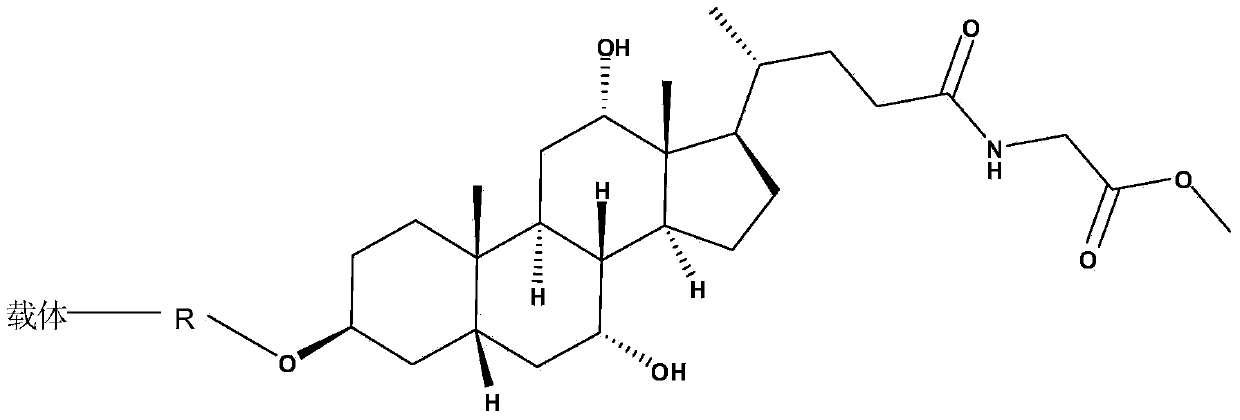
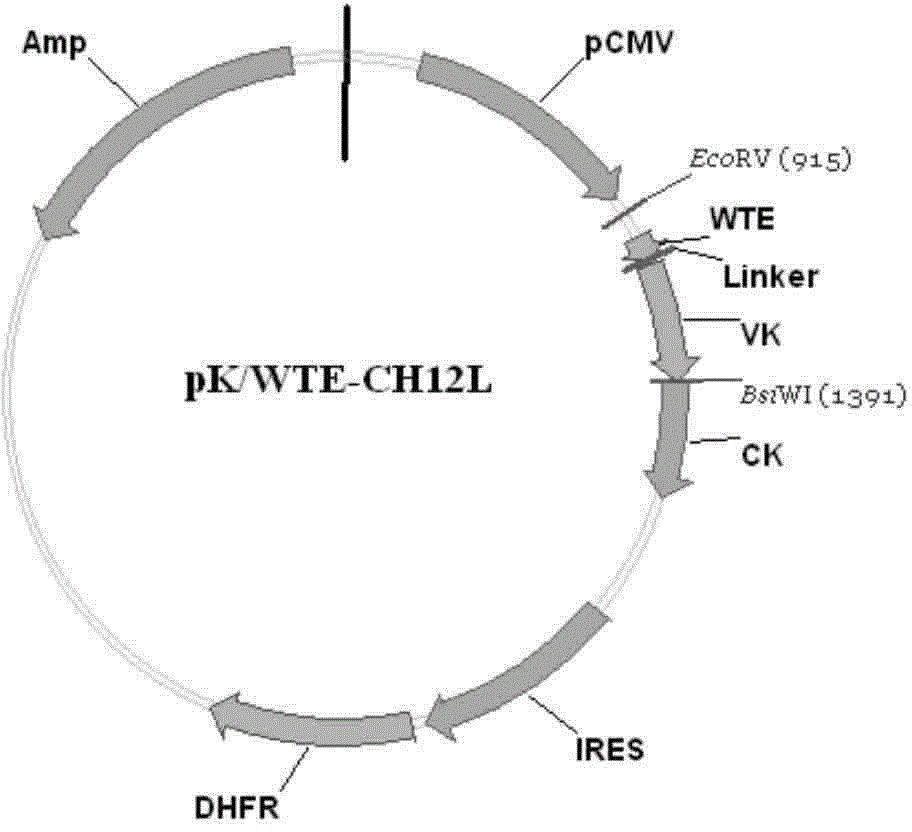
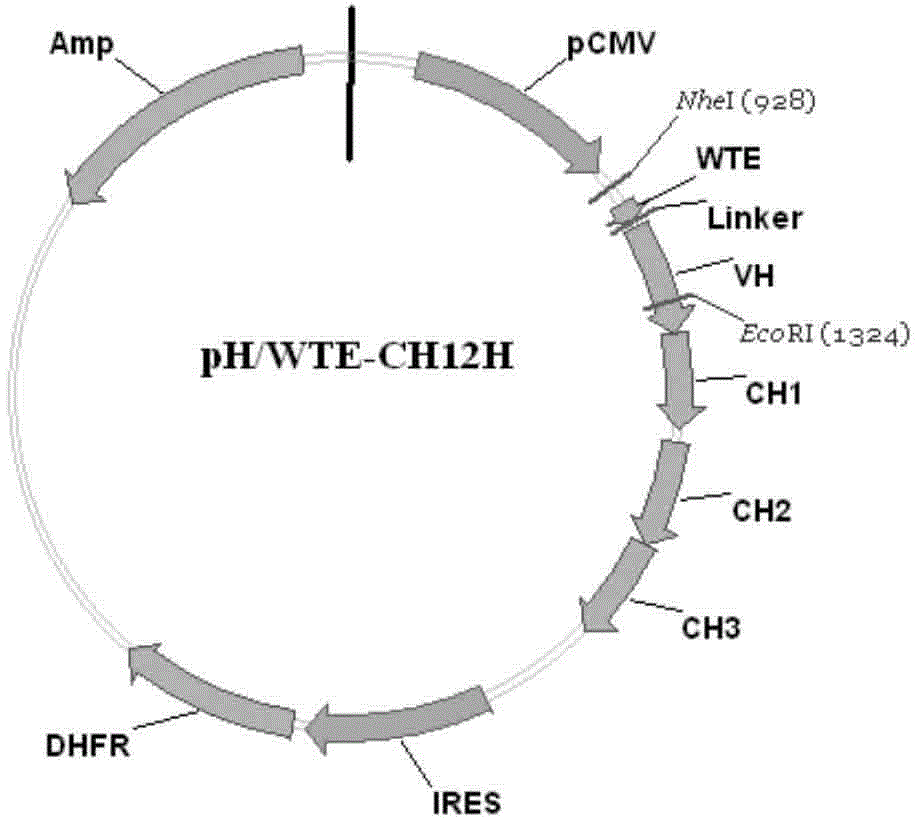
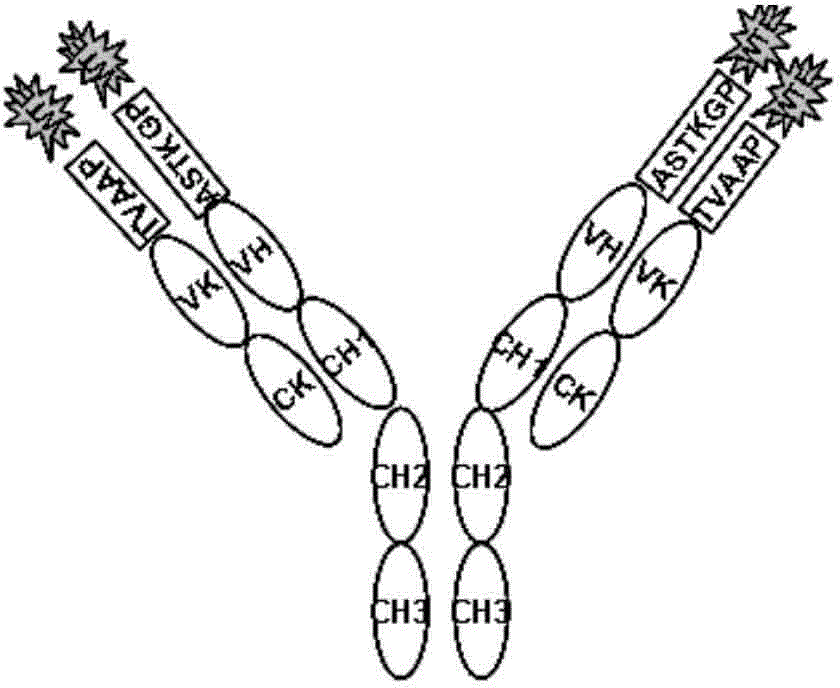
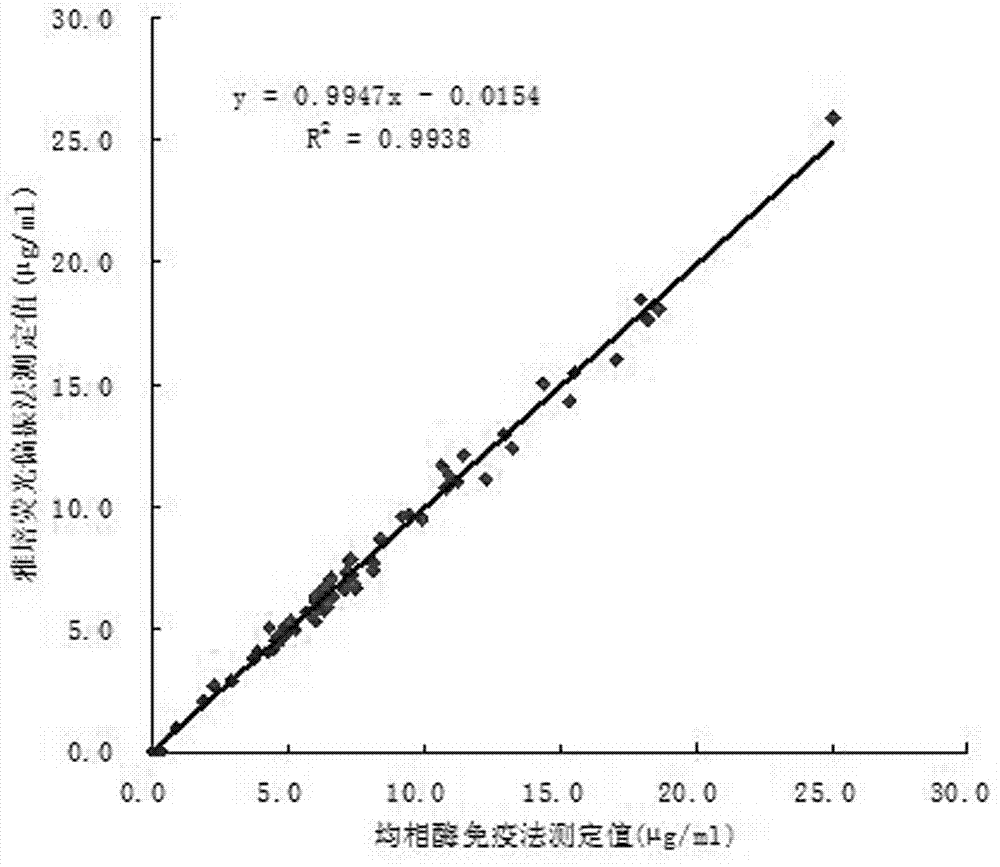
Patents
Literature
1516 results about "Cross reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of cross-reaction : reaction of one antigen with antibodies developed against another antigen : reaction of one antigen with antibodies developed against another antigen
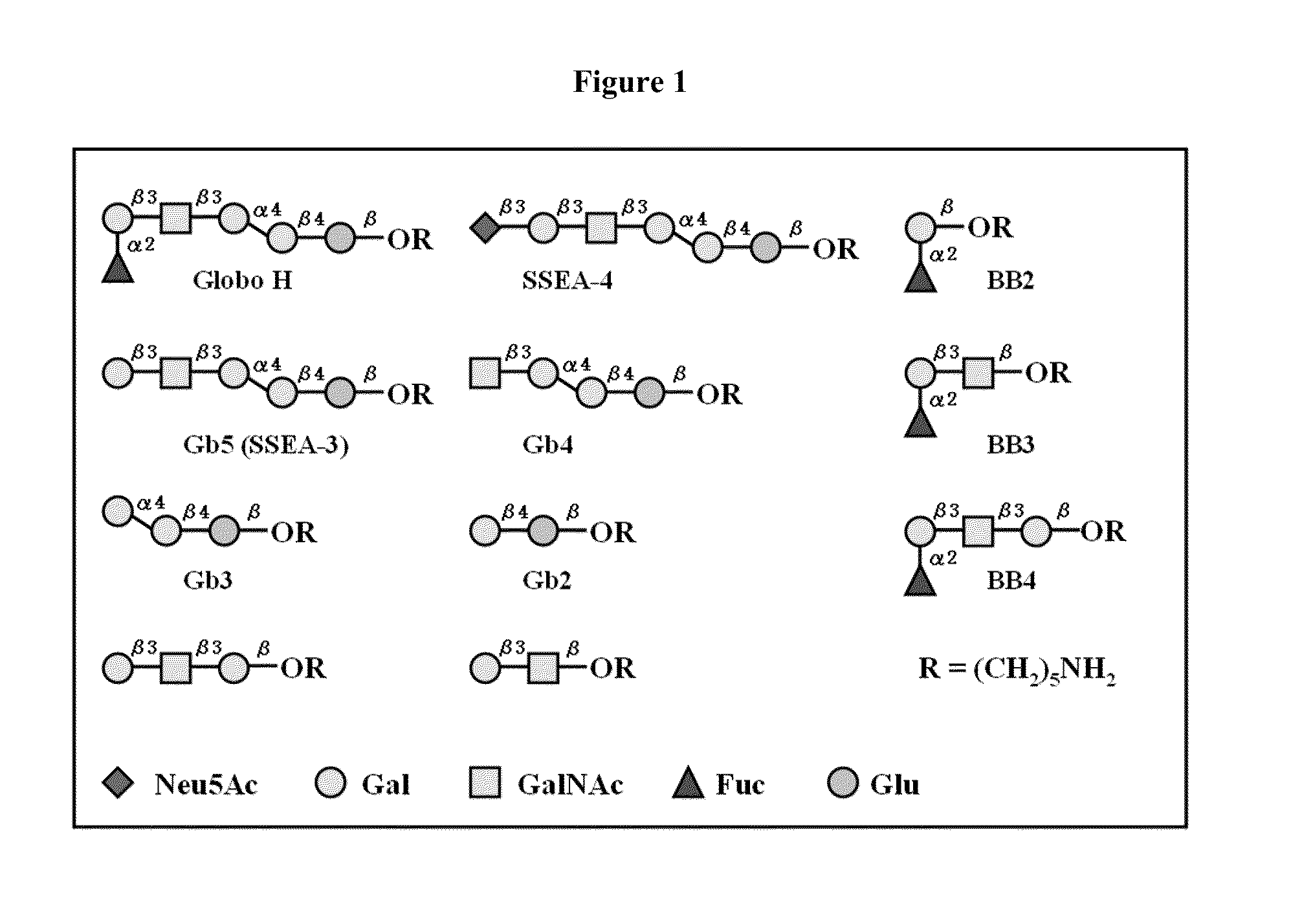
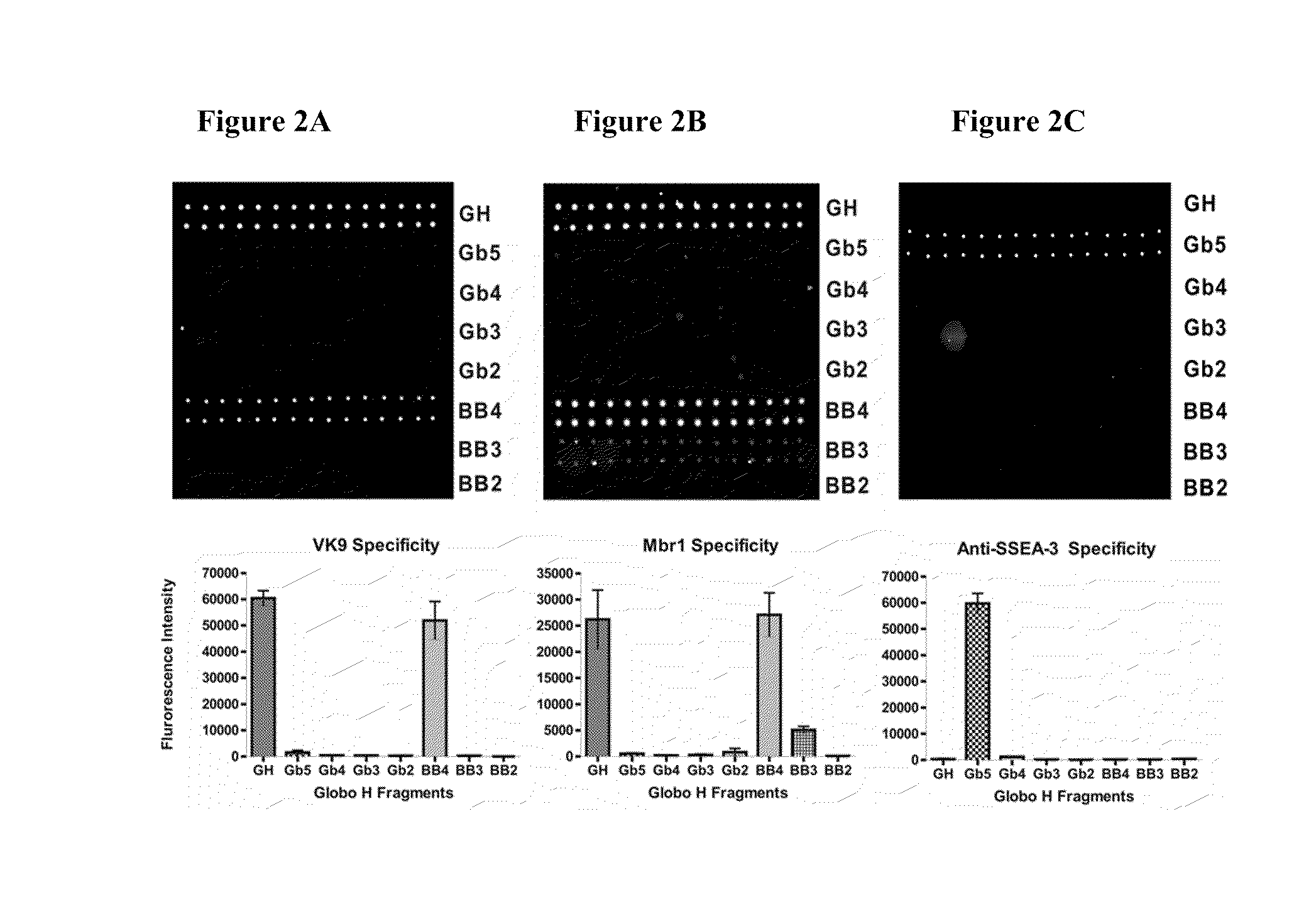
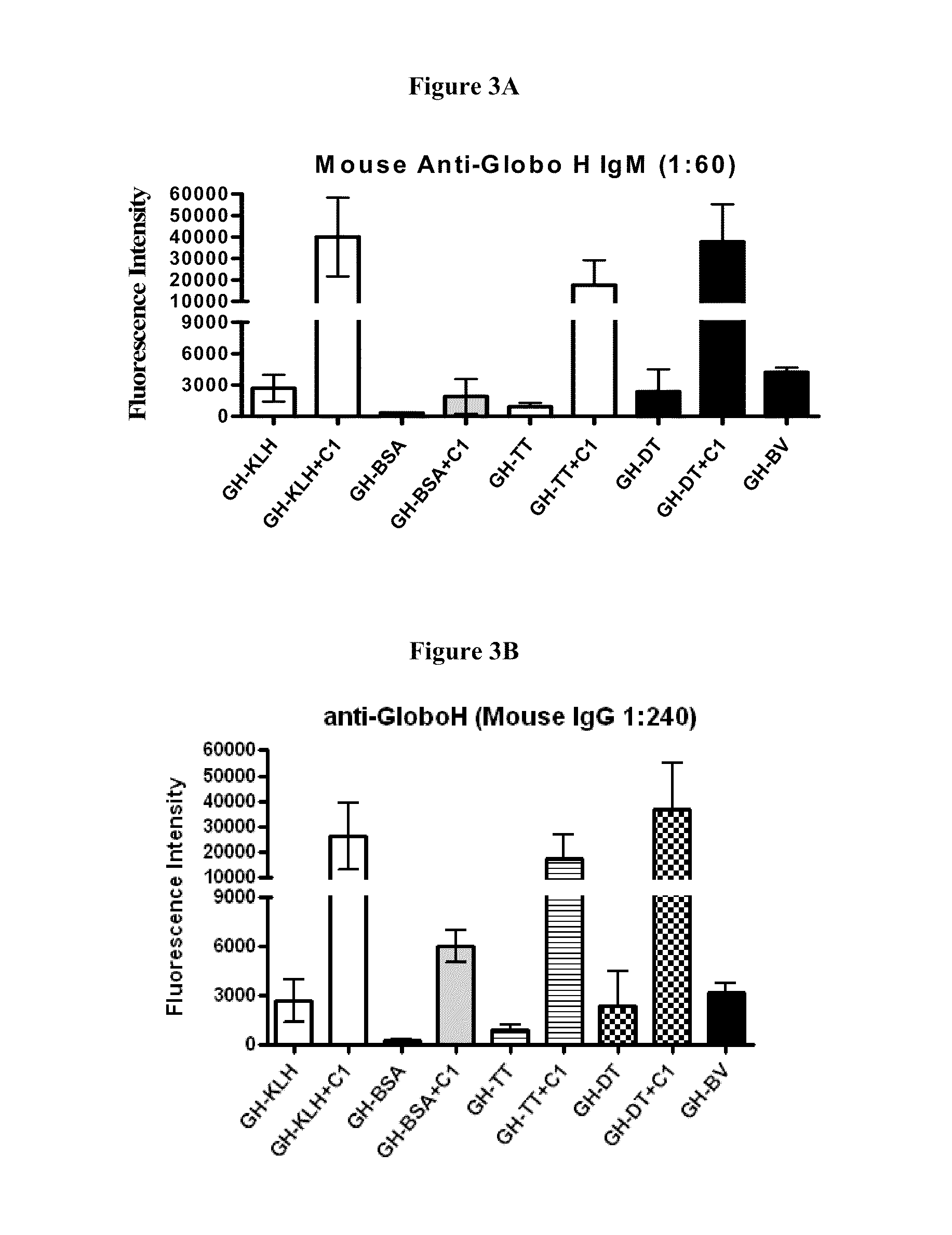
Globo h and related Anti-cancer vaccines with novel glycolipid adjuvants
ActiveUS20100136042A1Shrink tumorInhibit tumor growthOrganic active ingredientsSugar derivativesDendritic cellAdjuvant
Owner:ACAD SINIC
Aflatoxin nano antibody gene pool, construction method and application of aflatoxin nano antibody gene pool as well as aflatoxin B1 nano antibody 2014AFB-G15
ActiveCN103866401AEasy to buildResistant to organic reagentsImmunoglobulins against fungi/algae/lichensBiological testingGene poolAntibody variable region
The invention relates to an aflatoxin nano antibody gene pool, a construction method and application of the aflatoxin nano antibody gene pool as well as an aflatoxin B1 nano antibody 2014AFB-G15. The aflatoxin nano antibody gene pool is prepared by extracting RNA (ribonucleic acid) in alpaca blood after immunization of an aflatoxin B1 antigen, performing specific amplification on a variable region gene of an alpaca heavy chain antibody by adopting an RT-PCR (reverse transcription-polymerase chain reaction) method to obtain an aflatoxin nano antibody VHH gene, and then performing transformation after connecting with a pCANTAB5E (his) vector. The aflatoxin B1 nano antibody 2014AFB-G15 obtained by screening, disclosed by the invention, has the characteristics of organic reagent resistance, high temperature resistance and the like, and is good in stability; the IC50 (half maximal inhibitory concentration) of the aflatoxin B1 nano antibody 2014AFB-G15 to the aflatoxin B1 is 0.66ng / mL, and the cross reactivity of the aflatoxin B1 nano antibody 2014AFB-G15 to the aflatoxins B2, G1,G2 and M1 is 22.6%, 0.95%, 32.1% and 26% respectively.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
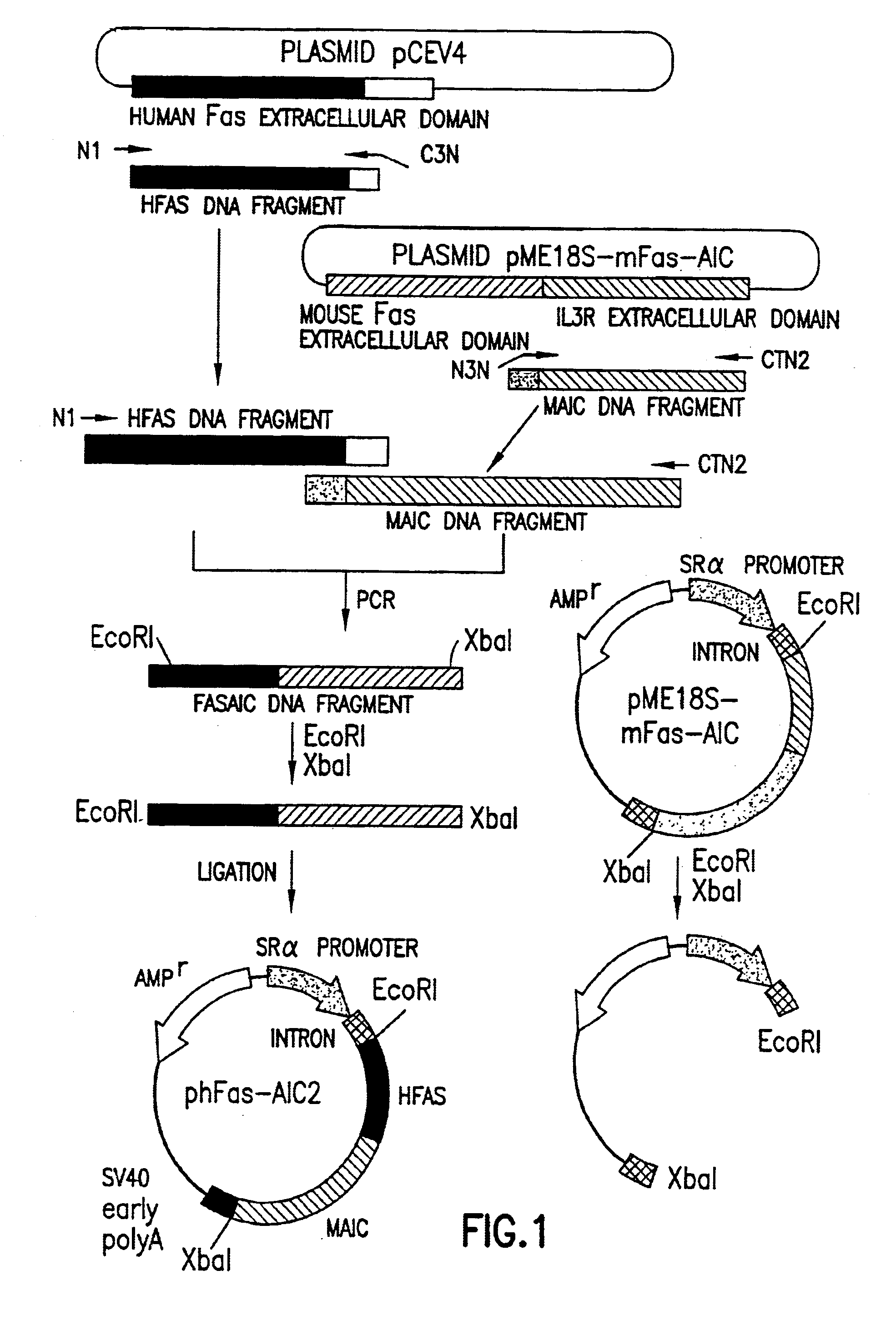
Anti-Fas antibodies
Anti-Fas antibodies which are cross-reactive with mouse and human Fas and are useful in the treatment of conditions attributable to abnormalities in the Fas / Fas ligand system.
Owner:SANKYO CO LTD
Hybridoma cell strain ST03, anti-aflatoxin biosynthesis precursor ST monoclonal antibody and application thereof
ActiveCN103849604ANo cross reactionHigh sensitivityTissue cultureImmunoglobulins against fungi/algae/lichensAbzymeAflatoxin biosynthesis
The invention relates to a hybridoma cell strain ST03, an anti-aflatoxin biosynthesis precursor ST monoclonal antibody and application thereof. The hybridoma cell strain ST03 with the preservation number of CCTCCNO.C2013187 can be used for preparing high-valence anti-aflatoxin biosynthesis precursor ST monoclonal antibody, wherein valence measured by an enzyme linked immunosorbent assay (ELISA) method of the anti-aflatoxin biosynthesis precursor ST mouse ascites antibody can reach 6.4*10<5>. The anti-aflatoxin biosynthesis precursor ST monoclonal antibody is high in sensitivity, has 50% inhibition concentration IC50 of 0.36 ng / mL on the aflatoxin biosynthesis precursor ST, has no cross reaction with the aflatoxin B1, aflatoxin B2, aflatoxin G1 and G2, and can be applied to measuring content of the aflatoxin biosynthesis precursor ST.
Owner:OIL CROPS RES INST CHINESE ACAD OF AGRI SCI
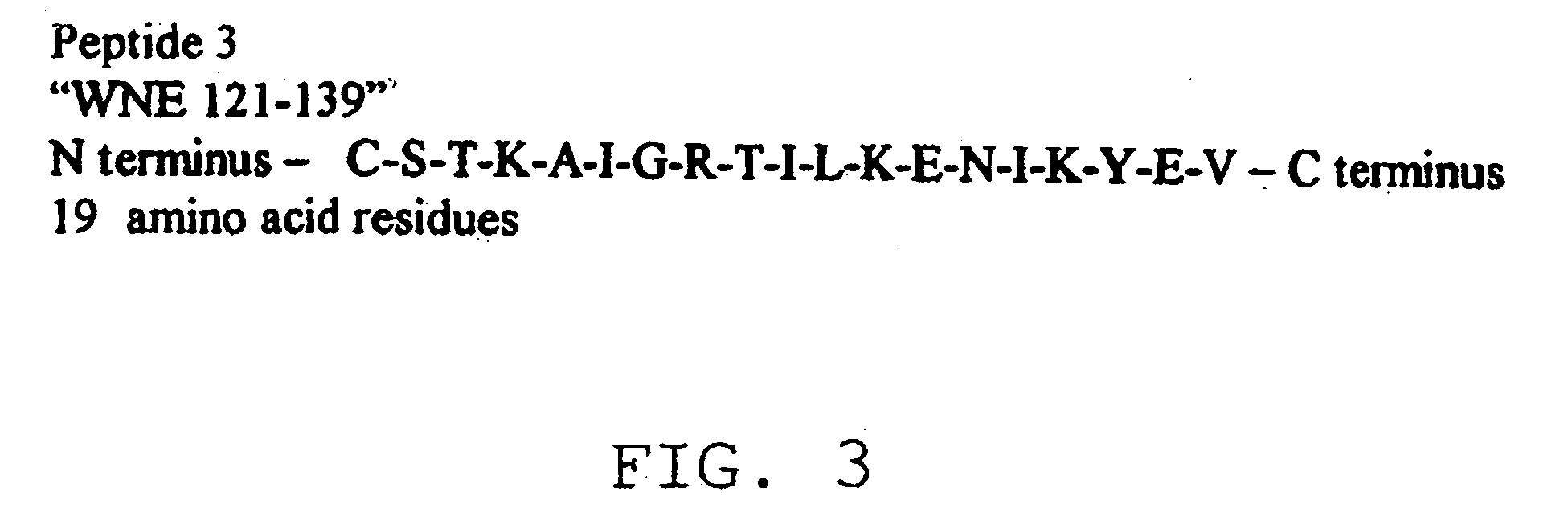
Diagnostic test for West Nile virus
InactiveUS20040197769A1More sensitiveEasy to useViral antigen ingredientsMicrobiological testing/measurementSt Louis encephalitis virusSerum ige
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result.
Owner:HEALTH RES INC
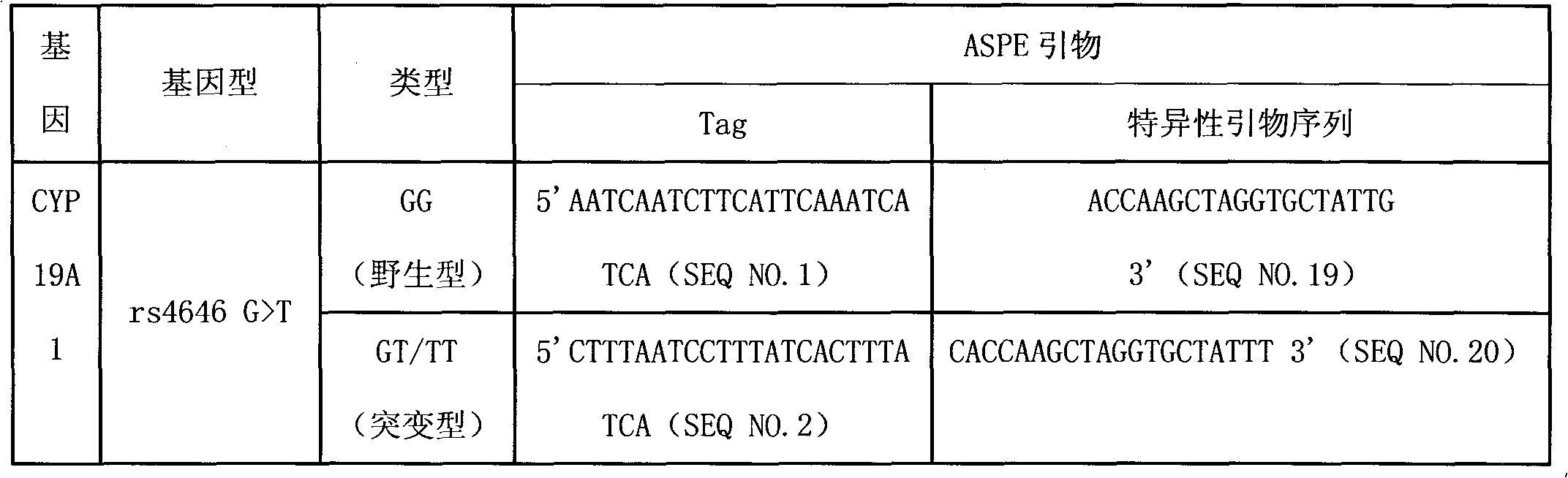
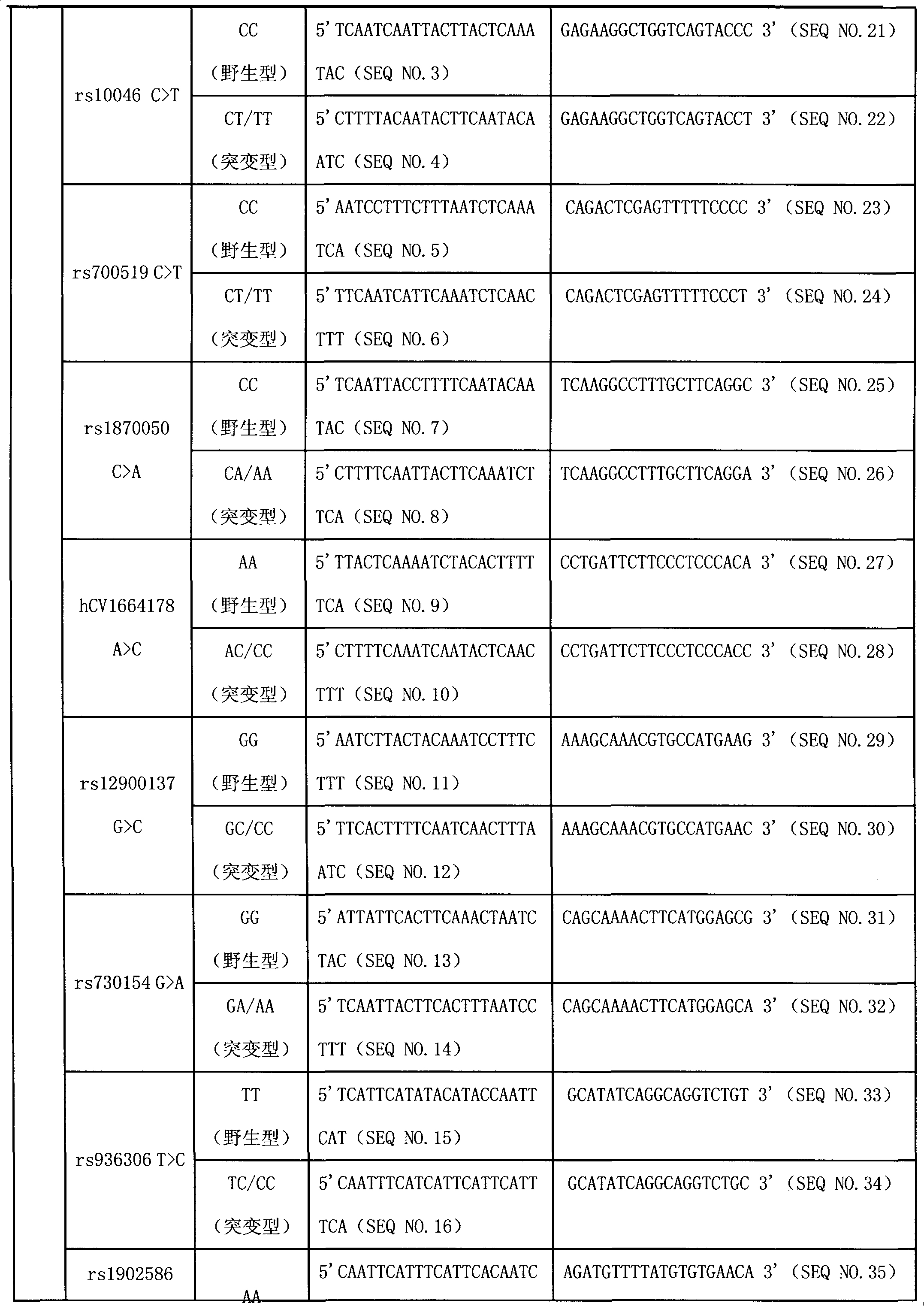
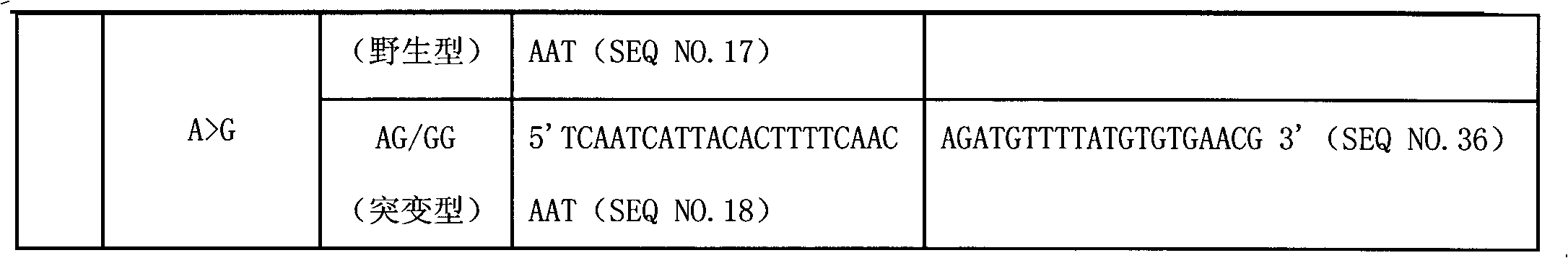
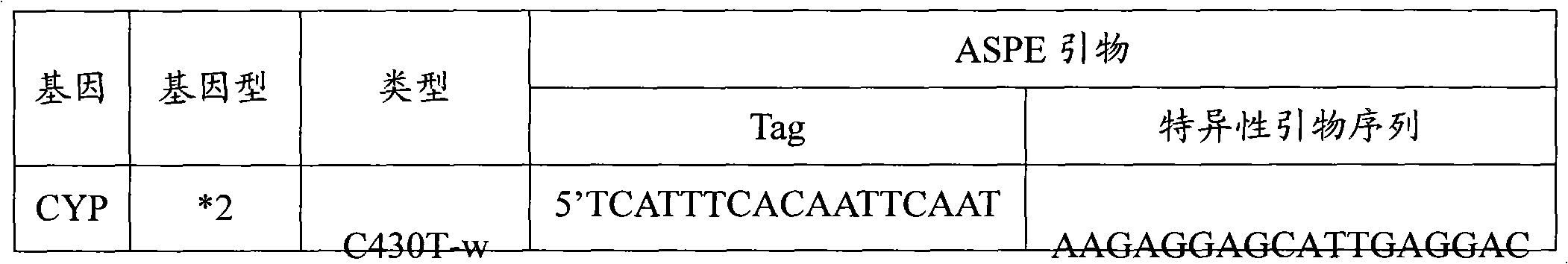
Liquid phase chip for CYP19A1 gene SNP (Single Nucleotide Polymorphism) detection and detection method thereof
ActiveCN101781684AImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementType specificWild type
The invention discloses a liquid phase chip for CYP19A1 gene SNP (Single Nucleotide Polymorphism) detection, which comprises wild type and mutant type specific ASPE primers designed targeting CYP19A1 gene SNPs of rs4646, rs10046C>T, rs700519C>T, rs1870050C>A, hCV1664178A>C, rs12900137G>C, rs730154G>A, rs936306T>C and rs1902586A>G, microballoon spheres respectively coated with specific anti-tag sequences and primers used for amplifying CYP19A1 gene SNPs with target sequences to be detected. Each ASPE primer comprises a tag sequence at 5' end and a specific primer sequence at 3' end, wherein the specific primers are selected from SEQ ID NO. 19-36 and the tag sequences are selected from SEQ ID NO.1-18; and the anti-tag sequences can be correspondingly in complementary pairing with the tag sequences. The invention also discloses a CYP19A1 gene SNP detection method. The coincidence rate of the detection method provided by the invention and a sequencing method is as high as 100%; and the prepared liquid phase chip for CYP19A1 gene SNP (Single Nucleotide Polymorphism) detection has excellent signal-to-noise rate, and basically no cross reaction exists between a design probe and the anti-tag sequences.
Owner:SUREXAM BIO TECH
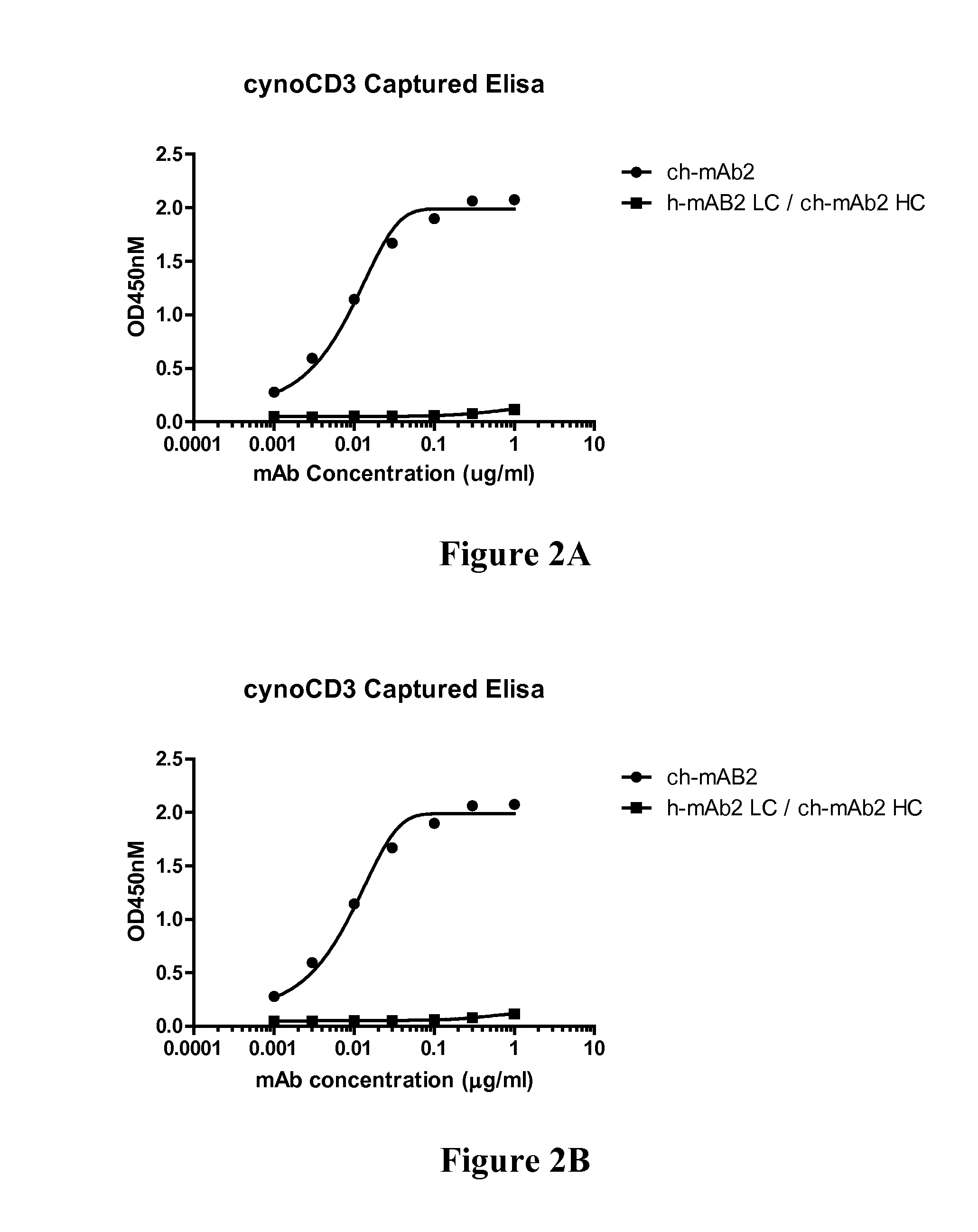
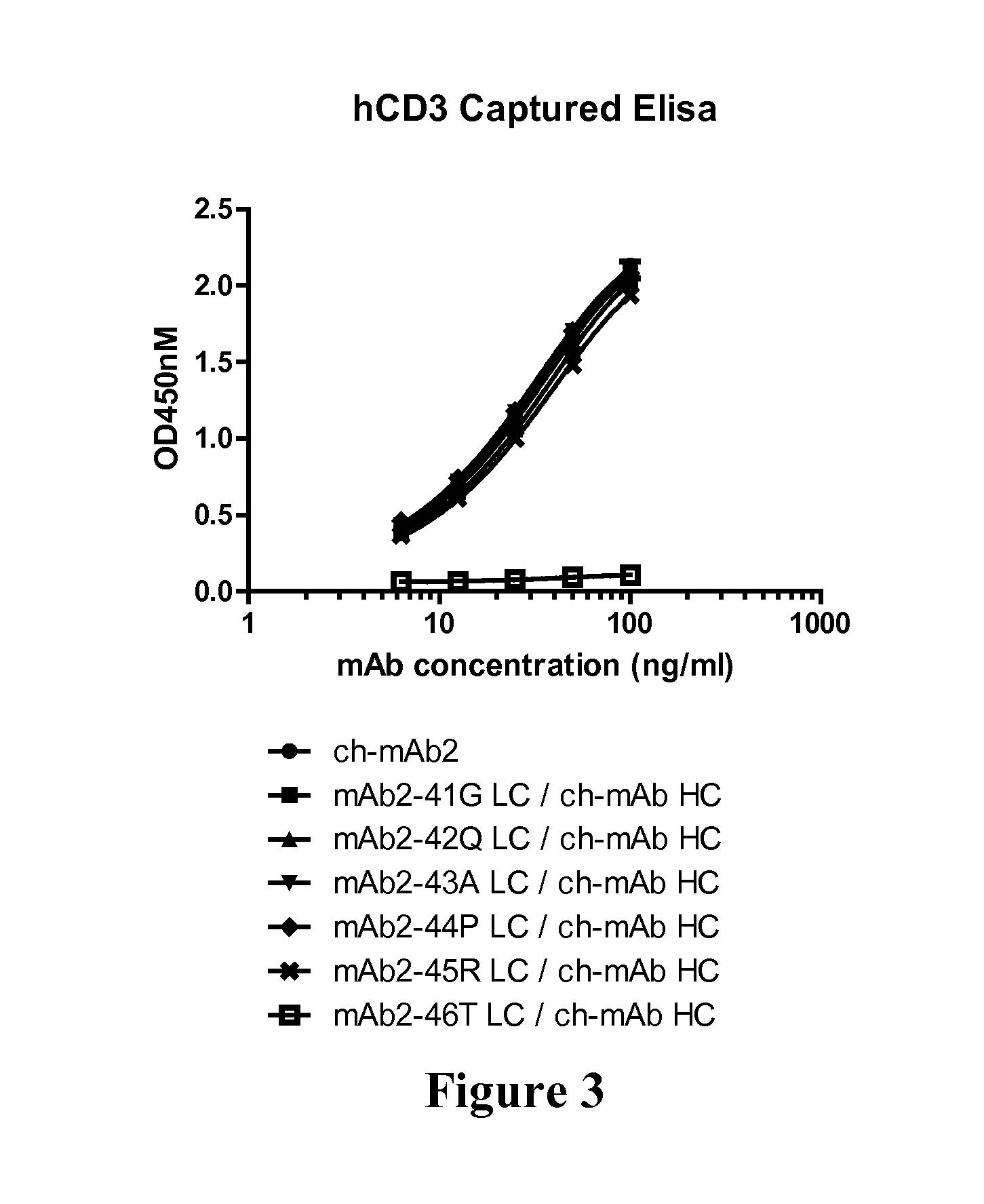
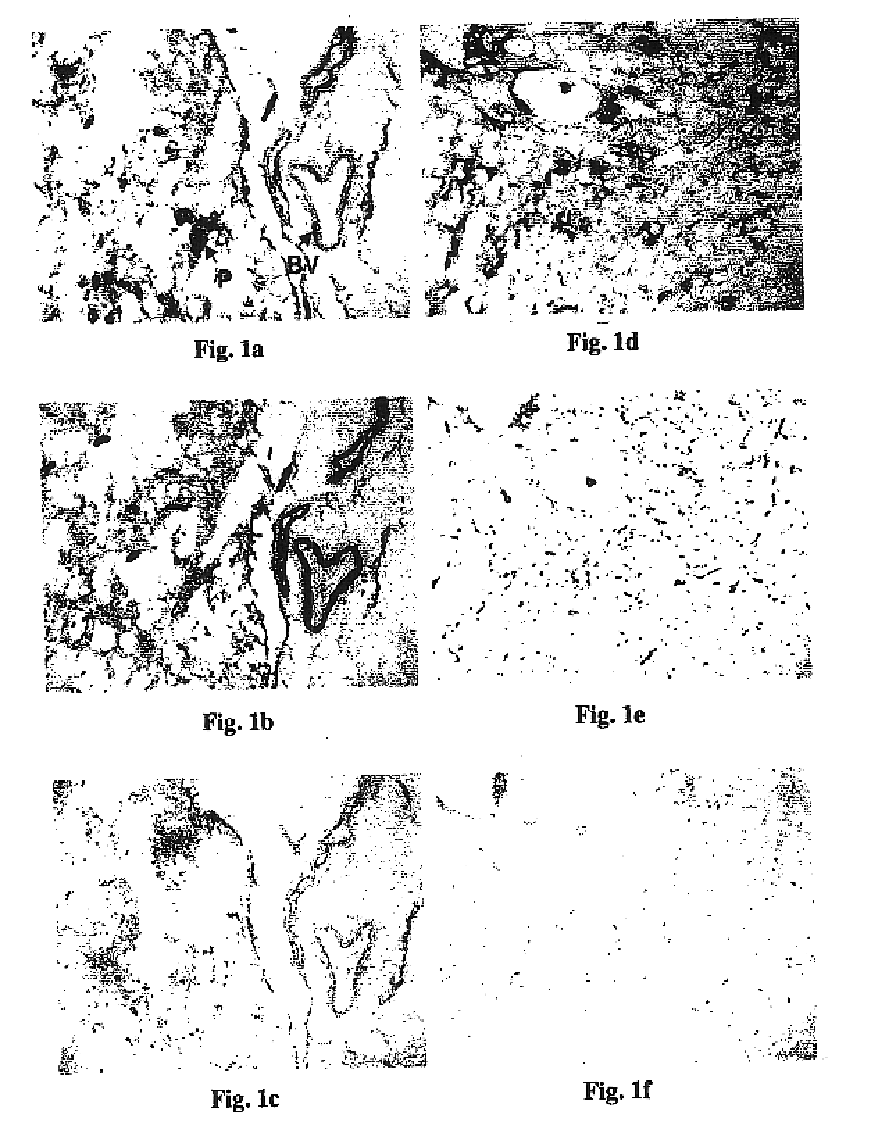
CD3-binding molecules capable of binding to human and non-human CD3
CD3-binding molecules capable of binding to human and non-human CD3, and in particular to such molecules that are cross-reactive with CD3 of a non-human mammal (e.g., a cynomolgus monkey) are presented. Uses of such antibodies and antigen-binding fragments in the treatment of cancer, autoimmune and / or inflammatory diseases and other conditions are presented.
Owner:MACROGENICS INC
Aflatoxin nano-antibody immunosorbent, immunoaffinity column and preparation methods and application of aflatoxin nano-antibody immunosorbent and immunoaffinity column
ActiveCN103861569AImprove stabilityHigh temperature resistantOther chemical processesComponent separationImmunosorbentsGene
The invention relates to an aflatoxin nano-antibody immunosorbent, an aflatoxin nano-antibody immunoaffinity column and preparation methods and application of the two. The immunosorbent comprises a solid phase carrier and an aflatoxin B1 nano-antibody 2014AFB-G15 coupled with the solid phase carrier, the half maximal inhibitory concentration (IC50) of the aflatoxin B1 nano-antibody 2014AFB-G15 to aflatoxin B1 is 0.66ng / mL, and the cross reaction rates of the aflatoxin B1 nano-antibody 2014AFB-G15 to aflatoxins B2, G1, G2 and M1 are 22.6%, 10.95%, 32.1% and 26%, respectively; an amino acid sequence of the aflatoxin B1 nano-antibody 2014AFB-G15 is represented by SEQIDNO:7, and a coding gene sequence of the aflatoxin B1 nano-antibody 2014AFB-G15 is represented by SEQIDNO:8. The aflatoxin nano-antibody immunoaffinity column disclosed by the invention can be used for purifying and concentrating a sample extracting solution before machinery detection and can be repeatedly used.
Owner:OIL CROPS RES INST CHINESE ACAD OF AGRI SCI
Immunogenic peptide composition comprising measles virus Fprotein Thelper cell epitope (MUFThl-16) and N-terminus of β-amyloid peptide
InactiveUS6906169B2Increase the gapHigh cross reactivityNervous disorderPeptide/protein ingredientsFibrilDisease patient
The present invention relates to a composition comprsing a peptide immunogen useful for the prevention and treatment of Alzheimer's Disease. More particularly, the peptide immunogen comprises a main functional / regulatory site, an N-terminal fragment of Amyloid β (Aβ) peptide linked to a helper T cell epitope (Th) having multiple class II MHC binding motifs. The peptide immunogen elicit a site-directed immune response against the main functional / regulatory site of the Aβ peptide and generate antibodies, which are highly cross-reactive to the soluble Aβ1-42 peptide and the amyloid plaques formed in the brain of Alzheimer's Disease patients. The antibodies elicited being cross reactive to the soluble Aβ1-42 peptide, promote fibril disaggregation and inhibit fibrillar aggregation leading to immunoneutralization of the “soluble Aβ-derived toxins”; and being cross-reactive to the amyloid plaques, accelerate the clearance of these plaques from the brain. Thus, the composition of the invention comprising the peptide immunogen is useful for the prevention and treatment of Alzheimer's Disease.
Owner:UNITED NEUROSCIENCE LIMITED
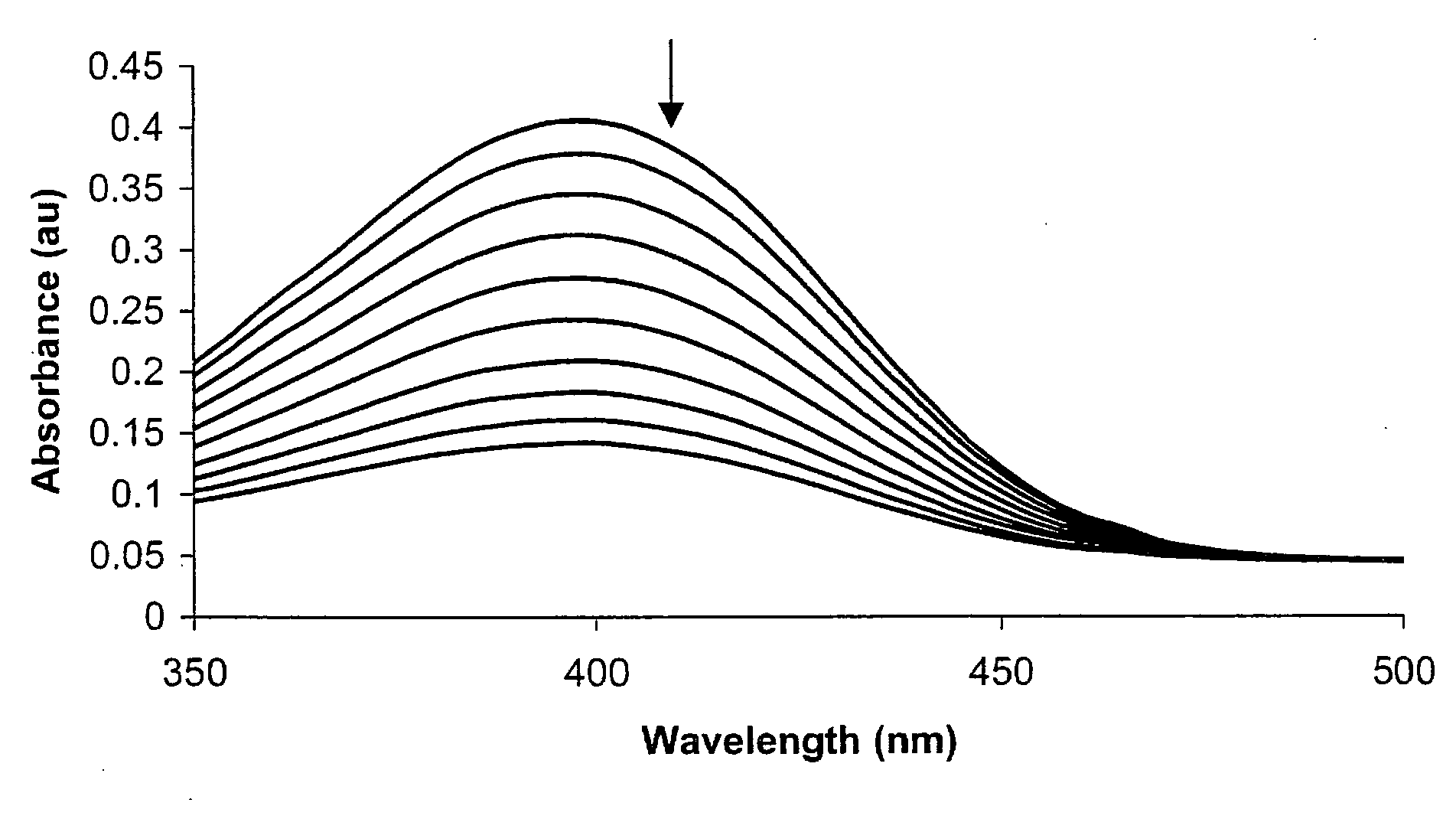
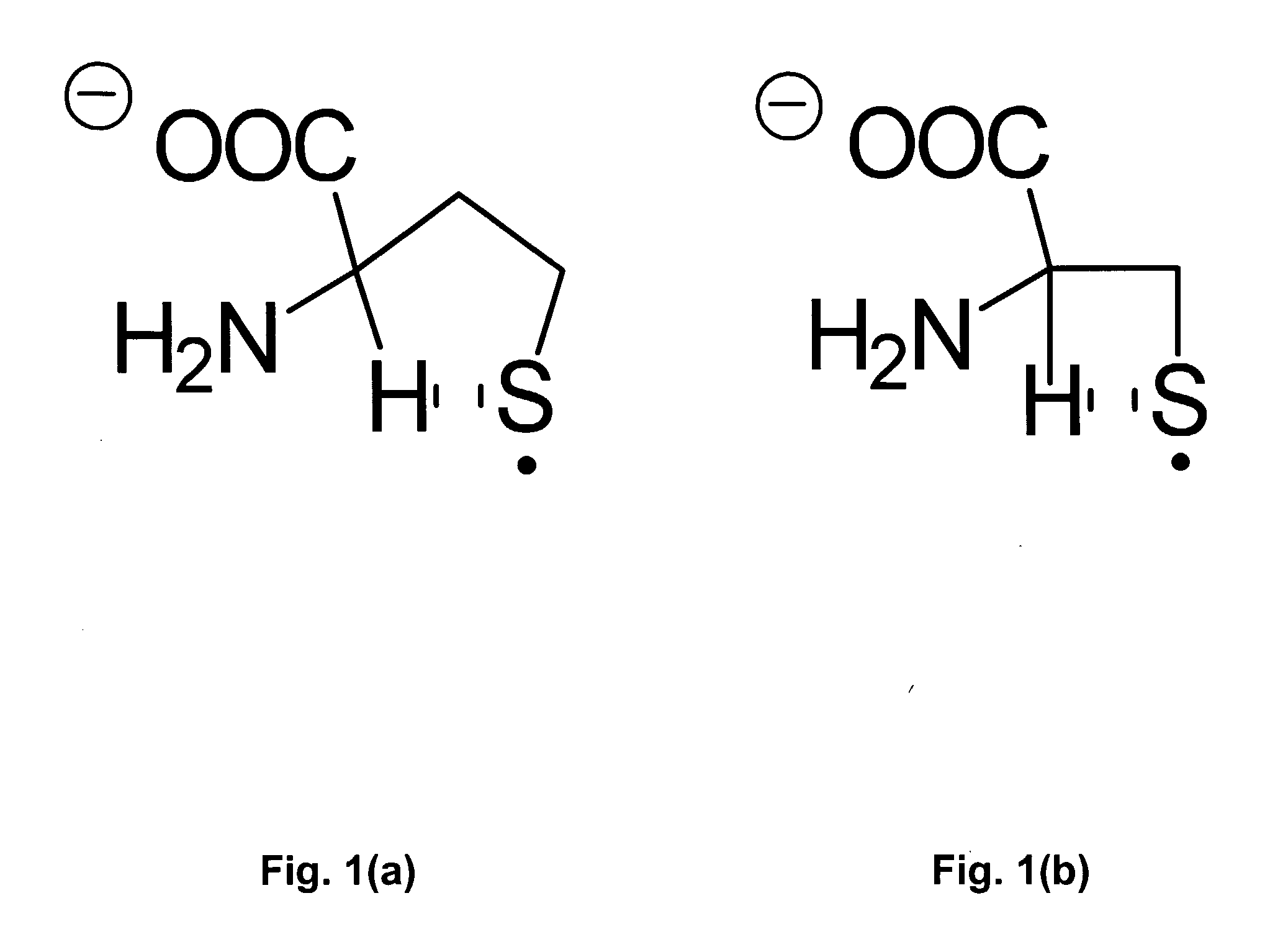
Colorimetric and Fluorometric Determination of Homocysteine and Cysteine
ActiveUS20080261315A1Material analysis by observing effect on chemical indicatorBiological testingFluorescenceCysteine thiolate
Colorimetric and fluorometric methods are disclosed for the rapid, accurate, selective, and inexpensive detection of homocysteine, or of homocysteine and cysteine, or of cysteine. The methods may be employed with materials that are readily available commercially. The novel methods are selective for homocysteine, for cysteine, or for total homocysteine and cysteine, and do not cross-react substantially with chemically-related species such as glutathione. The homocysteine-selective method does not have substantial cross-reactivity to the very closely related species cysteine. The cysteine-selective method does not have substantial cross-reactivity to the very closely related species homocysteine. The methods may be used, for example, in a direct assay of human blood plasma for homocysteine levels.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
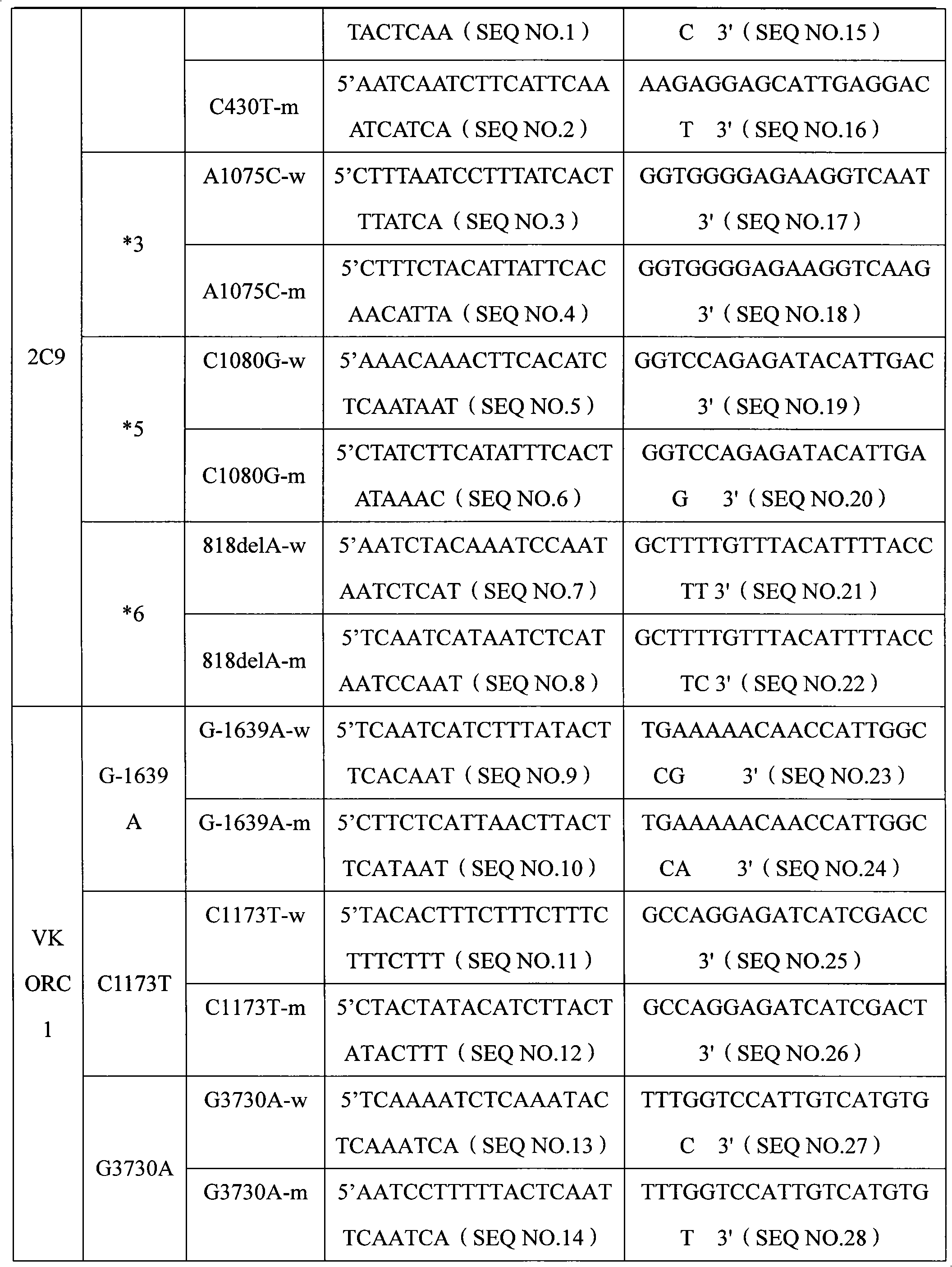
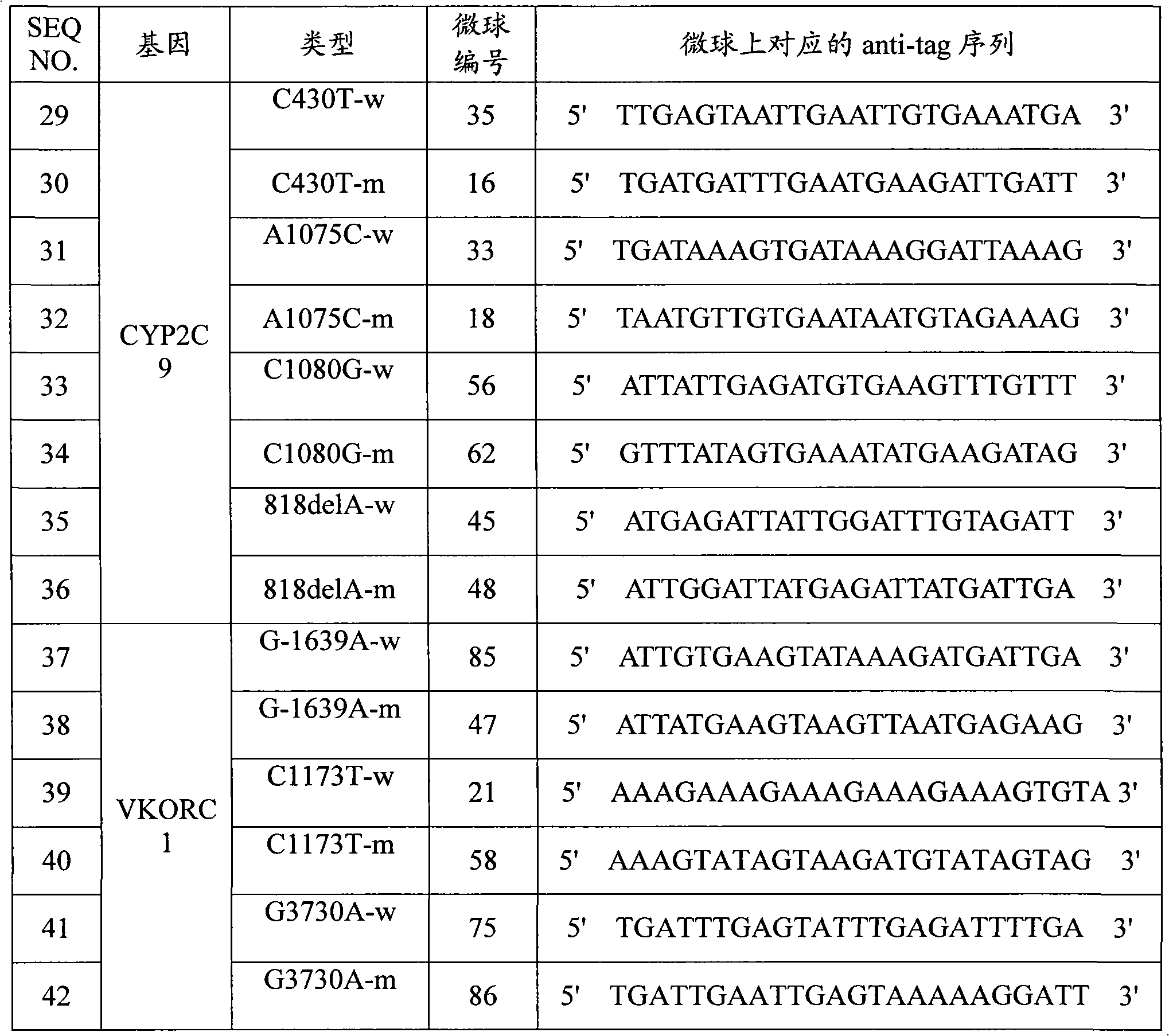
Specific primer, liquid-phase chip and method for SNP detection of CYP2C9 and VKORC1 genes
ActiveCN101824466AImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementDNA/RNA fragmentationVKORC1Microsphere
The invention discloses a specific primer, a liquid-phase chip and a method for SNP detection of CYP2C9 and VKORC1 genes. The liquid-phase chip comprises wild-type and mutable-type ASPE primer pairs and microspheres coated by a specific anti-tag sequence respectively, which are designed respectively aiming at each type of mutable loci, primers used for amplifying a CYP2C9 gene target sequence having CYP2C9*2, CYP2C9*3, CYP2C9*5 and CYP2C9*6SNP loci, and / or primers used for amplifying a VKORC1 gene target sequence having G1639A, G1173T and G3730A SNP loci. The liquid phase chip of the invention has a quite good signal-noise ratio, and the cross reaction does not happen between a designed probe and the anti-tag sequence basically; the ASPE primer designed by the invention has quite good specificity, and can accurately differentiate various types of mutable loci; and the detection method has the advantages that: a few steps are adopted, 7 types of SNP loci can be detected in one step, the operation is convenient, a lot of uncertain factors existing in a process of repeated operations can be avoided, and the detection accuracy is greatly improved.
Owner:SUREXAM BIO TECH
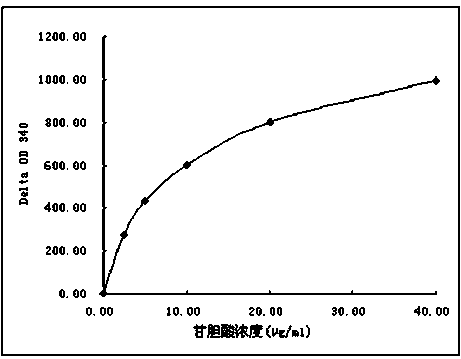
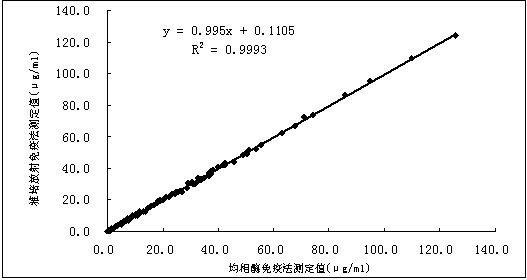
Glycocholic acid immunodetection reagent and preparing method and detecting method thereof
ActiveCN103760348AStrong immunogen specificityStrong specificityDisease diagnosisAntiendomysial antibodiesPharmaceutical drug
The invention relates to a glycocholic acid detecting reagent and a preparing method and a detecting method thereof, in particular to a glycocholic acid immunodetection reagent and a preparing method and a detecting method thereof. The glycocholic acid immunodetection reagent comprises a glycocholic acid specificity resisting antibody and an indicating reagent, wherein the indicating reagent is used for detecting the glycocholic acid specificity resisting antibody and a glycocholic acid composite; the glycocholic acid specificity resisting antibody is obtained from glycocholic acid immunogen immune animals. The glycocholic acid immunodetection reagent has the benefits that the glycocholic acid immunogen specificity is strong, the immunogenicity is high, and the prepared glycocholic acid specificity resisting antibody has strong specificity and high valence and does not have any cross reaction with 45 common drugs; a homogeneous enzyme immunodetection reagent containing the glycocholic acid specificity resisting antibody can conveniently, rapidly and accurately determine the content of glycocholic acid in a sample and can simultaneously test multiple samples on a fully-automatic biochemical analysis instrument; the high-throughout rapid measurement of the glycocholic acid is realized, the accuracy is high, the specificity is strong, and both the precision and the detection efficiency are greatly improved.
Owner:苏州博源医疗科技有限公司
System for inhibiting pathological target cells in space-time adjustable manner
ActiveCN105194661APolypeptide with localisation/targeting motifImmunoglobulin superfamilyEffector cellNormal tissue
The invention relates to a system for inhibiting pathological target cells in a space-time adjustable manner, and discloses a technical scheme based on the tumor specificity chimeric antigen receptor (CAR) technology. The CAR immunologic effector cells can target the pathological target cells only under a condition that a mediate material exists, so as to realize continuous multiplication and play the killing effect on tumor cells; under the condition that the mediate material does not exist, the CAR immunologic effector cells do not play the function. According to the system, a solution is provided for avoiding in-vivo continuous multiplication of the CAR immunologic effector cells and the toxic effect generated on normal tissue cross reaction of the CAR immunologic effector cells.
Owner:CARSGEN THERAPEUTICS
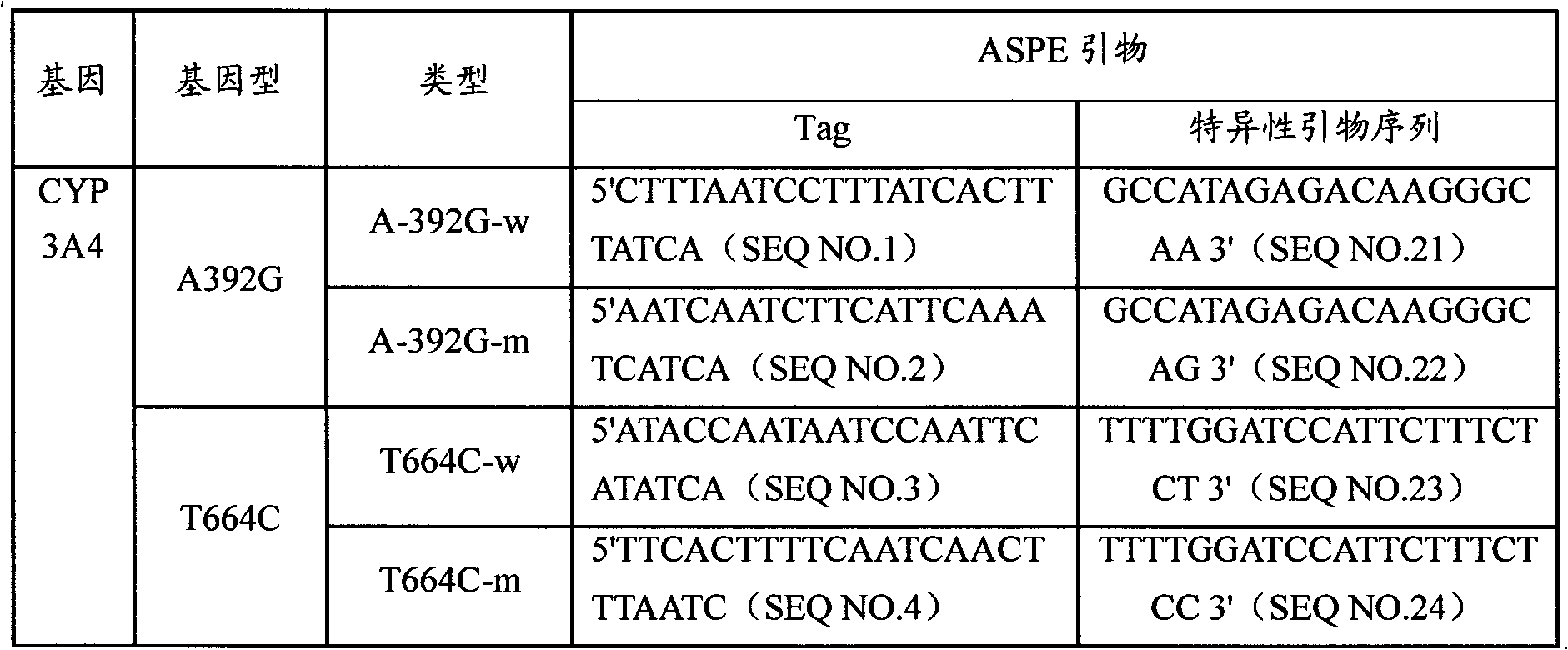
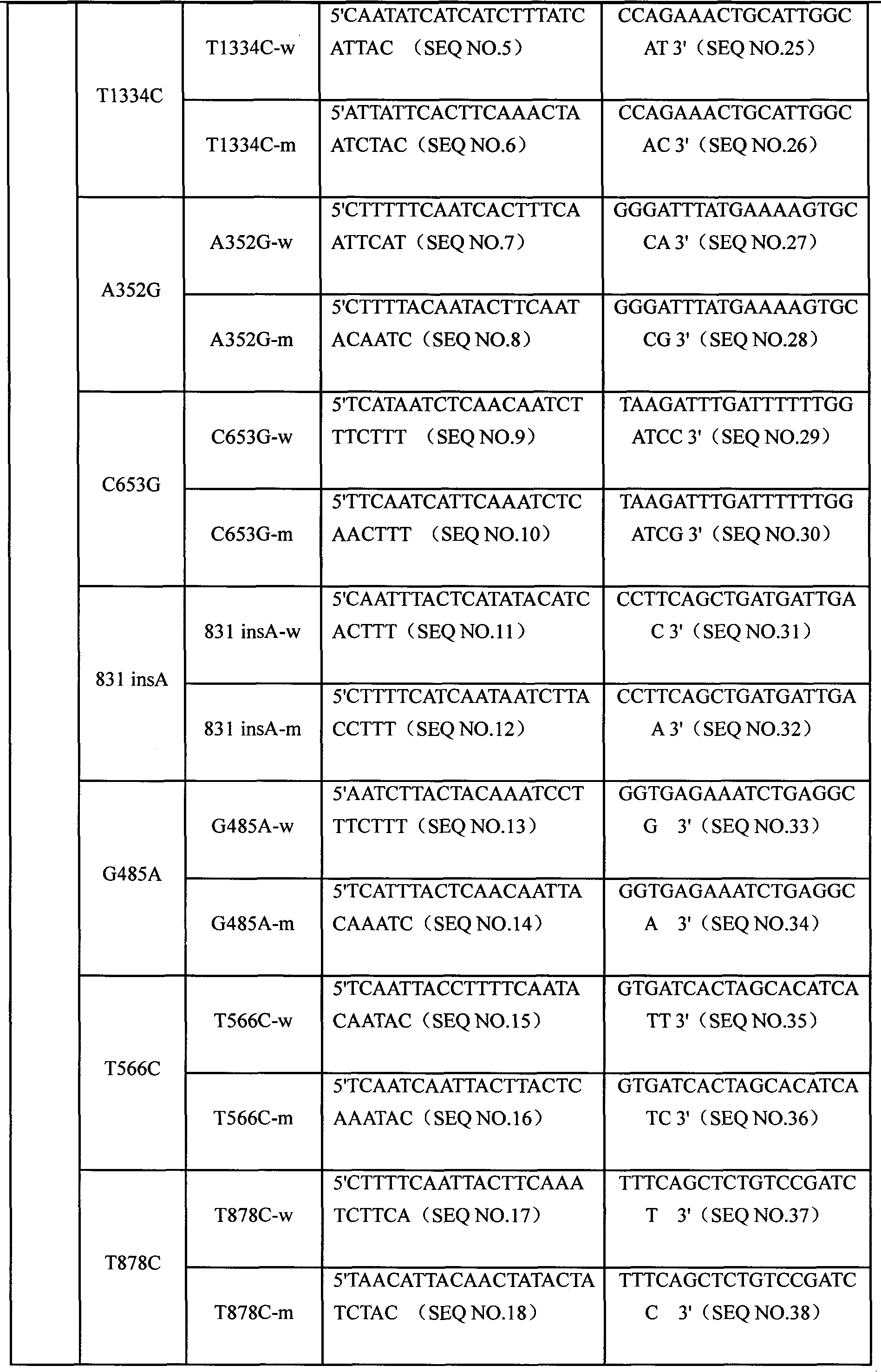
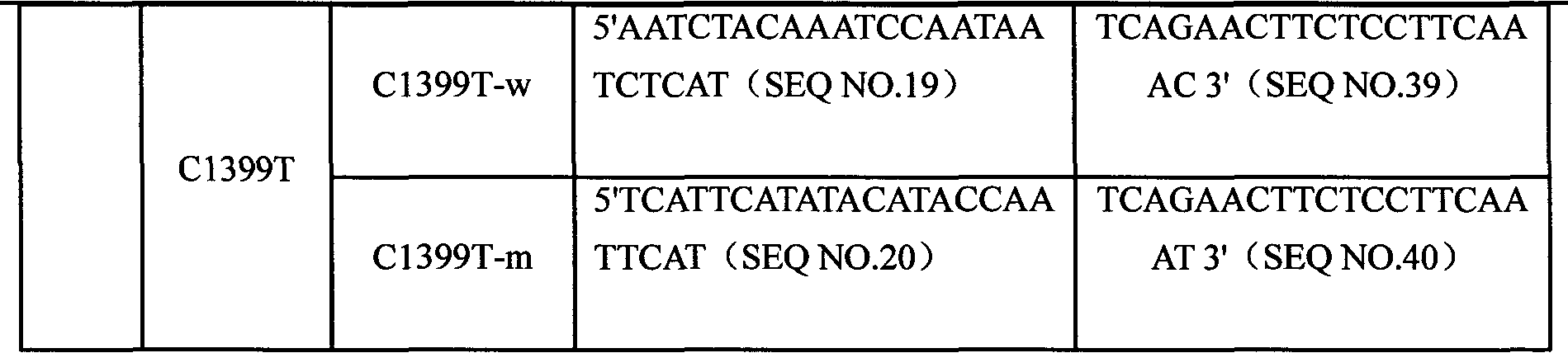
CYP3A4 gene SNP detection specific primer, liquid-phase chip and method
InactiveCN101812511AImplement parallel detectionImprove signal-to-noise ratioMicrobiological testing/measurementDNA/RNA fragmentationGene targetsMicrosphere
The invention discloses a CYP3A4 gene SNP detection specific primer, a liquid-phase chip and a method; and the liquid-phase chip comprises wild-type and mutant ASPE primers which are respectively designed for each type of mutation points, microspheres which are respectively coated with specific anti-tag sequences, and the primer which is used for amplifying a CYP3A4 gene target sequence with CYP3A4*1B, CYP3A4*2, CYP3A4*3, CYP3A4*4, CYP3A4*5, CYP3A4*6, CYP3A4*15, CYP3A4*17, CYP3A4*18 and / or CYP3A4*19SNP sites. The CYP3A4 gene SNP detection liquid-phase chip has very good signal-noise ratio, and a designed probe and the anti-tag sequences essentially have no cross reaction. The designed ASPE primers have very good specificity and can accurately distinguish all types of mutation points. The detection method has simple steps and ten types of SNP sites can be detected at one step, so that the operation is convenient, thereby preventing a plurality of uncertain factors in a plurality of operation processes, and greatly improving the detection accuracy rate.
Owner:SUREXAM BIO TECH
Immunodetection reagent of carbamazepine homogeneous enzyme and detection method thereof
The invention discloses a carbamazepine immunogen, a carbamazepine specific antibody directly obtained through the immunogen, a detection reagent containing the specific antibody and a method for detecting the content of the carbamazepine in samples to be tested. The immunodetection reagent and the detection method have the advantages that the carbamazepine immunogen is strong in specificity and high in immunogenicity; the prepared carbamazepine immunogen is strong in specificity and high in titer and has no cross reaction with 45 common drugs; the homogeneous enzyme immunodetection reagent containing the carbamazepine specific antibody can conveniently, fast and accurately determine the content of the carbamazepine in the samples and can measure a plurality of samples simultaneously on a full-automatic biochemical analyzer, so that high-throughput and fast measuring of the carbamazepine are achieved, the accuracy is high, the specificity is strong, the accuracy and the detection efficiency are greatly improved compared with the prior art, full automation in the detection process is achieved simultaneously, the requirements on detection staff is not high, and the immunodetection reagent and the detection method are easy to achieve, popularize and use.
Owner:苏州博源医疗科技有限公司
Hybridoma cell line 3G1 and anti-alfatoxin B1 monoclonal antibody produced by the same
ActiveCN102747043AHigh sensitivityStrong specificityBiological material analysisTissue cultureAflatoxin BELISA unit
The present invention relates to a hybridoma cell line 3G1 and an anti-alfatoxin B1 monoclonal antibody produced by the hybridoma cell line 3G1. The hybridoma cell line 3G1 (CCTCCNO.C201014) can be used for preparation of a high titer anti-aflatoxin B1 monoclonal antibody, wherein an enzyme-linked immunosorbent assay (ELISA) method is adopted to determine a titer, and the titer is 6.40*10<6>. The anti-aflatoxin B1 monoclonal antibody of the present invention has characteristics of high sensitivity and good specificity, wherein 50% inhibiting concentration on aflatoxin B1 by the monoclonal antibody is 1.6 ng / mL, cross reaction rate with aflatoxin B2 is 6.4%, and cross reaction rates with aflatoxin G1 and G2 are less than 1%. In addition, the anti-aflatoxin B1 monoclonal antibody of the present invention can be used for determination of aflatoxin B1.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Respiratory tract common pathogen multiple PT-PCR combined gene chip detection kit
ActiveCN107090519AAvoid non-specific amplificationAvoid non-specific hybridizationMicrobiological testing/measurementMicroorganism based processesPositive controlReaction system
The invention relates to a respiratory tract common pathogen multiple PT-PCR combined gene chip detection kit. The kit detects one or more of 22 respiratory tract common pathogens by the usage of multiple specific conservative degenerate primer combination and probe combination and provided with endogenous control, positive control and negative control at the same time. According to the detection kit, the coverage rate for a high mutant pathogen subject sequence is increased, the problem of nonspecific cross reaction between the multiple primers and the probe is avoided, one single reaction system can detect more than 20 respiratory tract common pathogens simultaneously, and a detection tool which is simple and sensitive, rapid and large in flux and capable of carrying out multi-index parallel detection is provided for respiratory tract common pathogen detection.
Owner:SUZHOU GENEWORKS TECH CO LTD
h5, h7, h9 subtype avian influenza virus detection kit
InactiveCN102260749AMicrobiological testing/measurementAvian influenza virusComplementary deoxyribonucleic acid
The invention discloses an H5, H7 and H9 subtype avian influenza virus detection kit. In the GenBank, three kinds of subtype HA gene sequences are searched, and specific primers are designed. By many tests and actual detections, three pairs of primers and reaction systems thereof are screened out and optimized, and the sizes of the amplified cDNA (complementary deoxyribonucleic acid) fragments are respectively 427bp, 312bp and 826bp. The result shows that by optimizing multiple RT-PCR (reverse transcription-polymerase chain reaction) amplification conditions, a multiple RT-PCR detection kit capable of detecting three kinds of subtype avian influenza viruses at the same time is prepared, and the minimum detectable quantity is 10pg. The detection kit does not have cross reaction to various kinds of chicken infectious diseases and normal structures, has strong specificity, and has consistent sensibility to three kinds of subtype detections. The avian influenza can be diagnosed in a shorttime in a primary laboratory and can be positioned in a subtype so as to gain the time for preventing and controlling the avian influenza, thus the spread of the avian influenza can be controlled in a small range as much as possible by adopting measures in time.
Owner:JILIN AGRICULTURAL UNIV
Method for detecting allergen almond component in foods by fluorescent PCR technology
InactiveCN101643786AAvoid complex processingReduce distractionsMicrobiological testing/measurementFluorescence/phosphorescenceFluorescent pcrBiology
The invention discloses a method for detecting an allergen almond component in foods by a fluorescent PCR technology, belonging to allergen detecting technologies, in particular a method for detectingan allergen almond component in foods by an exonuclease probe fluorescent PCR technology (TaqMan). Aiming at an allergen almond component Pru du 1.06B DNA sequence a primer and a TaqMan probe are designed, the fluorescent PCR detecting method is established. The method includes the designed primer and a probe of the almond component specificity, and the fluorescent PCR reaction condition matchedwith the primer and the probe. The method has no cross reaction with peanuts, hazelnuts, chestnuts, walnuts, pine nuts, macadamia nuts, and the like and has specificity; and the detecting sensitivitycan reach 5mg / kg. The method can be used for detecting the allergen in the foods and preventing anaphylactic reaction caused by the foods and has practical meanings.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Molecularly imprinted polymers (MIPs) for inspecting melamine and preparation method thereof
InactiveCN101559352AStrong specificityReduce polarityOther chemical processesPolymer scienceFunctional monomer
The invention discloses molecularly imprinted polymers (MIPs) for detecting melamine and a preparation method thereof. The MIPs are prepared by the steps as follows: cyromazine is used as a virtual moulding board and is polymerized with a functional monomer, a cross linker agent, a pore-foaming agent and an initiator at the temperature from 50 to 80 DEG C to obtain a bulk polymer; and after being processed by the operations of pulverization, screen separation, moulding board removal by acetic acid methanol and methanol, the bulk polymer is processed by vacuum drying to prepare the MIPs which have high cross reaction and outstanding selectivity to melamine. The invention selects the cyromazine as the virtual moulding board to prepare the MIPs, and avoids the problem that the melamine is hard to dissolve in a polymerization system and the problem that when the MIPs prepared by using the melamine as the moulding board is used for solid phase extraction of fill materials, moulding board leakage can lead to an inaccurate result; the invention selects the mixed system of methanol and water as the pore-foaming agent, and the prepared MIPs have high compatibility with the melamine in an aqueous solution; and used as a sample pre-processing material for analyzing the melamine, the prepared MIPs have broad application prospect.
Owner:SOUTH CHINA AGRI UNIV
Methods for isolating molecular mimetics of unique Neisseria meningitidis serogroup B epitopes
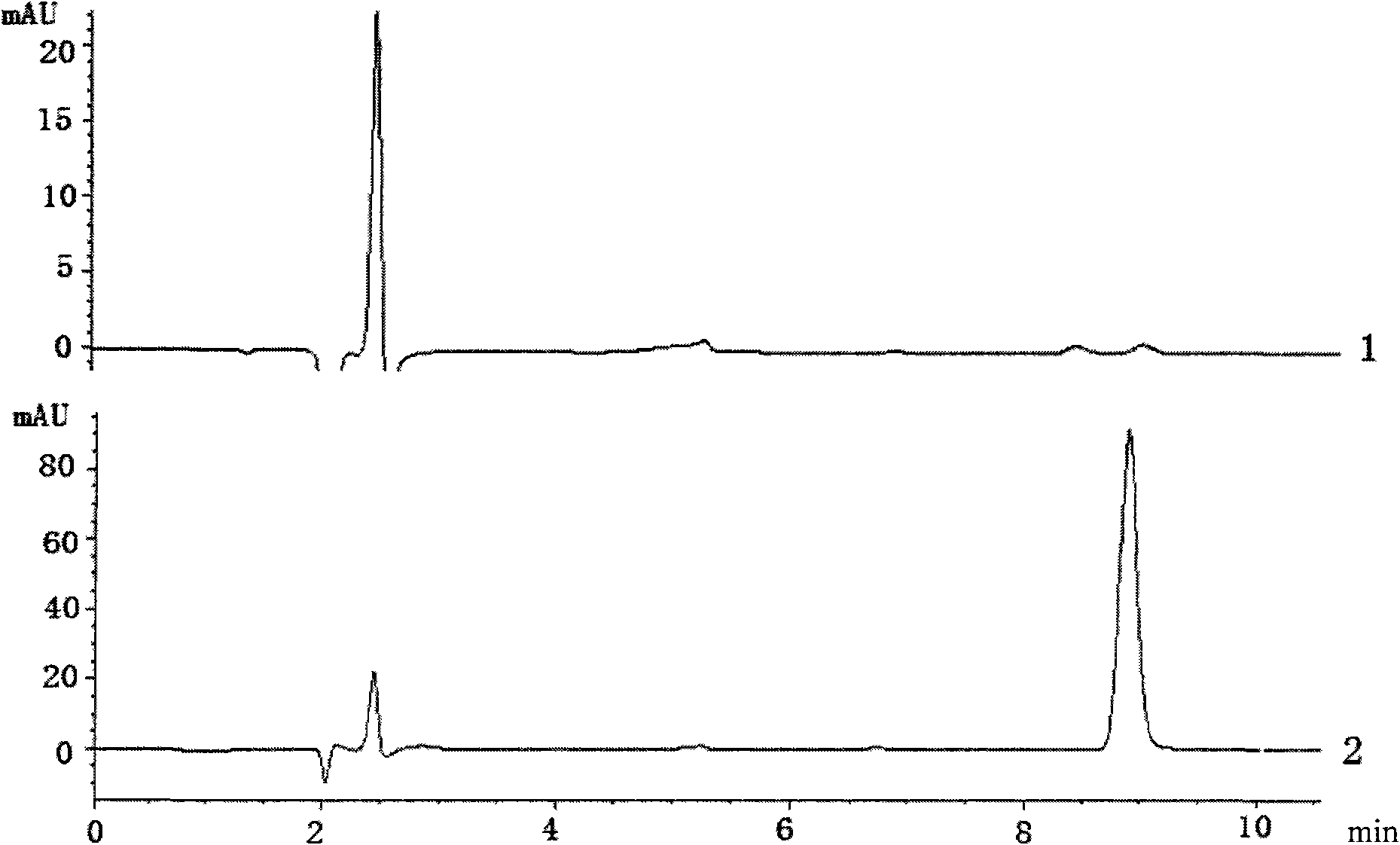
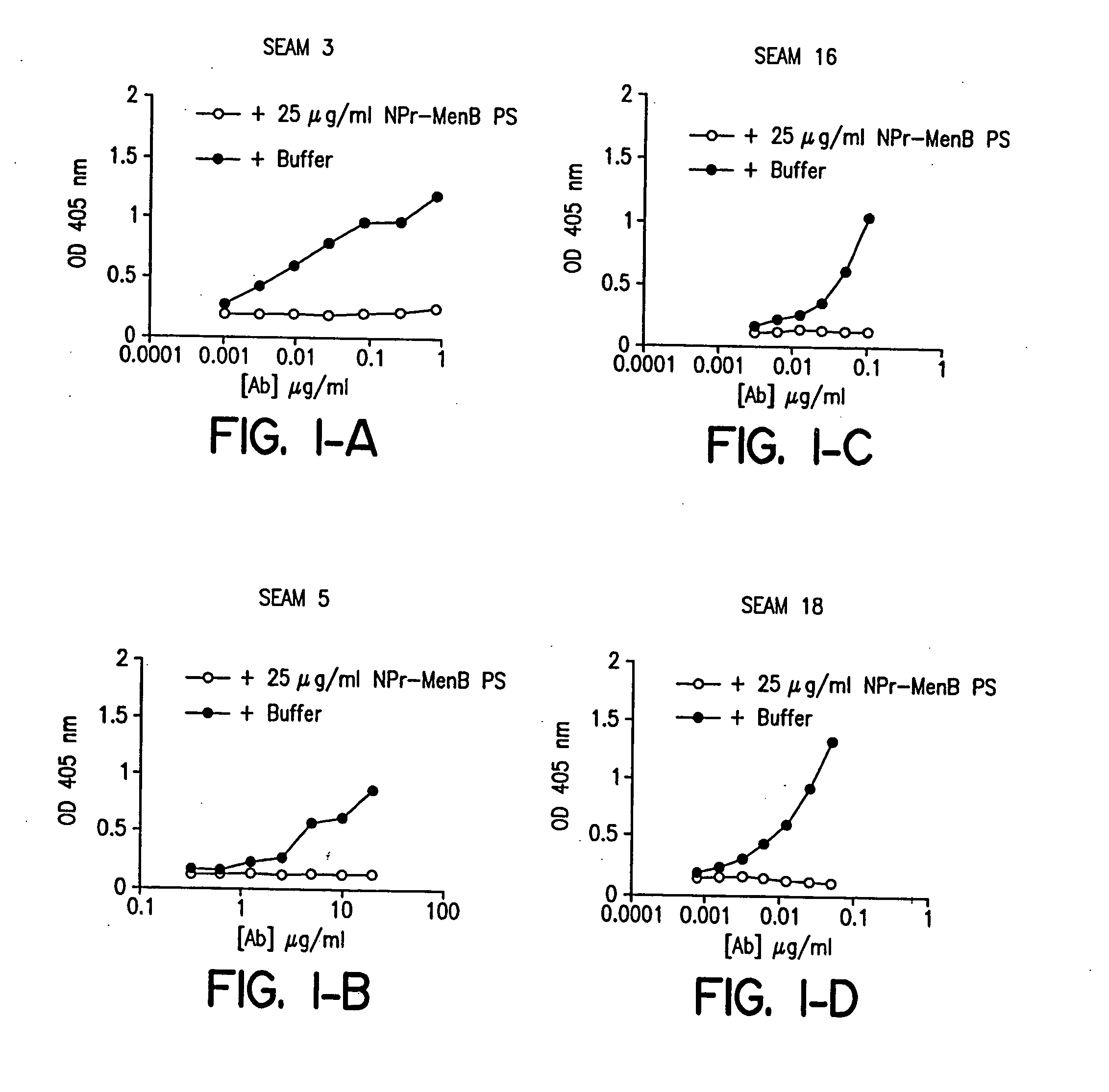
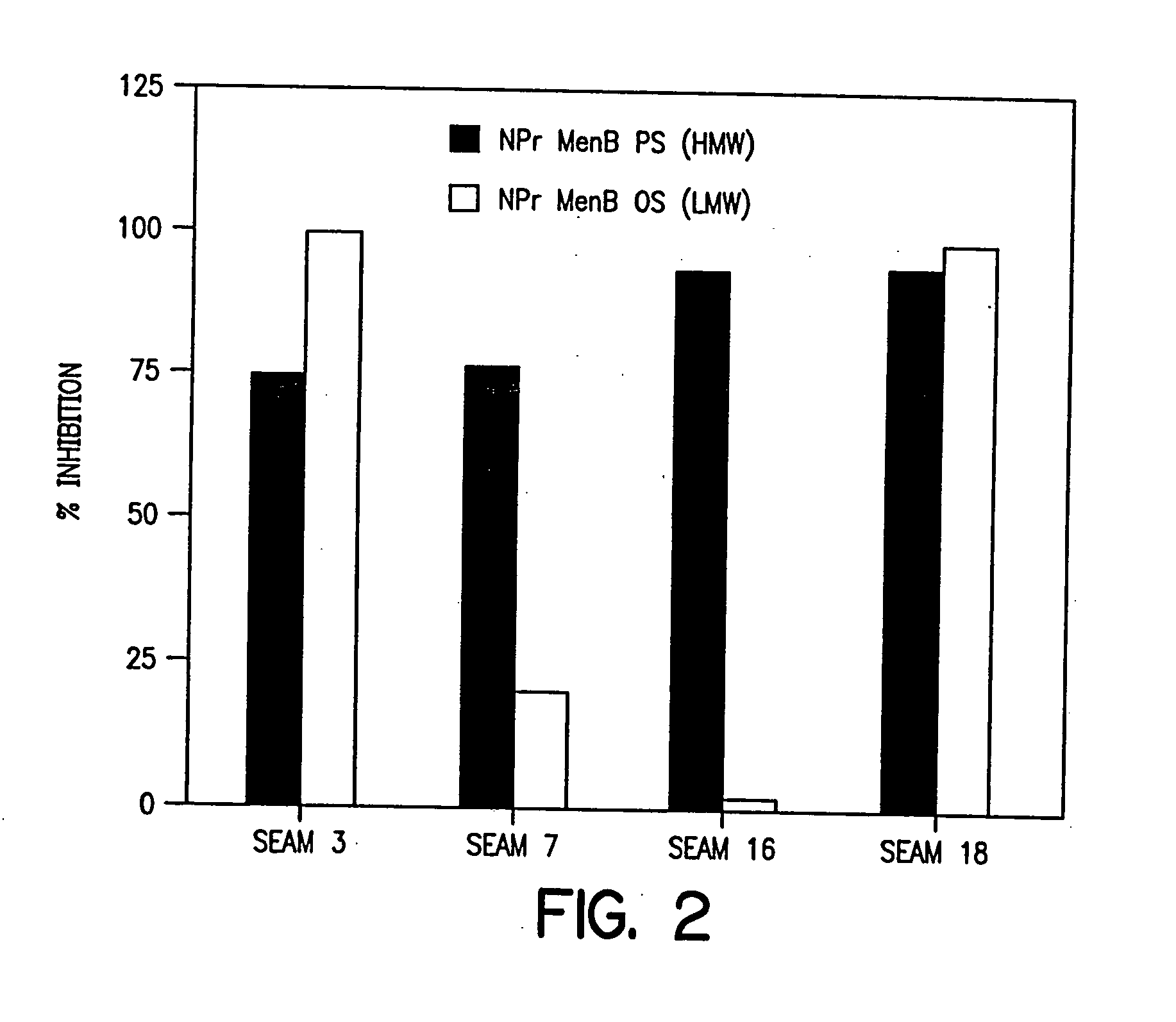
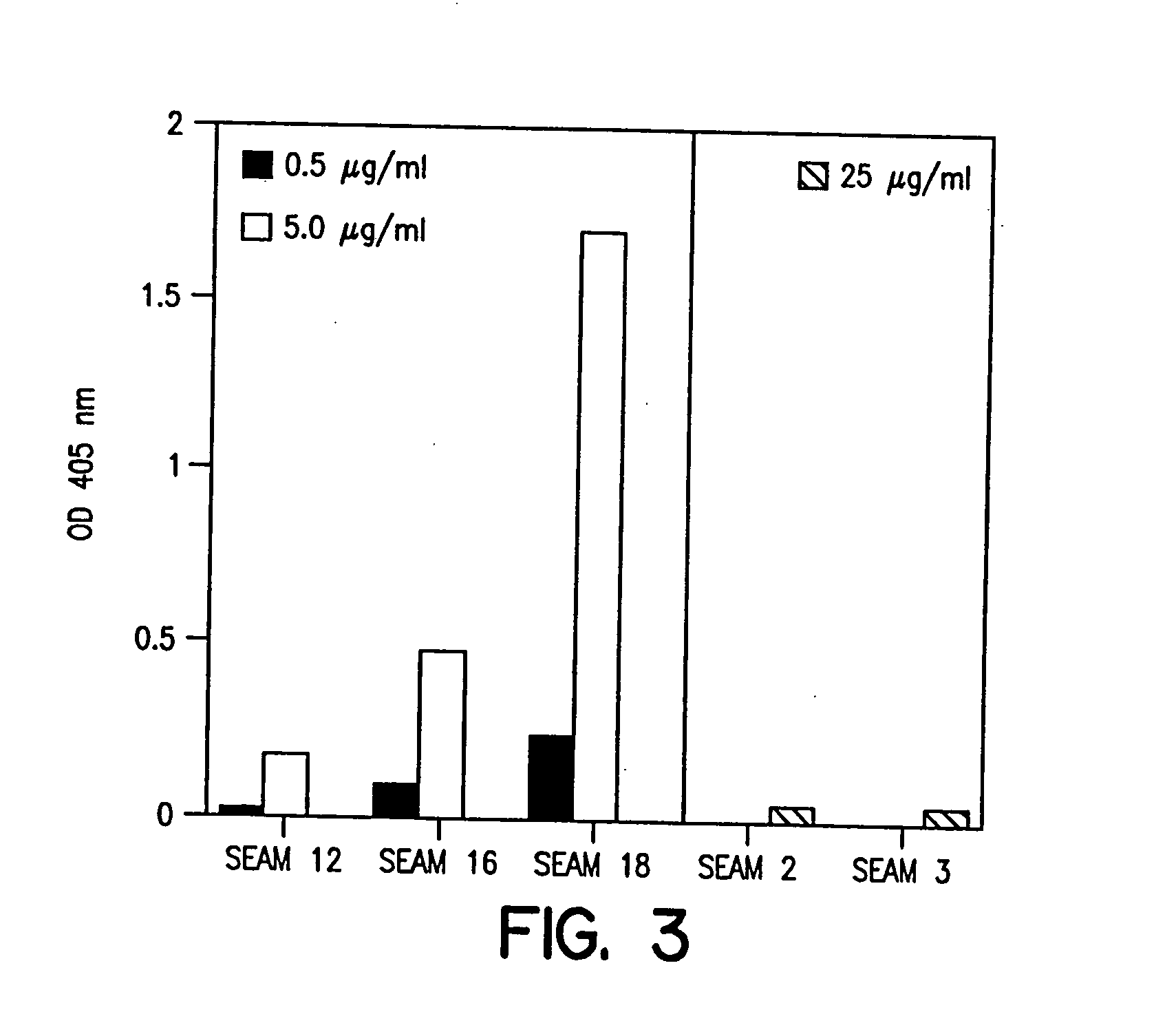
InactiveUS20060035284A1Determine autoreactivityLeast riskAntibacterial agentsAnimal cellsEscherichia coliSalmonella serotype typhi
Novel bactericidal antibodies against Neisseria meningitidis serogroup B (“MenB”) are disclosed. The antibodies either do not cross-react or minimally cross-react with host tissue polysialic acid and hence pose minimal risk of autoimmune activity. The antibodies are used to identify molecular mimetics of unique epitopes found on MenB or E. coli K1. Examples of such peptide mimetics are described that elicit serum antibody capable of activating complement-mediated bacteriolysis of MenB. Vaccine compositions containing such mimetics can be used to prevent MenB or E. coli K1 disease without the risk of evoking autoantibody.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Breaking immunological toterance with a genetically encoded unnatural amino acid
InactiveUS20090263376A1Improving immunogenicityStrong immune responseAntibacterial agentsNervous disorderDiseaseAntigenic variation
The present invention comprises methods and compositions for producing and / or enhancing an immunological response in a subject against a target moiety such as a disease-related moiety by administration of an antigenic version of the target moiety having one or more unnatural amino acid and / or by administration of an antibody against a version of a target moiety having one or more unnatural amino acid which antibody is cross reactive with the natural target moiety.
Owner:THE SCRIPPS RES INST
Fluorescent microsphere lateral chromatographic detection strip for multiple joint inspection of trace target substances as well as preparation method and application thereof
The invention discloses a fluorescent microsphere lateral chromatographic detection strip for multiple joint inspection of trace target substances as well as a preparation method and application thereof. The fluorescent microsphere lateral chromatographic detection strip is characterized by comprising a soleplate, wherein the top of the soleplate is sequentially provided with a sample absorption pad, a combination pad, a chromatographic film and a piece of water absorption paper from left end to right end, the right end of the sample absorption pad is pressed to be attached onto the left end of the combination pad, the right end of the combination pad is pressed to be attached onto the left end of the chromatographic film, the left end of the water absorption paper is pressed to be attached onto the right end of the chromatographic film to form a lateral chromatographic detection structure; the chromatographic film is provided with a detection line and a quality control line from left to right; the combination pad is formed by a polyester fiber film and fluorescent microspheres marked with different ligands on the polyester fiber film, and the chromatographic film consists of a nitrocellulose film and a plurality of detection lines which are arranged on the nitrocellulose film and contain different ligands which can be specifically combined with the target substances. The fluorescent microsphere lateral chromatographic detection strip can be used for performing multiple joint inspection on a plurality of trace target substances simultaneously, all display results have no cross reaction and are dependent from one another, the detection sensitivity and accuracy can be remarkably improved, rapidness and convenience in operation can be realized, and an important significance on biological detection and early diagnosis and treatment of diseases can be achieved.
Owner:HOSPITAL AFFILIATED TO GUANDONG MEDICAL COLLEGE
Amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains
PendingCN110184390ARapid identificationImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationTonsilAfrican swine fever
The invention provides an amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains. A P72 gene of ASFV and a 5'UTR noncoding region of CSFV arerespectively used as an amplification target area, a pair of specific primers and a TaqMan MGB probe (SEQ ID NO:1-6) are designed, a real-time fluorescent quantitation PCR(FQ-PCR) technique is used, and identification and detection of ASFV and CSFV are realized. The detection reagent kit provided by the invention is suitable for detecting viral nucleic acid in samples of serum, spleen, lymph nodes, tonsil, kidney and the like of suspected ASFV or CSFV infected pigs, the sensitivity can reach 1.0*10<1>copy / [mu]L, and the detection reagent kit does not have any cross reactions with other pathogens which are liable to be in mixed infection with the ASFV and the CSFV or of which the infection symptoms are similar such as PRRSV, PRV, PCV2, PPV, JEV and HPS.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Immunologic diagnosis kit for detecting type II dengue virus NS1 antigen
ActiveCN101226196AAccurate detectionQuick checkMaterial analysisAgainst vector-borne diseasesSerotypeElisa test
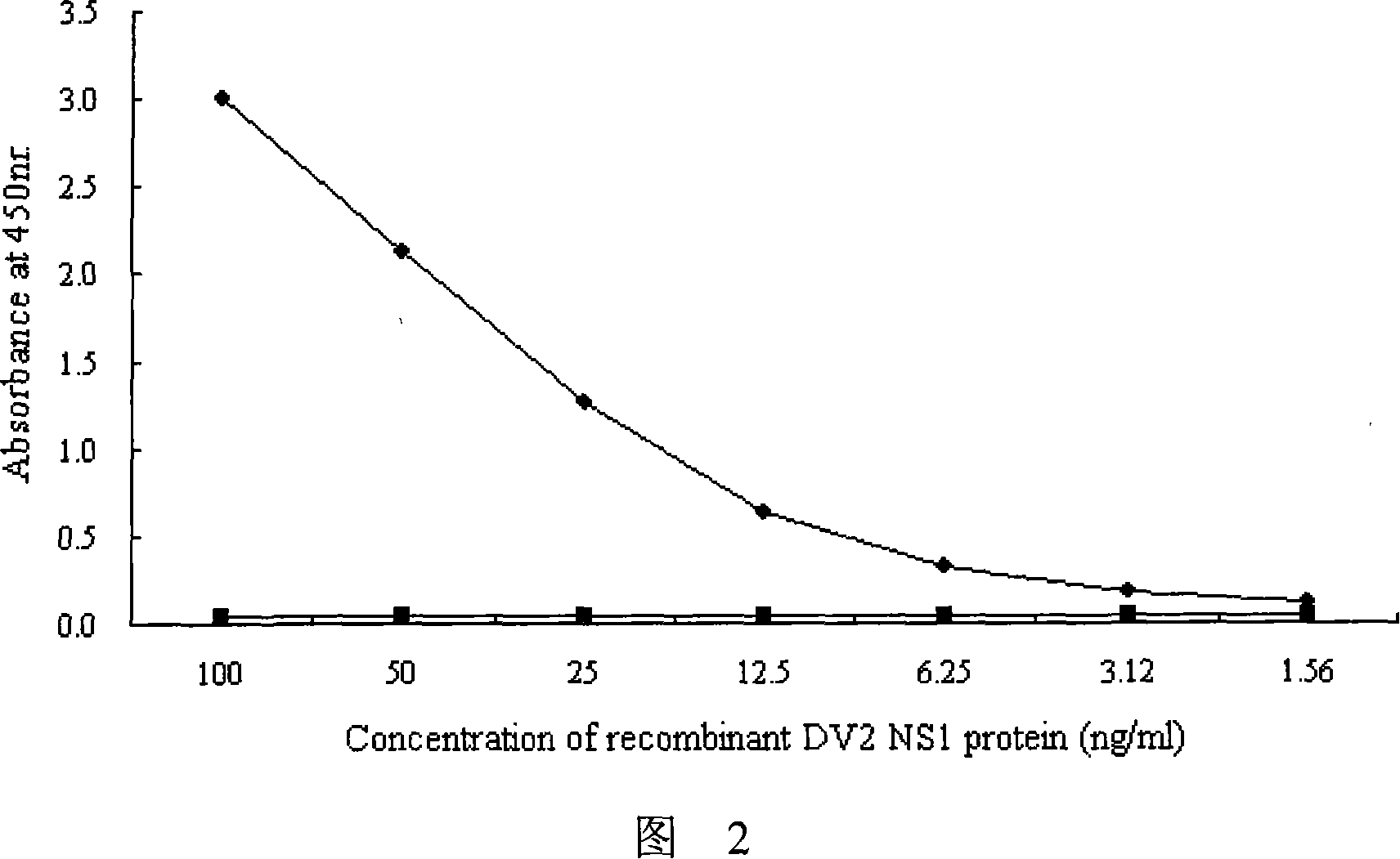
The invention provides an immunity diagnosis test kit for detecting II-type dengue virus antigen, which comprises a porous reaction plate covering monoclonal antibody DV2-M6, a sample treatment liquid, a monoclonal antibody DV2-M15 marked with a label, a positive contrast, a negative contrast, a concentration washing liquid, a develop liquid and a termination liquid, wherein the monoclonal antibodies DV2-M6 and DV2-M14 of the test kit can be specifically combined with NS1 protein of II-type dengue virus, without cross reaction with other three kinds of serotype dengue viruses NS1 and respectively combined with different antigen points of NS1, while the check sensitivity of NS1 protein of II-type dengue virus can reach 3ng / ml and the check sensitivity of culture supernatant of II-type dengue virus infection cell is 8 power of Pan-E dengue early elisa test kit, thereby improving the sensitivity of clinical serum sample check.
Owner:SOUTHERN MEDICAL UNIVERSITY
Lead molecule cross-reaction prediction and optimization system
InactiveUS20050170379A1Improve featuresFacilitate further optimizationCompound screeningApoptosis detectionBiopolymerRe engineering
A method for the prediction of adverse cross-reactions between lead candidate biomolecules and potential reactant molecules, often biopolymers, is described. In a computational system, reactions are modeled within a suitable environment, in order to determine a reaction profile between a lead candidate molecule and a potential reactant molecule. A risk assessment is then generated for each lead based on a plurality of reaction profiles for the lead with respect to a plurality of potential reactant molecules. The method includes provisions for redesign and optimization of the lead candidate, possibly iterative in nature, in order to avoid predicted adverse cross-reactions.
Owner:VERSEON INT CORP
Blocking ELISA kit for detecting NDV (Newcastle disease virus) antibody
ActiveCN106596933ASimple and fast operationEasy to operateBiological material analysisElisa kitPositive control
The invention discloses a blocking ELISA kit for detecting an NDV (Newcastle disease virus) antibody. The blocking ELISA kit for detecting the NDV antibody comprises an ELISA plate coated with an NDV inactivated antigen, an NDV positive control serum, an NDV negative control serum and a horseradish peroxidase labeled NDV NP protein monoclonal antibody, wherein the horseradish peroxidase labeled NDV NP protein monoclonal antibody is secreted by a hybridoma cell strain with the preservation number being CCTCC NO: C2016180. The blocking ELISA kit for detecting the NDV antibody can detect serum samples which are infected with the suspected NDV and are from different species, can distinguish an MG7-deficient vaccine from an NDV serum after being infected with a wild virus, and has no cross reaction with a common avian viral pathogen positive serum, thereby being high in sensitivity and specificity, good in reproducibility and suitable for high-throughput detection of serum samples.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
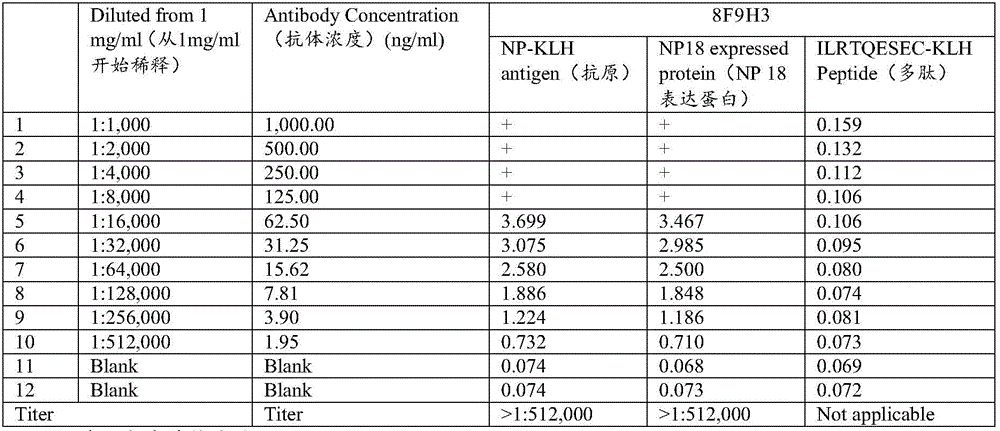
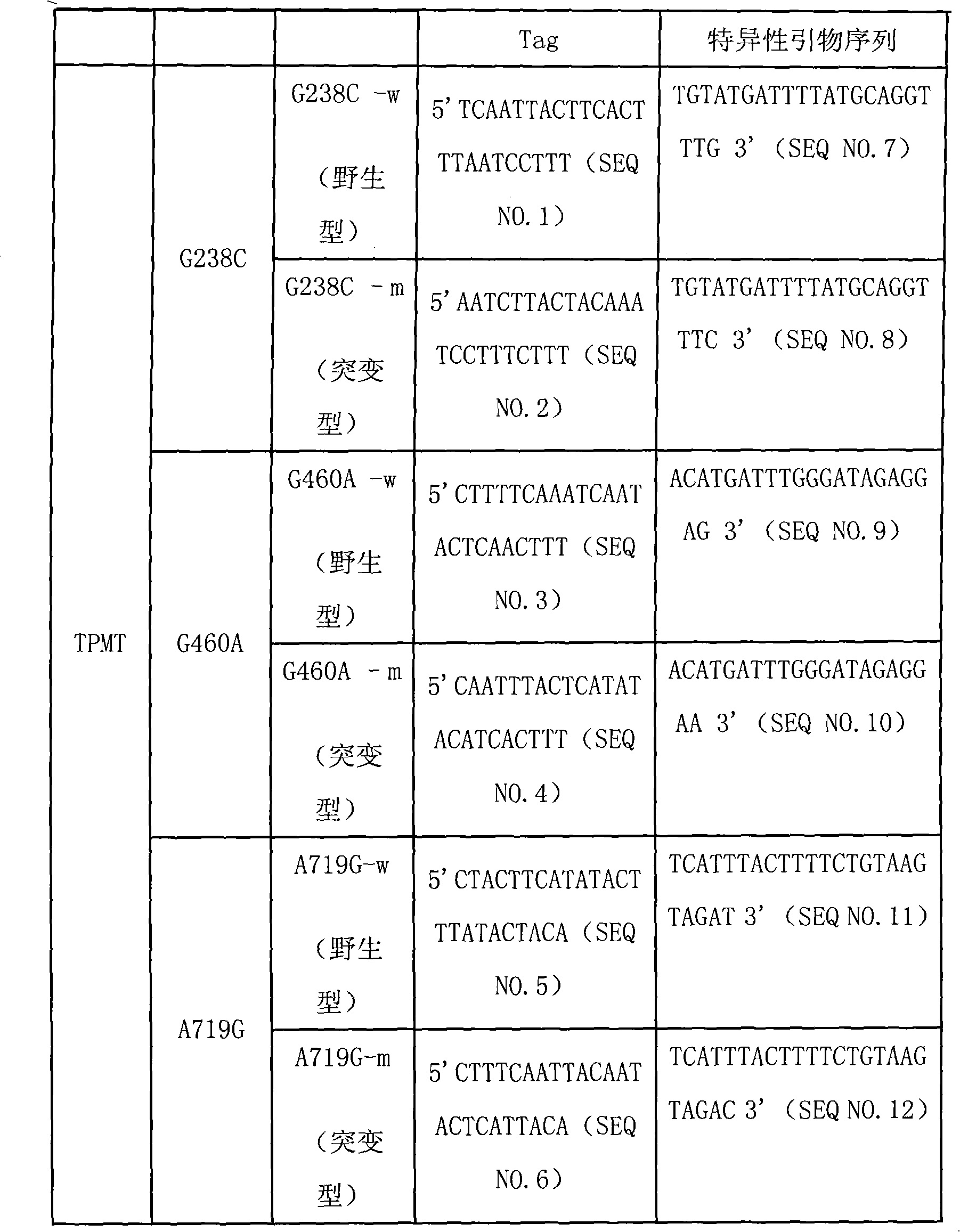
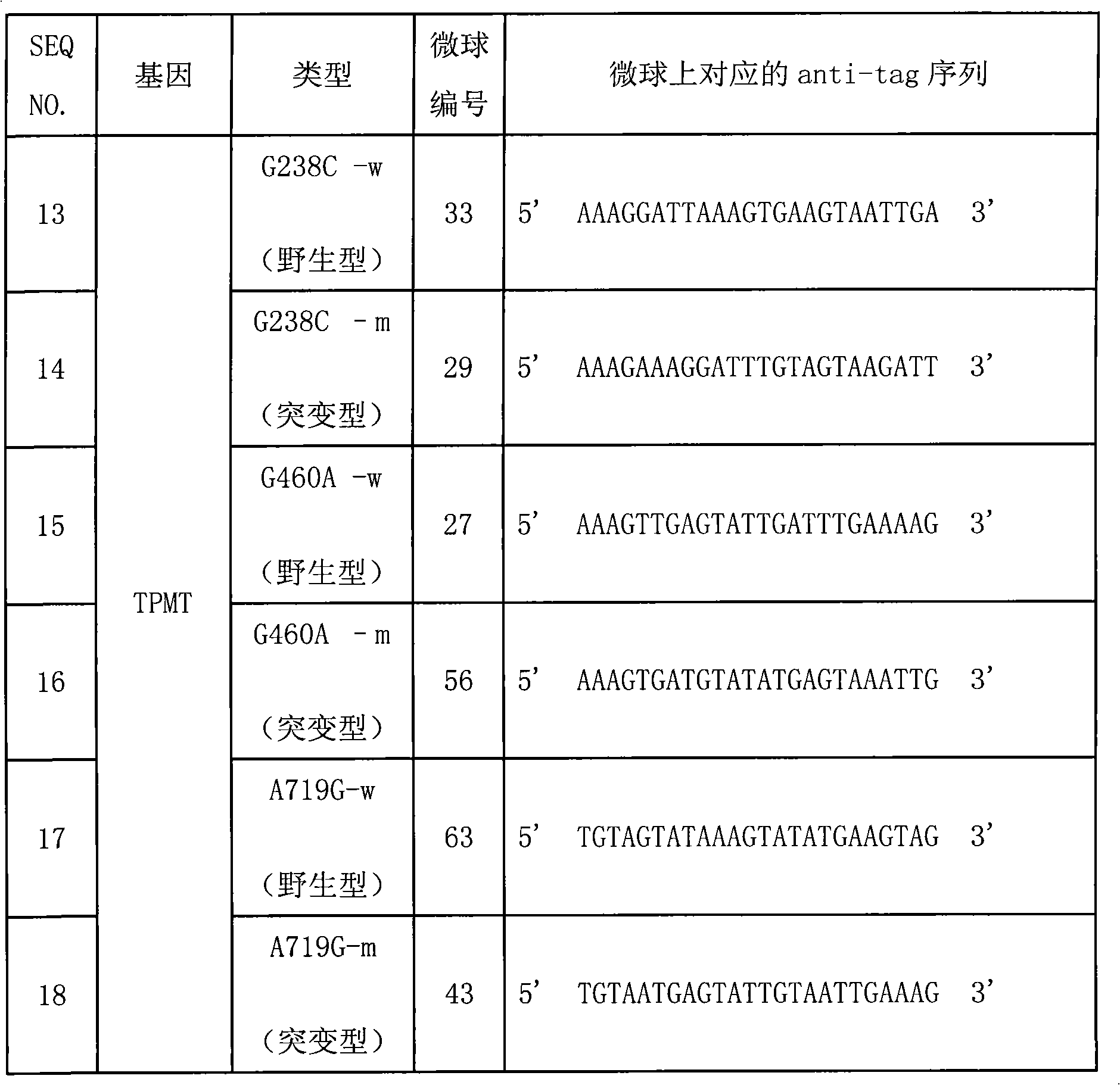
Specific sequence, liquid phase chip and method for SNP detection of TPMT gene
ActiveCN101671739AImprove signal-to-noise ratioNo cross-reactivityMicrobiological testing/measurementDNA/RNA fragmentationSignal-to-noise ratio (imaging)Type specific
The invention discloses a liquid phase chip, a specific prime and a method for the SNP detection of TPMT gene, the liquid phase chip comprises wild-type and mutant-type specific ASPE primer pairs designed respectively in accordance with mutant sites of each type, microspheres respectively enveloped with specific anti-tag sequences, and primers for amplifying target sequences having SNP sites of TMPT gene G238C, G460A and / or A719G. The prepared liquid phase chip for the SNP detection of TPMT gene has quite excellent signal-to-noise ratio and, basically, no cross reaction is present between thedesigned probe and the anti-tag sequence. The ASPE primers designed according to the invention has quite excellent specificity and can accurate distinguish the mutant sites of each type. The detectionmethod has simple steps and convenient operation, can complete the detection of 3 types of mutant sites in one step, and can avoid plenty of uncertain factors existing in the process of repeated operation, therefore, the accuracy of the detection can be enhanced enormously.
Owner:广州益善医学检验所有限公司
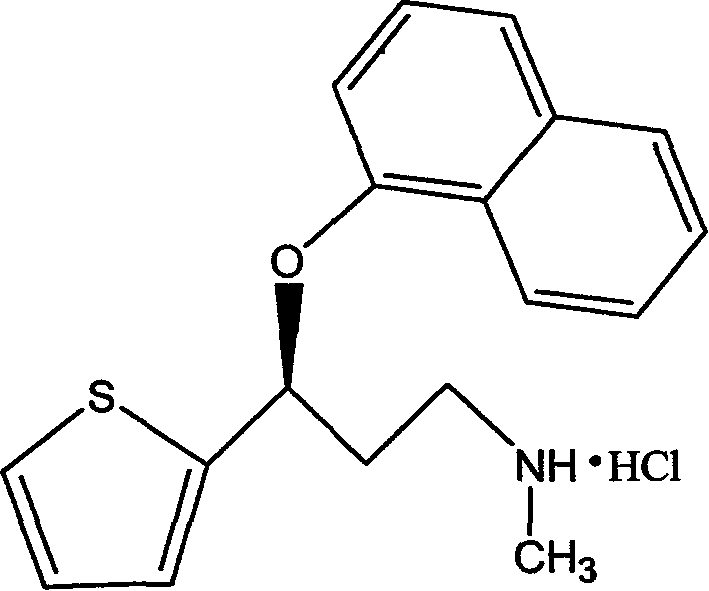
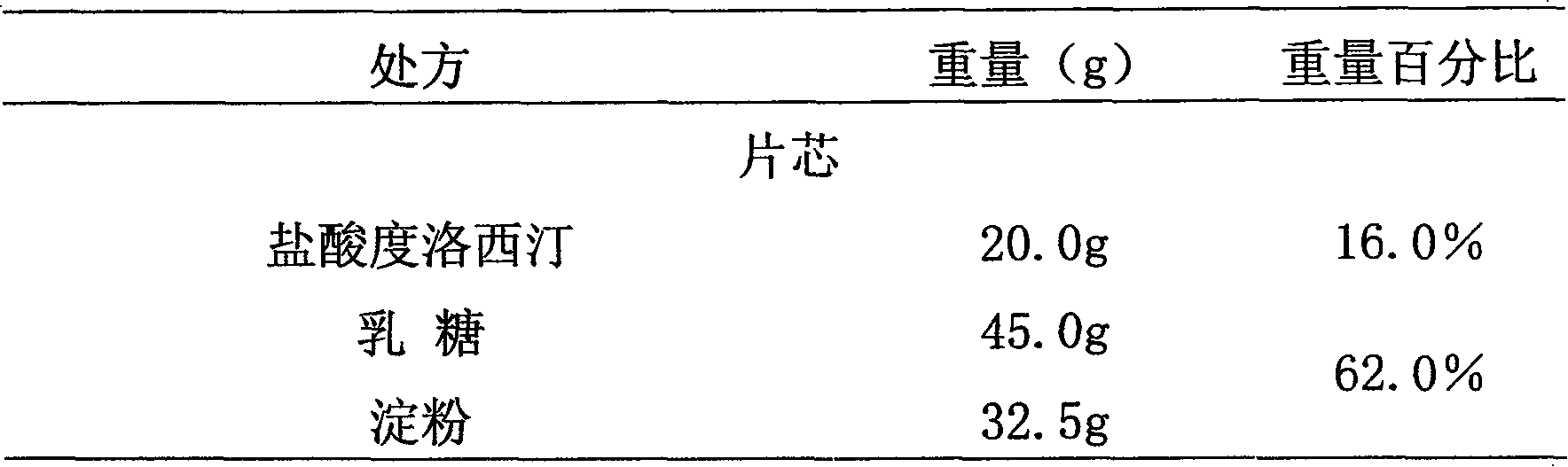
Medicinal preparations containing duloxetine hydrochloride and preparation method thereof
ActiveCN101190208AImpact releaseImprove toleranceOrganic active ingredientsNervous disorderIsolation layerPharmaceutical formulation
The invention relates to an enteric-coated tablet containing duloxetine hydrochloride and a preparation method thereof. The enteric-coated tablet of duloxetine hydrochloride consists of three parts: a tablet core, a gastric-coated isolation layer and an enteric-coated layer. The technical proposal of the invention can effectively avoid the cross reaction between the medicine and enteric coating material occurring during the releasing process of the medicine and affecting the release of the medicine; therefore, the stability of the medicine can be effectively improved. The preparation method is simple and is easy to be operated, which is suitable for industrialized production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
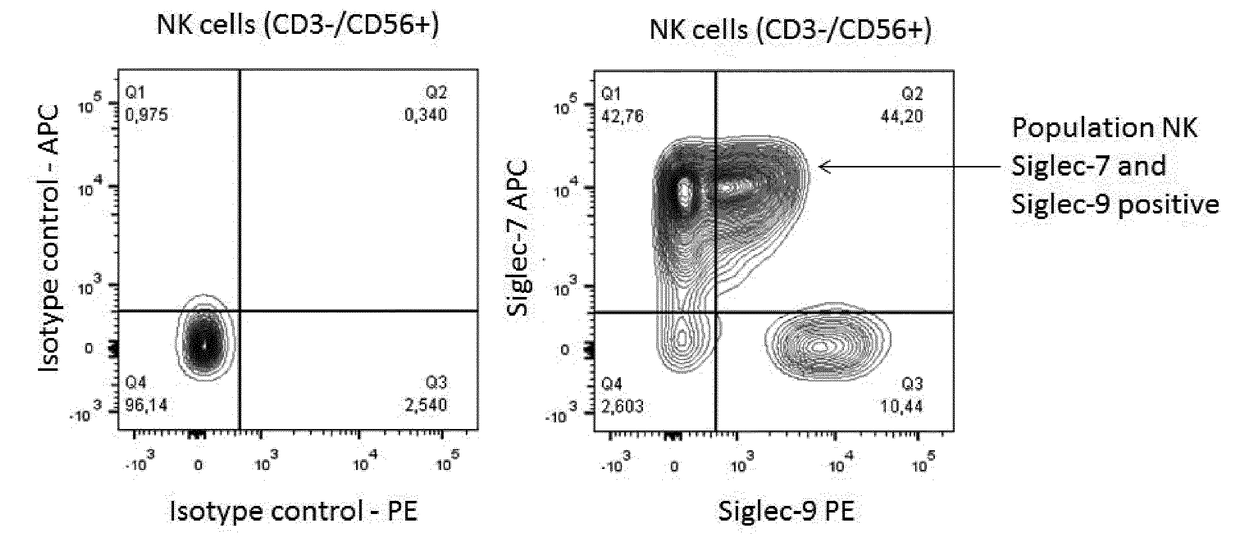
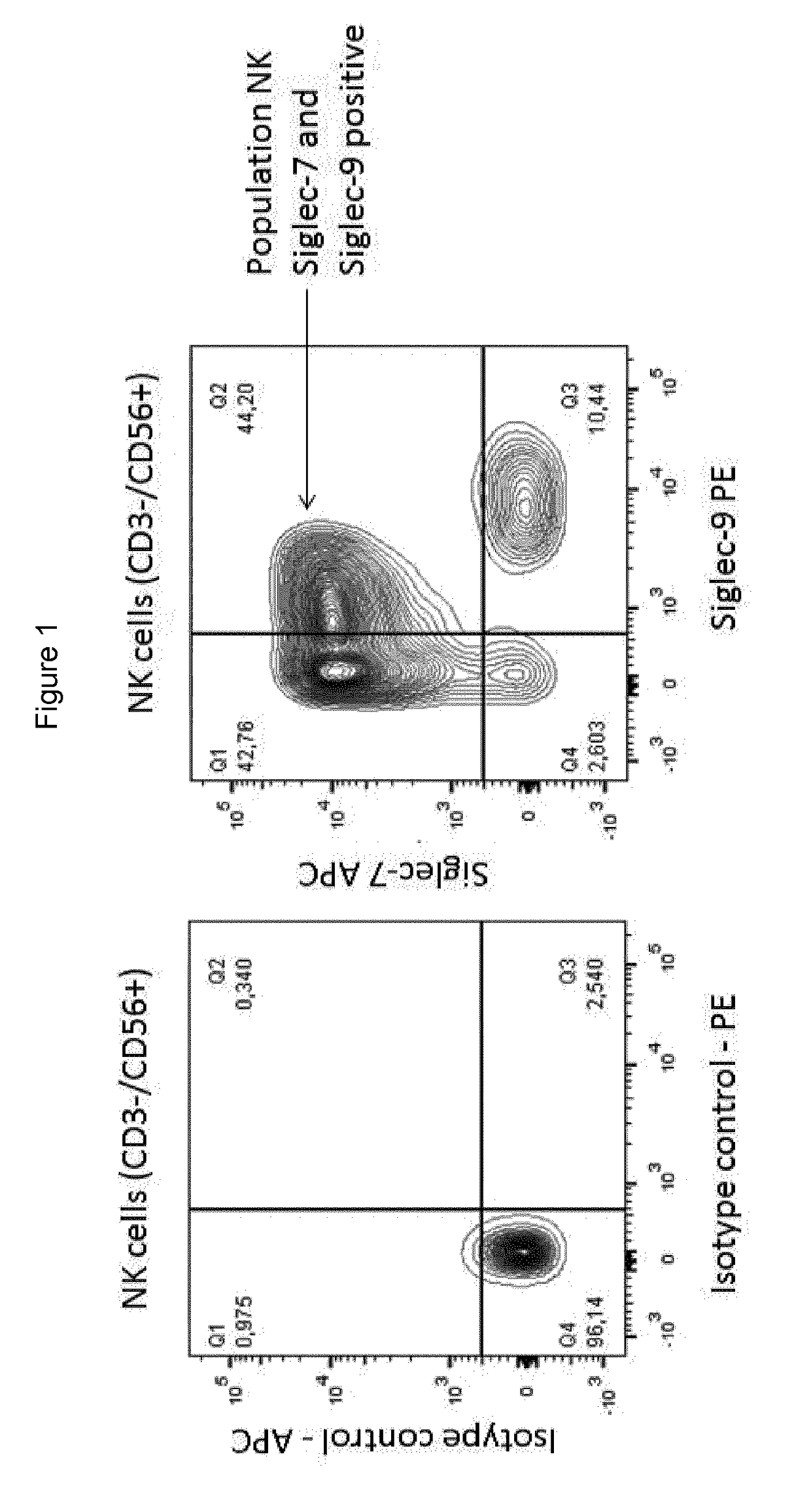
Cross reactive siglec antibodies
InactiveUS20170306014A1Improve abilitiesMaximally effectiveImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsLymphocyteSIGLEC
Owner:INNATE PHARMA SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com