System for inhibiting pathological target cells in space-time adjustable manner
A target cell, pathological technology, applied in the field of tumor immunology, can solve the problem that the toxic effect cannot be ignored
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] Conventional methods for preparing "chimeric antigen receptor immune effector cells" are known to those skilled in the art, including allowing them to express the intracellular domains of intracellular co-stimulatory cell molecules, such as CD28 (preferably including CD28a, CD28b) , CD137, CD27, CD3ζ (preferably CD3ζ intracellular domain), CD8, CD19, CD134, CD20, one or more of FcRγ. Through their combination with corresponding ligands, the second signal of immune effector cells is activated, the proliferation ability of immune cells and the secretion function of cytokines are enhanced, and the survival time of activated immune cells is prolonged.
[0061] In the present invention, the pathological target cells may be various harmful cells in the body that are not conducive to health and must be removed from the body. The pathological target cells include tumor cells. Any tumor known in the art can be included in the present invention, as long as the tumor can express ...
Embodiment 1
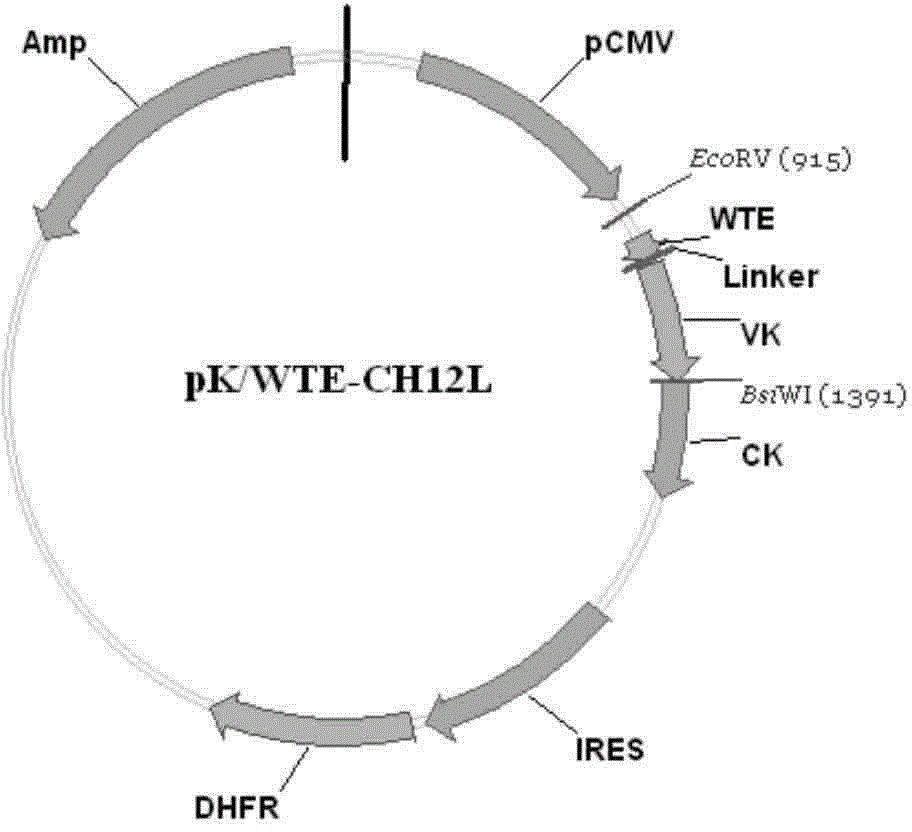
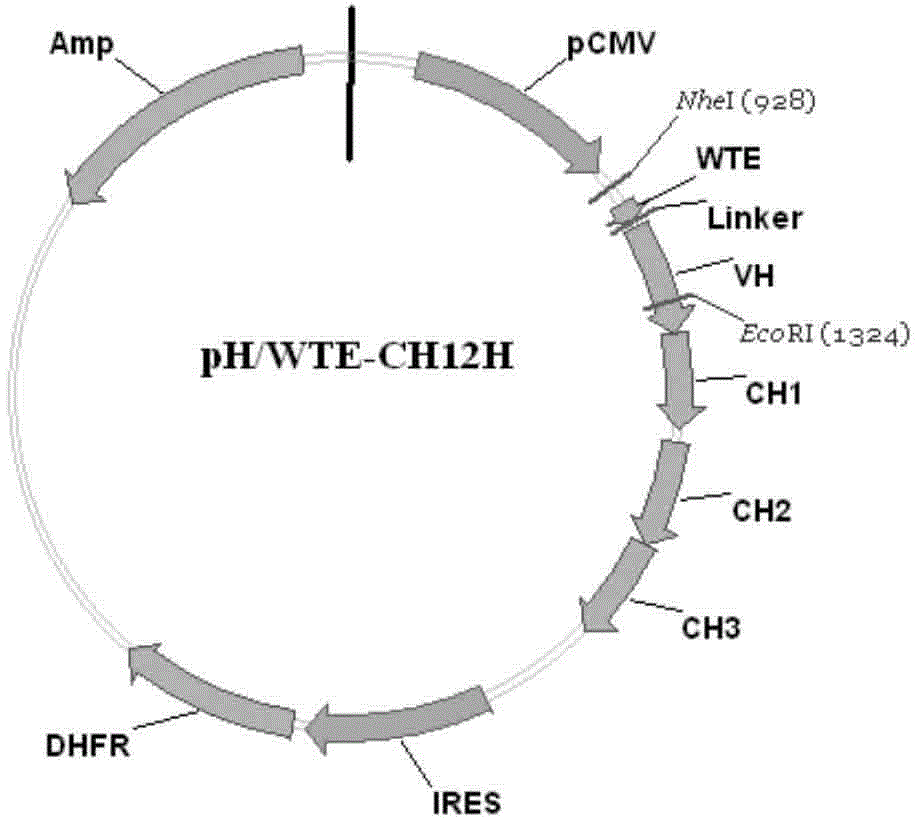
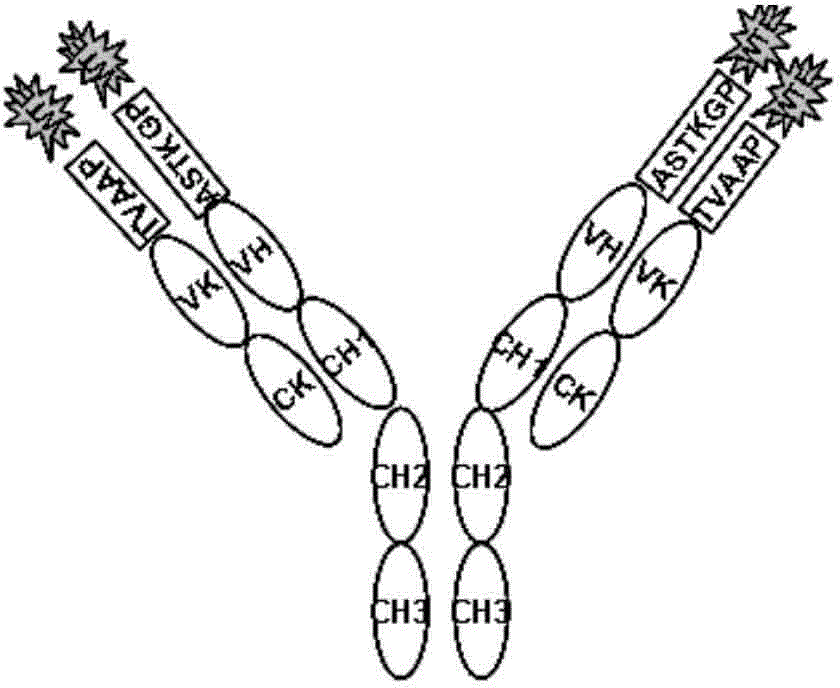
[0071] Example 1. Construction of anti-human EGFRVIIIWTE-CH12 antibody recombinant plasmid in the present invention
[0072] 1. Amplification of nucleic acid fragments
[0073] (1) Using the antibody pH / CH12 as a template (see SEQ ID NO: 36 for the sequence). The upstream primer 5'-gatgtgcagcttcaggagtcggg-3' (SEQ ID NO: 1) and the downstream primer 5'-acaataatatgtggctgtgtcc-3' (SEQ ID NO: 2) PCR amplified the CH12VH fragment, and the PCR amplification conditions were pre-denaturation: 94°C, 4min; denaturation: 94°C, 40s; annealing: 58°C, 40s; extension: 68°C, 40s; 27 cycles, and then a total extension of 68°C, 10min. The size of the amplified product was 288bp, which was in line with the expectation.
[0074] (2) Amplification of the heavy chain signal peptide-WTE fragment, the primers are as follows:
[0075] 5'-cctagctagccaccatgagagtgctgattcttttgtggctgttcacagcctttcct-3' (SEQ ID NO: 3),
[0076] 5'-agctgtggagccagacaggaaaccaggaaaggctgtgaacagccac-3' (SEQ ID NO: 4),
[0077] ...
Embodiment 2
[0101] Example 2, Expression and purification of anti-human EGFRvIIIWTE-CH12 antibody
[0102] 1. Expression of anti-human EGFRvIIIWTE-CH12 antibody
[0103] Free-Style293-F cells (purchased from Invitrogen) were used for antibody expression, and the suspension culture and transfection methods were in accordance with FreeStyle TM 293ExpressionSystem manual operation. Specifically, the cell density was adjusted to 1×10 before transfection. 6 Cells / mL, blow off and make the cells free of clumps, and use trypan blue staining to determine the cell viability > 95%. Transfection step: Dilute 52μgpH / WTE-CH12H and 48μgpK / WTE-CH12K (molar ratio 1:1) recombinant plasmids and 200μL Free-Style293-F cell liposome transfection reagent "293fectin" to 3.33mL with Opti-MEM After standing still for 5 minutes, the plasmid and transfection reagent were slowly mixed, and reacted at room temperature for 20 minutes to form a DNA-fectin mixture, and then added 93.3mL Free-Style293-F cells (density...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com