Modified hematopoietic stem/progenitor and non-t effector cells, and uses thereof
a technology of which is applied in the field of modified hematopoietic stem/progenitor and non-t effector cells, can solve the problems of lethal delay in treatment, and achieve the effect of removing delays and expense in treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
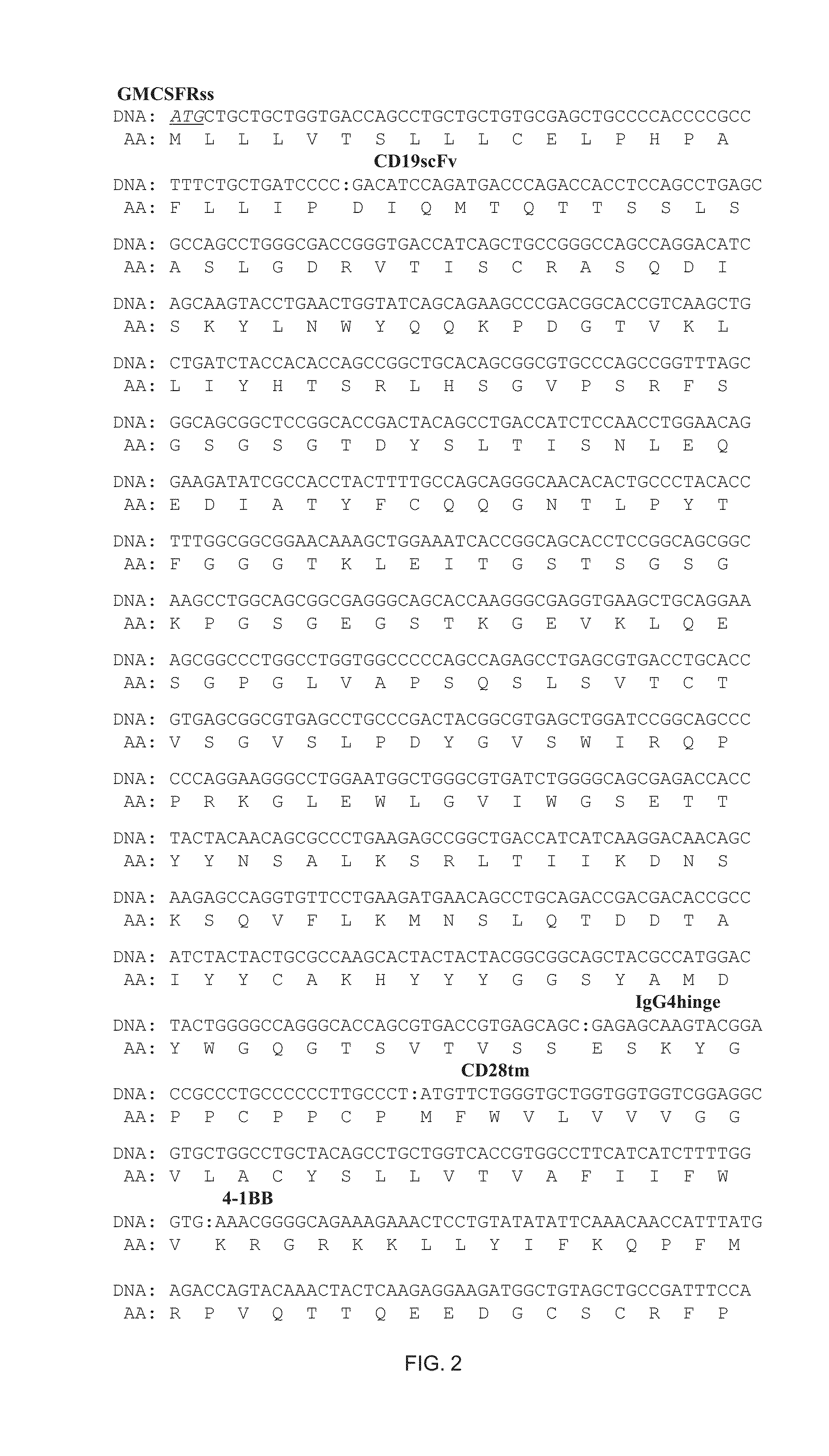
2. A HSPC of embodiment 1 wherein the ligand binding domain is a single chain Fv fragment (scFv) including a CDRL1 sequence of RASQDISKYLN (SEQ ID NO. 108), a CDRL2 sequence of SRLHSGV (SEQ ID NO. 111), a CDRL3 sequence of GNTLPYTFG (SEQ ID NO. 104), a CDRH1 sequence of DYGVS (SEQ ID NO. 103), a CDRH2 sequence of VTWGSETTYYNSALKS (SEQ ID NO. 114), and a CDRH3 sequence of YAMDYWG (SEQ ID NO. 115).
3. A HSPC of embodiments 1 or 2 wherein the spacer region is 12 amino acids or less.
4. A HSPC of any one of embodiments 1-3 wherein the spacer region includes SEQ ID NO: 47.
5. A non-T effector cell genetically modified to express (i) an extracellular component including a ligand binding domain that binds CD19; (ii) an intracellular component including an effector domain including a cytoplasmic domain of CD28 or 4-1BB; (iii) a spacer region including a hinge region of human IgG4; and (iv) a human CD4 or CD28 transmembrane domain.
embodiment 5
6. A non-T effector cell of embodiment 5 wherein the ligand binding domain is a single chain Fv fragment (scFv) including a CDRL1 sequence of RASQDISKYLN (SEQ ID NO. 108), a CDRL2 sequence of SRLHSGV (SEQ ID NO. 111), a CDRL3 sequence of GNTLPYTFG (SEQ ID NO. 104), a CDRH1 sequence of DYGVS (SEQ ID NO. 103), a CDRH2 sequence of VTWGSETTYYNSALKS (SEQ ID NO. 114), and a CDRH3 sequence of YAMDYWG (SEQ ID NO. 115).
7. A non-T effector cell of embodiments 5 or 6 wherein the spacer region is 12 amino acids or less.
8. A non-T effector cell of any one of embodiments 5-7 wherein the spacer region includes SEQ ID NO: 47.
9. A non-T effector cell of any one of embodiments 5-8 wherein the non-T effector cell is a natural killer cell.
10. A hematopoietic stem progenitor cell (HSPC) genetically modified to express a chimeric antigen receptor (CAR) of SEQ ID NO: 34, 53, 54, 55, 56, 57, or 58.
embodiment 10
11. A HSPC of embodiment 10 wherein the HSPC is CD34+.
12. A non-T effector cell genetically modified to express a CAR of SEQ ID NO: 34, 53, 54, 55, 56, 57, or 58.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com