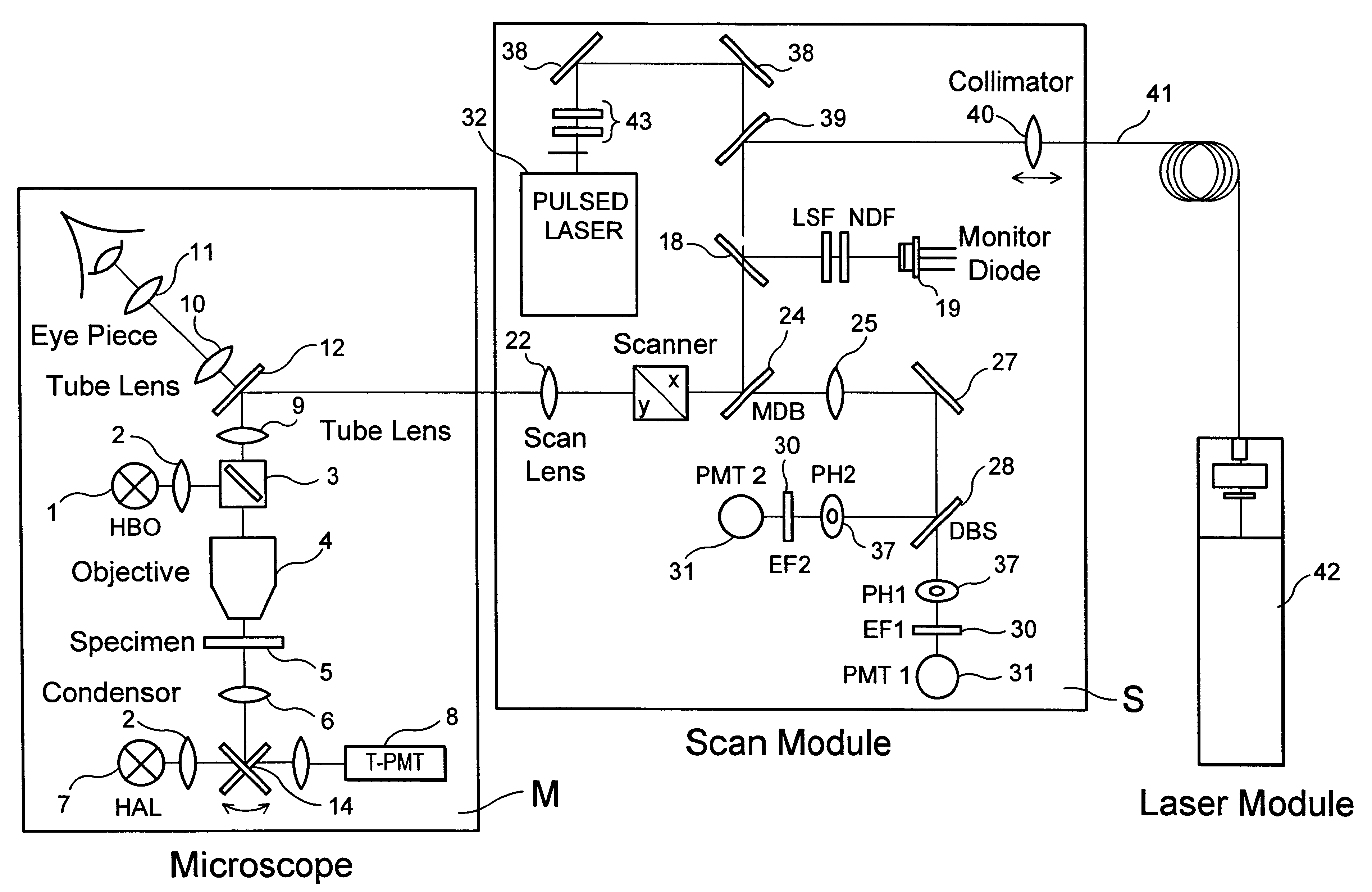
Highly compact laser scanning microscope with integrated short-pulse laser
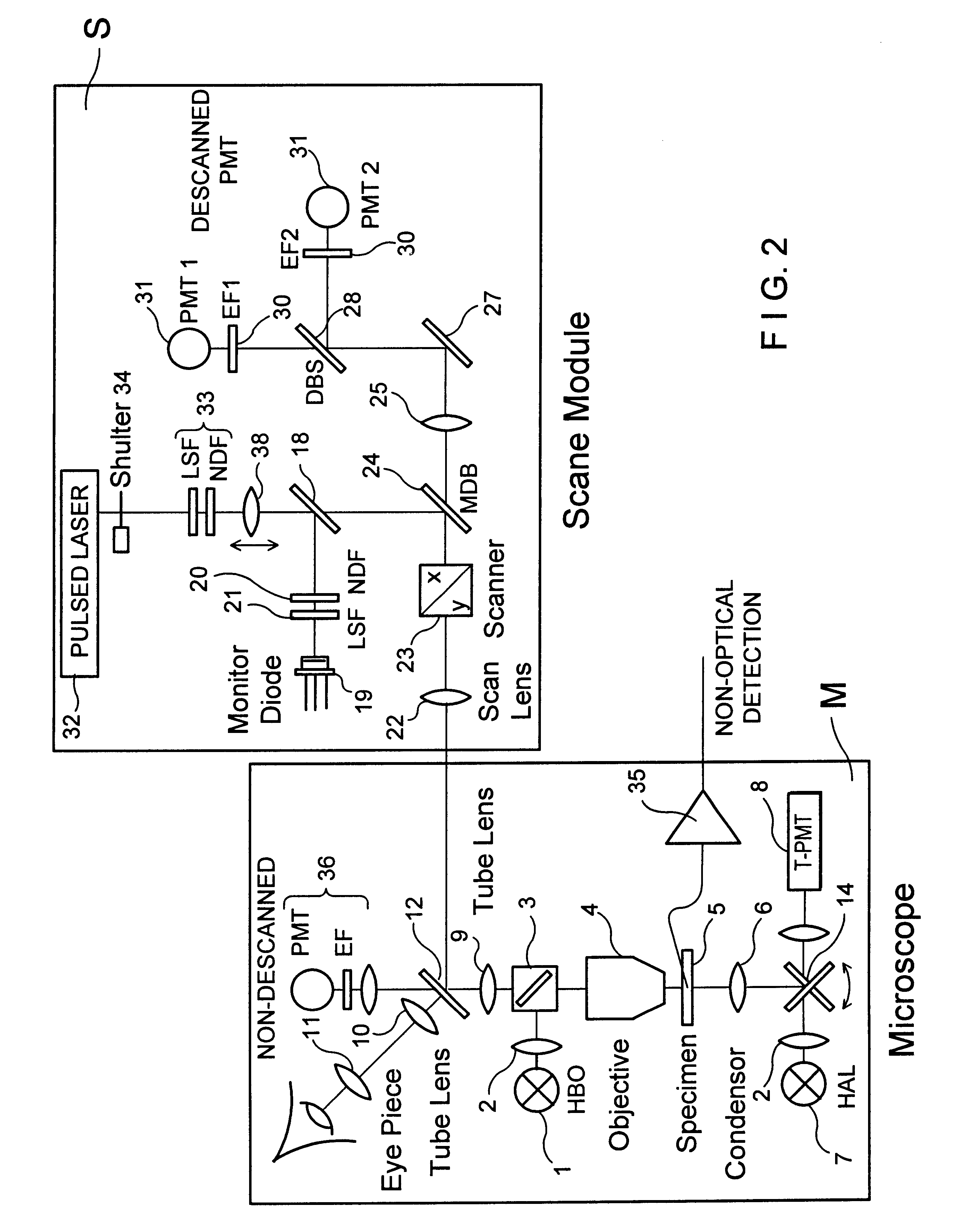
InactiveUS6356088B1Improve signal-to-noise ratioAttenuation bandwidthMaterial analysis using wave/particle radiationScanning probe techniquesLaser scanning microscopeDirect coupling
The invention describes a highly compact laser scanning microscope with integrated short-pulse laser. Direct coupling or a fiber coupling of the short-pulse laser with the laser scanning microscope is advantageously circumvented with this arrangement. This compact arrangement of the laser in the scan module of the laser scanning microscope can be used in a particularly advantageous manner, for example, in multiphoton microscopy for three-dimensionally resolved microscopic analysis, e.g., of biological specimens. Because of the inherent depth discrimination of the multiphoton technique, confocal pinholes can be entirely omitted in the detection beam path. Accordingly, the microscope system can be realized in a very simple manner with respect to engineering and is particularly simple to handle with respect to application. Through the use of a short-pulse laser system in which a plurality of wavelengths are available simultaneously, diverse applications can be realized in one and the same compact microscope system.
Owner:CARL ZEISS MICROSCOPY GMBH
Methods and Compositions for Preparing Biological Specimens for Microscopic Analysis
ActiveUS20150144490A1High optical claritySludge treatmentVolume/mass flow measurementOrganismAnalysis method
Methods and compositions are provided for preparing a biological specimen for microscopic analysis. These methods find many uses, for example in medicine and research, e.g., to diagnose or monitor disease or graft transplantation, to study healthy or diseased tissue, to screen candidate agents for toxicity and efficacy in disease modification. Also provided are reagents, devices, kits and systems thereof that find use in practicing the subject methods.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Specimen processing system and specimen container classifying apparatus
ActiveUS20100111767A1Material analysis by optical meansBiological testingSpecimen HandlingEngineering
A specimen processing system comprising: a specimen measuring section for measuring specimens accommodated in specimen containers; a transport section for transporting specimen containers to the specimen measuring section; a specimen container collect section for collecting specimen containers; an obtainer for obtaining shape information on specimen containers or state information on specimens accommodated in specimen containers; a supply judger configured for determining whether specimen containers are to be supplied to the specimen measuring section on the basis of the result obtained by the obtainer; and a delivery section for delivering specimen containers, which are determined to be supplied to the specimen measuring section by the supply judger, toward the transport section, and delivering specimen containers, which are determined not to be supplied to the specimen measuring section by the supply judger, toward the specimen container collect section, is disclosed. A specimen container classifying apparatus is also disclosed.
Owner:SYSMEX CORP
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com